Abstract
Disulfiram in conjunction with copper has been shown to be a potent anticancer agent. However, disulfiram’s therapeutic potential in prostate cancer is hindered by off-target effects due to its reactive and nucleophilic thiol-containing component, diethyldithiocarbamate (DTC). To minimize undesirable reactivity, we have strategically blocked the thiol moiety in DTC with a cleavable p-aminobenzyl (pAB) group linked to peptide substrates recognized by prostate specific antigen (PSA). Here we report the synthesis and evaluation in cancer cell models of two PSA-activatable prodrugs: HPD (Ac-HSSKLQL-pAB-DTC and RPD (RSSYYSL-pAB-DTC). In vitro exposure to PSA was found to trigger activation of HPD and RPD to release diethyldithiocarbamate, and both prodrugs were found to induce toxicity in prostate cancer cells, with HPD showing the most promising selectivity. With copper supplementation, the IC50 of HPD was 1.4 μM in PSA-expressing LNCaP cells, and 11 μM in PC3 cells that do not express PSA. These studies demonstrate the utility of using peptide recognition handles to direct the activity of dithiocarbamate prodrugs for selective cytotoxicity of cancer cells.
Keywords: Prostate Specific Antigen, Disulfiram, Diethyldithiocarbamate, Prochelator, copper
Graphical Abstract
Prostate cancer is the second most common cancer among men.1 The androgen receptor (AR) remains the major drug target for prostate cancer.2,3 While androgen deprivation therapy can be effective at initial stages, there are many cases where disease progresses to metastatic cancer.4,5 Although hormonal agents targeting AR such as enzalutamide and abiraterone acetate have improved treatment outcomes, the frequent occurrence of resistance to these treatments underscores a clear need for new prostate cancer therapies that act on targets other than AR.
The avidity of prostate cancer cells to accumulate copper (Cu) has been recognized to sensitize these cells toward cytotoxicity by compounds that act via a mechanism that relies on copper.6-9 Disulfiram, a drug commercially available as Antabuse and approved for alcohol aversion therapy, is a principle example. In the cancer setting, the activity of disulfiram has been attributed to its reduced version, diethyldithiocarbamate (DTC), which binds Cu and leads to generation of reactive oxygen species (ROS) and cell death.10,11 Indeed, co-administration of Cu(II) salts dramatically enhanced the efficacy of disulfiram to inhibit the growth of both androgen-responsive and castration-resistant prostate xenografts.12 Furthermore, the Cu(DTC)2 complex has been detected in mice fed with disulfiram, in human plasma of patients undergoing disulfiram treatment for alcoholism, and in tumor tissue after disulfiram administration.13
The promising anticancer activity of disulfiram, however, also carries many liabilities, as it relies on a reactive metabolite that binds non-specifically to metal ions, reacts non-specifically with protein and other cellular thiols, and further decomposes to form unwanted metabolites with off-target effects.14-16 To overcome these drawbacks, we devised a prochelator strategy that would generate the Cu(DTC)2 complex in situ in prostate cancer tissue.17 Prochelators are masked chelators that are activated by a trigger to release the metal-binding form specifically in the target region.18
Our first iteration of an enzyme-activatable DTC prodrug leveraged gamma glutamyl transferase (GGT) as the trigger.17 The prochelator, termed GGTDTC, selectively released DTC only in cells with measurable GGT activity; antiproliferative efficacy also correlated with cellular GGT activity, with 24 h IC50 values of 800 nM in prostate cancer cell lines but over 15 μM in normal prostate epithelial cells.17
While GGT is overexpressed in many aggressive cancer cells, it is also overexpressed in liver, especially during liver disease. To explore alternative trigger mechanisms, in our current work we chose prostate specific antigen (PSA) as the triggering enzyme. PSA is a serine protease that is expressed by prostate cells, is overexpressed in prostate cancer, is not expressed by other normal cells in humans, and is used as a biomarker for prostate cancer activity.19-22
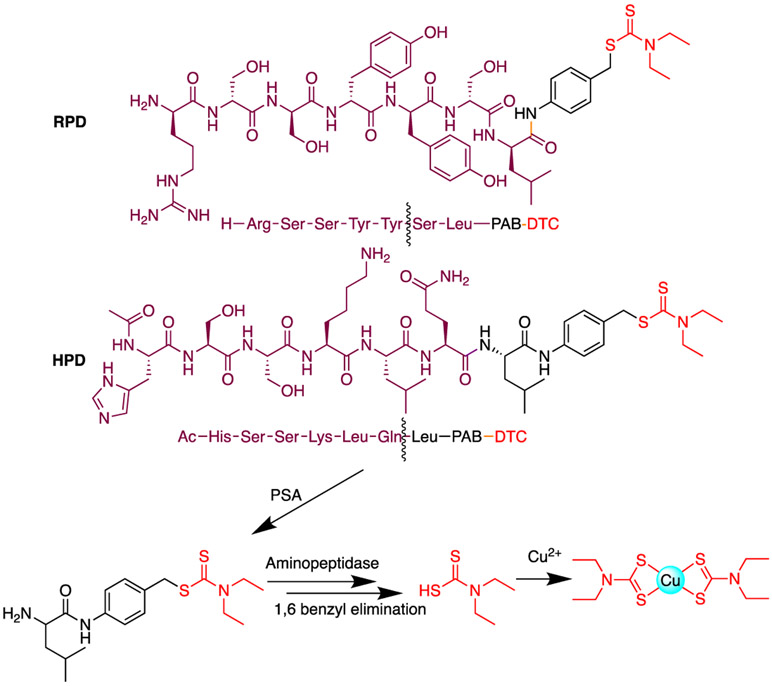
The natural proteolytic substrates for PSA, a member of the kallikrein enzyme family,23 are semenogelin proteins SgI and SgII found in semen.22,24 PSA is also enzymatically active in prostate tumor tissue, but is inactivated upon binding to α1-antichymotrypsin if released into the blood stream.25 The tumor-specific activity of PSA has been leveraged to develop prodrugs that have found success in pre-clinical models to release several cytotoxic agents, including vinblastine, thapsigargin, and doxorubicin, the latter having passed a Phase I clinical trial.26-28 In order to create a DTC-releasing prodrug, we chose two peptide sequences that are known PSA substrates optimized to release a drug upon PSA cleavage: RSSYYS and HSSKLQ.29,30 The former is derived from chymotrypsin substrates and has been used to develop doxorubicin prodrugs that demonstrated moderate selectivity for PSA-expressing vs. non-expressing cells.28,31 The HSSKLQ sequence represents the PSA cleavage sites in SgI and SgII proteins; it has been used to develop prodrugs of thapsigargin, doxorubicin and vinblastine.30 In this work, we conjugated DTC via a leucine spacer and a self-immolative p-aminobenzyl (pAB) linker to the C-terminus of these peptide sequences to generate Arg-Ser-Ser-Tyr-Tyr-Ser-Leu-pAB-DTC and His-Ser-Ser-Lys-Leu-Gln-Leu-pAB-DTC (RPD and HPD, respectively) as shown in Figure 1. Blocking the thiol of DTC prevents it from binding to Cu and hence is in an inactive form, as we have shown in our previous study.17 In the current design, PSA within tumor tissue is expected to cleave the peptides at the bonds highlighted in Figure 1, with non-specific cellular proteases like aminopeptidases trimming the final residues to initiate the 1,6-benzyl elimination of the pAB linker to release the DTC cargo. The released DTC is then available to capture Cu in the tumor microenvironment.
Figure 1.
Chemical structures of prodrugs RPD and HPD, with products from cleavage by PSA enzyme and subsequent amino acid trimming by non-specific aminopeptidases. Spontaneous 1,6 benzyl elimination of the PAB linker releases dithiocarbamic acid, which is then available for coordinating copper. Wavy lines indicate the position of cleavage by PSA.
The peptide-DTC conjugates were synthesized following the route summarized in Scheme 1, starting with coupling Fmoc-Leucine to p-aminobenzyl alcohol. The alcohol group of pAB was then converted to bromine to enable nucleophilic substitution to conjugate DTC. Deprotection of the fmoc group frees the amino group for further conjugation to the substrate peptides. For the case of HPD, N-acetylated and alloc-protected AcHSSK(alloc)LQ peptide was conjugated by using a peptide coupling protocol to generate 5, which was then deprotected and purified by HPLC to afford HPD (Scheme 1). For RPD, Fmoc-RSSYYS was coupled to 4 followed by fmoc deprotection and purification to give the final product (Scheme S1).
Scheme 1.
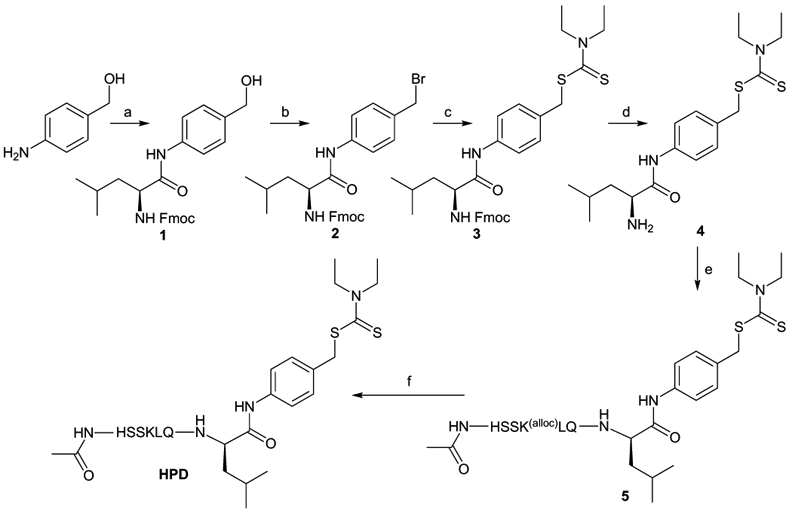
Synthesis of HPD, a PSA-targeted prochelator. a) FmocLeu-OH, 4% NMM in DMF, HBTU; b) Dry THF, PBr3; c) Sodium diethyldithiocarbamate, Dry THF; d) 20% piperidine in DMF; e) AcHSSK(alloc)LQ, EDC, HOBt, DMF; f) Pd(PPh3)4, PhSiH3, DCM
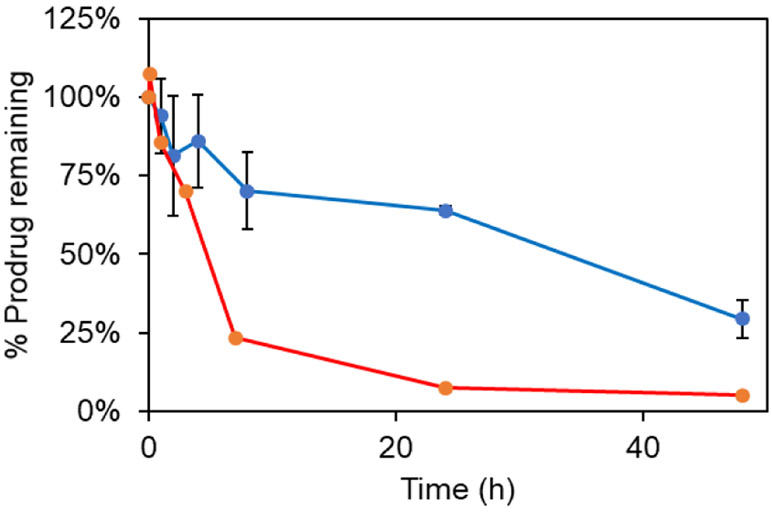
To check the cleavage by PSA enzyme the prodrugs HPD and RPD were treated with 20 μg/mL of PSA enzyme. PSA is expected to cleave between glutamine and leucine residues. The cleavage products were monitored via HPLC, with caffeine added as an internal standard. Intact HPD eluted at 10 min as confirmed by mass spectrometry (Figure S3). The relative intensity of the HPD peak eluting at 10 min decreased with time, consistent with gradual cleavage of the peptide (Figure 2). The cleavage product of this reaction with HPD was confirmed to be Leu-PAB-DTC by mass spectrometry (m/z 368 for [M+H]+ and 735 for [2M+H]+) (Figure S3). At least 30% of HPD remained even after 48 h treatment with enzyme. RPD on the other hand was cleaved completely within 8 h under these conditions. RPD was cleaved between Tyr and Ser to release cleavage product Ser-Leu-PAB-DTC as confirmed by mass spectrometry (m/z 477 for [M+Na]+ and m/z 909 for [2M+Na]+, Figure S4). These results are consistent with previous reports suggesting that the kinetics for cleavage of HSSKLQ is slower than that of RSSYYS by PSA enzyme.32
Figure 2.
% of HPD (blue) and RPD (red) remaining at different timepoints on treatment with 20 μg/mL PSA enzyme HPLC trace at λ 254 nm of HPD for 5 min, 1 h, 2 h, 4 h, 8 h, 24 h, 48 h.
To test the design element that general amino peptidases in the cell would trim the last amino acid remaining after PSA cleavage and activate the PAB linker to release DTC, HPD and RPD were treated with a mixture of PSA and leucine amino peptidase. DTC itself was not observable by our LC-MS conditions, but spiking the reaction mixture with CuSO4 and analyzing by LC-MS revealed a clean peak at 14.5 min with m/z of 358.87, consistent with the Cu(DTC)2 complex (Figure S5). An isomer construct of RPD where serine in the sixth position was replaced with d-serine was also prepared and tested in this same assay. The only observed product was a peak eluting at 9 min with m/z of 455.06, consistent with the PSA-cleaved product d-Ser-Leu-PAB-DTC; no Cu(DTC)2 peak was observed. This result confirms that amino peptidase activity is required to activate the PAB linker and release DTC.
To test the selectivity of the prodrugs in cells the cleavage of the prodrugs in cell lysate was studied by HPLC. We chose LNCaP as a PSA-expressing cell line and PC3 as a control cell line that does not express PSA. The cell lysates were treated with HPD or RPD and the cleavage was monitored over time with HPLC with caffeine as reference. RPD was completely cleaved by LNCaP lysate within 4 h. PC3 was also able to cleave more than 50% of the RPD prodrug within 4 h even though they do not express PSA enzyme. This result indicates that RPD is non-selectively cleaved by other proteases in cells. HPD was also completely cleaved in 4 h in the LNCaP lysate, but HPD was not cleaved in the PC3 lysate even up to 48 h (Figure 3 and S6-S9). Monitoring the cleavage in lysates shows that HPD is more selective toward PSA-expressing cells and is stable against nonselective proteases.
Figure 3.
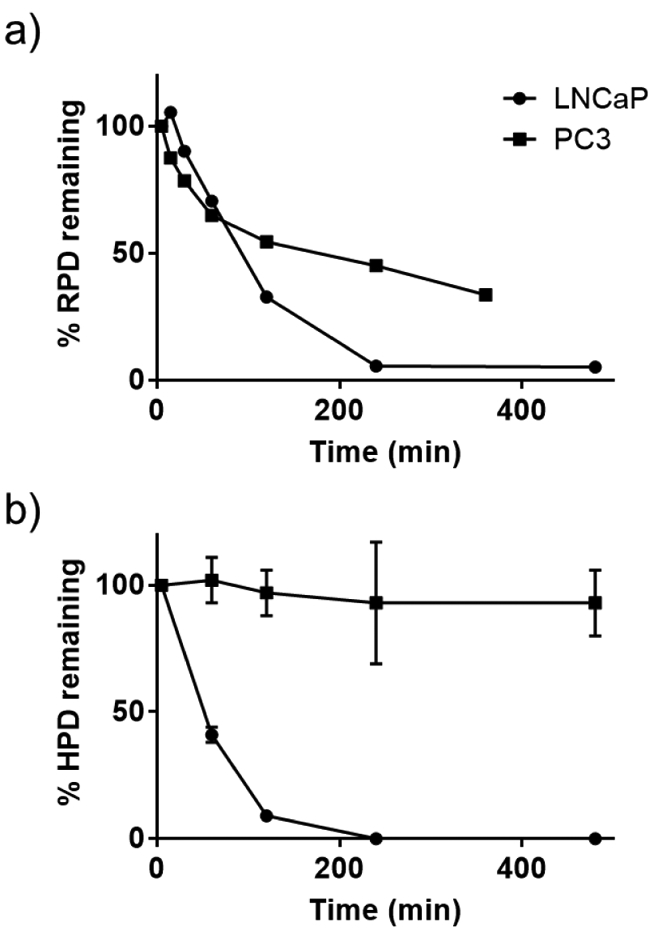
% Prodrugs remaining in LNCaP and PC3 cell lysate over time a) RPD and b) HPD.
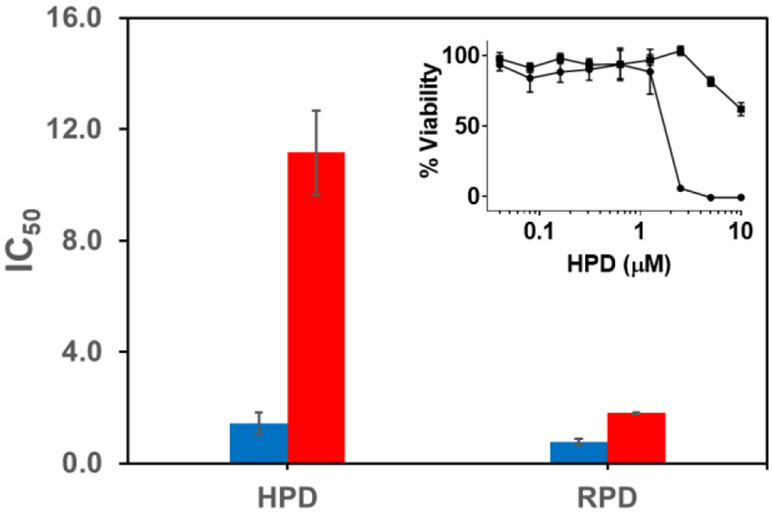
The anticancer activity of the prodrugs was evaluated in LNCaP and PC3 cell lines. The cells were treated with HPD or RPD and 1 μM CuSO4 since copper is essential for cytotoxicity induced by DTC (Figure S10).12 Inhibition of cell proliferation was monitored at 24 h using the resazurin assay. RPD displayed an IC50 of 0.77 μM in LNCaP cells and 1.8 μM in PC3 cells (Figure 4). The d-Ser variant of RPD did not show any considerable antiproliferative activity at the concentrations tested (Figure S10). These data confirm that neither the whole prodrug construct nor this level of supplemental Cu is toxic to these cells, and support the conclusion that toxicity is induced upon prodrug cleavage and subsequent interaction with Cu. Based on the cell lysate studies and above data we conclude that RPD is non-selectively cleaved by cellular proteases. Since RSSYYS peptide was derived from a chymotrypsin substrate it might be sensitive to other chymotrypsin-like serine proteases. On the other hand, HPD displayed an IC50 of 1.4 μM in LNCaP and 11.2 μM in PC3 cells. While RPD demonstrated a lower IC50, HPD demonstrated selectivity consistent with the lysate studies. With this approach we have successfully developed a PSA targeting prochelator of DTC.
Figure 4.
IC50 in μM of HPD and RPD in LNCaP (blue) and PC3 (red) cell lines. Inset: Representative plot of % viability vs prodrug concentration. The plot presented is for HPD in LNCaP (circle) and PC3 cell lines (square).
There are many metal ion chelators like dithiocarbamates that show potent anticancer activity but fail in clinical settings due to their off-target activities. The prochelator strategy reported in our previous and current work improves the efficacy of such chelators. We have previously reported a gamma glutamyl transferase targeted DTC prodrug. While this GGT targeted prodrug demonstrated excellent and selective anticancer activity in cell culture models, it would be desirable to have prodrugs that target biomarkers of prostate cancer like PSA for specific application in prostate cancers. The PSA-targeted DTC prodrugs HPD and RPD reported here were cleaved by enzymes and PSA expressing prostate cancer cells and showed desirable antiproliferative activity. Prodrug HPD gave a more selective response to PSA-producing prostate cancer cells, thereby emerging as a successful candidate for future in vivo testing. This versatile prochelator strategy can be further modified to attach peptides that could target other proteases overexpressed by different types and stages of cancer, particularly those found to be responsive to manipulation of Cu. It is not yet clear whether the released DTC pharmacophores bind extracellular Cu or interact with Cu already incorporated into the cell. Ongoing efforts to differentiate these mechanistic details are important for informing this promising strategy to overcome limitations of current prostate cancer therapies.
Supplementary Material
Acknowledgements
The authors acknowledge support from the Duke Cancer Institute P30 Cancer Center Support Grant (NIH CA014236), a Foerster-Bernstein Postdoctoral Fellowship for S.B., and a scholarship from the Royal Government of Thailand’s Development and Promotion of Science and Technology Talents Project for P.W.
Footnotes
Supplementary Data
Supplementary data (all experimental details and supporting data figures) associated with this article can be found, in the online version.
References
- (1).Bray F; Ferlay J; Soerjomataram I; Siegel RL; Torre LA; Jemal A CA Cancer J Clin 2018, 68, 394. [DOI] [PubMed] [Google Scholar]
- (2).de Bono JS; Logothetis CJ et al. N Engl J Med 2011, 364, 1995.21612468 [Google Scholar]
- (3).Scher HI; Fizazi K et al. N Engl J Med 2012, 367, 1187. [DOI] [PubMed] [Google Scholar]
- (4).Attard G; Parker C; Eeles RA; Schroder F; Tomlins SA; Tannock I; Drake CG; de Bono JS Lancet 2016, 387, 70. [DOI] [PubMed] [Google Scholar]
- (5).Ryan CJ, Smith MR, Fizazi K, Miller K, Mulders P, Sternberg CN, Saad F, Griffin T, De Porre P, Park YC, Li J, Kheoh T, Naini V, Molina A, Rathkopf D, Mills I In Eur Soc Med Onco Madrid, Spain, 2014. [Google Scholar]
- (6).Denoyer D; Pearson HB; Clatworthy SAS; Smith ZM; Francis PS; Llanos RM; Volitakis I; Phillips WA; Meggyesy PM; Masaldan S; Cater MA Oncotarget 2016, 7, 37064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Cater MA; Pearson HB; Wolyniec K; Klaver P; Bilandzic M; Paterson BM; Bush AI; Humbert PO; La Fontaine S; Donnelly PS; Haupt Y ACS Chem Biol 2013, 8, 1621. [DOI] [PubMed] [Google Scholar]
- (8).Piccardo A; Paparo F et al. J Nucl Med 2018, 59, 444. [DOI] [PubMed] [Google Scholar]
- (9).Hoberück S; Wunderlich G; Michler E; Hölscher T; Walther M; Seppelt D; Platzek I; Zöphel K; Kotzerke JJ Labelled Compd. Radiopharm 2019, 62, 523. [DOI] [PubMed] [Google Scholar]
- (10).Cen D; Brayton D; Shahandeh B; Meyskens FL; Farmer PJ J. Med. Chem 2004, 47, 6914. [DOI] [PubMed] [Google Scholar]
- (11).Lewis DJ; Deshmukh P; Tedstone AA; Tuna F; O'Brien P Chem. Commun 2014, 50, 13334. [DOI] [PubMed] [Google Scholar]
- (12).Safi R; Nelson ER; Chitneni SK; Franz KJ; George DJ; Zalutsky MR; McDonnell DP Cancer Res 2014, 74, 5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Skrott Z; Mistrik M et al. Nature 2017, 552, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Eneanya DI; Bianchine JR; Duran DO; Andresen BD Annu. Rev. Pharmacol. Toxicol 1981, 21, 575. [DOI] [PubMed] [Google Scholar]
- (15).Johansson B Acta Psychiatr Scand Suppl 1992, 369, 15. [DOI] [PubMed] [Google Scholar]
- (16).Schweizer MT; Lin J; Blackford A; Bardia A; King S; Armstrong AJ; Rudek MA; Yegnasubramanian S; Carducci MA Prostate Cancer Prostatic Dis 2013, 16, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bakthavatsalam S; Sleeper ML; Dharani A; George DJ; Zhang T; Franz KJ Angew. Chem. Int. Ed 2018, 57, 12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Wang Q; Franz KJ Acc Chem Res 2016, 49, 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Stamey TA; Yang N; Hay AR; McNeal JE; Freiha FS; Redwine E N Engl J Med 1987, 317, 909. [DOI] [PubMed] [Google Scholar]
- (20).Catalona WJ; Smith DS; Ratliff TL; Dodds KM; Coplen DE; Yuan JJJ; Petros JA; Andriole GL N Engl J Med 1991, 324, 1156. [DOI] [PubMed] [Google Scholar]
- (21).Lilja H J Clin Invest 1985, 76, 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lilja H; Abrahamsson PA; Lundwall A J Biol Chem 1989, 264, 1894. [PubMed] [Google Scholar]
- (23).Watt KW; Lee PJ; M'Timkulu T; Chan WP; Loor R Proc Natl Acad Sci U S A 1986, 83, 3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Akiyama K; Nakamura T; Iwanaga S; Hara M FEBS Lett. 1987, 225, 168. [DOI] [PubMed] [Google Scholar]
- (25).Ravery V; Boccon-Gibod L Eur Urol 1997, 31, 385. [DOI] [PubMed] [Google Scholar]
- (26).DeFeo-Jones D; Garsky VM et al. Nat. Med 2000, 6, 1248. [DOI] [PubMed] [Google Scholar]
- (27).Denmeade SR; Jakobsen CM; Janssen S; Khan SR; Garrett ES; Lilja H; Christensen SB; Isaacs JT J Natl Cancer Inst 2003, 95, 990. [DOI] [PubMed] [Google Scholar]
- (28).Elsadek B; Graeser R; Warnecke A; Unger C; Saleem T; El-Melegy N; Madkor H; Kratz F ACS Med Chem Lett 2010, 1, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).DeFeo-Jones D; Garsky VM et al. Nat. Med 2000, 6, 1248. [DOI] [PubMed] [Google Scholar]
- (30).Denmeade SR; Lou W; Lovgren J; Malm J; Lilja H; Isaacs JT Cancer Res 1997, 57, 4924. [PMC free article] [PubMed] [Google Scholar]
- (31).Coombs GS; Bergstrom RC; Pellequer JL; Baker SI; Navre M; Smith MM; Tainer JA; Madison EL; Corey DR Chem Biol 1998, 5, 475. [DOI] [PubMed] [Google Scholar]
- (32).LeBeau AM; Kostova M; Craik CS; Denmeade SR Biol Chem 2010, 391, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.