Abstract
Introduction:
E-cigarette (e-cig) use is widespread and may play an important role in facilitating smoking reduction. Racial/ethnic minorities are less likely than Whites to use e-cigs and suffer disproportionate tobacco-related disease, making them a priority for harm reduction. This paper explores factors associated with smoking reduction among African American (AA) and Latinx smokers enrolled in a trial assessing toxicant exposure in those assigned to e-cigs or smoking as usual.
Methods:
Participants were randomized to receive 6 weeks of JUUL e-cigs or continue smoking cigarettes as usual (N=187). This analysis focuses on 109 participants randomized to e-cigs. We modeled cigarettes smoked in the past week at baseline and week 6 as a function of a priori selected predictors (number of JUUL pods used throughout the study, baseline cigarette dependence, and baseline cotinine) using a Poisson model fit with generalized estimating equations.
Results:
Over the six-week study, cigarette smoking decreased from an average of 82.4 to 15.5 cigarettes per week. Greater numbers of JUUL pods used predicted a greater smoking reduction by week 6 (IRR = 0.94 [0.91, 0.96], p < 0.001). Higher baseline cigarette dependence (IRR = 1.03 [1.01, 1.05], p = 0.004), and baseline cotinine (IRR = 1.18 [1.03, 1.37], p = 0.020) predicted a lesser smoking reduction.
Conclusions:
AA and Latinx smokers reduced their cigarette consumption while using JUUL e-cigs. Higher e-cig use during an intervention to switch to e-cigs to reduce harm may facilitate a transition to smoking fewer cigarettes, offering an opportunity to narrow smoking-related health disparities.
Keywords: e-cigarettes, smoking, reduction, cessation, racial/ethnic minorities, health disparities
1. INTRODUCTION
While cigarette smoking among U.S. adults has decreased over the past 50 years to an all-time low prevalence of 13.7% (Creamer et al., 2019), smoking continues to be the leading nationwide cause of preventable death (Lariscy, 2019) with higher morbidity and mortality among racial/ethnic minorities (Trinidad, Pérez-Stable, White, Emery, & Messer, 2011). In recent years, the tobacco landscape has changed substantially to include increasingly popular non-combustible products including e-cigarettes (e-cigs). A national survey revealed that 21.6% of ever smokers reported current e-cig use in 2018 (Obisesan et al., 2020). Current cigarette smokers and those seeking to quit are especially likely to use e-cigs: nearly 90% of adult e-cig users are either current or former cigarette smokers ("Percentage of Adults Aged >/=18 Years Who Currently Use E-Cigarettes, by Sex and Age Group - NHIS, 2016," 2018).
The uptake of e-cigs among smokers has been shown to increase the odds of cigarette reduction and cessation; cigarette smokers who initiated daily e-cig use between Wave 1 (2013–2014) and 2 (2014–2015) of the nationally representative Population Assessment of Tobacco and Health (PATH) study were 7.9 times as likely to achieve 30-day cigarette cessation as non e-cig users at Wave 2 (Berry et al., 2019). Although smoking reduction rather than cessation is the outcome in the current analysis, data from smoking cessation research is presented here given the substantial overlap between the processes of reduction and cessation. Decades of research show that smoking reduction is beneficial to the health of smokers (USDHHS, 2014). Reducing cigarette consumption through e-cig use is linked with lower toxicant exposure and reduced short-term risks (Stratton, Kwan, & Eaton, 2018). While cessation of all tobacco products remains the ultimate goal for smokers, quitting smoking is difficult and relapse rates are high (Piasecki, 2006). Reducing smoking via substitution with nicotine-containing e-cigs may be an important harm reduction strategy for smokers who are unable to quit.
Racial/ethnic minority smokers face unique health risks and challenges in reducing their cigarette smoking. African American (AA) and Latinx smokers are less successful in attempts to reduce smoking and experience a disproportionate burden of smoking-related diseases despite generally smoking fewer cigarettes per day (CPD) and on fewer days than Whites ((Nollen et al., 2019; "Tobacco use among U.S. racial/ethnic minority groups--African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, Hispanics. A Report of the Surgeon General. Executive summary," 1998; Trinidad et al., 2011). Furthermore, AA and Latinx smokers, who belong to the two largest ethnic minority groups in the U.S., are less likely than their White counterparts to switch from combustibles to exclusive e-cig use (Friedman & Horn, 2019). If this trend continues, tobacco disparities will widen as disadvantaged groups continue to suffer from increased tobacco-related death and disease unmitigated by harm reduction strategies such as e-cig use (Giovenco, 2019). It is essential to focus research on racial/ethnic minority populations to narrow current disparities and mitigate future differences.
Cigarette reduction is an essential and understudied area of focus in e-cig studies. Although the aim of these studies is to facilitate an exclusive switch from combustibles, dual users of cigarettes and e-cigs who reduce their cigarette consumption may also experience benefits. An observational study showed that dual users had significantly lower levels of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL; a pulmonary carcinogen) than smokers (Piper, Baker, Benowitz, Kobinsky, & Jorenby, 2019). Furthermore, dual users in an RCT of JUUL e-cigs experienced a significant reduction in NNAL, CO, and self-reported respiratory symptoms at week 6 of the trial (Pulvers et al., 2020). Therefore, cigarette reduction via dual use of cigarettes and e-cigs or an exclusive switch to e-cigs may ultimately reduce harm among smokers, making it an important outcome to anticipate and measure in smokers who begin using e-cigs, especially racial/ethnic minorities who face potentially greater harms. Nevertheless, the long-term harm reduction effect of dual use is not yet known (Czoli, Fong, Goniewicz, & Hammond, 2019; Goniewicz et al., 2018; National Academies of Sciences et al., 2018).
The current study explores factors predictive of a reduction in the number of cigarettes smoked in the past week between baseline and week 6 in a recent trial among AA and Latinx cigarette smokers randomized to JUUL e-cigs and encouraged to switch exclusively. This study addresses current gaps in the literature by specifically focusing on racial/ethnic minority groups that are historically underrepresented in tobacco-related clinical research, in addition to analyzing a variety of demographic and tobacco-related variables that affect smoking reduction. Additionally, the JUUL e-cig used is a 4th generation device with more efficient delivery of nicotine than older e-cig devices used in previous studies. The aim of the RCT is that switching to JUUL will help smokers reduce their cigarette consumption, but it is important to recognize that some may benefit more from this intervention than others. Since there are no previous studies assessing predictors of smoking reduction among racial/ethnic minority smokers in an e-cig clinical trial, we base our hypotheses on data from (a) observational studies reporting factors associated with e-cig uptake or reduced smoking among smokers who initiate e-cig use (Berry et al., 2019; Russell, Haseen, & McKeganey, 2019) and, (b) RCTs assessing factors associated with smoking reduction and cessation (Berg et al., 2010; Nollen et al., 2006; Ussher, Kakar, Hajek, & West, 2016), which likely share many similarities with smoking reduction via e-cigs.
Motivated by this prior literature, we selected conceptually relevant variables to include in a model predicting cigarettes smoked. We hypothesized that lower cigarette dependence and lower baseline cotinine (metabolite of nicotine) would be associated with reduced smoking six weeks after randomization to the e-cig group. This is based on studies showing that lower dependence predicted smoking cessation (Ussher et al., 2016) and lower cotinine predicted smoking reduction (Berg et al., 2010). Additionally, we hypothesized that greater use of the JUUL e-cig (as quantified by the number of pods used) would reduce cigarette consumption at week 6, as previous research shows that heavier and more frequent e-cig use is associated with more successful smoking cessation (Berry et al., 2019; Russell et al., 2019) and smoking reduction (Berry et al., 2019) among smokers who begin using e-cigs.
2. METHODS
This is a secondary analysis of data from a randomized, unblinded clinical trial in which smokers were assigned to either receive 6 weeks of JUUL e-cigs or continue smoking cigarettes as usual (Pulvers et al.,2020). Recruitment began in May 2018 and primary follow-up was completed in May 2019. The study procedures were approved and monitored by the California State University San Marcos Institutional Review Board and the University of Kansas School of Medicine Institutional Review Board and all participants provided written informed consent. The following participants and procedures described refer to those in the overall RCT, with details pertaining to the current analysis noted as such.
2.1. Participants
Participants were recruited from two sites: Hispanic/Latinx participants from San Diego, CA and Black/AA participants from Kansas City, MO. Eligible participants were at least 21 years old, smoked at least five cigarettes per day, smoked on at least 25 of the past 30 days, and smoked for over six months. Other enrollment criteria were a baseline carbon monoxide reading greater than five parts per million (PPM) and blood pressure under 160 systolic and under 105 diastolic. All participants were fluent in English or Spanish, had regular access to a telephone, and were willing to switch from smoking cigarettes to e-cigs for six weeks.
Exclusion criteria included use of other tobacco products more than or equal to use of cigarettes, use of e-cigs on four or more of the past 30 days, or current enrollment in a smoking cessation program or other clinical trial. Other exclusion criteria were the use of nicotine replacement therapy or smoking cessation medication in the past 30 days, hospitalization for a psychiatric issue in the past 30 days, or a heart-related event in the past 30 days. Finally, those who resided with someone else in the study, were pregnant, breastfeeding, or planning to become pregnant in the next six months or had unstable mental or physical health status were excluded. Participants were ineligible if planning to move away from San Diego or Kansas City in the next six weeks. Those ineligible for the study were referred to smoking cessation resources. In total, 187 participants were enrolled. Of the 125 participants randomized to the JUUL condition (see Procedures), 114 (91.2%) completed week 6 follow-up and the 109 without missing data are included in these analyses.
2.2. Procedures
Participants were stratified by study site and randomly assigned to study condition (e-cig or the assessment-only control group) in a 2:1 ratio (Nollen et al., 2020) to enhance recruitment and gain information about e-cig use. Participants in the e-cig group received a JUUL e-cig device and nicotine pods (5% nicotine) in their choice of flavor (Virginia Tobacco, menthol, Cool Mint, or mango). They received education, training, and action planning supporting an exclusive switch from cigarettes to e-cigs. The e-cig group received a two-week supply of pods at baseline and a four-week supply of pods at the week 2 visit, with an allocation of one pod per pack of cigarettes based on cigarette use reported at baseline. At follow-up appointments on weeks 1, 2, and 4, barriers and benefits of switching to e-cigs were discussed and action planning for exclusive switching was revisited. Baseline, week 2, and week 6 follow-up appointments included a timeline follow back (TLFB) interview of cigarette and e-cig use, questionnaires, and biological measurements including carbon monoxide and providing a urine sample. Weeks 2 and 6 also included objective measurement of JUUL pods.
Total compensation for study participation was $120, distributed at increasing intervals (i.e. $20 at baseline, $40 at week 2, $60 at week 6). A portion of study compensation for the e-cig group participants was contingent on returning all used and un-used pods to the week 2 and Week 6 lab visits; 90% of distributed pods were returned.
2.3. Measures
The primary outcome of the current study was the reduction in cigarettes smoked between baseline and Week 6 (recorded as cigarettes smoked in the past week via TLFB interview) (Brown et al., 1998; Kari Jo Harris et al., 2009). The covariates were poverty level, gender, age, and race/ethnicity, while the predictors were cigarette dependence, cotinine level, and JUUL pods. The number of fully used JUUL pods were counted and partially used pods were weighed and converted into a proportion of pods used. Fully and partially used pods at week 2 and week 6 were summed to caculate the number of JUUL pods used throughout the entire study.
Demographic characteristics were measured at baseline. Age was reported in years, and current gender identity was self-reported as male, female, or transgender/gender nonconforming. Race/ethnicity corresponded to study site (AA or Latinx). Poverty level was dichotomized at 200% Federal Poverty Level, a measure based on self-reported yearly gross household income and number of people in the household (USA, 2018). Education level was dichotomized into high school diploma/GED or less vs. higher than high school diploma/GED.
Cigarette dependence was assessed at baseline using a 16-item measure of symptoms of tobacco dependence (Strong et al., 2017). This measure has been used in nationally representative surveys and has shown strong psychometric properties (α = 0.95). Questions 1–15 used Likert-type responses ranging from 1 (not true of me at all) to 5 (extremely true of me) and question 16 was a dichotomous question 1 (yes) or 0 (no). Responses were summed to create an index score ranging from 15–76 with higher scores representing higher cigarette dependence. Scores for cigarette dependence were prorated and included for participants who responded to at least 80% of questions in the scale (Graham, 2012; Parent, 2012). Baseline creatinine-adjusted cotinine and NNAL levels were measured in urine. Exhaled carbon monoxide was measured using a breath analysis monitor and recorded in parts per million (PPM).
2.4. Data Analysis
Means and frequencies were used to describe demographic and tobacco-related characteristics of the study sample. Demographic and tobacco-related characteristics of the 114 included participants and the 11 participants lost to follow-up were compared using chi-square tests and t tests. After examining descriptive statistics, four participants were excluded from models due to missing data for the selected variables: two were missing poverty level, one was missing cigarette dependence, and one was missing cotinine. Additionally, one participant identified as transgender/gender nonconforming and was excluded from analyses. These exclusions reduced the analytical sample to 109 (95.6%) from 114.
To identify predictors associated with reduced smoking in participants assigned to receive JUUL, we fit a generalized linear model of the count of cigarettes smoked at baseline and six weeks as a function of focal predictors and covariates. Counts were assumed to follow a Poisson distribution and were fit by generalized estimating equations (GEE) with an unstructured covariance matrix. To facilitate interpretability of model intercepts, we mean-centered all covariates. For binary variables (i.e., covariates FPL, gender, race/ethnicity), mean-centering results in a unit change continuing to represent the slope of moving from one category to the second category, but increases the interpretability of the model intercept (i.e., number of past 7-day cigarettes at mean levels of covariates, focal predictors, and at time 0). We regressed past 7-day cigarette use onto all covariates (FPL, gender, age, race/ethnicity), mean-centered focal predictors (cigarette dependence, cotinine, and JUUL pods), time (0 = baseline; 1 = week 6), and three interaction terms of time x focal predictor. We then exponentiated model estimates to compute incidence rate ratios (IRRs). Finally, we conducted follow-up simple slope analyses (Aiken, West, & Reno, 1991) of significant interactions by examining model-predicted past 7-day cigarettes at baseline and week 6, for mean, low (-1SD), and high (+1SD) levels of our focal predictors. Analyses were conducted using SPSS statistical software, version 25 (IBM 2017) and R (4.0.2) (Team, 2017) including the geepack library for GEE (Halekoh, 2006).
3. RESULTS
The mean age of participants randomized to e-cigs was 44.5 years old, with 41.3% identifying as male, 52.3% as AA, and 47.7% as Latinx. Over the six weeks of the study, participants decreased their cigarettes per week from an average of 82.4 to 15.5 (12 CPD to 2.8 CPD) and used an average of 0.51 (SD=0.42) JUUL pods per day. Of the 109 participants who reduced their smoking, 48 (44%) quit combustible cigarettes during the study while 61 (56%) partially reduced their smoking level. Participants with a high school education or below were more likely to completely eliminate combustible cigarette consumption (54.1%) than those with more than a high school education (31.3%). There were no other significant differences in demographic characteristics between those who completely eliminated versus those who reduced their cigarette consumption. Other demographic and tobacco-related characteristics of the sample are summarized in Table 1. Participants lost to follow-up were more likely to be Latinx and reported higher cotinine at baseline.
Table 1.
Demographic and tobacco-related measures (N=109)
| Demographic measures | |
| Age | 44.5 (12.8) |
| Male, n (%) | 45 (41.3) |
| African American, n (%) | 57 (52.3) |
| Latinx, n (%) | 52 (47.7) |
| Income Level, n (%) at or below 200% FPL | 80 (73.4) |
| Education Level, n (%) high school diploma or less | 61 (60.0) |
| Tobacco-related measures | |
| Baseline smoking within 30 mins of waking, n (%) | 78 (71.6) |
| Baseline Cigarette dependence, values range from 15–76 | 48.9 (14.5) |
| aBaseline Cotinine, ng/mg | 1222.1 (1420.5) |
| aBaseline NNAL, pg/mg | 128.9 (114.9) |
| Baseline Carbon Monoxide, parts per million | 17.7 (9.2) |
| Baseline cigarettes in past week | 82.4 (49.8) |
| Baseline days smoked in past week | 6.8 (0.5) |
| Baseline cigarettes per day | 12.0 (7.1) |
| Week 6 cigarettes smoked in past week | 15.5 (29.7) |
| Week 6 days smoked in past week | 2.4 (2.8) |
| Week 6 cigarettes per day | 2.8 (4.3) |
| JUUL pods used during 6-week study | 21.4 (17.7) |
| JUUL pods used per day | 0.51 (0.42) |
Note: All measures are reported as mean (SD) unless otherwise specified.
Values were creatinine-adjusted.
Results from the Poisson regression model predicting cigarettes smoked in the past week at baseline and week 6, including covariate effects, are presented in Table 2. At mean levels of baseline cigarette dependence, baseline cotinine, and JUUL pod use from baseline to week 6, past 7-day smoking declined by 89% between baseline and week 6 (IRR = 0.11 [0.08, 0.17], p < 0.001). Greater cigarette dependence was associated with more past 7-day smoking at baseline. Similarly, JUUL pod use during the study was associated with more past 7-day smoking at baseline (i.e., heavier smokers at baseline ended up using more JUUL pods over the course of the study). There was no significant relationship between baseline cotinine and baseline past 7 -day smoking.
Table 2.
IRRs for covariates and predictors of cigarettes smoked in past week between baseline and week 6 (n=109)
| Cigarettes smoked in past week, baseline and week 6 | ||
|---|---|---|
| Variables | Poisson IRR (95% CI) | p-value |
| Covariates | ||
| Age | 1.01 (1.00, 1.02) | .031 |
| Male | 0.90 (0.73, 1.11) | .331 |
| Latinx | 1.09 (0.857, 1.38) | .494 |
| Income above 200% FPL | 1.10 (0.892, 1.35) | .381 |
| Education above high school | 1.05 (0.858, 1.29) | .627 |
| Focal Effects | ||
| Cigarette dependence | 1.02 (1.01, 1,02) | <.001 |
| Baseline cotinine | 1.01 (0.96, 1.06) | .645 |
| JUUL pods used during study | 1.01 (1.00, 1.01) | .033 |
| Time | 0.11 (0.08, 0.17) | <.001 |
| Time × Cigarette dependence | 1.03 (1.01, 1.05) | .004 |
| Time × Cotinine | 1.18 (1.03, 1.37) | .020 |
| Time × JUUL pods used | 0.94 (0.91, 0.96) | <.001 |
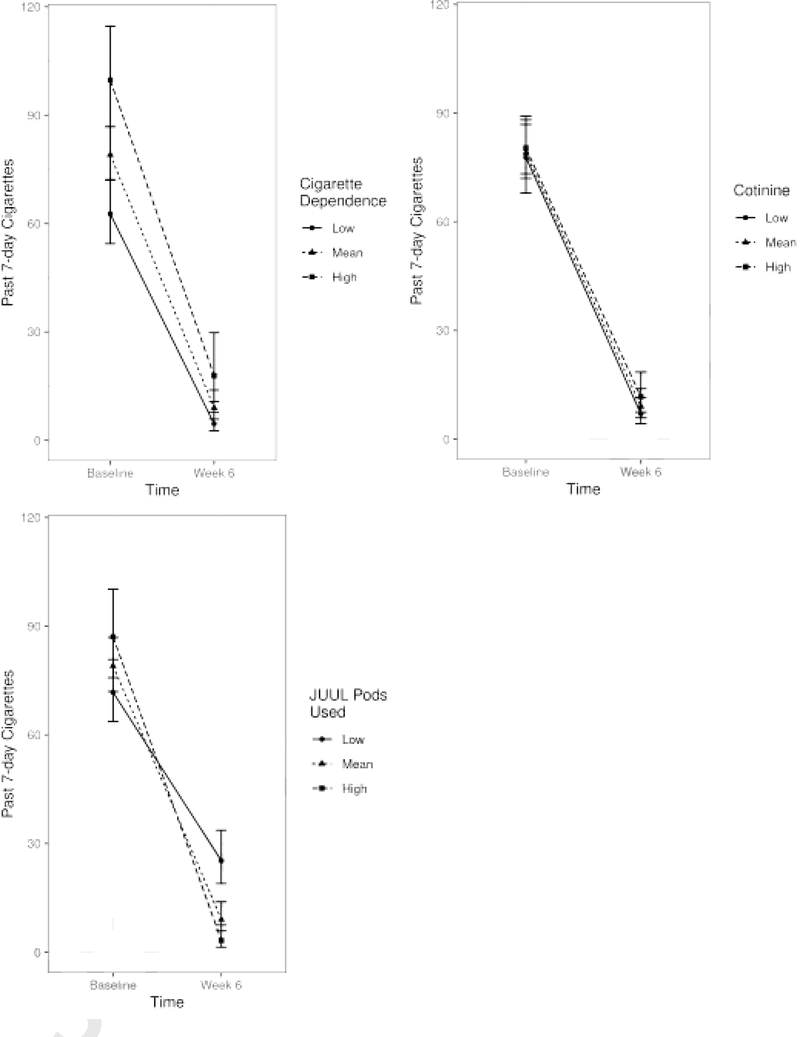
Note: Interactive effects and associated simple slopes are demonstrated in Figure 1.
These relationships were qualified by three significant interactions (see Figure 1). The results shown in Figure 1 are significant for interactions between time and past-week cigarettes and cigarette dependence, cotinine, and JUUL pods used as also shown in Table 2. Interaction terms between time and cigarette dependence and time and cotinine demonstrated that the negative slope of time was attenuated for greater levels of cigarette dependence and cotinine levels. In other words, greater levels of cigarette dependence and cotinine both predicted a lower relative decline (albeit a greater absolute decline) in 7-day cigarette use by week 6. On the other hand, higher JUUL pod use was associated with a significantly decreased slope of time (i.e., a greater reduction in cigarette smoking between baseline and week 6). Follow-up simple slopes analyses revealed significant effects of time across all levels of focal predictors (mean and ±1SD); model-predicted past 7-day cigarettes (i.e., intercepts) and their bootstrapped standard errors are presented in Figure 1.
Figure 1.
Simple slope analyses of focal predictors over time
4. DISCUSSION
This study analyzes factors associated with cigarette reduction in an RCT of JUUL e-cigs in racial/ethnic minorities. AA and Latinx smokers randomized to use JUUL e-cigs cut their average daily cigarette consumption by more than two-thirds over the 6-week intervention, which reduces health risk via decreased toxicant exposure (Stratton K, 2018; USDHHS, 2014). Poisson regression analysis indicated that baseline cigarette dependence, baseline cotinine, and JUUL pods used throughout the course of the study, significantly predicted smoking reduction over the six weeks of the study. The factors identified in our study are consistent with prior observational studies of e-cig use among smokers, as well as smoking cessation trials (Berg et al., 2010; Friedman & Horn, 2019;Harlow, Stokes,& Brooks,2019; Russell et al., 2019). Thus, this paper both strengthens and confirms existing work in addition to contributing novel information regarding racial/ethnic minorities and e-cigs.
The strongest predictor of smoking reduction was the number of JUUL pods used over the course of the study. Previous work similarly demonstrates that smokers who initiated daily e-cig use between PATH Waves 1 and 2 were more likely to reduce their average daily cigarette consumption by at least half than those who became nondaily, experimental, or nonusers of e-cigs (Berry et al., 2019). Likewise, in an online survey of over 15,000 adult smokers who recently purchased a JUUL starter kit, more days of JUUL use in the past 30 days was associated with increased odds of reporting 30-day cigarette abstinence at the 6-month mark (Russell et al., 2019). Additionally, a study comparing smoking and ex-smoking e-cig users found that dual users of cigarettes and e-cigs used significantly less e-liquid per week than ex-smokers who had switched to exclusive e-cig use (Adriaens, Van Gucht, & Baeyens, 2017). Our analyses, as well as these prior findings, demonstrate that heavier e-cig use can facilitate a more successful reduction in CPD. This pattern of heavier e-cig usage and reduced CPD may ultimately reduce tobacco-related harms; exclusive e-cig users and dual users of e-cigs and cigarettes experienced significant reductions in biomarkers of exposure and potential harm (Pulvers et al., 2020).
Additionally, lower baseline cigarette dependence scores and cotinine levels were both predictive of greater smoking reduction relative to baseline. Prior work in cigarette cessation (including in other trials focused on racial/ethnic minority smokers) consistently supports lower dependence and lower cotinine as predictors of cessation (Berg et al.,2010; Harris et al. 2004; Nollen et al., 2006; Ussher et al., 2016). Importantly, although the relative (i.e., proportional) reduction in smoking for those with high cigarette dependence at baseline was lower than for those with low cigarette dependence at baseline, it was nevertheless associated with a larger absolute decline in past 7-day cigarettes smoked suggesting that even highly dependent individuals may benefit from an e-cig intervention. This study notably extends the importance of these factors to racial/ethnic minority smokers seeking to reduce cigarette use via e-cigs. Nevertheless, despite the current study and prior work demonstrating the predictive nature of cotinine for longer term cigarette use, the relationship baseline cotinine and baseline past 7-day smoking was not significant. This is consistent with prior work showing that CPD predicts cotinine levels and other measures of smoke exposure more poorly for AAs than Whites (Benowitz, Dains, Dempsey, Wilson, & Jacob, 2011). There is therefore an important distinction between the association between cotinine and long-term smoking reduction potential and the association between cotinine and current smoking. While cotinine may be helpful in considering long-term smoking levels, it is not highly correlated with current CPD among AAs. Researchers and clinicians should therefore pay close attention to measures of smoke exposure and dependence among smokers to best offer tailored treatment options.
Special attention must be paid to the most dependent smokers, who are less likely to quit smoking and may receive the most benefit from e-cigarettes. Increased cigarette dependence has been shown to predict more frequent smoking and less frequent e-cig use among dual users (Morean, Krishnan-Sarin, & O'Malley, 2018), making smokers who are highly dependent or who have other markers of dependence, such as high cotinine levels, a target for specialized cessation treatment and support with e-cig adherence when working towards an exclusive switch or smoking reduction. Smokers with several factors that may decrease their likelihood of cessation, such as racial/ethnic minorities who are highly dependent, especially deserve unique attention in quit attempts and the use of novel products such as e-cigs to potentially reduce harm. Our findings suggest that despite evidencing lower relative reductions in smoking, smokers with high cigarette dependence demonstrated the greatest reductions in absolute smoking reinforcing the potential of e-cig substitution interventions for these individuals.
The current study has several limitations. The trial’s size limited the number of participants in the e-cig group to 109. In addition, our study population was limited to Latinx smokers in San Diego and AAs in Kansas City. There may be factors that differ based on location or race/ethnicity that cannot be identified in this study. Lastly, the timeline of the study took place over six weeks, which is a relatively short period of time to see the full effects of cigarette reduction and e-cig substitution. Future studies should continue to investigate the use of e-cigs for smoking reduction/cessation using larger sample sizes and a longer follow-up period.
In conclusion, African American and Latinx cigarette smokers may benefit from the opportunity to use e-cigs, a nicotine-delivering product of potentially lesser harm, to reduce their cigarette consumption. The current study identifies unique factors predictive of fewer CPD among AA and Latinx smokers in an RCT using JUUL e-cigs, which may inform future research and practice in this population. As it is important to better understand the differences between AA and Latinx smokers as well as other vulnerable populations, future studies with larger sample sizes should examine differences across racial/ethnic groups. This study notably identifies high e-cig usage as a predictor of smoking reduction, suggesting that optimizing adherence to e-cig intervention and cessation treatments is key to decreasing cigarette consumption across diverse populations of smokers. Future work can better characterize factors that are associated with adherence to e-cigs. As this study uses a 4th generation e-cig, it provides current information that can best help researchers and clinicians assess current treatment options. This study also includes the provision of education and action planning alongside the e-cig, which is shown to increase the efficacy of pharmacotherapeutic interventions for smoking cessation (Hartmann-Boyce, Hong, Livingstone-Banks, Wheat, & Fanshawe, 2019), so likely also improved the efficacy of the current study. Future research can examine the extent to which behavioral support impacts results of e-cig interventions. Researchers should continue to implement studies focusing on populations in the most need of smoking cessation/reduction interventions, such as racial/ethnic minorities, and clinicians should consider novel products such as e-cigs for smokers who are unable to quit using FDA approved cessation medications.
Highlights.
Minority smokers decreased cigarette consumption in a trial of e-cigarettes
Smoking reduction was associated with a higher number of JUUL pods used
Lower baseline cigarette dependence predicted greater smoking reduction
Lower baseline cotinine predicted greater smoking reduction
ACKNOWLEDGEMENTS
We thank Tricia Snow, Brian Hernandez, Michael Arnold, Ana Leon, Jennifer Mosley, Amanda Dean, Crystal Marez, Dalia Hipolito, Mirella Orozco, Justin Sanchez, Juan Alva, John Le, Madison Garrett, Nathan Au-Yeung, Jeremy Mills-Shimell, Shyla Everett, Alexis Osuna, Daniell Derry, Flavia Ponce, Kexin Qu, and staff at Neighborhood Healthcare, Mission Treatment Services, and Foundry Clinic.
FUNDING
This study was funded by 5SC3GM122628 to KP. JA and EA were supported in part by P20GM130414, a NIH funded Center of Biomedical Research Excellence (COBRE).
Footnotes
Declaration of Interests: One author serves as a consultant to Lucy Goods, a manufacturer of nicotine gum. The other authors have no conflicts of interest to declare.
DECLARATION OF INTERESTS
JA serves as a consultant to Lucy Goods, a manufacturer of nicotine gum. The other authors have no conflicts of interest to declare.
Conflicts of Interest
JA serves as a consultant to Lucy Goods, a manufacturer of nicotine gum. The other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriaens K, Van Gucht D, & Baeyens F (2017). Differences between dual users and switchers center around vaping behavior and its experiences rather than beliefs and attitudes. International Journal of Environmental Research and Public Health, 15(1), 12. 10.3390/ijerph15010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions. Sage. [Google Scholar]
- Berg CJ, Thomas JL, Guo H, An LC, Okuyemi KS, Collins TC, & Ahluwalia JS (2010). Predictors of smoking reduction among Blacks. Nicotine Tob Res, 12(4), 423–431. 10.1093/ntr/ntq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Dempsey D, Wilson M, & Jacob P (2011). Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine & Tobacco Research, 13(9), 772–783. 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry KM, Reynolds LM, Collins JM, Siegel MB, Fetterman JL, Hamburg NM, … Stokes A (2019). E-cigarette initiation and associated changes in smoking cessation and reduction: The Population Assessment of Tobacco and Health Study, 2013–2015. Tobacco Control, 28(1), 42–49. 10.1136/tobaccocontrol-2017-054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, & Miller IW (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 12(2), 101–112. [Google Scholar]
- Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, … Neff L (2019). Tobacco product use and cessation indicators among adults – United States, 2018. MMWR. Morbidity and mortality weekly report, 68(45), 1013–1019. 10.15585/mmwr.mm6845a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoli CD, Fong GT, Goniewicz ML, & Hammond D (2019). Biomarkers of exposure among “dual users” of tobacco cigarettes and electronic cigarettes in Canada. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 21(9), 1259–1266. 10.1093/ntr/nty174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AS, & Horn SJL (2019). Socioeconomic disparities in electronic cigarette use and transitions from smoking. Nicotine & Tobacco Research, 21(10), 1363–1370. 10.1093/ntr/nty120. [DOI] [PubMed] [Google Scholar]
- Giovenco DP (2019). Different smokes for different folks? E-Cigarettes and tobacco disparities. American Journal of Public Health, 109(9), 1162–1163. 10.2105/AJPH.2019.305250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, … Hyland AJ (2018). Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA network open, 1(8), e185937. 10.1001/jamanetworkopen.2018.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW (Ed.). (2012). Missing Data: Analysis and Design. New York, NY: Springer New York. [Google Scholar]
- Halekoh U, Højsgaard S, & Yan J (2006). The R package geepack for generalized estimating equations. Journal of Statistical Software, 15(2), 1–11. [Google Scholar]
- Harlow AF, Stokes A, & Brooks DR (2019). Socioeconomic and racial/ethnic differences in e-cigarette uptake among cigarette smokers: Longitudinal analysis of the population assessment of tobacco and health (PATH) Study. Nicotine & Tobacco Research, 21(10), 1385–1393. 10.1093/ntr/nty141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KJ, Okuyemi KS, Catley D, Mayo MS, Ge B, & Ahluwalia JS (2004). Predictors of smoking cessation among African-Americans enrolled in a randomized controlled trial of bupropion. Prev Med, 38(4), 498–502. 10.1016/j.ypmed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Golbeck AL, Cronk NJ, Catley D, Conway K, & Williams KB (2009). Timeline follow-back versus global self-reports of tobacco smoking: A comparison of findings with nondaily smokers. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors, 23(2), 368–372. 10.1037/a0015270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Boyce J, Hong B, Livingstone-Banks J, Wheat H, & Fanshawe TR (2019). Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews, 6. 10.1002/14651858.CD009670.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariscy JT (2019). Smoking-attributable mortality by cause of death in the United States: An indirect approach, 100349–100349 SSM – Population Health, 7. 10.1016/j.ssmph.2019.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean M, Krishnan-Sarin S, & O’Malley SS (2018). Comparing cigarette and e-cigarette dependence and predicting frequency of smoking and e-cigarette use in dual-users of cigarettes and e-cigarettes. Addictive Behaviors, 87, 92–96. https://doi. org/10.1016/j.addbeh.2018.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, E., Medicine, Health, Medicine, D., Board on Population, H., Public Health, P., & Committee on the Review of the Health Effects of Electronic Nicotine Delivery, S. (2018). In Eaton DL, Kwan LY, & Stratton K (Eds.), Public Health Consequences of E-Cigarettes. Washington (DC): National Academies Press (US). Copyright 2018 by the National Academy of Sciences. All rights reserved. [Google Scholar]
- Nollen NL, Mayo MS, Sanderson Cox L, Okuyemi KS, Choi WS, Kaur H, & Ahluwalia JS (2006). Predictors of quitting among African American light smokers enrolled in a randomized, placebo-controlled trial. J Gen Intern Med, 21(6), 590–595. 10.1111/j.1525-1497.2006.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen NL, Mayo MS, Sanderson Cox L, Benowitz NL, Tyndale RF, Ellerbeck EF, Scheuermann TS, & Ahluwalia JS (2019). Factors That Explain Differences in Abstinence Between Black and White Smokers: A Prospective Intervention Study. Journal of the National Cancer Institute, 111(10), 1078–1087. 10.1093/jnci/djz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen NL, Cox LS, Mayo MS, Ellerbeck EF, & Ahluwalia JS (2020). Counseling alone or in combination with nicotine replacement therapy for treatment of black non-daily smokers: a randomized trial. Addiction, 115(8), 1547–1560. 10.1111/add.14948. [DOI] [PubMed] [Google Scholar]
- Obisesan OH, Osei AD, Uddin SMI, Dzaye O, Mirbolouk M, Stokes A, & Blaha MJ (2020). Trends in e-cigarette use in adults in the United States, 2016–2018. JAMA Intern Med, 180(10), 1394–1398. 10.1001/jamainternmed.2020.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent MC (2012). Handling item-level missing data: simpler is just as good. The Counseling Psychologist, 41(4), 568–600. 10.1177/0011000012445176. [DOI] [Google Scholar]
- Percentage of Adults Aged >/=18 Years Who Currently Use E-Cigarettes, by Sex and Age Group - NHIS, 2016. (2018). MMWR Morbidity and Mortality Weekly Report, 66(51–52), 1412. doi: 10.15585/mmwr.mm665152a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM (2006). Relapse to smoking. Clinical Psychology Review, 26(2), 196–215. 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Piper ME, Baker TB, Benowitz NL, Kobinsky KH, & Jorenby DE (2019). Dual users compared to smokers: Demographics, dependence, and biomarkers. Nicotine & Tobacco Research, 21(9), 1279–1284. 10.1093/ntr/nty231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvers K, Nollen NL, Rice M, Schmid CH, Qu K, Benowitz NL, & Ahluwalia JS (2020). Effect of Pod e-Cigarettes vs Cigarettes on Carcinogen Exposure Among African American and Latinx Smokers: A Randomized Clinical Trial. JAMA network open, 3(11), Article e2026324. 10.1001/jamanetworkopen.2020.26324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C, Haseen F, & McKeganey N (2019). Factors associated with past 30-day abstinence from cigarette smoking in adult established smokers who used a JUUL vaporizer for 6 months, 59–59 Harm reduction journal, 16(1). 10.1186/s12954-019-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, & Eaton DL (2018). Public Health Consequences of e-Cigarettes: Consensus Study Report. National Academies Press. [PubMed] [Google Scholar]
- Strong DR, Pearson J, Ehlke S, Kirchner T, Abrams D, Taylor K, … Niaura R (2017). Indicators of dependence for different types of tobacco product users: Descriptive findings from Wave 1 (2013–2014) of the Population Assessment of Tobacco and Health (PATH) study. Drug and Alcohol Dependence, 178, 257–266. 10.1016/j.drugalcdep.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Team RC (2017). R: A Language and Environment for Statistical Computing. https://www.r-project.org.
- Tobacco use among U.S. racial/ethnic minority groups–African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, Hispanics. A Report of the Surgeon General. Executive summary. (1998). MMWR Recommendations and Reports, 47(Rr-18), v–xv, 1–16. [PubMed] [Google Scholar]
- Trinidad DR, Perez-Stable EJ, White MM, Emery SL, & Messer K (2011). A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. American Journal of Public Health, 101(4), 699–706. 10.2105/AJPH.2010.191668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USA F (2018). Table outlining the 2018 Federal Poverty Guidelines. Federal Poverty Guidelines. Retrieved from https://familiesusa.org/product/federal-povertyguidelines.
- USDHHS. (2014). The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. In (pp. 1–36): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- Ussher M, Kakar G, Hajek P, & West R (2016). Dependence and motivation to stop smoking as predictors of success of a quit attempt among smokers seeking help to quit. Addictive Behaviors, 53, 175–180. 10.1016/j.addbeh.2015.10.020. [DOI] [PubMed] [Google Scholar]