Abstract
Background
Streptococcus suis (Ss) is a Gram-positive and anaerobic zoonotic pathogen that is susceptible to all populations and can cause meningitis, septicemia, endocarditis and arthritis in humans.
Methods
In this study, patients with meningitis who were admitted to our hospital with negative blood and cerebrospinal fluid culture were divided into a next-generation sequencing group and a control group. In the next-generation sequencing group, we used the next-generation sequencing method to detect pathogenic bacteria in the patients’ cerebrospinal fluid. In the control group, we used blood and cerebrospinal fluid bacterial culture method to detect pathogenic bacteria in the patients' cerebrospinal fluid. The detection rates of pathogenic bacteria in the cerebrospinal fluid of the two groups were compared and analyzed.
Results
A total of 18 patients were included in this study, including 8 patients in the next-generation sequencing group and 10 patients in the control group. The mean age (P = 0.613) and mean disease duration (P = 0.294) were similar in both groups. Patients in the next-generation sequencing group had a leukocyte count of 13.13 ± 4.79 × 109, a neutrophil percentage of 83.39 ± 10.36%, and a C-reactive protein level of 134.95 ± 107.69 mg/L. Patients in the control group had a temperature of 38.32 ± 1.07, a leukocyte count of 8.00 ± 2.99 × 109, and a neutrophil percentage of 74.61 ± 8.89%, and C-reactive protein level was 4.75 ± 6.8 mg/L. The statistical results showed that the leukocytes (P = 0.013) and C-reactive protein levels (P = 0.001) were significantly higher in the patients of the next-generation sequencing group than in the control group. No statistically significant differences were seen in body temperature and neutrophil percentage between the two groups (P > 0.05). The incidence of intracranial pressure and meningeal irritation signs were similar in the two groups (P > 0.05). The detection rate of Streptococcus suis in the cerebrospinal fluid of patients in the next-generation sequencing group was 100%, and the detection rate of Streptococcus suis in the cerebrospinal fluid of the control group was 0%.
Conclusion
The detection rate of Streptococcus suis infection in cerebrospinal fluid by next-generation sequencing was significantly higher than that by blood and cerebrospinal fluid bacterial culture. Therefore, the diagnosis of porcine streptococcal meningitis by next-generation sequencing method is worthy of clinical promotion and application.
Keywords: Diagnosis, Next-generation sequencing, Streptococcal meningitis
Introduction
Streptococcus suis (Ss) is a Gram-positive and anaerobic zoonotic pathogen that is susceptible to all populations and can cause meningitis, septicemia, endocarditis and arthritis in humans. Among them, meningitis is the most common, and most of them can have severe sequelae of cochlear and vestibular nerve damage [1]. Its invasion of the central nervous system through the blood–brain barrier or blood-cerebrospinal fluid barrier via cerebral microvascular epithelial cells or choroidal epithelial cells leads to inflammation of the meningeal brain parenchyma [2], and the main causative risk factors include occupational exposure to pigs, raw pork, or consumption of raw pig meat products [3].
Cerebrospinal fluid pathogenic bacteria with next-generation sequencing (NGS) technology is an emerging molecular diagnostic method for rapid detection of intracranial pathogens [4], which has unique advantages in unexplained pathogenic infections. Therefore, this study intends to investigate the diagnostic value of next-generation sequencing (NGS) in the diagnosis of porcine streptococcal meningitis with negative blood and cerebrospinal fluid culture.
Data and methods
Study subjects
Patients with meningitis who were admitted to our hospital with negative blood and cerebrospinal fluid culture were the main subjects of this study, and were divided into next-generation sequencing group and control group. This study is in accordance with the Declaration of Helsinki of the World Medical Association and has been approved by the ethics committee of our hospital, and all patients signed an informed consent form.
Inclusion and exclusion criteria
Inclusion criteria are: (1) patients diagnosed with meningitis; (2) age > 18 years; and (3) patients who had signed the informed consent form. Exclusion criteria are: (1) patients with advanced malignancy and (2) patients with incomplete information.
Study methods
In the next-generation sequencing group, we used the next-generation sequencing method to detect pathogenic bacteria in the patients’ cerebrospinal fluid by next-generation sequencing equip (USA, Illumina Nextseq550). In the control group, we used blood and cerebrospinal fluid bacterial culture method to detect pathogenic bacteria in the patients’ cerebrospinal fluid. The detection rates of pathogenic bacteria in the cerebrospinal fluid of patients in the two groups were compared and analyzed.
Cerebrospinal fluid detection method
About 2–3 mL of cerebrospinal fluid was collected by lumbar puncture and stored at − 20 °C after aseptic sealing. DNA was extracted according to the DNA extraction kit (Tiangen (Beijing) Biotech Co., Ltd, Tiangen DNA Mini kit DP316). Next-generation sequencing (NGS) technology was used to detect pathogens in the cerebrospinal fluid.
Main observation indexes
The main observation indexes of this study included gender, age, disease duration, body temperature, leukocytes, neutrophil percentage, C-reactive protein level, intracranial pressure, and cerebrospinal fluid test.
Statistical analysis
SPSS 20.0 statistical software was used for data processing in this study, and the measurement data were expressed as mean ± standard deviation (). Count data were expressed as percentages (%). The t test was used for comparison between two groups obeying normal distribution; the non-parametric test was used for comparison between groups not obeying normal distribution. Counting data were tested by Chi-square test. P < 0.05 was considered a statistically significant difference.
Results
General information
A total of 18 patients were included in this study, including 8 patients in the next-generation sequencing group and 10 patients in the control group. The mean age of patients in the next-generation sequencing group was 54.25 ± 8.60 years, and the mean duration of disease was 5.88 ± 4.64 days. The mean age of patients in the control group was 50.10 ± 21.31 years, and the mean disease duration was 9.50 ± 8.46 days. There was no significantly difference about mean age (P = 0.613) and mean duration of illness (P = 0.294) between the two groups.
Comparison of infection indicators between the two groups of patients
The results showed that the white blood cell count was significantly higher in the diphtheria group than control group (13.13 ± 4.79 × 109 vs. 8.00 ± 2.99 × 109, P = 0.013), the C-reactive protein level was significantly higher in the diphtheria group than control group (134.95 ± 107.69 mg/L vs. 4.75 ± 6.8 mg/L, P = 0.001). The body temperature (38.95 ± 0.49 °C vs. 38.32 ± 1.07 °C, P = 0.143) and neutrophil percentage (83.39 ± 10.36% vs. 74.61 ± 8.89%, P = 0.071) were similar in both the groups (Figs. 1, 2).
Fig. 1.
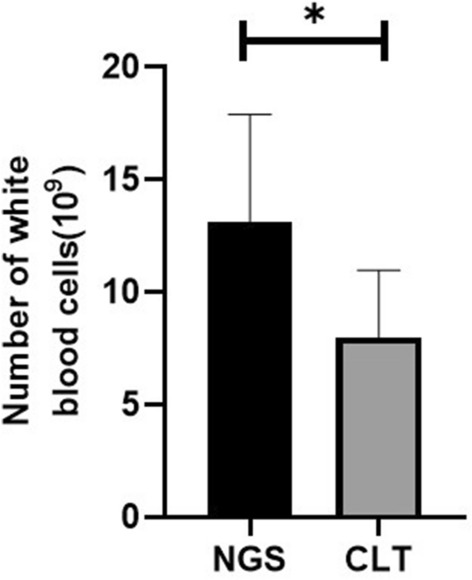
Comparison of leukocyte count between the two groups of patients
Fig. 2.
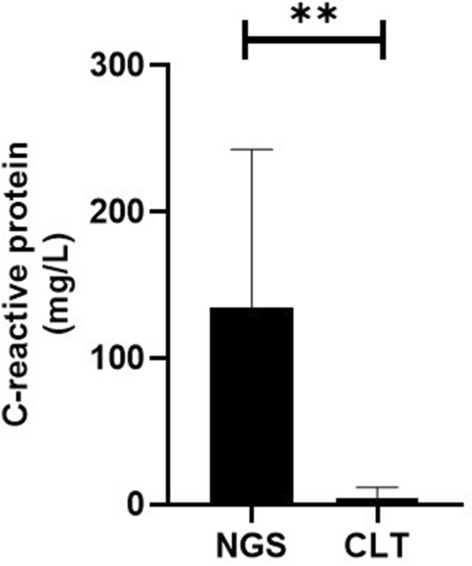
Comparison of C-reactive protein levels between the two groups of patients
Results of cerebrospinal fluid testing in the two groups of patients
The intracranial pressure of patients in the next-generation sequencing group was 205.63 ± 66.09 mmH2O, 190.70 ± 53.55 mmH2O in the control group. 100% of patients in the next-generation sequencing group and 80% of patients in the control group had meningeal irritation signs. The statistical results showed that the incidence of intracranial pressure and meningeal irritation signs were similar in the two groups (P > 0.05). The detection rate of Streptococcus suis in the cerebrospinal fluid of patients in the next-generation sequencing group was 100%, and the detection rate of Streptococcus suis in the cerebrospinal fluid of the control group was 0% (Fig. 3).
Fig. 3.
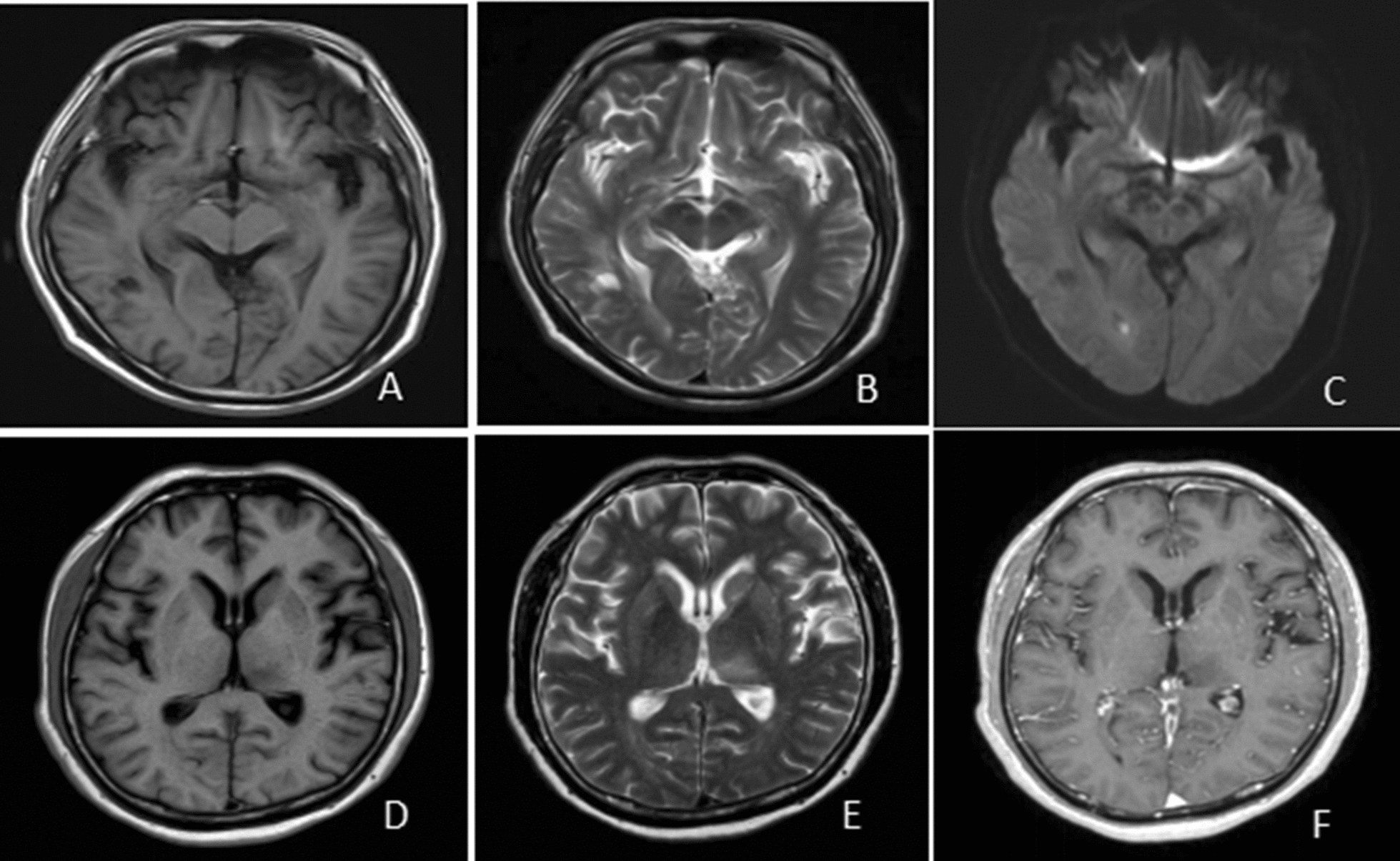
The results of cranial imaging of porcine streptococcal meningitis. A–C The cranial MRI results of one patient, showing an abnormal lesion in the right occipital lobe parietal ventricle with small abscess formation; D–F The cranial MRI results of another patient, showing inflammatory lesions in the left thalamus and basal ganglia region
Discussion
Porcine streptococcal meningitis is the most predominant type of human infection with porcine streptococcal disease, which has been outbreaks and epidemics in Sichuan and Jiangsu in China, but is disseminated in other provinces, and rare cases are reported in Hainan [5]. The diagnosis of this disease is based on the epidemiological history, clinical manifestations, and confirmation of diagnosis requires positive blood and cerebrospinal fluid culture of Streptococcus suis [6], but due to factors such as low blood and cerebrospinal fluid bacteria, insufficient sampling or pre-treatment with antibiotics, resulting in positive blood and cerebrospinal fluid culture is not high, the positive rate of blood bacterial culture is about 32%, the positive rate of cerebrospinal fluid bacterial culture is 18.5%, and negative blood and cerebrospinal fluid bacterial culture increases the difficulty of clinical diagnosis [7].
Common signs and symptoms of porcine streptococcal meningitis include fever, headache, and neck stiffness [8–11]. Bilateral hearing impairment occurs early in the disease and the incidence can be as high as 66.4% [12], mainly due to labyrinthitis. The cellular classification of the cerebrospinal fluid in this disease is dominated by multiple nucleated cells, which may be misdiagnosed as viral meningitis or tuberculous meningitis once it is quickly converted to single nucleated cells or lymphocytes predominant after antibiotic treatment [13].
Next-generation sequencing technology, as an emerging molecular diagnostic method in current clinical practice [14–18], has been applied in various infectious disease areas in clinical practice [19, 20]. Therefore, the present study was proposed to investigate the diagnostic value of next-generation sequencing (NGS) in blood and cerebrospinal fluid culture-negative porcine streptococcal meningitis. A total of 18 patients were included in this study, including 8 patients in the next-generation sequencing group and 10 patients in the control group. The results of the study showed that the detection rate of Streptococcus suis in the cerebrospinal fluid of patients in the next-generation sequencing group was 100%, and the detection rate of Streptococcus suis in the cerebrospinal fluid of the control group was 0%. Therefore, when the pathogenic bacteria cannot be detected by blood and cerebrospinal fluid bacterial culture methods, the pathogenic bacteria in cerebrospinal fluid can be detected by next-generation sequencing method. In this study, the results showed that the leukocytes and C-reactive protein levels were significantly higher in the patients of the next-generation sequencing group than control group. The reason maybe due to the specific clinical characteristics, such as complicated with other bacterial infections. However, other reasons still needs further research.
The present study still has the following shortcomings. First, this study is a single-center clinical study, and follow-up multicenter clinical studies are still needed for further investigation. Second, the sample size included in this study was small, and further studies with larger sample size are needed.
Conclusion
The detection rate of Streptococcus suis infection in cerebrospinal fluid by next-generation sequencing was significantly higher than that by blood and cerebrospinal fluid bacterial culture. Therefore, when clinically suspected streptococcal meningitis, but the cerebrospinal fluid culture is negative, we can use this next generation of sequencing to confirm the diagnosis, so as to improve the clinical diagnostic efficiency of streptococcal meningitis. The diagnosis of Streptococcus suis meningitis by next-generation sequencing method is worthy of clinical application.
Acknowledgements
Not applicable.
Abbreviations
- NGS
Next-generation sequencing
- Ss
Streptococcus suis
Authors’ contributions
ZEY and WDM conceived of the study and LN, HSX and ZZY participated in its design and coordination, and HSJ, HXY and WGQ helped to draft the manuscript. All the authors read and approved the final manuscript.
Funding
Supported by Scientific research projects of health industry in Hainan Province (No. 20A200223).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of our hospital. All the participants had signed the informed consent.
Consent for publication
Not applicable.
Competing interests
All the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Eryi Zhao and Daimei Wang contributed equally to this study
References
- 1.Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infectious Diseases. 2007;7:201–209. doi: 10.1016/S1473-3099(07)70001-4. [DOI] [PubMed] [Google Scholar]
- 2.Dutkiewicz J, Zając V, Sroka J, Wasiński B, Cisak E, Sawczyn A, et al. Streptococcus suis: a re-emerging pathogen associated with occupational exposure to pigs or pork products. Part II—Pathogenesis. Ann Agric Environ Med. 2018;25:186–203. doi: 10.26444/aaem/85651. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi D, Kerdsin A, Akeda Y, Chiranairadul P, Loetthong P, Tanburawong N, Areeratana P, et al. Impact of a food safety campaign on Streptococcus suis infection in humans in Thailand. Am J Trop Med Hyg. 2017;96:1370–1377. doi: 10.4269/ajtmh.16-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown JR, Bharucha T, Breuer J. Encephalitis diagnosis using metagenomics: application of next generation sequencing for undiagnosed cases. J Infect. 2018;76:225–240. doi: 10.1016/j.jinf.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hlebowicz M, Jakubowski P, Smiatacz T. Streptococcus suis meningitis: epidemiology, clinical presentation and treatment. Vector Borne Zoonotic Dis. 2019;19:557–562. doi: 10.1089/vbz.2018.2399. [DOI] [PubMed] [Google Scholar]
- 6.Sun YY, Liu HT, Du R, Li SG, Qu GG, Zhu RN, et al. Characteristic comparison of meningitis and non-meningitis of Streptococcus suis in an experimentally infected porcine model. Inflammation. 2018;5:887. doi: 10.1007/s10753-017-0692-4. [DOI] [PubMed] [Google Scholar]
- 7.Yang XX, Jiang N, Wu JY, Tang RZ, Wang SJ, Wang T, Luo WG, Su L, Yang Y, Yang WZ. Clinical analysis of 75 cases of human infection with Streptococcus suis. Chinese Journal of Internal Medicine. 2007;46:764–765. [Google Scholar]
- 8.Beek VD, Diederik S, Lodewijk DG. Streptococcus suis meningitis in the Netherlands. J Infect. 2015;71:602–604. doi: 10.1016/j.jinf.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14:319–338. doi: 10.1146/annurev-pathmechdis-012418-012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wertheim H, Nguyen HN, Taylor W, Lien TT, Ngo HT, Nguyen TQ, et al. Streptococcus suis, an important cause of adult bacterial meningitis in Northern Vietnam. PLoS ONE. 2009;4:e5973. doi: 10.1371/journal.pone.0005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschalk M, Segura M. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet Microbiol. 2000;76:259–272. doi: 10.1016/S0378-1135(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 12.Susilawathi NM, Adi Tarini NM, Dwi Fatmawati NN, Mayura PI, Ayu Suryapraba AA, Subrata M, et al. Streptococcus suis—associated meningitis, Bali, Indonesia. Emerg Infect Dis. 2019;25:2235–2242. doi: 10.3201/eid2512.181709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu LX, Li HF, Chen Y, Zhang AM. A case of septic shock secondary to Streptococcus suis infection with suspected viral meningitis. Chin J Neurol. 2017;50:681–683. [Google Scholar]
- 14.Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66:778–788. doi: 10.1093/cid/cix881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai YY, Chen L, Chang WJ, Lu HW, Cui P, Ma XL, et al. Culture-negative Streptococcus suis infection diagnosed by metagenomic next-generation sequencing. Front Public Health. 2019;7:379. doi: 10.3389/fpubh.2019.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barzon L, Lavezzo E, Militello V, Toppo S, Palù G. Applications of next-generation sequencing technologies to diagnostic virology. Int J Mol Sci. 2011;12:7861–7884. doi: 10.3390/ijms12117861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besser J, Carleton HA, Gerner-Smidt P, Lindsey RL, Trees E. Next-generation sequencing technologies and their application to the study and control of bacterial infections. Clin Microbiol Infect. 2017;11:987. doi: 10.1016/j.cmi.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capobianchi MR, Giombini E, Rozera G. Next-generation sequencing technology in clinical virology. Clin Microbiol Infect. 2013;19:15–22. doi: 10.1111/1469-0691.12056. [DOI] [PubMed] [Google Scholar]
- 19.Geng S, Mei Q, Zhu C, Fang XW, Yang TJ, Zhang L, et al. Metagenomic next-generation sequencing technology for detection of pathogens in blood of critically ill patients. Int J Infect Dis. 2020;15:447. doi: 10.1016/j.ijid.2020.11.166. [DOI] [PubMed] [Google Scholar]
- 20.Guo LY, Li YJ, Liu LL, Wu HL, Zhou JL, Zhang Y, et al. Detection of pediatric bacterial meningitis pathogens from cerebrospinal fluid by next-generation sequencing technology. J Infect. 2019;78:323–337. doi: 10.1016/j.jinf.2018.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


