Abstract
Objectives
Rheumatoid arthritis (RA) patients have an increased risk of cardiovascular disease (CVD). In the present study, we evaluated the inflammatory activity of the ascending aorta in RA patients who received biological treatment.
Methods
We assessed the aortic wall inflammation of RA patients using 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography before and after 6 months of biologic therapies. We also compared the inflammatory activity at the aortic wall in RA patients with remission or low disease activity (RLDA) and those with moderate or high disease activity (MHDA). The aortic uptake was measured by the standardized uptake value (SUV) and the target-to-background ratio (TBR).
Results
A total of 64 patients were included in the analysis (mean age, 58.4 ± 13.8 years old; female, 77%). The Disease Activity Score for 28 joints (DAS28) erythrocyte sedimentation rate (ESR) had significantly decreased after 6 months: from 5.0 ± 1.2 to 3.3 ± 1.2 (p < 0.001). The FDG uptake in the ascending aorta changed from baseline to 6 months, showing a maximum SUV (SUVmax) of 1.83 ± 0.34 to 1.90 ± 0.34 (p = 0.059) and TBR of 1.71 ± 0.23 to 1.75 ± 0.24 (p = 0.222). The SUVmax and TBR after 6 months were significantly higher in the RLDA group than in the MHDA group (2.05 ± 0.32 vs. 1.79 ± 0.33 (p = 0.002) and 1.89 ± 0.33 vs. 1.65 ± 0.20 (p = 0.001), respectively). The percentage of monocytes also significantly increased from baseline to 6 months: from 5.9 ± 1.6 to 6.9 ± 2.6 (p = 0.032).
Conclusion
The inflammation activity at the ascending aorta in RA patients did not change significantly after 6 months of biological treatment. RA patients with a low disease activity or in clinical remission after 6 months of biological treatment still had an increased inflammatory activity at the aortic wall.
Keywords: Aortic inflammation, FDG PET/CT, Biologic therapy, Rheumatoid arthritis
Background
Rheumatoid arthritis (RA) increases the risk of cardiovascular disease (CVD) [1–3]. An accelerated progression of atherosclerosis leads to an increased mortality in RA patients [4–10]. Because both an inflamed synovial membrane and atherosclerotic plaque share important common pathological processes, chronic systemic inflammation might accelerate the development of atherosclerosis in RA patients [10]. Previous studies have demonstrated that atherosclerosis is more prevalent in the RA population than in healthy individuals [4–9].
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) can be used to evaluate the joints in RA patients [11–16]. In addition, FDG PET/CT has also been widely used to evaluate atherosclerosis, since the 18F-FDG uptake reflects the glucose metabolism of macrophages in atherosclerotic plaque [17–21]. However, while FDG PET/CT is a highly reproducible method of evaluating arterial inflammation, there have been few reports regarding its utility in assessing aortic inflammation in RA patients.
In the present study, we assessed the ascending aortic wall inflammation in RA patients who received biologic therapies using 18F-FDG PET/CT. We also compared the inflammatory activity at the aortic wall in RA patients in remission or with a low disease activity (RLDA) and those with moderate or high disease activity (MHDA) after biologic treatment.
Materials and methods
Study population
Sixty-four RA patients who underwent whole-body 18F-FDG PET/CT at baseline and 6 months after the initiation of biologic therapies at Gunma University Hospital were enrolled in this study. All patients were diagnosed with RA according to the American College of Rheumatology criteria revised in 1987 and had a history of inadequate clinical response to previous treatments with nonbiological disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX). Therefore, these patients had been recommended for treatment with biologics.
The study protocol was approved by the Institutional Review Board of Gunma University Hospital. Written, informed consent was obtained from each patient before they participated in the study.
Imaging acquisition
Whole-body 18F-FDG PET/CT was performed using a PET/CT scanner (Biograph 16; Siemens Medical Solutions Inc., Malvern, PA, USA) before and 6 months after the initiation of biologic therapies. In brief, 18F-FDG (5 MBq/kg) was injected intravenously after at least 6 h of fasting. Patients were imaged approximately 60 min after FDG injection in the supine position. A non-contrast-enhanced CT scan (140 kV, 120–240 mAs) for attenuation correction and anatomic co-registration was obtained before PET imaging. PET imaging was performed in the 3-dimensional mode with 3 min per bed position and slice thickness of 3.27 mm. The PET images were reconstructed using an ordered-subsets expectation-maximization algorithm into 128 × 128 matrices as previously described [11, 13].
Imaging analyses
Image analyses were performed using the syngo.via software program (Siemens Healthcare, Erlangen, Germany). An independent radiologist blinded to the clinical data analyzed all of the PET/CT images. According to the recommendation of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) [22], we used the maximum standardized uptake value (SUVmax) and the target-to-background ratio (TBR) as parameters for the FDG uptake in the ascending aorta. The SUVmax and TBR correlate with histological markers of inflammation and have been used in several studies to measure the FDG uptake in the arterial wall [17, 18, 23, 24].
Ascending aorta inflammation was quantified within each region of interest (ROI), containing the arterial wall and the lumen, along the length of the ascending aorta. The ROIs were manually drawn every 5 mm on axial images, starting 1 cm above the origin of the left main coronary artery and ending at the merging point with the aortic arch. The SUV is determined as the radioactivity concentration (kBq/ml) within an ROI divided by the decay-corrected amount of injected dose per patient’s weight (kBq/g). The average of the SUVmax (mean SUVmax) for all ROIs of the entire ascending aorta was then calculated. Subsequently, the TBR was obtained as the mean SUVmax divided by the background SUVmax in the superior vena cava to correct for the blood activity.
Clinical and laboratory evaluations
RA disease activity was assessed using the Disease Activity Score for 28 joints (DAS28) based on the ESR at baseline and 6 months after the initiation of biologic therapies. We also measured the white blood cell (WBC) count, serum levels of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), matrix metalloproteinase-3 (MMP-3), anti-cyclic citrullinated peptide antibodies (ACPA), and rheumatoid factor antibodies (RF).
The patients were divided into 2 groups according to the DAS28-ESR at 6 months: RLDA in those with a DAS28-ESR < 3.2 and MHDA in those with a DAS28-ESR ≥ 3.2. The clinical parameters and FDG uptake for the ascending aorta at baseline were compared between these two groups.
Statistical analyses
Data analyses were performed using the IBM SPSS Statistics 25 software program (IBM Corp., Armonk, NY, USA). Data were expressed as the mean ± standard deviation for parametric variables, as the median and interquartile range for nonparametric variables, and as numbers and percentages for categorical variables. For the comparison of continuous data between two clinical response groups, an unpaired Student’s t-test was used. For the comparison of continuous data before and after treatment, paired Student’s t-test or Wilcoxon’s signed-rank test was used. For the comparison of categorical data, the chi-square test or Fisher’s exact test was used. A value of p < 0.05 was considered statistically significant.
Results
A total of 64 patients were included in the analysis (mean age, 58.4 ± 13.8 years old; females, 77%) (Table 1). The mean disease duration was 13.1 ± 11.8 years. A total of 70% of the patients received MTX, and 48% received prednisolone (PSL). Figure 1 shows the 18F-FDG PET/CT findings for a case at baseline and 6 months after the initiation of biologic therapy. The mean FDG uptake values for the ascending aorta and the clinical parameters at baseline and 6 months after the initiation of biologic treatments are shown in Table 2. Although all clinical parameters except for the ACPA were significantly decreased at 6 months compared with the baseline, the mean SUVmax and TBR were not significantly changed after 6 months (Table 2).
Table 1.
The demographic characteristics and medication use of patients at baseline
| Clinical characteristics | Values |
|---|---|
| Age (years) | 58.4 ± 13.8 |
| Female/male | 49/15 |
| Disease duration (years) | 13.1 ± 11.8 |
| BMI | 21.8 ± 4.0 |
| Smoking, n (%) | 8 (12.7) |
| Hypertension, n (%) | 23 (36.5) |
| Diabetes, n (%) | 6 (9.7) |
| Total cholesterol (mg/dL) | 197.4 ± 39.3 |
| LDL (mg/dL) | 114.9 ± 37.5 |
| HDL (mg/dL) | 59.6 ± 15.0 |
| Triglycerides (mg/dL) | 108.2 ± 46.0 |
| PSL use, n (%) | 31 (48.4) |
| PSL dose (mg/day) | 2.0 ± 2.3 |
| MTX use, n (%) | 45 (70.3) |
| MTX dose (mg/week) | 5.7 ± 3.5 |
| Biologics (IFX/ETN/ADA/GLM/TCZ/ABT) | 18/14/16/2/13/1 |
The data are expressed as the mean ± standard deviation
BMI body mass index; LDL low-density lipoprotein cholesterol; HDL high-density lipoprotein cholesterol; PSL prednisolone; MTX methotrexate; IFX infliximab; ETN etanercept; ADA adalimumab; GLM golimumab; TCZ tocilizumab; ABT abatacept
Fig. 1.
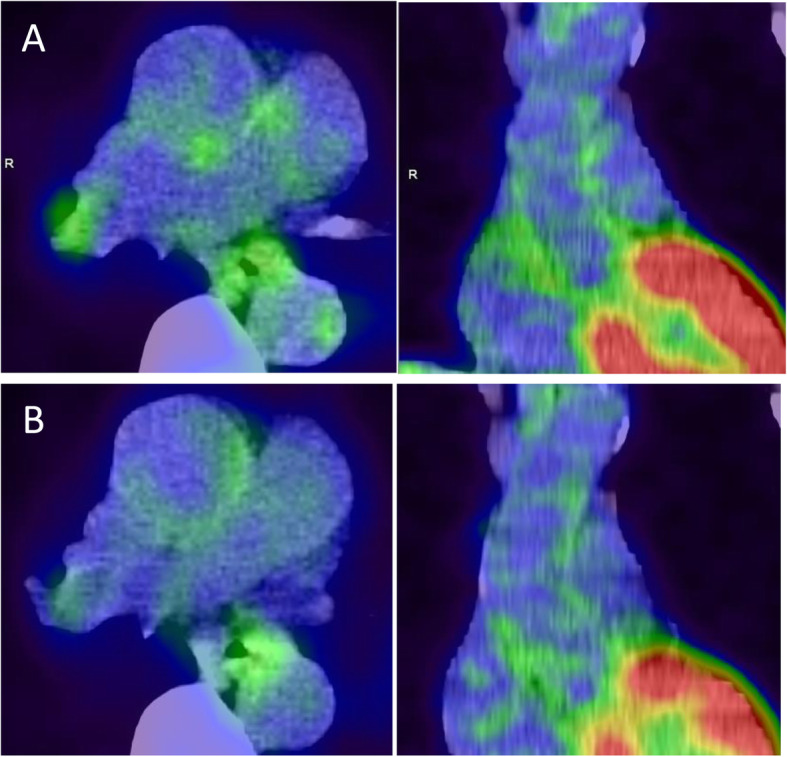
Typical 18F-FDG PET/CT images of the ascending aorta wall uptake at baseline and after 6 months of biologic therapy. Axial and coronal images of ascending aorta from a 65-year-old female patient who underwent treatment with infliximab. A At baseline, the mean SUVmax was 1.64, and the TBR was 1.63. B At 6 months, the mean SUVmax was 1.56, and the TBR was 1.44. SUVmax, maximum standardized uptake value; TBR, target-to-background ratio
Table 2.
Changes in FDG parameters and clinical parameters after treatment
| Parameters | Baseline | After 6 months | p value |
|---|---|---|---|
| FDG parameters | |||
| Mean SUVmax | 1.83 ± 0.34 | 1.90 ± 0.34 | 0.059 |
| TBR | 1.71 ± 0.23 | 1.75 ± 0.29 | 0.222 |
| Clinical parameters | |||
| ESR (mm/h) | 62.3 ± 32.2 | 36.5 ± 28.6 | < 0.001 |
| CRP (mg/dL)a | 1.3 (0.4–3.0) | 0.1 (0.0–0.6) | < 0.001 |
| MMP-3 (ng/mL)a | 153.6 (70.5–412.6) | 65.2 (39.3–133.4) | < 0.001 |
| WBC (/μL) | 6537.5 ± 2239.2 | 5609.4 ± 1992.5 | < 0.001 |
| % neutrophils | 68.7 ± 9.2 | 57.9 ± 12.7 | < 0.001 |
| % eosinophils | 2.3 ± 2.3 | 3.0 ± 2.6 | 0.004 |
| % basophils | 0.4 ± 0.2 | 0.5 ± 0.3 | < 0.001 |
| % monocytes | 5.3 ± 1.8 | 6.3 ± 2.2 | 0.003 |
| % lymphocytes | 22.2 ± 7.5 | 31.3 ± 11.6 | < 0.001 |
| ACPA (U/mL)a | 87.4 (12.1–100.0) | 55.8 (8.0–100.0) | 0.276 |
| ACPA positive, n (%) | 34 (53.1) | 29 (45.3) | < 0.001 |
| RF (U/mL)a | 49 (13–137) | 27 (10–79) | < 0.001 |
| RF positive, n (%) | 34 (53.1) | 25 (39.1) | < 0.001 |
| DAS28-ESR | 5.0 ± 1.2 | 3.3 ± 1.2 | < 0.001 |
The data are expressed as the mean ± standard deviation, with the exception of skewed variables (a), which are represented as medians (interquartile range)
SUVmax maximum standardized uptake value; TBR tissue-to-background ratio; ESR erythrocyte sedimentation rate; CRP C-reactive protein; WBC white blood cell count; MMP-3 matrix metalloproteinase-3; ACPA anti-cyclic citrullinated peptide antibody; RF rheumatoid factor; DAS28 Disease Activity Score in 28 joints
We compared the FDG uptake at the ascending aorta between the two groups (RLDA group vs. MHDA group) at baseline and after 6 months of treatment. Figure 2 shows the increases in the mean SUVmax and TBR values for the ascending aorta at 6 months after the initiation of treatment, especially in the RLDA group. The mean SUVmax value at 6 months was significantly higher in the RLDA group than in the MHDA group (2.05 ± 0.32 vs. 1.79 ± 0.33; p = 0.002). The TBR value at baseline was significantly higher in the RLDA group than in the MHDA group (1.79 ± 0.26 vs. 1.65 ± 0.19; p = 0.018). After 6 months, the TBR value was also significantly higher in the RLDA group than in the MHDA group (1.89 ± 0.33 vs. 1.65 ± 0.20; p = 0.001).
Fig. 2.
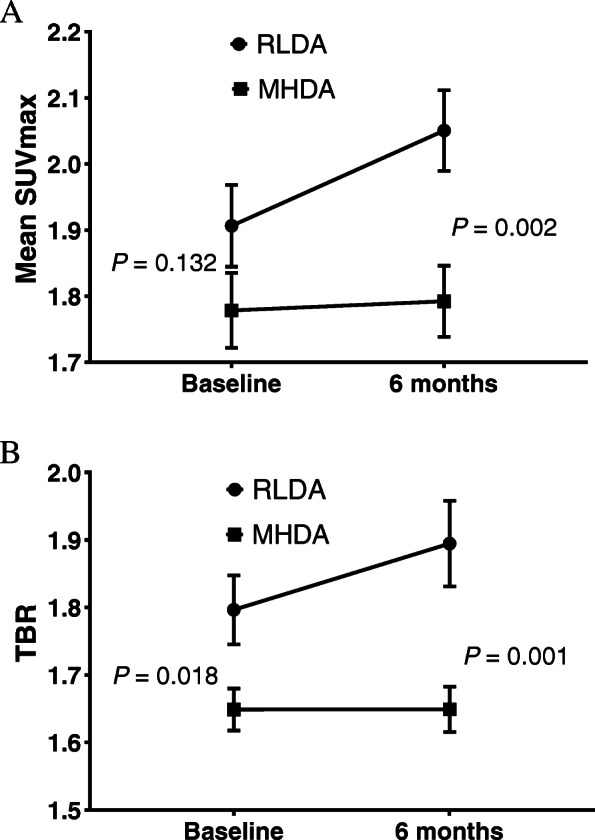
FDG uptake parameters of the ascending aorta for the two clinical response groups. The mean SUVmax and TBR values of the ascending aorta at 6 months after biologic treatment were significantly higher in the group with a DAS28-ESR < 3.2 (indicating remission or low disease activity) than in the group with a DAS28-ESR ≥ 3.2 (indicating moderate or high disease activity). SUVmax, maximum standardized uptake value; TBR, target-to-background ratio; DAS28, Disease Activity Score in 28 joints; RLDA, remission or low disease activity; MHDA, moderate or high disease activity
At baseline, the WBC count and percentage of monocytes were significantly higher in the RLDA group than in the MHDA group (p = 0.005 and p = 0.035) (Table 3). Regarding the WBC subtypes at 6 months, there were significantly lower percentages of neutrophils (p < 0.001) and significantly higher percentages of lymphocytes (p < 0.001) in the RLDA group than in the MHDA group (Table 4). The percentage of monocytes significantly increased from baseline to 6 months (5.9 ± 1.6 to 6.9 ± 2.6; p = 0.032).
Table 3.
The comparison of clinical parameters at baseline
| RLDA (n = 27) | MHDA (n = 37) | p value | |
|---|---|---|---|
| Age (years) | 56.3 ± 15.7 | 59.9 ± 12.1 | 0.334 |
| Female/male | 18/9 | 31/6 | 0.110 |
| Disease duration (years) | 10.3 ± 8.3 | 15.1 ± 13.6 | 0.082 |
| PSL dose (mg/day) | 2.2 ± 2.5 | 1.8 ± 2.1 | 0.483 |
| MTX dose (mg/week) | 5.2 ± 3.4 | 6.1 ± 3.6 | 0.321 |
| ESR (mm/h) | 53.1 ± 33.9 | 68.9 ± 29.5 | 0.056 |
| CRP (mg/dL)a | 1.7 (0.4–3.1) | 1.3 (0.6–2.9) | 0.807 |
| WBC (/μL) | 7429.6 ± 2078.1 | 5886.5 ± 2150.2 | 0.005 |
| % neutrophils | 68.3 ± 9.2 | 69.0 ± 9.3 | 0.775 |
| % eosinophils | 2.0 ± 1.1 | 2.6 ± 2.8 | 0.418 |
| % basophils | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.699 |
| % monocytes | 5.9 ± 1.6 | 4.9 ± 1.9 | 0.035 |
| % lymphocytes | 22.4 ± 7.9 | 22.0 ± 7.3 | 0.857 |
| MMP-3 (ng/mL)a | 158.0 (95.0–399.3) | 134.5 (69.1–500.0) | 0.579 |
| ACPA (U/mL)a | 61.4 (10.7–100.0) | 99.1 (15.1–100.0) | 0.393 |
| ACPA ≥ 60 U/mL, n (%) | 13 (50.0) | 21 (58.3) | 0.515 |
| RF (U/mL)a | 33 (10–100) | 61.5 (23.8–211.8) | 0.082 |
| RF ≥ 40 U/mL, n (%) | 12 (48.0) | 22 (64.7) | 0.199 |
| DAS28-ESR | 4.5 ± 1.4 | 5.3 ± 1.0 | 0.008 |
The data are expressed as the mean ± standard deviation, with the exception of skewed variables (a), which are represented as medians (interquartile range)
RLDA remission and low disease activity; MHDA moderate and high disease activity; PSL prednisolone; MTX methotrexate; ESR erythrocyte sedimentation rate; CRP C-reactive protein; WBC white blood cell count; MMP-3 matrix metalloproteinase-3; ACPA anti-cyclic citrullinated peptide antibody; RF rheumatoid factor; DAS28 Disease Activity Score in 28 joints
Table 4.
Counts for WBC subtypes at 6 months in the two groups
| RLDA (n = 27) | MHDA (n = 37) | p value | |
|---|---|---|---|
| WBC (/μL) | 5807.4 ± 1994.2 | 5465.0 ± 2006.1 | 0.501 |
| % neutrophils | 51.3 ± 12.0 | 62.8 ± 11.0 | < 0.001 |
| % eosinophils | 2.8 ± 2.0 | 3.0 ± 2.9 | 0.881 |
| % basophils | 0.6 ± 0.4 | 0.4 ± 0.2 | 0.114 |
| % monocytes | 6.9 ± 2.6 | 5.8 ± 1.7 | 0.065 |
| % lymphocytes | 37.7 ± 11.3 | 26.7 ± 9.5 | < 0.001 |
The data are expressed as the mean ± standard deviation
RLDA remission and low disease activity; MHDA moderate and high disease activity; WBC white blood cell
We compared the FDG uptake and clinical parameters between the groups with and without anti-tumor necrosis factor (TNF) drug treatment, but there were no significant differences between these two groups (data not shown).
Discussion
In the present study, we found that the inflammation activity of the ascending aorta in RA patients did not significantly change after 6 months of biologic treatment. RA patients with RLDA after 6 months had a significantly higher FDG uptake at the aortic wall than those with MDLA.
Biological agents showed beneficial effects on reducing RA symptoms and disease activity by suppressing inflammation. Since atherosclerosis is a chronic inflammation process, these drugs may be also effective in reducing CVD risk in RA. Previous reports have shown that 8-week anti-TNF-α therapy reduced aortic inflammation in RA patients [25]. However, the FDG uptake of the arterial wall after treatment was still significantly higher in RA patients than in the control group.
In our study, although the disease activity and serum markers of RA patients significantly decreased after 6 months of biologic treatment, the inflammatory activity of the ascending aorta still existed. This result indicated that the cardio-protective effects of biologic therapy might not apply to all RA patients at six months.
A cross-sectional study in the USA demonstrated that RA patients who needed anti-TNF therapy to continue their clinical remission had a greater FDG uptake at the ascending aorta than those receiving non-biological DMARDs [26]. In addition to the high disease activity before the biological treatment, our patients had a long disease duration after the onset of RA. These patients’ condition might have led to the remnant aortic inflammation after 6 months of biological treatment, even if their joints had a good clinical response to biological drugs. Our results indicated that if we were intending to use biological drugs to achieve a cardio-protective effect, we should ensure RA patients maintain a low disease activity for a long time.
The FDG uptake in the aortic wall was significantly higher in RA patients with RLDA than in those with MHDA at 6 months. This was a controversial result. However, as mentioned above, our follow-up period was only 6 months and thought to be insufficient to reduce systemic inflammation by RA. It is thus possible that a longer treatment period led to the reduced aortic inflammation in the RLDA group.
In addition, a 3-year, prospective, observational study in an Italian cohort clearly showed that the maintenance of remission was associated with a reduced risk of atherosclerosis [27]. Our results showed that while the use of biologic therapies had reduced the clinical symptoms at 6 months, the aortic wall inflammation in these patients persisted despite 6 months of biologic treatment. Therefore, tight control of the RA activity in these patients should be maintained, and their CVD risk should be carefully estimated.
In this study, we also noted an elevation of monocytes at baseline and at 6 months in the RLDA group. An increased activation of circulating monocytes has been reported in patients receiving anti-TNF therapy [26] and the elevations of monocyte subpopulation can be seen in RA patients with coronary artery atherosclerosis and are associated with an increased CVD risk [28, 29]. Furthermore, a previous in vivo imaging study reported that the peripheral blood mononuclear cell accumulation was correlated with the arterial wall inflammation assessed on hybrid single-photon emission computed tomography/CT [30]. These previous findings suggest that the increased percentage of monocytes might be involved in arterial wall inflammation in RA patients, even after biologic therapy.
Several limitations associated with the present study warrant mention. First, traditional cardiovascular risk factors could not be fully investigated because of the lack of such information. Second, the selection of biologic agents was left to each physician. Since different drugs have different mechanisms through which they act on RA pathogenesis, treatment bias may exist. Third, since FDG-PET/CT is a nuclear imaging modality, repeated examinations might lead to increased radiation exposure. Finally, this was a short-term study. To determine whether or not biological therapies are useful for reducing aortic inflammation and CVD risk in RA patients, further long-term follow-up research should be conducted.
Conclusions
We demonstrated that the inflammation activity of the ascending aorta in RA patients was not significantly altered after 6 months of biologic treatment. RA patients with a low disease activity or in clinical remission after 6 months of such treatment still had an increased inflammatory activity at the aortic wall.
Acknowledgements
None.
Abbreviations
- ACPA
Anti-cyclic citrullinated peptide antibody
- CRP
C-reactive protein
- DAS
Disease activity score
- DMARDs
Disease-modifying antirheumatic drugs
- CVD
Cardiovascular disease
- EANM
European association of nuclear medicine
- ESR
Erythrocyte sedimentation rate
- FDG-PET/CT
Fluorodeoxyglucose-positron emission tomography/computed tomography
- RLDA
RA patients in remission or with a low disease activity
- MHDA
RA patients with moderate or high disease activity
- MMP-3
Matrix metalloproteinase 3
- MTX
Methotrexate
- PSL
Prednisolone
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- ROI
Region of interest
- SUV
Standardized uptake value
- SUVmax
Maximum standardized uptake value
- TBR
Target-to-background ratio
- TNF
Tumor necrosis factor
- WBC
White blood cell
Authors’ contributions
DTT and KO made substantial contributions to the design of the study; acquisition, analysis, and interpretation of the data; and writing of the manuscript. TS and YY participated in the design of the study, acquired the data, helped to draft the manuscript, and contributed to the revising of the manuscript critically. HS and TN participated in acquiring the data and critically contributing to the revising of the manuscript. YT and HC critically contributed to the revising of the manuscript enhancing its intellectual content and approving the final content of the manuscript. All authors read and approved the final manuscript.
Funding
There has been no significant financial support for this work that could have influenced its outcome. The authors acknowledge the financial support provided by a UCB Japan Grant (UCBJ Grant) for the English proofreading and article processing charge of the journal.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the institutional review board of the Gunma University Hospital. Patients provided written informed consent before taking part in this study.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
D. A. M. Thuy Trang, Email: trangbeo.fsh@gmail.com.
Koichi Okamura, Email: kokamura@gunma-u.ac.jp.
Takahito Suto, Email: sdosdosdo11@gmail.com.
Hideo Sakane, Email: hsakane0614@gmail.com.
Yukio Yonemoto, Email: yukioyone@ybb.ne.jp.
Takahito Nakajima, Email: nakajima@md.tsukuba.ac.jp.
Yoshito Tsushima, Email: tsushima.rad.gunma@gmail.com.
Hirotaka Chikuda, Email: chikuda-tky@umin.ac.jp.
References
- 1.Peters MJL, van Halm VP, Voskuyl AE, Smulders YM, Boers M, Lems WF, Visser M, Stehouwer CDA, Dekker JM, Nijpels G, Heine R, Dijkmans BAC, Nurmohamed MT. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Care Res. 2009;61(11):1571–1579. doi: 10.1002/art.24836. [DOI] [PubMed] [Google Scholar]
- 2.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheumatism. 2001;44(12):2737–45. 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-#. [DOI] [PubMed]
- 3.Crowson CS, Liao KP, Davis JM, 3rd, Solomon DH, Matteson EL, Knutson KL, et al. Rheumatoid arthritis and cardiovascular disease. Am Heart J. 2013;166(4):622–628. doi: 10.1016/j.ahj.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skeoch S, Cristinacce PLH, Williams H, Pemberton P, Xu D, Sun J, James J, Yuan C, Hatsukami T, Hockings PD, Alexander MY, Waterton JC, Bruce IN. Imaging atherosclerosis in rheumatoid arthritis: evidence for increased prevalence, altered phenotype and a link between systemic and localised plaque inflammation. Sci Rep. 2017;7(1):827. doi: 10.1038/s41598-017-00989-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonsson SW, Backman C, Johnson O, Karp K, Lundstrom E, Sundqvist KG, et al. Increased prevalence of atherosclerosis in patients with medium term rheumatoid arthritis. J Rheumatol. 2001;28(12):2597–602. [PubMed]
- 6.Gonzalez-Juanatey C, Llorca J, Testa A, Revuelta J, Garcia-Porrua C, Gonzalez-Gay MA. Increased prevalence of severe subclinical atherosclerotic findings in long-term treated rheumatoid arthritis patients without clinically evident atherosclerotic disease. Medicine. 2003;82(6):407–413. doi: 10.1097/01.md.0000101572.76273.60. [DOI] [PubMed] [Google Scholar]
- 7.Hannawi S, Haluska B, Marwick TH, Thomas R. Atherosclerotic disease is increased in recent-onset rheumatoid arthritis: a critical role for inflammation. Arthritis Res Ther. 2007;9(6):R116. doi: 10.1186/ar2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aubry M-C, Maradit-Kremers H, Reinalda MS, Crowson CS, Edwards WD, Gabriel SE. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol. 2007;34(5):937–942. [PubMed] [Google Scholar]
- 9.Semb AG, Rollefstad S, Provan SA, Kvien TK, Stranden E, Olsen IC, Hisdal J. Carotid plaque characteristics and disease activity in rheumatoid arthritis. J Rheumatol. 2013;40(4):359–368. doi: 10.3899/jrheum.120621. [DOI] [PubMed] [Google Scholar]
- 10.Skeoch S, Bruce IN. Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat Rev Rheumatol. 2015;11(7):390–400. doi: 10.1038/nrrheum.2015.40. [DOI] [PubMed] [Google Scholar]
- 11.Okamura K, Yonemoto Y, Arisaka Y, Takeuchi K, Kobayashi T, Oriuchi N, Tsushima Y, Takagishi K. The assessment of biologic treatment in patients with rheumatoid arthritis using FDG-PET/CT. Rheumatology. 2012;51(8):1484–1491. doi: 10.1093/rheumatology/kes064. [DOI] [PubMed] [Google Scholar]
- 12.Okamura K, Yonemoto Y, Okura C, Higuchi T, Tsushima Y, Takagishi K. Evaluation of tocilizumab therapy in patients with rheumatoid arthritis based on FDG-PET/CT. BMC Musculoskelet Disord. 2014;15(1):393. doi: 10.1186/1471-2474-15-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suto T, Okamura K, Yonemoto Y, Okura C, Tsushima Y, Takagishi K. Prediction of large joint destruction in patients with rheumatoid arthritis using 18F-FDG PET/CT and Disease Activity Score. Medicine. 2016;95(7):e2841. doi: 10.1097/MD.0000000000002841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suto T, Yonemoto Y, Okamura K, Okura C, Kaneko T, Kobayashi T, Tachibana M, Tsushima Y, Takagishi K. Predictive factors associated with the progression of large-joint destruction in patients with rheumatoid arthritis after biologic therapy: a post-hoc analysis using FDG-PET/CT and the ARASHI (assessment of rheumatoid arthritis by scoring of large-joint destruction and healing in radiographic imaging) scoring method. Modern Rheumatol. 2017;27(5):820–827. doi: 10.1080/14397595.2016.1266132. [DOI] [PubMed] [Google Scholar]
- 15.Yonemoto Y, Okamura K, Takeuchi K, Kaneko T, Kobayashi T, Okura C, Tsushima Y, Takagishi K. [18F]fluorodeoxyglucose uptake as a predictor of large joint destruction in patients with rheumatoid arthritis. Rheumatol Int. 2016;36(1):109–115. doi: 10.1007/s00296-015-3331-0. [DOI] [PubMed] [Google Scholar]
- 16.Yonemoto Y, Okamura K, Kobayashi T, Kaneko T, Okura C, Suto T, Tachibana M, Tsushima Y, Takagishi K. Predictive factors related to shoulder joint destruction in rheumatoid arthritis patients treated with biologics: a prospective study. Mod Rheumatol. 2017;27(4):587–592. doi: 10.1080/14397595.2016.1245125. [DOI] [PubMed] [Google Scholar]
- 17.Rudd JHF, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, Johnström P, Davenport AP, Kirkpatrick PJ, Arch BN, Pickard JD, Weissberg PL. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–2711. doi: 10.1161/01.CIR.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 18.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. In Vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 19.Græbe M, Pedersen SF, Borgwardt L, Højgaard L, Sillesen H, Kjær A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET) Eur J Vasc Endovasc Surg. 2009;37(6):714–721. doi: 10.1016/j.ejvs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Paulmier B, Duet M, Khayat R, Pierquet-Ghazzar N, Laissy J-P, Maunoury C, et al. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nuclear Cardiol. 2008;15(2):209–217. doi: 10.1016/j.nuclcard.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, Nikolaou K, Reiser MF, Bartenstein P, Hacker M. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nuclear Med. 2009;50(10):1611–1620. doi: 10.2967/jnumed.109.065151. [DOI] [PubMed] [Google Scholar]
- 22.Bucerius J, Hyafil F, Verberne HJ, et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging. 2016;43(4):780–792. doi: 10.1007/s00259-015-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, Lawler MA, Grinspoon SK, Brady TJ, Nasir K, Hoffmann U, Tawakol A. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6(12):1250–1259. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JHF, Farkouh ME, Nunes IO, Beals CR, Shankar SS. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62(10):909–917. doi: 10.1016/j.jacc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 25.Maki-Petaja KM, Elkhawad M, Cheriyan J, Joshi FR, Ostor AJ, Hall FC, et al. Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473–2480. doi: 10.1161/CIRCULATIONAHA.112.120410. [DOI] [PubMed] [Google Scholar]
- 26.Bernelot Moens SJ, van der Valk FM, Strang AC, Kroon J, Smits LP, Kneepkens EL, Verberne HJ, van Buul JD, Nurmohamed MT, Stroes ESG. Unexpected arterial wall and cellular inflammation in patients with rheumatoid arthritis in remission using biological therapy: a cross-sectional study. Arthritis Res Ther. 2016;18(1):115. doi: 10.1186/s13075-016-1008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruscitti P, Cipriani P, Liakouli V, Iacono D, Pantano I, Margiotta DPE, Navarini L, Destro Castaniti GM, Maruotti N, di Scala G, Picciariello L, Caso F, Bongiovanni S, Grembiale RD, Atzeni F, Scarpa R, Perosa F, Emmi G, Cantatore FP, Guggino G, Afeltra A, Ciccia F, Giacomelli R. Subclinical and clinical atherosclerosis in rheumatoid arthritis: results from the 3-year, multicentre, prospective, observational GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) study. Arthritis Res Ther. 2019;21(1):204. doi: 10.1186/s13075-019-1975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winchester R, Giles JT, Nativ S, Downer K, Zhang H-Z, Bag-Ozbek A, Zartoshti A, Bokhari S, Bathon JM. Association of elevations of specific T cell and monocyte subpopulations in rheumatoid arthritis with subclinical coronary artery atherosclerosis. Arthritis Rheumatol. 2016;68(1):92–102. doi: 10.1002/art.39419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Limon P, Ortega-Castro R, Barbarroja N, Perez-Sanchez C, Jamin C, Patiño-Trives AM, et al. Molecular characterization of monocyte subsets reveals specific and distinctive molecular signatures associated with cardiovascular disease in rheumatoid arthritis. Front. Immunol. 10:1111. 10.3389/fimmu.2019.01111 [DOI] [PMC free article] [PubMed]
- 30.van der Valk FM, Kroon J, Potters WV, Thurlings RM, Bennink RJ, Verberne HJ, Nederveen AJ, Nieuwdorp M, Mulder WJM, Fayad ZA, van Buul JD, Stroes ESG. In vivo imaging of enhanced leukocyte accumulation in atherosclerotic lesions in humans. J Am Coll Cardiol. 2014;64(10):1019–1029. doi: 10.1016/j.jacc.2014.06.1171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information file.


