Abstract
Background:
The development of immunotherapy has dramatically changed the treatment of non-small-cell lung cancer. The negative association of antibiotics on the clinical activity of immune checkpoint inhibitors in patients with NSCLC is well known.
Methods:
PubMed, Embase, and Medline databases were searched until January 11, 2020. We included retrospective studies of ICIs (e.g., PD-1, PD-L1, and CTLA-4). The clinical outcomes were progression-free survival (PFS) and overall survival (OS). Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, and subgroup and sensitivity analyses were performed.
Results:
Our results indicated that the use of antibiotics reduced the survival of NSCLC patients treated with ICIs. The pooled HRs of PFS and OS were HR = 1.41 (95% CI = 1.23-1.61; P < 0.001) and HR = 2.16 (95% CI = 1.79-2.60; P < 0.001). We divided the studies into 5 subgroups according to antibiotic exposure time. Subgroup analysis showed that the patients that were administered antibiotics [−60 days; 0 days] or [−30 days; 0 days] before the initiation of ICIs treatment had a poorer OS rate, whereas those patients that were administered antibiotics [0 days; 30 days] after the initiation of ICIs treatment had a poorer PFS rate. In summary, ATB treatment in patients [−60 days; +30 days] near the initiation of ICIs treatment significantly reduced the survival in NSCLC patients.
Conclusion:
Our results indicated that ATB use is negatively associated with survival in NSCLC patients treated with ICIs immunotherapy. Similar studies involving a larger sample of cases are still being published. This meta-analysis identified that the timing of ATB treatment in NSCLC patients receiving ICIs immunotherapy has different effects on the OS and PFS of these patients. ATB treatment prior to the initiation of ICIs treatment affects OS, whereas ATB treatment after the initiation of ICIs treatment affects PFS.
Keywords: antibiotic, immune-checkpoint inhibitors, overall survival, progression-free survival, non-small-cell lung cancer (NSCLC)
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer deaths. 1 Non-small-cell lung cancer (NSCLC) is the most frequent histological subtype. Approximately 70% of patients with NSCLC are already in the advanced stage when diagnosed. Consequently, surgery is not a treatment option for these patients. Instead, radiotherapy and chemotherapy are the main treatment options. The 5-year survival rate for NSCLC is about 15%. 2,3 Recently, immunotherapy has evolved into a treatment modality with programed cell death-1 (PD-1) and programed cell death ligand-1 (PD-L1) inhibitors as the standard care for patients with NSCLC. 4 -6 Treatment of targeted immune checkpoints offers NSCLC patients both chemo-free treatment opportunities and long-term survival, creating a paradigm shift in NSCLC treatment. 7,8
Not all NSCLC patients benefit from treatment with immune checkpoint inhibitors (ICIs). Only 25%-30% of patients derive a durable benefit from immunotherapy. It is vital to identify prognostic factors and treatment-related factors associated with the response to ICIs. 9,10 The effects of immunotherapy are influenced by several factors, and the degree of influence is different. At present, clinical studies have reported that PD-L1 expression levels affect the efficacy of PD-1/PD-L1 inhibitors. High expression of PD-L1 has been shown to be a good predictive biomarker for OS and PFS. 11 As compared to proficient mismatch repair (pMMR)/microsatellite stable (MSS)in colorectal cancers (CRCs), deficient mismatch repair (dMMR)/ microsatellite instability (MSI) typically have an increased tumor mutational burden (TMB), lower response rate to 5-fluorouracil-based chemotherapy, distinctive immunological features, such as high tumor-infiltrating lymphocytes (TILs), and a better prognosis; therefore, the immunotherapy efficacy was better in colorectal cancer. 12 In a pan-cancer analysis of over 1,600 patients, a higher TMB in patients receiving ICIs therapy was associated with longer survival and higher response rates. 13,14 Surprisingly, A recent study showed that the sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS) patient categories had no effect on the use of ICIs in advanced cancer. There was no evidence of an association of sex, age (<65 vs. ≥65 years), or ECOG PS (0 vs ≥ 1) with a cancer immunotherapy survival benefit. 15
Current research has demonstrated that the intestinal microbiota of humans have a complicated relationship with ICIs immunotherapy and has generated a great deal of interest. A previous epidemiologic study showed that the risk of developing lung cancer increased by 1.4-fold with more than 5 courses of penicillin, which suggests a relationship between the Gut microbiota (GM) and carcinogenesis. 16 Several clinical research studies have demonstrated that ATB can cause changes in the GM that may influence the efficacy of ICIs in NSCLC. 17,18 A reasonable number of studies between ATB use and ICIs efficacy have been published. Most of these studies have calculated how the use of ATB may impair the efficacy of PD-L1, and clinical data supports this hypothesis. 19 -21 On the contrary, other studies did not find a correlation between ATB use and response to ICIs in patients with advanced NSCLC. 22,23 A recent large meta-analysis study revealed that antibiotic treatment around the initiation of ICIs immunotherapy was correlated with a lower OS and PFS in patients. Furthermore, the effect depended on the time of exposure, with stronger effects reported when the patients took antibiotics [−60 days; 60 days] around ICIs initiation. 21 Even now, similar studies involving a larger sample of NSCLC cases have been published. This study demonstrated that the timing of ATB treatment in NSCLC patients receiving ICIs immunotherapy has different effects on the OS and PFS of these patients.
Methods
Literature Search and Selection
We searched PubMed, EMBASE, and Cochrane databases for literature regarding the association between ATB use and ICIs efficacy in NSCLC patients through January11, 2020. The following search terms were used: “immune checkpoint inhibitor,” “PD-1 inhibitor,” “PD-L1 inhibitors,” “CTLA-4 inhibitors,” “immunotherapy,” “ICIs,” “antibiotic,” “antibiotics,” “macrobiotic,” “ATB,” “non-small-cell lung,” “non-small-cell lung cancer,” and “NSCLC.” Data were extracted independently by 2 investigators, and conflicts were adjudicated by a third investigator. For the selected studies, information on all available variables was extracted and entered into a Microsoft Excel spreadsheet (Microsoft, Redmond, WA, USA).
Eligibility Criteria and Data Extraction
Studies that met the following inclusion criteria were included in our meta-analysis: (1) patients: eligible patients were diagnosed with NSCLC and treated with ICIs monotherapy (PD-1, PD-L1, or CTLA-4 inhibitors) or in combination with systemic chemotherapy; (2) intervention: ATBs were used before and/or after the initiation of and/or during immunotherapy, regardless of time and dose; (3) comparison: the control group did not receive treatment with ATBs; (4) outcome: the 2 main outcomes were PFS and OS, and the outcome measures could be extracted. The data from the included studies were independently reviewed and extracted by 2 authors (KDH and ZJH). During the data extraction, the corresponding authors were contacted if any information was missing or needed clarification. The following data were extracted from each included study: first author, publication year and country, study design, the time of ATB use, type of ICIs used, sample size (for both the antibiotic exposure group and control group), and outcome measures.
We performed a subgroup analysis to determine the impact of the time of antibiotic exposure on ICIs, which was our main research goal. The patients were divided into the following groups: A [−60 days, 0]: used antibiotics within the 60 days before the initiation of ICIs immunotherapy: B [−30 days, 0]: used antibiotics within the 30 days before the initiation of ICIs immunotherapy; C [−60 days, 30 days]: used antibiotics within the 60 days before and 30 days after the initiation of ICIs immunotherapy; D [−30 days, 30 days]: used antibiotics within the 30 days before and 30 days after the initiation of ICIs immunotherapy; E [0, 30 days]: used antibiotics during the 30 days after the initiation of ICIs immunotherapy; F [During]: used antibiotics during the initiation of ICIs immunotherapy.
Statistical Analysis
Statistical analyses were performed using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. 24 The primary studied result was OS, and the secondary outcome was PFS. The correlation between the use of ATB and the efficacy of ICIs immunotherapy was determined by hazard ratios (HRs) and 95% confidence intervals (CIs). The overall analysis included all of the studies, and subgroup analyses were conducted based on the time of ATB use, type of ICIs drug, sample size, publication country, and study type.
We used I2 statistics and a Cochran Q test to evaluate heterogeneity among the studies. If significant heterogeneity was found among the included studies, then P < 0.10 and/or I2 >50%); otherwise P > 0.10 and I2 <50%) was applied. A random-effect model was used to pool the HRs if the heterogeneity was significant; otherwise, a fixed-effects model was used. 25 Begg’s and Egger’s tests were used to evaluate publication bias. 26 Sensitivity analysis was performed to assess the risk of bias in studies and to investigate the stability and consistency of our results. We also used a trim-and-fill method of testing and adjusting for publication bias in the meta-analysis. 27 All cases were statistically analyzed by Stata software (version 12.0, Stata Corporation, College Station, TX, USA). A 2-sided P value of < 0.05 was considered statistically significant.
Results
Identification of Eligible Studies
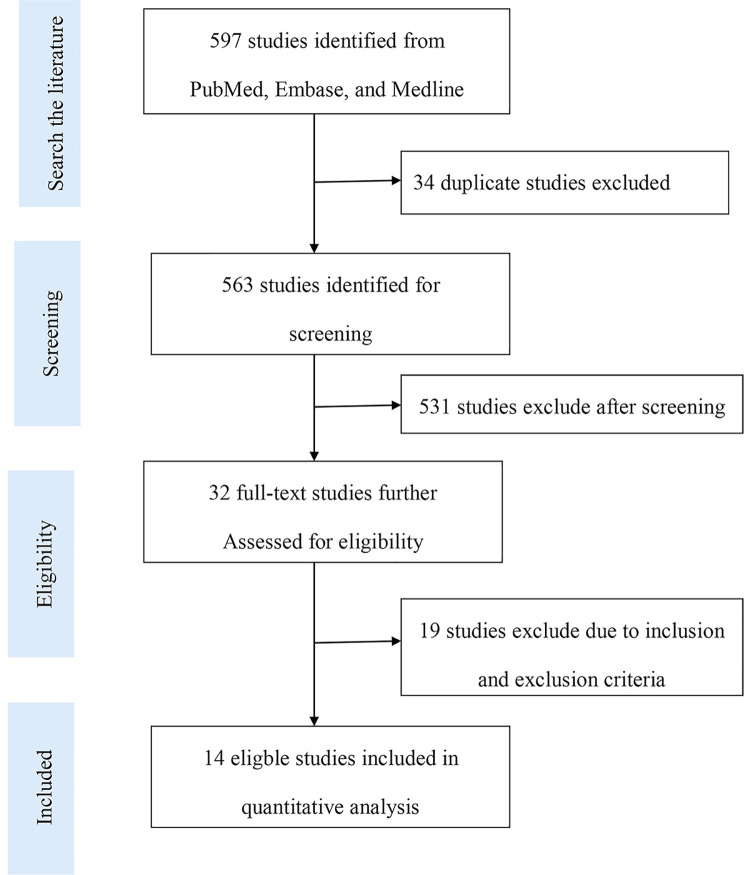
A total of 597 studies were identified from our literature search, among which 32 studies were remaining after duplicates, case reports, reviews, or unrelated studies were removed by assessing the titles and abstracts. Finally, 14 studies were included, while the other studies were excluded after further review because the overall study included NSCLC, but relevant data were not provided (Figure 1).
Figure 1.
A flow diagram illustrating the search strategy used in our meta-analysis.
Characteristics of Included Studies
A total of 1882 patients were included in our analysis. The sample sizes ranged from 30 to 243 among the included studies, and the median was 125. The included studies were published between 2017 and 2019, of which about 85.7% were published in 2018 and 2019 and were from 10 different countries. According to the time of antibiotic exposure, there were 6 studies that used antibiotics before ICIs initiation, 5 studies that used ATB before and after ICIs initiation, and 5 studies that used ATB during treatment. Six studies used anti-PD-1/PD-L1/CTLA-4 inhibitors, 6 studies used anti-PD-1 inhibitors, and 2 studies did not provide the type of ICIs drugs used. Eleven of the eligible studies were complete retrospective cohort studies, and 2 studies were only conference papers, but we contacted the corresponding author for specific information. The main features of the included studies are listed (Table 1).
Table 1.
The NSCLC Baseline Characteristics of Included Studies.
| Author | Year | Country | Type of study | Type of inhibitor | Sample (Y/N) | Time between ATB exposure and first ICIs | HR for PFS (95%CI) | HR for OS (95%CI) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beforea | Duringa | Aftera | |||||||||
| 1 | Kaderbhai | 2017 | France | Retrospective | Nivolumab | 74 (15/39) | [−90,0] | During | NA | 0.89 [0.47-1.86] | NA |
| 2 | Thompson | 2017 | United States | Retrospective | Nivolumab | 74 (18/56) | NA | NA | [0,45] | 2.5 [1.56-5.41] | 3.5 [1.49-8.21] |
| 3 | Derosa | 2018 | France | Retrospective | PD-L1/CTLA-4 | 239 (48/191) | [−30,0] | NA | NA | 1.3 [0.9-1.8] | 2.5 [1.6-3.7] |
| 243 (68/175) | [−60,0] | NA | NA | 1.2 [0.9-1.7] | 2.0 [1.3-3.2] | ||||||
| 4 | Huemer | 2018 | Austria | Retrospective | Nivolumab /Pembrolizumab | 30 (11/19) | [−30,0] | NA | [0,30] | 2.5 [1.08-5.78] | 4.29 [1.08-16.98] |
| 5 | Routy | 2018 | France | Retrospective | PD-1/PD-L1 | 140 (−/−) | [−60,0] | NA | [0,30] | 0.89 [0.58-1.34] | 2.31 [1.40-3.83] |
| 6 | Hakozaki | 2019 | Japan | Retrospective | Nivolumab | 90 (13/77) | NA | NA | [0,30] | 2.02 [0.70-5.83] | 2.03 [1.03-3.99] |
| 7 | Kim | 2019 | Korea | Retrospective | PD-1/PD-L1/CTLA-4 | 131 (60/71) | [−60,0] | NA | NA | 2.397 [1.28-4.42] | 3.843 [1.74-8.47] |
| 8 | Pinato | 2019 | United Kingdom | Retrospective | PD-1/PD-L1 | 119 (−/−) | [−30,0] | During | NA | NA | 3.40 [1.90-6.10] |
| 9 | Rounis | 2019 | Greece | Retrospective | ICIs | 44 (−/−) | [−30,0] | During | NA | 2.76 [1.80-6.40] | 4.6 [1.70-12.0] |
| 10 | Schett | 2019 | Switzerland | Retrospective | Nivolumab Pembrolizumab Atezolizumab |
218 (33/185) | [−60,0] | NA | NA | 2.20 [1.50-3.40] | 2.80 [1.70-4.50] |
| NA | During | NA | 0.86 [0.61-1.22] | 1.1 [0.75-1.63] | |||||||
| NA | NA | [0,30] | 1.15 [0.69-1.93] | 0.86 [0.46-1.59] | |||||||
| [−60,0] | During | NA | 1.27 [0.94-1.71] | 1.74 [0.75-3.99] | |||||||
| 11 | Zhao | 2019 | Chinese | Retrospective | PD-1 inhibitor | 109 (20/89) | [−30,0] | NA | [0,30] | 3.13 [1.70-5.56] | 2.86 [1.30-6.25] |
| 12 | Galli | 2019 | Italy | Retrospective | ICIs | 157 (27/30) | NA | During | NA | 1.5 [0.83,2.70] | 2.02 [0.61-6.65] |
| 13 | Ouaknine | 2019 | France | Retrospective | Nivolumab | 72 (30/42) | [−60,0] | NA | [0,30] | 1.6 [0.6-2.2] | 2.2 [1.10-4.80] |
| 14 | Huemer | 2019 | Austria | Retrospective | PD-1/PD-L1 | 142 (62/80) | [−30,0] | NA | [0,30] | 1.02 [0.69-1.50] | 0.91 [0.57-1.45] |
a Time between ATB exposure and first ICIs, before the ICI treatment initiation; after the ICI treatment initiation; during the ICI treatment initiation.
ATB Use and PFS
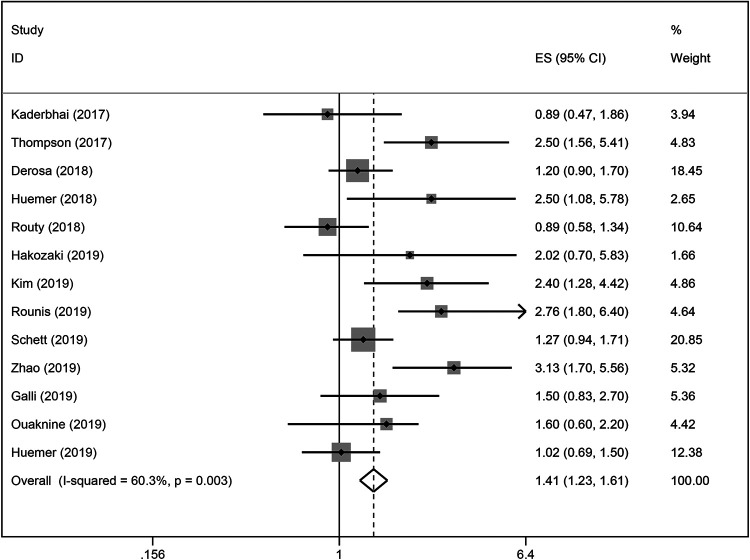
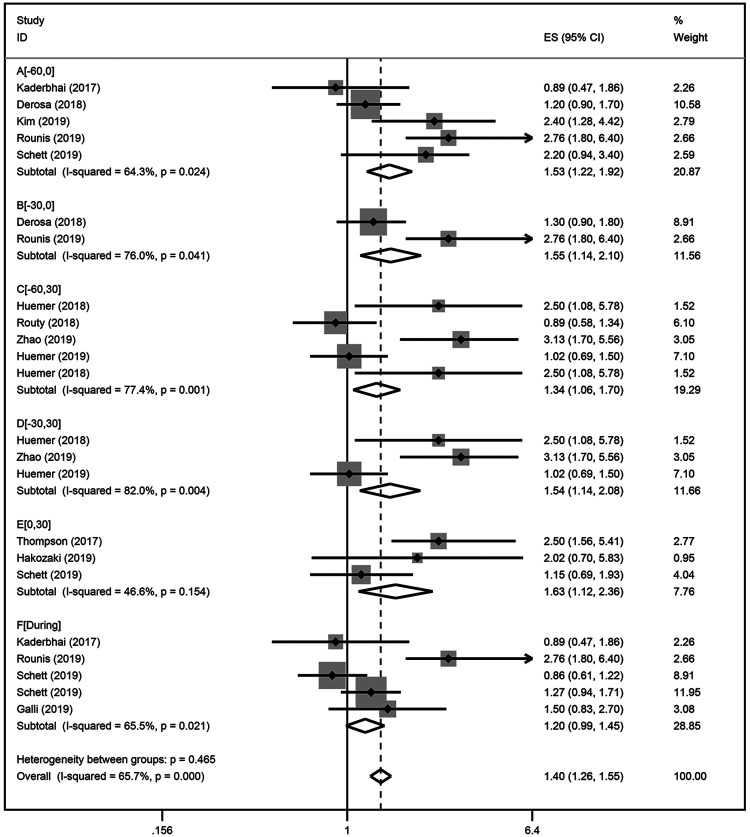
Our results indicated that ATB use significantly reduced the PFS of patients treated with ICIs (HR = 1.41; 95% CI, 1.32-1.61; P < 0.001; Figure 2), with heterogeneity among the studies (I2 = 60.3; P = 0.003). The sensitivity analysis was not affected by the single study (Figure S1). We conducted subgroup analyses for the time at which ATB were used and found that, in addition to the association with reduced PFS from ICIs immunotherapy, and ATB significantly influenced the PFS of patients treated with ICIs. NSCLC patients that were exposed to antibiotics [0 days; 30 days] (HR = 1.63; 95% CI [1.12-2.30]) after ICIs initiation displayed stronger effects on PFS than those patients that were exposed to antibiotics before [−30 days; 0 days] (HR = 1.55; 95% CI [1.14-2.10]) ICIs immunotherapy (Figure 3). Subgroup analysis results based on sample size (<100 or ≥ 100) and publication country (Asia or not-Asia) were statistically significant. All of the subgroup analyses indicated that ATB use was associated with a poorer PFS (Table 2).
Figure 2.
A forest plot of hazard ratios for the PFS of NSCLC patients exposed to antibiotics (ATB) as compared to NSCLC patients not exposed to antibiotics around ICIs treatment initiation.
Figure 3.
A forest plot of hazard ratios for the PFS of NSCLC patients exposed to antibiotics (ATB) as compared to NSCLC patients not exposed to antibiotics based on the exposure duration of antibiotics. Group A: antibiotic exposure in the following time window [−60 days; 0] relative to ICIs treatment initiation; Group B: [−30 days; 0]; Group C: [−60 days; 30 days]; Group D: [−30 days; 30 days]; Group E: [0; 30 days]; Group F: [During].
Table 2.
The Results for the Relationship Between ATB Use and PFS of Immune Checkpoint Inhibitors in NSCLC.
| N | Hazard ration (HR) | P for HR | Heterogeneity (P, I2) | Publication bias | |
|---|---|---|---|---|---|
| Progression-free survival | |||||
| Overall | 13 | 1.41 [1.23-1.61] | 0.000 | 0.003, 60.3% | Begg’s Test = 0.462 Egger’s test = 0.366 |
| Time of antibiotics use | |||||
| A group [−60, 0] | 5 | 1.53 [1.22-1.92] | 0.000 | 0.024, 64.3% | Begg’s Test = 1.000 Egger’s test =/ |
| B group [−30, 0 ] | 2 | 1.55 [1.14-2.10] | 0.005 | 0.041, 76.0% | Begg’s Test =/ Egger’s test =/ |
| C group [−60,30] | 5 | 1.34 [1.06-1.70] | 0.014 | 0.001, 77.4% | Begg’s Test = 0.602 Egger’s test =/ |
| D group [−30,30] | 3 | 1.54 [1.14-2.06] | 0.005 | 0.004, 82% | Begg’s Test = 1.000 Egger’s test =/ |
| E group [0 , 30 ] | 3 | 1.63 [1.12-2.369] | 0.010 | 0.154, 46.6% | Begg’s Test =/ Egger’s test =/ |
| F group [During] | 5 | 1.20 [0.99-1.45] | 0.067 | 0.021, 65.5% | Begg’s Test =/ Egger’s test =/ |
| ICI drug | |||||
| PD-1 inhibitor | 5 | 1.73 [1.25-2.40] | 0.001 | 0.241, 31.20% | Begg’s Test = 1.000 Egger’s test =/ |
| PD-1/PD-L1/CTLA-4 inhibitor | 8 | 1.35 [1.16-1.57] | 0.000 | 0.002, 68.9% | Begg’s Test = 1.000 Egger’s test = 0.408 |
| Sample size | |||||
| <100 | 6 | 1.91 [1.43-2.56] | 0.000 | 0.190, 32.8% | Begg’s Test = 1.000 Egger’s test = 0.695 |
| ≥100 | 7 | 1.29 [1.11-1.51] | 0.001 | 0.008, 65.4% | Begg’s Test = 1.000 Egger’s test =/ |
| Country | |||||
| Asia | 3 | 2.64 [1.77-3.92] | 0.000 | 0.721, 0.0% | Begg’s Test = 0.296 Egger’s test = 0.146 |
| No-Asia | 8 | 1.29 [1.12-3.92] | 0.000 | 0.028, 51.8% | Begg’s Test = 1.000 Egger’s test = 0.891 |
ATB Use and OS
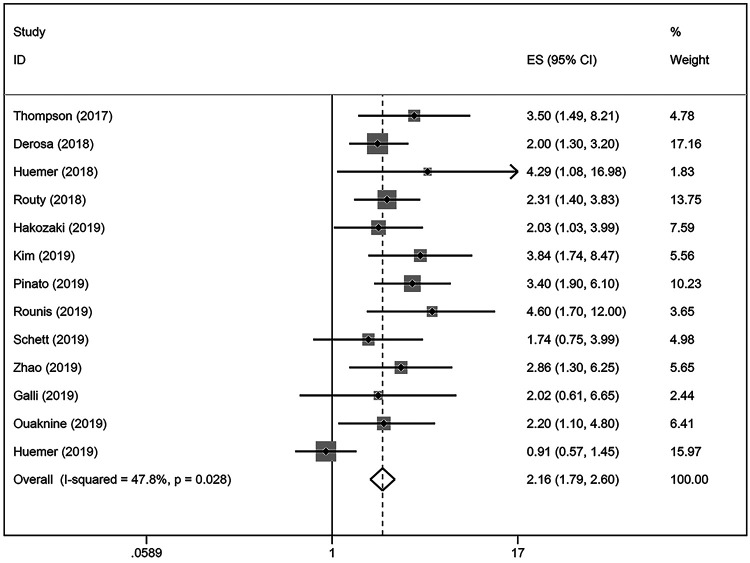
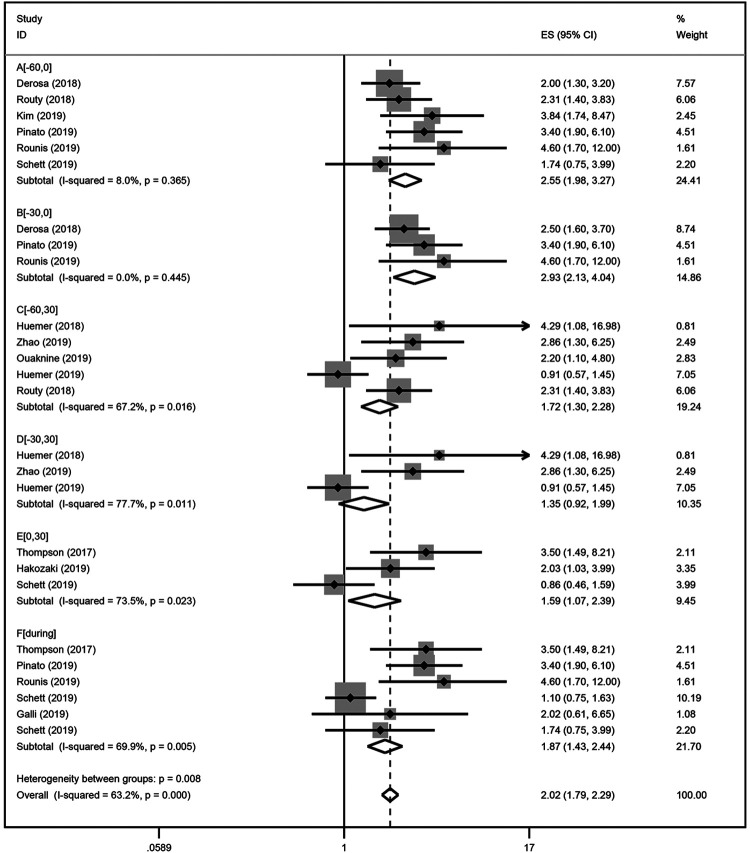
Our results indicated that ATB use was negatively correlated with the OS in NSCLC patients treated with ICIs (HR = 2.16; 95% CI = 1.79-2.60; P < 0.001; Figure 4). There was significant heterogeneity among the studies analyzed (I2 = 47.8%; P = 0.028). The stability of our results was confirmed by sensitivity analysis, and no single study substantially dominated the results (Figure S2). NSCLC patients that were exposed to antibiotics before [−30 days; 0 days] (HR = 2.93; 95% CI [2.13-4.04]) ICIs initiation had stronger effects than on PFS than those patients that were exposed to antibiotics after [0 days; 30 days] (HR = 1.59; 95% CI [1.07-2.39]) ICIs immunotherapy. In terms of the ATB exposure time, our results showed that antibiotic exposure around [−60 days; +30 days] ICIs initiation significantly reduced survival in NSCLC patients (Figure 5). Results for ICIs drugs, including PD-1 inhibitors, PD-1/PD-L1/CTLA-4 inhibitors, and ICIs monotherapy, were provided in 3, 7, and 2 studies, respectively. Similar results showed that NSCLC patients treated with ATBs had a poor OS without heterogeneity. The results of subgroup analysis, sample size, and publication country demonstrated that ATB use in NSCLC patients was associated with a decreased OS (Table 3).
Figure 4.
A forest plot of hazard ratios for the OS of NSCLC patients exposed to antibiotics (ATB) as compared to NSCLC patients not exposed to antibiotics around ICIs treatment initiation.
Figure 5.
A forest plot of hazard ratios for the OS of NSCLC patients exposed to antibiotics (ATB) as compared to NSCLC patients not exposed to antibiotics based on the exposure duration of antibiotics. Group A: antibiotic exposure in the following time window [−60 days; 0] relative to ICIs treatment initiation; Group B: [−30 days; 0]; Group C: [−60 days; 30 days]; Group D: [−30 days; 30 days]; Group E: [0; 30 days]; Group F: [During].
Table 3.
The Results for the Relationship Between ATB Use and OS of Immune Checkpoint Inhibitors in NSCLC.
| N | Hazard ration (HR) | P for HR | Heterogeneity (P, I2) | Publication bias | |
|---|---|---|---|---|---|
| Overall survival | |||||
| Overall | 13 | 2.16 [1.79-2.60] | 0.000 | 0.028, 22.9% | Begg’s Test = 0.474 Egger’s test = 0.565 |
| Time of antibiotics use | |||||
| A group [−60, 0] | 6 | 2.55 [1.98-3.27] | 0.000 | 0.365, 8.0% | Begg’s Test = 1.000 Egger’s test = 0.684 |
| B group [−30, 0 ] | 3 | 2.93 [2.13-4.04] | 0.0000 | 0.445, 0.0% | Begg’s Test = 0.296 Egger’s test = 0.457 |
| C group [−60,30] | 5 | 1.72 [1.30-2.28] | 0.000 | 0.016, 67.2% | Begg’s Test = 0308 Egger’s test = 0.398 |
| D group [−30,30] | 3 | 1.35 [0.92-1.99] | 0.124 | 0.011, 77.7% | Begg’s Test = 1.000 Egger’s test =/ |
| E group [0, 30 ] | 3 | 1.59 [1.07-2.39] | 0.023 | 0.023, 73.59% | Begg’s Test = 1.000 Egger’s test =/ |
| F group [During] | 6 | 1.74 [1.43-2.44] | 0.000 | 0.005, 69.9% | Begg’s Test = 1.000 Egger’s test = 0.600 |
| ICI drug | |||||
| PD-1 inhibitor | 4 | 2.50 [1.71-3.64] | 0.000 | 0.756, 0.0% | Begg’s Test = 0.308 Egger’s test = 0.050 |
| PD-1/PD-L1/CTLA-4 inhibitor | 9 | 2.06 [1.66-2.55] | 0.000 | 0.007, 62% | Begg’s Test = 1.000 Egger’s test = 0.955 |
| Sample size | |||||
| <100 | 5 | 2.76 [1.80-4.03] | 0.000 | 0.575, 0.0% | Begg’s Test = 0.462 Egger’s test = 0.222 |
| ≥100 | 8 | 2.00 [1.61-2.47] | 0000 | 0.012, 61% | Begg’s Test = 0.462 Egger’s test = 0.472 |
| Country | |||||
| Asia | 3 | 2.72 [1.77-4.18] | 0.000 | 0.481, 0.0% | Begg’s Test = 0.296 Egger’s test = 0.146 |
| No-Asia | 8 | 2.05 [1.66-2.52] | 0.000 | 0.017, 5.4% | Begg’s Test = 1.000 Egger’s test = 0.891 |
Assessment of Publication Bias
Publication bias was evaluated by funnel plots. There was no significant publication bias in PFS and OS. The results of Begg’s and Egger’s tests indicated that there was no significant publication bias in the subgroups (Tables 2 and 3).
Discussion
ICIs, including nivolumab, pembrolizumab, atezolizumab, and durvalumab, are currently approved for treating advanced stage NSCLC. CheckMate-017, CheckMate-057, KEYNOTE-010, OAK trials, and PACIFIC studies demonstrated the superiority of these agents over chemotherapy. 5 -7 ICIs have been shown to improve the response rates and survival in NSCLC patients. 9 However, the objective response rates were no more than 35%-44%, while secondary resistance rates approached 100%. 7,28,29 Both the primary or acquired resistance to ICIs and attenuation of the therapeutic efficacy of ICIs are caused by many factors, such as PD-L1 expression, tumor mutational load, the tumor immune infiltrate, environmental factors, and loss of diversity or a shift in the composition of the GM. 30,31 Patients exposed to ATBs during the entire ICIs period with a higher the median irAE was 4.2% and irAE had worse PFS and OS. 22 Currently, there are no efficient biomarkers to predict the efficacy of ICIs.
However, there are 4 major questions that need to be answered. (1) How to accurately screen the beneficiaries? (2) Whether to predict resistance and Immune-related adverse events (irAE)? (3) How the new joint strategy can improve treatment effectiveness? (4) How to modify an unfavorable ATB-associated dysbiosis to a “favorable” phenotype for enhancing ICIs efficacy? We look forward to answering these questions.
The lungs carry out the exchange of gas between the body and the environment. The principal site of infections is in the lung. 32 Now current literature indicates a link between the gut and lung microbiomes, which interact through multiple pathways. The bi-directional crosstalk between the gut and lung (termed as the gut-lung axis) is best exemplified by intestinal disturbances observed in lung diseases. 32,33 There are few studies regarding the effect of the pulmonary flora on the intestinal tract. Some external factors disrupt the airway microbiome, resulting in translocation of these bacteria into the bloodstream, which then disturbs the GM. 30,31 In pre-clinical models, resistance to ICIs can be attributed to an abnormal GM composition. 34 The microbiome governs the cancer-immune set point in cancer-bearing individuals, and manipulating the gut ecosystem to circumvent primary resistance to ICIs may be feasible. 35 Several studies have reported that the GM influences the anti-tumor response of ICIs immunotherapy, suggesting that loss of diversity and a shift in the composition of the GM can attenuate the therapeutic efficacy of ICIs. 36 In theory, ATB should be able to shift the microbiota composition temporally and impact the efficacy of ICIs.
Recently many studies have recognized the association between ATB use and the clinical efficacy of ICIs immunotherapy. In early 2013, animal experiments confirmed that mice with solid tumors grown in germ-free conditions or treated with broad-spectrum antibiotics had a poor response to immunotherapy. 37 Thus, multitudinous studies have demonstrated that ATB use may reduce the efficacy of ICIs immunotherapy in NSCLC patients by changing the diversity and composition of the GM. 35 Conversely, some studies have shown the exact opposite effect. 22,23 Although it is generally acknowledged that ATB use reduces the efficacy of ICIs immunotherapy by altering the diversity and composition of the gut-lung axis microbiota.
The chronological order of antibiotic exposure and immunotherapy affects the outcome of ICIs. The blood concentration of antibiotics, which depends on the duration, route, and type of ATB used, affects the diversity and composition of the GM. The use of ATBs may also change over time as the patient’s condition changes, and as such, it is very difficult for studies to evaluate the consistent and detailed definition of ATBs in immunotherapy. None of the analyses of the included studies state a unique definition for the time of ATB use. Many studies used a definition of 1 month before and/or after and during the initiation of ICIs immunotherapy. A study showed that in advanced NSCLC the impact of ATB 60 days before ICIs was not as potent as within the first 30 days before ICIs. 38 Another study demonstrated that modification of the microbiota by antibiotics (in the 3 months before the first nivolumab injection or during treatment) did not affect the efficacy of nivolumab in patients with NSCLC. 23 A recent study demonstrated that a PFS and OS benefit derived from ICIs may be attenuated by the administration of antibiotics in temporal proximity to the initiation of ICIs in patients with advanced NSCLC. 39,40 A current meta-analysis confirmed that patients exposed to antibiotics around [−60 days; +60 days] ICIs initiation had stronger poor effects on survival. 21 Even if this hypothesis is true, it should be interpreted with extreme caution, as the route and dosage of antibiotics also affects absorption, and if patients take other medications, such as ATB, proton pump inhibitors, steroids, or a combination, can also result in poor outcomes. Also, the drugs mentioned above has not been mechanistically and prospectively tested in cancer populations treated with ICIs immunotherapy. Thus, we need to further explore the relationship between ATB and immunotherapy in lung cancer.
Similar studies involving a larger sample of NSCLC cases have been published. 21 This study indicated that NSCLC patients exposed to antibiotics around [−60 days; +60 days] ICIs initiation had stronger worse effects on survival. This meta-analysis identified that the timing of ATB treatment in NSCLC patients receiving ICIs immunotherapy has different effects on the OS and PFS of these patients and ATB impair the efficacy of ICIs was illustrate the relationship in more detail became the biggest bright spot. Our results showed that the sequence of ATB exposure and initiation of ICIs immunotherapy has different effects on OS and PFS. Our subgroup analysis showed that the NSCLC patients exposed to antibiotics before [−60 days; 0 days] or [−30 days; 0 days] ICIs initiation had worse effects on OS. Additionally, NSCLC patients exposed to antibiotics after [0 days; 30 days] ICIs initiation had worse effects on PFS.
Our research had some limitations, and as such, we offer some suggestions for future research. First, in this meta-analysis most of the included studies did not provide detailed tumor characteristics and subgroup stratification, such as PD-L1 expression, lines of therapy, type of ICIs drug, epidermal growth factor receptor (EGFR) mutations, tumor node metastasis (TNM) stage, tumor mutational burden (TMB), Eastern Cooperative Oncology Group (ECOG) performance status score, tumor-infiltrating lymphocytes, mismatch repair gene (MMR), and irAE, which may influence the efficacy of ICIs. Second, ATB use and immune-related adverse events were not detailed in the trials involved, which may have different effects on the efficacy of ICIs. Third, the heterogeneity of PFS existed in the included studies; therefore, PFS results were less robust endpoints in our results. Different periods of immunotherapy, different intervals of administration, and different responses to drugs resulted in high heterogeneity of PFS. Heterogeneity also existed in subgroup analysis, which may have influenced the outcome. Moreover, patients were not excluded from taking antibiotics, corticosteroids, and/or proton pump inhibitors (PPI). These factors may lead to heterogeneity.
Immunotherapy has achieved long-term survival and protects NSCLC patients from the adverse reactions of chemotherapy. Immunotherapy is gradually changing and reshaping NSCLC treatment. Moreover, stratification according to antibiotic treatment status (e.g., use time, duration, route, and type of ATB) may be warranted in future trials investigating ICIs. We will continue to seek adequate biomarkers and optimized guidelines to reduce this impact and improve ICIs efficacy.
Conclusion
Excessive antibiotic use can disrupt the delicate balance of the gut-lung bacterial axis. Furthermore, antibiotic use has been associated with immune-related adverse events and the efficacy of ICIs immunotherapy. In this meta-analysis, we demonstrated that NSCLC patients who took antibiotics [−60 days; +30 days] around ICIs initiation had significantly reduced survival. Furthermore, our study showed that antibiotic exposure [−60 days; 0 days] or [−30 days; 0 days] before ICIs immunotherapy had strong effects on OS, while antibiotic exposure [0; 30 days] after the initiation of ICIs immunotherapy had worse effects on PFS. However, this conclusion has to be confirmed with a larger randomized clinical study. Clinical application of antibiotics should be further standardized and limited to strict indications in patients receiving ICIs.
Supplemental Material
Supplemental Material, sj-tif-1-tct-10.1177_15330338211033498 for How to Choose a Survival Period? The Impact of Antibiotic Use on OS or PFS in NSCLC Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis by Hua Chen, Ke-dong Han, Zhi-jiang He and Yi-sheng Huang in Technology in Cancer Research & Treatment
Supplemental Material, sj-tif-2-tct-10.1177_15330338211033498 for How to Choose a Survival Period? The Impact of Antibiotic Use on OS or PFS in NSCLC Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis by Hua Chen, Ke-dong Han, Zhi-jiang He and Yi-sheng Huang in Technology in Cancer Research & Treatment
Acknowledgments
We thank the members of the Second Tumor and Cardiology Department, Maoming People’s Hospital, for their fruitful discussions and technical assistance. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Abbreviations
- Non-small-cell lung cancer
NSCLC
- Immune checkpoint inhibitors
ICIs
- Antibiotics
ATB
- Monoclonal antibody
mAb
- Programed cell death-1
PD-1
- Cytotoxic T-Lymphocyte Antigen 4
CTLA-4
- Programed cell death ligand-1
PD-L1
- Progression-free survival
PFS
- Overall survival
OS
- Hazard ratios
HRs
- 95% confidence intervals
95% Cis
- Proficient mismatch repair
pMMR
- Microsatellite stable
MSS
- Colorectal cancers
CRCs
- Deficient mismatch repair
dMMR
- Microsatellite instability
MSI
- Tumor mutational burden
TMB
- Tumor-infiltrating lymphocytes
TILs
- Eastern Cooperative Oncology Group
ECOG
- Performance status
PS
- Gut microbiota
GM
- Immune-related adverse events
irAE
- Epidermal growth factor receptor
EGFR
- Tumor node metastasis
TNM
- Proton pump inhibitors
PPI
Authors’ Note: Hua Chen, Ke-dong Han, and Zhi-jiang He are co-first authors. The authors contributed equally to this work. Hua Chen and Yi-sheng Huang designed the study. Ke-dong Han, Zhi-jiang He collected, analyzed, and interpreted the data. Hua Chen and Ke-dong Han wrote and edited the manuscript. All of the authors read and approved the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was funded by the High-level Hospital Construction Research Project of Maoming People’s Hospital.
ORCID iD: Hua Chen  https://orcid.org/0000-0003-4681-6948
https://orcid.org/0000-0003-4681-6948
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Erratum: global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70(4):313. [DOI] [PubMed] [Google Scholar]
- 2. Sun Y, Guo X, Zhang L, Zhang W, Zuo Y. Evaluation of radiotherapy combined with targeted therapy and concurrent radiotherapy, chemotherapy in the treatment of non-small cell lung cancer with brain metastasis. Pak J Med Sci. 2020;36(3):322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383(7):640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carbone DP, Reck M, Paz-Ares L, et al. ; CheckMate 026 Investigators. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reck M, Taylor F, Penrod JR, et al. Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non-small cell lung cancer: results from the checkmate 017 study. J Thorac Oncol. 2018;13(2):194–204. [DOI] [PubMed] [Google Scholar]
- 6. Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, phase III trials checkmate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24. [DOI] [PubMed] [Google Scholar]
- 8. Zhang S, Bai X, Shan F. The progress and confusion of anti-PD1/PD-L1 immunotherapy for patients with advanced non-small cell lung cancer. Int Immunopharmacol. 2020;80:106247. [DOI] [PubMed] [Google Scholar]
- 9. Alexa T, Antoniu SA, Alexa I, et al. Checkpoint inhibitors in NSCLC for the elderly: current challenges and perspectives. Expert Rev Anticancer Ther. 2021;21(3):315–323. [DOI] [PubMed] [Google Scholar]
- 10. Koleczko S, Wolf J. Immune checkpoint inhibitors in lung cancer [in German]. Internist (Berl). 2020;61(7):676–681. [DOI] [PubMed] [Google Scholar]
- 11. Zhang B, Liu Y, Zhou S, Jiang H, Zhu K, Wang R. Predictive effect of PD-L1 expression for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatment for non-small cell lung cancer: a meta-analysis. Int Immunopharmacol. 2020;80:106214. [DOI] [PubMed] [Google Scholar]
- 12. Lizardo DY, Kuang C, Hao S, Yu J, Huang Y, Zhang L. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: from bench to bedside. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Killock D. TMB—a histology-agnostic predictor of the efficacy of ICIs? Nat Rev Clin Oncol. 2020;17(12):718. [DOI] [PubMed] [Google Scholar]
- 14. Lee M, Samstein RM, Valero C, Chan TA, Morris LGT. Tumor mutational burden as a predictive biomarker for checkpoint inhibitor immunotherapy. Hum Vaccine Immunother. 2020;16(1):112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang F, Markovic SN, Molina JR, et al. Association of sex, age, and Eastern Cooperative Oncology Group performance status with survival benefit of cancer immunotherapy in randomized clinical trials: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(8):e2012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boursi B, Mamtani R, Haynes K, Yang YX. Recurrent antibiotic exposure may promote cancer formation—another step in understanding the role of the human microbiota? Eur J Cancer. 2015;51(17):2655–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ubeda C, Pamer EG. Antibiotics, microbiota, and immune defense. Trend Immunol. 2012;33(9):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schett A, Rothschild SI, Curioni-Fontecedro A, et al. Predictive impact of antibiotics in patients with advanced non small-cell lung cancer receiving immune checkpoint inhibitors: antibiotics immune checkpoint inhibitors in advanced NSCLC. Can Chemother Pharmacol. 2020;85(1):121–131. [DOI] [PubMed] [Google Scholar]
- 20. Kim H, Lee JE, Hong SH, Lee MA, Kang JH, Kim IH. The effect of antibiotics on the clinical outcomes of patients with solid cancers undergoing immune checkpoint inhibitor treatment: a retrospective study. BMC Cancer. 2019;19(1):1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lurienne L, Cervesi J, Duhalde L, et al. A NSCLC immunotherapy efficacy and antibiotic use: a systematic review and meta-analysis. J Thorac Oncol. 2020;15(7):1147–1159. [DOI] [PubMed] [Google Scholar]
- 22. Galli G, Triulzi T, Proto C, et al. Association between antibiotic-immunotherapy exposure ratio and outcome in metastatic non small cell lung cancer. Lung Cancer. 2019;132:72–78. [DOI] [PubMed] [Google Scholar]
- 23. Kaderbhai C, Richard C, Fumet JD, et al. Antibiotic use does not appear to influence response to nivolumab. Anticancer Res. 2017;37(6):3195–3200. [DOI] [PubMed] [Google Scholar]
- 24. Jin Y, Sanger N, Shams I, et al. Does the medical literature remain inadequately described despite having reporting guidelines for 21 years?—A systematic review of reviews: an update. J Multidiscip Healthc. 2018;11:495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmidt FL, Oh IS, Hayes TL. Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol. 2009;62(pt 1):97–128. [DOI] [PubMed] [Google Scholar]
- 26. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 27. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 28. Huang Z, Su W, Lu T, et al. First-line immune-checkpoint inhibitors in non-small cell lung cancer: current landscape and future progress. Front Pharmacol. 2020;11:578091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onoi K, Chihara Y, Uchino J, et al. Immune checkpoint inhibitors for lung cancer treatment: a review. J Clin Med. 2020;9(5):1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang XZ, Gao P, Song YX, et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology. 2019;8(12):e1665973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maung TZ, Ergin HE, Javed M, Inga EE, Khan S. Immune checkpoint inhibitors in lung cancer: role of biomarkers and combination therapies. Cureus. 2020;12(5):e8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gong GC, Song SR, Su J. Pulmonary fibrosis alters gut microbiota and associated metabolites in mice: an integrated 16 S and metabolomics analysis. Life Sci. 2021;264:118616. [DOI] [PubMed] [Google Scholar]
- 33. Anand S, Mande SS. Diet, microbiota and gut-lung connection. Front Microbiol. 2018;9:2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sze MA, Tsuruta M, Yang SW, et al. Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS One. 2014;9(10):e111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. [DOI] [PubMed] [Google Scholar]
- 36. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. [DOI] [PubMed] [Google Scholar]
- 37. Nagasaka M, Sexton R, Alhasan R, Rahman S, Azmi AS, Sukari A. Gut microbiome and response to checkpoint inhibitors in non-small cell lung cancer—a review. Cri Rev Oncol Hematol. 2020;145:102841. [DOI] [PubMed] [Google Scholar]
- 38. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huemer F, Rinnerthaler G, Westphal T, et al. Impact of antibiotic treatment on immune-checkpoint blockade efficacy in advanced non-squamous non-small cell lung cancer. Oncotarget. 2018;9(23):16512–16520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huemer F, Lang D, Westphal T, et al. Baseline absolute lymphocyte count and ECOG performance score are associated with survival in advanced non-small cell lung cancer undergoing PD-1/PD-L1 blockade. J Clin Med. 2019;8(7):1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-tif-1-tct-10.1177_15330338211033498 for How to Choose a Survival Period? The Impact of Antibiotic Use on OS or PFS in NSCLC Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis by Hua Chen, Ke-dong Han, Zhi-jiang He and Yi-sheng Huang in Technology in Cancer Research & Treatment
Supplemental Material, sj-tif-2-tct-10.1177_15330338211033498 for How to Choose a Survival Period? The Impact of Antibiotic Use on OS or PFS in NSCLC Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis by Hua Chen, Ke-dong Han, Zhi-jiang He and Yi-sheng Huang in Technology in Cancer Research & Treatment