Abstract
Stroke represents the leading cause of disability and mortality amongst the elderly worldwide. Multiple risk factors, including both genetic and non-genetic components, as well as their interactions, are proposed as etiological factors involved in the development of ischemic stroke (IS). Promoter polymorphisms of the IL-6-174G/C (rs1800795) and TNF-α-308G/A (rs1800629) genes have been considered as predictive risk factors of IS; however, these have not yet been evaluated in a Thai population. The aims of this study were to investigate the association of IL-6-174G/C and TNF-α-308G/A polymorphisms with IS. Genomic DNA from 200 patients with IS and 200 controls were genotyped for IL-6-174G/C and TNF-α-308G/A polymorphisms using TaqMan™ SNP genotyping and quantitative PCR-high resolution melting analysis, respectively. It was found that the TNF-α-308 A allele was significantly associated with an increased risk of IS development compared with the G allele [odds ratio (OR)=2.044; 95% CI=1.154-3.620; P=0.014]. Moreover, the IS risk was significantly higher in the presence of TNF-α-308 GA or AA genotypes compared with that in the presence of GG genotypes with a dominant inheritance (OR=1.971; 95% CI=1.080-3.599; P=0.027). However, there was no association between IL-6-174G/C and the risk of IS development. The interaction study demonstrated that IL-6-174 GG and TNF-α-308 GG genotypes enhanced IS susceptibility when combined with hypertension, hyperlipidemia and alcohol consumption. Hypertensive and hyperlipidemic subjects with the TNF-α-308 GA and AA genotypes were more likely to develop IS compared with those who did not have these two conditions and had the GG genotype. In a matched study design (1:1), the IL-6-174 GC genotype was associated with higher IL-6 levels in the control group. Collectively, the present results highlight the utility of the TNF-α-308G/A polymorphism as a predictive genetic risk factor for development of IS.
Keywords: polymorphism, inflammation, cytokine, ischemic stroke
Introduction
Stroke is a significant cause of disability and mortality in the elderly (≥65 years) worldwide (1). Moreover, stroke incidence and mortality rates remain high in young adults (18-50 years) in developing and developed countries alike (2,3). In Thailand, the incidence of stroke-associated mortality per 100,000 individuals has continued to increase, reaching 53.0% in 2019, up from 47.1% in 2017 and 47.8% in 2018(4). Ischemic stroke (IS), involving the occlusion of a major cerebral artery or its branches in the brain, is the primary type of stroke globally (3). The development of stroke is affected by the interaction between multiple risk factors, involving both genetic and non-genetic factors (5,6). Chronic inflammatory diseases and unhealthy lifestyle habits are the most common risk factors associated with stroke (6). The inflammatory component has been considered as one of the possible etiological factors in the early stages of IS development, thereby promoting the formation of a cerebral thrombus in association with the endothelium and coagulation (7). After cerebral ischemia, a robust inflammatory response is triggered by ischemic neuronal cell death and the release of necrotic cell debris, which induces the production of inflammatory cytokines and chemokines from microglia and macrophage cells (8-10). A dysregulated immune response, accompanied by an increased number of inflammatory cells, high TNF-α production and the activation of microglia cells has been shown to enhance neuroinflammation and exacerbate ischemic brain injury in patients with IS and a mouse model of cerebral ischemia (11). Inflammatory cytokines and chemokines have been used as predictors of stroke incidence and outcomes (12,13). Thus, the genetic variation of inflammatory cytokine genes has become a critical issue in research relating to the predictive risk factors of IS occurrence (14,15), as well as its severity and outcome (16), and this provides potential therapeutic options for the treatment of inflammatory diseases (17,18).
IL-6 and TNF-α are potent pro-inflammatory cytokines that serve vital roles in the immune response during inflammation (19). IL-6 levels have been suggested as a potential biomarker that is associated with stroke outcomes (20), short-term acute ischemic stroke death (21) and post-stroke dementia (22). It has also been shown that the overexpression of TNF-α transcripts is an independent risk factor for the incidence of IS (13). Furthermore, high TNF-α levels post-stroke are associated with poor patient outcomes (23).
Single-nucleotide polymorphisms (SNPs) are the most commonly studied genetic variations for IS susceptibility (24), and functional SNPs located in a gene's promoter region regulate that gene's transcriptional expression (25,26). A meta-analysis revealed that the IL-6-174G/C polymorphism was significantly associated with IS risk in Asian populations in both dominant and recessive inheritance models (14). However, other studies reported that the IL-6-174G/C polymorphism was not associated with the risk of IS in North Indian (16,27) and Chinese populations (28). Moreover, no association of the IL-6-174G/C polymorphism was observed with the expression levels of IL-6 in the plasma of patients with IS and controls (29). With regard to TNF-α gene, it was found that the TNF-α-308G/A polymorphism was associated with a risk of IS development (15) and hypertension (30) in an Asian population. In addition, TNF-α-308 GA genotypes may be protective factors for IS occurrence in East Asian populations (31,32).
However, there remains considerable controversy surrounding the two SNPs, IL-6-174G/C and TNF-α-308G/A, which are located in the promoter region of the gene (14-16,32). These may be predictive risk factors of IS development and modify the gene's transcriptional activities, thereby contributing to changes in the gene's expression levels. Presumably, the association between these two SNPs and IS risk may be attributed to different ethnicities, environmental influences and experimental design. To the best of our knowledge, there have been no reports on the association of IL-6-174G/C and TNF-α-308G/A polymorphisms with the risk of IS and the expression levels of IL-6 and TNF-α in Thailand. Therefore, the present study aimed to investigate the association between IL-6-174G/C and TNF-α-308G/A polymorphisms with IS susceptibility, their interactions with clinical variables and the expression levels of IL-6 and TNF-α in a southern Thai population.
Materials and methods
Sample size calculation
A sample size calculation was performed using the freely available G*Power version 3.1.9.2 application power analysis (33). To achieve a power of 95% at an α of 0.05, the sample size was calculated based on the previous reports (14,15). The estimated sample sizes were 367 and 420 subjects for the IL-6 and TNF-α genotypes, respectively.
Study participants
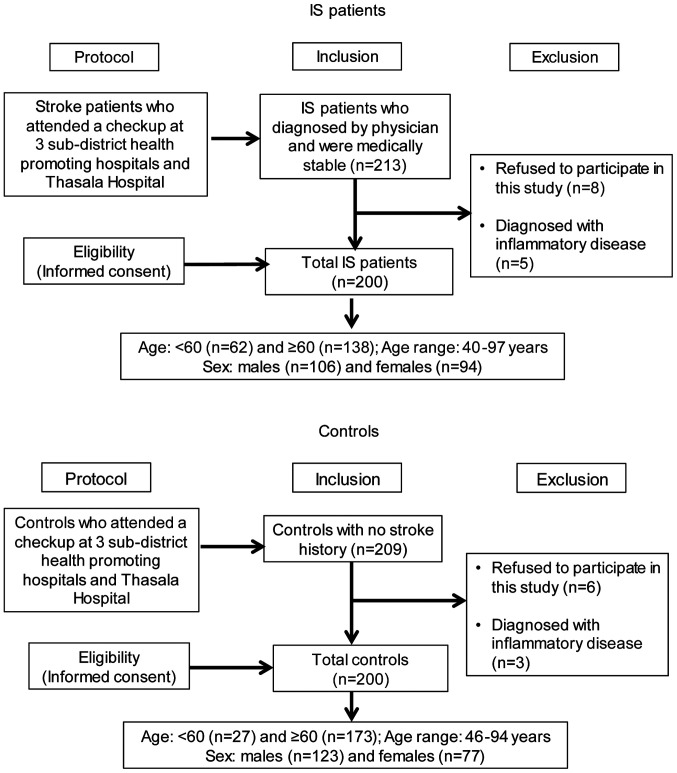
A total of 422 blood samples of Thai-Buddhist origin were collected from Thungchon, Bansakha and Thaiburi sub-district health promoting hospitals and the Thasala Hospital (Thasala, Nakhon Si Thammarat, Thailand) between November 2019 and August 2020. Patients with IS were recruited based on the physician's diagnosis, assessed using clinical symptoms, computed tomography scans and/or magnetic resonance imaging. Patients were all medically stable and received the same class of drug regimens. The controls were individuals from the same demographic who attended a checkup, but had no history of stroke. Clinical information, including age, sex, blood pressure, BMI, biochemical parameters (blood sugar and lipid profile), smoking habit and alcohol consumption, were collected from reviews of medical records. Subjects were excluded if they had a history of inflammatory diseases, acute heart failure, myocardial infarction (MI), hepatitis, cirrhosis, chronic renal failure and dialysis, autoimmune diseases, immune-suppressive therapy or cancer. Amongst the 422 subjects, 22 subjects did not fulfill the inclusion criteria (Fig. 1). Finally, a total of 400 blood samples were obtained for genotyping analyses. Samples were collected from 200 patients with IS (106 males and 94 females; age range 40-97, median age 67 years) and 200 controls (123 males and 77 females; age range 46-94, median age 66 years). The research protocols were performed in accordance with the Declaration of Helsinki guidelines (34) and approved by the Human Research Ethics Committee of Walailak University (approval no. WUEC-19-189-01). Informed consent was acquired from each participant in this study.
Figure 1.
Flow diagram defining the study participants with ischemic stroke and the controls.
Hypertension was defined as a resting systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, or a requirement of antihypertensive drugs (35). Hyperlipidemia was defined as a total cholesterol level of ≥240 mg/dl or a history of taking antihyperlipidemic drugs (36). Diabetes mellitus was defined as a fasting plasma glucose level ≥126 mg/dl or current use of glycemic control medication (37). Moreover, subjects that possessed ≥3 of the following five risk factors were diagnosed with metabolic syndrome (38,39): BMI ≥27.0 kg/m2 in men and ≥25.0 kg/m2 in women; triglycerides of ≥150 mg/dl; high-density lipoprotein cholesterol <40 mg/dl in men or <50 mg/dl in women; blood pressure ≥130/85 mmHg or patients currently taking hypertensive medication; and fasting plasma glucose levels of ≥100 mg/dl or patients currently taking insulin or hypoglycemic medication. Smoking status referred to subjects that were current smokers only. Subjects were considered alcohol drinkers if they consumed ≥100 ml alcohol >3 times per week.
IL-6 and TNF-α genotyping
Ethylenediamine tetra-acetic acid blood samples of 3-5 ml from the patients with IS and controls were collected only once at admission. Genomic DNA was extracted and purified using a genomic DNA Mini kit (Geneaid Biotech, Ltd.). Then, DNA concentration and purity were determined using a NanoDrop spectrophotometer (NanoDrop OneC; Thermo Fisher Scientific, Inc.) and stored at -20˚C until required for further analysis. IL-6-174G/C (rs1800795) genotyping was performed using a TaqMan™ SNP Genotyping assay, available with probe and primers (assay ID: C_1839697_20; cat. no. 4351379; Thermo Fisher Scientific, Inc.). Genotyping reactions were performed in a 10-µl reaction volume and the amplification was conducted according to the manufacturer's instructions as follows: Polymerase activation at 95˚C for 10 min, followed by 50 cycles of denaturation at 95˚C for 15 sec and annealing/extension at 60˚C for 1 min using a StepOnePlus™ Real-Time PCR system (Thermo Fisher Scientific, Inc.). Data were analyzed using StepOnePlus™ software (version 2.2.2; Thermo Fisher Scientific, Inc.).
For TNF-α-308G/A (rs1800629) genotyping, the primers were designed using the Primer3 and BLAST software (ncbi.nlm.nih.gov/tools/primer-blast) from the National Center for Biotechnology Information. The primer sequences were: Forward, 5'-CTGGTCCCCAAAAGAAATGGAG-3' and reverse, 5'-CTGGGCCACTGACTGATTTGTG-3' (product size, 96 bp). The total volume of the PCR reaction was 20 µl, which was composed of 1X HOT FIREPol® EvaGreen® HRM mix (ROX) (cat. no. 08-33-00001; Solis BioDyne), 250 nM forward and reverse primers and 20 ng DNA samples. A quantitative-(q)PCR was set up in a 96-well PCR plate and processed on a Quanstudio™ 5 Real-Time PCR system (Thermo Fisher Scientific, Inc.) with the following thermocycling conditions: Initial denaturation at 95˚C for 15 min, followed by 40 cycles of denaturation at 95˚C for 15 sec, annealing at 60˚C for 20 sec and elongation at 72˚C for 20 sec. The melt curve protocol was set as follows: Denaturation at 95˚C for 15 sec, reannealing at 60˚C for 20 sec and melting from 60˚C to 95˚C with a ramp rate of 0.05˚C/sec. Data were analyzed via high resolution melting (HRM) curve analysis (version 3.1; Thermo Fisher Scientific, Inc.). In total, 184 DNA samples were randomly analyzed for the first round of examination using HRM software. Among them, three different patterns of different plots were observed, and the samples were selected for DNA sequencing. The DNA sequencing results revealed 100% concordance with the results of the HRM analyses. The three different genotypes obtained from the first round were submitted as genotype controls and included in each plate. The qPCR and HRM analyses were reanalyzed for the first round of 184 samples and performed for all DNA samples.
DNA sequencing
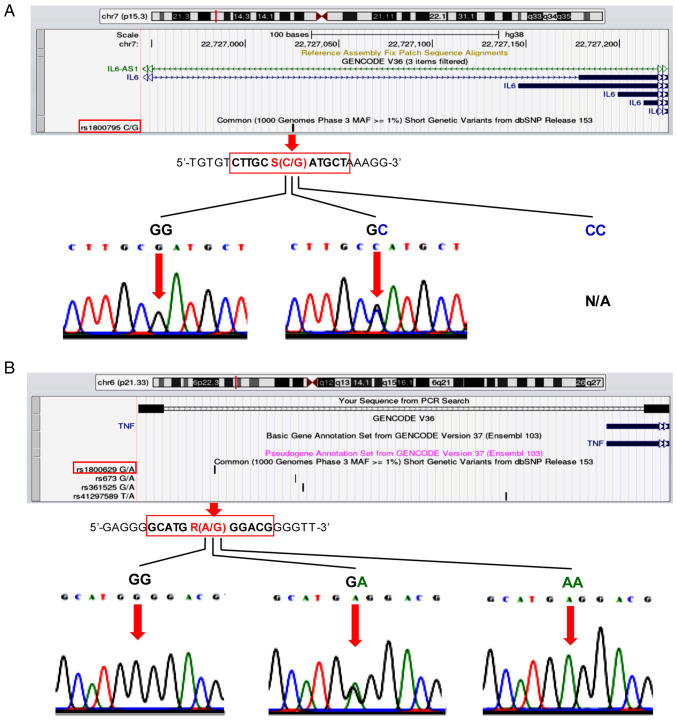
To validate the genotyping results of the IL-6-174G/C and TNF-α-308G/A polymorphisms, a random selection of 10% of samples for re-genotyping were subjected to DNA sequencing (Apical Scientific Sdn. Bhd.). Before re-genotyping, PCR amplification of the two SNPs was performed using 5X FIREPol® MasterMix (cat. no. 04-12-00125; Solis BioDyne). The IL-6-174G/C polymorphism was amplified using the redesigned forward primer, 5'-GTAAAACTTCGTGCATGACTTC-3' and reverse primer, 5'-AATCTTTGTTGGAGGGTGAG-3', to obtain a product size of 255 bp. To identify the TNF-α-308 polymorphism, the following redesigned primers were used: Forward, 5'-GCCCCTCCCAGTTCTAGTTC-3' and reverse, 5'-CATCAAGGACCCCTCACACTC-3', to obtain the extended PCR product size of 225 bp for DNA sequencing. The UCSC In-Silico PCR online informatics tool (genome.ucsc.edu/cgi-bin/hgPcr) was used to create the UCSC Genome Browser on Human Dec. 2013 (GRCh38/hg38) Assembly in order to visualize the position of the two polymorphisms in the predicted PCR product [Fig. 2A (IL-6, upper panel) and 2B (TNF-α, upper panel)]. After initial activation at 95˚C for 5 min, PCR cycling conditions of the two polymorphisms (95˚C for 15 sec, 54˚C for 30 sec and 72˚C for 30 sec) were carried out for 30 cycles on a GeneAmp® PCR System 9700 thermal cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.). The presence of bands of the expected size was confirmed via agarose gel electrophoresis. PCR products were visualized on 1.5% agarose gel stained with SYBR™ Safe DNA Gel Stain (cat. no. S33102; Thermo Fisher Scientific, Inc.). The DNA sequencing results highlighted that they were reproducible with no discrepancies.
Figure 2.
University of California, Santa Cruz Genome Browser views of the IL-6-174G/C and TNF-α-308G/A polymorphisms and representative electropherograms displaying the sequencing results for various genotypes of each SNP. (A) The -174G/C polymorphism (rs1800795) in the IL-6 promoter region and electropherograms of two different genotypes (GG and GC; SNP site indicated by red arrow). The IL-6-174 CC genotype was not detected in this population. (B) The -308G/A polymorphism (rs1800629) in the TNF-α promoter region and electropherograms of three distinct genotypes (GG, GA and AA; SNP site indicated by red arrow). N/A, not available; SNP, single nucleotide polymorphism.
ELISA for cytokine levels
A total of 268 samples from patients with IS and controls based on 1:1 matching of sex, age ±4 years and BMI ±2.5 were selected to measure IL-6 and TNF-α levels. Amongst these, the volume of plasma in 48 samples was not sufficient to perform the test. The remaining 220 samples [110 patients with IS (63 males and 47 females), age range 49-88 years, median age 67 years and 110 controls (63 males and 47 females), age range 47-88 years, median age 66 years] were subjected to ELISA. Centrifugation of blood samples was performed at 1,200 x g for 5 min at room temperature. Subsequently, plasma was aliquoted and stored at -80˚C for the assessment of cytokine levels. The levels of TNF-α and IL-6 were determined using commercially available ELISA MAX™ Deluxe Set Human IL-6 (cat. no. 430504) and TNF-α (cat. no. 430204) kits, which were conducted in accordance with the manufacturer's instructions (BioLegend, Inc.).
Statistical analysis
SPSS version 25 was used to analyze the data (IBM, Corp.). The normality of data was assessed using a Kolmogorov-Smirnov test. The baseline characteristics of the patients with IS and controls were examined using a χ2 test for categorical data and a Mann-Whitney U test for continuous data. Categorical variables are reported as proportions, whereas continuous variables are reported as the median [interquartile range (IQR)]. A binomial test was used to analyze the difference between the genotype and allele frequencies in patients with IS and controls. The association of the genetic polymorphisms with the risk of IS was determined using multivariate logistic regression. The Enter method, which is the default in SPSS Statistics, was used to enter all independent variables into the equation in one step (40). The Hardy-Weinberg equilibrium (HWE) was evaluated using a χ2 test. To investigate the interaction between the IS genetic polymorphism and clinical factors, multivariate logistic regression analysis was performed. The difference in plasma levels between patients with IS and controls were compared using a Mann-Whitney U test. The difference in plasma levels of cytokines among genotypes in patients with IS and controls was compared using a Mann-Whitney U tests for two groups and Kruskal-Wallis tests followed by a Dunn's posthoc test for three groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of patients with IS and controls
The baseline characteristics and clinical factors of patients with IS and controls are presented in Table I. There were no significant differences between the age or sex of patients between the IS and controls (P>0.05). Hypertension (patients with IS, 77.0% vs. controls, 39.0%), hyperlipidemia (patients with IS, 53.0% vs. controls, 25.5%), smoking (patients with IS, 28.5% vs. controls, 19.5%) and alcohol consumption (patients with IS, 22.0% vs. controls, 13.0%) were significantly more frequent in patients with IS than in the controls. IS patients had considerably lower total and low-density lipoprotein cholesterol levels than the controls (P<0.001). Lower cholesterol levels in patients with IS may be linked to the use of anti-lipidemic medications.
Table I.
Clinical parameters of patients with IS and controls.
| Variable | IS patients, n=200 | Controls, n=200 | P-value |
|---|---|---|---|
| Age, yearsd | 67 (57.2-76.0) | 66 (62.0-73.0) | 0.810 |
| Sex, n (%) | |||
| Male | 106 (53.0) | 123 (61.5) | 0.086 |
| Female | 94 (47.0) | 77 (38.5) | |
| Body mass index, kg/m2d | 23 (20.2-24.7) | 24 (20.7-26.8) | 0.016a |
| Blood pressured | |||
| Systolic blood pressure, mmHg | 146 (132.0-169.8) | 142 (130.0-155.0) | 0.012a |
| Diastolic blood pressure, mmHg | 81 (73.0-95.0) | 79 (72.0-88.0) | 0.003b |
| Clinical factors, n (%) | |||
| Hypertension | 154 (77.0) | 78 (39.0) | <0.001c |
| Hyperlipidemia | 106 (53.0) | 51 (25.5) | <0.001c |
| Diabetes mellitus | 72 (36.0) | 86 (43.0) | 0.152 |
| Metabolic syndrome | 79 (39.5) | 74 (37.0) | 0.607 |
| Smoking | 57 (28.5) | 39 (19.5) | 0.035a |
| Alcohol drinking | 44 (22.0) | 26 (13.0) | 0.018a |
| Blood testsd | |||
| Fasting blood glucose, mg/dl | 96 (88.0-109.0) | 97 (89.3-110.0) | 0.321 |
| Total cholesterol, mg/dl | 170 (141.0-203.0) | 203 (172.3-230.8) | <0.001c |
| Triglyceride, mg/dl | 130 (94.2-176.0) | 126 (94.3-168.8) | 0.801 |
| High-density lipoprotein cholesterol, mg/dl | 49 (40.0-59.0) | 48 (41.0-58.8) | 0.945 |
| Low-density lipoprotein cholesterol, mg/dl | 103 (79.0-136.0) | 126 (101.0-149.8) | <0.001c |
aP<0.05,
bP<0.01,
cP<0.001. P-values were calculated using a χ2 test for categorical variables and a Mann-Whitney U test for continuous data.
dMedian (IQR). IS, ischemic stroke.
Associations of IL-6 and TNF-α polymorphisms with the risk of IS development
The genotype distribution of IL-6-174G/C and TNF-α-308G/A between patients with IS and controls was in HWE (IL-6-174G/C, patients with IS, P=0.72 vs. controls, P=0.66; TNF-α-308G/A, patients with IS, P=0.27 vs. controls, P=0.48). It was identified that the IL-6-174 GG genotype and G allele were the most prevalent genotype in both patients with IS and controls, whilst the GC genotype and C allele were less common, and the CC genotype was not observed in the research samples (Table II). As illustrated in the lower panel of Fig. 2A, the representative electropherograms of the IL-6-174G/C sequencing data revealed only two distinct genotypes (GG and GC). There was no significant difference in the distribution of the IL-6-174G/C genotype as well as G and C alleles between patients with IS and controls. To determine whether the IL-6-174G/C polymorphism was associated with IS risk, multivariate logistic regression analysis was performed. The results indicated that there was no association between IL-6-174G/C polymorphism and the risk of IS occurrence in dominant and recessive inheritance, after adjustment for sex (Table III).
Table II.
Genotype and allele frequencies of IL-6 and TNF-α gene polymorphisms in the 200 patients with IS and the 200 control individuals, and their associations with the risk of IS susceptibility.
| A, IL-6-174G/C | |||||
|---|---|---|---|---|---|
| Polymorphism | IS patients, n (%) | Controls, n (%) | P-valueb | OR (95% CI) | P-valuec |
| Genotype | |||||
| GG | 190 (95.0) | 188 (94.0) | 0.959 | 1.000 | |
| GC | 10 (5.0) | 12 (6.0) | 0.832 | 0.825 (0.348-1.955) | 0.661 |
| CC | 0 (0.0) | 0 (0.0) | |||
| Allele | |||||
| G | 390 (97.5) | 388 (97.0) | 0.971 | 1.000 | |
| C | 10 (2.5) | 12 (3.0) | 0.832 | 0.829 (0.354-1.941) | 0.666 |
| MAF (%) | 2.5 | 3.0 | |||
| B, TNF-α-308G/A | |||||
| Genotype | |||||
| GG | 166 (83.0) | 181 (92.1) | 0.452 | 1.000 | |
| GA | 31 (15.5) | 19 (7.9) | 0.119 | 1.747 (0.951-3.211) | 0.072 |
| AA | 3 (1.5) | 0 (0.0) | 0.250 | ||
| GA+AA | 34 (17.0) | 19 (7.9) | 0.053 | 1.951 (1.071-3.554) | 0.029a |
| Allele | |||||
| G | 363 (90.7) | 381 (95.2) | 0.533 | 1.000 | |
| A | 37 (9.3) | 19 (4.8) | 0.022a | 2.044 (1.154-3.620) | 0.014a |
| MAF (%) | 9.3 | 4.8 | |||
aP<0.05.
bCalculated using a binomial test.
cCalculated using multivariate logistic regression. OR (95% CI) were calculated using a multivariate logistic regression model. IS, ischemic stroke; MAF, minor allele frequency; OR, odds ratio; CI, confidence interval.
Table III.
Associations of IL-6 and TNF-α gene polymorphisms with the risk of IS development according to different modes of inheritance.
| A, IL-6-174G/C | ||
|---|---|---|
| Polymorphism | Adjusted OR (95% CI) | P-value |
| C dominance, G wild type | ||
| GG | 1.000 | |
| GC or CC | 0.801 (0.337-1.907) | 0.617 |
| C recessive, G wild type | ||
| GG or GC | 1.000 | |
| CC | 1.162 (0.072-18.805) | 0.916 |
| B, TNF-α-308G/A | ||
| Polymorphism | Adjusted OR (95% CI) | P-value |
| A dominance, G wild type | ||
| GG | 1.000 | |
| GA or AA | 1.971 (1.080-3.599) | 0.027a |
| A recessive, G wild type | ||
| GG or GA | 1.000 | |
| AA | 4.138 (0.456-37.544) | 0.207 |
aP<0.05. P-values were calculated using multivariate logistic regression analysis. The adjusted OR (95% CI) was calculated using multivariate logistic regression after adjustment for sex. Variables in the model include IL-6-174G/C and TNF-α-308G/A. Method of variables entry in the model: Enter method. OR, odds ratio; CI, confidence interval.
For the TNF-α-308G/A polymorphism, the GG genotype was found most frequently, followed by the GA and AA genotypes, in the patients with IS; however, the AA genotype was not found in the controls (Table II). The TNF-α-308G/A representative electropherograms of the three genotypes (GG, GA and AA) were shown in Fig. 2B, lower panel. No significant difference in the distribution of the genotypes between patients with IS and controls was observed, whilst there was a significant difference (P=0.022) in the frequency of the TNF-α-308 A allele between patients with IS (9.3%) and controls (4.8%). Multivariate logistic regression analysis revealed that the GA and AA genotypes, and the A allele of TNF-α-308 increased the risk of IS development (GA and AA genotypes vs. GG genotype, OR=1.951; 95% CI=1.071-3.554; P=0.029; and A allele vs. G allele, OR=2.044; 95% CI=1.154-3.620; P=0.014). When the mode of inheritance was dominant, the GA or AA genotype had a 1.97-fold higher risk of IS development compared with the GG genotype, after adjusting for sex (adjusted OR=1.971; 95% CI=1.080-3.599; P=0.027), as shown in Table III. Therefore, the A allele appears to be a risk factor for IS development in a dominant mode of inheritance. However, when the mode of inheritance was recessive, the AA genotype was not an IS risk factor.
Interaction of IL-6 and TNF gene polymorphisms with clinical variables in IS susceptibility
To further investigate the interaction between genes and clinical factors, an interaction analysis of the IL-6-174G/C and TNF-α-308G/A polymorphisms with clinical factors was performed using multivariate logistic regression (Tables IV and V, respectively). Carriers of the IL-6-174 GG genotype who had hypertension, hyperlipidemia or consumed alcohol exhibited an increased risk for IS compared with those without these clinical features (hypertensive subjects, adjusted OR=4.095, 95% CI=2.604-6.441, P<0.001; hyperlipidemic subjects, adjusted OR=2.979, 95% CI=1.850-4.797, P<0.001; and alcohol consumption, adjusted OR=1.968, 95% CI=1.050-3.688; P=0.035; Table IV). Non-hypertensive subjects who had the IL-6-174 GC genotype were less likely to develop IS compared with those who had the GG genotype (adjusted OR=0.087, 95% CI=0.009-0.797; P=0.031).
Table IV.
Interaction of IL-6-174G/C polymorphism with clinical factors, as determined using multivariate logistic regression analysis.
| Polymorphism | Clinical factor | IS patients, n=200 | Controls, n=200 | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|---|
| IL-6-174G/C | Hypertension | ||||
| GG | No | 45 | 113 | 1.000 | |
| GC | No | 1 | 9 | 0.087 (0.009-0.797)c | 0.031a |
| GG | Yes | 145 | 75 | 4.095 (2.604-6.441)c | <0.001b |
| GC | Yes | 9 | 3 | 2.372 (0.571-9.852)c | 0.235 |
| IL-6-174G/C | Hyperlipidemia | ||||
| GG | No | 89 | 141 | 1.000 | |
| GC | No | 5 | 8 | 0.791 (0.219-2.852)d | 0.720 |
| GG | Yes | 101 | 47 | 2.979 (1.850-4.797)d | <0.001b |
| GC | Yes | 5 | 4 | 0.705 (0.164-3.030)d | 0.639 |
| IL-6-174G/C | Smoking | ||||
| GG | No | 138 | 152 | 1.000 | |
| GC | No | 5 | 9 | 0.449 (0.126-1.597)e | 0.216 |
| GG | Yes | 52 | 36 | 1.608 (0.929-2.784)e | 0.090 |
| GC | Yes | 5 | 3 | 1.763 (0.315-9.866)e | 0.519 |
| IL-6-174G/C | Alcohol drinking | ||||
| GG | No | 151 | 164 | 1.000 | |
| GC | No | 5 | 10 | 0.488 (0.140-1.705)f | 0.261 |
| GG | Yes | 39 | 24 | 1.968 (1.050-3.688)f | 0.035a |
| GC | Yes | 5 | 2 | 1.967 (0.317-12.193)f | 0.468 |
aP<0.05,
bP<0.001. P-values were calculated using multivariate logistic regression analysis. The adjusted OR (95% CI) was calculated via multivariate logistic regression analysis after adjustment for
csex, age, diabetes mellitus, metabolic syndrome, hyperlipidemia, smoking and drinking;
dsex, age, diabetes mellitus, metabolic syndrome, hypertension, smoking and drinking;
esex, age, diabetes mellitus, metabolic syndrome, hypertension, hyperlipidemia and drinking;
fsex, age, diabetes mellitus, metabolic syndrome, hypertension, hyperlipidemia and smoking. IS, ischemic stroke; OR, odds ratio; CI, confidence interval.
Table V.
Interaction of the TNF-α-308G/A polymorphism with clinical factors, as determined using multivariate logistic regression analysis.
| Polymorphism | Clinical factor | IS patients, n=200 | Controls, n=200 | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|---|
| TNF-α-308G/A | Hypertension | ||||
| GG | No | 42 | 110 | 1.000 | |
| GA+AA | No | 4 | 12 | 0.448 (0.133-1.513)c | 0.196 |
| GG | Yes | 124 | 71 | 2.655 (1.721-4.094)c | <0.001b |
| GA+AA | Yes | 30 | 7 | 4.585 (1.892-11.110)c | 0.001b |
| TNF-α-308G/A | Hyperlipidemia | ||||
| GG | No | 79 | 135 | 1.000 | |
| GA+AA | No | 15 | 14 | 1.291 (0.546-3.054)d | 0.560 |
| GG | Yes | 87 | 46 | 2.253 (1.401-3.624)d | 0.001b |
| GA+AA | Yes | 19 | 5 | 3.016 (1.027-8.862)d | 0.045a |
| TNF-α-308G/A | Smoking | ||||
| GG | No | 118 | 145 | 1.000 | |
| GA+AA | No | 25 | 16 | 1.466 (0.686-3.134)e | 0.324 |
| GG | Yes | 48 | 36 | 1.404 (0.800-2.462)e | 0.237 |
| GA+AA | Yes | 9 | 3 | 4.010 (0.889-18.092)e | 0.071 |
| TNF-α-308G/A | Alcohol drinking | ||||
| GG | No | 126 | 156 | 1.000 | |
| GA+AA | No | 30 | 18 | 1.650 (0.811-3.355)f | 0.167 |
| GG | Yes | 40 | 25 | 1.934 (1.037-3.608)f | 0.038a |
| GA+AA | Yes | 4 | 1 | 2.721 (0.288-25.693)f | 0.382 |
aP<0.05,
bP≤0.001. P-values were calculated using multivariate logistic regression analysis. The adjusted OR (95% CI) was calculated via multivariate logistic regression analysis after adjustment for
csex, age, diabetes mellitus, metabolic syndrome, hyperlipidemia, smoking and drinking;
dsex, age, diabetes mellitus, metabolic syndrome, hypertension, smoking and drinking;
esex, age, diabetes mellitus, metabolic syndrome, hypertension, hyperlipidemia and drinking;
fsex, age, diabetes mellitus, metabolic syndrome, hypertension, hyperlipidemia and smoking. IS, ischemic stroke; OR, odds ratio; CI, confidence interval.
In the present study, hypertensive and hyperlipidemic subjects carrying TNF-α-308 GA and AA genotypes had the highest risk of IS prevalence compared with non-hypertensive and non-hyperlipidemic subjects carrying the GG genotype (hypertensive subjects, adjusted OR=4.585, 95% CI=1.892-11.110, P=0.001; and hyperlipidemic subjects, adjusted OR=3.016, 95% CI=1.027-8.862, P=0.045) (Table V). Carriers of the GG genotype who had hypertension, hyperlipidemia and who consumed alcohol were susceptible to a higher risk of IS compared with subjects with the same genetic background but lacking these three clinical factors (hypertensive subjects, adjusted OR=2.655, 95% CI=1.721-4.094, P<0.001; hyperlipidemic subjects, adjusted OR=2.253, 95% CI=1.401-3.624, P=0.001; and alcohol drinkers, adjusted OR=1.934, 95% CI=1.037-3.608; P=0.038). Moreover, there was no interaction between the TNF-α-308 GA and AA genotype with smoking or alcohol consumption.
Associations of IL-6-174G/C and TNF-α-308G/A polymorphisms with plasma IL-6 and TNF-α levels
A total of 110 patients with IS and 110 controls were matched at 1:1, and their cytokine levels were measured. The median (IQR) levels of IL-6 [patients with IS, 30.5 (14.0-81.8) pg/ml vs. controls, 40.5 (17.0-110.3) pg/ml] and TNF-α [patients with IS, 28.0 (7.8-59.5) pg/ml vs. controls, 24.0 (14.0-41.3) pg/ml] were not significantly different between the two groups. Furthermore, the plasma IL-6 and TNF-α levels were not different amongst the various genotypes of patients with IS (Table VI). However, IL-6 levels were significantly increased in the IL-6-174 GC genotype carriers compared with the GG genotype carriers amongst controls (P=0.015). Thus, the IL-6 -174 GC genotype appeared to be associated with high levels of IL-6 in controls, but may not be associated with the IL-6 levels in patients with IS.
Table VI.
Plasma IL-6 and TNF-α levels among the genotypes of patients with IS and controls.
| Plasma level (pg/ml), median (IQR, n) | ||||
|---|---|---|---|---|
| Polymorphism | IS patients, n=110 | P-value | Controls, n=110 | P-value |
| IL-6-174G/C | ||||
| GG | 32.5 (14.8-81.8, n=106) | 0.307b | 36.0 (17.0-95.0, n=103) | 0.015a,b |
| GC | 14.0 (9.5-72.5, n=4) | 194.0 (81.0-195.0, n=7) | ||
| CC | N/A | N/A | ||
| TNF-α-308G/A | ||||
| GG | 29.0 (7.0-63.8, n=90) | 0.668c | 24.0 (14.3-41.8, n=100) | 0.803b |
| GA | 18.0 (8.5-42.5, n=17) | 25.0 (11.8-42.3, n=10) | ||
| AA | 26.0 (7.0-N/A, n=3) | N/A | ||
aP<0.05. P-values were calculated using a
bMann-Whitney U tests for comparisons between two groups and a
cKruskal-Wallis test for comparisons between three groups. IQR, interquartile range; N/A, not available; IS, ischemic stroke.
Discussion
Inflammation is considered a significant contributor to IS pathogenesis (7,8) and is proposed as a target of pharmacological therapy for inflammatory diseases (17,18). Inflammatory cytokines produced by immune and non-immune cells are upregulated upon cerebral ischemia and injury (8,41). Furthermore, polymorphisms of inflammatory cytokine genes at the promoter region may predict IS risk factors and outcomes (14-16). The present study evaluated the association of the genetic polymorphisms of IL-6-174G/C and TNF-α-308G/A with the risk of IS development. The current results indicated that subjects carrying the TNF-α-308 GA and AA genotypes and the A allele had a higher risk of IS development. In line with the current findings, previous reports have shown that the TNF-α-308 A allele was associated with a risk of cerebral infarction in Korean populations (42), and that North Indian subjects carrying TNF-α-308 GA and AA genotypes had a higher risk of coronary artery disease (43). By contrast, the TNF-α-308G/A polymorphism was not associated with IS risk in a Chinese population (44) but was associated with protection against IS in East Asians (31,32). With regards to the IL-6-174G/C polymorphism, the present study did not identify any association of IL-6-174G/C with the risk of IS, although this SNP has been revealed as a risk factor of IS in Asians in both the dominant mode (GG vs. GC + CC; OR=0.74, 95% CI=0.62-0.88; P=0.0005) and recessive mode of inheritance (CC vs. GG + GC; OR=1.61, 95% CI=1.17-2.21; P=0.003) (14). In addition, subjects carrying the IL-6-174 CC genotype were not observed in the present study population, which was consistent with findings of previous Chinese Han and Uyghur studies (28). The current finding demonstrated that there was no association of the IL-6-174G/C polymorphism with IS risk, which was in accordance with other studies of North Indian (16) and Chinese populations (28). Collectively, it was suggested that the association of these two SNPs with IS prevalence may be due to ethnic differences, methodological differences and variations in sample sizes.
In the present study, elevated IL-6 levels were associated with IL-6-174 GC genotypes in controls; however, no association between the TNF-α-308G/A polymorphism and TNF-α levels was found in patients with IS when compared with the controls. The functional SNP IL-6-174G/C in the GC genotype is associated with higher serum IL-6 levels, increased IS severity and poorer outcomes in patients with IS from North India (16). However, the IL-6-174G/C polymorphism is not associated with elevated serum IL-6 levels in young Indian patients with IS (aged 18-45 years) and controls (29). A meta-analysis revealed that TNF-α levels were significantly increased in patients with IS compared with control subjects (15). Moreover, amongst healthy individuals, TNF-α-308 GA and AA genotypes had lower TNF-α levels compared with the GG genotype (15). Inconclusive cytokine expression results may depend on multiple non-genetic and genetic factors, as well as their interaction, leading to variability in the expression levels (15,29). The interactions of other SNPs located in the inflammatory gene's promoter region (15) or gene-gene interactions (45) may affect the overall transcriptional activity of gene promoters. However, other SNPs located in the promoter region of the IL-6 and TNF-α genes and the interactions of each SNP are yet to be verified.
In previous studies, it was reported that inflammatory gene transcripts were upregulated following ischemic injury of the astrocytes in vitro (46) and the brain in vivo (47). The upregulation of inflammatory genes is mediated via the induction of the Toll-like receptor/NF-κB signaling pathway, which is the major contributor induced by cerebral ischemia/reperfusion (48,49). NF-κB acts as a critical transcription factor contributing to the transcription of various inflammatory genes, including IL-6 and TNF-α after cerebral ischemia-reperfusion injury (50). The DNA binding ability and transcriptional activity of NF-κB depend on the variants at the binding site of the NF-κB target gene, which modulate gene expression and influence disease risk (51). The molecular mechanism of how IL-6 and TNF-α gene polymorphisms influence the occurrence of IS remains to be elucidated. It has been suggested that functional polymorphisms at the promoter region of the IL-6 and TNF-α genes may increase the promoters' transcriptional activation by enhancing its responsiveness to inflammatory stimuli-mediated activation (52,53). Thus, modulation of gene expression may be the mechanism via which IL-6 and TNF-α genetic variations influence IS occurrence.
In the present analysis, the interactions between IL-6-174 GG and hypertension, hyperlipidemia and alcohol consumption were associated with a higher risk of IS. Notably, patients with IS had a higher prevalence of hypertension, hyperlipidemia and alcohol consumption compared with the controls. In a Chinese population, the IL-6-174G/C polymorphism was found to interact with hypertension and obesity, but not smoking, to elevate the risk of IS development (54). The genotypes in the current analysis differ from those in a Chinese study (54), in that the IL-6-174 GG genotype, rather than the CC genotype, interacted with hypertension and hyperlipidemia. Consistent with the current findings, it has been demonstrated that the combination of alcohol consumption and the IL-6-174G/C interaction increases the risk of carotid atherosclerosis, a risk factor for stroke in Western Germany (55). For the TNF-α-308G/A polymorphism, it was discovered that the GA and AA genotypes interacted with hypertension and hyperlipidemia to increase the risk of developing IS. The TNF-α-308 GA + AA genotypes were found to be associated with the risk factor of hypertension in the Asian population, which is consistent with the current findings (30). The TNF-α-308 A allele also appeared to have a higher risk of stroke when patients also had fever (56) and showed an increased risk of MI when combined with obesity (57). A previous study reported that serum high-density lipoprotein cholesterol levels in TNF-α-308 A allele carriers were negatively associated with polyunsaturated fatty acid intake (58). These studies support the current findings indicating increased risk of IS occurrence when the TNF-α-308 GA and AA genotypes were combined with hypertension and hyperlipidemia. The present study also identified no interaction between the TNF-α-308 GA and AA genotypes and smoking for IS risk. Consistent with the current results, the interaction of TNF-α-308G/A with smoking was not associated with the risk of MI (57). Thus, the current results provide the potential clinical utility of genetic variants as one of the risk predictions for IS development. This study can help to inform implementation strategies and support future research for IS prevention, lower stroke severity and improve therapeutic options to reduce IS incidence.
A limitation of the present study was that not all variants at the promoter regions of IL-6 and TNF-α genes were assessed. Complete sequencing may enable the identification of potentially causative mutations in the whole-gene function region of inflammatory genes. An interaction study of other variants at the promoter regions of IL-6 and TNF-α genes and the two variants presented in the current study may be required to determine the gene promoters' transcriptionally-related transcripts and the association with IS susceptibility. As the TNF-α-308G/A polymorphism has been proposed as a predictive risk factor of IS development in the current study, the mechanism of how the TNF-α-308G/A polymorphism regulates IS susceptibility should be confirmed in the future. Another limitation was the relatively small sample size used to analyze plasma IL-6 and TNF-α levels. Further investigations with additional samples from various study populations should be performed to confirm the current findings in the southern Thai population and other ethnic groups.
In conclusion, the present study demonstrated that the promoter polymorphism of TNF-α-308G/A was associated with a higher risk of IS occurrence, while there was no association between the IL-6-174G/C polymorphism and the risk of IS in this study population. It was suggested that the IL-6-174G/C and TNF-α-308G/A polymorphisms, combined with the three clinical variables of hypertension, hyperlipidemia and alcohol consumption, may enhance the risk of developing IS. Moreover, the high expression levels of IL-6 appeared to be associated with IL-6-174 GC genotype carriers in the control group. Collectively, to the best of our knowledge, the current findings provide the first report on the association of inflammatory cytokine gene polymorphism with the risk of IS susceptibility in a southern Thai population.
Acknowledgements
We are grateful to Assistant Professor Dr Wanida Limmun, Department of Mathematics and Statistics, School of Science, Walailak University for statistical analysis recommendations.
Funding Statement
Funding: The present study was supported by the Walailak University Fund (grant no. WU-IRG-62-034), Thailand Science Research and Innovation Fund (grant no. WU-FF64102) and the Development and Promotion of Science and Technology Talents Project (DPST).
Availability of data and materials
All data generated and/or analyzed during the current study are available in the GenBank Nucleotide Database repository [ncbi.nlm.nih.gov/genbank; accession nos. MZ379749-MZ379813 for IL-6-174G/C (rs1800795) (n=20) and MZ379814-MZ379833 for TNF-α-308G/A (rs1800629) (n=20)].
Authors' contributions
KK, NP, OM and WP performed the experiments and analyzed the data. KK, NP and PP contribute to data curation and validated the study. MN, PJ and WC participated in the study design and supervised the study. NK and JT aided in the experimental design and refined the manuscript. WC wrote and edited the manuscript, and was the corresponding author during the manuscript submission and revision. All the authors have read and approve to the final version of the manuscript. KK, NP, MN, PJ and WC confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki guidelines and approved by the Human Research Ethics Committee of Walailak University (approval no. WUEC-19-189-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kim J, Thayabaranathan T, Donnan GA, Howard G, Howard VJ, Rothwell PM, Feigin V, Norrving B, Owolabi M, Pandian J, et al. Global stroke statistics 2019. Int J Stroke. 2020;15:819–838. doi: 10.1177/1747493020909545. [DOI] [PubMed] [Google Scholar]
- 2.Boot E, Ekker MS, Putaala J, Kittner S, De Leeuw FE, Tuladhar AM. Ischaemic stroke in young adults: A global perspective. J Neurol Neurosurg Psychiatry. 2020;91:411–417. doi: 10.1136/jnnp-2019-322424. [DOI] [PubMed] [Google Scholar]
- 3.Avan A, Digaleh H, Di Napoli M, Stranges S, Behrouz R, Shojaeianbabaei G, Amiri A, Tabrizi R, Mokhber N, Spence JD, Azarpazhooh MR. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019;17(191) doi: 10.1186/s12916-019-1397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Thai Ministry of Public Health: Public Health Statistics A.D. 2019, 2020. Available from: https://bps.moph.go.th/new_bps/sites/default/files/statistic62.pdf. [Google Scholar]
- 5.Yamada Y, Kato K, Oguri M, Horibe H, Fujimaki T, Yasukochi Y, Takeuchi I, Sakuma J. Identification of nine genes as novel susceptibility loci for early-onset ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage. Biomed Rep. 2018;9:8–20. doi: 10.3892/br.2018.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Park A, Mun S, Kim HJ, Son H, Choi H, Kim D, Lee SJ, Kim JG, Kang HG. Proteomics-based identification of diagnostic biomarkers related to risk factors and pathogenesis of ischemic stroke. Diagnostics (Basel) 2020;10(340) doi: 10.3390/diagnostics10050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genchi A, Semerano A, Gullotta GS, Strambo D, Schwarz G, Bergamaschi A, Panni P, Simionato F, Scomazzoni F, Michelozzi C, et al. Cerebral thrombi of cardioembolic etiology have an increased content of neutrophil extracellular traps. J Neurol Sci. 2021;423(117355) doi: 10.1016/j.jns.2021.117355. [DOI] [PubMed] [Google Scholar]
- 8.Clausen BH, Wirenfeldt M, Høgedal SS, Frich LH, Nielsen HH, Schrøder HD, Østergaard K, Finsen B, Kristensen BW, Lambertsen KL. Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. Acta Neuropathol Commun. 2020;8(81) doi: 10.1186/s40478-020-00957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mo Y, Sun YY, Liu KY. Autophagy and inflammation in ischemic stroke. Neural Regen Res. 2020;15:1388–1396. doi: 10.4103/1673-5374.274331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(70) doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 11.Meng H, Zhao H, Cao X, Hao J, Zhang H, Liu Y, Zhu MS, Fan L, Weng L, Qian L, et al. Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc Natl Acad Sci USA. 2019;116:5558–5563. doi: 10.1073/pnas.1814394116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martha SR, Cheng Q, Fraser JF, Gong L, Collier LA, Davis SM, Lukins D, Alhajeri A, Grupke S, Pennypacker KR. Expression of cytokines and chemokines as predictors of stroke outcomes in acute ischemic stroke. Front Neurol. 2020;10(1391) doi: 10.3389/fneur.2019.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng PF, Liao FJ, Yin RX, Chen LZ, Li H, Nie RJ, Wang Y, Liao PJ. Genes associated with inflammation may serve as biomarkers for the diagnosis of coronary artery disease and ischaemic stroke. Lipids Health Dis. 2020;19(37) doi: 10.1186/s12944-020-01217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, Yang Y. A meta-analysis on associations of IL-6 and IL-10 polymorphisms with susceptibility to ischemic stroke. J Neuroimmunol. 2019;335(577004) doi: 10.1016/j.jneuroim.2019.577004. [DOI] [PubMed] [Google Scholar]
- 15.Cui G, Wang H, Li R, Zhang L, Li Z, Wang Y, Hui R, Ding H, Wang DW. Polymorphism of tumor necrosis factor alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, and cardiovascular risk factor for ischemic stroke. J Neuroinflammation. 2012;9(235) doi: 10.1186/1742-2094-9-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakraborty B, Chowdhury D, Vishnoi G, Goswami B, Kishore J, Agarwal S. Interleukin-6 gene -174 G/C promoter polymorphism predicts severity and outcome in acute ischemic stroke patients from north India. J Stroke Cerebrovasc Dis. 2013;22:683–689. doi: 10.1016/j.jstrokecerebrovasdis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Fabris M, Quartuccio L, Fabro C, Sacco S, Lombardi S, Ramonda R, Biasi D, Punzi D, Adami S, Olivieri I, et al. The -308 TNFα and the -174 IL-6 promoter polymorphisms associate with effective anti-TNFα treatment in seronegative spondyloarthritis. Pharmacogenomics J. 2016;16:238–242. doi: 10.1038/tpj.2015.49. [DOI] [PubMed] [Google Scholar]
- 18.Bank S, Julsgaard M, Abed OK, Burisch J, Broder Brodersen J, Pedersen NK, Gouliaev A, Ajan R, Nytoft Rasmussen D, Honore Grauslund C, et al. Polymorphisms in the NFkB, TNF-alpha, IL-1beta, and IL-18 pathways are associated with response to anti-TNF therapy in Danish patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:890–903. doi: 10.1111/apt.15187. [DOI] [PubMed] [Google Scholar]
- 19.Rincon M. Interleukin-6: From an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33:571–577. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Klimiec-Moskal E, Piechota M, Pera J, Weglarczyk K, Slowik A, Siedlar M, Dziedzic T. The specific ex vivo released cytokine profile is associated with ischemic stroke outcome and improves its prediction. J Neuroinflammation. 2020;17(7) doi: 10.1186/s12974-019-1691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiche EMV, Gelinksi JR, Alfieri DF, Flauzino T, Lehmann MF, de Araújo MCM, Lozovoy MAB, Simão ANC, de Almeida ERD, Maes M. Immune-inflammatory, oxidative stress and biochemical biomarkers predict short-term acute ischemic stroke death. Metab Brain Dis. 2019;34:789–804. doi: 10.1007/s11011-019-00403-6. [DOI] [PubMed] [Google Scholar]
- 22.Choi DW, Kim TS, Kim YS, Kim DJ. Elevated plasma biomarkers of inflammation in acute ischemic stroke patients with underlying dementia. BMC Neurol. 2020;20(293) doi: 10.1186/s12883-020-01859-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JW, Park MS, Kim JT, Kang HJ, Bae KY, Kim SW, Shin MG, Cho KH, Kim JM. The impact of tumor necrosis factor-α and interleukin-1β levels and polymorphisms on long-term stroke outcomes. Eur Neurol. 2018;79:38–44. doi: 10.1159/000484599. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan G, Debette S. Genetic risk factors for Ischemic and Hemorrhagic stroke. Curr Cardiol Rep. 2016;18(124) doi: 10.1007/s11886-016-0804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramazi S, Heydari-Zarnagh H, Goudarzian M, Khalaj-Kondori M, Bonyadi M. Thromboxane A synthase 1 gene expression and promotor haplotypes are associated with risk of large artery-atherosclerosis stroke in Iranian population. J Cell Biochem. 2019;120:15222–15232. doi: 10.1002/jcb.28787. [DOI] [PubMed] [Google Scholar]
- 26.Fan Y, Chen H, Li A, Shi Y, Zhang Y, Feng Q, Sun Y, Zheng H, He Y. A promoter polymorphism (rs17222919, -1316T/G) of ALOX5AP gene is associated with decreased risk of ischemic stroke in two independent Chinese populations. PLoS One. 2015;10(e0122393) doi: 10.1371/journal.pone.0122393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee I, Gupta V, Ahmed T, Faizaan M, Agarwal P, Ganesh S. Inflammatory system gene polymorphism and the risk of stroke: A case-control study in an Indian population. Brain Res Bull. 2008;75:158–165. doi: 10.1016/j.brainresbull.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Tong Y, Wang Z, Geng Y, Liu J, Zhang R, Lin Q, Li X, Huang D, Gao S, Hu D, et al. The association of functional polymorphisms of IL-6 gene promoter with ischemic stroke: Analysis in two Chinese populations. Biochem Biophys Res Commun. 2010;391:481–485. doi: 10.1016/j.bbrc.2009.11.084. [DOI] [PubMed] [Google Scholar]
- 29.Akhter MS, Biswas A, Abdullah SM, Hobani Y, Ranjan R, Behari M, Saxena R. Influence of interleukin-6 (IL-6) promoter gene polymorphisms (-174G>C, -572G>C, and -597G>A) on IL-6 plasma levels and their impact in the development of acute ischemic stroke in young Indians. Clin Appl Thromb Hemost. 2019;25(1076029619854136) doi: 10.1177/1076029619854136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao YS, Chang WW, Jin YL. Association between TNF-α promoter -308G/A polymorphism and essential hypertension in the Asian population: A meta-analysis. J Renin Angiotensin Aldosterone Syst. 2017;18(1470320317741066) doi: 10.1177/1470320317741066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong Y, Geng Y, Xu J, Wang Z, Zhang Y, Lin L, Zhang R, Deng P, Li Y, Hou W, et al. The role of functional polymorphisms of the TNF-alpha gene promoter in the risk of ischemic stroke in Chinese Han and Uyghur populations: Two case-control studies. Clin Chim Acta. 2010;411:1291–1295. doi: 10.1016/j.cca.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Song D, Cheng D. Associations of TNFα-308G/A and TNFα-238G/A polymorphisms with ischemic stroke in East Asians and non-East Asians: A meta-analysis. Genet Test Mol Biomarkers. 2017;21:10–16. doi: 10.1089/gtmb.2015.0265. [DOI] [PubMed] [Google Scholar]
- 33.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 34. World Medical Association (WMA): WMA Declaration of Helsinki-ethical principles for medical research involving human subjects, 2018. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. [Google Scholar]
- 35.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 36.Morgan JM, Capuzzi DM. Hypercholesterolemia. The NCEP Adult Treatment Panel III Guidelines. Geriatrics. 2003;58:33–38. [PubMed] [Google Scholar]
- 37.2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40 (Suppl 1):S11–S24. doi: 10.2337/dc17-S005. American Diabetes Association. [DOI] [PubMed] [Google Scholar]
- 38.Grundy SM. An International atherosclerosis society position paper: Global recommendations for the management of dyslipidemia. J Clin Lipidol. 2013;7:561–565. doi: 10.1016/j.jacl.2013.10.001. Expert Dyslipidemia Panel. [DOI] [PubMed] [Google Scholar]
- 39.Pongchaiyakul C, Nguyen TV, Wanothayaroj E, Karusan N, Klungboonkrong V. Prevalence of metabolic syndrome and its relationship to weight in the Thai population. J Med Assoc Thai. 2007;90:459–467. [PubMed] [Google Scholar]
- 40.Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: Logistic regression. Perspect Clin Res. 2017;8:148–151. doi: 10.4103/picr.PICR_87_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–1266. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Um JY, Kim HM. Tumor necrosis factor alpha gene polymorphism is associated with cerebral infarction. Brain Res Mol Brain Res. 2004;122:99–102. doi: 10.1016/j.molbrainres.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Kumari R, Kumar S, Ahmad MK, Singh R, Kant Kumar S, Pradhan A, Chandra S, Kumar S. Promoter variants of TNF-α rs1800629 and IL-10 rs1800871 are independently associated with the susceptibility of coronary artery disease in north Indian. Cytokine. 2018;110:131–136. doi: 10.1016/j.cyto.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 44.Gu L, Wu G, Su L, Yan Y, Liang B, Tan J, Cai H, Jiang H, Wei Q, Shen T, Wei A. TNF-a (-238G/A and -308G/A) gene polymorphisms may not contribute to the risk of ischemic stroke. Int J Neurosci. 2016;126:219–226. doi: 10.3109/00207454.2015.1010200. [DOI] [PubMed] [Google Scholar]
- 45.Linderson Y, Bresso F, Buentke E, Pettersson S, D'Amato M. Functional interaction of CARD15/NOD2 and Crohn's disease-associated TNFalpha polymorphisms. Int J Colorectal Dis. 2005;20:305–311. doi: 10.1007/s00384-004-0732-z. [DOI] [PubMed] [Google Scholar]
- 46.Yu AC, Lau LT. Expression of interleukin-1 alpha, tumor necrosis factor alpha and interleukin-6 genes in astrocytes under ischemic injury. Neurochem Int. 2000;36:369–377. doi: 10.1016/s0197-0186(99)00145-x. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi A, Jitsuishi T, Hozumi T, Iwanami J, Kitajo K, Yamaguchi H, Mori Y, Mogi M, Sawai S. Temporal expression profiling of DAMPs-related genes revealed the biphasic post-ischemic inflammation in the experimental stroke model. Mol Brain. 2020;13(57) doi: 10.1186/s13041-020-00598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye X, Kong D, Wang J, Ishrat T, Shi H, Ding X, Cui G, Hua F. MyD88 contributes to neuroinflammatory responses induced by cerebral ischemia/reperfusion in mice. Biochem Biophys Res Commun. 2016;480:69–74. doi: 10.1016/j.bbrc.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Hao T, Yang Y, Li N, Mi Y, Zhang G, Song J, Liang Y, Xiao J, Zhou D, He D, Hou Y. Inflammatory mechanism of cerebral ischemia-reperfusion injury with treatment of stepharine in rats. Phytomedicine. 2020;79(153353) doi: 10.1016/j.phymed.2020.153353. [DOI] [PubMed] [Google Scholar]
- 50.Zhai Y, Zhu Y, Liu J, Xie K, Yu J, Yu L, Deng H. Dexmedetomidine post-conditioning alleviates cerebral ischemia-reperfusion injury in rats by inhibiting high mobility group protein B1 group (HMGB1)/Toll-Like Receptor 4 (TLR4)/Nuclear Factor kappa B (NF-κB) Signaling Pathway. Med Sci Monit. 2020;26(e918617) doi: 10.12659/MSM.918617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang JH, Chen WT, Lee MC, Fang WH, Hsu YJ, Chin-Lin Chen HC, Chang HL, Chen CF, Tu MY, et al. Investigation of the variants at the binding site of inflammatory transcription factor NF-κB in patients with end-stage renal disease. BMC Nephrol. 2019;20(300) doi: 10.1186/s12882-019-1471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Liu RY, Yang L, Zhao J, Zhao X, Lu D, Yi N, Han B, Chen XF, Zhang K, et al. A two-SNP IL-6 promoter haplotype is associated with increased lung cancer risk. J Cancer Res Clin Oncol. 2013;139:231–242. doi: 10.1007/s00432-012-1314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiss-Toth E, Harlock E, Lath D, Quertermous T, Wilkinson JM. A TNF variant that associates with susceptibility to musculoskeletal disease modulates thyroid hormone receptor binding to control promoter activation. PLoS One. 2013;8(e76034) doi: 10.1371/journal.pone.0076034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang X, Feng L, Li C, Li Y. Association of IL-6-174G>C and -572C>G polymorphisms with risk of young ischemic stroke patients. Gene. 2014;539:258–262. doi: 10.1016/j.gene.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 55.Jerrard-Dunne P, Sitzer M, Risley P, Steckel DA, Buehler A, von Kegler S, Markus HS. Interleukin-6 promoter polymorphism modulates the effects of heavy alcohol consumption on early carotid artery atherosclerosis: The carotid atherosclerosis progression study (CAPS) Stroke. 2003;34:402–407. doi: 10.1161/01.str.0000053849.09308.b2. Carotid Atherosclerosis Progression Study. [DOI] [PubMed] [Google Scholar]
- 56.Lalouschek W, Schillinger M, Hsieh K, Endler G, Greisenegger S, Marculescu R, Lang W, Wagner O, Cheng S, Mannhalter C. Polymorphisms of the inflammatory system and risk of ischemic cerebrovascular events. Clin Chem Lab Med. 2006;44:918–923. doi: 10.1515/CCLM.2006.165. [DOI] [PubMed] [Google Scholar]
- 57.Padovani JC, Pazin-Filho A, Simões MV, Marin-Neto JA, Zago MA, Franco RF. Gene polymorphisms in the TNF locus and the risk of myocardial infarction. Thromb Res. 2000;100:263–269. doi: 10.1016/s0049-3848(00)00315-7. [DOI] [PubMed] [Google Scholar]
- 58.Fontaine-Bisson B, Wolever TM, Chiasson JL, Rabasa-Lhoret R, Maheux P, Josse RG, Leiter LA, Rodger NW, Ryan EA, Connelly PW, et al. Genetic polymorphisms of tumor necrosis factor-alpha modify the association between dietary polyunsaturated fatty acids and fasting HDL-cholesterol and apo A-I concentrations. Am J Clin Nutr. 2007;86:768–774. doi: 10.1093/ajcn/86.3.768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analyzed during the current study are available in the GenBank Nucleotide Database repository [ncbi.nlm.nih.gov/genbank; accession nos. MZ379749-MZ379813 for IL-6-174G/C (rs1800795) (n=20) and MZ379814-MZ379833 for TNF-α-308G/A (rs1800629) (n=20)].