Abstract
Menstrual blood-derived mesenchymal stem cells (MenSCs) have great potential in regenerative medicine. MenSC has received increasing attention owing to its impressive therapeutic effects in both preclinical and clinical trials. However, the study of MenSC-derived small extracellular vesicles (EVs) is still in its initial stages, in contrast to some common MSC sources (e.g., bone marrow, umbilical cord, and adipose tissue). We describe the basic characteristics and biological functions of MenSC-derived small EVs. We also demonstrate the therapeutic potential of small EVs in fulminant hepatic failure, myocardial infarction, pulmonary fibrosis, prostate cancer, cutaneous wound, type-1 diabetes mellitus, aged fertility, and potential diseases. Subsequently, novel hotspots with respect to MenSC EV-based therapy are proposed to overcome current challenges. While complexities regarding the therapeutic potential of MenSC EVs continue to be unraveled, advances are rapidly emerging in both basic science and clinical medicine. MenSC EV-based treatment has great potential for treating a series of diseases as a novel therapeutic strategy in regenerative medicine.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-021-02511-6.
Keywords: Menstrual blood-derived mesenchymal stem cell (MenSC), Small extracellular vesicle, Exosomes, Cell-free therapy, Regenerative medicine
Background
Mesenchymal stem cells (MSCs) are heterogeneous subsets of stromal/mesenchymal regenerative cells [1, 2]. They possess powerful self-renewal ability and multi-lineage differentiation potential via symmetric/asymmetric cell division [3–5]. Currently, MSC-based therapy has been diffusely exploited in the treatment of numerous diseases in basic science and clinical medicine [6–12]. Additionally, many clinical trials have proved that MSC infusion is safe and effective at various doses [13–18]. Currently, MSCs can be obtained from almost all parts of tissues/organs, including bone marrow, umbilical cord, adipose tissue, placenta, fetal tissue, Wharton’s jelly, induced pluripotent stem cell (iPSC), embryonic stem cell (ESC), cervical tissue placentae, periodontal ligaments amniotic membrane/fluid, endometrium, lung, liver, dental pulp, peripheral blood, dermal tissues, synovial membranes, and skeletal muscle tissue [19–22]. With the development of personalized medicine, some attractive treatment modalities should be considered to provide precise measures that reflect the underlying biological processes of the complex of diseases in each patient [23–25]. Moreover, with the exception of common sources of MSCs [including bone marrow (BM)-MSCs, umbilical cord (UC)-MSCs, and adipose tissue (AD)-MSCs], other sources should also be considered because these novel sources of MSCs may possess powerful merits in the treatment of corresponding diseases [10, 26–28]. Menstrual blood-derived mesenchymal stem cells (MenSCs) were first found by Meng et al. in 2007 [29]. Since then, MenSC has become a promising therapeutic strategy for the development of effective treatments [30–33]. Compared with other sources of MSCs, MenSCs have several advantages, including abundance, periodic acquisition, non-invasive isolation, high proliferation rate, low immunological rejection, and lack of ethical issues [34–36]. More importantly, MenSCs supply an alternative way that is both painless and free of ethical issues arising from BM-MSCs donations [36]. MenSCs possess a doubling time of approximately 19.4 h, twice as fast as that of BM-MSCs that is estimated at 40–45 h [29]. Menstrual blood in women can be obtained monthly from the age of 20 to 45 years [37–40]. This impressive source is superior to BM-MSCs, AD-MSCs, and UC-MSCs. Although extensive progress has been made in deciphering the immunosuppression/immunoregulation of MSCs, the study on the immunoregulation of MenSCs is still in its infancy [34]. It is only known that MenSCs do not express MHC-II. Therefore, the slow progress in the immunoregulation of MenSCs greatly limits the application of MenSCs. Based on these advantages, MenSCs have been continuously reported for treating various diseases in both basic science and clinical medicine [37, 40–43].
An increasing number of studies have demonstrated that the therapeutic benefits of MSCs are principally mediated via paracrine roles, through the secretion of growth factors, chemokines, and cytokines rather than their differential abilities or cellular replacements [5, 10, 44–53]. Therefore, researchers are increasingly interested in the therapeutic value of MSC-derived bioactive molecules, especially the secretome and extracellular vesicles (EVs), which are considered the key components of paracrine effect in the treatment of MSC-based therapy [54–56]. Furthermore, researchers have shown that MSC-conditioned medium induced repair of injured tissues in several animal models [47, 57]. Compared with MSC-based therapy, MSC EV-based therapy is highly recommended because it is less likely to trigger an immune-repulsion response and is safe to the host, not causing ethical problems [58, 59]. In addition, EVs have different routes of injections, including intranasal, oral, intravenous, intraperitoneal, and subcutaneous [60–64]. Thus, MenSC-derived EVs offer important application advantages. In this review, we systematically discuss the current progress of MenSC-derived EVs with regard to the identification of components, functions, and therapeutic potential in treating a series of diseases. Moreover, we highlight current challenges and promising perspectives of MenSC-derived EVs in regenerative medicine to guide future clinical applications.
The basic characteristics and biological functions of MSC-derived small EVs
EVs are generally released from the endosomal compartments, present in almost all body fluids, and released by all types of cells [65, 66]. They are involved in multiple pathological processes with cell-to-cell communication monitoring, showing promising therapeutic potential in different diseases [67–70]. Classically, EVs are generally divided into exosomes, microvesicles, and apoptotic bodies, based on their sizes, origins, biogenesis, and cargo: (1) exosomes, diameter of 30–150 nm, fused with the cell membrane through multivesicular bodies to deliver into the extracellular body; (2) microvesicles, diameter of 50–1000 nm, derived from the direct budding of the plasma membrane; (3) apoptotic bodies, a diameter of 100–5000 nm, displaying wide distributions [71–73]. Their biological functions are shown in Table 1.
Table 1.
Biological functions of extracellular vesicles (EVs) in body fluids
| EVs functions | Exosomes | Microvesicles | Apoptotic bodies |
|---|---|---|---|
| Origin | Endosomal multivesicular bodies | Cell surface | Apoptotic cell surfaces |
| Generation | Intracellular vesicle traffic | Plasma membrane | Plasma membrane |
| Size | 30–150 nm | 50–1000 nm | 100–5000 nm |
| Markers | Tetraspanins (CD9/63/81), Alix, HSP70/90, flotillin, TSG101, clathrin, GM130, MHC | Annexin V, selectins, integrins, flotillin-2, CD40, metalloproteinases | Histones, Annexin V |
| Cargos | Proteins, lipids, mRNA, miRNA, DNA, carbohydrates | Proteins, mRNA, miRNA | Proteins, mRNA, miRNA, fragment of DNA |
As consensus has not yet emerged on specific markers of EV subtypes, it is hard to distinguish exosomes or microvesicles; therefore, MSC exosomes or microvesicles are referred to as MSC-derived small EVs, following the classical references [74–77]. Small EVs consist of various biomolecules, such as regulatory proteins, small peptides, lipids, and some genetic materials (including mRNA, small RNA, long non-coding RNA, genomic DNA, complementary DNA, and mitochondrial DNA), which are delivered to a spectrum of recipient cell types [78–81]. Over the past decade, small EVs have emerged as major mediators of cell-free therapy and are a promising tool for a variety of diseases. In view of their exceptionally broad biological functions, small EVs can stimulate targeting cells, transfer membrane receptors, deliver proteins or genetic information, and eventually cause epigenetic differences in recipient cells [82–85]. In addition to cell communication, it is increasingly evident that small EVs have an important function in regulating different physiological processes, such as cell maintenance, immune surveillance, cell migration, tissue repair, glycometabolic regulation, cell differentiation, cancer therapy, hematopoietic engraftment, blood coagulation, and angiogenesis [86–92]. Thus, small EVs offer a unique platform for the development of a novel class of therapeutics for the treatment of various diseases.
Generally, MSC-derived small EVs share an evolutionarily conserved set of molecules, including membrane transport and fusion proteins (GTPases, annexins, and flotillin), heat shock protein (HSP) family (HSP20, HSP27, HSP40, HSP60, HSP70, and HSP90), tetraspanins (CD9, CD63, and CD81), multivesicular body biogenesis [ALG-2-interacting protein-X (Alix) and TSG101], as well as some lipid-related proteins and phospholipases [93–96]. The therapeutic potential of MSC-derived small EVs is usually elicited by delivering biologically relevant proteins and RNAs to recipient cells [97]. Accumulating evidence shows that MSC-derived small EVs are successfully applied as therapy of several disease models [98–105]. Recently, small EVs have been reported as the principal therapeutic agents with regenerative capabilities and immunomodulatory functions of MSC secretions [75, 80, 106, 107]. To date, MSC-derived small EVs have been isolated from a series of sources, including human/mouse/rat/canine/pig bone marrow [108–112], human/mouse/rat/canine/equine/mini-pig adipose tissue [112–117], mouse cardiac tissue [118], and human umbilical cord [119], ESC [120], iPSC [121], menstrual blood [122], Wharton’s jelly [123], placental and fetal tissue [124, 125], dental pulp [126], gastric cancer tissue [127], synovial membrane [128], corneal [129], fetal liver [130], oral mucosa [131], and amniotic fluid [132]. Detailed information on reports of MSC-derived small EVs from different sources is presented in Table 2. Although an increasing number of sources of MSCs are being evaluated for their role in exosomes, the underlying mechanism and appropriate source need to be further explored.
Table 2.
Different sources of MSC-derived small EVs
| Source of MSC-derived small EVs | Year | Reference |
|---|---|---|
| Human ESC | 2010 | [120] |
| Rat BM | 2012 | [108] |
| Mouse BM | 2012 | [109] |
| Human BM | 2013 | [110] |
| Human AD | 2013 | [113] |
| Human UC | 2013 | [119] |
| Human placental | 2013 | [124] |
| Human Wharton’s jelly | 2013 | [123] |
| Human gastric cancer tissue | 2014 | [127] |
| Fetal tissue | 2014 | [125] |
| Dental pulp | 2015 | [126] |
| Human iPSC | 2015 | [121] |
| Rat AD | 2016 | [114] |
| Mouse AD | 2016 | [115] |
| Human menstrual blood | 2016 | [122] |
| Mini-pig AD | 2016 | [116] |
| Human amniotic fluid | 2016 | [132] |
| Human synovial membrane | 2016 | [128] |
| Human Corneal | 2018 | [129] |
| Pig BM | 2018 | [111] |
| Mouse cardiac | 2018 | [118] |
| Human fetal liver | 2019 | [130] |
| Human oral mucosal | 2019 | [131] |
| Equine AD | 2019 | [117] |
| Canine BM and AD | 2019 | [112] |
Notes: ESC, embryonic stem cell; BM, bone marrow; AD, adipose; UC, umbilical cord; iPSC, induced pluripotent stem cell; AM, amniotic membrane
MenSC-derived small EVs were first reported by Lopez-Verrilli et al. in 2016 [122], and the authors revealed that MenSC-derived small EVs promote axonal regeneration after nerve injury in the central and peripheral nervous system. Previous studies showed that MenSC-derived small EVs express CD63 and TSG101 [133, 134], and other researchers further discovered that MenSC-derived small EVs present CD9, CD81, HSP70, and HSP90 [122, 135–140]. Additionally, MenSC-derived small EVs do not express Rab5 or calnexin [122, 136, 138, 140]. Thus, protein markers of MenSC-derived small EVs should include CD9, CD63, CD81, HSP70, HSP90, and TSG101 and exclude Rab5 and calnexin (Fig. 1). Although these markers are commonly studied, some other molecules (such as HSP60 and Alix) still need to be recognized in accordance with universal MSC-derived small EVs [94, 106, 141]. Moreover, serving as a unique tissue type source of MSCs, some representative markers from MenSC-derived small EVs should be identified to represent the specific source of MSCs. Although research on MenSC-derived small EVs is relatively new compared to common sources of MSCs, the basic definition and identification of MenSC-derived small EVs should be established for future research.
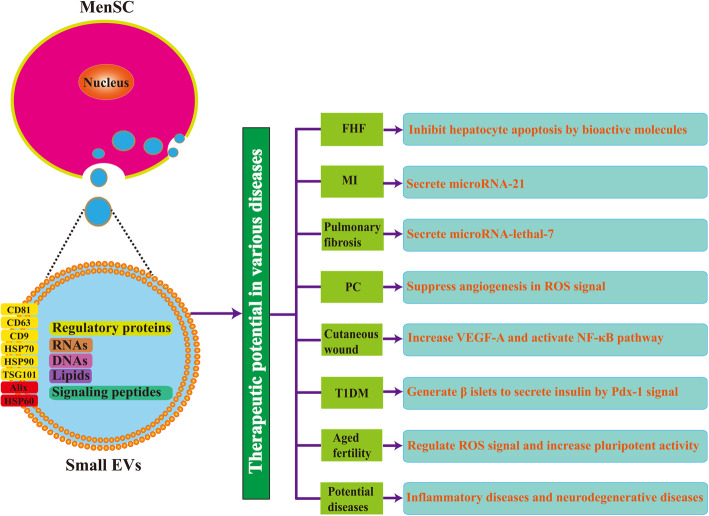
Fig. 1.
Identification of MenSC-derived small EVs and their therapeutic potentials for tissue repair in various diseases. Small EVs from MenSCs consist of regulatory proteins, RNAs, and DNAs, lipids, and siginaling peptides promoting regenerative repair of wounded cells and tissues. MenSC-derived small EVs are positive for the expression of CD9, CD63, CD81, HSP70, HSP90, and TSG101, and they are negative for Rab5 and calnexin. The expression of HSP60 and Alix, which are positive for universal MSC-derived small EVs, need to be recognized for further verification. The therapeutic potential of MenSC-derived small EVs in various diseases, including fulminant hepatic failure (FHF; via inhibition of hepatocyte apoptosis by bioactive molecules), myocardial infarction (MI, via secreted microRNA-21), pulmonary fibrosis (via secreted microRNA-lethal-7), prostate cancer (PC; via suppression of angiogenesis by ROS signaling), cutaneous wound (via increase in VEGF-A and activation of NF-κB pathway), type-1 diabetes mellitus (T1DM; via generation of β islets to secrete insulin by Pdx-1 signaling), aged fertility (via regulation of ROS signaling and increase in pluripotent activity), and some potential diseases (such as inflammatory and neurodegenerative diseases)
Therapeutic potential of MenSC-derived small EVs in treating various diseases
In contrast to numerous studies on small EVs from common sources of MSCs (such as BM-MSCs, AD-MSCs, and UC-MSCs), the research on the therapeutic potential and underlying mechanisms of MenSC-derived small EVs are still in an initial stage. In this context, although the therapeutic effect of MenSC has been demonstrated since 2007 [29], the study on MenSC-derived small EVs was first reported in 2016 [122]. Owing to the superiority of MenSC gradually emerging in recent years [31, 33, 34, 142], studies on MenSC-derived small EVs have great potential and profound significance in regenerative medicine, as shown in Fig. 1.
MenSC-derived small EVs for fulminant hepatic failure (FHF)
FHF, also termed acute liver failure (ALF), is a progressive, life-threatening, and sharp pathological reaction characterized by hepatic dysfunction [143]. Currently, orthotopic liver transplantation (OLT) is the most effective treatment for FHF. However, because of the shortage of donor organs, high transplantation costs, and accurate expertise needed for the surgery, an increasing number of researchers are seeking other available methods to treat FHF. It has been verified that MenSC-derived small EVs have an effect in suppressing hepatocyte apoptosis in a D-galactosamine (D-GalN)/lipopolysaccharide (LPS)-induced FHF model in mice [133]; also, the expression of tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β was evidently reduced in co-culture of alpha mouse liver 12 (AML12) hepatocytes with MenSC-derived small EVs in vitro. Additionally, the effective bioactive molecules for ameliorating FHF were mainly mediated by MenSC exosomes of angiopoietin-2, intercellular adhesion molecule-1 (ICAM-1), anexelekto, IL-6, osteoprotegerin, IL-8, insulin-like growth factor-binding protein-6 (IGFBP-6), and angiogenin [133].
MenSC-derived small EVs for myocardial infarction
Myocardial infarction (MI), a type of coronary artery disease, is caused by apoptosis of cardiomyocytes due to excessive ischemic conditions [144]. Because MI has a long-term undiscovered period, it usually leads to severe hemodynamic deterioration and sudden death. Thus, a novel therapeutic strategy is required to treat MI. Wang et al. discovered that transplantation of MenSC-derived small EVs significantly improved cardiac function in infarcted rat hearts [134]. The authors further found that microRNA (miR)-21 secreted from MenSC-derived small EVs played a dominant role in improving MI in the animals. The exosomal miR array showed that miR-21 targets phosphatase and tensin homolog (PTEN) and the downstream molecule of AKT/PKB (protein kinase B) to trigger signal cascades. This result showed that MenSC-derived small EVs ameliorate the damaged cardiac function in MI primarily through the paracrine function on excretive miR-21.
MenSC-derived small EVs for pulmonary fibrosis
Pulmonary fibrosis is a chronic problem that is of widespread concern [145]. Lung transplantation is currently the optimal treatment for this disease, but it is limited by the lack of donors; thus, an alternative method is required for pulmonary fibrosis treatment. Sun et al. verified that transplantation of MenSC-derived small EVs significantly ameliorated bleomycin-induced pulmonary fibrosis by repairing alveolar epithelial cell injury in a mouse model in vivo and in vitro [146]. Further investigation revealed that miR lethal-7 (let-7) of MenSC-derived small EVs enhanced the ability of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) to inhibit the activation of reactive oxygen species (ROS) and mitochondrial-DNA damage by regulating NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) signaling pathway. Thus, targeting miRs (such as let-7) of MenSC-derived small EVs is a promising approach for the treatment of pulmonary fibrosis.
MenSC-derived small EVs for prostate cancer
Prostate cancer (PC) is an epithelial malignancy that occurs in the prostate and is the third-leading cause of cancer mortality in men [147]. Although comprehensive treatments (such as surgery radiotherapy, endocrine therapy, and radiation) are used in PC patients, the practical effect is still far away from curing the disease [148]. Some researchers have found that MSC-derived small EVs have the ability to ameliorate the tumor microenvironment by limiting tumor growth, angiogenesis, and metastasis, mainly targeting fibroblasts, endothelial cells, and immune cells [149]. Recently, Alcayaga-Miranda et al. proved that MenSC-derived small EVs significantly inhibited tumor angiogenesis in the PC3 tumors model in mice [135]. Moreover, the antitumor effect contributed to a decrease in vascular density and tumor hemoglobin content. MenSC-derived small EVs inhibited the secretion of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) and reduced the activity of nuclear factor kappa B (NF-κB). The authors further proved that MenSC-derived small EVs lowered reactive oxygen species (ROS) production in PC3 cells. In this context, a previous study showed that ROS regulates angiogenesis and tumor development through HIF-1α and VEGF in PC3 cells [150]. Therefore, these results indicate that MenSC-derived small EVs act as a blocker of tumor-induced PC angiogenesis by suppressing tumor-induced angiogenesis via a ROS-dependent mechanism.
MenSC-derived small EVs for cutaneous wounds
Cutaneous wounds commonly occur via loss of structures and appendages by externally acute stimulants (such as extensive burns, scalds, trauma, or diabetic ulcers) that induce chronic wounds/scars [151]. MSC-derived small EVs have therapeutic potential in cutaneous repair and regeneration [152]. Dalirfardouei et al. showed that MenSC-derived small EVs significantly reduced cutaneous damage in diabetic foot ulcers in mice [140]. Wound healing mainly contributes to the polarization of M1-M2 macrophages by increasing VEGF-A to promote angiogenesis and activating NF-κB to alleviate local inflammation.
MenSC-derived small EVs for type-1 diabetes mellitus (T1DM)
T1DM is caused by multiple factors that lead to an increase in blood glucose concentration and a severe decrease in insulin secretion [153]. Currently, transplantation of islets is the most effective treatment; however, it is restricted owing to the lack of sufficient pancreatic donors. The therapeutic potential of MenSCs for treating T1DM has been verified [154]. Mahdipour et al. demonstrated that MenSC-derived small EVs have a therapeutic function, improving T1DM in rats [138]. The authors also found that administration of MenSC-derived small EVs improved the regenerative capacity of β islets and facilitated the production of insulin through the pancreatic and duodenal homeobox 1 (Pdx-1) signaling cascade.
MenSC-derived small EVs for aged fertility
With social and financial pressure, an increasing number of women have postponed motherhood after the age of thirty-five. However, because of the poor quality and insufficient quantity of oocytes, the overall pregnancy rate and fertility level is low [155, 156]. Therefore, improving the quality of oocytes or activating aging oocytes is a viable route to improve the fertility of aged women [157]. Different sources of MSC-derived small EVs play a vital role in improving ovarian insufficiency age-related fertility [139]. Moreover, EVs can be used to improve the quality of embryos during assisted reproduction [157]. Marinaro et al. found that MenSC-derived small EVs increased embryo quality and quantity by regulating antioxidant enzymes and increasing pluripotent activity in an aged mouse model [139]. Additionally, MenSC-derived small EVs showed the ability to increase the developmental level of in vitro fertilization-derived embryos via an ROS-dependent approach in aged female mice [137]. Based on the proteomics analysis of murine blastocysts, some core genes related to cellular response to oxidative stress (Gpx1 and Sod1), metabolism (Acaca and Gapdh), placentation (Pgf, VEGF-A), and trophectoderm/inner cell mass formation (Pou5f1 and Sox2) are the most likely candidates for improving embryo quality and quantity [137, 139]. Other researchers found that miR-17-5P, miR-223-3P, miR-146a-5p, and miR-21-5p from UC-MSC-derived small EVs are possible contributors to improving ovarian insufficiency or age-related fertility [158–160]. Additionally, Zhao et al. revealed that increased expression of integrin-β3, leukemia inhibitory factor, and VEGF in AD-MSC-derived small EVs may promote endometrial regeneration and fertility restoration [161].
MenSC-derived small EVs for potential diseases
Although many studies have focused on the mutual effect between MenSC-derived small EVs and specific disease models, the study of the interaction between MenSC-derived small EVs and pro-inflammatory conditions also provides a direction for regenerative medicine. Marinaro et al. used a comprehensive proteomics and transcriptomics analysis and found that some functionally immunomodulatory proteins [including colony-stimulating factor-1, PYCARD (PYD and CARD domain), and endoplasmic reticulum aminopeptidase 1 (ERAP1)] regulate immune responses in interferon (IFN)-γ primed MenSC-derived small EVs [162]. Thus, MenSC-derived small EVs have a promising immunomodulatory potential for treating inflammation-related diseases in future studies. Additionally, Lopez-Verrilli et al. found that MenSC-derived small EVs effectively enhanced the growth of primary neuronal cells [122]. The authors showed that MenSC-derived small EVs have superior potential when compared with MSC-derived small EVs from other sources (including bone marrow, umbilical cord, and chorion) in neurodegenerative diseases.
Current challenges of MenSC-derived small EVs for tissue repair
Although MenSC-derived small EVs have been described in several studies, the effective elements of small MenSC-derived EVs remain a mystery. Small EVs contain bioactive molecules that affect the characteristics of target cells [82, 98]. Additionally, the involvement of miRNAs in the cellular and molecular mechanisms of MenSC-derived small EVs is of great importance, but to date, only a few miRNAs (miR-21and let-7) have been explored [134, 146]. In fact, MenSC expresses octamer-binding transcription factor 4 (OCT-4), which is a marker of ESC [154], a distinct marker compared with other sources of MSCs. Research on MenSC-derived small EVs is relatively limited compared with MSC-derived small EVs from other sources (such as bone marrow, adipose tissue, and umbilical cord). Currently, the similarity of therapeutic mechanisms between MenSC-derived and other sources of small EVs is mainly due to the secretion of effective bioactive molecules and production of miRNAs [163]. The miR-21, miR-27a, miR-196a, and miR-206 are abundant in EVs from BM-MSCs and are responsible for pro-regenerative and immunomodulatory effects [164–166]; miR-20, miR-21, miR-23a, miR-125b, miR-326, and miR-145 are profuse in EVs from UC-MSCs and are responsible for mediation of apoptosis, regulation of autophagy, inhibition of neddylation, and suppression of myofibroblast differentiation [167–169]; let-7, miR148a, miR378, and miR532-5p are abundant in EVs from AD-MSCs and are responsible for angiogenesis, cellular transport, apoptosis, and proteolysis [170, 171]; and let-7 and miR21 are abundant in EVs from MenSCs and are responsible for regulating mitochondrial-DNA damage and enhancing cell survival rate [134, 146]. Several studies explored MSC-derived small EVs signaling pathways [64, 160, 172, 173], supporting that a thorough database of small EVs from MenSCs is needed to further assess their therapeutic potential. Additionally, current studies about small EVs from MenSCs are relatively few and most of them are preliminary, the further in-depth comparisons are necessary between MenSC-derived and other sources of MSC-derived small EVs. And distinct bioactive elements and special signaling pathways from MenSC-derived small EVs are needed to be explored in the future.
Determining the optimal dose and appropriate time points for the administration of small EVs without adverse effects are vital issues. The quality control of MenSC-derived small EVs is an important factor, an indispensable link in the process for the final approval of MenSC-derived small EV therapy. The quality of small EVs mainly includes characteristics, purity, efficacy, safety, and stability based on a large amount of data to establish the standards of consistency and stability. Although MenSC is a heterogeneous cell population, as a minimum standard catalog, it must follow the current guidelines of the International Society for Cellular Therapy [174]. Different methods to separate and quantify MenSC-derived small EVs with different identification standards may cause controversy and reduce reliability in experimental conclusions. It is difficult to analyze and compare exosomes from different sources because the corresponding contents are also discrepant. Therefore, establishing a unified standard of MenSC-derived small EVs will facilitate their clinical application.
The long-term effect of MenSC-derived small EVs is a vital issue that needs to be addressed in regenerative medicine. There are few studies concerning the sustained therapeutic effects. Current purification and enrichment strategies (including ultracentrifugal collection, tandem filtration, and polyethylene glycol precipitation) of MSC-derived small EVs originate from the manufacturing methods of viruses or viral-like particles. The stabilization of the purity and physiological function of MenSC-derived small EVs remains a problem. Therefore, if any viral-related products (including lentiviral and adenoviral vectors of gene editing) are present in the conditioned medium or recipient cell, they will be enriched in the final exosome extraction, which is a potential risk for safe use. In addition, small EVs contain abundant small RNAs. These small RNAs may increase the instability of nucleic acid chains or cause structural changes in partial tissues along with some complications [175, 176]. Therefore, before MenSC-derived small EVs are applied in clinical medicine, more studies are required with a large number of basic medicine and clinical trials to assess their long-term safety.
Future perspectives of MenSC-derived small EVs in regenerative medicine
As there is great potential for the clinical application of MenSC-derived small EVs, novel strategies should be developed to expedite this process. Future perspectives of MenSC-derived small EVs with regard to regenerative medicine will be devoted to the aspects subsequently described (Fig. S1).
Engineered MenSC-derived small EVs
Currently, genome editing is a novel technology widely applied in genetic modifications, functional genomics, transcriptional regulations, and stem-cell therapies. With the rapid development of CRISPR/Cas9, engineered MSC-derived small EVs are a powerful tool [94, 177, 178]. This modification can be achieved by overexpressing proteins or modifying miRs in MSCs to achieve changes in exosomes [179]. These engineered MSC-derived small EVs have a higher therapeutic potential than the initial MSC-derived small EVs. This has been proven for small EVs from miRs (including miR-92a-3p, miR-133b, miR-181-5p, miR-22-3p, miR-31, miR-466, and miR-584)-engineered MSCs [180–186]. Additionally, small EVs from proteins (including SDF-1, TRAIL, TIMP2, P53, IDO1, and PEDF)-engineered MSCs also improved the treatment outcome in regenerative medicine [187–192]. Owing to the therapeutic potential of MenSC-derived small EVs in several diseases, some engineered small EVs of MenSC are establishing a foundation for clinical trials and clinical medicine. As there are only sporadic studies on miR-21 engineered MenSC-derived small EVs in treating MI [134], more engineered MenSC-derived small EVs should be explored.
Hypoxia-treated MenSC-derived small EVs
Hypoxia is an important feature of various tumors. It can maintain the survival of tumor cells and has a strong correlation with tumor invasion and poor prognosis [193]. Hypoxic cells undergo extensive intracellular molecular and metabolic regulation to create a tumor microenvironment that is conducive to their survival and growth. Cells secrete various cytokines, exosomes, proteins, nucleic acids, and lipids during hypoxia. In fact, hypoxia-treated MSC-derived exosomes have a better effect in treating diseases. Small EVs from hypoxia-treated human AD-MSCs have a high ability to increase angiogenesis through VEGF/VEGF-receptor and protein kinase A (PKA) signaling pathways [194, 195]. Zhu et al. discovered that BM-MSC-derived small EVs effectively protected the cardiac function through miR-125b in a hypoxia-induced MI mouse model [196]. Cheng et al. found that BM-MSC-derived small EVs restrained apoptosis to improve myocyte protection in a hypoxia-challenged MI rat model, partially owing to exosomes containing miR-210 [197]. Thus, hypoxia-treated MenSC-derived small EVs could be a strong candidate for enhancing the cardiac function.
MenSC-derived small EVs combined with targeting drugs
Small EVs have a series of advantages as drug carriers, such as unique structure and physicochemical properties, effective cell access, low immunogenicity and toxicity, and natural capacity to cross organism barriers [198, 199]. Additionally, MSC-derived small EVs can deliver drugs to recipient cells in a highly selective manner [98, 200]. In other words, MSC-derived small EVs are an ideal delivery system for small molecular drugs. Chang et al. found that AD-MSC-derived small EVs combined with 50 mg/kg/day melatonin improved acute inflammatory colitis in a rat model [201]. Kalimuthu et al. verified that paclitaxel (25, 50, and 100 mg/mL) mixed with BM-MSC-derived small EVs were more powerful than single BM-MSC-derived small EVs in inhibiting breast cancer [202]. The authors revealed that the loading efficiency was 38.9, 76.1, and 74.22 ng/mg for 25, 50, and 100 mg/mL of paclitaxel, respectively. Currently, targeting drugs vary with specificity and uptake efficiency of recipient cells; thus, further investigation is needed to confirm the optimum dose of each qualified drug. We believe that targeting drugs combined with MenSC-derived small EVs is promising to exert a stronger role than that of MenSC-derived small EVs alone.
MenSC-derived small EVs from three-dimensional cultures
Three-dimensional (3D) tissue-specific cultures have been a powerful tool in disease therapy in recent years, and a large number of studies have been conducted on various diseases [203]. Currently, 3D structures can be derived from pluripotent stem cells (including ESCs and iPSCs) or adult stem cells (including epithelial cells and MSCs [204, 205]. 3D culture can provide researchers with precise control over spatial heterogeneity within the tumor microenvironment by spatially depositing predefined biobanks that contain multiple stem-cell types, biochemical factors, and ECMs [206, 207]. Kim et al. found that 3D-cultured MSCs significantly enhanced the secretion efficiency of exosomes and their production [208]. Furthermore, exosomes from 3D-cultured BM-MSCs [209] and UC-MSCs [210] showed a powerful regeneration capacity. Although 3D culture from single-cultured MSCs has not been systematically reported, small EVs from 3D-cultured MenSCs would produce abundant bioactive molecules to meet the dose requirements for clinical medicine.
MenSC-derived small EVs for cancer immunotherapy
The successful application of immune checkpoint inhibitors of cytotoxic T lymphocyte antigen-4, programmed cell death protein 1 (PD-1), and programmed cell death protein ligand 1 (PD-L1) in various diseases has attracted interest in the field of immunotherapy, especially cancer immunotherapy [211–213]. The underlying function of small EVs has been explored in cancer immunotherapy as a novel therapeutic strategy [214, 215]. The immune-modulation of MSC-derived small EVs has been applied, for example, to improve skin regeneration [216], protect against hearing loss [217], prevent inflammation, or induce remyelination in multiple sclerosis [218], graft-versus-host disease [219], and asthma [220]. Marinaro et al. revealed that MenSC-derived small EVs exert immunomodulatory effects in the treatment of inflammatory conditions by immunomodulatory proteins and several miRNAs using proteomics and genomics analyses [162]. Thus, MenSC-derived small EVs may be a competitive candidate for future cancer immunotherapy owing to their outstanding immunomodulatory role.
MenSC-derived small EVs immobilized in hydrogel
The use of chemical materials with biological functions may be an interesting candidate to transfer MSC-derived small EVs [221]. Biomaterials can provide matrix interaction, enhancing the transmission effect of MSC-derived small EVs and affect secretion characteristics through signal transmission from outside to inside. Currently, well-defined synthetic hydrogels are promising carriers for the delivery of stem cells [222, 223]. Shi et al. found that the combination of human gingival exocrine MSCs and hydrogel can effectively alleviate skin wound healing in diabetic rats by improving collagen epithelialization, deposition, and remodeling and increase angiogenesis and neuron growth [224]. Zhang et al. verified that chitosan hydrogel combined with MSC-derived small EVs significantly enhanced the therapeutic roles of hindlimb ischemia, via firefly luciferase imaging of angiogenesis [225]. Zhao et al. found that chitosan hydrogel-encapsulated MSC-derived small EVs significantly prolonged the aging of skin processes by improving the function of old dermal fibroblasts [226]. Li et al. established a system for human MSC-derived small EVs immobilized in an exosome peptide-modified adhesive hydrogel (Exo-pGel), which effectively migrated to the spinal cord injury microenvironment and exerted evident nerve recovery and urinary tissue preservation through relieving inflammation and oxidation [227]. Thus, the function of MenSC-derived small EVs may effectively enhance immobilization in hydrogels, and this may be a promising strategy in future regenerative medicine.
Conclusions
MenSC-derived small EVs deliver a large amount of regulatory proteins and mRNAs to improve the regenerative repair of wounded cells and tissues. While complexities about their therapeutic potential continue to be unraveled, advances are continuously found in both basic science and clinical medicine. Novel techniques (including engineered molecules, hypoxia-treated conditions, targeting drugs, 3D culture, cancer immunotherapy, and hydrogel) with respect to MenSC-derived small EVs may further promote translational medicine. Additionally, a strategy for developing the clinical use of MenSC-derived small EVs was proposed (Fig. 2). Rapid progress in separation techniques and combinations are available in MSC-derived small EVs, as important sources of MSC, MenSC, and MenSC-derived small EVs should be explored in the future. Additionally, the function of MenSC-derived small EVs also needs to be investigated for further comparisons with other sources of MSC-derived small EVs. In summary, although more research is needed, MenSC-derived small EV-based therapy has great potential for treating various diseases in regenerative medicine.
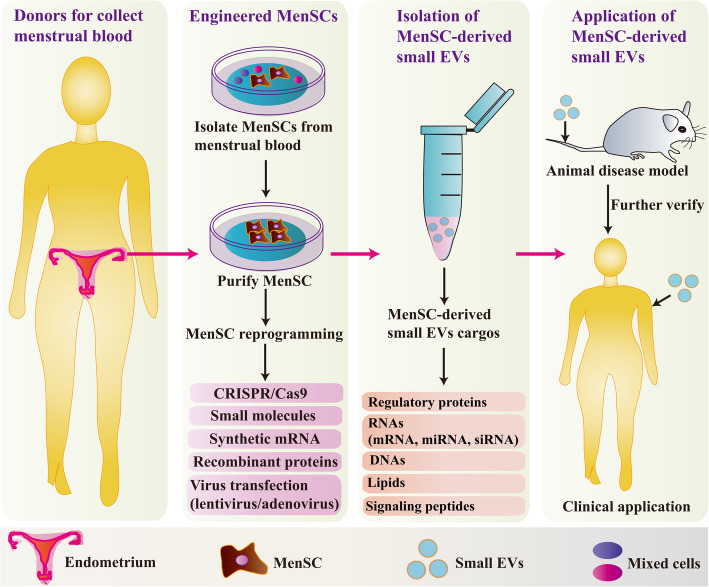
Fig. 2.
The strategy for developing clinical applications of MenSC-derived small EVs. Tissue donors should be selected and examined for MenSC-derived small EVs production. Donors can be autologous or allogeneic obtained from menstrual blood. MenSCs modification by bioengineering [CRISPR/Cas9, small molecules, synthetic mRNA, virus transfection (lentivirus/adenovirus), recombinant proteins] may be considered to improve therapeutic efficacy of MenSC-derived small EVs. Therapeutic effects of engineered MenSC may be further improved by encapsulating miRNA or siRNA in them. The application of MenSC-derived small EVs first evaluated the safety and effectiveness through an animal disease model. Then, MenSC-derived small EVs were employed to treat a variety of diseases in the clinic
Supplementary Information
Additional file 1: Fig. S1. Perspectives of MenSC-derived small EVs in regenerative medicine. The electron microscopy of MenSC-derived small EVs are labelled with a yellow arrow (the scale is 200 nm). As the great potential of the clinical application of MenSC-derived small EVs, novel strategies with regard to future perspectives of MenSC-derived small EVs are shown in: (1) engineered MenSC-derived small EVs, which is contributed by gene editing (such as CRISPR/Cas9, overexpression and RNA interference); (2) hypoxia-treated MenSC-derived small EVs, which improves the microenvironment conducive to their survival and growth; (3) MenSC-derived small EVs combined with targeting drug, which enhances the delivering efficiency of drugs to recipient cells; (4) 3D-culture of MenSC-derived small EVs, which increases the secreting efficiency of small EVs; (5) MenSC-derived small EVs for cancer immunotherapy, which enhances the immunomodulatory role; (6) MenSC-derived small EVs immobilized in hydrogel, which enhances the transmission effect.
Acknowledgements
The authors thank Prof. Jinfu Wang from the College of Life Sciences, Zhejiang University for critical review of this manuscript, and Prof. Ting Xie from Stowers Institute for Medical Research for technical help. Additionally, we would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- AD
Adipose tissue
- ALF
Acute liver failure
- Alix
ALG-2-interacting protein-X
- AML12
Alpha mouse liver 12
- BM
Bone marrow
- ESC
Embryonic stem cell
- ERAP1
Endoplasmic reticulum aminopeptidase 1
- EV
Extracellular vesicle
- Exo-pGel
Exosome peptide-modified adhesive hydrogel
- FHF
Fulminant hepatic failure
- HIF-1α
Hypoxia-inducible factor-1α
- HSP
Heat shock protein
- ICAM-1
Intercellular adhesion molecule-1
- IFN
Interferon
- IGFBP-6
Insulin-like growth factor-binding protein-6
- IL
Interleukin
- iPSC
Induced pluripotent stem cell
- let-7
Lethal-7
- LPS
Lipopolysaccharide
- LOX-1
Lectin-like oxidized low-density lipoprotein receptor-1
- MenSC
Menstrual blood-derived mesenchymal stem cell
- MI
Myocardial infarction
- miR
MicroRNA
- MSC
Mesenchymal stem cell
- NF-κB
Nuclear factor kappa B
- NLRP
NOD-, LRR-, and pyrin domain-containing protein 3
- OLT
Orthotopic liver transplantation
- PC
Prostate cancer
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death protein ligand 1
- Pdx-1
Pancreatic and duodenal homeobox 1
- PK
Protein kinase
- PTEN
Phosphatase and tensin homolog
- ROS
Reactive oxygen species
- TNF-α
Tumor necrosis factor-α
- T1DM
Type-1 diabetes mellitus
- UC
Umbilical cord
- VEGF
Vascular endothelial growth factor
- 3D
Three-dimensional
Authors’ contributions
Charlie Xiang designed the manuscript and approved the final manuscript for publication; Lijun Chen and Jingjing Qu wrote the manuscript; Quanhui Mei, Xin Chen, Yangxin Fang, Lu Chen, and Yifei Li collected the references and modified the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81900563 and 81802278), the National Major Scientific and Technological Special Project for “Significant New Drugs Development” of China (2018ZX09201002-005), the Independent Task of State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, and National Key R&D Program of China (2017YFA0105701).
Availability of data and materials
Please contact the corresponding author for data requests.
Declarations
Ethics approval and consent to participate:
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lijun Chen and Jingjing Qu are equally first authors.
References
- 1.Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells. 2019;37(7):855–864. doi: 10.1002/stem.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galipeau J, Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl Med. 2018;7(9):651–663. doi: 10.1002/sctm.18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alfaifi M, Eom YW, Newsome PN, Baik SK. Mesenchymal stromal cell therapy for liver diseases. J Hepatol. 2018;68(6):1272–1285. doi: 10.1016/j.jhep.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 8.Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323–3348. doi: 10.1007/s00018-019-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Blanc K, Davies LC. MSCs-cells with many sides. Cytotherapy. 2018;20(3):273–278. doi: 10.1016/j.jcyt.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77(14):2771–2794. doi: 10.1007/s00018-020-03454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeken LJ, Ting DSJ, Sidney LE. Potential of mesenchymal stem cells as topical immunomodulatory cell therapies for ocular surface inflammatory disorders. Stem Cells Transl Med. 2021;10(1):39–49. doi: 10.1002/sctm.20-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther. 2020;11(1):345. doi: 10.1186/s13287-020-01855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller CA, Gonwa TA, Hodge DO, Hei DJ, Centanni JM, Zubair AC. Feasibility, safety, and tolerance of mesenchymal stem cell therapy for obstructive chronic lung allograft dysfunction. Stem Cells Transl Med. 2018;7(2):161–167. doi: 10.1002/sctm.17-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6(2):613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, Chen L, Gao H, Lu X, Yu L, et al. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering (Beijing) 2020;6(10):1153–1161. doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl Med. 2020;9(1):17–27. doi: 10.1002/sctm.19-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, Wang S, Zhang C, Yuan X, Xu Z, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6(1):58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, Alvarez Gil A, Poggioli R, Ruiz P, Marttos AC, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13(9):1738–1755. doi: 10.1002/term.2914. [DOI] [PubMed] [Google Scholar]
- 20.Musial-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28(7):801–812. doi: 10.1177/0963689719837897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128–1139. doi: 10.1038/cdd.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camernik K, Mihelic A, Mihalic R, Haring G, Herman S, Marolt Presen D, Janez A, Trebse R, Marc J, Zupan J. Comprehensive analysis of skeletal muscle- and bone-derived mesenchymal stem/stromal cells in patients with osteoarthritis and femoral neck fracture. Stem Cell Res Ther. 2020;11(1):146. doi: 10.1186/s13287-020-01657-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeggini E, Gloyn AL, Barton AC, Wain LV. Translational genomics and precision medicine: Moving from the lab to the clinic. Science. 2019;365(6460):1409–1413. doi: 10.1126/science.aax4588. [DOI] [PubMed] [Google Scholar]
- 24.Muller H, Dagher G, Loibner M, Stumptner C, Kungl P, Zatloukal K. Biobanks for life sciences and personalized medicine: importance of standardization, biosafety, biosecurity, and data management. Curr Opin Biotechnol. 2020;65:45–51. doi: 10.1016/j.copbio.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Ho D, Quake SR, McCabe ERB, Chng WJ, Chow EK, Ding X, Gelb BD, Ginsburg GS, Hassenstab J, Ho CM, et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol. 2020;38(5):497–518. doi: 10.1016/j.tibtech.2019.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin I, Galipeau J, Kessler C, Le Blanc K, Dazzi F. Challenges for mesenchymal stromal cell therapies. Sci Transl Med. 2019;11(480):eaat2189. [DOI] [PubMed]
- 28.Petrenko Y, Vackova I, Kekulova K, Chudickova M, Koci Z, Turnovcova K, Kupcova Skalnikova H, Vodicka P, Kubinova S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci Rep. 2020;10(1):4290. doi: 10.1038/s41598-020-61167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv H, Hu Y, Cui Z, Jia H. Human menstrual blood: a renewable and sustainable source of stem cells for regenerative medicine. Stem Cell Res Ther. 2018;9(1):325. doi: 10.1186/s13287-018-1067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Niu R, Li W, Lin J, Stamm C, Steinhoff G, Ma N. Therapeutic potential of menstrual blood-derived endometrial stem cells in cardiac diseases. Cell Mol Life Sci. 2019;76(9):1681–1695. doi: 10.1007/s00018-019-03019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Chen X, Zhao Y, Wang Y, Li Y, Xiang C. Genome-wide DNA methylation and hydroxymethylation analysis reveal human menstrual blood-derived stem cells inhibit hepatocellular carcinoma growth through oncogenic pathway suppression via regulating 5-hmC in enhancer elements. Stem Cell Res Ther. 2019;10(1):151. doi: 10.1186/s13287-019-1243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Qu J, Cheng T, Chen X, Xiang C. Menstrual blood-derived stem cells: toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res Ther. 2019;10(1):406. doi: 10.1186/s13287-019-1503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khoury M, Alcayaga-Miranda F, Illanes SE, Figueroa FE. The promising potential of menstrual stem cells for antenatal diagnosis and cell therapy. Front Immunol. 2014;5:205. doi: 10.3389/fimmu.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu B, Ding F, Hu D, Zhou Y, Long C, Shen L, Zhang Y, Zhang D, Wei G. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-kappaB signaling pathway in vivo and in vitro. Stem Cell Res Ther. 2018;9(1):7. doi: 10.1186/s13287-017-0760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Qu J, Xiang C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 2019;10(1):1. [DOI] [PMC free article] [PubMed]
- 37.Xu X, Jiang W, Chen L, Xu Z, Zhang Q, Zhu M, Ye P, Li H, Yu L, Zhou X, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clin Transl Med. 2021;11(2):e297. doi: 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X, Wang Y, Zhang B, Lan X, Lu S, Sun P, Li X, Shi G, Zhao Y, Han H, et al. Treatment of experimental colitis by endometrial regenerative cells through regulation of B lymphocytes in mice. Stem Cell Res Ther. 2018;9(1):146. doi: 10.1186/s13287-018-0874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, Xu X, Kong L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman's syndrome. Hum Reprod. 2016;31(12):2723–2729. doi: 10.1093/humrep/dew235. [DOI] [PubMed] [Google Scholar]
- 40.Khanmohammadi M, Golshahi H, Saffarian Z, Montazeri S, Khorasani S, Kazemnejad S. Repair of osteochondral defects in rabbit knee using menstrual blood stem cells encapsulated in fibrin glue: a good stem cell candidate for the treatment of osteochondral defects. Tissue Eng Regen Med. 2019;16(3):311–324. doi: 10.1007/s13770-019-00189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo Y, Zhang Z, Xu X, Xu Z, Wang S, Huang D, Li Y, Mou X, Liu F, Xiang C. Menstrual blood-derived stem cells as delivery vehicles for oncolytic adenovirus virotherapy for colorectal cancer. Stem Cells Dev. 2019;28(13):882–896. doi: 10.1089/scd.2018.0222. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Zhang C, Chen L, Wang X, Xiang B, Wu X, Guo Y, Mou X, Yuan L, Chen B, et al. Human menstrual blood-derived stem cells ameliorate liver fibrosis in mice by targeting hepatic stellate cells via paracrine mediators. Stem Cells Transl Med. 2017;6(1):272–284. doi: 10.5966/sctm.2015-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang QY, Zhang SW, Li PP, Yuan ZW, Tan JC. Safety of menstrual blood-derived stromal cell transplantation in treatment of intrauterine adhesion. World J Stem Cells. 2020;12(5):368–380. doi: 10.4252/wjsc.v12.i5.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Wang Y, Wang Z, Gutkind JS, Wang Z, Wang F, Lu J, Niu G, Teng G, Chen X. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells. 2015;33(2):456–467. doi: 10.1002/stem.1878. [DOI] [PubMed] [Google Scholar]
- 45.Seo Y, Kang MJ, Kim HS. Strategies to potentiate paracrine therapeutic efficacy of mesenchymal stem cells in inflammatory diseases. Int J Mol Sci. 2021;22(7):1619. [DOI] [PMC free article] [PubMed]
- 46.Shammaa R, El-Kadiry AE, Abusarah J, Rafei M. Mesenchymal Stem Cells Beyond Regenerative Medicine. Front Cell Dev Biol. 2020;8:72. doi: 10.3389/fcell.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 48.Chang C, Yan J, Yao Z, Zhang C, Li X, Mao HQ. Effects of mesenchymal stem cell-derived paracrine signals and their delivery strategies. Adv Healthc Mater. 2021;10(7):e2001689. [DOI] [PMC free article] [PubMed]
- 49.Maacha S, Sidahmed H, Jacob S, Gentilcore G, Calzone R, Grivel JC, Cugno C. Paracrine mechanisms of mesenchymal stromal cells in angiogenesis. Stem Cells Int. 2020;2020:4356359. doi: 10.1155/2020/4356359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu H, Lee CW, Wang YF, Huang S, Shin LY, Wang YH, Wan Z, Zhu X, Yung PSH, Lee OK. The role of paracrine regulation of mesenchymal stem cells in the crosstalk with macrophages in musculoskeletal diseases: a systematic review. Front Bioeng Biotechnol. 2020;8:587052. doi: 10.3389/fbioe.2020.587052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razavi M, Rezaee M, Telichko A, Inan H, Dahl J, Demirci U, Thakor AS. The Paracrine Function of Mesenchymal Stem Cells in Response to Pulsed Focused Ultrasound. Cell Transplant. 2020;29:963689720965478. doi: 10.1177/0963689720965478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obendorf J, Fabian C, Thome UH, Laube M. Paracrine stimulation of perinatal lung functional and structural maturation by mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):525. doi: 10.1186/s13287-020-02028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parate D, Kadir ND, Celik C, Lee EH, Hui JHP, Franco-Obregon A, Yang Z. Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Stem Cell Res Ther. 2020;11(1):46. doi: 10.1186/s13287-020-1566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Goncalves RM. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front Immunol. 2018;9:2837. doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 2015;23(5):812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang B, Tian X, Hao J, Xu G, Zhang W. Mesenchymal stem cell-derived extracellular vesicles in tissue regeneration. Cell Transplant. 2020;29:963689720908500. doi: 10.1177/0963689720908500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu CH, Tseng YW, Chiou CY, Lan KC, Chou CH, Tai CS, Huang HD, Hu CW, Liao KH, Chuang SS, et al. Bone marrow concentrate-induced mesenchymal stem cell conditioned medium facilitates wound healing and prevents hypertrophic scar formation in a rabbit ear model. Stem Cell Res Ther. 2019;10(1):275. doi: 10.1186/s13287-019-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park KS, Bandeira E, Shelke GV, Lasser C, Lotvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10(1):288. doi: 10.1186/s13287-019-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Manrique P, Gutierrez G, Blanco-Lopez MC. Fully Artificial Exosomes: Towards New Theranostic Biomaterials. Trends Biotechnol. 2018;36(1):10–14. doi: 10.1016/j.tibtech.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Losurdo M, Pedrazzoli M, D'Agostino C, Elia CA, Massenzio F, Lonati E, Mauri M, Rizzi L, Molteni L, Bresciani E, et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Transl Med. 2020;9(9):1068–1084. doi: 10.1002/sctm.19-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan Y, Jiang W, Tan Y, Zou S, Zhang H, Mao F, Gong A, Qian H. Xu W: hucMSC exosome-derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther. 2017;25(2):465–479. doi: 10.1016/j.ymthe.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Braun RK, Chetty C, Balasubramaniam V, Centanni R, Haraldsdottir K, Hematti P, Eldridge MW. Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem Biophys Res Commun. 2018;503(4):2653–2658. doi: 10.1016/j.bbrc.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Z, Ge L, Zhang S, Wang J, Jiang W, Xin Q, Luan Y. The protective effects of MSC-EXO against pulmonary hypertension through regulating Wnt5a/BMP signalling pathway. J Cell Mol Med. 2020;24(23):13938–13948. doi: 10.1111/jcmm.16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 66.Gandham S, Su X, Wood J, Nocera AL, Alli SC, Milane L, Zimmerman A, Amiji M, Ivanov AR. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020;38(10):1066–1098. doi: 10.1016/j.tibtech.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stevic I, Buescher G, Ricklefs FL. Monitoring therapy efficiency in cancer through extracellular vesicles. Cells. 2020;9(1):130. doi: 10.3390/cells9010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paolicelli RC, Bergamini G, Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience. 2019;405:148–157. doi: 10.1016/j.neuroscience.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Valter M, Verstockt S, Finalet Ferreiro JA, Cleynen I. Extracellular vesicles in inflammatory bowel disease: small particles, big players. J Crohns Colitis. 2021;15(3):499–510. doi: 10.1093/ecco-jcc/jjaa179. [DOI] [PubMed] [Google Scholar]
- 70.Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, Zambirinis CP, Rodrigues G, Molina H, Heissel S, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182(4):1044–1061. doi: 10.1016/j.cell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, Buzas EI, Lotvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2:20677. doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21(10):585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agrahari V, Agrahari V, Burnouf PA, Chew CH, Burnouf T. Extracellular microvesicles as new industrial therapeutic Frontiers. Trends Biotechnol. 2019;37(7):707–729. doi: 10.1016/j.tibtech.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 74.Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M, Hill AF, De Kleijn D, Koh M, Lai RC, et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8(1):1609206. doi: 10.1080/20013078.2019.1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woo CH, Kim HK, Jung GY, Jung YJ, Lee KS, Yun YE, Han J, Lee J, Kim WS, Choi JS, et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J Extracell Vesicles. 2020;9(1):1735249. doi: 10.1080/20013078.2020.1735249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang SB, Zhang HY, Wang C, He BX, Liu XQ, Meng XC, Peng YQ, Xu ZB, Fan XL, Wu ZJ, et al. Small extracellular vesicles derived from human mesenchymal stromal cells prevent group 2 innate lymphoid cell-dominant allergic airway inflammation through delivery of miR-146a-5p. J Extracell Vesicles. 2020;9(1):1723260. doi: 10.1080/20013078.2020.1723260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiklander OPB, Brennan MA, Lotvall J, Breakefield XO, El Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci Transl Med. 2019;11(492):eaav8521. doi: 10.1126/scitranslmed.aav8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Latifkar A, Hur YH, Sanchez JC, Cerione RA, Antonyak MA. New insights into extracellular vesicle biogenesis and function. J Cell Sci. 2019;132(13):jcs222406. doi: 10.1242/jcs.222406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hur YH, Cerione RA, Antonyak MA. Extracellular vesicles and their roles in stem cell biology. Stem Cells. 2020;38(4):469–476. doi: 10.1002/stem.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sung BH, Parent CA, Weaver AM. Extracellular vesicles: Critical players during cell migration. Dev Cell. 2021;56(13):1861-74. [DOI] [PMC free article] [PubMed]
- 82.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [DOI] [PMC free article] [PubMed]
- 83.Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, Zhao Y, Zhao X, Wang X, Ma Y, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4(1):69–83. doi: 10.1038/s41551-019-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu HK, Chen LJ, Zhou SN, Li YF, Xiang C. Multifunctional role of microRNAs in mesenchymal stem cell-derived exosomes in treatment of diseases. World J Stem Cells. 2020;12(11):1276–1294. doi: 10.4252/wjsc.v12.i11.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He X, Zhong X, Hu Z, Zhao S, Wei P, Li D. An insight into small extracellular vesicles: Their roles in colorectal cancer progression and potential clinical applications. Clin Transl Med. 2020;10(8):e249. doi: 10.1002/ctm2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54(Suppl 2):789–792. doi: 10.1038/s41409-019-0616-z. [DOI] [PubMed] [Google Scholar]
- 87.Fafian-Labora JA, Rodriguez-Navarro JA, O'Loghlen A. Small extracellular vesicles have GST activity and ameliorate senescence-related tissue damage. Cell Metab. 2020;32(1):71–86. doi: 10.1016/j.cmet.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harada Y, Nakajima K, Suzuki T, Fukushige T, Kondo K, Seino J, Ohkawa Y, Suzuki T, Inoue H, Kanekura T, et al. Glycometabolic regulation of the biogenesis of small extracellular vesicles. Cell Rep. 2020;33(2):108261. doi: 10.1016/j.celrep.2020.108261. [DOI] [PubMed] [Google Scholar]
- 89.Moller A, Lobb RJ. The evolving translational potential of small extracellular vesicles in cancer. Nat Rev Cancer. 2020;20(12):697–709. doi: 10.1038/s41568-020-00299-w. [DOI] [PubMed] [Google Scholar]
- 90.Preciado S, Muntion S, Sanchez-Guijo F. Improving hematopoietic engraftment: potential role of mesenchymal stromal cell-derived extracellular vesicles. Stem Cells. 2021;39(1):26–32. doi: 10.1002/stem.3278. [DOI] [PubMed] [Google Scholar]
- 91.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49(3):347–360. doi: 10.1016/j.devcel.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 92.Li S, Yi M, Dong B, Jiao Y, Luo S, Wu K. The roles of exosomes in cancer drug resistance and its therapeutic application. Clin Transl Med. 2020;10(8):e257. doi: 10.1002/ctm2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 94.Elahi FM, Farwell DG, Nolta JA, Anderson JD. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells. 2020;38(1):15–21. doi: 10.1002/stem.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Joo S, Lee B. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21(3):727. doi: 10.3390/ijms21030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. 2020;9(3):660. [DOI] [PMC free article] [PubMed]
- 98.Baek G, Choi H, Kim Y, Lee HC, Choi C. Mesenchymal stem cell-derived extracellular vesicles as therapeutics and as a drug delivery platform. Stem Cells Transl Med. 2019;8(9):880–886. doi: 10.1002/sctm.18-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hong P, Yang H, Wu Y, Li K, Tang Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019;10(1):242. doi: 10.1186/s13287-019-1358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, Kourembanas S. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019;4(21):e128060. doi: 10.1172/jci.insight.128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X, Thomsen P. Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration. Basic Clin Pharmacol Toxicol. 2021;128(1):18–36. doi: 10.1111/bcpt.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen A, Tang S, He J, Li X, Peng G, Zhang H, Chen J, Chen L, Chen X. Small extracellular vesicles from human adipose-derived mesenchymal stromal cells: a potential promoter of fat graft survival. Stem Cell Res Ther. 2021;12(1):263. doi: 10.1186/s13287-021-02319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi J, Zhao YC, Niu ZF, Fan HJ, Hou SK, Guo XQ, Sang L, Lv Q. Mesenchymal stem cell-derived small extracellular vesicles in the treatment of human diseases: Progress and prospect. World J Stem Cells. 2021;13(1):49–63. doi: 10.4252/wjsc.v13.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shah TG, Predescu D, Predescu S. Mesenchymal stem cells-derived extracellular vesicles in acute respiratory distress syndrome: a review of current literature and potential future treatment options. Clin Transl Med. 2019;8(1):25. doi: 10.1186/s40169-019-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bodart-Santos V, de Carvalho LRP, de Godoy MA, Batista AF, Saraiva LM, Lima LG, Abreu CA, De Felice FG, Galina A, Mendez-Otero R, et al. Extracellular vesicles derived from human Wharton’s jelly mesenchymal stem cells protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. Stem Cell Res Ther. 2019;10(1):332. doi: 10.1186/s13287-019-1432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Allan D, Tieu A, Lalu M, Burger D. Mesenchymal stromal cell-derived extracellular vesicles for regenerative therapy and immune modulation: Progress and challenges toward clinical application. Stem Cells Transl Med. 2020;9(1):39–46. doi: 10.1002/sctm.19-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li M, Soder R, Abhyankar S, Abdelhakim H, Braun MW, Trinidad CV, Pathak HB, Pessetto Z, Deighan C, Ganguly S, et al. WJMSC-derived small extracellular vesicle enhance T cell suppression through PD-L1. J Extracell Vesicles. 2021;10(4):e12067. doi: 10.1002/jev2.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reis LA, Borges FT, Simoes MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7(9):e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126(22):2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9(1):17. doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Villatoro AJ, Alcoholado C, Martin-Astorga MC, Fernandez V, Cifuentes M, Becerra J. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vet Immunol Immunopathol. 2019;208:6–15. doi: 10.1016/j.vetimm.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 113.Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197. doi: 10.1038/srep01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin KC, Yip HK, Shao PL, Wu SC, Chen KH, Chen YT, Yang CC, Sun CK, Kao GS, Chen SY, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–185. doi: 10.1016/j.ijcard.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 115.Matula Z, Nemeth A, Lorincz P, Szepesi A, Brozik A, Buzas EI, Low P, Nemet K, Uher F, Urban VS. The Role of extracellular vesicle and tunneling nanotube-mediated intercellular cross-talk between mesenchymal stem cells and human peripheral T cells. Stem Cells Dev. 2016;25(23):1818–1832. doi: 10.1089/scd.2016.0086. [DOI] [PubMed] [Google Scholar]
- 116.Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, Shao PL, Chen YL, Chai HT, Lin KC, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7(46):74537–74556. doi: 10.18632/oncotarget.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klymiuk MC, Balz N, Elashry MI, Heimann M, Wenisch S, Arnhold S. Exosomes isolation and identification from equine mesenchymal stem cells. BMC Vet Res. 2019;15(1):42. doi: 10.1186/s12917-019-1789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ju C, Li Y, Shen Y, Liu Y, Cai J, Liu N, Ma G, Tang Y. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes for Angiogenesis. J Cardiovasc Transl Res. 2018;11(5):429–437. doi: 10.1007/s12265-018-9824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22(6):845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 121.Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi: 10.1186/s12967-015-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129–139. doi: 10.1016/j.neuroscience.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 123.Wu S, Ju GQ, Du T, Zhu YJ, Liu GH. Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PLoS One. 2013;8(4):e61366. doi: 10.1371/journal.pone.0061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, Rice GE. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8(7):e68451. doi: 10.1371/journal.pone.0068451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5(3):76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jarmalaviciute A, Tunaitis V, Pivoraite U, Venalis A, Pivoriunas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. 2015;17(7):932–939. doi: 10.1016/j.jcyt.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 127.Wang M, Zhao C, Shi H, Zhang B, Zhang L, Zhang X, Wang S, Wu X, Yang T, Huang F, et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110(5):1199–1210. doi: 10.1038/bjc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guo SC, Tao SC, Yin WJ, Qi X, Sheng JG, Zhang CQ. Exosomes from human synovial-derived mesenchymal stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head in the rat. Int J Biol Sci. 2016;12(10):1262–1272. doi: 10.7150/ijbs.16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, Eslani M, Djalilian AR. Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2018;59(12):5194–5200. doi: 10.1167/iovs.18-24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fan Y, Herr F, Vernochet A, Mennesson B, Oberlin E, Durrbach A. Human fetal liver mesenchymal stem cell-derived exosomes impair natural killer cell function. Stem Cells Dev. 2019;28(1):44–55. doi: 10.1089/scd.2018.0015. [DOI] [PubMed] [Google Scholar]
- 131.Li W, Han Y, Zhao Z, Ji X, Wang X, Jin J, Wang Q, Guo X, Cheng Z, Lu M, et al. Oral mucosal mesenchymal stem cellderived exosomes: A potential therapeutic target in oral premalignant lesions. Int J Oncol. 2019;54(5):1567–1578. doi: 10.3892/ijo.2019.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiao GY, Cheng CC, Chiang YS, Cheng WT, Liu IH, Wu SC. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep. 2016;6:23120. doi: 10.1038/srep23120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen L, Xiang B, Wang X, Xiang C. Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res Ther. 2017;8(1):9. doi: 10.1186/s13287-016-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, Zhao J, Wang L, Wang Y, Zhong Z, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Transl Med. 2017;6(1):209–222. doi: 10.5966/sctm.2015-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alcayaga-Miranda F, Gonzalez PL, Lopez-Verrilli A, Varas-Godoy M, Aguila-Diaz C, Contreras L, Khoury M. Prostate tumor-induced angiogenesis is blocked by exosomes derived from menstrual stem cells through the inhibition of reactive oxygen species. Oncotarget. 2016;7(28):44462–44477. doi: 10.18632/oncotarget.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dalirfardouei R, Jamialahmadi K, Mahdipour E. A feasible method for the isolation of mesenchymal stem cells from menstrual blood and their exosomes. Tissue Cell. 2018;55:53–62. doi: 10.1016/j.tice.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 137.Marinaro F, Pericuesta E, Sanchez-Margallo FM, Casado JG, Alvarez V, Matilla E, Hernandez N, Blazquez R, Gonzalez-Fernandez L, Gutierrez-Adan A, et al. Extracellular vesicles derived from endometrial human mesenchymal stem cells improve IVF outcome in an aged murine model. Reprod Domest Anim. 2018;53(Suppl 2):46–49. doi: 10.1111/rda.13314. [DOI] [PubMed] [Google Scholar]
- 138.Mahdipour E, Salmasi Z, Sabeti N. Potential of stem cell-derived exosomes to regenerate beta islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J Cell Physiol. 2019;234(11):20310–20321. doi: 10.1002/jcp.28631. [DOI] [PubMed] [Google Scholar]
- 139.Marinaro F, Macias-Garcia B, Sanchez-Margallo FM, Blazquez R, Alvarez V, Matilla E, Hernandez N, Gomez-Serrano M, Jorge I, Vazquez J, et al. Extracellular vesicles derived from endometrial human mesenchymal stem cells enhance embryo yield and quality in an aged murine modeldagger. Biol Reprod. 2019;100(5):1180–1192. doi: 10.1093/biolre/ioy263. [DOI] [PubMed] [Google Scholar]
- 140.Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–568. doi: 10.1002/term.2799. [DOI] [PubMed] [Google Scholar]
- 141.Toh WS, Lai RC, Zhang B, Lim SK. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans. 2018;46(4):843–853. doi: 10.1042/BST20180079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ren H, Sang Y, Zhang F, Liu Z, Qi N, Chen Y. Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy. Stem Cells Int. 2016;2016:1–13. doi: 10.1155/2016/3516574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Squires JE, McKiernan P, Squires RH. Acute Liver Failure: An Update. Clin Liver Dis. 2018;22(4):773–805. doi: 10.1016/j.cld.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 144.Lu L, Liu M, Sun R, Zheng Y, Zhang P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem Biophys. 2015;72(3):865–867. doi: 10.1007/s12013-015-0553-4. [DOI] [PubMed] [Google Scholar]
- 145.Laporta Hernandez R, Aguilar Perez M, Lazaro Carrasco MT, Ussetti Gil P. Lung Transplantation in Idiopathic Pulmonary Fibrosis. Med Sci (Basel). 2018;6(3):68. [DOI] [PMC free article] [PubMed]
- 146.Sun L, Zhu M, Feng W, Lin Y, Yin J, Jin J, Wang Y. Exosomal miRNA Let-7 from menstrual blood-derived endometrial stem cells alleviates pulmonary fibrosis through regulating mitochondrial DNA damage. Oxidative Med Cell Longev. 2019;2019:4506303. doi: 10.1155/2019/4506303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mohler JL, Antonarakis ES, Armstrong AJ, D'Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington TA, Higano CS, et al. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2019;17(5):479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 148.Tharmalingam H, Choudhury A, Van Herk M, McWilliam A, Hoskin PJ. New approaches for effective and safe pelvic radiotherapy in high-risk prostate cancer. Nat Rev Urol. 2019;16(9):523–538. doi: 10.1038/s41585-019-0213-3. [DOI] [PubMed] [Google Scholar]
- 149.Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol. 2018;35:69–79. doi: 10.1016/j.smim.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhou Q, Liu LZ, Fu B, Hu X, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate insulin-induced VEGF and HIF-1alpha expression through the activation of p70S6K1 in human prostate cancer cells. Carcinogenesis. 2007;28(1):28–37. doi: 10.1093/carcin/bgl085. [DOI] [PubMed] [Google Scholar]
- 151.Wier EM, Garza LA. Through the lens of hair follicle neogenesis, a new focus on mechanisms of skin regeneration after wounding. Semin Cell Dev Biol. 2020;100:122–129. doi: 10.1016/j.semcdb.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20(3):291–301. doi: 10.1016/j.jcyt.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 153.Di Gialleonardo V, de Vries EF, Di Girolamo M, Quintero AM, Dierckx RA, Signore A. Imaging of beta-cell mass and insulitis in insulin-dependent (Type 1) diabetes mellitus. Endocr Rev. 2012;33(6):892–919. doi: 10.1210/er.2011-1041. [DOI] [PubMed] [Google Scholar]
- 154.Wu X, Luo Y, Chen J, Pan R, Xiang B, Du X, Xiang L, Shao J, Xiang C. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev. 2014;23(11):1245–1257. doi: 10.1089/scd.2013.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Qiao J, Wang ZB, Feng HL, Miao YL, Wang Q, Yu Y, Wei YC, Yan J, Wang WH, Shen W, et al. The root of reduced fertility in aged women and possible therapentic options: current status and future perspects. Mol Asp Med. 2014;38:54–85. doi: 10.1016/j.mam.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 156.Donnez J, Dolmans MM. Fertility Preservation in Women. N Engl J Med. 2017;377(17):1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 157.Giacomini E, Makieva S, Murdica V, Vago R, Vigano P. Extracellular vesicles as a potential diagnostic tool in assisted reproduction. Curr Opin Obstet Gynecol. 2020;32(3):179–184. doi: 10.1097/GCO.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 158.Ding C, Zhu L, Shen H, Lu J, Zou Q, Huang C, Li H, Huang B. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells. 2020;38(9):1137–1148. doi: 10.1002/stem.3204. [DOI] [PubMed] [Google Scholar]
- 159.Xin L, Lin X, Zhou F, Li C, Wang X, Yu H, Pan Y, Fei H, Ma L, Zhang S. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta Biomater. 2020;113:252–266. doi: 10.1016/j.actbio.2020.06.029. [DOI] [PubMed] [Google Scholar]
- 160.Yang W, Zhang J, Xu B, He Y, Liu W, Li J, Zhang S, Lin X, Su D, Wu T, et al. HucMSC-derived exosomes mitigate the age-related retardation of fertility in female mice. Mol Ther. 2020;28(4):1200–1213. doi: 10.1016/j.ymthe.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao S, Qi W, Zheng J, Tian Y, Qi X, Kong D, Zhang J, Huang X. Exosomes derived from adipose mesenchymal stem cells restore functional endometrium in a rat model of intrauterine adhesions. Reprod Sci. 2020;27(6):1266–1275. doi: 10.1007/s43032-019-00112-6. [DOI] [PubMed] [Google Scholar]
- 162.Marinaro F, Gomez-Serrano M, Jorge I, Silla-Castro JC, Vazquez J, Sanchez-Margallo FM, Blazquez R, Lopez E, Alvarez V, Casado JG. Unraveling the molecular signature of extracellular vesicles from endometrial-derived mesenchymal stem cells: potential modulatory effects and therapeutic applications. Front Bioeng Biotechnol. 2019;7:431. doi: 10.3389/fbioe.2019.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ghafouri-Fard S, Niazi V, Taheri M. Role of miRNAs in conveying message of stem cells via extracellular vesicles. Exp Mol Pathol. 2020;117:104569. doi: 10.1016/j.yexmp.2020.104569. [DOI] [PubMed] [Google Scholar]
- 164.Kim H, Lee MJ, Bae EH, Ryu JS, Kaur G, Kim HJ, Kim JY, Barreda H, Jung SY, Choi JM, et al. Comprehensive molecular profiles of functionally effective MSC-derived extracellular vesicles in immunomodulation. Mol Ther. 2020;28(7):1628–1644. doi: 10.1016/j.ymthe.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Deng CL, Hu CB, Ling ST, Zhao N, Bao LH, Zhou F, Xiong YC, Chen T, Sui BD, Yu XR, et al. Photoreceptor protection by mesenchymal stem cell transplantation identifies exosomal MiR-21 as a therapeutic for retinal degeneration. Cell Death Differ. 2021;28(3):1041–1061. doi: 10.1038/s41418-020-00636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961. doi: 10.1038/srep21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang L, Song Y, Chen L, Li D, Feng H, Lu Z, Fan T, Chen Z, Livingston MJ, Geng Q. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J Cell Physiol. 2020;235(4):3698–3710. doi: 10.1002/jcp.29264. [DOI] [PubMed] [Google Scholar]
- 168.Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, Qian X, Wu M, Ji K, Zhao Y, et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-beta/SMAD2 Pathway During Wound Healing. Stem Cells Transl Med. 2016;5(10):1425–1439. doi: 10.5966/sctm.2015-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang G, Yuan J, Cai X, Xu Z, Wang J, Ocansey DKW, Yan Y, Qian H, Zhang X, Xu W, et al. HucMSC-exosomes carrying miR-326 inhibit neddylation to relieve inflammatory bowel disease in mice. Clin Transl Med. 2020;10(2):e113. doi: 10.1002/ctm2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O'Brien D, van Wijnen AJ, Lerman LO. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hao X, Guo Y, Wang R, Yu X, He L, Shu M. Exosomes from adipose-derived mesenchymal stem cells promote survival of fat grafts by regulating macrophage polarization via let-7c. Acta Biochim Biophys Sin. 2021;53(4):501–510. doi: 10.1093/abbs/gmab018. [DOI] [PubMed] [Google Scholar]
- 172.Haider KH, Aramini B. Mircrining the injured heart with stem cell-derived exosomes: an emerging strategy of cell-free therapy. Stem Cell Res Ther. 2020;11(1):23. [DOI] [PMC free article] [PubMed]
- 173.de Araujo FV, O'Valle F, Serrano-Saenz S, Anderson P, Andres E, Lopez-Penalver J, Tovar I, Nieto A, Santos A, Martin F, et al. Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol Cancer. 2018;17(1):122. doi: 10.1186/s12943-018-0867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 175.Li Y, Yin Z, Fan J, Zhang S, Yang W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct Target Ther. 2019;4:47. doi: 10.1038/s41392-019-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cai Q, He B, Weiberg A, Buck AH, Jin H. Small RNAs and extracellular vesicles: New mechanisms of cross-species communication and innovative tools for disease control. PLoS Pathog. 2019;15(12):e1008090. doi: 10.1371/journal.ppat.1008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kostyushev D, Kostyusheva A, Brezgin S, Smirnov V, Volchkova E, Lukashev A, et al. Gene Editing by Extracellular Vesicles. Int J Mol Sci. 2020;21(19):7362. [DOI] [PMC free article] [PubMed]
- 178.Yao X, Lyu P, Yoo K, Yadav MK, Singh R, Atala A, Lu B. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J Extracell Vesicles. 2021;10(5):e12076. doi: 10.1002/jev2.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 2018;7(1):1522236. doi: 10.1080/20013078.2018.1522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9(1):247. doi: 10.1186/s13287-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li D, Zhang P, Yao X, Li H, Shen H, Li X, Wu J, Lu X. Exosomes Derived From miR-133b-Modified Mesenchymal Stem Cells Promote Recovery After Spinal Cord Injury. Front Neurosci. 2018;12:845. doi: 10.3389/fnins.2018.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim R, Lee S, Lee J, Kim M, Kim WJ, Lee HW, Lee MY, Kim J, Chang W. Exosomes derived from microRNA-584 transfected mesenchymal stem cells: novel alternative therapeutic vehicles for cancer therapy. BMB Rep. 2018;51(8):406–411. doi: 10.5483/BMBRep.2018.51.8.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, Lu L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017;21(10):2491–2502. doi: 10.1111/jcmm.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shi MM, Zhu YG, Yan JY, Rouby JJ, Summah H, Monsel A, Qu JM. Role of miR-466 in mesenchymal stromal cell derived extracellular vesicles treating inoculation pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. Clin Transl Med. 2021;11(1):e287. doi: 10.1002/ctm2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang K, Li F, Yuan Y, Shan L, Cui Y, Qu J, Lian F. Synovial Mesenchymal Stem Cell-Derived EV-Packaged miR-31 Downregulates Histone Demethylase KDM2A to Prevent Knee Osteoarthritis. Mol Ther Nucleic Acids. 2020;22:1078–1091. doi: 10.1016/j.omtn.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang X, Wang Y, Zhao H, Han X, Zhao T, Qu P, Li G, Wang W. Extracellular vesicle-encapsulated miR-22-3p from bone marrow mesenchymal stem cell promotes osteogenic differentiation via FTO inhibition. Stem Cell Res Ther. 2020;11(1):227. doi: 10.1186/s13287-020-01707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gong XH, Liu H, Wang SJ, Liang SW, Wang GG. Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. J Cell Physiol. 2019;234(8):13878–13893. doi: 10.1002/jcp.28070. [DOI] [PubMed] [Google Scholar]
- 188.Shamili FH, Bayegi HR, Salmasi Z, Sadri K, Mahmoudi M, Kalantari M, Ramezani M, Abnous K. Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. Int J Pharm. 2018;549(1-2):218–229. doi: 10.1016/j.ijpharm.2018.07.067. [DOI] [PubMed] [Google Scholar]
- 189.Ni J, Liu X, Yin Y, Zhang P, Xu YW, Liu Z. Exosomes Derived from TIMP2-Modified Human Umbilical Cord Mesenchymal Stem Cells Enhance the Repair Effect in Rat Model with Myocardial Infarction Possibly by the Akt/Sfrp2 Pathway. Oxidative Med Cell Longev. 2019;2019:1958941. doi: 10.1155/2019/1958941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mao J, Liang Z, Zhang B, Yang H, Li X, Fu H, Zhang X, Yan Y, Xu W, Qian H. UBR2 Enriched in p53 Deficient Mouse Bone Marrow Mesenchymal Stem Cell-Exosome Promoted Gastric Cancer Progression via Wnt/beta-Catenin Pathway. Stem Cells. 2017;35(11):2267–2279. doi: 10.1002/stem.2702. [DOI] [PubMed] [Google Scholar]
- 191.He JG, Xie QL, Li BB, Zhou L, Yan D. Exosomes Derived from IDO1-Overexpressing Rat Bone Marrow Mesenchymal Stem Cells Promote Immunotolerance of Cardiac Allografts. Cell Transplant. 2018;27(11):1657–1683. doi: 10.1177/0963689718805375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Huang X, Ding J, Li Y, Liu W, Ji J, Wang H, Wang X. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp Cell Res. 2018;371(1):269–277. doi: 10.1016/j.yexcr.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 193.Kumar A, Deep G. Hypoxia in tumor microenvironment regulates exosome biogenesis: Molecular mechanisms and translational opportunities. Cancer Lett. 2020;479:23–30. doi: 10.1016/j.canlet.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 194.Xue C, Shen Y, Li X, Li B, Zhao S, Gu J, Chen Y, Ma B, Wei J, Han Q, et al. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis Through the PKA Signaling Pathway. Stem Cells Dev. 2018;27(7):456–465. doi: 10.1089/scd.2017.0296. [DOI] [PubMed] [Google Scholar]
- 195.Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59–68. doi: 10.1016/j.biocel.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 196.Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163–6177. doi: 10.7150/thno.28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cheng H, Chang S, Xu R, Chen L, Song X, Wu J, Qian J, Zou Y, Ma J. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res Ther. 2020;11(1):224. doi: 10.1186/s13287-020-01737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang XX, Sun C, Wang L, Guo XL. New insight into isolation, identification techniques and medical applications of exosomes. J Control Release. 2019;308:119–129. doi: 10.1016/j.jconrel.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 199.Chen BY, Sung CW, Chen C, Cheng CM, Lin DP, Huang CT, Hsu MY. Advances in exosomes technology. Clin Chim Acta. 2019;493:14–19. doi: 10.1016/j.cca.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 200.Zhang G, Huang X, Xiu H, Sun Y, Chen J, Cheng G, Song Z, Peng Y, Shen Y, Wang J, et al. Extracellular vesicles: Natural liver-accumulating drug delivery vehicles for the treatment of liver diseases. J Extracell Vesicles. 2020;10(2):e12030. doi: 10.1002/jev2.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chang CL, Chen CH, Chiang JY, Sun CK, Chen YL, Chen KH, Sung PH, Huang TH, Li YC, Chen HH, et al. Synergistic effect of combined melatonin and adipose-derived mesenchymal stem cell (ADMSC)-derived exosomes on amelioration of dextran sulfate sodium (DSS)-induced acute colitis. Am J Transl Res. 2019;11(5):2706–2724. [PMC free article] [PubMed] [Google Scholar]
- 202.Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW, Gopal A, Baek SH, Jeong SY, Lee SW, et al. A New Approach for Loading Anticancer Drugs Into Mesenchymal Stem Cell-Derived Exosome Mimetics for Cancer Therapy. Front Pharmacol. 2018;9:1116. doi: 10.3389/fphar.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lerman MJ, Lembong J, Gillen G, Fisher JP. 3D printing in cell culture systems and medical applications. Appl Phys Rev. 2018;5(4):041109. doi: 10.1063/1.5046087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Brassard JA, Lutolf MP. Engineering Stem Cell Self-organization to Build Better Organoids. Cell Stem Cell. 2019;24(6):860–876. doi: 10.1016/j.stem.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 205.Salaris F, Rosa A. Construction of 3D in vitro models by bioprinting human pluripotent stem cells: Challenges and opportunities. Brain Res. 2019;1723:146393. doi: 10.1016/j.brainres.2019.146393. [DOI] [PubMed] [Google Scholar]
- 206.Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng. 2020;4(4):370–380. doi: 10.1038/s41551-019-0471-7. [DOI] [PubMed] [Google Scholar]
- 207.Ong CS, Yesantharao P, Huang CY, Mattson G, Boktor J, Fukunishi T, Zhang H, Hibino N. 3D bioprinting using stem cells. Pediatr Res. 2018;83(1-2):223–231. doi: 10.1038/pr.2017.252. [DOI] [PubMed] [Google Scholar]
- 208.Kim M, Yun HW, Park DY, Choi BH, Min BH. Three-Dimensional Spheroid Culture Increases Exosome Secretion from Mesenchymal Stem Cells. Tissue Eng Regen Med. 2018;15(4):427–436. doi: 10.1007/s13770-018-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen P, Zheng L, Wang Y, Tao M, Xie Z, Xia C, Gu C, Chen J, Qiu P, Mei S, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yan L, Wu X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol Toxicol. 2020;36(2):165–178. doi: 10.1007/s10565-019-09504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang X, Wang F, Zhong M, Yarden Y, Fu L. The biomarkers of hyperprogressive disease in PD-1/PD-L1 blockage therapy. Mol Cancer. 2020;19(1):81. doi: 10.1186/s12943-020-01200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.de Miguel M, Calvo E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell. 2020;38(3):326–333. doi: 10.1016/j.ccell.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 213.Gide TN, Quek C, Menzies AM, Tasker AT, Shang P, Holst J, Madore J, Lim SY, Velickovic R, Wongchenko M, et al. Distinct Immune Cell Populations Define Response to Anti-PD-1 Monotherapy and Anti-PD-1/Anti-CTLA-4 Combined Therapy. Cancer Cell. 2019;35(2):238–255. doi: 10.1016/j.ccell.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 214.Xie F, Zhou X, Fang M, Li H, Su P, Tu Y, Zhang L, Zhou F. Extracellular Vesicles in Cancer Immune Microenvironment and Cancer Immunotherapy. Adv Sci (Weinh) 2019;6(24):1901779. doi: 10.1002/advs.201901779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nam GH, Choi Y, Kim GB, Kim S, Kim SA, Kim IS. Emerging Prospects of Exosomes for Cancer Treatment: From Conventional Therapy to Immunotherapy. Adv Mater. 2020;32(51):e2002440. doi: 10.1002/adma.202002440. [DOI] [PubMed] [Google Scholar]
- 216.Ha DH, Kim HK, Lee J, Kwon HH, Park GH, Yang SH, et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells. 2020;9(5):1157. [DOI] [PMC free article] [PubMed]
- 217.Warnecke A, Harre J, Staecker H, Prenzler N, Strunk D, Couillard-Despres S, Romanelli P, Hollerweger J, Lassacher T, Auer D, et al. Extracellular vesicles from human multipotent stromal cells protect against hearing loss after noise trauma in vivo. Clin Transl Med. 2020;10(8):e262. doi: 10.1002/ctm2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hosseini Shamili F, Alibolandi M, Rafatpanah H, Abnous K, Mahmoudi M, Kalantari M, Taghdisi SM, Ramezani M. Immunomodulatory properties of MSC-derived exosomes armed with high affinity aptamer toward mylein as a platform for reducing multiple sclerosis clinical score. J Control Release. 2019;299:149–164. doi: 10.1016/j.jconrel.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 219.Dal Collo G, Adamo A, Gatti A, Tamellini E, Bazzoni R, Takam Kamga P, Tecchio C, Quaglia FM, Krampera M. Functional dosing of mesenchymal stromal cell-derived extracellular vesicles for the prevention of acute graft-versus-host-disease. Stem Cells. 2020;38(5):698–711. doi: 10.1002/stem.3160. [DOI] [PubMed] [Google Scholar]
- 220.Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner DE, Coffey A, Antunes M, Robinson KL, Mitsialis SA, Kourembanas S, et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles Ameliorates Aspergillus Hyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem Cells Transl Med. 2015;4(11):1302–1316. doi: 10.5966/sctm.2014-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Khayambashi P, Iyer J, Pillai S, Upadhyay A, Zhang Y, Tran SD. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int J Mol Sci. 2021;22(2):684. [DOI] [PMC free article] [PubMed]
- 222.Wechsler ME, Rao VV, Borelli AN, Anseth KS. Engineering the MSC secretome: a hydrogel focused approach. Adv Healthc Mater. 2021;10(7):e2001948. [DOI] [PMC free article] [PubMed]
- 223.Hasani-Sadrabadi MM, Sarrion P, Pouraghaei S, Chau Y, Ansari S, Li S, et al. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci Transl Med. 2020;12(534):eaay6853. [DOI] [PubMed]
- 224.Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H, Xu J, Guo X. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol. 2017;8:904. doi: 10.3389/fphys.2017.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang K, Zhao X, Chen X, Wei Y, Du W, Wang Y, Liu L, Zhao W, Han Z, Kong D, et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for hindlimb ischemia treatment. ACS Appl Mater Interfaces. 2018;10(36):30081–30091. doi: 10.1021/acsami.8b08449. [DOI] [PubMed] [Google Scholar]
- 226.Zhao X, Liu Y, Jia P, Cheng H, Wang C, Chen S, Huang H, Han Z, Han ZC, Marycz K, et al. Chitosan hydrogel-loaded MSC-derived extracellular vesicles promote skin rejuvenation by ameliorating the senescence of dermal fibroblasts. Stem Cell Res Ther. 2021;12(1):196. doi: 10.1186/s13287-021-02262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li L, Zhang Y, Mu J, Chen J, Zhang C, Cao H, Gao J. Transplantation of human mesenchymal stem-cell-derived exosomes immobilized in an adhesive hydrogel for effective treatment of spinal cord injury. Nano Lett. 2020;20(6):4298–4305. doi: 10.1021/acs.nanolett.0c00929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Perspectives of MenSC-derived small EVs in regenerative medicine. The electron microscopy of MenSC-derived small EVs are labelled with a yellow arrow (the scale is 200 nm). As the great potential of the clinical application of MenSC-derived small EVs, novel strategies with regard to future perspectives of MenSC-derived small EVs are shown in: (1) engineered MenSC-derived small EVs, which is contributed by gene editing (such as CRISPR/Cas9, overexpression and RNA interference); (2) hypoxia-treated MenSC-derived small EVs, which improves the microenvironment conducive to their survival and growth; (3) MenSC-derived small EVs combined with targeting drug, which enhances the delivering efficiency of drugs to recipient cells; (4) 3D-culture of MenSC-derived small EVs, which increases the secreting efficiency of small EVs; (5) MenSC-derived small EVs for cancer immunotherapy, which enhances the immunomodulatory role; (6) MenSC-derived small EVs immobilized in hydrogel, which enhances the transmission effect.
Data Availability Statement
Please contact the corresponding author for data requests.