Abstract
Objective
This study aimed to examine whether prenatal low-dose aspirin (LDA) therapy affects risk of cesarean versus vaginal delivery.
Study Design
This study is a secondary analysis of the randomized clinical effects of aspirin in gestation and reproduction (EAGeR) trial. Women received 81-mg daily aspirin or placebo from preconception to 36 weeks of gestation. Mode of delivery and obstetric complications were abstracted from records. Log-binomial regression models estimated relative risk (RR) of cesarean versus vaginal delivery. Data were analyzed among the total preconception cohort, as well as restricted to women who had a live birth.
Results
Among 1,228 women, 597 had a live birth. In the intent-to-treat analysis, preconception-initiated LDA was not associated with risk of cesarean (RR = 1.02; 95% confidence interval [CI]: 0.98–1.07) compared with placebo. Findings were similar in just women with a live birth and when accounting prior cesarean delivery and parity.
Conclusion
Preconception-initiated daily LDA was not associated with mode of delivery among women with one to two prior losses.
Keywords: low-dose aspirin, spontaneous vaginal delivery, cesarean delivery, induction, placentation
Maternal morbidity and mortality vary significantly by mode of delivery.1 While one-third of pregnancies in the United States are delivered through cesarean section, this mode of delivery is associated with increased maternal and neonatal complications.1–5 Women with a spontaneous vaginal delivery (SVD) have the lowest number of complications overall, whereas women with primary cesarean deliveries have the highest number of complications, accounting for over 50% of postpartum transfusions and over 38% of postpartum intensive care unit (ICU) admissions.6
Even more concerning, women with repeat cesareans have the highest rate of uterine rupture and unplanned hysterectomy2 often due to rupture of the prior uterine scar or placenta accreta spectrum. Further, the risk of bleeding increases in cesarean delivery and a hysterectomy is more likely to be performed at the time of delivery for excessive and uncontrolled bleeding.2 Given the increased complication rates associated with cesarean deliveries compared with SVDs,1,3 there are ongoing efforts to increase the latter over the former.
Low-dose aspirin (LDA) reduces the risk of developing preeclampsia.7 Aspirin improves blood flow and reduces inflammation in reproductive organs and taken during preconception has been shown to improve endometrial growth and vascularization.7–11 Particularly, use of aspirin in pregnancy was highly suggested by the United States preventative task force guidelines as it carried such low risks in pregnancy, as demonstrated in another secondary analysis of the EAGeR trial.12 The effects of aspirin in gestation and reproduction (EAGeR) trial has shown that LDA may also be associated with a higher live birth rate and lower risk of preterm birth in women with a single recent pregnancy loss.13 While there are reports of increased risk of placental abruption,7–11 which may lend itself to increased risk of cesarean delivery, there are no conclusive data on the impact of LDA to the mode of delivery, either increasing risk of cesarean or improving likelihood of vaginal delivery. Thus, our objective was to examine the effect of daily prenatal LDA therapy on mode of delivery.
Materials and Methods
This is a secondary analysis of the EAGeR trial, a multicenter, double-blind, block-randomized, placebo-controlled trial evaluating the effects of LDA on live birth. Details of this trial have been outlined previously.14,15 Briefly, 1,228 women aged 18 to 40 years with a history of one to two previous pregnancy losses were included from 2006 to 2012 at four U.S. clinical centers. Exclusion criteria included a history of infertility, pelvic inflammatory disease, tubal occlusion, endometriosis, anovulation, uterine abnormalities, polycystic ovarian syndrome, and contraindications to aspirin. Institutional review board authorization was obtained for the data coordinating center and at all clinical centers. At the University of Utah Medical Center, the institutional review board (IRB) of Inter-mountain Healthcare Office of Research approved IRB no.: 1002521-EAGeR on November 4, 2010. At the University of Buffalo, the Health science IRB approved HSIRB project no.: SPM0900107A on September 18, 2007. For the Denver review, the COMIRB number was 08–0982 and the Wright Center for Graduate Medical Education Institutional Review Board approved Protocol No: HHSN275200403394 on March 6, 2013. Patient safety was optimized by a Data Safety and Monitoring Board (DSMB) and the trial was registered with ClinicalTrials.gov, number NCT00467363.
Randomization to Low-Dose Aspirin
Women were randomized 1:1 to take daily aspirin of 81 mg with folic acid of 400 μg or placebo with folic acid of 400 μg. Participants began taking LDA or placebo on days 2 to 4 of the first menstrual cycle of follow-up, and continued for up to six menstrual cycles of attempting pregnancy and, if they became pregnant, through 36 weeks’ gestation.
Mode of Delivery
The primary outcome for this analysis was mode of delivery. Of 1,228 women enrolled, 597 delivered a live-born infant and were evaluated for mode of delivery. Mode of delivery was collected as part of the study design using medical record abstraction. Patients were categorized as having a cesarean versus vaginal delivery. Differences in cesarean versus vaginal delivery were further evaluated by parity and history of prior cesarean delivery (Fig. 1).
Fig. 1.
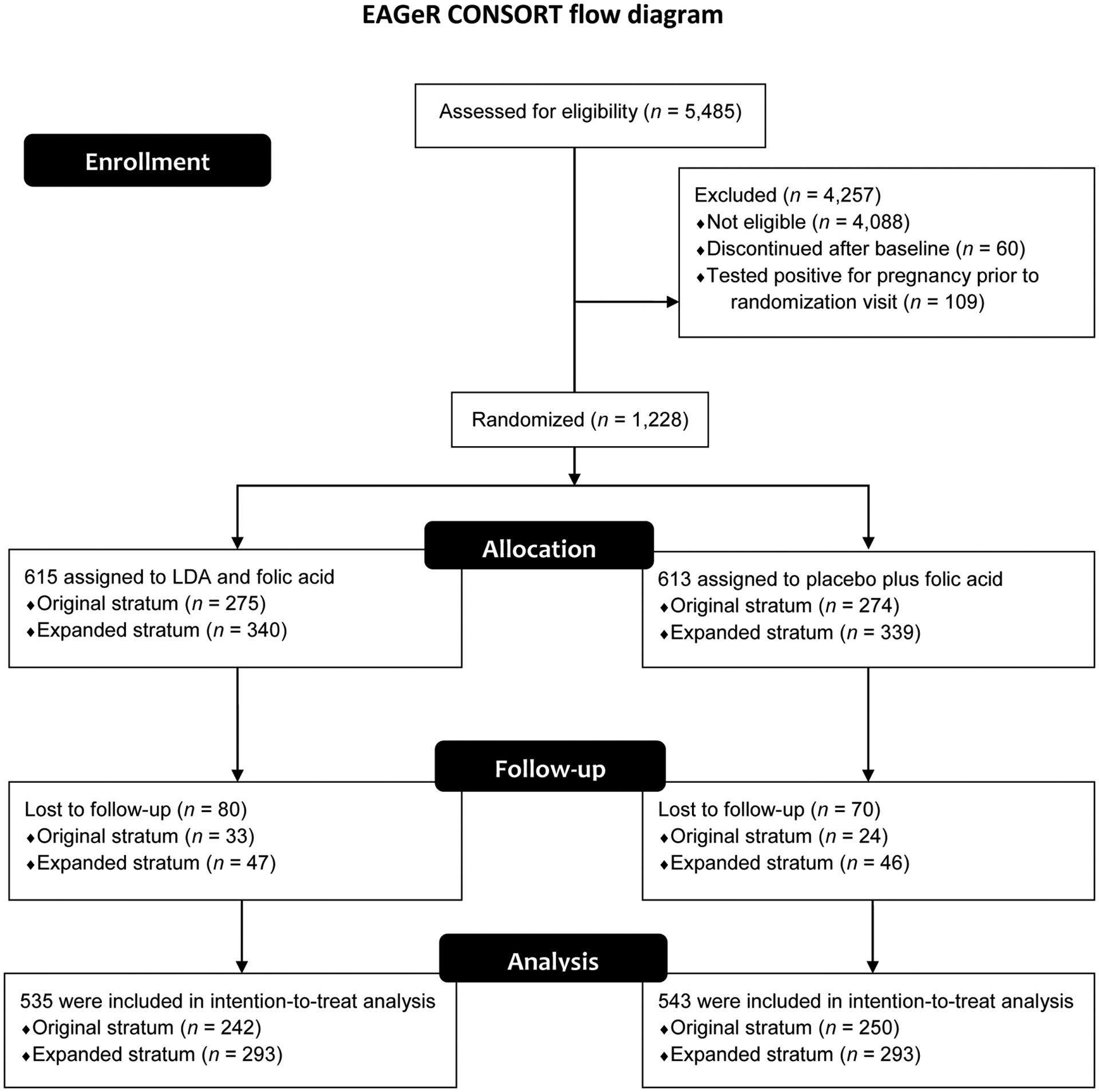
CONSORT flow diagram. EAGeR, effects of aspirin in gestation and reproduction; LDA, low-dose aspirin.
Other Covariates
Participant characteristics were collected at enrollment into the study, including age, prepregnancy body mass index (BMI), race/ethnicity (white versus non-White), education (<high school, high school, and >high school), household income (<$40,000, 40,000 to <100,000, and ≥100,000), smoking in the past year (yes vs. no), number of previous pregnancies (1–4), number of previous losses (1–2), number of previous live births (0–2), and number of previous vaginal deliveries and cesarean deliveries. Complications in the current pregnancy were assessed through medical record abstraction and include preterm delivery, premature rupture of membranes, gestational diabetes, and hypertensive disorders of pregnancy.
Statistical Analyses
Descriptive statistics were summarized as counts and percentages or means and standard deviations. Differences in descriptive statistics across LDA and placebo groups were calculated using Fisher’s exact test for categorical variables and t-tests for continuous variables. Participants missing information on mode of delivery (n = 19; 8 in LDA group and 11 in placebo group) were excluded from analysis. Log-binomial regression was used to estimate the relative risk (RR) and 95% confidence intervals (CIs) for delivery outcomes among those assigned to LDA versus placebo. We first used an intent-to-treat approach, evaluating the association of LDA with mode of delivery among all 1,088 participants with complete follow-up data, therefore excluding the 19 patients with missing information. We then evaluated the relationship of LDA with mode of delivery among the 597 participants who had a live birth, addressing the potential for selection bias by excluding participants who became pregnant or who experienced a loss by weighting models for the inverse probability of having a live birth given the following predictors: treatment assignment, number of prior live births, number of prior losses, number of prior cesarean sections, age, BMI, race/ethnicity and cigarette smoking prior to pregnancy. In both approaches, we evaluated the effect of LDA treatment with cesarean versus vaginal delivery, and by history of cesarean delivery and parity through inclusion of interaction terms.
We additionally conducted a sensitivity analysis to determine whether missing information on mode of delivery may have biased our findings. Participants with missing data were randomly assigned to (1) spontaneous or induced, meaning requiring an induction of labor, vaginal delivery versus cesarean; or (2) SVD versus induced vaginal delivery or cesarean, with the proportion assigned to each group ranging from 0 to 1.0 in intervals of 0.2. To evaluate the potential for differential bias by assignment to LDA versus placebo, all potential combinations of proportions across groups were evaluated. All analyses were conducted in SAS 9.4 (Cary, NC).
Results
Of the 1,228 participants who enrolled in the study, 597 had a live birth (309 assigned to LDA and 288 to placebo). Mean age was 28.7 (standard deviation [SD] = 4.8) years and mean prepregnancy BMI = 26.3 (SD = 6.5) kg/m2 (Table 1). A total of 417 (34.0%) patients had one pregnancy, 447 (36.4%) two pregnancies and 364 (29.6%) three or more pregnancies prior to enrollment. Most patients only had one prior loss (76.2%) and about half had no prior live birth (47.0%). Of those with prior live births, about half had a single vaginal delivery (48.7%) and one quarter (24.1%) had two prior vaginal deliveries. Only approximately 27% of this population had a one or two prior cesarean deliveries. Mean age, prepregnancy BMI, and number of prior pregnancies did not differ between the LDA and placebo groups. Rates of pregnancy complications overall were low, with approximately 10% developing any hypertension in pregnancy, 9% delivering preterm, and 4% developing premature rupture of membrane (PROM) or gestational diabetes (Table 2).
Table 1.
Demographics of participants in the EAGeR Cohort (n = 1228)
| Total (n = 1,228) n (%) |
LDA (n = 615) n (%) |
Placebo (n = 613) n (%) |
p-Value | |
|---|---|---|---|---|
| Age (y) Mean, (SD) |
28.7 (4.8) | 28.8 (4.9) | 28.7 (4.7) | 0.68 |
| Prepregnancy BMI (kg/m2) Mean (SD) |
26.3 (6.5) | 26.2 (6.6) | 26.4 (6.4) | 0.45 |
| Race/ethnicity | 0.13 | |||
| White | 1,162 (94.6) | 576 (93.7) | 586 (95.6) | |
| Non-White | 66 (5.4) | 39 (6.3) | 27 (4.4) | |
| Education | 0.89 | |||
| < high school | 25 (2.0) | 13 (2.1) | 12 (2.0) | |
| High school | 145 (11.8) | 75 (12.2) | 70 (11.4) | |
| ≥ high school | 1,057 (86.2) | 526 (85.7) | 531 (86.6) | |
| Household income ($) | 0.44 | |||
| < 40,000 | 208 (33.9) | 198 (32.3) | 406 (33.1) | |
| 40,000– < 100,000 | 155 (25.3) | 175 (28.5) | 330 (26.9) | |
| ≥ 100,000 | 250 (40.8) | 241 (39.3) | 491 (40.0) | |
| Smoking in the past year | 0.22 | |||
| Yes | 150 (12.3) | 79 (13.0) | 71 (11.7) | |
| No | 529 (87.7) | 529 (87.0) | 538 (88.3) | |
| Number of previous pregnancies | 0.84 | |||
| 1 | 417 (34.0) | 206 (33.5) | 211 (34.4) | |
| 2 | 447 (36.4) | 230 (37.4) | 217 (35.4) | |
| 3 | 272 (22.2) | 136 (22.1) | 136 (22.2) | |
| 4 | 92 (7.5) | 43 (7.0) | 49 (8.0) | |
| Number of previous losses | 0.28 | |||
| 1 | 825 (76.2) | 422 (68.6) | 403 (65.7) | |
| 2 | 403 (32.8) | 193 (31.4) | 210 (34.3) | |
| Number of previous live births | 0.88 | |||
| 0 | 577 (47.0) | 287 (46.7) | 290 (47.3) | |
| 1 | 438 (35.7) | 218 (35.5) | 220 (35.9) | |
| 2 | 213 (17.4) | 110 (17.9) | 103 (16.8) | |
| Number of previous vaginal deliveries | 0.74 | |||
| 0 | 754 (61.4) | 371 (60.3) | 383 (62.5) | |
| 1 | 317 (25.8) | 163 (26.5) | 154 (25.1) | |
| 2 | 157 (12.8) | 81 (13.2) | 76 (12.4) | |
| Number of previous cesarean deliveries | 0.96 | |||
| 0 | 1,053 (85.8) | 529 (86.0) | 524 (85.5) | |
| 1 | 135 (11.0) | 66 (10.7) | 69 (11.3) | |
| 2 | 40 (3.3) | 20 (3.3) | 20 (3.3) |
Abbreviations: BMI, body mass index; EAGeR, effects of aspirin in gestation and reproduction; LDA, low-dose aspirin; SD, standard deviation.
Table 2.
Pregnancy outcomes of EAGeR Cohort (n = 578 excluding 19 with missing data on mode of delivery)
| Total (n = 578) n (%) |
LDA (n = 301) n (%) |
Placebo (n = 277) n (%) |
p-Value | |
|---|---|---|---|---|
| Preterm delivery (<37 weeks of gestation) | 52 (9.0) | 22 (7.3) | 30 (10.8) | 0.14 |
| PROM | 25 (4.3) | 14 (4.7) | 11 (4.0) | 0.69 |
| Gestational diabetes | 21 (3.6) | 10 (3.3) | 11 (4.0) | 0.68 |
| Hypertensive disorder of pregnancy | 60 (10.4) | 31 (10.3) | 29 (10.5) | 0.96 |
Abbreviations: EAGeR, effects of aspirin in gestation and reproduction; LDA, low-dose aspirin; PROM, premature rupture of membrane.
Of participants with complete follow-up (n = 1069), 422 (39.5%) had a vaginal delivery, with 30 having an operative vaginal delivery, including 13 Forceps deliveries, 16 vacuum deliveries, and 1 delivery that included both interventions. A total of 156 (14.6%) had a cesarean delivery, with 67 patients having an elective repeat low transverse cesarean delivery. Of the 25 patients who attempted trial of labor after cesarean section (TOLAC) during this trial, 13 (52%) had a successful vaginal birth after cesarean (VBAC; Table 3).
Table 3.
Pregnancy outcomes of EAGeR cohort (n = 578 excluding 19 with missing data on mode of delivery)
| Total (n = 578) n (%) |
LDA(n = 301) n (%) |
Placebo (n = 277) n (%) |
p-Value | |
|---|---|---|---|---|
| Mode of delivery | 0.88 | |||
| Spontaneous vaginal delivery | 265 (45.9) | 135 (44.9) | 130 (46.9) | |
| Induced vaginal delivery | 157 (27.2) | 83 (27.6) | 74 (26.7) | |
| Cesarean | 156 (27.0) | 83 (27.6) | 73 (26.4) | |
| Mode of delivery by prior cesarean | ||||
| No prior cesarean delivery | 0.75 | |||
| Spontaneous vaginal delivery | 255 (52.5) | 130 (51.2) | 125 (53.9) | |
| Induced vaginal delivery | 154 (31.7) | 81 (31.9) | 73 (31.5) | |
| Cesarean | 77 (15.8) | 43 (16.9) | 34 (14.7) | |
| Prior cesarean delivery | 0.86 | |||
| Spontaneous vaginal delivery | 10 (10.9) | 5 (10.6) | 5 (11.1) | |
| Induced vaginal delivery | 3 (3.3) | 2 (4.3) | 1 (2.2) | |
| Cesarean | 79 (85.9) | 40 (85.1) | 39 (86.7) |
Abbreviations: EAGeR, effects of aspirin in gestation and reproduction; LDA, low-dose aspirin.
The mode of delivery was not affected by treatment assignment. In the intent-to-treat analysis, a total of 83 (15.7%) women in the LDA group and 73 (13.5%) women in the placebo group progressed to a cesarean delivery (RR = 1.02, 95% CI: 0.98–1.07, p = 0.31; Table 4). Risk of cesarean delivery was similar for women with (RR = 1.03, 95% CI: 0.88–1.20) and without (RR = 1.02, 95% CI: 0.99–1.06) a prior cesarean delivery, as well as for nulliparous (RR = 1.06, 95% CI: 1.00–1.12, p = 0.07), and multiparous (RR = 0.99, 95% CI: 0.94–1.06) women.
Table 4.
LDA and mode of delivery: intent-to-treat analysis, participants with complete follow-up (n = 1,069)
| Percent with cesarean by treatment group | ||||
|---|---|---|---|---|
| LDA % (n/N) |
Placebo % (n/N) |
RR (95% CI) | p-Value | |
| Cesarean versus vaginal birth | 15.7 (83/529) | 13.5 (73/540) | 1.02 (0.98, 1.07) | 0.31 |
| Cesarean versus vaginal birth by prior cesarean | ||||
| Prior cesarean | 50.0 (40/80) | 47.0 (39/83) | 1.03 (0.88, 1.20) | 0.70 |
| No prior cesarean | 9.6 (43/449) | 7.4 (34/457) | 1.02 (0.99, 1.06) | 0.25 |
| Cesarean versus vaginal birth by parity | ||||
| Nulliparous | 15.1 (36/239) | 9.6 (24/249) | 1.06 (1.00, 1.12) | 0.07 |
| Parous | 16.2 (47/290) | 16.8 (49/291) | 0.99 (0.94, 1.06) | 0.84 |
Abbreviations: CI, confidence interval; LDA, low-dose aspirin; n, number with cesarean delivery; N, total number of live births; RR, relative risk.
In the weighted analysis restricted to participants who had a live birth (n = 578), findings were similar to the intent-to-treat analysis (Table 5). For example, LDA was associated with a 12% higher risk (95% CI: 0.87–1.44) of cesarean delivery overall, but confidence intervals were wide. Further, for patients who underwent prior cesarean, LDA remained unassociated with risk of repeat cesarean (RR = 0.98, 95% CI: 0.84–1.15).
Table 5.
LDA and mode of delivery among those achieving a live birth, weighted analysis (n = 578): weights include treatment assignment, number of prior live births, number of prior cesareans, age, race/ethnicity, BMI, number of prior losses and smoking
| Percent with cesarean by treatment group | ||||
|---|---|---|---|---|
| LDA (n = 301) % (n/N) |
Placebo (n = 277) % (n/N) |
RR (95% CI) | p-Value | |
| Cesarean versus vaginal birth | 27.6 (83/301) | 26.4 (73/277) | 1.12 (0.87, 1.44) | 0.37 |
| Cesarean versus vaginal birth by prior cesarean | ||||
| Prior cesarean | 85.1 (40/47) | 86.7 (39/45) | 0.98 (0.84, 1.15) | 0.83 |
| No prior cesarean | 16.9 (43/254) | 14.7 (34/232) | 1.25 (0.86, 1.83) | 0.24 |
| Cesarean versus vaginal birth by parity | ||||
| Nulliparous | 28.4 (36/127) | 22.2 (24/108) | 1.28 (0.81, 2.00) | 0.29 |
| Parous | 27.0 (47/174) | 29.0 (49/169) | 0.93 (0.66, 1.31) | 0.68 |
Abbreviations: BMI, body mass index; CI, confidence interval; LDA, low-dose aspirin; n, number with cesarean delivery; N, total number of live births; RR, relative risk.
In a sensitivity analysis evaluating whether missing data on mode of delivery (n = 19) may have biased findings, we observed that even the most extreme cases of misclassification did not lead to a meaningful difference in findings (Supplementary Table S1, available in the online version). For example, when all participants in the LDA group with missing outcome were assigned to cesarean delivery and all participants in the placebo group with missing outcome were assigned to vaginal delivery, the relative risk of cesarean delivery increased to 1.16 (95% CI: 0.89–1.51). Conversely, when all participants with missing outcome in the LDA group were assigned to vaginal delivery and all in the placebo group to cesarean delivery, the relative risk of cesarean delivery decreased to 0.92 (95% CI: 0.71–1.19).
Comment
Principal Findings
Daily LDA taken from preconception through 36 weeks of gestation did not affect the mode of delivery. Findings were similar about those with and without prior cesarean delivery, as well as by parity, and findings were robust to missing outcome. Finding suggest that LDA does not increase risk of cesarean delivery among women with low-to-moderate risk pregnancies, which is particularly important given the increasing number of indications for prescription of LDA during the prepregnancy and antepartum period.
Given the lack of association between LDA and mode of delivery, our findings further support that LDA is likely a safe medication in the prepregnancy and antepartum period. It is important to evaluate the risks and benefits associated with this now widely used medication, to better make clinical decisions for patients and counseling patients on risks.
Results
While there are some reports that the use of aspirin in pregnancy can increase the risk of placental abruptions and bleeding, there are other reports that do not support this outcome.16 which would theoretically increase the risk of cesarean deliveries; however, the differences in rates were not found to be statistically significant (RR = 1.17, 95% CI: 0.93–1.48, I2 = 36.4%), therefore this likely did not contribute to the lack of differences in cesarean deliveries12(RR = 1.17, 95% CI: 0.93–1.48, I2 = 36.4%; RR = 1.17, 95% CI: 0.93–1.48, I2 = 36.4%).
However, what is known, is that women who develop preeclampsia with severe features are more likely to undergo a cesarean delivery overall.17 For example, one study reported cesarean rates of 30% with chronic hypertension alone, 40% with superimposed preeclampsia without severe features, and 56% with superimposed preeclampsia with severe features.17 If LDA is utilized to help keep high-risk women from developing preeclampsia, it is important to ensure that there are no additional risks associated with delivery method.18 This study suggests that the risk of cesarean delivery is not affected by the addition of LDA among a healthy population of women with one to two prior pregnancy losses.
Research Implications
While our study population had a history of one to two prior losses, we excluded participants who self-reported current treatment for a chronic health condition. Given that women with chronic health conditions have a higher risk of cesarean delivery, the association of aspirin with mode of delivery among women with preexisting conditions is an important point for further research.3
Strengths and Limitations
Our study was unique in randomizing participants to LDA during preconception and continuing throughout pregnancy, allowing for an examination of the effect of LDA on mode of delivery among a healthy cohort of women with exposure to aspirin throughout pregnancy. While we relied on medical record abstraction for defining mode of delivery, there are multiple studies that demonstrate the strong validity of medical record abstraction for assessing birth outcomes, especially mode of delivery.19,20 Further, baseline risk factors for cesarean delivery include advanced maternal age, elevated prepregnancy BMI, nulliparity, gestational diabetes, induction of labor from low Bishop’s score, tobacco use, and intrauterine growth restriction.1,2 All of these were accounted for by randomization to the treatment and placebo groups in EAGeR.13 Limitations of this study include that the majority of patients were Caucasian with higher incomes and education, limiting generalizability to other populations with different patterns of risk factors. Further, risk factors for cesarean delivery are more common in non-White populations.6 Finally, to examine the concern for increased risk of abruption was difficult to do in this study, as it is a rare outcome and this study was not powered to examine a rare outcome.
Conclusion
Overall, we found that daily LDA taken from preconception through 36 weeks was not associated with mode of delivery; this did not change in preterm versus term deliveries. There was no effect on success of TOLAC or differences by parity. However, future studies should evaluate whether maternal morbidity and mortality are decreased with the use of LDA in patients with elevated risk for abnormal placentation and placental insufficiency including preeclampsia,21–23 other hypertensive disorders of pregnancy, and assisted reproductive technology. This study is also not able to generalize to high-risk patients including those with risk of preeclampsia or gestational diabetes. In all, our findings suggest a lack of association between LDA and mode of delivery in preconception and pregnancy and thus may provide reassurance to women who may benefit from the medication and the physicians who prescribe them.
Supplementary Material
Key Points.
Aspirin was not associated with risk of cesarean section.
Aspirin was not associated with mode of delivery.
No increased risk of bleeding with use of aspirin.
Acknowledgments
The authors thank all the EAGeR participants for their commitment to the study, the EAGeR investigators and staff, and the members of the data safety monitoring board.
Funding
This research was supported by the International Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (National Institutes of Health, Bethesda, MD, USA; contract numbers: HHSN267200603423, HHSN267200603424, and HHSN267200603426). J.G.R have been funded by the NIH Medical Research Scholars Program, a public-private partnership jointly supported by the NIH and generous contributions to the Foundation for the NIH by the Doris Duke Charitable Foundation (Grant no.: 2014194), the American Association for Dental Research, the Colgate Palmolive Company, Genentech, Elsevier and other private donors. For a complete list, visit the foundation website at http://www.fnih.org.
Footnotes
Conflict of Interest
None declared.
The authors of this paper have no disclosures other than the advertised funding. The opinions expressed in this manuscript are those of the authors and do not represent the Department of Health and Human Services or the Department of Defense.
References
- 1.Spong CY, Berghella V, Wenstrom KD, Mercer BM, Saade GR. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver national institute of child health and human development, society for maternal-fetal medicine, and American college of obstetricians and gynecologists workshop. Obstet Gynecol 2012;120(05):1181–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 115: Vaginal birth after previous cesarean delivery. Obstet Gynecol 2010;116(2 Pt 1):450–463 [DOI] [PubMed] [Google Scholar]
- 3.Curtin SC, Gregory KD, Korst LM, Uddin SF. Maternal morbidity for vaginal and cesarean deliveries, according to previous cesarean history: new data from the birth certificate, 2013. Natl Vital Stat Rep 2015;64(04):1–13 [PubMed] [Google Scholar]
- 4.Dodd JM, Crowther CA, Huertas E, Guise JM, Horey D. Planned elective repeat caesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev 2004;(04):CD004224. [DOI] [PubMed] [Google Scholar]
- 5.Guise J-M, Eden K, Emeis C, et al. Vaginal birth after cesarean: new insights. Evid Rep Technol Assess (Full Rep) 2010;191(191):1–397 [PMC free article] [PubMed] [Google Scholar]
- 6.Edmonds JK, Yehezkel R, Liao X, Moore Simas TA. Racial and ethnic differences in primary, unscheduled cesarean deliveries among low-risk primiparous women at an academic medical center: a retrospective cohort study. BMC Pregnancy Childbirth 2013;13 (01):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson JT, O’Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia. Ann Intern Med 2014;161(08):613–614 [DOI] [PubMed] [Google Scholar]
- 8.Hauth JC, Goldenberg RL, Parker CR Jr, et al. Low-dose aspirin therapy to prevent preeclampsia. Am J Obstet Gynecol 1993;168 (04):1083–1091, discussion 1091–1093 [DOI] [PubMed] [Google Scholar]
- 9.Rotchell YE, Cruickshank JK, Gay MP, et al. Barbados low dose aspirin study in pregnancy (BLASP): a randomised trial for the prevention of pre-eclampsia and its complications. Br J Obstet Gynaecol 1998;105(03):286–292 [DOI] [PubMed] [Google Scholar]
- 10.Sibai BM, Caritis SN, Thom E The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units, et al; Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. N Engl J Med 1993;329 (17):1213–1218 [DOI] [PubMed] [Google Scholar]
- 11.Subtil D, Goeusse P, Puech F; Essai Régional Aspirine Mère-Enfant (ERASME) Collaborative Group, et al. Aspirin (100 mg) used for prevention of pre-eclampsia in nulliparous women: the Essai Régional Aspirine Mère-Enfant study (Part 1). BJOG 2003;110 (05):475–484 [DOI] [PubMed] [Google Scholar]
- 12.LeFevre ML U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;161(11):819–826 [DOI] [PubMed] [Google Scholar]
- 13.Schisterman EF, Silver RM, Lesher LL, et al. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014;384(9937):29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schisterman EF, Silver RM, Perkins NJ, et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatr Perinat Epidemiol 2013;27(06):598–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mumford SL, Silver RM, Sjaarda LA, et al. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum Reprod 2016;31(03): 657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberge S, Bujold E, Nicolaides KH. Meta-analysis on the effect of aspirin use for prevention of preeclampsia on placental abruption and antepartum hemorrhage. Am J Obstet Gynecol 2018;218(05): 483–489 [DOI] [PubMed] [Google Scholar]
- 17.Moussa HN, Leon MG, Marti A, et al. Pregnancy outcomes in women with preeclampsia superimposed on chronic hypertension with and without severe features. Am J Perinatol 2017;34 (04):403–408 [DOI] [PubMed] [Google Scholar]
- 18.Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn 2014;34(07):642–648 [DOI] [PubMed] [Google Scholar]
- 19.Mi MY, Collins JE, Lerner V, Losina E, Katz JN. Reliability of medical record abstraction by non-physicians for orthopedic research. BMC Musculoskelet Disord 2013;14(01):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meredith Nahm. Data accuracy in medical record abstraction. Available at: https://digitalcommons.library.tmc.edu/cgi/view-content.cgi?referer=https://www.google.com/&httpsre-dir=1&article=1018&context=uthshis_dissertations#:~:text=articles%20and%20pooled.-,Our%20analysis%20demon-strates%20that%20the%20accuracy%20associated%20with%20data%20processing,5019%20errors%20per%2010%2C000%20fields). Accessed September 21, 2020
- 21.Ahrens KA, Silver RM, Mumford SL, et al. Complications and safety of preconception low-dose aspirin among women with prior pregnancy losses. Obstet Gynecol 2016;127(04):689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies NJ, et al. Low-dose aspirin in the prevention of hypertensive disorders of pregnancy in relatively low-risk nulliparous women. Hypertens Pregnancy 1995;14(01):49–55 [Google Scholar]
- 23.Iriye BK, Gregory KD, Saade GR, Grobman WA, Brown HL. Quality measures in high-risk pregnancies: executive summary of a cooperative workshop of the Society for Maternal-Fetal Medicine, National Institute of Child Health and Human Development, and the American College of Obstetricians and Gynecologists. Am J Obstet Gynecol 2017;217(04):B2–B25 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


