Abstract
Obesity and type 2 diabetes are major factors in COVID-19 causing a progression to excessive morbidity and mortality. An important characteristic of these conditions is poor glycaemic control leading to inappropriate chemical reactions and the production of glycated proteins in which positively charged lysine and arginine residues are neutralised. We propose that this protein glycation primes the inflammatory system as the presence of aspartate and glutamate residues in any glycated zwitterionic protein will thus increase its anionic characteristics. As a result, these macromolecules will be recognised by the innate immune system and identified as originating from infection or cell damage (sterile inflammation). Many proteins in the body exist to non-specifically target these anionic macromolecules and rely heavily on positively charged (cationic) binding-sites to produce a relatively non-specific interaction as the first step in the body’s response. Proteins involved in this innate immunity are collectively referred to as damage associated molecular pattern molecules or pathogen associated molecular pattern molecules. A crucial player in this process is RAGE (Receptor for Advanced Glycation End products). RAGE plays a central role in the inflammatory response and on ligand binding stimulates many aspects of inflammation including the production of the key inflammatory mediator NF-κB, and the subsequent production of inflammatory cytokines. This process has the potential to show a positive feedback loop resulting in a dramatic response within the tissue. We propose that protein glycation primes the inflammatory system by generating negatively charged surfaces so that when a SARS-Cov-2 infection occurs within the lung the further release of negatively-charged macromolecules due to cell damage results in a potentially catastrophic inflammatory response resulting in the cytokine storm associated with COVID-19 morbidity and mortality. That part of the population who do not suffer from inflammatory priming (Phase 1), such as the young and the non-obese, should not be subjected to the catastrophic inflammatory response seen in others (Phase 2). This hypothesis further highlights the need for improved dietary intake to minimise the inflammatory priming resulting from poor glycaemic control.
Keywords: Obesity, Diabetes, Innate-immunity, SARS-Cov-2, Glycation, COVID-19, RAGE
The hypothesis presented in this paper is that age, compounded by obesity and hyperglycaemia produces an inflammatory environment that is in part driven by increased glycation of proteins and the accumulation of these now negatively charged macromolecules within the body. These anionic macromolecules are seen by the host as a threat and are removed by innate immunity processes, in which the receptor for advanced glycation end products (RAGE) plays a primary role in binding these negatively charged macromolecules and subsequently initiating an inflammatory response. This response produces a state of basal inflammation within the body, particularly the lung where RAGE is constitutively highly expressed in the adult. A subsequent viral infection compounds the innate immune response and thus may tip the body into an inflammatory spiral producing a cytokine storm and possibly death. The pulmonary target of this response to SARS-Cov-2 is likely due to the enhanced presence of RAGE in lung cells in combination with the presence of the SARS-CoV-2 receptor in this tissue.
Innate immunity is the body’s first line of defence against infection and tissue damage. It is a rapid and non-specific process that targets structures not normally seen in the extra-cellular environment. The defence proteins (alarmins) that are involved in this response are referred to as damage associated molecular pattern molecules (DAMPs) or pathogen associated molecular pattern molecules (PAMPS). The term sterile inflammation is used to describe the phenomenon associated with tissue damage which induces the release of DAMPS [1]. Fundamental to initiating the innate response is the presence of macromolecules or membrane surfaces rich in negative charge. Such surfaces are not normally present in the extracellular environment but are found within cells as a part of normal cell function.
Much of innate immunity utilises blood proteins or membrane receptors that express cationic domains. These domains or patches undergo relatively non-specific electrostatic binding to the foreign structures that present anionic surfaces. The classic example of this is the blood clotting cascades that are initiated by the exposure of anionic surfaces including the presence of the anionic phospholipid, phosphatidylserine [2] on the rupture of the endothelial lining of the blood vessels. Blood proteins involved in innate immunity include the acute phase proteins, the levels of which often rise dramatically with the inflammatory response to infection and tissue damage.
We have previously highlighted the role of the human acute phase protein group IIa PLA2 [3], [4]. This protein is possibly unique in being highly cationic with a global distribution of surface positively charged arginine and lysine residues. This structure supports two functions of the enzyme. Firstly, an anti-bacterial role where the enzyme is targeted to the anionic cell membrane of Gram-positive bacteria and phospholipid hydrolysis assists in bacterial killing [5], [6]. In this example of innate immunity, the changing of up to five positively charged amino acids to negatively charged ones on the surface of this PLA2 by site-directed mutagenesis results in a loss in the ability of the enzyme to bind to and penetrate the negatively charged bacterial cell wall [5]. Secondly, a non-catalytic role is proposed in which the protein forms supramolecular aggregates with anionic phospholipid vesicles or debris. It is proposed that the aggregates are then internalised via interactions with cell surface heparan sulphate proteoglycans and micropinocytosis for disposal by macrophages [3], [4].
The presentation of anionic surfaces or patches is characteristic of other physiological and pathological events. A much-studied phenomenon is a type of protein modification which is a non-enzymatic process (the Maillard reaction) [7] in which sugars or sugar derived metabolites are covalently attached to positively-charged protein amino (lysine) or guanidino (arginine) side chains on the surface of proteins. The phenomenon is referred to as protein glycation and is implicated with ageing and diseases associated with ageing and metabolic disorders [7], [8]. As a result of such chemical events the positively charged lysine and arginine residues are normally neutralised and the protein will acquire increasingly negative surface charge due to the resulting predominance of the negatively-charged glutamic and aspartic residues also present on the surface of proteins. Thus, the protein becomes more anionic in character and gains the characteristic negative surface charge associated with bacteria and cell debris, including cell debris resulting from viral infection. The extent of the chemical reaction involving the aldehyde (carbonyl) group of glucose and other metabolites will be a function of the concentration of such metabolites and time. Hence glycated protein will be enhanced in uncontrolled diabetes where glucose concentration is raised and will also increase with age. The poorer prognosis of diabetics who fail to regulate the blood glucose levels by insulin or diet is due to the excessive glycation of tissue proteins leading to, for example, visual, kidney and cardiovascular problems. The gold standard for glucose compliance by the patient is the measurement of glycated haemoglobin (HbAc1), a protein that is more anionic than normal haemoglobin. It has been suggested that elevated circulating glycated proteins may provide the link between obesity and the development of metabolic syndrome [9]. Our hypothesis focuses on protein glycation because of hyperglycaemia, a condition that is linked to age and obesity. However, other events linked to oxidative stress and protein carbonylation may also result in the formation of anionic patches on proteins. For example, lipid peroxidation and the formation of reactive lipid aldehydes because of oxidative stress can result in protein carbonylation including surface lysine modification [10].
Such anionic glycated proteins are perceived as foreign and are normally removed using processes involving receptor proteins. The major player in this process is the Receptor for Advanced Glycation End products (RAGE) [8], [11]. In the adult, the receptor is constitutively expressed in type 1 epithelial cells of the lung [12] and may also be expressed in type 2 cells [13]. It is believed to be a major mediator of pulmonary inflammatory response [8], [12], [14], [15], [16], [17], [18]. The crystal structure has shown that the interaction of glycated proteins (AGEs) with RAGE involves non-specific electrostatic interaction between anionic patches on the ligand (protein) and cationic patches on the ligand binding domain of RAGE. Such cationic patches on RAGE will also bind DNA and RNA [19], [20] as well as to lipids such as phosphatidylserine [21] and lysophosphatidic acid [22] and bacterial lipopolysaccharide [23]. RAGE will also bind other key regulatory proteins [8], particularly the nucleic acid binding HMGB1 that can be released from damaged cells and bind extracellular DNA or RNA as part of the process of innate immunity [24], [25]. Due to the binding of multiple ligands to RAGE and the strong electrostatic nature of ligand RAGE multimers, it has been proposed that these receptor–ligand complexes are tightly bound and lead to sustained activation of RAGE ligand signalling [8], [11], [26].
The role of RAGE in downstream signalling leading to an inflammatory response is of considerable interest [8], [12], [14], [15], [16], [17], [18]. The sustained activation of the NF-κB pathway leading to cytokine production is of particular importance. This response increases NF-κB in a process that produces a positive feedback loop between RAGE and NF-κB that contributes to chronic, pathological inflammation in many tissues [8], [11], [14] including the lung where RAGE is found in high abundance in the adult. The positive feedback loop involves RAGE stimulating its own expression to produce more RAGE. Thus, in the presence of an excess of anionic ligands for RAGE, the inflammatory situation will be accelerated [8], [11].
Glycated proteins, produced as a result of obesity and diabetes, as well as a normal consequence of ageing, provide the necessary anionic surfaces to illicit an innate immune response. Such a response raises the inflammatory sensitivity of target tissues thus priming the system. Because of the distribution of RAGE, the lung is a primary target for this inflammation. We would describe this overall process as Phase 1 of the inflammatory response that results in COVID-19 morbidity and mortality. We envisage a situation where the lung may be primed for a further and possibly catastrophic inflammatory response.
Viral infection is very well documented as a stimulant of innate immunity. Such infection will give rise to cell derived anionic macromolecules including RNA and DNA resulting from cell destruction by the virus. Viruses themselves may also contribute by providing these negatively-charged molecules [8] while it is proposed that massive amounts of cellular RNA are liberated during infections [27]. Cells normally affected would be those allowing virus uptake via a cell surface receptor. In the case of SARS-CoV-2, this is primarily attributed to Angiotensin Converting Enzyme 2 (ACE2) [28], a receptor found in a number of tissues including heart, kidney and pancreas and both type 1 and type 2 alveolar cells in the lungs [29]. We would suggest that RNA and DNA along with other anionic macromolecules derived from damaged cells or possibly RNA from the virus, may provide a major contribution to the innate response by interacting with RAGE either directly or by binding to HMGB1 in the first instance. We would refer to this viral stage as Phase 2 involving further stimulation of RAGE and downstream inflammatory pathways.
Whereas the Phase 1 response to anionic glycated proteins directly involved RAGE, the Phase 2 response involving cell damage involves the release of RNA and DNA. Phosphatidylserine exposed during apoptosis [21] may also contribute to this process. These anionic molecules directly interact with HMGB1, a partner of RAGE. Therefore, a different inflammatory response might be anticipated. Indeed, such pathways have been discussed in terms of the response of the pulmonary system to HMGB1 [24], [25]. Thus, the Phase 2 response may not be a simple amplification of Phase 1 but involve the recruitment of different inflammatory pathways in lung tissue. The complexity of the response as a result of RAGE-HMGB1-DNA/RNA interactions has been considered [8].
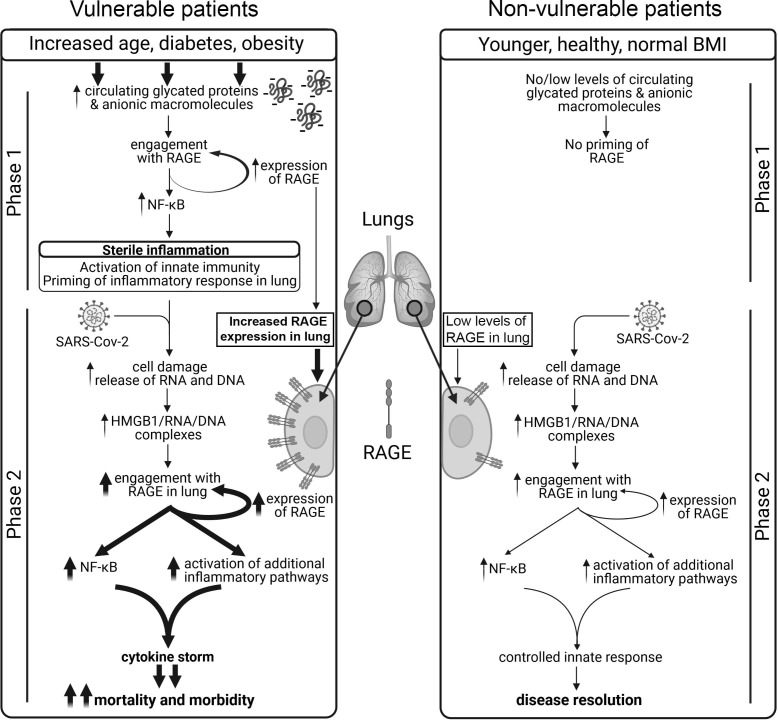
The involvement of a Phase 1 (pre-existing inflammatory condition resulting from sterile immunity priming the system) and phase 2 (viral infection and tissue damage) could produce a tipping point in the system for inflammation in which the contribution of each phase will depend on individual circumstances. The overall result will be the triggering of a potentially devasting positive feed-back stimulation of the inflammatory response in the lung that is consistent with the catastrophic cytokine storm [30] associated with severe cases of COVID-19 (Fig. 1 ). Thus, it is the presence of RAGE and ACE2 in the lung that is probably critical in the process. This series of events would explain the morbidity and mortality from COVID-19 seen in patients of advanced age and/or suffering from underlying problems, especially obesity and diabetes [31], [32], [33]. A further factor that will add to the problem would be high viral load as part of phase 2. This would increase the probability of a severe clinical outcome for those exposed to high levels of the virus, particularly frontline health workers.
Fig. 1.
Proposed 2 phase model of innate immune priming in response to SARS-Cov-2 infection in aging, diabetic and/or obese patients. Comparison of the sequence of events between vulnerable (left sequence) and non-vulnerable (right sequence) patients. Vulnerable patients show increased glycation of proteins because of age, obesity and hyper glycaemia resulting in the generation of circulating negatively charged macromolecules, specifically proteins. In these vulnerable patients the lungs are primed to produce an inflammatory state as the result of the interaction of these circulating anionic macromolecules with RAGE. This further upregulates expression of RAGE in the lungs of these patients in a positive feedback loop. Patients with this primed inflammatory state subsequently over-react to viral infection, inducing an inflammatory spiral in Phase 2 resulting in a cytokine storm and enhanced morbidity and mortality. In non-vulnerable patients there is no phase 1 and the patient recovers from viral infection (phase 2) with little or no symptoms. Thicker arrows highlight pathways that are upregulated in vulnerable patients.
In summary, innate immunity is driven to a large extent by the presentation of anionic (negatively charged) surfaces or macromolecules. Such surfaces are not normally present in the extracellular environment of a healthy individual but are presented as a result of cell damage or infection. Such surfaces occur naturally within the cell as part of normal cell function. High levels of reactive carbonyl-containing molecules including glucose and glucose-derived metabolites, can also give rise to anionic surfaces on zwitterionic macromolecules, particularly proteins, thus mimicking infection. This is because the chemical neutralisation of positively charged lysine and arginine residues on the protein surface due to glycation will result in an excess of negatively charged residues, namely aspartate and glutamate. These processes, which are associated with ageing, will invoke an innate immune reaction that will generate an inflammatory response. The extent of this sterile inflammation (Phase 1) and also virus load leading to cell damage (Phase 2) can precipitate a tipping point leading to a cytokine storm resulting in serious morbidity or mortality in the case of SARS-CoV-2 infections. The priming effect of Phase 1 would predict adverse outcomes in other infectious diseases including viral infections. This is well established in the case of obese patients [34]. Since writing, and in support of this hypothesis, a comprehensive review of RAGE and COVID-19 has appeared [16] that also looks at the COVID-19 problem in terms of the direct consequence of viral infection and a pre-existing inflammatory situation. Our proposed hypothesis takes this further and highlights the implications for public health strategies for combating SARS-Cov-2, particularly those relating to nutrition [35], to reduce obesity and accompanying diabetes in future populations. SARS-CoV-2 is multifaceted in terms of how it can affect body physiology, both short and long term. It can have other actions such as effects on taste and smell. These effects may reflect the location of SARS-CoV-2 receptors on other cells.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Professor Muhammad Akhtar FRS for his support and critically reading the manuscript. Charles Birts is supported by the Against Breast Cancer Lectureship and gratefully acknowledges a programme grant from Against Breast Cancer (www.againstbreastcancer.org.uk; UK Charity 1121258).
References
- 1.Chen G.Y., Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leventis P.A., Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. doi: 10.1146/annurev.biophys.093008.131234. [DOI] [PubMed] [Google Scholar]
- 3.Birts C.N., Barton C.H., Wilton D.C. A catalytically independent physiological function for human acute phase protein group IIA phospholipase A2: Cellular uptake facilitates cell debris removal. J Biol Chem. 2008;283:5034–5045. doi: 10.1074/jbc.M708844200. [DOI] [PubMed] [Google Scholar]
- 4.Birts C.N., Barton C.H., Wilton D.C. Catalytic and non-catalytic functions of human IIA phospholipase A2. Trends Biochem Sci. 2010;35:28–35. doi: 10.1016/j.tibs.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Beers S.A., Buckland A.G., Koduri R.S., Cho W., Gelb M.H., Wilton D.C. The Antibacterial Properties of Secreted Phospholipases A2: A major physiological role for the group iia enzyme that depends on the very high pi of the enzyme to allow penetration of the bacterial cell wall. J Biol Chem. 2002;277:1788–1793. doi: 10.1074/jbc.M109777200. [DOI] [PubMed] [Google Scholar]
- 6.Buckland A.G., Wilton D.C. The antibacterial properties of secreted phospholipases A(2) Biochim Biophys Acta. 2000;1488:71–82. doi: 10.1016/s1388-1981(00)00111-6. [DOI] [PubMed] [Google Scholar]
- 7.Rowan S., Bejarano E., Taylor A. Mechanistic targeting of advanced glycation end-products in age-related diseases. Biochim Biophys Acta (BBA) – Mol Basis Disease. 2018;1864:3631–3643. doi: 10.1016/j.bbadis.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson B.I., Lippman M.E. Targeting RAGE signaling in inflammatory disease. Annu Rev Med. 2018;69:349–364. doi: 10.1146/annurev-med-041316-085215. [DOI] [PubMed] [Google Scholar]
- 9.Uribarri J., Cai W., Woodward M., et al. Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: A link between healthy and unhealthy obesity? J Clin Endocrinol Metab. 2015;100:1957–1966. doi: 10.1210/jc.2014-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauck A.K., Bernlohr D.A. Oxidative stress and lipotoxicity. J Lipid Res. 2016;57:1976–1986. doi: 10.1194/jlr.R066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz G. RAGE: a single receptor fits multiple ligands. Trends Biochem Sci. 2011;36:625–632. doi: 10.1016/j.tibs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Uchida T., Shirasawa M., Ware L.B., et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsuoka F., Kawakami Y., Arai T., et al. Type II alveolar epithelial cells in lung express receptor for advanced glycation end products (RAGE) gene. Biochem Biophys Res Commun. 1997;238:512–516. doi: 10.1006/bbrc.1997.7263. [DOI] [PubMed] [Google Scholar]
- 14.Oczypok E.A., Perkins T.N., Oury T.D. All the “RAGE” in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr Respir Rev. 2017;23:40–49. doi: 10.1016/j.prrv.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas A., Gonzalez I., Morales M.A. SARS-CoV-2-mediated inflammatory response in lungs: should we look at RAGE? Inflamm Res. 2020;69:641–643. doi: 10.1007/s00011-020-01353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy D., Ramasamy R., Schmidt A.M. Journey to a receptor for advanced glycation end products connection in severe acute respiratory syndrome coronavirus 2 infection. Arterioscler Thromb Vasc Biol. 2021;41:614–627. doi: 10.1161/ATVBAHA.120.315527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerkeni M., Gharbi J. RAGE receptor: May be a potential inflammatory mediator for SARS-COV-2 infection? Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiappalupi S., Salvadori L., Vukasinovic A., Donato R., Sorci G., Riuzzi F. Targeting RAGE to prevent SARS-CoV-2-mediated multiple organ failure: Hypotheses and perspectives. Life Sci. 2021;272 doi: 10.1016/j.lfs.2021.119251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch M., Chitayat S., Dattilo B.M., et al. Structural basis for ligand recognition and activation of RAGE. Structure. 2010;18:1342–1352. doi: 10.1016/j.str.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park H., Boyington J.C. The 1.5Å crystal structure of human receptor for advanced glycation endproducts (RAGE) ectodomains reveals unique features determining ligand binding. J Biol Chem. 2010;285:40762–40770. doi: 10.1074/jbc.M110.169276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He M., Kubo H., Morimoto K., et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12:358–364. doi: 10.1038/embor.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rai V., Touré F., Chitayat S., et al. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J Exp Med. 2012;209:2339–2350. doi: 10.1084/jem.20120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto Y., Harashima A., Saito H., et al. Septic shock is associated with receptor for advanced glycation end products ligation of LPS. J Immunol. 2011;186:3248. doi: 10.4049/jimmunol.1002253. [DOI] [PubMed] [Google Scholar]
- 24.Andersson U., Ottestad W., Tracey K.J. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol Med. 2020;26:42. doi: 10.1186/s10020-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson U., Tracey K.J. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kierdorf K., Fritz G. RAGE regulation and signaling in inflammation and beyond. J Leukoc Biol. 2013;94:55–68. doi: 10.1189/jlb.1012519. [DOI] [PubMed] [Google Scholar]
- 27.Preissner K.T., Fischer S., Deindl E. Extracellular RNA as a versatile DAMP and alarm signal that influences leukocyte recruitment in inflammation and infection. Front Cell Dev Biol. 2020;8:1618–1642. doi: 10.3389/fcell.2020.619221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann M., Kleine-Weber H., Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78:779–784. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holly J.M.P., Biernacka K., Maskell N., Perks C.M. Obesity, diabetes and COVID-19: An infectious disease spreading from the east collides with the consequences of an unhealthy Western Lifestyle. Front Endocrinol. 2020;11:665–678. doi: 10.3389/fendo.2020.582870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim S., Bae J.H., Kwon H.-S., Nauck M.A. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drucker D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning. Cell Metab. 2021;33:479–498. doi: 10.1016/j.cmet.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milner J.J., Beck M.A. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71:298–306. doi: 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calder P.C. Nutrition, immunity and COVID-19. BMJ Nut Prevent Health. 2020:74–92. doi: 10.1136/bmjnph-2020-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]