Abstract
Objective
Well-controlled glucose levels (ie, 70-180 mg/dL) have been associated with lower mortality from COVID-19. The addition of dexamethasone to COVID-19 treatment protocols has raised concerns about the potential negative consequences of dexamethasone-induced hyperglycemia.
Methods
We developed a protocol to guide the management of dexamethasone-induced hyperglycemia in hospitalized patients with COVID-19. Two of the 4 medical teams managing patients with COVID-19 at a tertiary center in Saudi Arabia used the protocol and the other 2 teams continued to manage hyperglycemia at the discretion of the treating physicians (protocol and control groups, respectively). The glycemic control and clinical outcomes in 163 patients hospitalized with COVID-19 and dexamethasone-induced hyperglycemia between July 5th and September 30th, 2020, were retrospectively compared between the 2 groups.
Results
Compared to the control group, the protocol group had higher proportions of patients with well-controlled glucose across all premeals and bedtime glucose readings throughout the hospital stay. The differences in glycemic control between the 2 groups were statistically significant for fasting glucose on days 4, 5, and the discharge day; prelunch glucose on the discharge day; predinner glucose on days 3, 5, and the discharge day; and bedtime glucose on day 1 (all P < .05). After adjusting for age, sex, nationality, body mass index, Charlson score, and diabetes status, patients in the protocol group were more likely to have well-controlled glucose levels compared with those in the control group. Moreover, the in-hospital mortality was significantly lower in the protocol group (12.93%) compared to the control group (29.93%) (P < .01).
Conclusion
The implementation of a protocol to manage dexamethasone-induced hyperglycemia in hospitalized patients with COVID-19 resulted in more patients achieving well-controlled glucose levels and was associated with lower mortality from COVID-19.
Key words: hyperglycemia, dexamethasone, COVID-19, diabetes mellitus, protocol
Abbreviations: BMI, body mass index; ICU, intensive care unit; IV, intravenous; OR, odds ratio
Introduction
The global pandemic of the novel coronavirus has placed a major burden on health care systems worldwide. The first case of COVID-19 in Saudi Arabia was reported in March 2020. As of this moment, the numbers of confirmed cases in Saudi Arabia have surpassed 395 000, cases including more than 6700 deaths.1 The factors associated with worse outcomes of COVID-19 include older age and the presence of underlying chronic diseases, such as diabetes, hypertension, obesity, and chronic respiratory diseases.2 Several studies hallmark diabetes and hyperglycemia as important risk factors of morbidity and mortality from COVID-19.3, 4, 5, 6, 7, 8 Moreover, early-onset hyperglycemia and hyperglycemia upon presentation to the hospital, has been associated with increased mortality from COVID-19 in patients with and without diabetes.9 , 10
After the promising results of the RECOVERY trial, dexamethasone emerged as a promising treatment of COVID-19, with a significant reduction in mortality in hospitalized patients with hypoxemia.11 The dexamethasone dose used in the trial was 6 mg daily for 10 days; a dose approximately 6 to 8 times the physiological equivalent of glucocorticoid production, and is associated with a high risk of steroid-induced hyperglycemia and other metabolic sequelae in patients with and without diabetes.12 This, along with the deleterious effect of COVID-19 on insulin sensitivity and beta-cell function (as a result of a cytokine storm) can lead to severe hyperglycemia, and subsequently, may instigate acute glycemic complications such as diabetic ketoacidosis and a hyperglycemic hyperosmolar state.13, 14, 15, 16, 17
Hyperglycemia on hospital admission, as well as during the first few days of admission, has been linked to severe illness and death among patients with COVID-19. 8 , 9 , 18 A study conducted in Wuhan, China showed reduced mortality in patients admitted with COVID-19 who had glucose levels between 70 and 180 mg/dL throughout the hospital stay compared to those who had glucose levels >180 mg/dL.18 Achieving glucose level targets when managing hospitalized patients with COVID-19 has been a challenging task during the pandemic. The shortage of staff and personal protective equipment, along with the need to minimize staff exposure during the outbreak, have hindered glycemic control in patients with COVID-19. In addition, health care providers from various specialties, including those who may not be very familiar with glucose management and steroid-induced hyperglycemia, have been called to manage patients with COVID-19. As a result, many patients with COVID-19 have had inadequate glycemic control during their hospital stay, particularly after the addition of dexamethasone to treatment protocols of severe COVID-19.19 In many instances, these patients could have benefited from more aggressive glucose management to maintain glucose levels between 70 and 180 mg/dL.
Here, we present a simplified, yet comprehensive, protocol to guide the management of dexamethasone-induced hyperglycemia in patients hospitalized with COVID-19. In addition, we evaluate the clinical effectiveness of this protocol in managing dexamethasone-induced hyperglycemia during the peak of COVID-19 cases in a tertiary center in Saudi Arabia.
Methods
Study Design
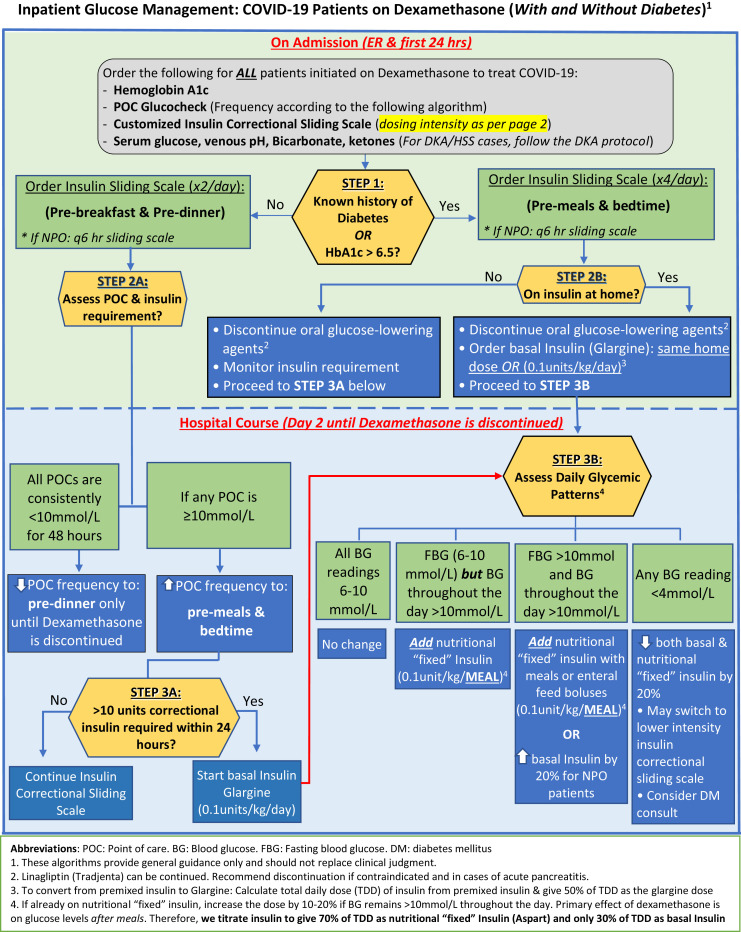
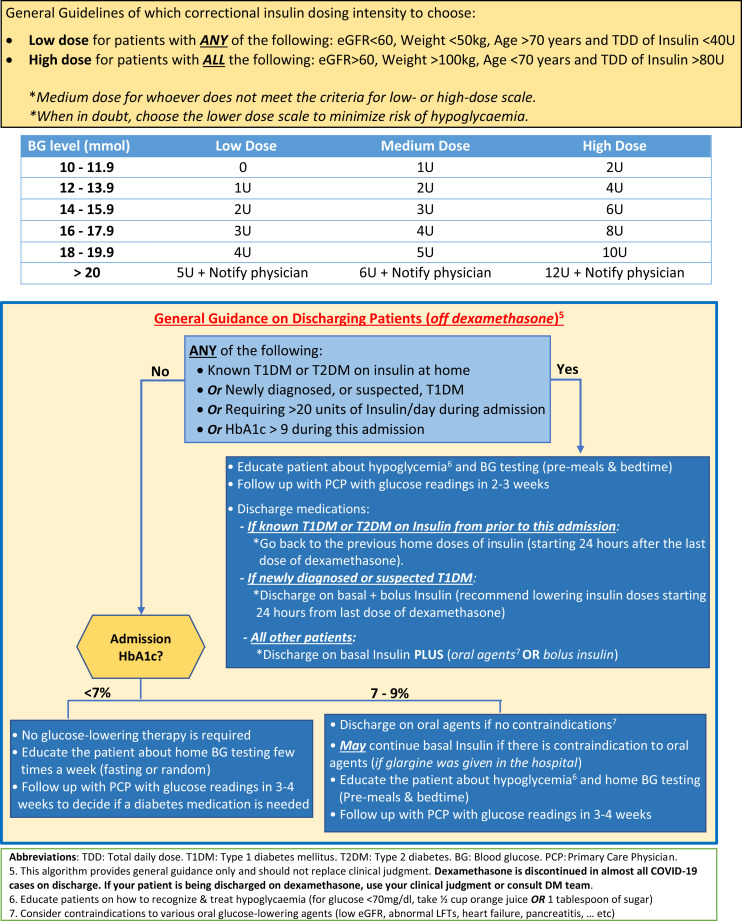
The study was conducted at King Saud Universiy (KSU) Medical City, a tertiary center in Saudi Arabia that was designated to manage patients with COVID-19 during the pandemic. We developed a protocol on how to manage dexamethasone-induced hyperglycemia as a quality improvement project that was approved by the institutional review board at KSU (Fig. 1 ). The protocol was disseminated to 2 of the 4 medical teams that were managing hospitalized patients with COVID-19 in a non-intensive care unit (ICU) setting between July 5, 2020, and September 30, 2020. We retrospectively reviewed the electronic medical records and glucose levels of all adult patients with COVID-19 who were treated with dexamethasone as part of the COVID-19 treatment protocol, and who were managed by one of the 4 medical teams at our hospital during the study period. Of these patients, we excluded those without diabetes and maintained glucose levels <180 mg/dL throughout the hospitalization period, as well as those who required intravenous (IV) insulin infusion within the first 3 days of hospitalization. We only included patients with COVID-19 who were treated with dexamethasone and had any of the following: known history of diabetes, previously undiagnosed diabetes (defined as having an A1C ≥ 6.5 on admission), and no diabetes, but developed hyperglycemia after the initiation of dexamethasone (defined as having ≥1 glucose level(s) >180 mg/dL). In this study, we refer to all 3 groups as patients with dexamethasone-induced hyperglycemia.
Fig. 1.
Protocol to manage dexamethasone-induced hyperglycemia among patients with COVID-19.
Protocol Description
The protocol guides health care professionals through all the steps needed to manage dexamethasone-induced hyperglycemia; from the time of admission (ie, in the emergency room) to the time of discharge and/or discontinuation of dexamethasone. This protocol is activated upon the initiation of dexamethasone in patients with COVID-19 and starts by determining the diabetes status, estimated glomerular filtration rate, weight, and age of the patient; to decide the frequency of point-of-care testing and dose intensity of the insulin correctional scale. Basal insulin is recommended for those who use insulin at home. Otherwise, patients who do not use insulin at home (including those without a prior history of diabetes) are managed with insulin corrections only and are initiated on basal insulin therapy if the daily requirement of insulin corrections exceeds 10 units. Linagliptin requires no renal dose adjustment and is the only dipeptidyl peptidase-4 inhibitor available in our hospital; therefore, our protocol offers the continuation of linagliptin in the inpatient setting as an option for patients who have no contraindications. In addition, this protocol guides health care professionals through the daily assessment of glycemic patterns and how to titrate basal, nutritional, and correctional insulin doses accordingly. The last section of this protocol provides general guidelines for a safe discharge process to avoid hypoglycemia after the discontinuation of dexamethasone, and to ensure a close follow-up plan (Fig. 1).20
Study Groups
The protocol group included patients admitted under the care of the 2 medical teams that had received and implemented the protocol for the management of dexamethasone-induced hyperglycemia. The control group included patients admitted under the care of the other 2 medical teams that had not received nor implemented the protocol. Dexamethasone-induced hyperglycemia in the control group was managed according to the usual care and at the discretion of the treating physicians. The 4 COVID-19 medical teams at our institution were comparable in the number and level of training of their members, as well as the acuity of patients admitted to each team. They were run by medical specialists only and each team consisted of a medical attending, a senior medical resident or registrar, and 3 junior medical residents. The senior medical residents and registrars were responsible for the protocol implementation in the 2 teams assigned to use the protocol. The other 2 medical teams continued to manage dexamethasone-induced hyperglycemia according to the usual care at the discretion of the treating physicians and without using the protocol.
Outcomes and Covariates
We collected data from the electronic medical records including age, sex, nationality, body mass index (BMI); vital signs, comorbidities (diabetes, hypertension, dyslipidemia, chronic lung disease, chronic kidney disease, ischemic heart disease, and heart failure), calculated Charlson, quick sequential organ failure assessment, confusion, urea, respiratory rate, blood pressure, and ≥65 years of age scores, clinical outcomes (including rates of in-hospital mortality, length of hospital stay, need for invasive and noninvasive ventilation, need for IV insulin infusion, and rates of 30-day and 6-month hospital readmission), and point-of-care glucose levels (premeals and bedtime) during the first 5 days of hospitalization, as well as on the day of discharge for those hospitalized for longer than 5 days. Well-controlled glucose levels were defined as having point-of-care glucose levels between 70 and 180 mg/dL throughout the day.8 , 18 Mild hypoglycemia was defined as a glucose level between 54 and 70 mg/dL, whereas severe hypoglycemia was defined as a glucose level of ≤54 mg/dL.
Statistical Analysis
All significance testing was 2-tailed with α of .05, and data were analyzed using Stata Statistical Software (release 15). Variables distributions were examined for normality using the Shapiro-Wilk test and visual examination of histograms. The nonnormally distributed variables of BMI, maximum temperature, respiratory rate, and Charlson score are presented as medians with interquartile ranges. Baseline characteristics and differences in glycemic control measures and clinical outcomes were compared by use of protocol using the t test for continuous and normally distributed data, Kruskal-Wallis test for continuous and nonnormally distributed data, and χ2 test of homogeneity for categorical variables. Logistic regression analysis was used to examine the association between the use of protocol and achieving well-controlled glucose levels in patients with dexamethasone-induced hyperglycemia before and after adjusting for age, sex, nationality, BMI, Charlson score, and diabetes status.
Results
Population Characteristics
The study included 163 patients with COVID-19 and dexamethasone-induced hyperglycemia, of whom 116 were managed using the protocol (protocol group), and 47 were managed without using the protocol at the discretion of the treating physician (control group). Upon admission, there were no significant differences in age, sex, BMI, the prevalence of diabetes, nationality, quick sequential organ failure assessment, confusion, urea, respiratory rate, blood pressure, and ≥65 years of age score, and Charlson score between the protocol and control groups (all P > .05) (Table 1 ).
Table 1.
Characteristics of Study Participants by Protocol Use
| Variable | All (N = 163) | Protocol (n = 116) | Control (n = 47) | P value |
|---|---|---|---|---|
| Age, mean (SD), y | 56.3 (15.8) | 56.27 (16.19) | 56.38 (14.94) | .97 |
| Saudi, % | 87 (53.37) | 66 (56.9) | 21 (44.68) | .16 |
| Female, % | 51 (31.29) | 37 (31.9) | 14 (29.79) | .79 |
| BMI, median (25th, 75th), kg/m2 | 29 (25.77, 33.33) | 29 (25.95, 33.62) | 27 (25.77, 32.27) | .47 |
| Vital signs on admission | ||||
| Tmax, median (25th, 75th), °C | 37.5 (36.9, 38.3) | 37.45 (36.9, 38.15) | 37.8 (37, 38.5) | .15 |
| Oxygen saturation on RA, mean (SD), % | 85.03 (9.74) | 85.80 (9.67) | 83.11 (9.75) | .11 |
| HR, mean (SD) | 98 (17.19) | 97 (17.62) | 101 (16.02) | .26 |
| RR, median (25th, 75th) | 26 (22, 32) | 25 (22, 30) | 27 (23, 35) | .05 |
| SBP, mean (SD), mm Hg | 126 (23.59) | 127 (24.45) | 122 (21.14) | .22 |
| DBP, mean (SD), mm Hg | 73 (17.97) | 74 (19.6) | 69 (12.66) | .12 |
| Oxygen delivery upon admission, n (%) | ||||
| Room air | 16 (9.82) | 14 (12.07) | 2 (4.26) | .25 |
| Noninvasive ventilation | 146 (89.57) | 101 (87.07) | 45 (95.74) | |
| Invasive ventilation (intubation) | 1 (0.61) | 1 (0.86) | 0 (0) | |
| Comorbidities, n (%) | ||||
| DM | 62 (38.04) | 41 (35.34) | 21 (44.68) | .27 |
| HTN | 64 (39.26) | 43 (37.07) | 21 (44.68) | .37 |
| DLP | 29 (17.79) | 18 (15.52) | 11 (23.4) | .23 |
| Respiratory disease | 20 (12.27) | 18 (15.52) | 2 (4.26) | .05 |
| CKD | 12 (7.36) | 10 (8.62) | 2 (4.26) | .4 |
| IHD | 6 (3.68) | 4 (3.45) | 2 (4.26) | .8 |
| Laboratory results on admission | ||||
| CRP, median (25th, 75th), (n = 157), mg/L | 101 (56, 169) | 102 (61, 154) | 99.5 (53, 180) | .78 |
| Procalcitonin, median (25th, 75th), (n = 134), ng/mL | 0.15 (0.06, 0.35) | 0.15 (0.07, 0.34) | 0.17 (0.06, 0.4) | .66 |
| ESR, median (25th, 75th), (n = 104), mm/h | 70 (47, 92) | 68 (42.5, 92) | 71.5 (52, 95) | .52 |
| Serum glucose, mean (SD), mg/dL | ||||
| Patients with diabetes (n = 62) | 231 (105) | 228 (113) | 237 (89) | .75 |
| Patients without diabetes (n = 101) | 135 (69) | 128 (67) | 157 (73) | .06 |
| Disease severity scores | ||||
| qSOFA score, median (25th, 75th) | 1 (1, 1) | 1 (1, 1) | 1 (1, 2) | .18 |
| CURB-65 score, median (25th, 75th) | 1 (0, 2) | 1 (0, 2) | 1 (1, 2) | .19 |
| Charlson score, median (25th, 75th) | 2 (1, 4) | 2 (0.5, 4) | 2 (1, 3) | .62 |
Abbreviations: BMI = body mass index; CKD = chronic kidney disease; CRP = C-reactive protein; CURB-65 = confusion, urea, respiratory rate, blood pressure, and ≥65 years of age; DBP = diastolic blood pressure; DLP = dyslipidemia; DM = diabetes mellitus; ESR = erythrocyte sedimentation rate; HR = heart rate; HTN = hypertension; IHD = ischemic heart disease; NC = nasal cannula; qSOFA = quick sequential organ failure assessment; RA = room air; RR = respiratory rate; SBP = systolic blood pressure; Tmax = maximum temperature.
Inpatient Glucose Level Control by Use of Protocol
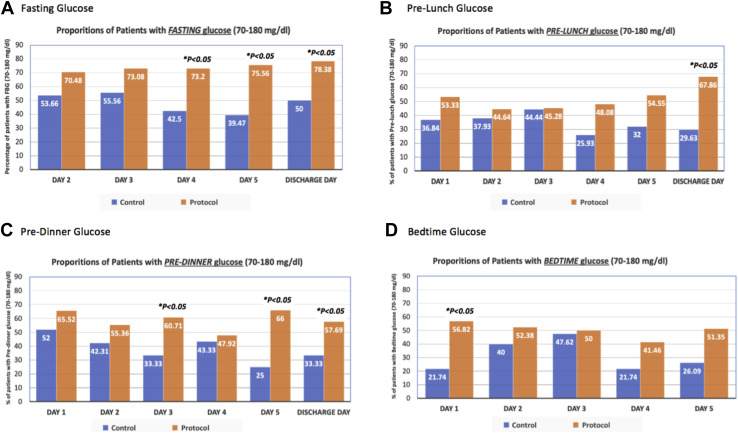
Fasting, prelunch, predinner, and bedtime point-of-care glucose level readings were compared between the protocol and control groups throughout the first 5 days of admission and on the day of discharge. Fasting glucose level readings on the first day were discarded, as it was unknown if the patients were fasting at time of admission. The protocol group had significantly lower fasting glucose levels than the control group on day 3 (135 mg/dL vs 160.2 mg/dL, respectively; P < .05), day 4 (134.1 mg/dL vs 203.4 mg/dL, respectively; P < .01), day 5 (129.6 mg/dL vs 206.1 mg/dL, respectively; P < .01) and on the day of discharge (133.2 mg/dL vs 186.3 mg/dL, respectively; P < .01). Prelunch glucose levels were also significantly lower in the protocol group compared to the control group on day 4 (183.6 mg/dL vs 243 mg/dL, respectively; P < .05), day 5 (162 mg/dL vs 212.4 mg/dL, respectively; P < .05), and on the day of discharge (145.8 mg/dL vs 246.6 mg/dL, respectively; P < .01). Similarly, predinner glucose levels on day 3, day 5, and bedtime glucose level on day 1 were all significantly lower in patients in the protocol group compared to patients the control group (all P < .05) (Table 2 ).
Table 2.
Comparison of Glucose Levels During Hospital Stay in Those Who Were Managed Using the Protocol Versus Those Who Were Not (Protocol vs Control) (N = 163)a
| Fasting glucose level (mg/dL) |
Prelunch glucose level (mg/dL) |
Predinner glucose level (mg/dL) |
Bedtime glucose level (mg/dl) |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | Protocol | Control | Protocol | Control | Protocol | Control | Protocol | |
| Day 1 | NA | NA | 214.2 (156.6, 297) n = 19 | 169.2 (126, 288) n = 45 | 180 (140.4, 112) n = 25 | 153 (124.2, 223.2) n = 58 | 223.2 (194.4, 279) n = 23 | 172.8 (134.1, 236.7)bn = 44 |
| Day 2 | 175.5 (124.2, 232.2) n = 41 | 151.2 (115.92, 198) n = 105 | 219.6 (151.2, 291.6) n = 29 | 197.1 (134.1, 278.1) n = 56 | 196.2 (144, 279) n = 26 | 175.5 (139.5, 240.3) n = 56 | 201.6 (147.6, 270) n = 25 | 180 (138.6, 243) n = 42 |
| Day 3 | 160.2 (120.6, 216) n = 45 | 135 (108, 182.7)bn = 104 | 194.4 (135, 275.4) n = 27 | 187.2 (132.3, 235.8) n = 52 | 221.4 (154.8, 307.8) n = 27 | 162 (129.6, 235.8)cn = 55 | 190.8 (147.6, 243) n = 21 | 180 (133.2, 253.8) n = 41 |
| Day 4 | 203.4 (135, 253.8) n = 40 | 134.1 (108, 181.8)cn = 96 | 243 (180, 295.2) n = 27 | 183.6 (131.4, 252)bn = 51 | 213.3 (149.4, 270) n = 30 | 181.8 (138.6, 239.4) n = 47 | 232.2 (187.2, 291.6) n = 23 | 198 (145.8, 250.2) n = 40 |
| Day 5 | 206.1 (144, 286.2) n = 38 | 129.6 (109.8, 180)cn = 89 | 212.4 (174.6, 277.2) n = 25 | 162 (127.8, 223.2)bn = 43 | 213.3 (171.9, 280.8) n = 28 | 142.2 (120.6, 203.4)cn = 49 | 214.2 (167.4, 293.4) n = 23 | 180 (135, 244.8) n = 36 |
| Discharge day | 186.3 (108, 237.6) n = 42 | 133.2 (96.12, 162)cn = 107 | 246.6 (144, 284.4) n = 27 | 145.8 (116.1, 180)cn = 52 | 226.8 (104.4, 250.2) n = 12 | 144 (117, 198) n = 22 | 217.8 (129.6, 280.8) n = 7 | 187.2 (126, 244.8) n = 14 |
Data are presented as medians (25th, 75th percentiles) and differences between the protocol and control groups were examined using Kruskal-Wallis tests.
P < .05 compared to the control group.
P < .01 compared to the control group.
Patients in the protocol group were more likely to have well-controlled glucose levels (ie, 70-180 mg/dL) across all premeal and bedtime values, and throughout the study period compared with patients in the control group. These differences were statistically significant for fasting glucose level on days 4, 5, and the day of discharge; prelunch glucose level on the day of discharge, predinner glucose level on days 3, 5, and the day of discharge; and bedtime glucose level on day 1 (all P < .05) (Fig. 2 ). A sensitivity analysis showed no major differences in the performance of the protocol among patients with diabetes versus patients without diabetes (data not shown).
Fig. 2.
Proportion of patients with well-controlled glucose levels (ie, 70-180 mg/dL) throughout the hospital stay (control vs protocol) (N = 163).
Clinical Outcomes by Use of Protocol
The protocol group had a significantly lower in-hospital mortality than the control group (12.93% vs 29.93%, respectively; P = .01). Moreover, patients in the protocol group were less likely to require ICU admission, IV insulin infusion, or mechanical ventilation compared to those in the control group—although none of these differences were statistically significant. Likewise, there were no statistically significant differences in the numbers of patients who developed ≥1 event of mild or severe inpatient hypoglycemia between the protocol and the control groups, although a slightly higher number of patients in the protocol group developed hypoglycemia during the first 5 days of hospitalization (ie, insulin titration period), and a slightly higher number of patients in the control group developed hypoglycemia after day 5 of hospitalization (Table 3 ).
Table 3.
Outcomes in Hospitalized Patients With COVID-19 and Dexamethasone-Induced Hyperglycemia (Protocol vs Control)
| Variable | All (N = 163) | Protocol (n = 116) | Control (n = 47) | P value |
|---|---|---|---|---|
| Discharged alive, n (%) | 133 (81.61) | 100 (86.21) | 33 (70.21) | .02 |
| In-hospital mortality, n (%) | 29 (17.79) | 15 (12.93) | 14 (29.93) | .01 |
| Length of hospital stay, mean (SD), d | 17 (23) | 16 (25) | 17 (17) | .80 |
| Mechanical ventilation (intubation), n (%) | 32 (19.63) | 19 (16.38) | 13 (27.66) | .10 |
| ICU admission, n (%) | 71 (43.56) | 47 (40.52) | 24 (51.06) | .22 |
| Length of hospital stay, mean (SD), d | 16.71 (22.50) | 16.41 (24.56) | 17.43 (16.57) | .80 |
| 30-day hospital readmission, n (%)a | 10 (7.52) | 8 (8.00) | 2 (6.06) | .71 |
| 6-month hospital readmission, n (%)a | 15 (11.28) | 12 (12.00) | 3 (9.09) | .65 |
| Glucose related outcomes | ||||
| Required IV insulin infusion, n (%)b | 25 (15.34) | 15 (12.93) | 10 (21.28) | .18 |
| Mild hypoglycemia event within the first 5 days of hospitalization, n (%)c | 4 (2.45) | 4 (3.45) | 0 (0) | .20 |
| Severe hypoglycemia within the first 5 days of hospitalization, n (%)c | 7 (4.29) | 7 (6.03) | 0 (0) | .09 |
| Mild hypoglycemia after day 5 of hospitalization, n (%)c | 16 (9.82) | 10 (8.62) | 6 (12.77) | .42 |
| Severe hypoglycemia after day 5 of hospitalization, n (%)c | 14 (8.59) | 7 (6.03) | 7 (14.89) | .07 |
Abbreviations: ICU = intensive care unit; IV = intravenous.
Analysis was limited to patients who were discharged from the hospital.
Required IV insulin therapy after day 3 of admission. Those who were started on IV insulin within the first 3 days of hospitalization were excluded from the study.
Number of patients with ≥1 inpatient hypoglycemia event (mild hypoglycemia is defined as a glucose level between 54-70 mg/dL and severe hypoglycemia is defined as a glucose level of ≤54 mg/dL).
Univariate and Multivariate Logistic Regression Analyses
The results of univariate and multivariate logistic regression analyses, examining the relationship between the use of inpatient management protocol of dexamethasone-induced hyperglycemia and having a well-controlled glucose level (ie, 70-180 mg/dL) among patients with COVID-19, are shown in Table 4 . Compared to patients in the control group, those who were managed using the protocol were 2- to 4- times more likely to have well-controlled fasting glucose levels on days 3, 4, 5, and the day of discharge in the unadjusted model (odds ratio [OR] [95% CI]: 2.17 (1.05, 4.51); 3.70 (1.71, 8.00); 4.74 (2.11, 10.64); 3.63 (1.70, 7.71), respectively). After adjusting for age, sex, nationality, BMI, Charlson score, and diabetes status, the odds of having fasting glucose levels within the target value remained significantly higher in the protocol group compared to the control group on days 4, 5, and the day of discharge (OR [95% CI]: 7.17 (2.4, 21.45); 6.95 (2.34, 20.66); 3.84 (1.67, 8.82), respectively). Prelunch glucose levels were more than twice as likely to be within target in the protocol group compared to the control group on day 5 and the day of discharge in the adjusted model (OR [95% CI]; 4.02 (1.19, 13.63); 6.02 (1.89, 19.18), respectively). Similarly, patients managed using the protocol were more likely to have predinner and bedtime glucose level readings within the target value throughout the study period compared to control (Table 4).
Table 4.
Logistic Regression Analysis of the Relationship Between the Use of the Protocol and Having Well-Controlled Glucose Levels (ie, 70-180 mg/dL) Among Patients With COVID-19 (N = 163)
| Unadjusted model |
Adjusted modela |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| FBG | ||||
| Day 2 FBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 2.06 (0.98, 4.34) | .06 | 1.74 (0.64, 4.72) | .28 |
| Day 3 FBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 2.17 (1.05, 4.51) | .04 | 2.29 (0.90, 5.84) | .08 |
| Day 4 FBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 3.70 (1.71, 8.00) | <.01 | 7.17 (2.40, 21.45) | <.01 |
| Day 5 FBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 4.74 (2.11, 10.64) | <.01 | 6.95 (2.34, 20.66) | <.01 |
| Discharge day FBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 3.63 (1.70, 7.71) | <.01 | 3.84 (1.67, 8.82) | <.01 |
| PLBG | ||||
| Day 1 PLBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 1.96 (0.65, 5.89) | .23 | 2.29 (0.67, 7.78) | .19 |
| Day 2 PLBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 1.32 (0.53, 3.30) | .55 | 1.10 (0.41, 2.98) | .85 |
| Day 3 PLBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 1.03 (0.41, 2.63) | .94 | 0.88 (0.28, 2.75) | .83 |
| Day 4 PLBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 2.65 (0.96, 7.32) | .06 | 2.18 (0.67, 7.08) | .20 |
| Day 5 PLBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 2.55 (0.91, 7.13) | .08 | 4.02 (1.19, 13.63) | .03 |
| Discharge day PLBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 5.01 (1.85, 13.61) | <.01 | 6.02 (1.89, 19.18) | <.01 |
| PDBG | ||||
| Day 1 PDBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 1.75 (0.68, 4.55) | .25 | 2.56 (0.74, 8.81) | .14 |
| Day 2 PDBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 1.70 (0.66, 4.33) | .27 | 1.52 (0.54, 4.30) | .43 |
| Day 3 PDBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 3.09 (1.18, 8.10) | .02 | 3.67 (1.21, 11.12) | .02 |
| Day 4 PDBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 1.20 (0.48, 3.01) | .69 | 0.88 (0.28, 2.75) | .82 |
| Day 5 PDBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 5.82 (2.07, 16.42) | <.01 | 13.80 (3.04, 62.63) | <.01 |
| Discharge day PDBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 2.73 (0.65, 11.40) | .17 | 4.04 (0.61, 26.84) | .15 |
| BTBG | ||||
| Day 1 BTBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 4.74 (1.49, 15.06) | <.01 | 4.97 (1.10, 22.57) | .04 |
| Day 2 BTBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 1.65 (0.60, 4.50) | .33 | 1.35 (0.43, 4.17) | .61 |
| Day 3 BTBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 1.1 (0.39, 3.14) | .86 | 1.07 (0.33, 3.39) | .91 |
| Day 4 BTBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 2.55 (0.79, 8.21) | .12 | 1.87 (0.49, 7.16) | .36 |
| Day 5 BTBG | ||||
| Control group | Reference | Reference | ||
| Protocol group | 2.99 (0.96, 9.28) | .06 | 3.27 (0.79, 13.53) | .10 |
Abbreviations: BTBG = bedtime blood glucose; FBG = fasting blood glucose; OR = odds ratio; PDBG = predinner blood glucose; PLBG = prelunch blood glucose.
Logistic regression analysis adjusted for age, sex, nationality, diabetes status, and the Charlson score.
Discussion
Our study highlights the clinical effectiveness of a simple, yet comprehensive, protocol developed during the peak period of COVID-19 cases in Saudi Arabia to guide the management of dexamethasone-induced hyperglycemia among hospitalized patients with severe COVID-19. Patients who were managed using this protocol were more likely to attain glucose levels between 70 and 180 mg/dL throughout the hospital stay; a glucose level range that has been previously linked to lower mortality of COVID-19 among hospitalized patients.18 The most prominent improvement in glucose level control using our protocol was noted in the fasting and predinner glucose levels. This is likely explained by the relatively early initiation of basal insulin in the protocol group, as recommended by our protocol, compared to standard practice. In addition, dexamethasone exerts its peak effect on glucose levels approximately 9 to 12 hours after the dose administration.21 , 22 It is our facility’s practice to administer most medications, including dexamethasone, at around 9 AM; the effect of dexamethasone on glucose level control appears to peak around the predinner time (ie, approximately 6 pm). This is evident in the control group, which had a progressive worsening of glucose level control throughout the hospital stay; particularly, at the predinner time. In contrast, the protocol group had a continuous improvement of glucose level control throughout their hospital stay, including at predinner time.
The association between hyperglycemia upon admission, as well as during the hospital stay, poor outcomes of COVID-19, and mortality has been shown in multiple studies.3, 4, 5, 6, 7, 8, 9, 10 In our study, patients who were managed using the protocol had significantly lower in-hospital mortality compared to those managed using the standard practice. Moreover, the protocol group trended toward a lower risk of ICU admission, initiation of IV insulin infusion, and need for mechanical ventilation. Whether hyperglycemia is an independent predictor of severe COVID-19 and mortality or is simply a marker of severe illness in hospitalized patients with COVID-19, remains largely unknown. Previous randomized controlled studies, albeit in non-COVID-19 patients, have shown a strong association between better glycemic control during the first days of hospitalization and better outcomes in patients admitted to the ICU, surgical, or medical wards.23, 24, 25 A recent study examined the temporality of the association between poor glucose level control upon admission, and throughout the first few days of hospitalization and the outcomes of COVID-19, and found a 7-fold increase in mortality risk in patients with COVID-19 and severe hyperglycemia.26 These findings highlight the importance of maintaining well-controlled glucose levels in patients with COVID-19. This calls for the implementation of glucose level management protocols, such as ours, to help health care providers caring for patients with COVID-19 and dexamethasone-induced hyperglycemia accomplish this goal as early as possible during the patient’s hospital stay.
To account for the severity of chronic medical conditions upon admission as a potential confounder, we adjusted for the Charlson score in the model; examining the association between the use of protocol and achieving well-controlled glucose levels, and the findings remained the same. Our protocol is designed to improve the patients’ glycemic control without compromising the patients’ or health care providers’ safety. It is designed to improve the patients’ glucose level control without significantly increasing the risk of insulin-induced hypoglycemia. As we were developing the protocol, we took into consideration staff shortages, the need to minimize nurses’ exposure to patients with COVID-19, and the fact that patients with COVID-19 were less frequently checked upon due to the low nurse-to-patient ratios during the pandemic. Minimizing the risk of hypoglycemia was a priority in our protocol, particularly when managing patients who were unable to express symptoms of hypoglycemia due to severe respiratory illness, altered mental status, sedation, or other factors. Therefore, we recommend a relatively low dose of 0.1 units per kilogram per day as the starting dose of basal insulin, with a gradual titration based on glucose levels afterward. The conservative nature of the protocol may explain why it took 2 days for the differences in glucose levels between the protocol and control groups to become statistically significant. For a more rapid improvement in glycemic control, it might be reasonable to start with a basal insulin dose of 0.2 units per kilogram per day in patients who are deemed low-risk of hypoglycemia.
To the best of our knowledge, this is the first study to examine the effectiveness of a steroid-induced hyperglycemia management protocol among hospitalized patients with COVID-19. The strengths of our study include the simplicity and comprehensiveness of our protocol, which makes it easy to implement and more likely to be adhered to in most health care facilities that care for patients with COVID-19 and steroid-induced hyperglycemia. Our findings have clinical implications during ordinary times and in times of disaster when the shortage of staff is a major limiting factor to achieving inpatient target glucose levels. Moreover, we were able to assess the severity of COVID-19 in our study participants using the Charlson score, a comorbidity index that predicts the 10-year survival in patients with multiple comorbidities, and adjusting for any potential confounding effect of illness severity on the association between use of the protocol and glycemic control. Our findings are limited to patients admitted to a non-ICU medical setting. This, along with the fact that the study was carried out in a single tertiary center in Saudi Arabia, limits the generalizability of our findings. The retrospective nature of our analysis may have resulted in a selection bias with “healthier” patients being more likely to be managed by their physicians using our protocol. However, this seems to have not been the case, as both study groups had comparable quick sequential organ failure assessment scores and other markers of disease severity at baseline. Moreover, the limited number of point-of-care glucose level readings at certain time points may have contributed to the lack of significant differences in glycemic control between the protocol and control groups at those time points. This can be addressed with the use of continuous glucose level monitors in an inpatient setting for future studies. In addition, our hospital has no standardized protocols for the inpatient management of diabetes, which may have negatively impacted the glycemic control in the control group. Finally, we do not have data to confirm that the use of our protocol resulted in less exposure of staff to patients with COVID-19. However, we believe that it did so through offering the option of 2 point-of-care glucose level checks per day when appropriate, minimizing the risk of frequent hyper- and/or hypoglycemia that often prompt a glucose level check with or without the administration of correctional insulin or dextrose; and minimizing the rates of IV insulin initiation to manage hyperglycemia in the protocol group.
In conclusion, the implementation of a simplified protocol for the management of dexamethasone-induced hyperglycemia in hospitalized patients with COVID-19 resulted in better glycemic control and was associated with better clinical outcomes and lower mortality of COVID-19. Future randomized controlled studies are needed to compare the inpatient glycemic control, preferably using continuous glucose level monitors, and mortality among patients with steroid-induced hyperglycemia treated with our protocol versus a control group treated with a standardized protocol for the management of inpatient hyperglycemia.
Acknowledgment
We would like to thank the COVID medical teams at King Saud University Medical City for their efforts in implementing the dexamethasone-induced hyperglycemia protocol in their patient care.
Disclosure
The authors have no multiplicity of interest to disclose.
References
- 1.COVID-19 Dashboard: Saudi Arabia. Accessed April 9, 2021. https://covid19.moh.gov.sa/
- 2.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., et al. China medical treatment expert group for COVID-19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Zangrillo A., Zanella A., et al. COVID-19 Lombardy ICU network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GuoW, Li M, DongY,et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. Published online 2020. https://doi.org/10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed]
- 6.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. Published correction appears in Lancet Respir Med. 2020;8:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klonoff D.C., Umpierrez G.E. COVID-19 in patients with diabetes: risk factors that increase morbidity. Metabolism. 2020;108:154224. doi: 10.1016/j.metabol.2020.154224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alguwaihes A.M., Al-Sofiani M.E., Megdad M., et al. Diabetes and COVID-19 among hospitalized patients in Saudi Arabia: a single-centre retrospective study. Cardiovasc Diabetol. 2020;19(1):205. doi: 10.1186/s12933-020-01184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazori A.Y., Bass I.R., Chan L., et al. Hyperglycemia is associated with increased mortality in critically ill patients with COVID-19. Endocr Pract. 2021;27(2):95–100. doi: 10.1016/j.eprac.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. Accessed July 6, 2020. https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-by-up-to-one-third-in-hospitalised-patients-with-severe-respiratory-complications-of-covid-19
- 12.Corsino L., Dhatariya K., Umpierrez G. In: Endotext [Internet] Feingold K.R., Anawalt B., Boyce A., et al., editors. MDText.com, Inc; 2000-2017. Management of diabetes and hyperglycemia in hospitalized patients; pp. 1–33. [Google Scholar]
- 13.Bornstein S.R., Rubino F., Khunti K., et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palermo N.E., Sadhu A.R., McDonnell M.E. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J Clin Endocrinol Metab. 2020;105(8):1–11. doi: 10.1210/clinem/dgaa360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suwanwongse K., Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: causality or coincidence? A report of three cases. J Med Virol. 2021;93(2):1150–1153. doi: 10.1002/jmv.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935–1941. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman N., Fink D., Cai J., Lee Y.N., Davies Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract. 2020;166:108291. doi: 10.1016/j.diabres.2020.108291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu L., She Z.G., Cheng X., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saand A.R., Flores M., Kewan T., et al. Does inpatient hyperglycemia predict a worse outcome in COVID-19 intensive care unit patients? J Diabetes. 2021;13(3):253–260. doi: 10.1111/1753-0407.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inpatient glucose control protocol. Accessed June 11, 2021. https://covidindiabetesmedia.azureedge.net/docs/AlSofiani_2020_Dexamethasone_Induced_Hyperglycemia_Protocol_KSU.pdf
- 21.Polderman J.A.W., Farhang-Razi V., van Dieren S., et al. Adverse side-effects of dexamethasone in surgical patients—an abridged cochrane systematic review. Anaesthesia. 2019;74(7):929–939. doi: 10.1111/anae.14610. [DOI] [PubMed] [Google Scholar]
- 22.Lukins M.B., Manninen P.H. Hyperglycemia in patients administered dexamethasone for craniotomy. Anesth Analg. 2005;100(4):1129–1133. doi: 10.1213/01.ANE.0000146943.45445.55. [DOI] [PubMed] [Google Scholar]
- 23.Sharif K., Ghadir S., Jakubowicz D., et al. Improved outcome of patients with diabetes mellitus with good glycemic control in the cardiac intensive care unit: a retrospective study. Cardiovasc Diabetol. 2019;18(1):4. doi: 10.1186/s12933-019-0810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Targher G., Dauriz M., Tavazzi L., et al. Prognostic impact of in-hospital hyperglycemia in hospitalized patients with acute heart failure: results of the IN-HF (Italian Network on Heart Failure) outcome registry. Int J Cardiol. 2016;203:587–593. doi: 10.1016/j.ijcard.2015.10.207. [DOI] [PubMed] [Google Scholar]
- 25.Gebreegziabher Y., McCullough P.A., Bubb C., et al. Admission hyperglycemia and length of hospital stay in patients with diabetes and heart failure: a prospective cohort study. Congest Heart Fail. 2008;14(3):117–120. doi: 10.1111/j.1751-7133.2008.07569.x. [DOI] [PubMed] [Google Scholar]
- 26.Klonoff D.C., Messler J.C., Umpierrez G.E., et al. Association between achieving inpatient glycemic control and clinical outcomes in hospitalized patients with COVID-19: a multicenter, retrospective hospital-based analysis. Diabetes Care. 2021;44(2):578–585. doi: 10.2337/dc20-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]