ABSTRACT
The effects of inactivated SARS-CoV-2 vaccine (CoronaVac) on previously naturally infected individuals are unknown. This study compared immunogenicity and reactogenicity of CoronaVac in once naturally infected health-care workers (HCWs) and uninfected HCWs. All HCWs were immunized with two doses of CoronaVac (600 U/0.5 ml) intramuscularly at a 28-day interval. Adverse reactions were obtained by web-based questionnaires or telephone calls seven days after each vaccine dose. Detection of antibody levels against the receptor-binding domain (RBD) of SARS-CoV-2 spike protein was done four weeks after the second dose of the vaccine. We enrolled 103 previously naturally infected and 627 uninfected HCWs. The mean time for vaccination after the first nasopharyngeal SARS-CoV-2 positivity was 64 days (range: 15–136 days) in previously naturally infected HCWs. Among the previously naturally infected HCWs, 41 (40%) were asymptomatic, 52 (50%) had mild upper respiratory tract infections, 10 (105) had pneumonia, and only 6 (5%) were hospitalized. Any reported adverse reactions, either from the first dose or the second dose of vaccine administration, did not differ between previously infected and uninfected HCWs. Anti-RBD antibody titers were obtained in 50 (51%) of 103 previously infected HCWs and 142 (23%) of 627 uninfected HCWs. Anti-RBD antibody titers were significantly higher in HCWs with a previous natural infection (median 1220 AU/ml, range: 202–10328 AU/mL) than in uninfected HCWs (median: 913 AU/ml, range: 2.8–15547 AU/mL, p = .032). CoronaVac administration was safe and may elicit higher antibody responses in previously naturally infected individuals.
KEYWORDS: SARS-coV-2 inactivated virus vaccine, CoronaVac, health care workers, vaccine antibody response, vaccine adverse effects, Turkey
Introduction
COVID-19 began in late 2019 and has spread worldwide and caused social and economic destruction in many countries. Health workers are among the most affected groups. Some studies reported that health-care workers who have intense and close contact with infected individuals can suffer from COVID-19 disease more than once.1 Safe and effective COVID-19 treatments have yet to be developed, but vaccination is an effective strategy in stopping the spread of SARS-CoV-2. Several vaccines have become available for use in different parts of the world: Over 40 candidate vaccines are in human trials, and over 150 are in preclinical studies.2
In Turkey, the SARS-CoV-2 vaccination program started on January 11, 2021, with priority given to HCWs and then to high-risk groups. This strategy uses two doses of CoronaVac 600 U/0.5 mL (Sinovac Life Science Co, Ltd, Beijing, China) given 28 days apart intramuscularly.3 The BNT162b2 vaccine (Pfizer-BioNTech) was later introduced to the immunization program with two doses given at four-week intervals.3 The total number of vaccines given in Turkey is 18,724,856; 7,619,467 have received the second dose.3 Previously, SARS-CoV-2 infected people are thought to have protective immunity and memory responses for at least six months.4 However, the ideal vaccination time and regimens have not yet been clarified in previously infected individuals. It is also reasonable for such individuals to delay any vaccine receipt for a few months after infection to allow others to get vaccinated sooner as the risk of reinfection appears extremely low in this period. The USA Centers for Disease Control and Prevention (CDC) also suggest that individuals who received monoclonal antibodies or convalescent plasma for COVID-19 should delay vaccination for at least 90 days from the time of treatment.5 The Turkish Ministry of Health recommended SARS-CoV-2 vaccination at least one month after COVID-19 infection in HCWs and six months later in high-risk group individuals. Individuals with a history of SARS-CoV-2 may also be more likely to experience local and systemic adverse reactions.5,6 However, the responses to SARS-CoV-2 inactivated virus vaccine (CoronaVac, Sinovac Life Science Co., Ltd, Beijing, China) in previously naturally infected individuals have not yet been assessed in clinical trials. Therefore, this study compared antibody response and adverse reactions between previously SARS-CoV-2 naturally infected and uninfected health-care workers (HCWs) after two doses of SARS-CoV-2 vaccine (CoronaVac) administration.
Materials and methods
This study was a nested case–control analysis of 103 HCWs with previous natural SARS-CoV-2 infection during the last four months before administering the first dose of SARS-CoV-2 inactivated virus vaccine (CoronaVac, Sinovac Life Science Co, Ltd, Beijing, China); there were also 627 infection-naive HCWs. All work was done between January 11 and February 25, 2021. This study was done at Memorial Istanbul Ataşehir Hospital and Memorial Istanbul Şişli Hospital. To investigate vaccine-related adverse reactions, we made an online web-based questionnaire using The Turkish Pediatric Workshop telegram group.7 Clinical features and antibody titers results were obtained from participating hospitals’ infection control unit records. Vaccine-related adverse reactions were collected seven days after each vaccine-dose administration via web-based questionnaires. Antibody titers were measured four weeks after the second dose of the vaccine. Antibodies against the receptor-binding domain (RBD) of SARS-CoV-2 spike protein were measured with a SARS-CoV-2 IgG II Quant Reagent Kit (Abbott Ireland Diagnostics Division, Finisklin Business Park, Sligo, Ireland).
CoronaVac is an inactivated virus vaccine with an alum adjuvant. The SARS-CoV-2 strain CN2 was extracted from bronchoalveolar lavage (BAL) of a hospitalized patient in Wuhan, cultured in Vero cells, harvested, inactivated using β-propiolactone, and purified before being absorbed into aluminum hydroxide.8 Each 0.5-mL vaccine vial contains 600 SU SARS-CoV-2 antigens, sodium chloride (9 mg/ml), disodium hydrogen phosphate (1.16 mg/ml), monosodium hydrogen phosphate, sodium hydroxide, and sterile water. All HCWs received two doses of CoronaVac at least 28 days apart, and blood was drawn for detection of anti-RBD antibody four weeks after the second dose of the vaccine. All HCWs provided informed consent. This study was approved by the COVID-19 scientific research commission of the Turkish Ministry of Health and ethically approved by the Istanbul Memorial Şişli Hospital ethics committee. Statistical analysis was performed with jamovi (version 1.6, computer software retrieved from https://jamovi.org.) Antibody titers between groups were tested using the two-tailed Mann-Whitney U-test, Student’s t-test, and Pearson χ2 test for categorical and continuous variables. A P-value <0.05 was considered significant.
Results
Of the 730 HCWs enrolled in the survey, 103 (14%) HCWs had a previous laboratory-confirmed mild or asymptomatic SARS-CoV-2 infection as diagnosed with positive nasopharyngeal aspiration (NP) swab PCR (only one HCW had a negative PCR result but positive anti-SARS-CoV2 IgM antibody); 627 (86%) HCWs were previously uninfected as shown by PCR. Demographic and clinical features of the study population are shown in Table 1. Among the previously naturally SARS-CoV-2 infected HCWs, 41 (40%) of them were asymptomatic, 52 (50%) had mild upper respiratory tract infection, 10 (10%) of them had pneumonia, and only 6 (5%) were hospitalized. None of the previously naturally SARS-CoV-2 infected HCWs died. The mean time for vaccination from the first nasopharyngeal SARS-CoV-2 positivity was 64 days (range: 15–136 days) in previously naturally SARS-CoV-2 infected HCWs. None of the HCWs received steroids or other immune-suppressive drugs for the treatment of SARS-CoV-2 infection.
Table 1.
Demographic and clinical features of study population
| Previously SARS-CoV-2 infected n = 103 |
SARS-CoV-2 uninfected n = 627 |
P value | |
|---|---|---|---|
| Age, median (range), years | 36 (22–68) | 41 (22–72) | <.001 |
| Sex | |||
| Male | 40 (37%) | 247 (39%) | .9 |
| Female | 63 (63%) | 380 (61%) | |
| Clinic severity | |||
| Asymptomatic | 41 (40%) | - | |
| URTI | 52 (50%) | - | |
| Pneumonia | 10 (10%) | - | |
| Hospitalization | 6 (5%) | - | |
| Days from NP SARS-CoV-2 PCR + to vaccination mean (range) | 64 (15–136) | - | |
| Days from 2nd dose vaccination to collecting blood for antibody mean; (range) | 28 days (13–34) | 28 days (15–36) | .8 |
| Any adverse Reactions after 1st dose of vaccine | 44 (42%) | 309 (43%) | .15 |
| Any adverse Reactions after 2nd dose of vaccine | 34 (35%) | 214 (34%) | .25 |
| Number of vaccinated individuals with available antibody result | 50 (51%) | 142 (23%) | - |
| Number of vaccinated subjects with undetectable antibody titers | 0 (0%) | 2 (%1) | - |
Any reported adverse reactions – whether from the first or second dose of vaccine administration – did not differ between previously infected and uninfected HCWs (Table 1). The most common self-reported vaccine-related adverse effects after the first dose of the vaccine were local injection site pain (41%), myalgia (19%), and headache (13%) in previously uninfected HCWs; injection site pain (44%) and myalgia (13%) were seen in once-infected HCWs. The most common self-reported vaccine-related adverse effects after the second dose of the vaccine were local injection site pain (26%), headache (12%), and myalgia (3%) in previously uninfected HCWs, and injection site pain (30%), and myalgia (3%) in previously infected HCWs. Self-reported adverse reactions for the second dose were lower in both groups than the first dose (Table 1). Interestingly, sleepiness was reported after the first dose of vaccine in 14% of previously infected HCWs and 16% of previously uninfected HCWs; the rate of sleepiness decreased to 7% in previously infected HCWs and decreased to 10% in uninfected HCWs after the second dose. The reported sleepiness rate, whether after the first dose or second dose of the vaccine administration, did not differ between previously infected and uninfected HCWs (p > .05, respectively).
The study included 103 previously infected HCWs and 627 uninfected HCWs. Anti-RBD-antibody (SARS-CoV-2 IgG) titers were obtained in 50 (51%) of 103 previously infected HCWs and 142 (23%) of 627 uninfected individuals; 190 (98%) of seroprevalent patients reached an assay detectable response (SARS-CoV-2 IgG index value ≥50 AU/mL). Only two (2%) HCWs who were 53 and 52 years of age with no previous-SARS-CoV-2 infection had an undetectable antibody level despite vaccination. Anti-RBD antibody titers were significantly higher in HCWs with previous natural infection (median 1220 AU/ml, range: 202–10328 AU/mL) than in uninfected HCWs (median: 913 AU/ml, range: 2.8–15547 AU/mL, p = .032) (Figure 1).
Figure 1.
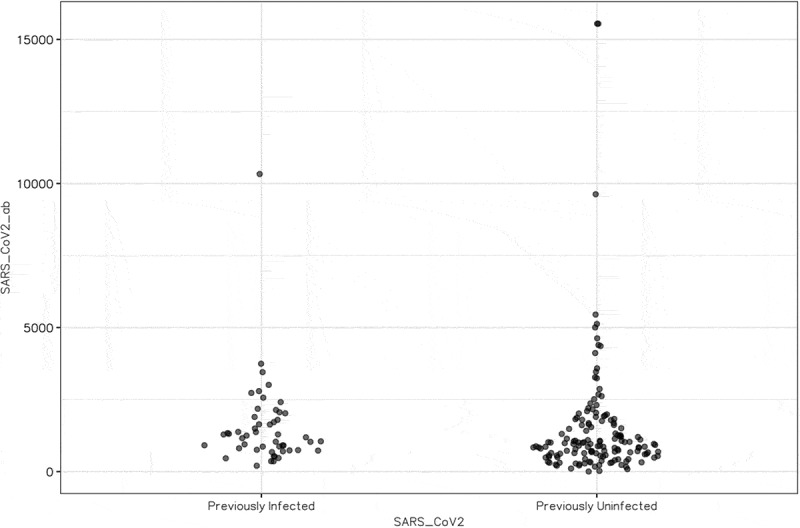
Anti-SARS-CoV-2 antibody responses after 2 doses of vaccine in health care workers concerning previous infection status.
Anti-RBD antibody (Arbitrary unit per ml)
Discussion
To the best of our knowledge, this is the first study to investigate reactogenicity and immunogenicity of inactivated SARS-CoV-2 vaccine (CoronaVac) in previously naturally infected individuals. Studies with inactivated SARS-CoV-2 vaccine (CoronaVac, Sinovac Life Science Co., Ltd., Beijing, China) have shown that most adverse reactions were mild. The most common symptom was injection-site pain, which agrees with previous studies. Previously, phase 1–2 clinical trials of CoronaVac among healthy adults aged 18–59 years showed that the vaccine was well tolerated, and seroconversion rates were 97–100% 28 days after the second dose of vaccine depending on the amount of antigen.8
Our study is in parallel with phase 1 and 2 studies of inactivated SARS-CoV-2 vaccine (CoronaVac, Sinovac Life Science Co., Ltd., Beijing, China); 98% of vaccinated HCWs had a detectable antibody response. This study’s main finding is that HCWs with previous SARS-CoV-2 infection had a higher antibody titer response to two doses of inactivated SARS-CoV-2 vaccine (CoronaVac, Sinovac Life Science Co, Ltd, Beijing, China) than those who were not previously infected. The median anti-RBD antibody titers were significantly higher in HCWs with previous natural infection (median 1220 AU/ml, range: 202–10328 AU/mL) than in uninfected HCWs (median: 913 AU/ml, range: 2.8–15547 AU/mL, p = .032).
To the best of our knowledge, there is no reported research either investigating the safety or immunogenicity of inactivated SARS-CoV-2 vaccine (CoronaVac, Sinovac Life Science Co., Ltd., Beijing, China) in previously naturally infected individuals. As a result, we cannot compare our findings to the literature. We examined studies done with other SARS-CoV-2 vaccines: Higher antibody titers after a single dose of mRNA vaccines were seen in previously naturally infected HCWs in many studies.6,9–14
Prendecki et al. reported that anti-S titers were significantly higher in HCWs with previous natural infection than in infection-naive HCWs after a single-dose of BNT 161b2 mRNA vaccine (Pfizer-BioNTech, Mainz, Germany) (median 16353 AU per mL [IQR 4741–28 581] vs. 615 · 1 AU/mL (286 · 4–1491)) [10]. Manisty et al. also compared a single dose of BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech, Mainz, Germany) responses in HCWs.10 They reported that among previously uninfected, seronegative individuals, anti-S titers after one vaccine dose were comparable to peak anti-S titers in individuals with a previous natural infection who had not yet been vaccinated. Among those with previous SARS-CoV-2 infection, vaccination increased anti-S titers more than 140-fold from peak pre-vaccine levels. This increase appears to be at least one order of magnitude greater than values reported after a conventional prime-boost vaccine strategy in previously uninfected individuals.10
Saadat et al. also investigated antibody responses after single-dose mRNA vaccines (either the Pfizer-BioNTech or Moderna vaccine) in 17 antibody-negative subjects, 16 asymptomatic SARS-CoV-2-infected subjects, and 26 symptomatic SARS-CoV-2-infected HCWs. HCWs with previous COVID-19 infection had higher antibody titer responses to a single dose of mRNA vaccines than those who were not previously infected based on laboratory-confirmed serology testing.
Antibody titers started peaking at seven days and achieved higher titers and neutralization rates in 14 days than antibody-negative volunteers.11 Bradley et al. determined antibody levels at baseline and three weeks after the first dose of the BNT162b2 SARS-CoV-2 mRNA vaccine (Pfizer-BioNTech, Mainz, Germany) in 36 HCWs who received laboratory confirmation of SARS-CoV-2 infection 30 to 60 days before they received the vaccine as well as 152 HCWs without a history of SARS-CoV-2 infection.12 They showed that three weeks after a single vaccination, HCWs with recent SARS-CoV-2 infection or seropositive status had higher antibody levels to SARS-CoV-2 antigens and higher levels of antibodies with neutralizing characteristics than those without a history of infection.12
Krammer et al. investigated antibody responses after mRNA vaccines (BNT162b2 [Pfizer] and mRNA-1273 [Moderna]) in 67 SARS-CoV-2 seronegative individuals and 43 seropositive individuals.6 They reported that the antibody titers of vaccines with preexisting SARS-CoV-2 antibody were 10 to 45 times as high as those vaccinated without preexisting antibodies at the same time points after the first vaccine dose. Seropositive patients also exceeded the median antibody titers measured in participants without preexisting antibodies after the second vaccine dose by more than a factor of 6.6 In addition, Ebinger et al. compared antibody responses to BNT162b2 (Pfizer–BioNTech) mRNA vaccine in individuals with previous SARS-CoV-2 infection (n = 35) versus infection-naive (n = 228) individuals.13 They reported that individuals previously infected with SARS-CoV-2 developed vaccine-induced antibody responses after a single dose of the BNT162b2 (Pfizer–BioNTech) mRNA vaccine that was similar to the antibody responses seen after a two-dose vaccination course administered to infection-naive individuals.13
In contrast, Tauzin et al. investigated humoral and T cell immune responses in cohorts of SARS-CoV-2 naive (n = 16) and naturally infected individuals (n = 16) prior and three weeks after the BNT162b2 (Pfizer–BioNTech) mRNA vaccine. They found that no neutralizing activity was seen in SARS-CoV-2-naive individuals three weeks after the first dose of vaccine. They still detected strong anti-RBD and spike antibodies with Fc-mediated effector functions and cellular responses dominated by the CD4+ T cell component. Moreover, after a single dose of the vaccine, a significant increase in preexisting humoral immunity, neutralization, and all T-cell responses were observed in SARS-CoV-2 naturally infected individuals.14
Covaxin was developed by the Indian pharmaceutical company Bharat Biotech in collaboration with the Indian Council of Medical Research (a government-funded biomedical research institute), and its subsidiary the National Institute of Virology; 800 participants have been enrolled in ongoing phase III trials since November 25, 2020. Bharat Biotech released interim efficacy data on March 3, 2021, which showed a clinical efficacy of 81%.15
This study shows that any adverse reactions after inactivated SARS-CoV-2 vaccine (CoronaVac, Sinovac Life Science Co, Ltd, Beijing, China) administration did not differ between previously infected and uninfected individuals. Healthy adults aged 18–89 years easily tolerated the vaccine in phase 1–2 clinical trials of inactivated SARS-CoV-2 vaccine (CoronaVac, Sinovac Life Science Co., Ltd., Beijing, China). In the phase 1 trial, 38% of subjects in the high-dose vaccine group reported adverse reactions. The most common symptom was injection site pain and the most adverse reactions were mild (grade 1) similar to our observations. The literature shows that previously infected individuals experienced significant post-vaccine symptoms more frequently than infection-naïve individuals after the first dose of BNT162b2 (Pfizer–BioNTech) mRNA vaccine. This difference was not observed after the second dose; naive individuals reported higher reactogenicity than previously infected individuals.13 Krammer et al. reported higher frequencies of any adverse reactions and systemic side effects after mRNA vaccines (BNT162b2 [Pfizer] and mRNA-1273 [Moderna]) in vaccine recipients with preexisting immunity.6 Prendecki et al., Manisty et al., Saadat et al., and Bradly et al. did not mention adverse vaccine reactions in their reports.9–12
Our study’s limitations are a small sample size, lack of pre-vaccination antibody titers of participants, lack of investigation of cellular immune responses, demonstration of vaccine efficacy, and potential enrollment bias. Because of ongoing worldwide vaccine shortages, this study’s results might lead to suggestions on a single-dose vaccination strategy for those with previous SARS-CoV-2 infection but this needs further study.
In conclusion, we showed that the CoronaVac vaccine elicits antibody responses in both SARS-CoV-2-uninfected and previously naturally infected individuals; the median antibody responses were higher in previously infected individuals. Furthermore, there was no difference in vaccine-related adverse reactions between previously infected and uninfected individuals either in the first or second dose. However, further study is needed to clarify if a single-dose of CoronaVac is sufficient for previously infected individuals.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Atici S, ÖF E, Yildiz MS, Şikgenç MM, Güzel E, Soysal A.. Symptomatic recurrence of SARS-CoV-2 infection in healthcare workers recovered from COVID-19. J Infect Dev Ctries. 2021. Jan 31;15(1):69–72. doi: 10.3855/jidc.14305. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Draft landscape of COVID-19 candidate vaccines. [accessed 2020 Oct 20]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- 3.The Turkish Ministry of Health Covid19 information page. [accessed 13 Apr 2021]. https://covid19asi.saglik.gov.tr/?_Dil=2.
- 4.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021. Feb 5;371(6529):eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States. [accessed 2021 Mar 3]. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html.
- 6.Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, Bermúdez-González MC, Bielak DA, Carreño JM, Chernet RL, et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021. Apr 8;384(14):1372–74. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gönüllü E, Soysal A, Can İ, Tutak E, Tunç T, Yıldız İ, Yeşilbaş O, Öner N, Anarat A, Soysal FG, et al. The use of social network in daily pediatric practice and education: Turkish pediatric atelier. Int J Pediatr. 2020. Sep 21;2020:7301309. doi: 10.1155/2020/7301309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021. Feb;21(2):181–92. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prendecki M, Clarke C, Brown J, Cox A, Gleeson S, Guckian M, Randell P, Pria AD, Lightstone L, Xu XN, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, Noursadeghi M, Boyton RJ, Semper A, Moon JC, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021. Mar 20;397(10279):1057–58. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saadat S, Rikhtegaran Tehrani Z, Logue J, Newman M, Frieman MB, Harris AD,Sajadi MM. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021. Apr 13;325(14):1467–69. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley T, Grundberg E, Selvarangan R, LeMaster C, Fraley E, Banerjee D, Belden B, Louiselle D, Nolte N, Biswell R, et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021. May 20;384(20):1959–61. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, Frias EC, Stewart JL, Van Eyk JE, Braun JG, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021. Apr 1;27:981–84. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tauzin A, Nayrac M, Benlarbi M, Gong SY, Gasser R, Beaudoin-Bussières G, Brassard N, Laumaea A, Vézina D, Prévost J et al. A single BNT162b2 mRNA dose elicits antibodies with Fc-mediated effector functions and boost pre-existing humoral and T cell responses. bioRxiv [Preprint]. 2021. Mar 18:2021.03.18.435972.
- 15.Thiagarajan K. What do we know about India’s Covaxin vaccine? BMJ 2021. Apr 20;373:n997. doi: 10.1136/bmj.n997. [DOI] [PubMed] [Google Scholar]


