Abstract
Background:
Gastrointestinal bleeding is the most common clinical manifestation of gastrointestinal stromal tumor. It is of great significance to the prognosis of patients. But the results are controversial. The purpose of this study was to evaluate the relationship between gastrointestinal bleeding and clinical prognosis in patients with GIST.
Methods:
A systematic literature search was performed in Pumbed, Cochrane Library, EMBASE, ClinicalTrials.gov, CNKI, VIP and wanfang databases with the pattern of unlimited languages. 12 studies with 2781 individuals were included in the final analysis. The overall survival (OS), recurrence-free survival/disease-free survival (RFS/DFS) and related factors affecting bleeding in patients with gastrointestinal stromal tumor (GIST) were extracted. Hazard ratio (HR) and 95% confidence interval (CI) were used for in the meta-analysis.
Results:
A total of 12 articles were included in the study, including 2781 patients with GIST, including 845 patients with gastrointestinal bleeding. The OS of GIST patients with gastrointestinal bleeding was significantly worse (HR = 2.54, 95% CI = 1.13-5.73, P = 0.025). But there was no significant difference in RFS between gastrointestinal bleeding patients and non-bleeding patients (HR = 1.35, 95% CI = 0.70-2.61, P = 0.371). Further analysis of the related factors of GI bleeding in GIST patients was observed, besides the aging factor (HR = 1.02, 95% CI = 0.69-1.50, P = 0.929), Small intestinal stromal tumor (HR = 0.56, 95% CI = 0.41-0.76, P < 0.001), tumor diameter ≥ 5 cm (HR = 2.09, 95% CI = 1.20-3.63, P = 0.009), Mitotic index ≥ 5/50 HPF (HR = 1.66, 95% CI = 1.11-2.49, P = 0.014) and tumor rupture (HR = 2.04, 95% CI = 1.0-3.82, P = 0.026) all increased the risk of GI bleeding in patients with GIST.
Conclusions:
The OS of GIST patients with GI bleeding was worse than non-GI bleeding, but had no significant effect on RFS. Nevertheless the aging factor, the location of GIST in the small intestine, tumor diameter ≥ 5 cm, Mitotic index ≥ 5/50 HPF and tumor rupture all increased the risk of GI bleeding in patients with GIST.
Keywords: gastrointestinal stromal tumors, gastrointestinal bleeding, prognosis, meta-analysis, value
Introduction
Gastrointestinal stromal tumors (GIST) are the most common mesenchymal tumors in the digestive tract, 1,2 accounting for about 1%-2% 3 of gastrointestinal tumors, the most common of which are in the stomach, small intestine and colorectum respectively. 4,5 About 69% of the patients with gastrointestinal stromal tumors may have clinical symptoms in the early stage. Gastrointestinal bleeding, as the most common clinical symptom, 2,6 is of great significance to the prognosis of GIST. Gastrointestinal stromal tumor has the possibility of malignancy, and the prognosis is affected by many factors. The modified National Institutes of Health risk classification scheme (NIH) included tumor location, tumor size, mitotic count, and tumor rupture in the high risk classification of recurrence. 2 However, the effect of gastrointestinal bleeding on the prognosis of GIST is not clear.
Currently, it has been found that the recurrence free survival of GIST patients with gastrointestinal bleeding is significantly shorter than that of non-gastrointestinal bleeding patients, 7 which is contrary to the conclusion of Wan et al. 7 -9 Therefore, the effect of gastrointestinal bleeding on the prognosis of GIST remains to be explored. The purpose of this study was to evaluate the relationship between gastrointestinal bleeding and clinical prognosis in patients with GIST.
Materials and Methods
Search Strategy
This study was performed according to the PRISMA guideline. 10 I have registered my study with INPLASY and the registration number is INPLASY202160027. The relevant literatures before August 2019 were searched in Pubmed, Cochrane Library, EMBASE, ClinicalTrials.gov, CNKI, VIP and wanfang databases with the pattern of unlimited languages. According to the PICOS principle, the retrieval words including: Gastrointestinal stromal tumors or GIST, bleeding, following the Cochrane Handbook. The search terms were subject words and free words. According to the inclusion criteria and exclusion criteria, we filtered the retrieved literatures and enlarged search for included literature.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) literature published in China and abroad; (2) the object of study is gastrointestinal stromal tumors; (3) bleeding on the prognosis of patients; (4) the statistics record of at least one of the following indicators: prognostic indicators of OS, DFS or RFS; (5) the risk ratio HR and 95% confidence interval or survival curve were reported to extract data. (6) If the articles of the same author were selected, Selection of highly rated literature according to the Newcastle-Ottawa scale. Exclusion criteria: (1) if the objective of the study was non-gastrointestinal stromal tumor; (2) Non-comparative study; (3) no observation index was reported; (4) complete clinical data was not provided, contact first author and no response was received; (5) repeated literature.
Data Extraction and Quality Assessment
According to the inclusion criteria and exclusion criteria, the retrieved literature is read independently by 2 authors, when there are different views, the third author participated in the discussion and finally determines the inclusion of the literature. The 2 authors extracted the data separately and checked the consistency of extracted data. Extracted data: title, author, years of publication, years of study, ages of patients, sample size, OS, RFS or DFS, related indicators affecting bleeding, HR and 95% CI of related observation indicators. According to NCI Dictionaries (https://www.cancer.gov/publications/dictionaries/cancer-terms/def/relapse-free-survival), RFS or DFS had the same Statistical significance and combined analysis. If the HR and 95% CI of observation indicators were not directly given in the literature, Engauge Digitizer 4.1 is used for data extraction. 11,12 The quality of the included literature was evaluated according to the Newcastle-Ottawa Scale (NOS) Literature quality Assessment scale. 13 The evaluation items included selection, exposure and comparability, with a full score of 9 * and ≥6 * as high-quality literature.
Statistical Analysis
The authors extracted HR and 95% CI from each study to evaluate the prognostic role of gastrointestinal bleeding in patients with GIST. Q test was used to analyze the heterogeneity in the study. When I2 ≥ 50% and P ≤ 0.05, the random effect model was used to analyze the heterogeneity, and the fixed effect model was used to analyze the heterogeneity when I2 < 50%. Begg’s test and Egger’s test were used to evaluate publication bias. Sensitivity analysis was performed to assess the stability of the results. All statistical analysis was carried out with STATA 15.0 (StataCorp, College Station, TX, USA), When the P ≤ 0.05 the results were deemed statistically significant.
Result
The Characteristics of Study
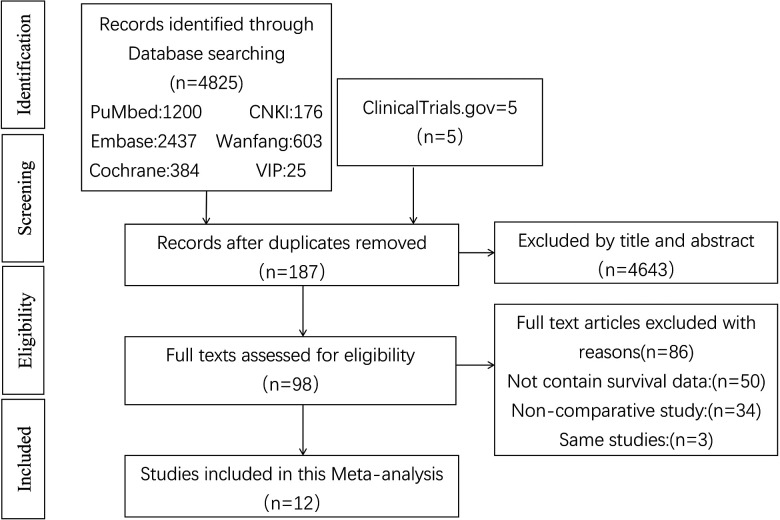
A total of 4825 related literatures and 5 clinical trials (ClinicalTrials.gov) were searched in the database. After reading the title and abstract, 4643 articles were excluded. Then 89 duplicated articles were deleted, and 86 articles were excluded according to the inclusion criteria and exclusion criteria. In the end, a total of 12 articles 7 -9,14 -22 were included in the study, including 2781 patients with GIST, including 845 patients with gastrointestinal bleeding. Among the articles included, 8 articles reported that RFS/DFS, 8 articles reported that OS; 11 research was carried out in China and 1 study was carried out in South Korea. The quality of the studies assessed by the Newcastle-Ottawa Quality Assessment Scale ranged from 6 to 8, with scores of 6 in 6 articles, 7 in 1 article, and 8 in 2 articles. The screening process is shown in Figure 1, and the general characteristics of the literature and the NOS quality score are shown in Table 1.
Figure 1.
Flow chart showing the process for selecting the included studies.
Table 1.
The Scores and Basic Characteristics of the Included Studies.
| Study | Country | Age (Years) | Included period | Study type | No. of sample | Male/female | No. of bleeding | No. of negative | Follow-up (Months) | Survival | Hazard ratios | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang | China | 60.26 ± 11.01 | 2016-2017 | RS | 140 | 72/68 | 47 | 93 | 4-40 | RFS, OS | Estimated | 6 |
| Wan | China | 54.9 ± 11.4 | 2009-2017 | RS | 333 | 176/157 | 164 | 169 | 4-100 | RFS, OS | Reported in text | 8 |
| Pih | Korea | 60 (51-67) | 1998-2015 | RS | 697 | 350/347 | 46 | 651 | 67 (34-105) | OS | Reported in text | 8 |
| Huang | China | 54.9 ± 11.4 | 2009-2017 | RS | 333 | 176/157 | 164 | 169 | 41 (4-100) | RFS | Reported in text | 7 |
| Liu | China | 58.27 ± 10.54 | 2007-2016 | RS | 170 | 89/81 | 63 | 107 | 3-70 | DFS, OS | Reported in text | 6 |
| Yin | China | 54.5 (21-84) | 2005-2015 | RS | 526 | 274/252 | 163 | 363 | 4-128 | RFS | Reported in text | 7 |
| Li | China | 59 (23-82) | 2008-2014 | RS | 200 | 80/120 | 57 | 143 | 1-80 | OS | Estimated | 6 |
| Wang | China | 61.5 (23-78) | 2005-2011 | RS | 84 | 46/38 | 24 | 60 | 40.3(4-97) | RFS | Reported in text | 7 |
| Xiao | China | 51 (36-66) | 1986-2010 | RS | 21 | 15/6 | 7 | 14 | 65 (4-140) | DFS, OS | Estimated | 6 |
| Su | China | 58 (29-83) | 2004-2014 | RS | 118 | 67/51 | 30 | 88 | 36.3 (4-115) | OS | Estimated | 6 |
| Zhou | China | 78.27 ± 9.28 | 2011-2015 | RS | 118 | 47/71 | 34 | 84 | NA | RFS | Reported in text | 6 |
| Gao | China | 55 (22-78) | 2005-2010 | RS | 41 | 22/19 | 16 | 25 | 35 (5-87) | OS | Reported in text | 7 |
Abbreviations: NA, not reported; RCT, Randomized Controlled Trial; OS, overall survival; PFS, relapse-free survival; DFS, disease-free survival; NOS, Newcastle-Ottawa Scale.
Meta-Analysis Results
Overall survival
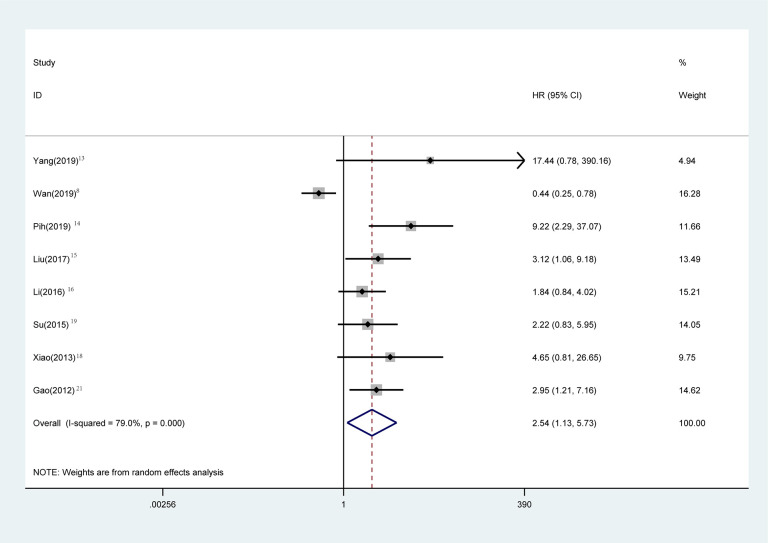
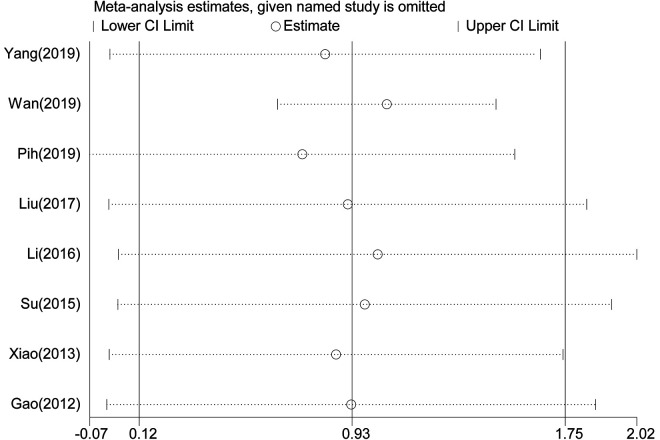
OS statistical analysis was performed in 1720 patients in 8 studies, 8,14 -17,19,20,22 and Meta-analysis showed that the OS of GIST patients with gastrointestinal bleeding was significantly worse (Figure 2) (HR = 2.54, 95% CI = 1.13-5.73, P = 0.025). The heterogeneity of each study was obvious (P < 0.001, I2: 79.0%). The random effect model was used for analysis. Sensitivity analysis was performed to see whether some studies affected the final results, and the results showed (Figure 3) that the deletion of any literature did not significantly affect the final results, indicating that our conclusions were reliable.
Figure 2.
Meta-analysis of the association between bleeding and OS in GIST patients. Results are presented as individual and pooled hazard ratios (HRs) with 95% confidence intervals (CIs).
Figure 3.
Sensitivity analysis on the relationship between bleeding and OS.
OS publication bias
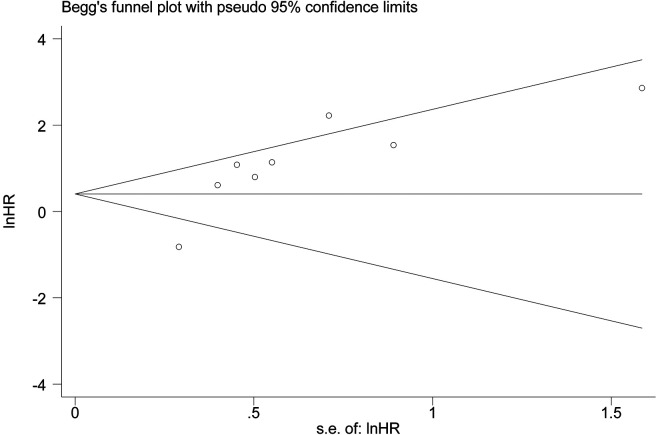
The Begg and Egger tests is used to evaluate publication bias in this Meta-analysis (Figure 4). The P values for OS were 0.108 (Begg’s test) and 0.048 (Egger’s test) hence no publication bias.
Figure 4.
Funnel plot for OS publication bias.
RFS/DFS
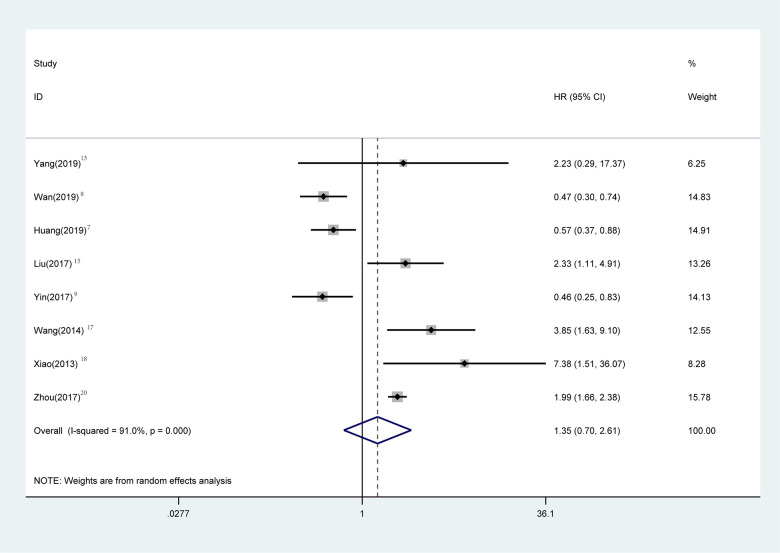
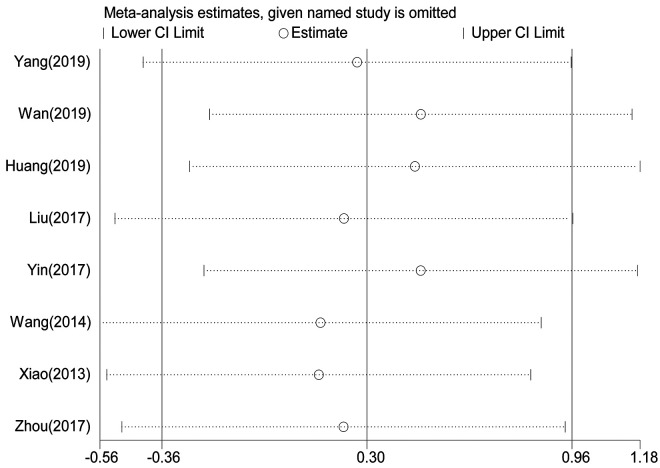
RFS statistical analysis was performed in 1725 patients in 8 studies. 7 -9,14,16,18,19,21 Meta-analysis showed that there was no significant difference in RFS between gastrointestinal bleeding patients and non-bleeding patients (Figure 5) (HR = 1.35, 95% CI = 0.70-2.61, P = 0.371). The heterogeneity of each study was obvious (P < 0.001, I2: 91%). The random effect model was used for Meta-analysis. Sensitivity analysis was performed to see whether some studies affected the final results, and the results showed (Figure 6) that the deletion of any literature did not significantly affect the final results, indicating that our conclusions were reliable.
Figure 5.
Meta-analysis of the association between bleeding and RFS in GIST patients. Results are presented as individual and pooled hazard ratios (HRs) with 95% confidence intervals (CIs).
Figure 6.
Sensitivity analysis on the relationship between bleeding and RFS.
RFS publication bias
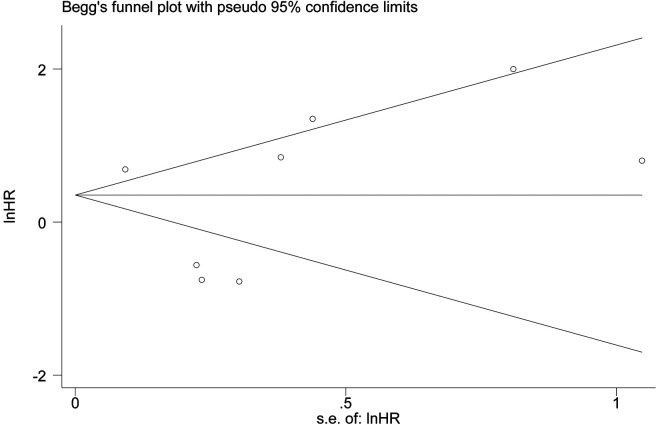
Begg and Egger tests were used to evaluate publication bias in this study (Figure 7). The P values for OS were 0.711 (Begg’s test) and 0.276 (Egger’s test) and there was no publication bias.
Figure 7.
Funnel plot for RFS publication bias.
Analysis of related factors affecting digestive tract hemorrhage
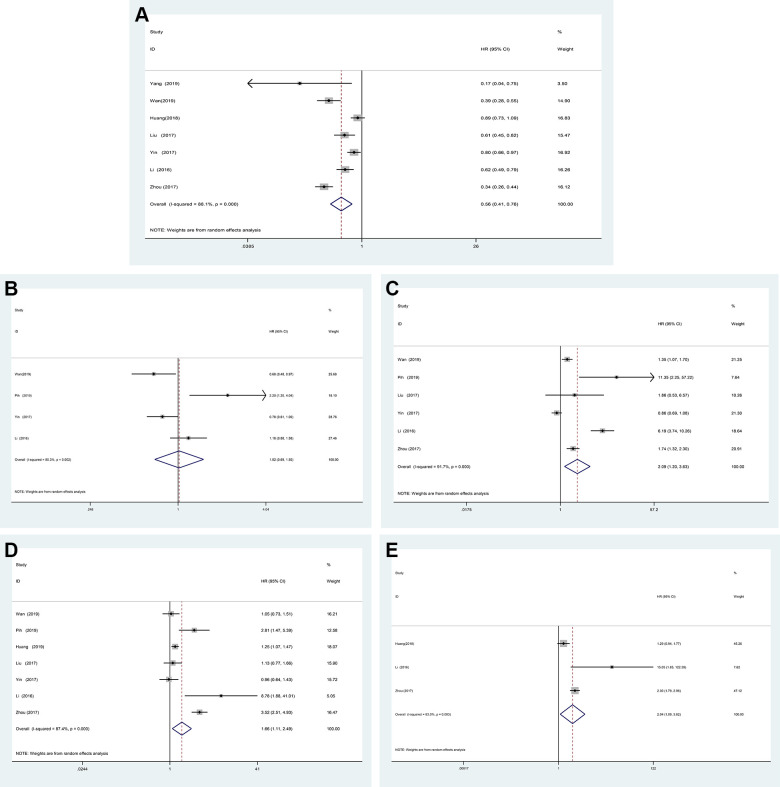
From the above analysis, it can be seen that gastrointestinal bleeding does not affect the RFS of GIST patients, but the OS is significantly worse, therefore the factors affecting gastrointestinal bleeding were to be further analyzed. A total of 7 studies were conducted to analyze the difference between gastrointestinal bleeding in 1820 patients in gastric stromal tumor and intestinal stromal tumor. The random effect model was used to analyze and Meta-analysis showed that the difference between gastric stromal tumor and intestinal stromal tumor. The risk of gastrointestinal bleeding in small intestinal stromal tumors was significantly higher than that in gastric stromal tumors (Figure 8A) (HR = 0.56, 95% CI = 0.41-0.76, P < 0.001). A total of 4 studies were conducted to analyze the aging difference between gastrointestinal bleeding in 1756 patients. 60 years old was taken as the interception value. The random effect model was used to analyze and Meta-analysis showed that the risk of gastrointestinal bleeding in patients with GIST was not affected by aging ≥60 years old (Figure 8B) (HR = 1.02, 95% CI = 0.69-1.50, P = 0.929). A total of 6 studies were conducted to analyze the tumor size difference between gastrointestinal bleeding in 1681 patients, using 5 cm as the interception value. The random effect model was used to analyze and Meta-analysis showed that the tumor size ≥ 5 cm increased the risk of gastrointestinal bleeding in patients with GIST (Figure 8C) (HR = 2.09, 95% CI = 1.20-3.63, P = 0.009). A total of 7 studies were conducted to analyze the mitotic index difference between gastrointestinal bleeding in 2377 patients. The mitotic index ≥ 5/50 high powered fields (5/50 HPF) was taken as the interception value. The random effect model was used to analyze and Meta-analysis showed that the mitotic index ≥ 5 HPF increased the risk of gastrointestinal bleeding in patients with GIST (Figure 8D) (HR = 1.66, 95% CI = 1.11-2.49, P = 0.014). A total of 3 studies were conducted to analyze tumor rupture difference between gastrointestinal bleeding in 651 patients. The random effect model was used to analyze and Meta-analysis showed that tumor rupture increased the risk of gastrointestinal bleeding in patients with GIST (Figure 8E) (HR = 2.04, 95% CI = 1.0-3.82, P = 0. 026).
Figure 8.
Forest plot of the association between bleeding and GIST site, aging, size mitotic and tumor rupture.
Discussion
The clinical manifestations of GIST are various, the size, and the location of the tumor can affect the clinical manifestations of the patients. 23 The most common clinical manifestations are gastrointestinal (GI) bleeding and abdominal discomfort. Patients with GI bleeding usually need emergency surgery, so it is of great significance to analyze the effect of GI bleeding on the prognosis of patients with GIST.
However, whether GI bleeding increases the risk of recurrence and affects the overall survival rate is still unclear. We performed the first systematic review and Meta-analysis to evaluate the relationship between GI bleeding and prognosis in patients with GIST. There are 3 included literatures reported that GI bleeding could reduce the risk of recurrence in patients, 7 -9 and the other 9 articles considered that GI bleeding could increase the risk of recurrence in patients. Through our meta-analysis, we found that there was no significant difference in the risk of recurrence between patients with GI bleeding and non-GI bleeding (HR = 1.35, P = 0.371, 95% CI = 0.70-2.61). However, the OS of GIST patients with the clinical manifestations of GI bleeding was significantly worse than that of non-bleeding group (HR = 2.54, P = 0.025, 95% CI = 1.13-5.73). Furthermore analysis of the related factors of GI bleeding in GIST patients showed that: be siding the aging factor the aging factor, Small intestinal stromal tumor, tumor diameter ≥ 5 cm, Mitotic index ≥ 5/50HPF and tumor rupture all increased the risk of GI bleeding in patients with GIST.
The effect of GI bleeding on RFS in patients with GIST is controversial. Yang et al 14 believe that GI bleeding can be used as an independent predictor of RFS and an important indicator of poor prognosis. However, Wan 8 collected the clinical data of their hospital and propensity score matching (PSM), it was found that the patients in the bleeding group had superior RFS. It may be that GIST patients with clinical symptoms of GI bleeding are more likely to be detected and diagnosed, and then received further treatment and obtain superior RFS. Our meta-analysis found that GI bleeding did not increase the risk of recurrence in patients with GIST. Patients with GI bleeding may have a larger tumor size and a greater likelihood of tumor rupture, and they are regarded as an important assessment of the NIH risk classification scheme, which may lead to a worse OS in patients with GI bleeding. It has even been suggested that GI bleeding be used to modify and perfect NIH risk classification scheme. 17,18
We further analyzed the causes of GI bleeding in patients with GIST. Taking the age of 60 years as the interception value, it was found that the aging factor did not increase the risk of GI bleeding in GIST patients. The location of the tumor is closely related to the clinical manifestations of the patients. Due to the narrow intestinal lumen or the thinner intestinal wall than the gastric wall, 24,25 GIST in the intestine is more likely to occur GI bleeding. When the tumor diameter ≥ 5 cm, the possibility of malignancy is greatly increased in GIST, and the smaller masses usually grow in the lumen, but when the tumor volume is larger than a certain extent, it will show the tendency of exogenous growth, 8,26 and it is more likely to develop ulcers or break through the intestinal cavity. In other studies, the microvessel density and vascular endothelial growth factor in tumors tumor diameter ≥ 5 cm GIST were higher than those tumor diameter < 5 cm. 27,28 All these factors may lead to GI bleeding in GIST patients with tumor diameter ≥ 5 cm.
Both NIH risk classification scheme and AFIP risk assessment scheme regard Mitotic ≥ 5/50 HPF as the key index of classification. 29 However, the literature between Mitotic and GI bleeding is still lacking. Through our meta-analysis, we found that Mitotic index ≥ 5/50 HPF can significantly increase the risk of GI bleeding in GIST. Some scholars also believe that the causes of GI bleeding in patients with GIST are caused by tumor rupture, 16 tumor invasion of mucosal or submucosal blood vessels, following tumor rupture, tumor dissemination, tumor recurrence and worse OS. It has been reported that 39.6% of GIST patients can find bleeding causing by mucosal ulcer 30 or rupture, resulting in a worse prognosis. Small intestinal tumor rupture is the most likely in all patients with GIST, 31,32 which may indicate that the prognosis of small intestinal GIST patients is worse. But we do not have enough data for meta-analysis in this study. Pih study confirms this result: tumor rupture is also regarded as an independent prognostic factor for RFS and OS. 31
However, there are still some shortcomings in our meta-analysis: (1) the literature included Asian population, which has certain regional restrictions, and the conclusion is more applicable to Asian population; (2) heterogeneity among studies was present in our Meta analysis, so the random effect model is chosen; (3) some literature reports do not directly give the HR and 95% CI of observation index; (4) all the studies included were retrospective studies, and there was a certain selection bias.
Conclusion
The OS of GIST patients with GI bleeding was worse than non-GI bleeding, but had no significant effect on RFS. Besides the aging factor, the location of GIST in the small intestine, tumor diameter ≥ 5 cm, Mitotic index ≥ 5/50 HPF and tumor rupture all increased the risk of GI bleeding in patients with GIST. In the future, we need further high-quality research design to analyze the impact of GI bleeding on the prognosis of GIST patients, and better guide the clinical work.
Footnotes
Authors’ Note: Xin Fan and He Han contributed equally to this work. This article did not require an ethical board approval because the study data were downloaded from the open database.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by The Foundation for Young Scientists of Affiliated Hospital of Jiangsu University (Grant number: JDFYRC2016002) and Jiangsu Provincial Medical Youth Talent (Grant number: QNRCQ2016839), and Zhenjiang Science and Technology Pillar Program (Grant number: SH2018082).
ORCID iD: He Han, PhD  https://orcid.org/0000-0002-0048-811X
https://orcid.org/0000-0002-0048-811X
References
- 1. Rammohan A, Sathyanesan J, Rajendran K, et al. A gist of gastrointestinal stromal tumors: a review. World J Gastrointest Oncol. 2013;5(6):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382(9896):973–983. [DOI] [PubMed] [Google Scholar]
- 3. Beltran MA, Cruces KS. Primary tumors of jejunum and ileum as a cause of intestinal obstruction: a case control study. Int J Surg (London, England). 2007;5(3):183–191. [DOI] [PubMed] [Google Scholar]
- 4. Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of Gastrointestinal Stromal Tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46. [DOI] [PubMed] [Google Scholar]
- 5. Mucciarini C, Rossi G, Bertolini F, et al. Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. BMC Cancer. 2007;7(1):230–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joensuu H, Martin-Broto J, Nishida T, Reichardt P, Schöffski P, Maki RG. Follow-up strategies for patients with gastrointestinal stromal tumour treated with or without adjuvant imatinib after surgery. Eur J Cancer. 2015;51(12):1611–1617. [DOI] [PubMed] [Google Scholar]
- 7. Huang Y, Rui Z, Cui Y, et al. Effect of gastrointestinal bleeding on gastrointestinal stromal tumor patients: a retrospective cohort study. Med Sci Monit. 2018;24:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wan W, Xiong Z, Zeng X, et al. The prognostic value of gastrointestinal bleeding in gastrointestinal stromal tumor: a propensity score matching analysis. Cancer Med. 2019;8(9):4149–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin Z, Gao J, Liu W, et al. Clinicopathological and prognostic analysis of primary gastrointestinal stromal tumor presenting with gastrointestinal bleeding: a 10-year retrospective study. J Gastrointest Surg. 2017;21(5):792–800. [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 11. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou ZR, Zhang TS, Bo LI, Mao Z, Zeng XT, Liu SX. Extracting and transforming of appropriate data of meta-analysis in survival curve. Chin J Evid Based Cardiovasc Med. 2014;6(3):243–247. [Google Scholar]
- 13. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 14. Yang Z, Wang F, Liu S, Guan W. Comparative clinical features and short-term outcomes of gastric and small intestinal gastrointestinal stromal tumours: a retrospective study. Sci Rep. 2019;9(1):10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pih GY, Jeon SJ, Ahn JY, et al. Clinical outcomes of upper gastrointestinal bleeding in patients with gastric gastrointestinal stromal tumor. Surg Endosc. 2019;(4):1–11. [DOI] [PubMed] [Google Scholar]
- 16. Liu Q, Li Y, Dong M, Kong F, Dong Q. Gastrointestinal bleeding is an independent risk factor for poor prognosis in GIST patients. Biomed Res Int. 2017;2017:7152406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li RT, Zhang GJ, Fu WH, Li WD. Prognostic analysis of gastrointestinal stromal tumors complicated with gastrointestinal bleeding. Zhonghua Zhong Liu Za Zhi. 2016;38(5):377. [DOI] [PubMed] [Google Scholar]
- 18. Wang H, Chen P, Liu XX, et al. Prognostic impact of gastrointestinal bleeding and expression of PTEN and Ki-67 on primary gastrointestinal stromal tumors. World J Surg Oncol. 2014;12(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao CC, Zhang S, Wang MH, et al. Clinicopathological features and prognostic factors of rectal gastrointestinal stromal tumors. J Gastrointest Surg. 2013;17(4):793–798. [DOI] [PubMed] [Google Scholar]
- 20. Dong-Liang SU, You-Wei K, Qiang WJCJoCAiGS. Effective predictors of the primary tumor location and gastrointestinal bleeding in the prognosis of gastrointestinal stromal tumor after surgery. Chin J Curr Adv Gen Surg. 2015;18(006):463–466. [Google Scholar]
- 21. Zhou HX, zhang DX, Mai LR. Survival status and influencing factors in elderly patients with gastrointestinal stromal tumor complicated with gastrointestinal bleeding. Chin J Gerontol. 2017;37(14):3517–3519. [Google Scholar]
- 22. Gao YP, Kou Y, Fu Q, Xie J. Analysis of prognostic factors in 41 patients of small intestine stromal tumors. Chin J Gen Surg. 2012;27(12):970–973. [Google Scholar]
- 23. Liu Q, Kong F, Zhou J, Dong M, Dong Q. Management of hemorrhage in gastrointestinal stromal tumors: a review. Cancer Manag Res. 2018;10:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nylund K, Hausken T, Odegaard S, Eide GE, Gilja OH. Gastrointestinal wall thickness measured with transabdominal ultrasonography and its relationship to demographic factors in healthy subjects. Ultraschall Med. 2012;33(07):E225–E232. [DOI] [PubMed] [Google Scholar]
- 25. Atkinson NS, Bryant RV, Dong Y, et al. How to perform gastrointestinal ultrasound: anatomy and normal findings. World J Gastroenterol. 2017;28(38):6931–6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang WM, Park HN, Min BH, Choi D, Kim KM, Kim S. Preoperative predictive factors for gastrointestinal stromal tumors: analysis of 375 surgically resected gastric subepithelial tumors. J Gastrointest Surg. 2015;19(4):631–638. [DOI] [PubMed] [Google Scholar]
- 27. Imamura M, Yamamoto H, Nakamura N, et al. Prognostic significance of angiogenesis in gastrointestinal stromal tumor. Mod Pathol. 2007;20(5):529–537. [DOI] [PubMed] [Google Scholar]
- 28. Yin YN. Prognosis Analysis of Clinical Features of Gastrointestinal Stromal Tumors and Study of Lymphangiogenesis and Angiogenesis. Central South University; 2012. [Google Scholar]
- 29. Sander B, Cameron S, Gunawan B, Füzesi L. Optimal thresholds of risk parameters for gastrointestinal stromal tumors. Eur J Surg Oncol. 2020;46(1):180–188. [DOI] [PubMed] [Google Scholar]
- 30. Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013;42(2):399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nishida T, Cho H, Hirota S, et al. Clinicopathological features and prognosis of primary GISTs with tumor rupture in the real world. Ann Surg Oncol. 2018;25(7):1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boye K, Berner JM, Hompland I, et al. Genotype and risk of tumour rupture in gastrointestinal stromal tumour. Br J Surg. 2018;105(2):e169–e175. [DOI] [PubMed] [Google Scholar]