Abstract
Objective:
Organoids have recently been used as in vitro models to screen chemotherapy drugs in combination with hyperthermia treatment in colorectal cancer. Our research aimed to establish a library of patient-derived colorectal cancer organoids to evaluate synergism between chemotherapy drugs and hyperthermia; validate an index of the hyperthermia chemotherapy sensitization enhancement ratio (HCSER) to identify the chemotherapeutics most enhanced by hyperthermia; and recommend chemotherapy drugs for hyperthermic intraperitoneal treatment.
Methods:
Organoids were grown from cells extracted from colorectal cancer patient samples or colorectal cancer cell lines. Cells from both sources were encapsulated in 3D Matrigel droplets, which were formulated in microfluidics and phase-transferred into identical cell-laden Matrigel microspheres. The microspheres were seeded in 96-well plates, with each well containing a single microsphere that developed into an organoid after 7 days. The organoids were used to evaluate the efficacy of chemotherapy drugs at both 37 °C as a control and 43 °C for 90 min to examine hyperthermia synergism. Cell viability was counted with 10% CCK8.
Results:
We successfully established a library of colorectal cancer organoids from 22 patient parental tumors. We examined the hyperthermia synergism of 7 commonly used hyperthermic intraperitoneal chemotherapy drugs. In 11 of the 22 patient organoids, raltitrexed had significant hyperthermia synergism, which was indexed as the highest HCSER score within each patient group.
Conclusions:
Our results primarily demonstrated the use of patient-derived colorectal cancer organoids as in vitro models to evaluate hyperthermia synergistic chemotherapeutics. We found that hyperthermia enhanced the effect of raltitrexed the most among the common anti-colorectal cancer drugs.
Keywords: Colorectal cancer, organoids, hyperthermia chemotherapy sensitization enhancement ratio, raltitrexed
Introduction
Colorectal cancer is the second most common cause of cancer deaths worldwide1, and the median life expectancy for metastatic colorectal cancer is only 16.3 months2. Among colorectal cancer metastases, peritoneal carcinomatosis (PC) is associated with particularly poor prognosis3. Hyperthermic intraperitoneal chemotherapy (HIPEC) was first introduced to treat PC in the 1980s4. Spratt and Sugarbaker4,5 then combined HIPEC with cytoreductive surgery, thus significantly improving prognosis in patients with metastatic colorectal cancer. HIPEC shows good treatment outcomes in PC caused by abdominal malignant tumors, such as colorectal cancer and ovarian cancer. In 2018, van Driel et al.6 demonstrated that, in patients with stage III epithelial ovarian cancer, cytoreductive surgery followed by HIPEC treatment significantly improves both recurrence-free and overall survival. HIPEC has become an accepted and commonly used adjunct in treating PC caused by colorectal cancer, gastric cancer, ovarian cancer, and other cancers7.
Hyperthermia is a method used to kill tumor cells by treating the tumor area or the whole body at an elevated temperature, usually between 39 °C and 48 °C, for one to several hours. Hyperthermia has a direct cytotoxic effect on tumor cells, as well as complementary and synergistic additive effects when combined with chemotherapy. However, no index is available to quantify the synergistic effects between individual drugs and hyperthermia.
Preclinical models that accurately reflect the biological characteristics of parental tumors are essential in evaluating and screening cancer therapeutics, particularly in personalized therapy8,9. Thus, colorectal cancer cell lines play a major role in colorectal cancer biology. However, these cell lines are limited because they lack the tumor microenvironment and have permanent gene alterations. Patient-derived xenografts solve the problem of the tumor microenvironment, but they require long times to establish, have low success rates, and are expensive. Meanwhile, whether mouse-based models can accurately simulate human diseases is a matter of debate. In this regard, the use of patient-derived organoids (PDOs) has led to the rapid development of precision cancer medicine10–12.
Organoids recapitulate the characteristics of tumors and their microenvironment in artificially engineered conditions, and they can overcome the shortcomings of cell lines in anticancer drug testing. They maintain homology with their primary tumors for as long as 1 year and more than 20 generations13. Organoid technology has developed into a high-throughput human biology analytic tool and is widely used to search for effective personalized treatment methods11. In the present study, we validated the application of PDOs in preclinical therapy screening for patient-variant colorectal cancer. We also identified raltitrexed as a chemotherapeutic drug that shows synergistic effects with hyperthermic intraperitoneal treatment.
Materials and methods
Human specimens and colorectal cancer cell lines
The present study was approved by the ethics committee of the Affiliated Cancer Hospital and Institute of Guangzhou Medical University. The colorectal cancer PDOs were derived from patients in the Affiliated Cancer Hospital and Institute of Guangzhou Medical University, between May and August 2020. The patients with colorectal cancer were diagnosed on the basis of pathology results, treated with cytoreductive surgery, and followed up with or without hyperthermic intraperitoneal chemotherapy. All colorectal cancer cell lines used in this study, including SW620, SW480, DLD-1, and COLO 205, were purchased from Procell Life Science & Technology Co. Ltd.
Establishing colorectal cancer organoids
Colorectal cancer PDOs and colorectal cancer cell line organoids were harvested and dissociated into single cells according to the passaging procedure described below. The patient tumor specimens were immersed in an organic preservative solution (AQIX, UK) and transported from the hospital to the laboratory at 4 °C. The specimens were used to extract cells for fabricating colorectal cancer organoids.
Tumor tissues were washed 3 times in cold PBS (1×) solution containing 3.0% penicillin-streptomycin (v/v; Gibco). They were then cut into small pieces, and the tissues were digested with 1.0 mg/mL collagenase type I (Sigma-Aldrich), with 3.0% penicillin-streptomycin and 2.0% FBS (v/v, Invitrogen Tech), on an orbital shaker at 37 °C and for 1–2 h. After digestion, the tissues were sheared with 5 mL plastic pipettes. The suspension was passed through a 100 μm filter (Falcon) and centrifuged at 1,000 rpm for 5 min. The pellet was resuspended in Dulbecco’s modified Eagle’s medium/F12 medium. The number of extracted cells depended on the size of each tumor specimen—approximately 4.0 × 106 cells were extracted from a fingernail-sized segment of tissue. The cells were counted with a hemocytometer.
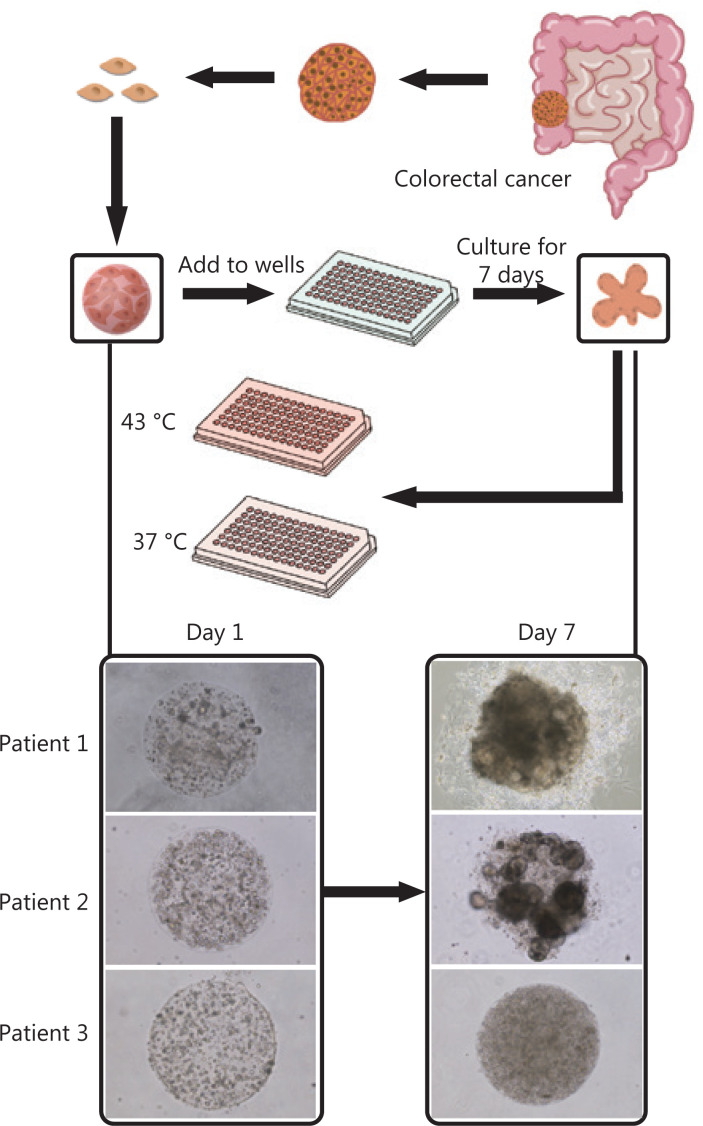
The cells were then suspended in growth factor-reduced Matrigel (Corning) at a density of 2.0 × 107 cells/mL. The Matrigel phase was subsequently loaded into a 1 mL syringe and installed in an injection pump. A fluorocarbon oil (HFE-7000; 3M Novec) was loaded into a 10 mL injection syringe and installed in another injection pump. Both pumps were placed in a refrigerator at 4 °C. These 2 phases were co-injected through polytetrafluoroethylene tubing (inner diameter, 600 μM; Woer) into a third piece of polytetrafluoroethylene tubing via a 3-way, handmade polydimethylsiloxane connector. The Matrigel phase was injected at a flow rate of 20 μL/min and subsequently sheared into monodisperse droplets by the fluorocarbon oil at a flow rate of 30 μL/min. The tubing carrying the Matrigel droplets, which was approximately 10 m long, was heated to 37 °C with a small water bath. The Matrigel droplets were circulated in the warmed tubing for 10 min before reaching the outlet, which was connected to the droplet printer head. The Matrigel beads were gelled from droplets and printed onto a 96-well plate. The printing sequences of the beads matched their sequences in the tubing. After printing, the beads were further incubated at 37 °C for 20 min to complete solidification. Next, 200 μL of culture medium was added to each well containing one cell-laden Matrigel bead, which constituted an organoid precursor. The culture medium consisted of 20% FBS, 1% penicillin-streptomycin, 100 ng/mL noggin (MCE), 100 ng/mL R-spondin 1 (MCE), 5 ng/mL epidermal growth factor (Peprotech), 10 ng/mL fibroblast growth factor-basic (MCE), 1× GlutaMAX supplement (Thermo Fisher Scientific), 10 mM HEPES (Thermo Fisher Scientific), 1× B-27 Supplement (Thermo Fisher Scientific), 5 mM nicotinamide (Sigma-Aldrich), 1.25 mM N-acetylcysteine (Sigma-Aldrich), 5 μM Y-27632 (Abmole), and 1× FibrOut™ (Chi Scientific). The preparation was then cultured at 37 °C in an incubator supplied with 5% CO2. The medium was changed every 3 days. Images of the organoids were acquired on days 1 and 7. The organoids were then harvested for further analysis or conditioned with drugs. Details on organoid fabrication and printing are described in another manuscript currently under review.
Cell viability assay
Cell viability was assessed with Cell Counting Kit-8 (CCK-8) assays (Dojindo, Kumamoto, Japan). The cell suspension (100 μL) was dispensed into 96-well plates. CCK-8 solution (10 μL/well) was then added, and the preparation was incubated for 60 min. The absorbance was measured at 450 nm with a microplate reader. Cell viability was calculated as a percentage of the values for untreated controls. Half-maximal inhibitory concentration (IC50) values for each chemotherapy drug were calculated on the basis of cell viability curves in SPSS software (Version 26.0, USA).
Drug screening by using colorectal cancer organoids
The colorectal cancer PDOs were established within 7 days. Cell line-derived organoids were established 3 days after the cell suspension was obtained. Subsequently, the culture medium was removed and replaced with 200 μL of drug-conditioned complete human organoid medium in each culturing well. The drug-conditioned medium was removed after 2 days and replaced with 100 μL of complete human organoid medium containing 10% CCK-8. The plates were placed back in the incubator (Thermo Fisher Scientific, Heraeus BB15). In most experiments, at least 3 replicates of each viability reading were acquired at each data point. Seven chemotherapy drugs were evaluated: oxaliplatin, lobaplatin, 5-fluorouracil, gemcitabine, mitomycin, raltitrexed, and abraxane. The concentration of each drug in the conditioned medium ranged from 0 to 1,000 μM (Table 1).
Table 1.
Information on the chemodrugs used in this study
| Drug name | Drug brand | Drug specifications |
|---|---|---|
| Raltitrexed | NANJING CHIA TAI TIANQING | 2 mg |
| Mitomycin | HANHUI PHARMA | 10 mg |
| Oxaliplatin | SANOFI | 50 g |
| 5-fluorouracil | PUDEP HARMA | 0.25 g |
| Lobaplatin | Hainan Changan International Pharmaceutical Co. Ltd. | 50 mg |
| Gemcitabine | NANJING CHIA TAI TIANQING | 0.2 g |
| Abraxane | CSPC | 100 mg |
Hyperthermia treatment of organoids
The tested organoids were seeded in 96-well plates and treated with hyperthermia. Specifically, the cells were heated in a cell incubator set at 43 °C for 90 min. They were then incubated at 37 °C in a regular cell culture incubator for another 48 h. Viability was determined with CCK-8 assays. The absorbance of the solution was measured at 450 nm, and the IC50, producing 50% cell death, was calculated via curve fitting in SPSS 26.0 software (Version 26.0, USA).
Western blot
Total protein extracts were obtained from 6 colorectal cancer tissues with lysis buffer containing protease inhibitor. The protein concentrations were then determined with a bicinchoninic acid protein assay kit (KenGEN BioTECH, Nanjing, China). First, 30-μg protein samples were separated with SDS-PAGE and transferred to polyvinylidene fluoride membranes. The membranes were then blocked with 8% skimmed milk for 2 h at room temperature and incubated in antibody at 4 °C overnight. After being washed 3 times with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h. GAPDH was used as an internal control. Protein expression was visualized with a chemiluminescence system (Tanon 5200; Tanon Science & Technology Co. Ltd., Shanghai, China).
The information on primary antibodies and the respective applications in this study are shown in Supplementary Table S1.
HIPEC procedure in patients
After surgical cytoreduction, HIPEC was administered for 90 min with chemotherapy drugs in 4–6 L of saline solution maintained at 43 °C and infused at a flow rate of 400–600 mL/min. The temperature of the perfusate was monitored with precision probes at multiple sites, including the entrance and exit ports, the tumor lesions, and the normal tissues. After perfusion was complete, the perfusate was drained. The fascia and skin were then closed according to standard surgical protocols.
Statistical analysis
Statistical analysis was performed in SPSS 26.0 and GraphPad Prism 7.0 software (GraphPad Software Inc., La Jolla, CA, USA). IC50 calculations were obtained with 4–6 concentrations of each compound and generated in SPSS 26.0 Logit. All data are expressed as mean ± standard deviation. Student’s unpaired t-test were used to compare continuous variables between two groups, whereas Pearson’s χ2 test or Fisher’s exact test were used to compare categorical variables between two groups. P < 0.05 indicated a statistically significant difference.
Results
Establishment of PDOs by using in vitro tumor models of colorectal cancer
The colorectal cancer organoids were derived from the surgical specimens of 22 patients. Colorectal cancer cell lines, including SW620, SW480, DLD-1, and COLO 205, were also used to construct organoids for parallel studies. The cells from both sources were encapsulated into monodisperse, cell-laden Matrigel tumor microspheres (diameter, 600 μm) and then cultured as suspensions in medium in 96-well plates. Cell line-derived organoids or PDOs were obtained after cell proliferation and self-organization in the individual microspheres for 3 days (for cell lines) or 1 week (for PDOs) (Figure 1). According to the 2019 version of the consensus reached by Chinese medical experts on the clinical applications of HIPEC7, the commonly used HIPEC drugs for colorectal cancer include mitomycin, oxaliplatin, and 5-fluorouracil. Other chemotherapy drugs are on the recommended list but require further exploration. Notably, the recommended concentrations of chemotherapy drugs in HIPEC are usually 1–2 orders of magnitude higher than those used in intravenous chemotherapy. On this basis, we designed an investigation portfolio comprising these 3 common drugs and 4 other pending drugs, with dose variations. The median doses were arbitrarily chosen; however, they were generally 1–2 orders of magnitude higher than the commonly responsive doses reported for organoid screening (Table 2). The organoids were conditioned with single drugs at the concentrations listed in Table 2 at 37 °C and 43 °C for 90 min.
Figure 1.
Establishment of patient-derived organoids as in vitro tumor models for colorectal cancer. Morphology of patient-derived organoids (PDOs) on days 1 and 7. After 7-day culture, the organoids were used to evaluate hyperthermia and chemotherapy. The figure shows a flowchart of hyperthermia and chemotherapy evaluation. PDOs are bifurcated to evaluate the following chemotherapy drugs at 37 °C and 43 °C: mitomycin, abraxane, 5-fluorouracil, oxaliplatin, raltitrexed, gemcitabine, and lobaplatin.
Table 2.
Drug doses used clinically and in experiments
| Drug name | Recommended dose in clinically of single chemotherapy | Intraperitoneal perfusion concentration | Experimental setting |
|---|---|---|---|
| Raltitrexed | 3 mg/m2 | 2.8 μM | 0/0.2/2/20/200/2,000 μM |
| Mitomycin | 6–8 mg | 5.9 μM | 0/0.05/0.5/5/50/500 μM |
| Oxaliplatin | 130 | 139 μM | 0/0.1/1/10/100/1,000 μM |
| 5-Fu | 500–600 mg/m2 | 1.96 μM | 0/0.02/0.2/2/20/200 μM |
| Lobaplatin | 50 mg/m2 | 53.5 μM | 0/0.05/0.5/5/50/500 μM |
| Gemcitabine | 1,000/1,250 mg/m2 | 1.8 μM | 0/0.02/0.2/2/200/2,000 μM |
| Abraxane | 260 mg/m2 | 122.9 μM | 0/0.1/1/10/100/1,000 μM |
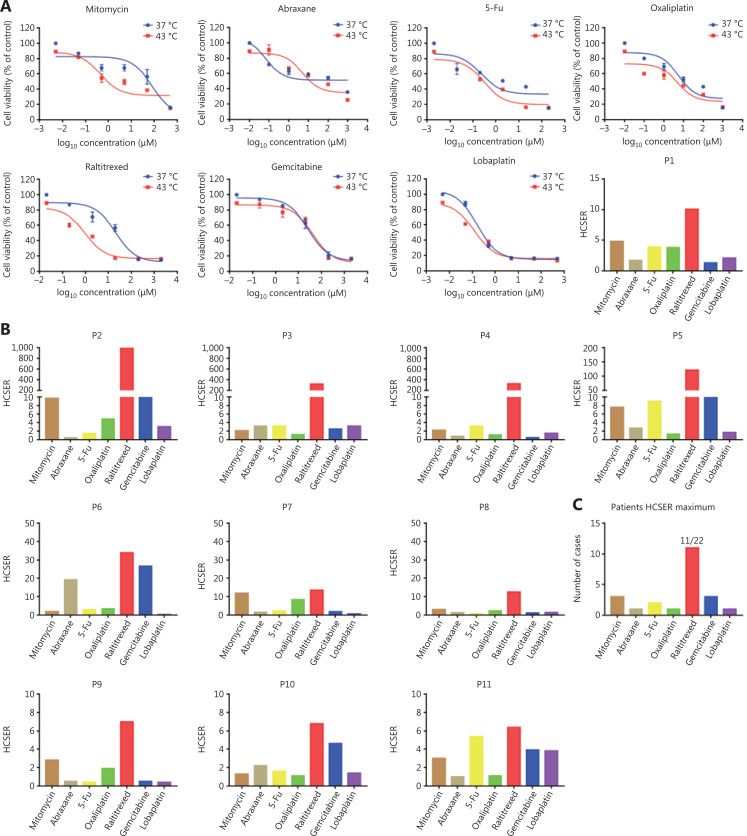
Raltitrexed has the highest hyperthermia chemotherapy sensitization enhancement ratio score among the screened chemotherapy drugs
To assess whether our colorectal cancer organoids were applicable as in vitro tumor models to evaluate patient-dependent variance in chemotherapy drug sensitivity, we generated dose-response curves for the 7 drugs and calculated their half-maximal inhibitory concentrations (IC50). The drug library was composed of oxaliplatin, lobaplatin, 5-fluorouracil, gemcitabine, mitomycin, raltitrexed, and abraxane. The hyperthermia chemotherapy sensitization enhancement ratio (HCSER) was used to evaluate the sensitizing effect of hyperthermia on chemotherapy. The HCSER is the ratio of the concentration of a drug applied to the conditions of chemotherapy alone or in combination with hyperthermia therapy that results in the same consequence of killing tumor cells, for example, 50% residual viability. The formula was HCSER = IC50 (37 °C)/IC50 (43 °C). In 11 of 22 patient organoids, raltitrexed had the highest HCSER score within each patient group. The differences in the hyperthermia chemotherapy sensitization enhancement ratio were statistically significant (P < 0.01) between the high-HCSER group and the low-HCSER group (Table 3). All IC50 values, HCSER scores, and their standard deviations are provided in Supplementary Table S2.
Table 3.
Comparison of HCSER between the high-HCSER group and the low-HCSER group
| High-HCSER group |
Low-HCSER group |
||||
|---|---|---|---|---|---|
| Cases | Average | SD | Cases | Average | SD |
| 11 | 167.37 | 297.4 | 11 | 4.89 | 5.90 |
| P | 0.0019 | ||||
The High-HCSER group contains patients 1–11, and the low-HCSER group contains patients 12–22. SD, standard deviation. P < 0.05 was considered statistically significant.
Raltitrexed is an anti-metabolic folate analog that specifically inhibits thymidylate synthase (TS)14,15, a key enzyme in the synthesis of thymidine triphosphate, a nucleotide necessary for DNA synthesis. Inhibition of TS can lead to DNA breakage and apoptosis16. Raltitrexed is a direct and specific TS inhibitor that can enhance the inhibition ability of TS and prolong the inhibition time17.
Organoids derived from patient 1 were tested with the 7 single drugs at both 37 °C and 43 °C in parallel. Raltitrexed had the highest HCSER of 10.1 (Figure 2A). Among the 22 patients investigated, raltitrexed had the highest HCSER score under hyperthermia synergism in 11 patients (Figure 2B and 2C). In the other 11 patients, the drugs with the highest HCSER scores obtained from the PDO library were mitomycin (3 of 22 patients), gemcitabine (3 of 22 patients), 5-Fu (2 of 22 patients), abraxane (1 of 22 patients), oxaliplatin (1 of 22 patients), and lobaplatin (1 of 22 patients) (Supplementary Figure S1).
Figure 2.
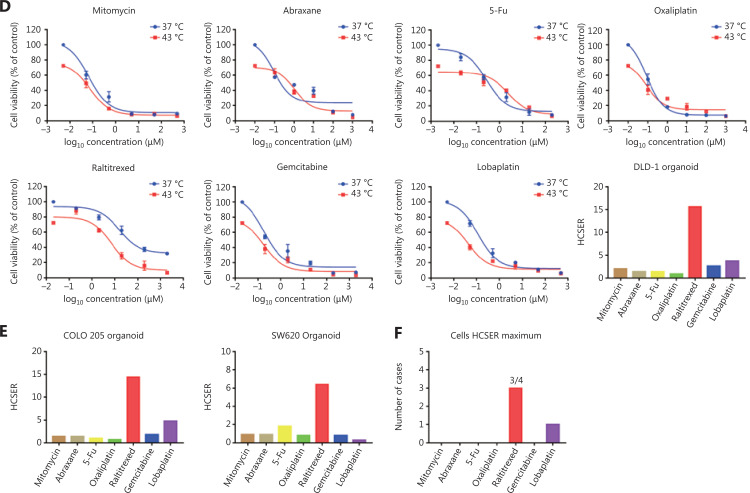
Raltitrexed has the highest hyperthermia chemotherapy sensitization enhancement ratio score among the screened chemotherapy drugs. (A) The dose-response curves of organoids derived from patient 1 after treatment with different drugs for 90 min at 37 °C and 43 °C. Cell viability at 37 °C was measured with 10% CCK-8 after 2 days under drug-free conditions after drug treatment. The hyperthermia chemotherapy sensitization enhancement ratio (HCSER) score was obtained by calculating the ratio of the IC50 values between 37 °C and 43 °C for each drug. That is, HCSER = IC50 (37 °C)/IC50 (43 °C). (B) The HCSER scores of organoids derived from the other 10 patients. Raltitrexed had the highest HCSER score. (C) The highest HCSER scores of the 7 chemotherapy drugs in organoids from 22 patients. (D) Dose-response curves of organoids derived from DLD-1 cell lines, and the HCSER summary for all evaluated drugs. (E) The HCSER scores of the organoids derived from another 2 colorectal cancer cell lines. Raltitrexed showed the highest HCSER scores. (F) Counts of the highest HCSER scores of the 7 chemotherapy drugs in the 4 colorectal cancer cell lines.
We performed a similar evaluation with colorectal cancer cell lines. Raltitrexed showed the highest HCSER scores in most of the cell lines investigated, including DLD-1, SW620, and COLO 205, although lobaplatin had the highest HCSER score in the SW480 cell line (Figures 2D–2F and Supplementary S2). These findings suggest that raltitrexed has a high level of hyperthermia synergism in the treatment of colorectal cancers, and that this drug should be included on the recommendation list for clinical administration of HIPEC.
Effects of raltitrexed on biological processes in colorectal cancer
Next, to ascertain how raltitrexed confers hyperthermia sensitization, we performed transcriptomic analysis with RNA sequencing (RNA-seq) of 5 primary tumors that were distinctively sensitive or non-sensitive to raltitrexed. The analysis included tumors from 3 patients (P15, P20, and P22) who were insensitive to raltitrexed (HCSER < 2) and 2 patients (P5 and P10) who were highly sensitive (HCSER > 6). More than 1,200 differentially expressed genes were identified between the raltitrexed sensitive and insensitive groups, with screening criteria of log2 (fold change) > 0.5 or < −0.5, and a P-value ≤ 0.05.
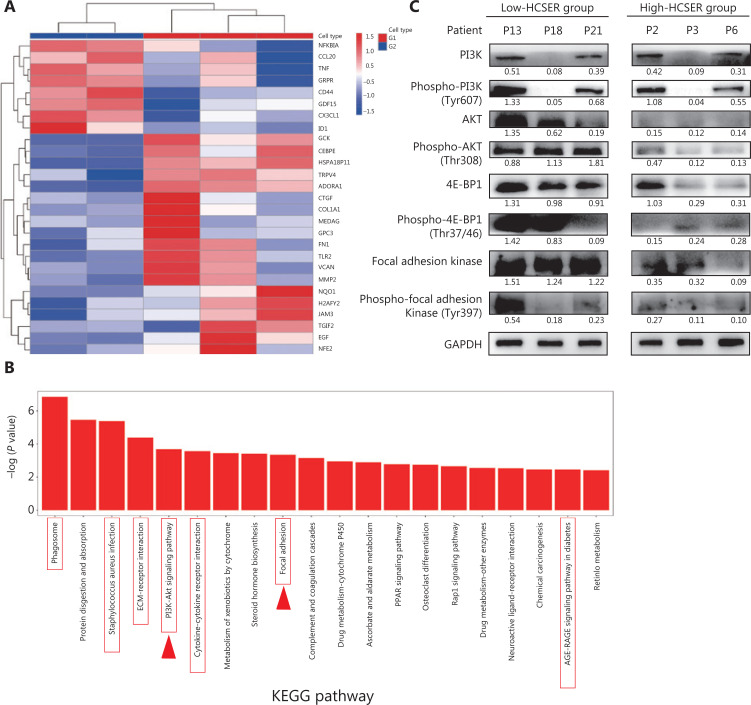
Twenty-seven genes showed a synergistic correlation between hyperthermia and chemotherapy (Table 4). According to the differentially expressed genes screened among the sampled groups, the genes and samples were clustered bidirectionally and displayed on a heat map (Figure 3A). We performed KEGG pathway analysis, which showed that numerous pathways might contribute to the differential sensitivity, including PI3K-AKT signaling18 and focal adhesion19 pathways that affect hyperthermia (Figure 3B). To investigate whether hyperthermia enhanced raltitrexed treatment by regulating the PI3K/AKT/mTOR and Focal Adhesion Kinase signaling pathways, we performed Western blot on tumors from 3 patients (P13, P18, and P21) with high HCSER scores and another 3 patients (P2, P3, and P6) with low HCSER scores. The expression levels of PI3K, p-PI3K, AKT, p-AKT, 4E-BP1, p-4E-BP1, Focal Adhesion Kinase, and p-Focal Adhesion Kinase were downregulated in the high-HCSER group. These findings suggested that hyperthermia may enhance raltitrexed in colorectal cancer by inhibiting the PI3K/AKT/mTOR and Focal Adhesion Kinase signaling pathways (Figure 3C).
Table 4.
Information on 27 genes previously reported to be associated with hyperthermia
| Gene | Title of related article | PMID |
|---|---|---|
| NFKBIA | Short-term hyperthermia prevents activation of proinflammatory genes in fibroblast-like synoviocytes by blocking the activation of the transcription factor NF-kappaB. | 16955275 |
| CCL20 | Local hyperthermia decreases the expression of CCL-20 in condyloma acuminatum. | 21050487 |
| TNF | Comparative in vitro studies of the potentiation of tumor necrosis factor (TNF)-alpha, TNF-beta, and TNF-SAM2 cytotoxicity by hyperthermia. | 1571335 |
| GRPR | How gastrin-releasing peptide receptor (GRPR) and α(v)β(3) integrin expression reflect reorganization features of tumors after hyperthermia treatments. | 28761146 |
| CD44 | CD44-targeted magnetic nanoparticles kill head and neck squamous cell carcinoma stem cells in an alternating magnetic field. | 31571863 |
| GDF15 | Involvement of ERK1/2 signalling and growth-related molecules’ expression in response to heat stress-induced damage in rat jejunum and IEC-6 cells. | 20707649 |
| CX3CL1 | Heat therapy promotes the expression of angiogenic regulators in human skeletal muscle. | 27357800 |
| ID1 | Correlation between the expression of Id-1 and hyperthermia-associated molecules in oral squamous cell carcinoma. | 23723304 |
| GCK | Cellular signalling after in vivo heat shock in the liver. | 10772775 |
| CEBPE | Gene networks involved in apoptosis induced by hyperthermia in human lymphoma U937 cells. | 19732844 |
| HSPA8P11 | The cellular and molecular basis of hyperthermia. | 12098606 |
| TRPV4 | Ischemic brain injury leads to brain edema via hyperthermia-induced TRPV4 activation. | 29793978 |
| ADORA1 | Activation of central adenosine A(2A) receptors lowers the seizure threshold of hyperthermia-induced seizure in childhood rats. | 21144776 |
| CTGF | Role of CTGF in sensitivity to hyperthermia in ovarian and uterine cancers. | 27806300 |
| COL1A1 | Molecular pathology of vertebral deformities in hyperthermic Atlantic salmon (Salmo salar). | 20604915 |
| MEDAG | Hyperthermia severely affects the vascular effects of MDMA and metabolites in the human internal mammary artery in vitro. | 28084566 |
| GPC3 | Glypican-3 (GPC3) targeted Fe3O4 core/Au shell nanocomplex for fluorescence/MRI/photoacoustic imaging-guided tumor photothermal therapy. | 31603456 |
| FN1 | Role of CTGF in sensitivity to hyperthermia in ovarian and uterine cancers. | 27806300 |
| TLR2 | Extracellular heat shock protein 70 mediates heat stress-induced epidermal growth factor receptor transactivation in A431 carcinoma cells. | 17126326 |
| VCAN | Versican and vascular endothelial growth factor expression levels in peritoneal metastases from colorectal cancer are associated with survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. | 26873137 |
| MMP2 | Hyperthermia inhibits the motility of gemcitabine-resistant pancreatic cancer PANC-1 cells through the inhibition of epithelial-mesenchymal transition. | 29568909 |
| NQO1 | Anti-cancer effect of bio-reductive drug beta-lapachon is enhanced by activating NQO1 with heat shock. | 18283592 |
| H2AFY2 | Proteomic and bioinformatic analysis of condyloma acuminata: mild hyperthermia treatment reveals compromised HPV infectivity of keratinocytes via regulation of metabolism, differentiation and anti-viral responses. | 30909744 |
| JAM3 | Core temperature correlates with expression of selected stress and immunomodulatory genes in febrile patients with sepsis and noninfectious SIRS. | 19496026 |
| TGIF2 | Involvement of ERK1/2 signalling and growth-related molecules’ expression in response to heat stress-induced damage in rat jejunum and IEC-6 cells. | 20707649 |
| EGF | Effect of hyperthermia on invasion ability and TGF-β1 expression of breast carcinoma MCF-7 cells. | 21455587 |
| NFE2 | Hyperthermia and protein homeostasis: cytoprotection and cell death. | 32716865 |
Figure 3.
Gene expression profiles of raltitrexed hyperthermia sensitization. (A) Clustered heat map of differentially expressed genes. G1 denotes patients insensitive to raltitrexed (P15, P20, and P22). G2 denotes patients sensitive to raltitrexed (P5 and P10). (B) Enrichment in KEGG pathway terms for differentially expressed genes between the G1 and G2 groups. The horizontal axis denotes the enriched KEGG pathways, and the vertical axis displays the −log10 (P-value). The red boxes represent the 27 genes participating in these pathways, and the red arrows represent pathways reported to be associated with hyperthermia. (C) Western blot analysis was conducted to assay the downregulated expression of PI3K, p-PI3K, AKT, p-AKT, 4E-BP1, p-4E-BP1, Focal Adhesion Kinase, and p-Focal Adhesion Kinase in the raltitrexed high-HCSER group. The low-HCSER group contains patients 13, 18, and 21, and the high-HCSER group contains patients 2, 3, and 6.
Colorectal cancer PDOs reflect the clinical outcomes of representative patients with colorectal cancer
We collected surgically removed colorectal cancer tissues from 22 patients (Table 5), all of whom had undergone radical colorectal cancer resection, and 6 of whom had received HIPEC treatment after surgery. The drugs for HIPEC included raltitrexed, mitomycin, and oxaliplatin.
Table 5.
Clinical and pathological features of colorectal cancer patients in 2 groups
| Characteristics | Surgery (n = 16) | Surgery + HIPEC (n = 6) | Total (n = 22) |
|---|---|---|---|
| Age | |||
| Mean ± SD | 58.1 ± 13.5 | 54.2 ± 3.5 | 57.0 ± 11.7 |
| Range | 37–82 | 50–60 | 37–82 |
| Gender | |||
| Male | 10 | 3 | 13 |
| Female | 6 | 3 | 9 |
| Primary tumor site | |||
| Ascending colon | 3 | 1 | 4 |
| Transverse colon | 1 | 0 | 1 |
| Descending colon | 1 | 0 | 1 |
| Sigmoid colon | 4 | 3 | 7 |
| Rectum | 7 | 2 | 9 |
| Drug used in HIPEC | |||
| Mitomycin | 0 | 3 | 3 |
| Raltitrexed | 0 | 1 | 1 |
| Raltitrexed + mitomycin + oxaliplatin | 0 | 2 | 2 |
| Surgical modality | |||
| Radical surgery | 14 | 3 | 17 |
| Palliative surgery | 2 | 3 | 5 |
| TNM stage | |||
| Stage I | 2 | 1 | 3 |
| Stage II | 4 | 2 | 6 |
| Stage III | 8 | 0 | 8 |
| Stage IV | 2 | 3 | 5 |
| Metastatic sites | |||
| Liver | 2 | 2 | 4 |
| Adrenal gland | 1 | 0 | 1 |
| Abdominal cavity | 0 | 1 | 1 |
| CEA (normal: < 5.0 ng/mL) | |||
| Normal | 9 | 3 | 12 |
| Abnormal | 7 | 3 | 10 |
| CA19-9 (normal: < 30 U/mL) | |||
| Normal | 13 | 4 | 17 |
| Abnormal | 3 | 2 | 5 |
| CA72-4 (normal: < 6.9 U/mL) | |||
| Normal | 11 | 4 | 15 |
| Abnormal | 5 | 2 | 7 |
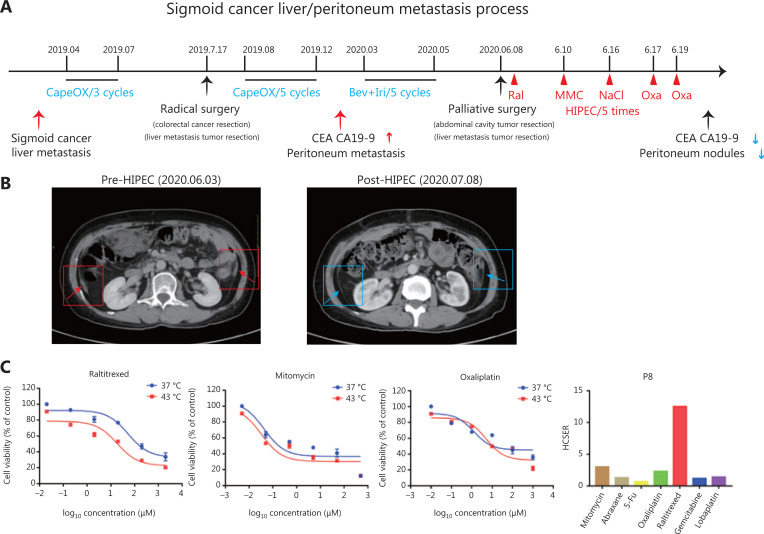
Patient 8 was diagnosed with sigmoid cancer combined with live metastasis in April 2019. She received 3 rounds of CapeOX (capecitabine and oxaliplatin) to reduce the tumor size in preparation for surgery. On July 17, 2019, the patient underwent colorectal cancer resection and liver metastasis tumor resection surgery, followed by 5 rounds of CapeOX (Figure 4A). However, 7 months after surgery, she experienced peritoneal metastasis. Computed tomography (CT) showed peritoneal thickening, and the expression of tumor markers (CEA and CA19-9) increased. After 5 rounds of chemotherapy, her overall condition had not improved. On June 6, 2020, she underwent palliative resection of an abdominal cavity tumor and a liver metastatic tumor. Liver metastases and metastasis nodules were found in the omentum and abdominal wall.
Figure 4.
Patient-derived organoids reflect personal response to chemotherapy in combination with hyperthermic intraperitoneal chemotherapy. (A) Treatment and procedure timeline in patient 8. The values highlighted in blue indicate the administration of chemotherapy. The values highlighted in red indicate the efficacy of hyperthermic intraperitoneal chemotherapy. (B) Left: CT image of patient 8 before surgery. Right: CT image of patient 8 at 1 month after surgery. The small nodules and peritoneal fluid decreased, as compared with those in the left CT image. (C) Cell viability of organoids derived from patient 8 in response to raltitrexed, mitomycin, and oxaliplatin, and the HCSER for those drugs.
Small intraperitoneal metastatic lesions were difficult to remove by surgery. Therefore, after the palliative resection surgery, Patient 8 received 5 rounds of HIPEC treatment with raltitrexed, mitomycin, and oxaliplatin on day 1 (24 h), day 2, day 8, day 9, and day 11. The second HIPEC therapy did not use any drug, but only 0.9% NaCl. At 1 month after surgery, CT revealed small nodules and reduced peritoneal fluid, thus indicating that HIPEC had relieved the symptoms in this patient (Figure 4B). The drug responses of the organoids derived from the same patient showed that raltitrexed, mitomycin, and oxaliplatin had high HCSER scores, thereby validating our PDOs for screening personalized chemotherapy drugs in HIPEC therapies (Figure 4C). These results also indicated that hyperthermia enhanced the effect of raltitrexed the most among the common anti-colorectal cancer drugs.
Discussion
A worldwide consensus on chemotherapy drugs in HIPEC currently exists20. Mitomycin and oxaliplatin are the most frequently used drugs in clinical and research protocols. However, because of the absence of effective pre-clinical models to evaluate drug efficacy, the performance and clinical recommendations for these drugs remain under debate21. Moreover, other clinically approved drugs should be added to HIPEC regimens to expand the selection pool and allow surgeons to match inter-patient variations in drug sensitivity. Hence, in the present study, we introduced PDOs as pre-clinical tumor models to evaluate HIPEC-associated chemotherapy drugs. We validated the reliability of PDOs for this purpose in a small library of samples from patients with colorectal cancer. In conjunction with PDO evaluation, we introduced the HCSER to evaluate HIPEC-associated drug choice. The HCSER was calculated with the IC50 of a drug at 37 °C and 43 °C treatment. In this way, it was used to examine the hyperthermia sensitization of chemotherapy drugs.
We found that raltitrexed had the highest HCSER score among the screened drugs, thus implying that it should be included in the clinical recommendations for HIPEC treatment. However, further pre-clinical studies should be conducted before such action is taken, because the current study has some limitations. For example, the patient cohort was fairly small. To further validate our findings, an expanded library with a richer diversity of samples should be established. For example, patients should be recruited from different regions of China or even worldwide. The performance of this treatment regimen in other cancers should also be studied to draw conclusions beyond the scope of colorectal cancer.
Further mechanistic study of hyperthermia sensitization is required for therapeutic improvement. We performed a sequence study on 2 samples that were sensitive to raltitrexed and 3 that were insensitive. The RNA sequencing results showed that 8 hyperthermia-related genes were upregulated in the raltitrexed-sensitive group, and 19 hyperthermia- related genes were downregulated. KEGG analysis showed that the PI3K-AKT and focal adhesion signaling pathways might be involved in the hyperthermia sensitization to raltitrexed. Huang et al.19 have reported that hyperthermia may disrupt the integrin-mediated actin cytoskeleton in thyroid carcinoma by affecting focal adhesions. Fang et al.18 have confirmed that hyperthermia improves the sensitivity of Raji cells to chemotherapy drugs by blocking the PI3K/AKT pathway and downregulating HSP70. However, further studies must be performed to ascertain the mechanism.
Personalized precision treatment of cancer has become a focus of medical research and clinical practice worldwide. PDOs have been suggested as personalized cancer screening models that can reflect inter-patient variability in drug sensitivity22–25. Furthermore, they are promising in the evaluation of personalized synergism between hyperthermia and chemotherapy. The present study indicated that PDOs are beneficial in evaluating personalized chemotherapy drug selection in HIPEC therapy. Additional clinical validation will be performed in follow-up studies.
Conclusions
In brief, our research primarily demonstrated that colorectal cancer PDOs can be used as in vitro cancer models to evaluate hyperthermia synergistic chemotherapeutics. We found that hyperthermia enhanced the effect of raltitrexed the most among the common anti-colorectal cancer drugs.
Supporting Information
Footnotes
Conflict of interest statement No potential conflicts of interest are disclosed.
Grant support
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81972918 and 61971255), Shenzhen Science and Technology Innovation Committee (Grant No. KQJSCX20180327143623167), NANJING CHIA TAI TIANQING Company, Foundation for Young Innovative Talents in Education of Guangdong (Grant No. 2017KQNCX161), Natural Science Foundation of Guangdong Province (Grant No. 2018A030310249), and Key Clinical Technique of Guangzhou (Grant No. 2019ZD16).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol. 2016;17:1709–19. doi: 10.1016/S1470-2045(16)30500-9. [DOI] [PubMed] [Google Scholar]
- 3.Vassos N, Piso P. Metastatic colorectal cancer to the peritoneum: current treatment options. Curr Treat Options Oncol. 2018;19:49. doi: 10.1007/s11864-018-0563-8. [DOI] [PubMed] [Google Scholar]
- 4.Spratt JS, Adcock RA, Sherrill W, Travathen S. Hyperthermic peritoneal perfusion system in canines. Cancer Res. 1980;40:253–5. [PubMed] [Google Scholar]
- 5.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230–40. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 7.Shuzhong C. Chinese expert consensus on the clinical application of hyperthermic intraperitoneal chemotherapy (2019 version) Natl Med J China. 2020;100:89–96. (in Chinese) [Google Scholar]
- 8.Koga Y, Ochiai A. Systematic review of patient-derived xenograft models for preclinical studies of anti-cancer drugs in solid tumors. Cells. 2019;8:418. doi: 10.3390/cells8050418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jo Y, Choi N, Kim K, Koo HJ, Choi J, Kim HN. Chemoresistance of cancer cells: requirements of tumor microenvironment-mimicking in vitro models in anti-cancer drug development. Theranostics. 2018;8:5259–75. doi: 10.7150/thno.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu HD, Xia BR, Jin MZ, Lou G. Organoid of ovarian cancer: genomic analysis and drug screening. Clin Transl Oncol. 2020;22:1240–51. doi: 10.1007/s12094-019-02276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernandez-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920–6. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopper O, de Witte CJ, Lohmussaar K, Valle-Inclan JE, Hami N, Kester L, et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med. 2019;25:838–49. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 13.He C, Ruan F, Jiang S, Zeng J, Yin H, Liu R, et al. Black phosphorus quantum dots cause nephrotoxicity in organoids, mice, and human cells. Small. 2020;16:e2001371. doi: 10.1002/smll.202001371. [DOI] [PubMed] [Google Scholar]
- 14.Zalcberg JR, Cunningham D, Van Cutsem E, Francois E, Schornagel J, Adenis A, et al. Zd1694: a novel thymidylate synthase inhibitor with substantial activity in the treatment of patients with advanced colorectal cancer. Tomudex colorectal study group. J Clin Oncol. 1996;14:716–21. doi: 10.1200/JCO.1996.14.3.716. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham D, Zalcberg JR, Rath U, Olver I, Van Cutsem E, Svensson C, et al. ‘Tomudex’ (zd1694): results of a randomised trial in advanced colorectal cancer demonstrate efficacy and reduced mucositis and leucopenia. The ‘tomudex’ colorectal cancer study group. Eur J Cancer. 1995;31A:1945–54. doi: 10.1016/0959-8049(95)00502-1. [DOI] [PubMed] [Google Scholar]
- 16.Tsavaris N, Kosmas C, Vadiaka M, Koufos C. Raltitrexed (tomudex) administration in patients with relapsed metastatic colorectal cancer after weekly irinotecan/5-fluorouracil/leucovorin chemotherapy. BMC cancer. 2002;2:2. doi: 10.1186/1471-2407-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei N, Zhang B, Wang Y, He XH, Xu LC, Li GD, et al. Transarterial chemoembolization with raltitrexed-based or floxuridine-based chemotherapy for unresectable colorectal cancer liver metastasis. Clin Transl Oncol. 2019;21:443–50. doi: 10.1007/s12094-018-1942-0. [DOI] [PubMed] [Google Scholar]
- 18.Fang X, Jiang Y, Feng L, Chen H, Zhen C, Ding M, et al. Blockade of PI3K/AKT pathway enhances sensitivity of Raji cells to chemotherapy through down-regulation of HSP70. Cancer Cell Int. 2013;13:48. doi: 10.1186/1475-2867-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang SH, Yang KJ, Wu JC, Chang KJ, Wang SM. Effects of hyperthermia on the cytoskeleton and focal adhesion proteins in a human thyroid carcinoma cell line. J Cell Biochem. 1999;75:327–37. [PubMed] [Google Scholar]
- 20.Eng OS, Turaga KK. Cytoreduction and hyperthermic intraperitoneal chemotherapy in metastatic colorectal cancer. J Sur Oncol. 2019;119:613–5. doi: 10.1002/jso.25438. [DOI] [PubMed] [Google Scholar]
- 21.Wisselink DD, Braakhuis LLF, Gallo G, van Grevenstein WMU, van Dieren S, Kok NFM, et al. Systematic review of published literature on oxaliplatin and mitomycin c as chemotherapeutic agents for hyperthermic intraperitoneal chemotherapy in patients with peritoneal metastases from colorectal cancer. Crit Rev Oncol Hematol. 2019;142:119–29. doi: 10.1016/j.critrevonc.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Aboulkheyr Es H, Montazeri L, Aref AR, Vosough M, Baharvand H. Personalized cancer medicine: an organoid approach. Trends Biotechnol. 2018;36:358–71. doi: 10.1016/j.tibtech.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Soragni A, Janzen DM, Johnson LM, Lindgren AG, Thai-Quynh Nguyen A, Tiourin E, et al. A designed inhibitor of p53 aggregation rescues p53 tumor suppression in ovarian carcinomas. Cancer cell. 2016;29:90–103. doi: 10.1016/j.ccell.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crespo M, Vilar E, Tsai SY, Chang K, Amin S, Srinivasan T, et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med. 2017;23:878–84. doi: 10.1038/nm.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill SJ, Decker B, Roberts EA, Horowitz NS, Muto MG, Worley MJ, Jr, et al. Prediction of DNA repair inhibitor response in short-term patient-derived ovarian cancer organoids. Cancer Discov. 2018;8:1404–21. doi: 10.1158/2159-8290.CD-18-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.