Abstract
Background
The variability of coronavirus disease 2019 (COVID-19) illness severity has puzzled clinicians and has sparked efforts to better predict who would benefit from rapid intervention. One promising biomarker for in-hospital morbidity and mortality is cardiac troponin (cTn).
Methods
A retrospective study of 1331 adult patients with COVID-19 admitted to the Rush University System in Illinois, USA was performed. Patients without cTn measurement during their admission or a history of end stage renal disease or stage 5 chronic kidney disease were excluded. Using logistic regression adjusted for baseline characteristics, pre-existing comorbidities, and other laboratory markers of inflammation, cTn was assessed as a predictor of 60-day mortality and severe COVID-19 infection, consisting of a composite of 60-day mortality, need for intensive care unit, or requiring non-invasive positive pressure ventilation or intubation.
Results
A total of 772 patients met inclusion criteria. Of these, 69 (8.9%) had mild cTn elevation (> 1 to < 2x upper limit of normal (ULN)) and 46 (6.0%) had severe cTn elevation (≥ 2x ULN). Regardless of baseline characteristics, comorbidities, and initial c-reactive protein, lactate dehydrogenase, and ferritin, when compared to the normal cTn group, mild cTn elevation and severe cTn elevation were predictors of severe COVID-19 infection (adjusted OR [aOR] aOR 3.00 [CI: 1.51 – 6.29], P < 0.01; aOR 9.96 [CI: 2.75 – 64.23], P < 0.01, respectively); severe cTn elevation was a predictor of in-hospital mortality (aOR 2.42 [CI: 1.10 – 5.21], P < 0.05) and 60-day mortality (aOR 2.45 [CI: 1.13 – 5.25], P < 0.05).
Conclusion
In our cohort, both mild and severe initial cTn elevation were predictors of severe COVID-19 infection, while only severe cTn elevation was predictive of 60-day mortality. First cTn value on hospitalization is a valuable longitudinal prognosticator for COVID-19 disease severity and mortality.
Keywords: COVID-19, SARS-CoV-2, Troponin, Biomarker, Mortality
1. Introduction
COVID-19 is responsible for a global pandemic with greater than 100 million cases and 2.3 million deaths worldwide as of February 2021 [1].
Due to the wide spectrum in severity of presentation ranging from completely asymptomatic to acute hypoxic respiratory failure, a large body of research has focused on predictors of poor outcomes in an attempt to risk stratify patients. While comorbidities and chest imaging can provide valuable prognostic information, laboratory biomarkers would provide a quantitative method that is overall easier to obtain and less expensive than imaging [2], [3], [4], [5]. To date, multiple biomarkers have been found to be helpful in prognosticating poor outcomes in patients with COVID-19 including hematologic markers (e.g., lymphocyte count and platelet count), inflammatory markers (e.g., interleukin-6 and C-reactive protein [CRP]), biochemical markers (e.g., lactate dehydrogenase [LDH] and creatinine kinase [CK]), and markers of coagulopathy (e.g., prothrombin time and D-Dimer) [6], [7], [8], [9]. A variety of molecular changes have also been associated with the SARS-CoV-2 virus, and subsequently, novel biomarkers are also being tested [9,10].
Cardiac injury, one of multiple cardiovascular complications of COVID-19, occurs in 8 to 28% of patients and is known to increase overall mortality, which makes cardiac biomarkers a particular area of interest [11], [12], [13], [14]. A meta-analysis of cardiac biomarkers found that cardiac troponin (cTn) and creatinine kinase (CK) levels were higher in non-survivors versus survivors of COVID-19, while no significant difference was found in the levels of brain natriuretic peptide (BNP) between these two groups [15]. In this study, cTn emerged as a particularly promising cardiac biomarker as it was elevated in the combined outcome of critical illness and mortality, which was not the case for CK [15].
The data supporting the prognostic role of cTn is robust as summarized by another meta-analysis of twenty-one studies and 6297 patients that found those with cardiac injury are 16.79 times more likely to suffer all-cause mortality [11,13,[16], [17], [18], [19], [20]]. Despite this mounting evidence, some have been hesitant in recommending its use in risk stratification likely due to its long-standing association with ischemic disease rather than as a biomarker for critical illness [21,22].
This retrospective study sought to add to the body of literature regarding the utility of cTn as a biomarker. Specifically, we wanted to compare COVID-19 disease severity, 60-day mortality, and 60-day complications in those with normal versus elevated cTn levels. We then sought to assess these outcomes by level of cTn elevation to further probe the potential benefits of cTn as a biomarker.
2. Methods
We conducted a retrospective cohort study of adult patients ≥ 18 years old with polymerase chain reaction confirmed COVID-19 between March to June 2020 who were admitted to Rush University System for Health (RUSH), which includes Rush University Medical Center, a 664-bed tertiary academic medical center; Rush Oak Park Hospital, a 201-bed affiliated community hospital; and Rush Copley Medical Center, a 210-bed suburban affiliated community hospital in Illinois, US. A combination of automatic and manual data extraction data collection was performed. Patient's medical records were followed and reviewed for a minimum of 60 days after the date of their initial COVID-19 admission through chart review of several local hospitals.
Those without a cTn measurement were excluded (Fig. 1 ). When multiple cTns were measured, only the first cTn measurement was used. The cTn assay used at our institution is Troponin I with normal values of 0.00 - 0.09 ng/mL. Cardiac injury was defined as a cTn value greater than this upper limit of normal (ULN). Patients were additionally subcategorized into normal cTn level, mild cTn elevation (> 1 to < 2x ULN), and severe cTn elevation (≥ 2x ULN).
Fig. 1.
Inclusion criteria to define study population.
Prior studies have suggested that the standard laboratory cutoff for various cardiac biomarkers should be redefined to enhance prognostic ability for COVID-19. 23 Specifically, Qin et al. found that reducing the cutoff for high sensitivity cTn by 49.0% improved mortality prediction [23]. To test this further, a subcategory was created for light cTn elevation, defined as cTn > 0.5x to ≥ 1x ULN. Normal cTn was redefined as ≤ 0.5x ULN, but this was only used when light cTn elevation was specifically being analyzed.
Additionally, those with end stage renal disease and stage 5 chronic kidney disease (CKD) were excluded as renal disease is a known to influence cTn measurement (Fig. 1) [24].
The primary outcome was 60-day mortality. Two secondary outcomes of particular interest were in-hospital mortality and a composite outcome for severe infection that consisted of 60-day mortality, intensive care unit (ICU) requirement, and need for non-invasive positive pressure ventilation or intubation. Other secondary outcomes included readmission, intubation, extracorporeal membrane oxygenation (ECMO), tracheostomy, vasopressor requirement, and inotrope requirement.
Patient charts were also reviewed for pre-selected major adverse events (MAE) up to 60 days from their hospital admission, which included life-threatening arrhythmia, deep venous thrombosis, symptoms of acute HF, acute renal failure requiring renal replacement therapy, stroke, and pulmonary embolism. During this subsequent chart review, data was also collected on hospital readmission and mortality.
3. Statistical analysis
All data analysis, including statistical analyses, was performed using RStudio version 1.3 (Boston, Massachusetts). Kaplan-Meier survival estimates were generated and plotted with the survival and survminer packages, respectively.
Continuous variables are reported with mean and standard deviation for normally distributed variables and with median and interquartile range for variables not normally distributed. Categorical variables are reported as counts and proportions. Continuous variables were compared with t-tests or Wilcoxon rank-sum test, and categorical variables with the Pearson chi-square test.
Logistic regression was performed between the level of cTn elevation as a predictor variable for severe infection and 60-day mortality, respectively. All logistic regression models were adjusted for baseline characteristics of age, sex, and body mass index, pre-existing history of coronary artery disease, atrial fibrillation, chronic obstructive pulmonary disorder, and diabetes mellitus, and the initial values of CRP, LDH, and ferritin during the index hospitalization. Odds ratios (OR) with 95% confidence intervals (CI) are reported for logistic regression. The threshold for statistical significance was set to a p-value < 0.05.
4. Results
Of the 1331 patients available in our dataset, 772 met inclusion criteria. Only 3 patients (0.4%) in our cohort had a cardiac catheterization during their hospitalization. Two of these patients were diagnosed with acute coronary syndrome with one having an ST-elevation myocardial infarction with percutaneous coronary intervention and the other having a type I non-ST segment elevation myocardial infarction with visible plaque rupture on the angiogram. The last patient's angiogram revealed chronic stable coronary artery disease requiring no intervention.
The median cTn level was 0.02 with a first and third quartile of 0.00 and 0.04, respectively. The overall distribution was heavily skewed to higher values (range: 0.00 to 68.86).
A total of 115 patients (14.9%) in our cohort had an elevated cTn level. Of these, 69 patients (60.0%) had mild cTn elevation, and 46 patients (40.0%) had severe cTn elevation.
Compared to the baseline characteristics of patients with normal cTn levels, those with increased cTn measurements were older and have a variety of comorbidities including a history of atrial fibrillation, coronary artery disease, hypertension, chronic kidney disease, cancer, ventricular arrhythmia, stroke, acute myocardial infarction, and prior deep venous thrombosis or pulmonary embolism (Table 1 ). Additionally, the elevated cTn group had higher measured values of white blood cells, creatinine, CRP, ferritin, and LDH but lower hemoglobin and temperature measurements.
Table 1.
Baseline characteristics by presence or absence of cardiac troponin elevation
| Normal Troponin,≤ 1 ULN | Elevated Troponin,> 1 ULN | P-value | |
|---|---|---|---|
| n | 657 | 115 | |
| Age* | 58 (46 - 69) | 66 (55.5 - 76) | <0.001 |
| Male (%) | 387 (58.9) | 69 (60.0) | 0.906 |
| BMI* | 28.10 (24.20 - 33.40) | 28.25 (23.77 - 34.72) | 0.646 |
| Race (%) | 0.062 | ||
| White | 186 (30.7) | 29 (26.4) | |
| Other | 204 (33.7) | 29 (26.4) | |
| African American | 215 (35.5) | 52 (47.3) | |
| Comorbidities | |||
| Current Smoker (%) | 32 (5.4) | 6 (6.5) | 0.837 |
| Atrial Fibrillation (%) | 92 (14.0) | 37 (32.2) | <0.001 |
| Coronary Artery Disease (%) | 156 (23.7) | 60 (52.2) | <0.001 |
| Hypertension (%) | 397 (60.4) | 99 (86.1) | <0.001 |
| Chronic Kidney Disease (%) | <0.001 | ||
| CKD stage 1 | 1 (0.2) | 0 (0.0) | |
| CKD stage 2 | 11 (1.7) | 8 (7.0) | |
| CKD stage 3 | 60 (9.1) | 25 (21.7) | |
| CKD stage 4 | 5 (0.8) | 4 (3.5) | |
| Unknown | 17 (2.6) | 4 (3.5) | |
| COPD (%) | 48 (7.3) | 15 (13.0) | 0.059 |
| Diabetes Mellitus (%) | 289 (44.0) | 60 (52.2) | 0.127 |
| Asthma (%) | 91 (13.9) | 12 (10.4) | 0.398 |
| Cancer (%) | 67 (10.2) | 20 (17.4) | 0.037 |
| Ventricular Arrhythmia (%) | 29 ( 4.4) | 14 (12.2) | 0.002 |
| Stroke (%) | 87 (13.2) | 28 (24.3) | 0.003 |
| Acute Myocardial Infarction (%) | 86 (13.1) | 47 (40.9) | <0.001 |
| DVT or Pulmonary Embolism (%) | 81 (12.3) | 30 (26.1) | <0.001 |
| Initial Labs* | |||
| Troponin (ng/mL) | 0.01 (0.00 - 0.02) | 0.22 (0.14 - 0.51) | <0.001 |
| White Blood Cell Count (cells/uL) | 6.90 (5.21 - 9.33) | 8.40 (6.10 - 12.85) | <0.001 |
| Lymphocyte Number (cells/uL) | 1.00 (0.70 - 1.30) | 1.01 (0.64 - 1.50) | 0.778 |
| Hemoglobin (g/dL) | 13.40 (12.20 - 14.60) | 13.20 (11.70 - 14.20) | 0.024 |
| Platelet Count (cells/uL) | 206 (162 - 270) | 213 (161.5 - 282) | 0.967 |
| Creatinine (mg/dL) | 1.01 (0.82 - 1.32) | 1.52 (1.08 - 2.26) | <0.001 |
| CRP (mg/dL) | 112.00 (54.90 - 184.60) | 165.00 (76.10 - 266.80) | <0.001 |
| Ferritin (ng/mL) | 732.00 (356.88 - 1543.00) | 949.00 (416.25 - 1941.25) | 0.041 |
| LDH (U/L) | 396.00 (302.00 - 523.00) | 520.00 (356.75 - 752.50) | <0.001 |
| Initial Vital Signs* | |||
| Systolic BP | 131 (115 - 145) | 124 (110.5 - 147) | 0.241 |
| Diastolic BP | 74 (65 - 86) | 76 (62 - 87) | 0.863 |
| Heart Rate | 99 (87 - 112) | 100 (84 - 116.5) | 0.725 |
| Respiratory Rate | 21 (19 - 25) | 22 (20 - 28) | 0.080 |
| Pulse Oximetry | 93 (88 - 96) | 94.00 (87.5 - 97.5) | 0.762 |
| Temperature | 99.0 (98.1 - 100.3) | 98.5 (97.8 - 99.85) | 0.030 |
ULN = upper limit of normal; BMI = body mass index; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disorder; DVT = deep venous thrombosis; CRP = c-reactive protein; LDH = lactate dehydrogenase; BP = blood pressure
Variables expressed as median and interquartile range
5. Primary survival outcome
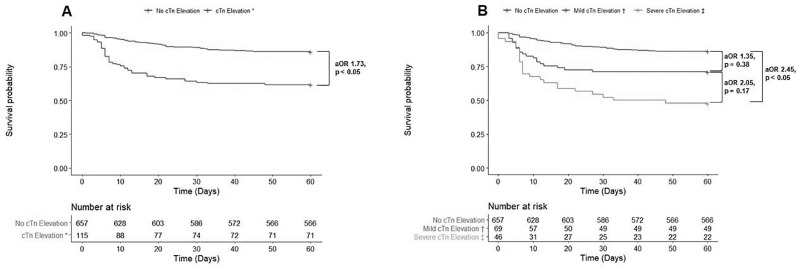
In our cohort, 135 (17.5%) patients suffered 60-day mortality with the median time of death of day 12 after hospitalization (interquartile range [IQR] 6 – 22). Compared to those without cTn elevation, patients with cTn elevation were significantly more likely to suffer 60-day mortality (adjusted OR [aOR] 1.73 [CI: 1.00 – 2.94]; P < 0.05) (Fig. 2 ).
Fig. 2.
Kaplan Meier Survival Estimates by First Cardiac Troponin Measurement
A. Comparison of survival rates with adjusted odds ratios by presence or absence of cardiac troponin elevation. B. Comparison of survival rates with adjusted odds ratios by level of cardiac troponin elevation
cTn = cardiac troponin; aOR = adjusted odds ratio
*Defined as cardiac troponin > 1x upper limit of normal
†Defined as cardiac troponin > 1 to < 2x upper limit of normal
‡Defined as cardiac troponin ≥ 2x upper limit of normal
When subclassifying cTn by the level of elevation, 91 (13.9%), 20 (29.0%), and 24 (52.2%) patients died within 60 days in the normal, mild cTn elevation, and severe cTn elevation groups, respectively. Those with severe cTn elevation (aOR 2.45 [CI: 1.13 – 5.25]; P < 0.05), but not those with those with mild cTn elevation (aOR 1.35 [CI: 0.68 – 2.60]; P = 0.38), were at increased risk for 60-day mortality compared to those with normal cTn levels (Fig. 2, Table 2 ). When compared to those with mild cTn elevation, patients with severe cTn elevation were at no higher risk of 60-day mortality (aOR 2.05 [CI: 0.74 – 5.78]; P = 0.17).
Table 2.
Cardiac Troponin Level as a Predictor for 60-Day Mortality and Severe COVID-19 Infection. All models were adjusted for baseline characteristics, pre-existing comorbidities, and initial values of c-reactive protein, lactate dehydrogenase, and ferritin during the index hospitalization
| Normal cTn, ≤ 1x ULN (n = 657) |
Mild cTn Elevation, > 1 to < 2x ULN (n = 69) |
P-value | Severe cTn Elevation, ≥ 2x ULN (n = 46) |
P -value | |
|---|---|---|---|---|---|
| 60-Day Mortality | |||||
| Incidence (%) | 91 (13.9) | 20 (29.0) | 24 (52.2) | - | |
| Model 1 Adjusted OR* (95% CI) | Ref | 1.35 (0.68 – 2.60) | 0.38 | 2.45 (1.13 – 5.25) | < 0.05 |
| Model 2 Adjusted OR† (95% CI) | - | Ref | - | 2.05 (0.74 – 5.78) | 0.17 |
| Severe Infection‡ | |||||
| Incidence (%) | 288 (43.7) | 49 (71.0) | 43 (93.5) | - | |
| Model 3 Adjusted OR*(95% CI) | Ref | 3.00 (1.51 – 6.29) | < 0.01 | 9.96 (2.75 – 64.23) | < 0.01 |
| Model 4 Adjusted OR† (95% CI) | - | Ref | - | 5.97 (0.97 – 72.35) | 0.09 |
cTn = cardiac troponin; ULN = upper limit of normal; OR = odds ratio; CI = confidence interval; Ref = reference
Model 1 and Model 3 compared both mild and severe cTn elevation to those with normal cTn levels.
Model 2 and Model 4 compared severe cTn elevation those with mild cTn elevation.
Severe infection was defined through a composite consisting of 60-day mortality, ICU requirement, and need for non-invasive positive pressure ventilation or intubation
6. In-Hospital mortality outcome
The in-hospital mortality rate was 13.6% (105 of 772). The odds of in-hospital mortality was 1.93 times higher in patients with elevated versus normal cTn (aOR 1.93 [CI: 1.09 – 3.36]; P < 0.05).
In analyzing by level of cTn elevation, 69 (10.5%), 16 (23.2%), and 20 (43.5%) patients suffered in-hospital mortality in the normal, mild cTn elevation, and severe cTn elevation groups, respectively. Patients in the severe (aOR 2.42 [CI: 1.10 – 5.21]; P < 0.05) cTn elevation group were at increased risk of in-hospital mortality when compared to those with normal cTn levels; no statistically increased risk was found in those with mild cTn elevation compared to those with normal cTn levels (aOR 1.62 [CI: 0.79 – 3.22]; P = 0.17). When only analyzing those with elevated cTn levels, patients with severe cTn elevation were no more likely to suffer in-hospital mortality (aOR 1.53 [CI: 0.57 – 4.09]; P = 0.40) versus those with only mild cTn elevation.
7. Composite outcome for severe infection
A total of 379 (49.1%) patients in our cohort had a severe COVID-19 infection as defined by the composite outcome. Those with cTn elevation were significantly more likely to have a severe COVID-19 infection compared to those a normal initial cTn level (aOR 3.99 [CI: 2.16 – 7.76]; P < 0.001).
When subclassifying cTn by the level of elevation, 288 (43.7%), 49 (71.0%), and 43 (93.5%) patients suffered severe infection in the normal, mild cTn elevation, and severe cTn elevation groups, respectively. Both those with mild cTn elevation (aOR 3.00 [CI: 1.51 – 6.29]; P < 0.01) and those with severe cTn elevation (aOR 9.96 [CI: 2.75 – 64.23]; P < 0.01) were at increased risk for the composite outcome compared to those with normal cTn levels (Table 2). When compared to those with mild cTn elevation, patients with severe cTn elevation (aOR 5.97 [CI: 0.97 – 72.35]; P = 0.09) were not at increased risk of severe infection. The incidence of the individual components of the composite outcome by level of cTn elevation is shown in Supplemental Table 1.
Supplemental Table.
1: Individual Components of the Composite Outcome for Severe COVID-19 Infection by Level of Cardiac Troponin Elevation
| Normal cTn,≤ 1x ULN | Mild cTn Elevation,> 1 to < 2x ULN | Severe cTn Elevation,≥ 2x ULN | P -value | |
|---|---|---|---|---|
| n | 658 | 69 | 46 | |
| 60-Day Mortality (%) | 91 (13.8) | 20 (29.0) | 24 (52.2) | <0.001 |
| Need for ICU (%) | 268 (40.7) | 47 (68.1) | 41 (89.1) | <0.001 |
| NIPPV (%) | 40 (6.1) | 1 (1.4) | 6 (13.0) | 0.039 |
| Mechanical Ventilation (%) | 170 (25.8) | 33 (47.8) | 33 (71.7) | <0.001 |
| Overall Composite (%) | 288 (43.8) | 49 (71.0) | 43 (93.5) | <0.001 |
cTn = cardiac troponin; ULN = upper limit of normal; ICU = intensive care unit; NIPPV = non-invasive positive pressure ventilation
8. Secondary outcomes by presence/absence of cTn elevation
When compared to those with normal cTn levels, patients with elevated cTn were more likely to be intubated (57.4% vs 25.7%, P < 0.001), require tracheostomy (9.6% vs 3.2%, P < 0.01), need ECMO (5.3% vs 1.5%, P < 0.05), have a pressor requirement (56.5% vs 24.2%, P < 0.001), have an inotrope requirement (13.0% vs 1.5%, P < 0.001), and require admission to the ICU (63.5% vs 21.8%, P < 0.001) or ICU level of care during their hospitalization (76.5% vs 40.6%, P < 0.001) (Table 3 ). Those with elevated cTn also had a higher incidence of a combined outcome of in-hospital mortality or readmission compared to those with normal cTn levels (41.7% vs 21.6%, P < 0.001). There was no significant difference in the rates of readmission alone between the groups.
Table 3.
Secondary Outcomes by Presence/Absence of Cardiac Troponin Elevation
| Normal Troponin, ≤ 1x ULN | Elevated Troponin, > 1x ULN | P -value | |
|---|---|---|---|
| n | 657 | 115 | |
| Readmitted (%) | 72 (11.0) | 12 (10.4) | 0.997 |
| In-Hospital Mortality or Readmission (%) | 142 (21.6) | 48 (41.7) | <0.001 |
| Admitted to ICU (%) | 143 (21.8) | 73 (63.5) | <0.001 |
| Required ICU During Admission (%) | 267 (40.6) | 88 (76.5) | <0.001 |
| ECMO (%) | 10 ( 1.5) | 6 ( 5.3) | 0.027 |
| Tracheostomy (%) | 21 ( 3.2) | 11 ( 9.6) | 0.004 |
| Required Pressors (%) | 159 (24.2) | 65 (56.5) | <0.001 |
| Required Inotropes (%) | 10 ( 1.5) | 15 (13.0) | <0.001 |
| Total 60-Day MAE (%) | 104 (15.8) | 57 (49.6) | <0.001 |
| PE (%) | 17 ( 2.6) | 12 (10.4) | <0.001 |
| DVT (%) | 16 ( 2.4) | 11 ( 9.6) | <0.001 |
| Renal Replacement Therapy (%) | 42 ( 6.4) | 25 (21.7) | <0.001 |
| Life-threatening Arrhythmia (%) | 43 ( 6.5) | 24 (20.9) | <0.001 |
| Stroke (%) | 1 ( 0.2) | 0 ( 0.0) | 1.000 |
| Acute HF Exacerbation (%) | 9 ( 1.4) | 10 ( 8.7) | <0.001 |
ULN = upper limit of normal; ICU = intensive care unit; ECMO = extracorporeal membrane oxygenation; MAE = major adverse events; PE = pulmonary embolism; DVT = deep vein thrombosis; HF = heart failure
The median time of the first MAE was 5 days after hospitalization (IQR 1 – 12). Those with elevated cTn levels were more likely to suffer a MAE in general compared to those with normal cTn measurement (49.6% vs 15.8%, P < 0.001). For individual MAE, elevated cTn was associated with increased rates of pulmonary embolism (10.4% vs 2.6%, P < 0.001), deep venous thrombosis (9.6% vs 2.4%, P < 0.001), acute kidney injury requiring renal replacement therapy (21.7% vs 6.4%, P < 0.001), life-threatening arrhythmia (20.9% vs 6.5%, P < 0.001), and acute heart failure exacerbation (8.7% vs 1.4%, P < 0.001). There was no significant difference between the groups in the incidence of stroke.
9. Secondary outcomes by level of cTn elevation
Those with severe cTn elevation were more likely to be intubated (71.7% vs 47.8%, P < 0.05), require the intensive care unit on admission (76.1% vs 55.1%, P < 0.05) or at some point during the hospitalization (89.1% vs 68.1%, P < 0.05), and need vasopressor (76.1% vs 43.5%, P < 0.01) and inotropic agents (23.9% vs 5.8%, P < 0.05) (Table 4 ). Significant differences between the mild and severe cTn groups were not present in the incidence of readmission, ECMO, tracheostomy, or a composite of both in-hospital mortality or readmission.
Table 4.
Secondary Outcomes by Level of Cardiac Troponin Elevation
| Mild cTn Elevation, > 1 to < 2x ULN | Severe cTn Elevation, ≥ 2x ULN | P -value | |
|---|---|---|---|
| n | 69 | 46 | |
| Readmitted (%) | 10 (14.5) | 2 (4.3) | 0.152 |
| In-Hospital Mortality or Readmission (%) | 26 (37.7) | 22 (47.8) | 0.375 |
| Admitted to ICU (%) | 38 (55.1) | 35 (76.1) | 0.036 |
| Required ICU During Admission (%) | 47 (68.1) | 41 (89.1) | 0.017 |
| ECMO (%) | 3 (4.3) | 3 (6.7) | 0.910 |
| Tracheostomy (%) | 8 (11.6) | 3 (6.7) | 0.585 |
| Required Pressors (%) | 30 (43.5) | 35 (76.1) | 0.001 |
| Required Inotropes (%) | 4 (5.8) | 11 (23.9) | 0.011 |
| Total 60-Day MAE (%) | 30 (43.5) | 27 (58.7) | 0.159 |
| PE (%) | 5 (7.2) | 7 (15.2) | 0.290 |
| DVT (%) | 4 (5.8) | 7 (15.2) | 0.174 |
| Renal Replacement Therapy (%) | 11 (15.9) | 14 (30.4) | 0.106 |
| Life-threatening Arrhythmia (%) | 12 (17.4) | 12 (26.1) | 0.373 |
| Stroke (%) | 0 (0.0) | 0 (0.0) | 1.000 |
| Acute HF Exacerbation (%) | 4 (5.8) | 6 (13.0) | 0.311 |
cTn = cardiac troponin; ULN = upper limit of normal; ICU = intensive care unit; ECMO = extracorporeal membrane oxygenation, MAE = major adverse event; PE = pulmonary embolism; DVT = deep venous thrombosis
There were also no significant differences between the incidence of MAE between the severe and mild cTn groups.
10. Light cTn elevation
In this cohort, 64 patients (8.3%) had light cTn elevation. When compared to cTn ≤ 0.5x ULN, those with light cTn elevation were more likely to have severe COVID-19 infection (61.5% vs 41.8%, P < 0.01), in-hospital mortality (18.5% vs 9.6%, P < 0.05), and 60-day mortality (24.6 vs 12.6%, P < 0.05).
11. Comparing those with vs without cTn measurement
The 820 patients who had cTn measured during their hospitalization were compared with the 512 patients without measurement regardless of chronic kidney disease status. In comparing baseline characteristics, the cTn group was more likely to be older and male with a higher median body mass index (Supplemental Table 2 ). Patients with cTn measured were also more likely to have a prior history of most comorbidities tested including atrial fibrillation, coronary artery disease, hypertension, and prior myocardial infarction (P < 0.001). The cTn group also had significantly higher incidence of 60-day mortality (17.1% vs 3.1%, P < 0.001) and severe COVID-19 infection (48.9% vs 19.9%, P < 0.001) when compared to those with no cTn measurement during their index hospitalization.
Supplemental Table.
2: Comparison of baseline characteristics, comorbidities, and outcomes in patients with and without cardiac troponin measurement
| No cTn Measured | cTn Measured | P-value | |
|---|---|---|---|
| n | 512 | 820 | |
| Age (median [IQR]) | 54.00 [42.00, 66.00] | 59.50 [47.00, 72.00] | <0.001 |
| Male (%) | 254 (49.6) | 485 (59.1) | 0.001 |
| BMI (median [IQR]) | 27.10 [23.15, 31.70] | 28.00 [24.10, 33.45] | 0.004 |
| Comorbidities | |||
| Atrial Fibrillation (%) | 42 (8.2) | 141 (17.2) | <0.001 |
| Coronary Artery Disease (%) | 87 (17.0) | 241 (29.4) | <0.001 |
| Hypertension (%) | 283 (55.3) | 540 (65.9) | <0.001 |
| Chronic Kidney Disease (%) | 102 (19.9) | 225 (27.4) | 0.002 |
| COPD (%) | 22 (4.3) | 67 (8.2) | 0.008 |
| Diabetes Mellitus (%) | 215 (42.0) | 389 (47.4) | 0.059 |
| Asthma (%) | 66 (12.9) | 105 (12.8) | 1.000 |
| Cancer (%) | 47 (9.2) | 92 (11.2) | 0.275 |
| Stroke (%) | 52 (10.2) | 126 (15.4) | 0.008 |
| Myocardial Infarction (%) | 38 (7.4) | 145 (17.7) | <0.001 |
| Venous Thromboembolism (%) | 48 (9.4) | 121 (14.8) | 0.005 |
| Outcomes | |||
| 60-Day Mortality (%) | 16 (3.1) | 140 (17.1) | <0.001 |
| Severe COVID-19 Infection (%) | 102 (19.9) | 401 (48.9) | <0.001 |
cTn = cardiac troponin; IQR = interquartile range; BMI = body mass index; COPD = chronic obstructive pulmonary disorder
12. Comparing initial to maximum cTn measurement
A total of 218 patients had multiple cTn measurements during their hospitalization. The median delta of the maximum cTn value minus the initial cTn value was 0.040 (IQR 0.016 – 0.323). On average, the maximum cTn value was obtained on hospital day 4 (IQR 1 – 9). No statistical analysis was performed using the delta or the maximum cTn value as the focus of this study was on the prognostic role of initial cTn measurement.
13. Discussion
To date, prior studies have demonstrated the prognostic role of cTn level measurement in predicting COVID-19 severity and mortality [11,13,[16], [17], [18], [19], [20]]. However, many of these studies only measured complications and mortality during a patient's hospitalization without post-discharge follow up [13,16,17]. Our study supports prior work and provides additional longitudinal outcomes with our 60 day follow up post hospital discharge.
In our cohort, both severe and mild cTn elevation were significant predictors of severe COVID-19 infection compared to those with normal cTn levels. Additionally, severe cTn elevation, but not mild cTn elevation, was also predictive of both in-hospital and 60-day mortality. Those with an elevated cTn level were also at increased risk for 60-day MAE in virtually every individual outcome including pulmonary embolism, deep venous thrombosis, life-threatening arrhythmia, and acute heart failure exacerbation. The role of cTn as a biomarker in predicting in-hospital mortality and morbidity has been elucidated in earlier studies [11,17,18]. Our study takes this further by establishing cTn's role in longitudinal prognostication of patients hospitalized with COVID-19 disease, up to 60 days after initial presentation regardless of medical comorbidities or other markers of inflammation including CRP, LDH, and ferritin [23].
In patients who have recovered from acute COVID-19 infection, the majority will have the persistence of at least one symptom with a significant fraction even having post-infection pulmonary or extrapulmonary organ dysfunction [25,26]. While there is still much to learn about the long-term effects of COVID-19 infection, some hospitals have established outpatient COVID-19 clinics to address the volume and unique needs of this group of patients [26]. While previous studies have focused on cTn's prognostic role for in-hospital events, our data on 60-day mortality and 60-day MAE indicates that the prognostic value of initial cTn measurement appears to extend beyond the acute COVID-19 hospitalization. Therefore, those with elevated cTn levels represent a high-risk group that should be closely followed even after their index hospitalization.
Our cohort does have key differences from previously published cTn data. Our positive cTn percentage (14.9%) was less than that found in Majure et al. in New York (30%) and Shah at al. in Georgia (37.5%) but within published estimates from China (7-28%) [16,19]. Furthermore, the mean age of our cohort (59) was younger than both Majure et al (66) and Shah et al (63) [16,19]. Our hospital also checked cTn on a greater percentage of those admitted with COVID-19 than did Majure et al (61% vs 56%)[19].
The data is mounting that the role of cTn should be expanded beyond the previous engrained notion as a test for myocardial ischemia. Even prior to COVID-19, cTn has been found to be a predictor of both in-hospital and long-term mortality in intensive care unit patients with acute respiratory disease [27]. In addition to myocardial infarction, the possible causes of elevated cTn in COVID-19 are multiple including chronic disease-causing myocardial injury, acute non-ischemic cardiac injury, and acute non-ischemic non-cardiac injury such as sepsis or pulmonary embolism [22].
As advances continue to be made in the therapeutic options for COVID-19 treatment, it is essential to continue building stronger risk stratification methods to rapidly determine who would most benefit from these therapies [28], [29], [30], [31]. Further studies should be focused on prospectively verifying the role of cTn as a predictor, and if those results support the current literature, cTn should be more aggressively considered as a routine prognostic lab in COVID-19 patients.
14. Study limitations
In this cohort study, a significant limitation is the selection bias of those who had cTn measured during their hospitalization. When tested, those with cTn measurement had increased frequency of most baseline comorbidities tested and an increased incidence of both severe COVID-19 infection and 60-day mortality when compared to those without cTn measurement who were excluded from our study. Additionally, while those with end-stage kidney disease or CKD Stage V were excluded, those with lesser degree of chronic kidney disease (CKD stages 1-4) were included. Given that any degree of renal dysfunction can affect cTn level, this could have affected cTn assessment to a certain level. Furthermore, our institution does not use high sensitivity cTn, which serves as an even more promising biochemical biomarker for COVID-19 due to the assay's ability to detect even smaller changes in cTn level. Lastly, as this study was conducted within the centers of the Rush University System for Health (RUSH) alone, it is possible that some post-discharge complications and deaths were not collected in our dataset as we only had access to local area hospitals that used the EPIC electronic medical record.
15. Conclusion
In our cohort, both mild and severe initial cTn elevation were predictors of severe COVID-19 infection, while only severe cTn elevation was predictive of 60-day mortality. First cTn value on hospitalization is a valuable longitudinal prognosticator for COVID-19 disease severity and mortality.
Conflict of interest
None
Footnotes
Funding: The authors received no financial support for the research, authorship, or publication of this manuscript.
Disclosures: The authors have no conflicts of interest to disclose
References
- 1.Johns Hopkins Coronavirus Resource Center. Available at: https://coronavirus.jhu.edu/. Accessed March 1, 2021.
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, Du B, Li L-J, Zeng G, Yuen K-Y, Chen R-C, Tang C-L, Wang T, Chen P-Y, Xiang J, Li S-Y, Wang J-L, Liang Z-J, Peng Y-X, Wei L, Liu Y, Hu Y-H, Peng P, Wang J-M, Liu J-Y, Chen Z, Li G, Zheng Z-J, Qiu S-Q, Luo J, Ye C-J, Zhu S-Y, Zhong N-S. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Nie C, Xu Q, Xie H, Wang M, Yu C, Hou X. Prognostic value of initial chest CT findings for clinical outcomes in patients with COVID-19. International journal of medical sciences. 2021;18:270–275. doi: 10.7150/ijms.48281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agricola E, Beneduce A, Esposito A, Ingallina G, Palumbo D, Palmisano A, Ancona F, Baldetti L, Pagnesi M, Melisurgo G, Zangrillo A, de Cobelli F. Heart and Lung Multimodality Imaging in COVID-19. JACC Cardiovascular imaging. 2020;13:1792–1808. doi: 10.1016/j.jcmg.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19 - A systematic review. Life sciences. 2020;254 doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danwang C, Endomba FT, Nkeck JR, Wouna DLA, Robert A, Noubiap JJ. A meta-analysis of potential biomarkers associated with severity of coronavirus disease 2019 (COVID-19) Biomarker research. 2020;8:37. doi: 10.1186/s40364-020-00217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, Gabrilove JL, Sacks H. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ evidence-based medicine. 2020 doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Critical reviews in clinical laboratory sciences. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen B, Yi X, Sun Y, Bi X, Du J, Zhang C, Quan S, Zhang F, Sun R, Qian L, Ge W, Liu W, Liang S, Chen H, Zhang Y, Li J, Xu J, He Z, Chen B, Wang J, Yan H, Zheng Y, Wang D, Zhu J, Kong Z, Kang Z, Liang X, Ding X, Ruan G, Xiang N, Cai X, Gao H, Li L, Li S, Xiao Q, Lu T, Zhu Y, Liu H, Chen H, Guo T. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell. 2020;182 doi: 10.1016/j.cell.2020.05.032. 59-72.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA cardiology. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu PP, Blet A, Smyth D, Li H. The Science Underlying COVID-19: Implications for the Cardiovascular System. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 13.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA cardiology. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19) JAMA cardiology. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson D, Dominic P, Sheth A, Modi M. Prognostic value of Cardiac Biomarkers in COVID-19 Infection: A Meta-analysis. Research square. 2020 doi: 10.21203/rs.3.rs-34729/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah P, Doshi R, Chenna A, Owens R, Cobb A, Ivey H, Newton S, Mccarley K. Prognostic Value of Elevated Cardiac Troponin I in Hospitalized Covid-19 Patients. The American journal of cardiology. 2020;135:150–153. doi: 10.1016/j.amjcard.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, van Vleck T, Vaid A, Chaudhry F, de Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. Journal of the American College of Cardiology. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. European heart journal. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majure DT, Gruberg L, Saba SG, Kvasnovsky C, Hirsch JS, Jauhar R. Usefulness of Elevated Troponin to Predict Death in Patients With COVID-19 and Myocardial Injury. The American journal of cardiology. 2021;138:100–106. doi: 10.1016/j.amjcard.2020.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu L, Liu X, Su Y, Ma J, Hong K. Prevalence and impact of cardiac injury on COVID-19: A systematic review and meta-analysis. Clinical cardiology. 2020 doi: 10.1002/clc.23540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Januzzi Jr. JL. Troponin and BNP Use in COVID-19. Available at: https://www.acc.org/latest-in-cardiology/articles/2020/03/18/15/25/troponin-and-bnp-use-in-covid19. Accessed January 9, 2020.
- 22.Sandoval Y, Jaffe AS. Key Points About Myocardial Injury and Cardiac Troponin in COVID-19. Available at: https://www.acc.org/latest-in-cardiology/articles/2020/07/17/08/00/key-points-about-myocardial-injury-and-cardiac-troponin-in-covid-19. Accessed January 9, 2020.
- 23.Qin J-J, Cheng X, Zhou F, Lei F, Akolkar G, Cai J, Zhang X-J, Blet A, Xie J, Zhang P, Liu Y-M, Huang Z, Zhao L-P, Lin L, Xia M, Chen M-M, Song X, Bai L, Chen Z, Zhang X, Xiang D, Chen J, Xu Q, Ma X, Touyz RM, Gao C, Wang H, Liu L, Mao W, Luo P, Yan Y, Ye P, Chen M, Chen G, Zhu L, She Z-G, Huang X, Yuan Y, Zhang B-H, Wang Y, Liu PP, Li H. Redefining Cardiac Biomarkers in Predicting Mortality of Inpatients With COVID-19. Hypertension (Dallas, Tex : 1979) 2020;76:1104–1112. doi: 10.1161/HYPERTENSIONAHA.120.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.deFilippi C, Seliger SL, Kelley W, Duh S-H, Hise M, Christenson RH, Wolf M, Gaggin H, Januzzi J. Interpreting cardiac troponin results from high-sensitivity assays in chronic kidney disease without acute coronary syndrome. Clinical chemistry. 2012;58:1342–1351. doi: 10.1373/clinchem.2012.185322. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet (London, England) 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortinovis M, Perico N, Remuzzi G. Long-term follow-up of recovered patients with COVID-19. Lancet (London, England) 2021;397:173–175. doi: 10.1016/S0140-6736(21)00039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasile VC, Chai H-S, Khambatta S, Afessa B, Jaffe AS. Significance of elevated cardiac troponin T levels in critically ill patients with acute respiratory disease. The American journal of medicine. 2010;123:1049–1058. doi: 10.1016/j.amjmed.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 28.The Writing Committee for the REMAP-CAP Investigators Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libster R, G Pérez Marc, Wappner D, Coviello S, Bianchi A, Braem V, Esteban I, Caballero MT, Wood C, Berrueta M, Rondan A, Lescano G, Cruz P, Ritou Y, V Fernández Viña, D Álvarez Paggi, Esperante S, Ferreti A, Ofman G, Á Ciganda, Rodriguez R, Lantos J, Valentini R, Itcovici N, Hintze A, Oyarvide ML, Etchegaray C, Neira A, Name I, Alfonso J, López Castelo R, Caruso G, Rapelius S, Alvez F, Etchenique F, Dimase F, Alvarez D, Aranda SS, Sánchez Yanotti C, de Luca J, Jares Baglivo S, Laudanno S, Nowogrodzki F, Larrea R, Silveyra M, Leberzstein G, Debonis A, Molinos J, González M, Perez E, Kreplak N, Pastor Argüello S, Gibbons L, Althabe F, Bergel E. Polack FP. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. The New England journal of medicine. 2021 doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Soleimani J, Herasevich S, Pinevich Y, Pennington KM, Dong Y, Pickering BW, Barwise AK. Clinical Characteristics, Treatment, and Outcomes of Critically Ill Patients With COVID-19: A Scoping Review. Mayo Clinic proceedings. 2021;96:183–202. doi: 10.1016/j.mayocp.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M-D, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC. Remdesivir for the Treatment of Covid-19 - Final Report. The New England journal of medicine. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]