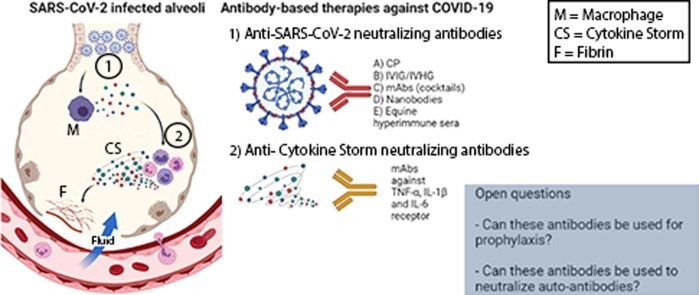
Graphical abstract
SARS-CoV-2 infected alveoli and the different types of the two antibody-based treatments discussed in this review. Can antibodies be used to protect against COVID-19 and neutralize autoantibodies?
Keywords: COVID-19, SARS-CoV-2, Spike, Passive immunotherapy, Neutralizing, Antibody-based treatments, Cytokine storm
Abbreviations: ABT, Antibody-based treatments; ACE2, Angiotensin-converting enzyme 2; ADE, Antibody-dependent enhanced disease; CLO, Chloroquine; COVID-19, Coronavirus disease 2019; CP, Convalescent plasma; FDA, US Food and Drug Administration; HCLO, Hydroxychloroquine; IFN, Interferon; IL, Interleukin; IVHI, Intravenous hyperimmune immunoglobulin; IVIG, Intravenous immune globulin; mAbs, Monoclonal antibodies; MERS-CoV, Middle East respiratory syndrome coronavirus; nAbs, Neutralizing antibodies; NTD, N-terminal domain; RBD, Receptor-binding domain; RBM, Receptor-binding motif; S, Spike virus protein; SARS-CoV, Severe acute respiratory syndrome coronavirus; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; TNF, Tumor necrosis factor; WHO, World Health Organization
Abstract
Coronavirus disease 2019 (COVID-19) has been declared by the World Health Organization (WHO) as a pandemic since March 2020. This disease is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The only available tools to avoid contamination and transmission of this virus are physical distancing, the use of N95 and surgical masks, and hand hygiene. Vaccines are another essential tool to reduce the impact of the pandemic, though these present challenges in terms of production and logistics, particularly in underdeveloped and developing countries. One of the critical early research findings is the interaction of the spike virus protein with the angiotensin-converting enzyme 2 (ACE2) human receptor. Developing strategies to block this interaction has therefore been identified as a way to treat this infection. Neutralizing antibodies (nAbs) have emerged as a therapeutic approach since the pandemic started. Infected patients may be asymptomatic or present with mild symptoms, and others may evolve to moderate or severe disease, leading to death. An immunological phenomenon known as cytokine storm has been observed in patients with severe disease characterized by a proinflammatory cytokine cascade response that leads to lung injury. Thus, some treatment strategies focus on anti-cytokine storm nAbs. This review summarizes the latest advances in research and clinical trials, challenges, and perspectives on antibody-based treatments (ABT) as therapies against COVID-19.
1. Introduction
A pandemic was declared in March 2020 by the World Health Organization (WHO). Since then, coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has intrigued researchers, medical doctors, and the general population.
The first disease cases were characterized as atypical pneumonia in Wuhan, China, in December 2019 [1]. Coronaviruses are known to have caused pneumonia epidemics in humans, such as SARS-CoV (related to SARS-CoV-2) and Middle East Respiratory Syndrome (MERS-CoV) [2], [3]. However, the origin of SARS-CoV-2, the most infectious coronavirus known, is still under investigation by the WHO.
Since no specific treatment has been approved so far, the only available tools to prevent the transmission of SARS-CoV-2 are physical distancing, the use of N95 and surgical masks, and hand hygiene [4]. Besides the Regeneron antibody cocktail, which has been approved by the US Food and Drug Administration (FDA) [5], emergency FDA approval has also been issued for an antibody cocktail from Eli Lilly and is now revoked [6]. An equine hyperimmune serum in Argentina has also received emergency approval from the Argentinian regulatory agency [7]. However, these treatments currently lack both usage data and scalability and are not being used for prevention. Currently, health practitioners rely on strategies known to work against other infections, such as dexamethasone [8], oxygen therapy, anti-coagulation drugs, and intensive care unit interventions like tracheostomy and mechanical respiration [9].
Vaccines are another essential tool to reduce the impact of the pandemic. As a result of the global race for a vaccine [10], the rate of vaccine development has been the fastest in human history. Some have already been approved, bringing hope for an end to the pandemic. However, vaccinating billions of people represents a production and logistics challenge, particularly in underdeveloped and developing countries, which have the less economic power to purchase vaccine doses [11]. In addition, anti-vaccine and anti-science movements represent a critical risk to the success of vaccination campaigns, as do the denialist positions that some leaders and their followers support [12]. These challenges highlight the ongoing importance of research on COVID-19 in order to save lives, especially in severe cases.
One of the critical early research findings is the interaction of spike virus protein with angiotensin-converting enzyme 2 (ACE2) human receptor, which is more robust against SARS-CoV-2 than SARS-CoV [13]. This characteristic is responsible for SARS-CoV-2 being more infectious and transmissible [13]. Thus, developing strategies to block this interaction can help treat infections. Neutralizing antibodies (nAbs) have therefore emerged as a therapeutic approach since the beginning of the pandemic, and their use continues to the present date.
As of July 27, 2021, COVID-19 was present in 192 countries, with 194,909,258 total cases and 4,171,772 total deaths [14]. Patients infected with SARS-CoV-2 can be asymptomatic or present with mild symptoms, though these may also progress to moderate or severe disease, potentially leading to death [15]. Some severely ill patients experience an immunological phenomenon known as cytokine storm, characterized by a proinflammatory cytokine cascade response that leads to lung injury [16]. Some treatment strategies have therefore focused specifically on anti-cytokine storm nAbs [16], [17], [18], [19], [20], [21].
This review summarizes new developments in research and clinical trials, challenges, and perspectives on antibody-based treatments (ABT) as therapies against COVID-19. We separate this review into four major parts: 1) spike virus glycoprotein/ACE2 human receptor interaction nAbs; 2) cytokine storm nAbs; 3) challenges; 4) perspectives.
2. SARS-CoV-2 and the COVID-19 pandemic: A brief characterization
SARS-CoV-2, the etiological agent of COVID-19, belongs to the subfamily of β-coronaviruses that infect humans and may cause severe disease and lead to death [22]. This genome is approximately 80% identical to SARS-CoV, the first coronavirus outbreak in China in 2002 [2], which killed 774 people.
SARS-CoV-2 is 87.23% identical to the corona-like bat virus bat-SL-CoVZXC21, and 87.99% identical to bat-SLCoVZC45, which has led to speculation SARS-CoV-2 originated in bats [23], though its zoonotic origins have yet to be determined. There is evidence that pangolins are an intermediate host, which could have initially transmitted the disease to humans at an open seafood market in Wuhan, China [24]. However, the WHO is still investigating the origins of transmission. Determining the outbreak's origin has proven challenging as some of the first patients did not go to the market.
In terms of clinical presentations, 80% of COVID-19 patients have asymptomatic to mild symptoms (fever, head and body pain, loss of smell and taste, diarrhea), and 20% have moderate to severe symptoms (pneumonia, dyspnea, secondary infections, renal or cardiac failures, and coagulation), which can lead to death [15]. The majority of the virus contamination occurs by aerial transmission, which has been documented more recently, but it also may occur less frequently by fomite droplets and contaminated surfaces [15].
A recent study by Flora and collaborators (2021) [25] at Bauru hospital in Brazil has provided insights into the differential protein expression during each stage of SARS-CoV-2 infection. The authors identified changes in plasma proteins related to complement activation, blood coagulation, antimicrobial humoral response, acute inflammatory, and endopeptidase inhibitor activity. Specifically, patients with mild symptoms had higher levels of the Iron-responsive element-binding protein 2 (IREB2), Gelsolin (GELS), DNA-directed RNA polymerase III subunit RPC 4 (POLR3D), Serum paraoxonase/arylesterase 1 (PON1), and UL16-binding protein 6 (ULBP6) proteins. Increased expression of Galectin-10 (Gal-10) was found in critical and severe patients [25]. In another robust genomic study in 208 intensive care units (ICUs) in the United Kingdom, called the Genetics of Mortality in Critical Care (GenOMICC), by Pairo-Castineira and collaborators (2021) [26], discovered susceptibility markers to severe COVID-19 development: low expression of the interferon receptor gene IFNAR2 and high expression of tyrosine kinase 2 (TYK2), as well as increased expression of the monocyte/macrophage chemotactic receptor CCR2 in the lung [26]. Such studies are critical to new pharmaceutical research and development related to SARS-CoV-2 since they help inform therapies that can target these genetic/blood markers.
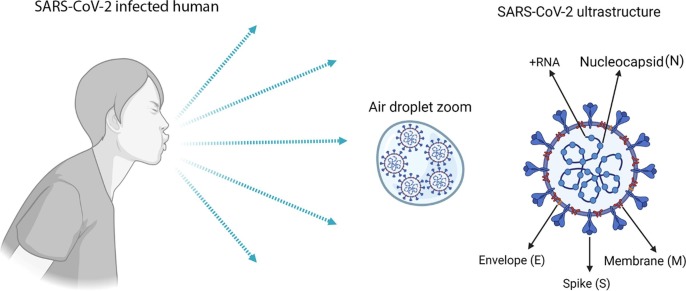
In terms of structure and organization, SARS-CoV-2 follows the structural protein forms of coronaviruses: envelope, spike (S) glycoprotein and membrane, and the non-structural nucleocapsid protein plus RNA-positive genome (26.2 to 31.7 kilobases) from the ribonucleocapsid complex (RNP), which are encapsulated in the envelope (Fig. 1 ) [22].
Fig. 1.
General SARS-CoV-2 proteins and genomic RNA from an infected human. After coughing in the air, the virus droplets can infect another person. The figure shows the general ultrastructural protein and genomic RNA of SARS-CoV-2, including the nucleocapsid (N), envelope (E), membrane (M), spike (S), and genomic + RNA, respectively. The figure was generated using BioRender software.
3. Interaction of the human receptor ACE2 with the virus spike glycoprotein for SARS-CoV-2 virus entry as the primary therapeutic target for neutralizing antibodies (nAbs)
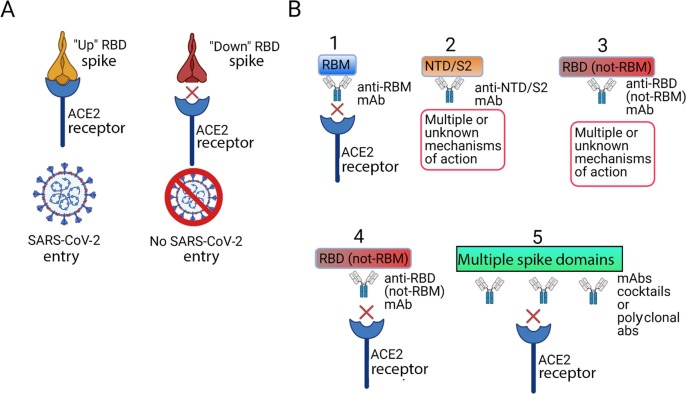
As thoroughly described in the literature, the virus S glycoprotein of SARS-CoV-2 is responsible for host cell entry after interacting with the human receptor ACE2 (Fig. 2 A) [13], [27], [28], [29]. Nevertheless, it is essential to know that inside the S1 monomer, a receptor-binding domain (RBD) is responsible for receptor interaction [26]. More specifically, the receptor-binding motif (RBM) contains the residues that actively bind to ACE2 [29], as in Fig. 2A. Structurally, the spike protein has two conformational states: “up” and “down” [5]. Significantly, the RBD only interacts with and binds to ACE2 when the spike protein is in the “up” conformation state (Fig. 2A) [5].
Fig. 2.
SARS-CoV-2 spike (S) glycoprotein interaction with ACE2 host receptor and antibodies against S protein can prevent virus entry. A) Spike has two conformational states: “up” and “down.” RDB only interacts and binds to ACE2 when spike is in the “up” conformation. B) Types of antibody-mediated blockage of virus entry: 1) nAbs bind to RBM, avoiding interaction with ACE2 and resulting in no entry and no infection; 2) nAbs bind to other spike domains, such as the N-terminal domain (NTD) and S2, with multiple mechanisms of action or with mechanisms of action that remain unknown; 3) nAbs bind to RBD (not RBM) and do not compete with ACE2 binding, with multiple mechanisms of action or with mechanisms of action that remain unknown; 4) nAbs bind to RBD (not RBM) and compete with ACE2 binding, resulting in no entry and no infection; and 5) nAbs bind to two or more regions (cocktails and polyclonal antibodies), resulting in no entry and no infection. The figure was generated using BioRender software.
The interaction of SARS-CoV-2 with ACE-2 is stronger than that of SARS-CoV due to mutations in RBM, such as the insertion of Gly-Val-Glu-Gly (GVEG) in residues 482–485 [13]. Outside of the RBD domain, a Pro-Arg-Arg-Ala-Arg (PRRAR) insertion, a second additional site for protease action and S2 monomer assembly for virus or RNA internalization, is a furin protease site that is another critical difference between SARS-CoV-2 and SARS-CoV and is involved in the increased pathogenesis [30]. Both mutations contribute to the greater transmissibility of SARS-CoV-2 and can be used as targets for treatments.
At the onset of the pandemic, the predominant strategies involved using available medicines to treat or even prevent infection. This strategy had a misleading success at the beginning, as some chemical compounds tested in vitro and in vivo presented evidence of early treatment and prophylaxis [31], such as the promising and novel use of clofazimine [32] and molnupiravir [33], as well as compounds that have already used for similar applications, such as chloroquine (CLO), hydroxychloroquine (HCLO), ivermectin, azithromycin and some others [8], including ABT [34], [35], [36], [37], [38].
However, in human clinical randomized studies, CLO, HCLO, and ivermectin present a risk of substantial collateral effects such as toxic hepatitis, which has been another pandemic burden. Indeed, the pharmaceutical producer of ivermectin, Merck, published a note concluding that the drug should not be used to prevent or treat COVID-19 [39]. Another risk of ivermectin usage is the false sense of protection, which leads people to avoid prevention actions of contamination. Meanwhile, the use of azithromycin can lead to microbial resistance and, ultimately, sepsis [40]. Furthermore, these medicines were found to offer no clinical benefits in randomized studies [41].
The amount of time necessary for widespread, global vaccination and the possibility of spreading novel variants highlights the need for continued research on therapeutic methods. Thus, ABT, which is specific, more manageable, safer, and faster to produce and use, has emerged as a possible alternative against SARS-CoV-2 [35].
ABT passive immunotherapy is based on nAbs to keep pathogens from entering the host cell. ABT can be divided into five categories: convalescent plasma (CP), intravenous immunoglobulin (IVIG), intravenous hyperimmune immunoglobulin (IVHI), monoclonal antibodies (mAbs) – alone or in cocktails – and nanobodies [34], [35], [36], [37], [38]. Major nAbs against SARS-CoV-2 act against the S glycoprotein RBD [42]. As postulated by the literature, five possible groups could be used based on the targeting region and mechanism of neutralization [42], as follows: 1) nAbs bind to RBM, avoiding interaction with ACE2 and resulting in no entry and no infection; 2) nAbs bind to other S glycoprotein domains, such as N-terminal binding domain (NTD) and S2, with multiple mechanisms of action or with mechanisms of action that remain unknown; 3) nAbs bind to RBD (not RBM) and do not compete with ACE2 binding, with multiple mechanisms of action or with mechanisms of action that remain unknown; 4) nAbs bind to RBD (not RBM) and compete with ACE2 binding, resulting in no entry and no infection; 5) nAbs bind to two or more regions (cocktails and polyclonal antibodies), resulting in no entry and no infection (Fig. 2B).
Three classes were mentioned in another more recent classification by Finkelstein and collaborators (2020) [34] of SARS-CoV-2 nAbs that mediate RBM biding to ACE2 by epitope blocking: class I, the largest structurally characterized mAbs, which are direct ACE2 competitors that bind only to “up” RBD; class II, which binds to “up” and “down” RBD, and may stabilize spike in a conformation that prevents ACE2 binding; class III, which blocks ACE2 with quaternary epitopes (NTD, non-RBM) by potentially locking the spike in a closed conformation, thus preventing access to the ACE2 binding site [34].
Hence, advances in the technology used to produce nAbs for HIV treatment, such as structural characterization of the host receptor-virus ligand and production of mAbs to block this ligation, allowed the fastest discovery of ABT for SARS-CoV and SARS-CoV-2 in science history [43].
4. Anti-SARS-CoV-2 neutralizing antibodies (nAbs) as a therapeutic approach against COVID-19
4.1. SARS-CoV and SARS-CoV-2 cross-reacted nAbs
Due to their genomic proximity and the similar virus entry mechanism (spike × ACE2 interaction) in SARS-CoV and SARS-CoV-2, one of the first strategies involving ABT to treat SARS-CoV-2 was the usage of mAbs (detailed below) and nAbs against SARS-CoV. As previously reviewed [42], many SARS-CoV nAbs were tested against SARS-CoV-2, both in vitro and in vivo [44], [45], [46]. In these studies, cross-reaction and little or weak neutralization were observed [44], [45], [46]. The best result was for VIR-7831, derived from the non-RBM S309 (clinical trial NCT04545060, Vir Biotechnology, and GlaxoSmithKline (GSK) Inc. collaboration, USA), which is currently at the clinical trial stage (Table 1 ), alone [47] or combination with other antibodies. This antibody is currently awaiting FDA emergency approval after showing 85% efficacy in clinical studies. One accepted explanation of the unsuccessful use of SARS-CoV nAbs against SARS-CoV-2 is the structural differences between the spike and ACE2 interaction.
Table 1.
Antibody-based treatments against SARS-CoV-2 proteins.
| ABT approach | nAb name/target | Stage | Origin | Reference(s) |
|---|---|---|---|---|
| SARS-CoV cross-reaction with SARS-CoV-2 | VIR-7831/spike | Clinical studies, waiting for FDA emergency approval | USA | [47] |
| CP | -/spike | Clinical studies /FDA emergency approval with some concerns / needs additional results | USA, UK, Italy, Spain, China, India, Brazil, South Africa, and others | [48], [53] |
| IVIG/ IVHG | -/spike | Clinical studies | Various | [51], [56] |
| mAbs | REGNCoV2 cocktail (Regeneron)/spike | FDA approved | USA | [5] |
| mAbs | LY-CoV555 in combination with LY-CoV016 (Eli Lilly)/spike | Emergency FDA authorization revoked | USA | [6] |
| Nanobodies | -/spike | Preclinical studies | Various | [61], [62], [63] |
| Horse hyperimmune serum | -/spike | Preclinical studies | China | [68] |
| Horse hyperimmune serum | -/spike | Awaiting ANVISA approval to initiate clinical studies | Brazil | [70] |
| Horse hyperimmune serum | -/whole inactivated virus | Clinical studies | Brazil | [73] |
| Horse hyperimmune serum | -/spike | Emergency Anmat authorization/ needs additional results | Argentina | [7], [69] |
| Horse hyperimmune serum | -/spike and mix | Preclinical studies | Costa Rica | [71] |
4.2. SARS-CoV-2 convalescent plasma, purified IgGs and mAbs/nAbs
The use of CP from recovered COVID-19 patients has emerged as a fast and safe treatment strategy due to the presence of nAbs against the spike glycoprotein [48]. The FDA has approved the emergency administration of CP to study its efficacy [49]. Some concerns have to be considered with respect to CP, such as 1) CP nAbs titles should be a minimum of 1:80 [50]; 2) randomized clinical trials should be undertaken to determine CP efficacy [51]; 3) pre-existing infections or diseases in donors should be documented, as well as the time to recover from COVID-19 [52]; 4) clinical structures and specialized nursing staff are required to stock and apply CP [48].
As recently reviewed by Devarasetti and collaborators (2021) [53], few randomized clinical trials focused on the use of CP have been carried out [53], some with contradictory conclusions (Table 1). A recent study from the Indian Council of Medical Research (ICMR) indicated that the best results and benefits were found: 1) in the early administration within five days of the first symptoms; 2) no CP administration to critical patients; 3) when donors exhibited ideal conditions, such as high nAbs titers and donation within 45 days of recovery from SARS-CoV-2 infections [50]. More standardized and multicenter randomized clinical trials should be done to understand the prophylactic benefit of CP therapy for SARS-CoV-2 patients.
Limitations of CP application include the difficulty in storage and donors’ availability. Some approaches could help, including IgG-specific S glycoprotein nAbs being isolated and purified from CP, termed IVIG nAbs (or IVHI if isolated from a concentrated pool of CPs) [54]. Due to higher titers, they could be used in a great number of patients. However, these approaches are time-consuming, expensive, and do not offer proinflammatory protection. Moreover, they could lead to antibody-dependent enhanced disease (ADE), such as dengue hemorrhagic fever, due to the presence of non-neutralizing antibodies [55]. As reviewed recently, little new research is available on IVIG or IVHI [51], [56], and few clinical studies are currently being performed.
mAbs and nAbs have emerged as promising alternatives that could solve some of the previously mentioned challenges. mAbs mostly come from immortalized hybridoma clones resulting from the fusion of B cell mAb producing cells with myeloma cancer cell lines [57]. The B cells could be obtained from recovered COVID-19 patients (using B cell isolation), phage-display libraries, or immunized animals (mouse, rabbit, and others) [58], [59]. Since the cell lines are immortalized, there is no need for other donors/animals. As a result of careful screening of nAb-specific B cell line isolation, ADE is less likely to occur. However, their production is more expensive than the purification of hyperimmune IgGs. Additionally, non-human mAbs need to be humanized in Fc portions for the best avidity results [60]. Another concern about mAbs is the escape of mutant variants due to their unique epitope response, which could be reverted by implementing mAbs cocktails with no competitive epitopes [47].
Two mAbs cocktails recently received FDA approval or emergency use authorization: REGN10933 in combination with REGN10987, named REGNCoV 2 (Regeneron) was approved [5] and, more recently, LY-CoV555 in combination with LY-CoV016 (Eli Lilly, USA) received an emergency use authorization [6] (Table 1). However, the Eli Lilly mAbs emergency approval was revoked after the announcement of the loss of efficacy against variants. Other mAbs (alone or in combination) are in different clinical evaluation phases [61], [62], [63]. More long-term studies are needed to verify their effectiveness not only for SARS-CoV-2 but, principally, for the variants. Thus, there is a demand for platforms that could predict mutations and combine them through genetic engineering to produce more efficient mABs cocktails.
4.3. SARS-CoV-2 camelid nanobodies and equine hyperimmune nAbs
Other technologies to develop therapeutic antibodies could be used for COVID-19 therapies. Nanobodies are smaller than antibodies due to the absence of an Fc domain and are produced primarily in camelids [64]. The advantage of these molecules is the opportunity to produce them recombinantly in bacterial platforms, which is less costly than other approaches. Since they are smaller, they could be administered by inhalation/nebulization and be directed to the lungs [37]. Indeed, nanobodies against S glycoprotein of SARS-CoV-2 have been produced, resulting in powerful nAbs such as synthetic humanized or yeast libraries, which block the interaction of the virus with ACE2 via distinct mechanisms, or camelid immunized with RBD, the epitope of which can recognize both “up” and “down” spike proteins and other non-overlapping epitopes with pico and femtomolar affinities [60], [65], [66], [67]. In spite of their importance, the technology for producing them has only been recently developed. Therefore, all possible nanobodies are in discovery or preclinical studies at the moment (Table 1).
Another ABT approach for SARS-CoV-2 is hyperimmune serum from horses, recently reviewed by Costa and collaborators (2021) from Vital Brazil Institute, Rio de Janeiro, Brazil [12]. The advantage of using equine serum lies in better production at higher volumes than with other species [12]. To date, four studies have been published: one in China [68], one in Argentina [69], one in Brazil [70], and one in Costa Rica [71]. They all used enzymatically digested IgG with pepsin or papain to obtain Fab or F(ab) 2 fractions, respectively. The nAbs production was 50–150 times higher in titles than CP [68], [69], [70]. Argentina's hyperimmune serum has recently received emergency approval, with some caveats, from the country’s regulatory agency (Anmat) since clinical studies showed a 45% reduction in mortality [7] (Table 1). Meanwhile, the use of Vital Brazil’s hyperimmune serum still requires authorization from Brazil’s regulatory agency (ANVISA) to start clinical studies (Table 1). No updates from the Chinese study have been published or announced after the first publication, so we consider it to be in the preclinical stage (Table 1). Furthermore, the Butantan Institute in Brazil is also developing an equine serum against SARS-CoV-2 using the whole inactivated virus as antigen. This study received authorization from ANVISA to start clinical studies (Table 1) [72]. Such an approach could help these countries combat the disease until vaccination rates increase.
5. SARS-CoV-2 cytokine storm and antibody-based treatments against key cytokines
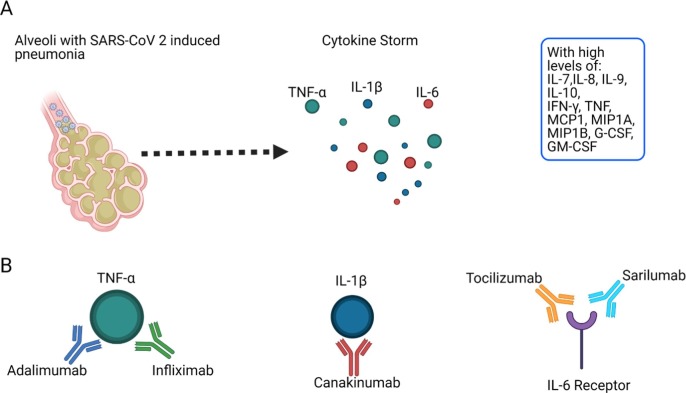
As recently reviewed by Sette and Crotty (2021), rapid viral clearance is due to T cell responses against SARS-CoV-2. An extended period of innate immune response is associated with severe/acute disease [73]. Another critical finding associated with severe/acute disease is the ineffective IFN I and III innate immunity, which leads to innate cell immunopathology and cytokine storm [74], [75]. The SARS-CoV-2 cytokine storm, induced by macrophages and other innate immune cells, is characterized by high levels of key cytokines such as TNF-α, IL-1β, and IL-6 [13], as well as IL-7, IL-8, IL-9, IL-10, IFN-γ, TNF, MCP1, MIP1A, MIP1B, G-CSF, GM-CSF (Fig. 3 A) [76]. The signature of these proinflammatory cytokines is very clearly observed in COVID-19 pneumonia patients, with other severity-associated symptoms such as coagulation [16]. Furthermore, inflammations may survive for months after virus clearance, as observed in many recovered patients [77] as the so-called “post-COVID-19 syndrome”, characterized by the persistence of symptoms such as fatigue and tiredness [78].
Fig. 3.
SARS-CoV-2 alveolar pneumonia induced by Cytokine Storm and antibody-based treatments against crucial cytokines. A) SARS-CoV-2 alveolar pneumonia induced by cytokine storm proinflammatory response caused by macrophages and other innate immune cells. The key cytokines are TNF-α IL-1β and IL-6. B) Antibody-based treatments against the key cytokines: TNF-α (adalimumab and infliximab), IL-1β (canakinumab), and IL-6 receptor (tocilizumab and sarilumab). The figure was generated using BioRender software.
Identifying the key cytokines may therefore enable the development of therapies to block them. Indeed, dexamethasone, an anti-inflammatory corticosteroid, has been shown to significantly reduce mortality among patients hospitalized with or without mechanical ventilation [79]. ABT against cytokine storm markers such as TNF-α, IL-1β, and IL-6 is an essential approach at the moment to stop a proinflammatory response to SARS-CoV-2 (Fig. 3B).
Specific particularities of the antibodies mentioned above can be found in the most recent review by Yakota et al. (2021) [16]. Table 2 shows the current clinical stages of these antibodies. Adalimumab, sarilumab, and infliximab are in clinical trials; canakinumab is in clinical trials and needs more randomized studies; and tocilizumab, the most promising, is approved in the UK [37] and a clinical study with remdesivir is ongoing with pneumonia patients in the USA (Table 2).
Table 2.
Recent advances in antibody-based treatments against SARS-CoV-2 cytokine storm.
| Antibody Name and origin | Target | Stage | Reference(s) |
|---|---|---|---|
| Adalimumab/ Humira®, AbbVie Inc., USA | TNF-α | Clinical trials | [16], [17], [80] |
| Infliximab/ Remicade®, Johnson & Johnson, USA | TNF-α | Clinical trials | [16] |
| Canakinumab/ Ilaris®, Novartis International, Switzerland | IL-1β | Clinical trials need additional randomized studies | [16], [18] |
| Tocilizumab/ Actemra®, Chugai Pharmaceutical, Japan | IL-6 receptor | Approved in the UK; part of a clinical study with remdesivir for pneumonia patients in the USA | [19], [20], [21], [37], [81], [82], [83], [84], [85] |
| Sarilumab/ Kevzara®, Sanofi S.A., France | IL-6 receptor | Clinical trials | [16], [20] |
6. Challenges
SARS-CoV-2 presents a multitude of challenges, three of which are particularly related to the context of this review: 1) reinfection with SARS-CoV-2 and SARS-CoV-2 variants due to the duration or escape of nAbs or memory cells, respectively; 2) ADE; 3) autoantibodies.
Reinfection, the ability of SARS-CoV-2 to infect previously infected patients, is a significant concern with SARS-CoV-2, as it can promote escape from vaccines and reduce the quality of life of re-infected people [86]. Asymptomatic and mildly symptomatic patients appear to be more susceptible to reinfection due to a lower memory B and T cell response and less long-term duration of nAbs. Moderate and severe patients are less vulnerable to reinfection due to the intermediate to long-term memory or duration of nAbs from 3 to 8 months [87], [88]. However, more robust cohort studies are needed to estimate the time of memory and nAbs in recovered patients.
Some SARS-CoV-2 variants that escape from nAbs/memory cells are an emerging issue at the moment. There are a lot of SARS-CoV-2 variants [89], but four are more transmissible and dangerous than the original SARS-CoV-2 and need more concern: B.117 from the United Kingdom (UK), P1 from Brazil, and 501Y.V2 (or B.1.351) from South Africa [89], and the most recently emerged, B.1.617.2 from India [90]. Until now, there has been no evidence of a loss of vaccine efficacy in response to the UK variant [89]. On the other hand, the South African variant could escape from CP [91]. The Brazilian and Indian variants are more recent than others, and little information is available. However, as with B.1.351, the Brazilian variant is highly transmissible and appears to re-infect people who have recovered from the original virus [92]. More studies are crucial to determine whether the variants are more lethal than the original virus and induce more reinfections and escape from vaccines. On May 31, 2021, WHO announced a new and more straightforward nomenclature for the variants, divided into “variants of concern” and “variants of interest”. The four above-mentioned variants of concern are now Alpha (former B.117 British variant), Beta (former B.351 South African variant), Gamma (former P1 Brazilian variant), and Delta (former B.1.617.2 Indian variant) [93].
ADE is characterized by a more potent disease after a second exposure to a pathogen [55]. It could be an issue in ABTs, principally those involving CP and hyperimmune serum, due to the presence of non-neutralizing antibodies together with the nAbs [55]. ADE has been observed in MERS and SARS-CoV [55]. However, no ADE has been confirmed for SARS-CoV-2, and IgG isolation, purification, mAb technology, and recombinant modification in glycosylation of the Fc domain could help avoid ADE [56].
Another antibody-mediated pathogenesis in COVID-19 patients is the production of autoantibodies in severe/critically ill patients, as suggested by the observation that these patients were reported to have antiphospholipid and anti-β2-glycoprotein I (β2GPI) IgA, IgM, and IgG antibodies [94], [95], [96], [97]. Some autoantibodies or factors related to autoimmune rheumatic diseases have been found in COVID-19 patients without a disease history [95], [98]. Another study showed that critically ill COVID-19 patients display lupus-like hallmarks, such as activating extrafollicular B cells [99]. These extrafollicular responses induce antibody-secreting cells. Thus, SARS-CoV-2 infection may lead to autoimmune disease induction by producing and amplifying autoantibodies [56].
As recently shown by Liu and collaborators (2021) [100] in a review, other autoantibodies have also been detected in COVID-19 patients [100], such as cold agglutinins, which cause hemolytic anemia and complicate laboratory assessment and renal replacement therapy [101], [102]; anti-Ro/SSA antibodies, which may be associated with severe pneumonia [103]; anti-Caspr2 antibodies [104], anti-GD1b antibodies [103], anti-MOG antibodies [105], and red cell-bound antibodies, associated with the anemia severity [106]. In addition, the American Journal of Nursing published a NewsCAP (March 2021) suggesting that autoantibodies across a wide range of immunological targets in COVID-19 patients are also related to long COVID-19 or post-COVID-19 syndrome [107]. More studies are needed to understand the risks of COVID-19 in patients with pre-existing autoimmune conditions or if COVID-19 disease generated this autoimmune disease.
7. Perspectives
Due to the challenges discussed previously, ABT for SARS-CoV-2 treatment may be discarded. However, as treatments involving ABT have been simpler, faster, and safer than vaccines, ABT using variants as targets could be used as alternative treatment until effective vaccines against variants are developed. In addition, mAbs cocktails or polyclonal antibodies could be used to block virus ABT escape. Research advances in molecular biology could discard ADE induced by ABT. Furthermore, we propose that ABT be used not only for treatment but also for prevention, as reinfection and poorly understood immune responses are significant issues for COVID-19. ABT could also block autoantibodies in severe/critical patients, and more efforts could be made along these lines.
In conclusion, more actions should be taken by the research community and pharmaceutical industry to make ABT less expensive and scalable, which would allow more access to these therapies, principally in underdeveloped countries. This technology could be used for other future pandemics, as ABT could be applied to block the pathogenesis of infection, reinfection, cytokine storm, and autoimmune antibodies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to acknowledge Maria Luiza Ferreira dos Santos, Nicole Cristine Kerkhoven, and Viviana Stephanie Costa Gagosian for critically reading this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. Hayden FG, et al, A novel coronavirus outbreak of global health concern. The Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F., Lau S.K., To K.K., et al. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. https://cmr.asm.org/content/28/2/465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. https://www.nejm.org/doi/full/10.1056/NEJMoa1211721 [DOI] [PubMed] [Google Scholar]
- 4.Central Disaster and Safety Countermeasure Headquarters of the Republic of Korea, Rules and guidelines for distancing in daily life to control coronavirus disease 2019 in Korea: 3rd version, announced on July 3, 2020, J. Educ. Eval. Health. Prof. (2020) 17-20. https://www.jeehp.org/DOIx.php?id=10.3352/jeehp.2020.17.20. [DOI] [PMC free article] [PubMed]
- 5.Wu B.Y., Wang F., Shen C., et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2021;368(2020):1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.E.L.A. Company, Lilly Announces Proof of Concept Data for Neutralizing Antibody LY-CoV555 in the COVID-19 Outpatient Setting. https://investor.lilly.com/news-releases/news-release-details/lilly-announces-proof-concept-data-neutralizing-antibody-ly 2020 (accessed 12 May 2021).
- 7.Lopardo G., Belloso W.H., Nanninid E., et al. RBD-specific polyclonal F(ab_)2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: A randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial. EClinicalMedicine. 2021;34 doi: 10.1016/j.eclinm.2021.100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baroutjian Amanda, Sanchez Carol, Boneva Dessy, McKenney Mark, Elkbuli Adel. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. A. J. Emerg. Med. 2020;38(11):2405–2415. doi: 10.1016/j.ajem.2020.08.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Yang, Lu Xiaofan, Li Yongsheng, Chen Hui, Chen Taige, Su Nan, Huang Fang, Zhou Jing, Zhang Bing, Yan Fangrong, Wang Jun. Clinical Course and Outcomes of 344 Intensive Care Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020;201(11):1430–1434. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.S.U. Kumar, N. Madhana Priya, S.R. Nithya, et al, (2021). A review of novel coronavirus disease (COVID-19): based on genomic structure, phylogeny, current shreds of evidence, candidate vaccines, and drug repurposing. Biotech.11, 198. https://doi.org/10.1007/s13205-021-02749-0. [DOI] [PMC free article] [PubMed]
- 11.Sampath Vanitha, Rabinowitz Grace, Shah Mihir, Jain Surabhi, Diamant Zuzana, Jesenak Milos, Rabin Ronald, Vieths Stefan, Agache Ioana, Akdis Mübeccel, Barber Domingo, Breiteneder Heimo, Chinthrajah Sharon, Chivato Tomas, Collins William, Eiwegger Thomas, Fast Katharine, Fokkens Wytske, O'Hehir Robyn E., Ollert Markus, O'Mahony Liam, Palomares Oscar, Pfaar Oliver, Riggioni Carmen, Shamji Mohamed H, Sokolowska Milena, Jose Torres Maria, Traidl‐Hoffmann Claudia, Zelm Menno, Wang De Yun, Zhang Luo, Akdis Cezmi A., Nadeau Kari C. Vaccines and Allergic reactions: the past, the current COVID-19 pandemic, and future perspectives. Allergy. 2021;76(6):1640–1660. doi: 10.1111/all.v76.610.1111/all.14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Costa Camila B.P., Martins Francislene J., da Cunha Luis E.R., Ratcliffe Norman A., Cisne de Paula Rafael, Castro Helena C. COVID-19 and Hyperimmune sera: A feasible plan B to fight against coronavirus. I. Immunopharm. 2021;90:107220. doi: 10.1016/j.intimp.2020.107220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang Jian, Ye Gang, Shi Ke, Wan Yushun, Luo Chuming, Aihara Hideki, Geng Qibin, Auerbach Ashley, Li Fang. Structural basis of receptor recognition bySARS-CoV-2. Nat. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johns Hopkins University. Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html, 2021 (accessed 27 July 2021).
- 15.Harrison Andrew G., Lin Tao, Wang Penghua. Mechanisms of SARS-CoV-2 Transmission and pathogenesis. T. in Immunol. 2020;41(12):1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokota Shumpei, Miyamae Takako, Kuroiwa Yoshiyuki, Nishioka Kusuki. Novel Coronavirus Disease 2019 (COVID-19) and Cytokine Storms for More Effective Treatments from anInflammatory Pathophysiology. J. Clin. Med. 2021;10(4):801. doi: 10.3390/jcm10040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L., Xu H. A clinical study for the efficacy and safety of dalimumab injection in the treatment of patients with severe novel coronavirus pneumonia (COVID-19) Trials. 2020;21:574. doi: 10.1186/s13063-020-04475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landi L., Ravaglia C., Russo E., et al. Blockage of interleukin-1 with canakinumab in patients with Covid-19. Sci. Rep. 2020;10:21775. doi: 10.1038/s41598-020-78492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone J.H., Frigault M.J., Serling-Boyd N.J., et al. Efficacy of Tocilizumab in Patients Hospitalised with Covid-19. N. Engl. J. Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. https://www.nejm.org/doi/10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cauchois Raphaël, Koubi Marie, Delarbre David, Manet Cécile, Carvelli Julien, Blasco Valery Benjamin, Jean Rodolphe, Fouche Louis, Bornet Charleric, Pauly Vanessa, Mazodier Karin, Pestre Vincent, Jarrot Pierre-André, Dinarello Charles A., Kaplanski Gilles. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. PNAS. 2020;117(32):18951–18953. doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wise J. Covid-19: Arthritis drugs improve survival in intensive care patients, shows study. BMJ. 2021;372 doi: 10.1136/bmj.n61. [DOI] [PubMed] [Google Scholar]
- 22.Rabaan A.A., Al-Ahmed S.H., Haque S., et al. SARS-CoV-2, SARS-CoV, and MERS-CoV: a comparative overview. L. Inf. in Med. 2020;2:174–184. [PubMed] [Google Scholar]
- 23.R. Lu, X. Zhao, J. Li, et al, Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet.395 (2020) 565-574. https://doi.org/10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed]
- 24.Y. Cao, K. Cai, L. Xiong, Coronavirus disease 2019: A new severe acute respiratory syndrome from Wuhan in China, A. Virol.64 (2020) 245-250. doi:10.4149/av_2020_201. [DOI] [PubMed]
- 25.D.C. Flora, A.D. Valle, H.A.B.S. Pereira, et al. (2021). Quantitative plasma proteomics of survivor and non-survivor COVID-19 patients admitted to hospital unravels potential prognostic biomarkers and therapeutic targets. medRxiv 2020.12.26.20248855. https://doi.org/10.1101/2020.12.26.20248855.
- 26.E. Pairo-Castineira, S. Clohisey, L. Klaric, et al, Genetic mechanisms of critical illness in COVID-19, Nat.591 (2021) 92–98. https://doi.org/10.1038/s41586-020-03065-y. [DOI] [PubMed]
- 27.Scialo Filippo, Daniele Aurora, Amato Felice, Pastore Lucio, Matera Maria Gabriella, Cazzola Mario, Castaldo Giuseppe, Bianco Andrea. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung. 2020;198(6):867–877. doi: 10.1007/s00408-020-00408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L., Xiang Y. Spike Glycoprotein-Mediated Entryof SARS Coronaviruses. Viruses. 2020;12:1289. doi: 10.3390/v12111289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.H.T. Nguyen, S. Zhang, Q. Wang, et al. (2020). Spike glycoprotein and host cell determinants. SARS-CoV-2 entry and cytopathic effects. J Virol.11, 95e02304-20. https://doi.org/10.1128/JVI.02304-20. [DOI] [PMC free article] [PubMed]
- 30.Johnson B.A., Xie X., Kalveram B., et al. Furin cleavage site is key to SARS-CoV 2 pathogenesis. BioRxiv. 2020 doi: 10.1101/2020.08.26.268854. [DOI] [Google Scholar]
- 31.Al-Beltagi Sarah, Preda Cristian Alexandru, Goulding Leah V., James Joe, Pu Juan, Skinner Paul, Jiang Zhimin, Wang Belinda Lei, Yang Jiayun, Banyard Ashley C., Mellits Kenneth H., Gershkovich Pavel, Hayes Christopher J., Nguyen-Van-Tam Jonathan, Brown Ian H., Liu Jinhua, Chang Kin-Chow. Thapsigargin Is a Broad-Spectrum Inhibitor of Major Human Respiratory Viruses: Coronavirus, Respiratory Syncytial Virus and Influenza A Virus. Viruses. 2021;13(2):234. doi: 10.3390/v13020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.S. Yuan, X. Yin, X. Meng, et al. (2021). Clofazimine broadly inhibits coronaviruses including SARS-CoV-2. Nat.https://doi.org/10.1038/s41586-021-03431-4. [DOI] [PubMed]
- 33.W.P. Painter, W. Holman, J.A. Bush, et al. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-2 Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2. Antimicrob. Agents Chemother. doi:10.1128/AAC.02428-20. [DOI] [PMC free article] [PubMed]
- 34.Finkelstein Maxwell T., Mermelstein Adam G., Parker Miller Emma, Seth Paul C., Stancofski Erik-Stephane D., Fera Daniela. Structural Analysis of Neutralizing Epitopes of the SARS-CoV-2 Spike to Guide Therapy and Vaccine Design Strategies. Viruses. 2021;13(1):134. doi: 10.3390/v13010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.J. Hussen, M. Kandeel, M.G. Hemida, et al. (2020) Antibody-Based Immunotherapeutic Strategies for COVID-19. Pathogens.9, 917. https://doi.org/10.3390/pathogens9110917. [DOI] [PMC free article] [PubMed]
- 36.Jiang Shibo, Zhang Xiujuan, Yang Yang, Hotez Peter J., Du Lanying. Neutralizing antibodies for the treatment ofCOVID-19. Nat. Biomed. Eng. 2020;4(12):1134–1139. doi: 10.1038/s41551-020-00660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.F. Levi-Schaffer, A. de Marco. (2021). COVID-19 and the revival of passive immunization: Antibody therapy for inhibiting SARS-CoV-2 and preventing host cell infection: IUPHAR review: 31. Br. J. Pharmacol.https://doi.org/10.1111/bph.15359. [DOI] [PubMed]
- 38.Focosi Daniele, Franchini Massimo. COVID-19 neutralizing antibody-based therapies in humoral immune deficiencies: A narrative review. Transfus. Apher. Sci. 2021;60(3):103071. doi: 10.1016/j.transci.2021.103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Statement on ivermectin use. https://www.merck.com/news/merck-statement-on-ivermectin-use-during-the-covid-19-pandemic/, 2021 (accessed in 12 May 2021).
- 40.Lucien Mentor Ali Ber, Canarie Michael F., Kilgore Paul E., Jean-Denis Gladzdin, Fénélon Natael, Pierre Manise, Cerpa Mauricio, Joseph Gerard A., Maki Gina, Zervos Marcus J., Dely Patrick, Boncy Jacques, Sati Hatim, Rio Ana del, Ramon-Pardo Pilar. Antibiotics and antimicrobial resistance in the COVID-19 era: Perspective from resource-limited settings. Int. J. Infect. Dis. 2021;104:250–254. doi: 10.1016/j.ijid.2020.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., Zhou Y., Zhang M., et al. Updated approaches against SARS-CoV-2. Antimicrob. Agents Chemother. 2020;64:e00483–e520. doi: 10.1128/AAC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavor E., Choong Y.K., Er S.Y., et al. Structural Basis of SARS-CoV-2 and SARS-CoV Antibody Interactions. Trends in Immunology. 2020;41:1006–1022. doi: 10.1016/j.it.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendoza P., Lorenzi J.C.C., Gaebler C. COVID-19 antibody development fueled by HIV-1 broadly neutralizing antibody research. Curr. Opin. HIV AIDS. 2021;16:25–35. doi: 10.1097/COH.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Z. Zhiqiang, M.V. Marthe, M.S. Sebastian, et al. (2020). Monoclonal antibodies for the S2 subunit of spike of SARS-CoV-1 cross-react with the newly-emerged SARS-CoV-2. Euro. Surveill. 25, 2000291. https://doi.org/10.2807/1560-7917.ES.2020.25.28.2000291. [DOI] [PMC free article] [PubMed]
- 45.Yu Fei, Xiang Rong, Deng Xiaoqian, Wang Lili, Yu Zhengsen, Tian Shijun, Liang Ruiying, Li Yanbai, Ying Tianlei, Jiang Shibo. Receptor-binding domain-specific human neutralizing monoclonal antibodies against SARS-CoV and SARS-CoV-2. Sig. Transduc. and Targ. Ther. 2020;5(1) doi: 10.1038/s41392-020-00318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D.E. Anderson, C.W. Tan, W.N. Chia, et al, Lack of cross-neutralization by SARS patient sera towards SARSCoV- 2, Emerg. Micro. & Inf. 9 (2020) 900-902. https://doi.org/10.1080/22221751.2020.1761267. [DOI] [PMC free article] [PubMed]
- 47.Pinto Dora, Park Young-Jun, Beltramello Martina, Walls Alexandra C., Tortorici M. Alejandra, Bianchi Siro, Jaconi Stefano, Culap Katja, Zatta Fabrizia, De Marco Anna, Peter Alessia, Guarino Barbara, Spreafico Roberto, Cameroni Elisabetta, Case James Brett, Chen Rita E., Havenar-Daughton Colin, Snell Gyorgy, Telenti Amalio, Virgin Herbert W., Lanzavecchia Antonio, Diamond Michael S., Fink Katja, Veesler David, Corti Davide. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 48.Thijssen Marijn, Devos Timothy, Ejtahed Hanieh-Sadat, Amini-Bavil-Olyaee Samad, Pourfathollah Ali Akbar, Pourkarim Mahmoud Reza. Convalescent Plasma against COVID-19: A Broad-Spectrum Therapeutic Approach for Emerging Infectious Diseases. Microorganisms. 2020;8(11):1733. doi: 10.3390/microorganisms8111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.USFDA. Investigational COVID-19 convalescent plasma: guidance for industry.https://www.fda.gov/media/13678/download, 2020 (accessed 12 May 2021).
- 50.ICMR. Evidence based advisory to address inappropriate use of convalescent plasma in COVID-19 patients. ICMR ADVISORY Convalescent plasma. https://www.icmr.gov.in/pdf/covid/techdoc/ICMR_ADVISORY_Convalescent_plasma_17112020_v1.pdf, 2020 (accessed 12 May 2021).
- 51.Rojas Manuel, Anaya Juan-Manuel. Why will it never be known if convalescent plasma is effective for COVID-19. J. Trans. Autoim. 2020;3:100069. doi: 10.1016/j.jtauto.2020.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.WHO. Use of Convalescent Whole Blood or Plasma Collected from Patients Recovered from Ebola Virus Disease for Transfusion, as an Empirical Treatment During Outbreaks: Interim Guidance for National Health Authorities and Blood Transfusion Services; World Health Organization: Geneva, Switzerland. https://apps.who.int/iris/handle/10665/135591, 2014 (accessed in 12 May 2021).
- 53.P.K. Devarasetti, L. Rajasekhar, R. Baisya et al, A review of COVID-19 convalescent plasma use in COVID-19 with focus on proof of efficacy, Immunol. Res.69 (2021) 18-25. https://doi.org/10.1007/s12026-020-09169-x. [DOI] [PMC free article] [PubMed]
- 54.Perricone Carlo, Triggianese Paola, Bursi Roberto, Cafaro Giacomo, Bartoloni Elena, Chimenti Maria Sole, Gerli Roberto, Perricone Roberto. Intravenous Immunoglobulins at the Crossroad of Autoimmunity and Viral Infections Microorganisms. 2021;9(1):121. doi: 10.3390/microorganisms9010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arvin Ann M., Fink Katja, Schmid Michael A., Cathcart Andrea, Spreafico Roberto, Havenar-Daughton Colin, Lanzavecchia Antonio, Corti Davide, Virgin Herbert W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nat. 2020;584(7821):353–363. doi: 10.1038/s41586-020-2538-8. [DOI] [PubMed] [Google Scholar]
- 56.Lu Ligong, Zhang Hui, Zhan Meixiao, Jiang Jun, Yin Hua, Dauphars Danielle J., Li Shi-You, Li Yong, He You-Wen. Antibody response and therapy in COVID-19 patients: what can be learned for vaccine development? Sci. China Life Sci. 2020;63(12):1833–1849. doi: 10.1007/s11427-020-1859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.H.A. Parray, S. Shukla 1, S. Samal 1, et al. (2020). Hybridoma technology a versatile method for isolation of monoclonal antibodies, it is applicability across species, limitations, advancement and future perspectives. Int. Immunopharmacol. 85:106639. https://doi.org/10.1016/j.intimp.2020.106639. [DOI] [PMC free article] [PubMed]
- 58.Cruz-Teran Carlos, Tiruthani Karthik, McSweeney Morgan, Ma Alice, Pickles Raymond, Lai Samuel K. Challenges and opportunities for antiviral monoclonal antibodies as COVID-19 therapy. Ad. Drug Del. Rev. 2021;169:100–117. doi: 10.1016/j.addr.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abraham Jonathan. Passive antibody therapy in COVID-19. Nat. Rev. Immunol. 2020;20(7):401–403. doi: 10.1038/s41577-020-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.M. Schoof, B. Faust, R.A. Saunders et al. (2020). An ultra-high affinity synthetic nanobody blocks SARS-CoV-2 infection by locking Spike into an inactive conformation. bioRxiv 2020.08.08.238469. https://doi.org/10.1101/2020.08.08.238469.
- 61.Ju B., Zhang Q., Ge J., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nat. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 62.Rogers T.F., Zhao F., Huang D., et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Sci. 2020;369:956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Lihong, Wang Pengfei, Nair Manoj S., Yu Jian, Rapp Micah, Wang Qian, Luo Yang, Chan Jasper F.-W., Sahi Vincent, Figueroa Amir, Guo Xinzheng V., Cerutti Gabriele, Bimela Jude, Gorman Jason, Zhou Tongqing, Chen Zhiwei, Yuen Kwok-Yung, Kwong Peter D., Sodroski Joseph G., Yin Michael T., Sheng Zizhang, Huang Yaoxing, Shapiro Lawrence, Ho David D. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nat. 2020;584(7821):450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 64.Dong Jianbo, Huang Betty, Jia Zhejun, Wang Bo, Gallolu Kankanamalage Sachith, Titong Allison, Liu Yue. Development of multi-specific humanized llama antibodies blocking SARS-CoV-2/ACE2 interaction with high affinity and avidity. Emerg. Microbes Infect. 2020;9(1):1034–1036. doi: 10.1080/22221751.2020.1768806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chi Xiaojing, Liu Xiuying, Wang Conghui, Zhang Xinhui, Li Xiang, Hou Jianhua, Ren Lili, Jin Qi, Wang Jianwei, Yang Wei. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting the spike receptor binding domain. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-18387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanke Leo, Vidakovics Perez Laura, Sheward Daniel J., Das Hrishikesh, Schulte Tim, Moliner-Morro Ainhoa, Corcoran Martin, Achour Adnane, Karlsson Hedestam Gunilla B., Hällberg B. Martin, Murrell Ben, McInerney Gerald M. An alpaca nanobody neutralizes SARS-CoV-2 by blocking receptor interaction. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-18174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiang Y., Nambulli S., Xiao Z., et al. Versatile, multivalent nanobody cocktails efficiently neutralize SARS-CoV-2. Sci. 2020;370:1479–1484. doi: 10.1126/science.abe4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan Xiaoyan, Zhou Pengfei, Fan Tiejiong, Wu Yan, Zhang Jing, Shi Xiaoyue, Shang Weijuan, Fang Lijuan, Jiang Xiaming, Shi Jian, Sun Yuan, Zhao Shaojuan, Gong Rui, Chen Ze, Xiao Gengfu. Immunoglobulin fragment F(ab’)2 against RBD potently neutralizes SARS-CoV-2 in vitro. Antivir. Research. 2020;182:104868. doi: 10.1016/j.antiviral.2020.104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zylberman V., Sanguineti S., Pontoriero A.V., et al. Development of a hyperimmune equine serum therapy for covid-19 in Argentina. Med. (Buenos Aires) 2020;80:1–6. [PubMed] [Google Scholar]
- 70.L.E.R. Cunha, M.A. Strauch, V.A.R. Pereira, et al. (2020). Equine hyperimmune globulin raised against the SARS-Cov 2 spike glycoglobulin has extremely high neutralizing titers. BioRxiv. 2020.08.17.254375. https://doi.org/10.1101/2020.08.17.254375.
- 71.León Guillermo, Herrera María, Vargas Mariángela, Arguedas Mauricio, Sánchez Andrés, Segura Álvaro, Gómez Aarón, Solano Gabriela, Corrales-Aguilar Eugenia, Risner Kenneth, Narayanan Aarthi, Bailey Charles, Villalta Mauren, Hernández Andrés, Sánchez Adriana, Cordero Daniel, Solano Daniela, Durán Gina, Segura Eduardo, Cerdas Maykel, Umaña Deibid, Moscoso Edwin, Estrada Ricardo, Gutiérrez Jairo, Méndez Marcos, Castillo Ana Cecilia, Sánchez Laura, Sánchez Ronald, Gutiérrez José María, Díaz Cecilia, Alape Alberto. Development and characterization of two equine formulations towards SARS-CoV-2 proteins for the potential treatment of COVID-19. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-89242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.FAPESP (Fundação de Amparo à Pesquisa do estado de São Paulo) research. Butantan equine serum notice by FAPESP. https://revistapesquisa.fapesp.br/butantan-desenvolve-soro-contra-o-novo-coronavirus/ 2021 (accessed in 12 May 2021).
- 73.Sette Alessandro, Crotty Shane. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Q. Zhang, P. Bastard, Z. Liu, et al. (2020). Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Sci. 370, eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed]
- 75.Bastard Paul, Rosen Lindsey B., Zhang Qian, Michailidis Eleftherios, Hoffmann Hans-Heinrich, Zhang Yu, Dorgham Karim, Philippot Quentin, Rosain Jérémie, Béziat Vivien, Manry Jérémy, Shaw Elana, Haljasmägi Liis, Peterson Pärt, Lorenzo Lazaro, Bizien Lucy, Trouillet-Assant Sophie, Dobbs Kerry, de Jesus Adriana Almeida, Belot Alexandre, Kallaste Anne, Catherinot Emilie, Tandjaoui-Lambiotte Yacine, Le Pen Jeremie, Kerner Gaspard, Bigio Benedetta, Seeleuthner Yoann, Yang Rui, Bolze Alexandre, Spaan András N., Delmonte Ottavia M., Abers Michael S., Aiuti Alessandro, Casari Giorgio, Lampasona Vito, Piemonti Lorenzo, Ciceri Fabio, Bilguvar Kaya, Lifton Richard P., Vasse Marc, Smadja David M., Migaud Mélanie, Hadjadj Jérome, Terrier Benjamin, Duffy Darragh, Quintana-Murci Lluis, van de Beek Diederik, Roussel Lucie, Vinh Donald C., Tangye Stuart G., Haerynck Filomeen, Dalmau David, Martinez-Picado Javier, Brodin Petter, Nussenzweig Michel C., Boisson-Dupuis Stéphanie, Rodríguez-Gallego Carlos, Vogt Guillaume, Mogensen Trine H., Oler Andrew J., Gu Jingwen, Burbelo Peter D., Cohen Jeffrey I., Biondi Andrea, Bettini Laura Rachele, D'Angio Mariella, Bonfanti Paolo, Rossignol Patrick, Mayaux Julien, Rieux-Laucat Frédéric, Husebye Eystein S., Fusco Francesca, Ursini Matilde Valeria, Imberti Luisa, Sottini Alessandra, Paghera Simone, Quiros-Roldan Eugenia, Rossi Camillo, Castagnoli Riccardo, Montagna Daniela, Licari Amelia, Marseglia Gian Luigi, Duval Xavier, Ghosn Jade, Tsang John S., Goldbach-Mansky Raphaela, Kisand Kai, Lionakis Michail S., Puel Anne, Zhang Shen-Ying, Holland Steven M., Gorochov Guy, Jouanguy Emmanuelle, Rice Charles M., Cobat Aurélie, Notarangelo Luigi D., Abel Laurent, Su Helen C., Casanova Jean-Laurent. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Sci. 2020;370(6515):eabd4585. doi: 10.1126/science:abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Chaolin, Wang Yeming, Li Xingwang, Ren Lili, Zhao Jianping, Hu Yi, Zhang Li, Fan Guohui, Xu Jiuyang, Gu Xiaoying, Cheng Zhenshun, Yu Ting, Xia Jiaan, Wei Yuan, Wu Wenjuan, Xie Xuelei, Yin Wen, Li Hui, Liu Min, Xiao Yan, Gao Hong, Guo Li, Xie Jungang, Wang Guangfa, Jiang Rongmeng, Gao Zhancheng, Jin Qi, Wang Jianwei, Cao Bin. novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.A. Nalbandian, K. Sehgal, A. Gupta et al, Post-acute COVID-19 Syndrome. Nat. Med.27 (2021) 601-615. https://doi.org/10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed]
- 78.Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.A. Fakharian, S. Barati, M. Mohamadi, et al. (2021). Successful Management of COVID-19 with Adalimumab in a Post-Coronary Artery Bypass Graft Surgery Patient. J. Cardiothorac. Vasc. Anesth. 7, S1053-0770(20)31370-7. https://doi.org/10.1053/j.jvca.2020.12.023. [DOI] [PMC free article] [PubMed]
- 80.Zhang Chi, Wu Zhao, Li Jia-Wen, Zhao Hong, Wang Gui-Qiang. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Xiaoling, Han Mingfeng, Li Tiantian, Sun Wei, Wang Dongsheng, Fu Binqing, Zhou Yonggang, Zheng Xiaohu, Yang Yun, Li Xiuyong, Zhang Xiaohua, Pan Aijun, Wei Haiming. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bennardo Francesco, Buffone Caterina, Giudice Amerigo. New therapeutic opportunities for COVID-19 patients with Tocilizumab: Possible correlation of interleukin-6 receptor inhibitors with osteonecrosis of the jaws. Oral Oncol. 2020;106:104659. doi: 10.1016/j.oraloncology.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D. Wang, B. Fu, and Z. Peng, et al. (2021) Tocilizumab Ameliorates the Hypoxia in COVID-19 Moderate Patients with Bilateral Pulmonary Lesions: A Randomized, Controlled, Open-Label, Multicenter Trial. .Available at http://dx.doi.org/10.2139/ssrn.3667681.
- 84.Salama C., Han J., Yau L., et al. Tocilizumab in Patients Hospitalised with Covid-19 Pneumonia. N. Engl. J. Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.P. Simmonds, S. Williams, H. Harvala. Understanding the outcomes of COVID-19 – does the current model of an acute respiratory infection really fit? J. Gen. Virol.102. https://doi.org/10.1099/jgv.0.001545. [DOI] [PMC free article] [PubMed]
- 86.Annen K., Morrison T.E., DomBourian M.G., et al. Presence and short-term persistence of SARS-CoV-2 neutralizing antibodies in COVID-19 convalescent plasma donors. Transfusion. 2021;61:1148–1159. doi: 10.1111/trf.16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nag D., Chaudhry R., Mishra M., et al. A Prospective Study on Rapidly Declining SARS-CoV-2 IgG Antibodies Within One to Three Months of Testing IgG Positive: Can It Lead to Potential Reinfections? Cureus. 2020;12 doi: 10.7759/cureus.11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gómez C.E., Perdiguero B., Esteban M. Emerging SARS-CoV-2 Variants and Impact in Global Vaccination Programs against SARS-CoV-2/COVID-19. Vaccines. 2021;9:243. doi: 10.3390/vaccines9030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen Xiaoying, Tang Haili, McDanal Charlene, Wagh Kshitij, Fischer William, Theiler James, Yoon Hyejin, Li Dapeng, Haynes Barton F., Sanders Kevin O., Gnanakaran Sandrasegaram, Hengartner Nick, Pajon Rolando, Smith Gale, Glenn Gregory M., Korber Bette, Montefiori David C. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral Spike vaccines. C. Host Microb. 2021;29(4):529–539.e3. doi: 10.1016/j.chom.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.J. Singh, S.A. Rahman, N.Z. Ehtesham, et al. (2021). SARS-CoV-2 variants of concern are emerging in India. Nat. Med. https://doi.org/10.1038/s41591-021-01397-4. [DOI] [PubMed]
- 91.Wibmer Constantinos Kurt, Ayres Frances, Hermanus Tandile, Madzivhandila Mashudu, Kgagudi Prudence, Oosthuysen Brent, Lambson Bronwen E., de Oliveira Tulio, Vermeulen Marion, van der Berg Karin, Rossouw Theresa, Boswell Michael, Ueckermann Veronica, Meiring Susan, von Gottberg Anne, Cohen Cheryl, Morris Lynn, Bhiman Jinal N., Moore Penny L. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021;27(4):622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- 92.Hoffmann Markus, Arora Prerna, Groß Rüdiger, Seidel Alina, Hörnich Bojan F., Hahn Alexander S., Krüger Nadine, Graichen Luise, Hofmann-Winkler Heike, Kempf Amy, Winkler Martin S., Schulz Sebastian, Jäck Hans-Martin, Jahrsdörfer Bernd, Schrezenmeier Hubert, Müller Martin, Kleger Alexander, Münch Jan, Pöhlmann Stefan. S.ARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184(9):2384–2393.e12. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.WHO variants nomenclature announcement. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed in 10 June 2021).
- 94.Sung J., Anjum S. Coronavirus disease 2019 (COVID-19) infection associated with antiphospholipid antibodies and fourextremity deep vein thrombosis in a previously healthy female. Cureus. 2020;12 doi: 10.7759/cureus.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vlachoyiannopoulos Panayiotis G, Magira Eleni, Alexopoulos Haris, Jahaj Edison, Theophilopoulou Katerina, Kotanidou Anastasia, Tzioufas Athanasios G. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann. Rheum. Dis. 2020;79(12):1661–1663. doi: 10.1136/annrheumdis-2020-218009. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Yan, Xiao Meng, Zhang Shulan, Xia Peng, Cao Wei, Jiang Wei, Chen Huan, Ding Xin, Zhao Hua, Zhang Hongmin, Wang Chunyao, Zhao Jing, Sun Xuefeng, Tian Ran, Wu Wei, Wu Dong, Ma Jie, Chen Yu, Zhang Dong, Xie Jing, Yan Xiaowei, Zhou Xiang, Liu Zhengyin, Wang Jinglan, Du Bin, Qin Yan, Gao Peng, Qin Xuzhen, Xu Yingchun, Zhang Wen, Li Taisheng, Zhang Fengchun, Zhao Yongqiang, Li Yongzhe, Zhang Shuyang. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N. Engl. J. Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zuo Yu, Estes Shanea K., Ali Ramadan A., Gandhi Alex A., Yalavarthi Srilakshmi, Shi Hui, Sule Gautam, Gockman Kelsey, Madison Jacqueline A., Zuo Melanie, Yadav Vinita, Wang Jintao, Woodard Wrenn, Lezak Sean P., Lugogo Njira L., Smith Stephanie A., Morrissey James H., Kanthi Yogendra, Knight Jason S. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020;12(570):eabd3876. doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mobini M., Ghasemian R., Larijani L.V., et al. Vasculitis-associated auto-antibodies and complement levels in patients with COVID-19 infection. R. Square. 2020 doi: 10.21203/rs.3.rs-30488/v1. [DOI] [Google Scholar]
- 99.Woodruff Matthew C., Ramonell Richard P., Nguyen Doan C., Cashman Kevin S., Saini Ankur Singh, Haddad Natalie S., Ley Ariel M., Kyu Shuya, Howell J. Christina, Ozturk Tugba, Lee Saeyun, Suryadevara Naveenchandra, Case James Brett, Bugrovsky Regina, Chen Weirong, Estrada Jacob, Morrison-Porter Andrea, Derrico Andrew, Anam Fabliha A., Sharma Monika, Wu Henry M., Le Sang N., Jenks Scott A., Tipton Christopher M., Staitieh Bashar, Daiss John L., Ghosn Eliver, Diamond Michael S., Carnahan Robert H., Crowe James E., Hu William T., Lee F. Eun-Hyung, Sanz Ignacio. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020;21(12):1506–1516. doi: 10.1038/s41590-020-00814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liua Y., Sawalha A.H., Lua Q. COVID-19 and autoimmune diseases Curr. Opin. Rheumatol. 2021;33:155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jensen Christopher E., Wilson Samuel, Thombare Aparna, Weiss Susan, Ma Alice. Cold agglutinin syndrome as a complication of Covid-19 in two cases. Clin. Infect. Pract. 2020;7-8:100041. doi: 10.1016/j.clinpr.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maslov Diana V., Simenson Victoria, Jain Suma, Badari Ambuga. COVID-19 and cold agglutinin hemolytic anemia. TH Open. 2020;04(03):e175–e177. doi: 10.1055/s-0040-1715791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fujii Hiroyuki, Tsuji Taisuke, Yuba Tatsuya, Tanaka Shunya, Suga Yoshifumi, Matsuyama Aosa, Omura Ayaka, Shiotsu Shinsuke, Takumi Chieko, Ono Seiko, Horiguchi Masahito, Hiraoka Noriya. High levels of anti-SSA/Ro antibodies in COVID-19 patients with severe respiratory failure: a case-based review. Clin. Rheumatol. 2020;39(11):3171–3175. doi: 10.1007/s10067-020-05359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guilmot Antoine, Maldonado Slootjes Sofia, Sellimi Amina, Bronchain Maroussia, Hanseeuw Bernard, Belkhir Leila, Yombi Jean Cyr, De Greef Julien, Pothen Lucie, Yildiz Halil, Duprez Thierry, Fillée Catherine, Anantharajah Ahalieyah, Capes Antoine, Hantson Philippe, Jacquerye Philippe, Raymackers Jean-Marc, London Frederic, El Sankari Souraya, Ivanoiu Adrian, Maggi Pietro, van Pesch Vincent. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2021;268(3):751–757. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pinto Ashwin A., Carroll Liam S., Nar Vijay, Varatharaj Aravinthan, Galea Ian. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19 Neurol. Neuroimmunol. Neuroinflamm. 2020;7(5):e813. doi: 10.1212/NXI.0000000000000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.A. Berzuini, C. Bianco, C. Paccapelo, et al, Red cell–bound antibodies and transfusion requirements in hospitalized patients with COVID-19, Blood.136 (2020) 766–768. doi: https://doi.org/10.1182/blood.2020006695. [DOI] [PMC free article] [PubMed]
- 107.American Journal of Nursing. NewsCAP: Autoantibody reactivity implicated in ‘long’ COVID-19. https://journals.lww.com/ajnonline/Fulltext/2021/03000/NewsCAP__Autoantibody_reactivity_implicated_in.11.aspx, 2021 (accessed in 12 May 2021). [DOI] [PubMed]