Abstract
Background
Dual antiplatelet therapy (DAPT) has important implications for clinical outcomes in coronary disease. However, the optimal DAPT duration remains uncertain.
Methods and results
We searched four major databases for randomised controlled trials comparing long-term (≥12 months) with short-term (≤6 months) or shorter (≤3 months) DAPT in patients with coronary syndromes. The primary outcome was all-cause mortality. Secondary outcomes were any bleeding and major bleeding (safety), cardiac death, myocardial infarction, stent thrombosis, revascularisation and stroke (efficacy). Nineteen randomised controlled trials (n=60 111) satisfied inclusion criteria, 8 assessed ≤3 months DAPT. Compared with long-term (≥12 months), short-term DAPT (≤6 months) was associated with a trend towards reduced all-cause mortality (RR: 0.90, 95% CI: 0.80 to 1.01) and significant bleeding reduction (RR: 0.68, 95% CI: 0.55 to 0.83 and RR: 0.66, 95% CI: 0.56 to 0.77 for major and any bleeding, respectively). There were no significant differences in efficacy outcomes. These associations persisted in sensitivity analysis comparing shorter duration DAPT (≤3 months) to long-term DAPT (≥12 months) for all-cause mortality (RR: 0.91, 95% CI: 0.79 to 1.05). In subgroup analysis, short-term DAPT was associated with lower risk of bleeding in patients with acute or chronic coronary syndromes (RR: 0.66, 95% CI: 0.54 to 0.81 and RR: 0.53, 95% CI: 0.33 to 0.65, respectively), but higher risk of stent thrombosis in acute coronary syndrome (RR: 1.49, 95% CI: 1.02 to 2.17 vs RR: 1.25, 95% CI 0.44 to 3.58).
Conclusion
Our meta-analysis suggests that short (≤6 months) and shorter (≤3 months) durations DAPT are associated with lower risk of bleeding, equivalent efficacy and a trend towards lower all-cause mortality irrespective of coronary artery disease stability.
Keywords: acute coronary syndrome, angina pectoris, pharmacology, clinical
Key messages.
What is already known about this subject?
Dual antiplatelet therapy (DAPT) is a central component of the modern management of acute and chronic coronary syndromes following percutaneous coronary intervention. Despite substantial evidence supporting its use, there remains major uncertainty regarding the optimal duration of therapy.
What does this study add?
Short-term (≤6 months) and shorter durations (≤3 months) of DAPT are associated with significantly lower risk of bleeding, equivalent efficacy and a trend towards lower all-cause mortality compared with long-term DAPT (≥12 months) irrespective of coronary artery stability.
This meta-analysis highlights the paucity of randomised controlled trial evidence to guide DAPT in acute coronary syndrome patients who are managed without percutaneous coronary intervention such as those receiving medical therapy only or those undergoing coronary artery bypass grafting.
How might this impact on clinical practice?
Shorter durations of DAPT in patients with acute or chronic coronary syndrome undergoing percutaneous coronary intervention may be the best balance between efficacy and safety outcomes as shown by all-cause mortality which tended to favour 3 months of therapy.
Introduction
Dual antiplatelet therapy (DAPT) is a central component of the modern management of acute coronary syndromes (ACS). The aim of DAPT is to reduce the risk of recurrent atherothrombotic events by suppressing thrombus formation related to disrupted atherosclerotic plaque.1 2 Despite substantial evidence supporting its use, there remains major uncertainty regarding the optimal duration of therapy. While clinical guidelines on the management of ACS recommend a default duration of 12 months of DAPT with aspirin and a P2Y12 receptor antagonist, they also advise consideration of short-term DAPT (≤6 months) for patients at a high risk of bleeding.3 4
Previous systematic reviews and meta-analyses concluded that shorter durations of DAPT may be superior to standard care in most patients, with apparent small reductions in all-cause mortality.5 6 This suggests that the risk of major bleeding outweighs any benefit gained from the reduction in future atherothrombotic events. These meta-analyses reviewed trials which, for the most part, evaluated DAPT following percutaneous coronary intervention with drug-eluting stents in patients with chronic coronary syndromes. Recently, there have been several large-scale randomised controlled trials evaluating shorter durations of DAPT (≤3 months) in the setting of ACS.7–9
Here, we perform an updated systematic review and meta-analysis comparing outcomes in long-term DAPT (≥12 months) with short-term (≤6 months) and shorter (≤3 months) durations of DAPT incorporating the latest randomised controlled trial evidence.
Methods
Data sources and search strategy
This systematic review and meta-analysis followed the Cochrane Collaboration guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (online supplemental research checklist) and was performed according to a prespecified analysis plan (online supplemental appendix).10 11 Two independent investigators (MM and AB) performed the literature search using four major databases: Central, Embase, Medline and Web of Science from 1950 to February 2020. In addition, online resources including ClinicalTrials.gov and proceedings from major cardiovascular conferences were also screened. The search strategy was individually tailored to each database (online supplemental S1 table). Relevant search items such as: ‘coronary syndrome’, ‘antiplatelet therapy’, ‘platelet aggregation inhibitor’, ‘drug eluting stent’, ‘coronary intervention’ were included in the Medical Subject Heading search.
openhrt-2021-001707supp001.pdf (11.5MB, pdf)
Study selection
Randomised controlled trials comparing different durations of DAPT, irrespective of presentation (acute or chronic coronary syndromes), or the management strategy (percutaneous coronary intervention, coronary artery bypass graft surgery or medical therapy alone) that assessed at least one of the prespecified outcomes of interest were included in this systematic review and meta-analysis. The DAPT durations of interest were ≤6 months (short-term) versus ≥12 months (long-term). Studies which compared mid-term DAPT (>6 but<12 months) to long-term (≥12 months) or standard term (12 months) to longer-term (>18 months) DAPT were excluded.12–15 Cross-sectional studies, observational studies, case reports or case series were also excluded.
Quality assessment and data extraction
Two investigators (MM and AB) independently screened article titles and abstracts to exclude any trials which did not match the research question of interest. Subsequently, the two reviewers independently screened the eligible full-text articles to identify randomised controlled trials which met the prespecified inclusion criteria. The reference lists of the relevant studies were manually checked to identify potentially missed studies. Data extraction was conducted independently by two authors (MM and AB) and any conflicts related to data extraction were resolved through discussion and review of data or consensus from a third author (KKL).
Data extraction included study characteristics (trial registration number, trial name, trial period, study centre(s), year of publication, first author, randomisation arms (intervention vs control), study population according to randomisation arm, treatment strategy according to randomisation arm, randomisation time, follow-up duration, outcome measures including primary, secondary outcomes and relevant definitions (table 1). Baseline characteristics for study population (age, sex, ACS at presentation, patients with background history of diabetes mellitus, ischaemic heart disease, peripheral vascular disease, renal impairment and cardiovascular risk factors) (online supplemental S2 table) were collected where available, and relevant risk estimates for the primary trial outcome and meta-analysis outcomes of interest (online supplemental S3 table).
Table 1.
Study characteristics according to randomisation arm
| Study | DAPT duration (months) | Total population | ACS population | CCS population | DAPT regimen | Randomisation | Follow-up | Primary outcome |
| CREDO* (Steinhubl et al39 2002) | 1 | 1063 | 703 (66%) | 360 (34%) | Aspirin 81–325 mg plus clopidogrel 75 mg | Prior to index PCI | 12 months | Composite of death, myocardial infarction (MI) and stroke in the intention-to-treat population. |
| 12 | 1053 | 704 (67%) | 349 (33%) | Aspirin 81–325 mg plus placebo | ||||
| DAPT-STEMI (Kedhi et al40 2018) | 6 | 433 | 433 (100%) | 0 (0%) | Aspirin 75–100 mg | Six months following index PCI | 24 months | Composite of all-cause mortality, any MI, any revascularisation, stroke or thrombolysis. |
| 12 | 437 | 437 (100%) | 0 (0%) | Aspirin 75–100 mg plus prasugrel 10 mg or 5 mg/ticagrelor 90 mg/clopidogrel 75 mg | ||||
| EXCELLENT (Gwon et al41 2012) | 6 | 722 | 369 (51%) | 353 (49%) | Aspirin 100–200 mg | At index PCI | 12 months | Composite of cardiac death, MI or target vessel revascularisation. |
| 12 | 721 | 375 (52%) | 346 (48%) | Aspirin 100–200 mg plus clopidogrel 75 mg | ||||
| GLOBAL LEADERS* (Vranckx et al7 2018) | 1 | 7980 | 3750 (47%) | 4230 (53%) | Ticagrelor 90 mg | At index PCI | 24 months | Composite of all-cause death or new Q-wave MI. |
| 12 | 7988 | 3737 (47%) | 4251 (53%) | Ticagrelor 90 mg or clopidogrel 75 mg and aspirin 75–100 mg | ||||
| I-LOVE-IT-2 (Han et al42 2016) | 6 | 909 | 752 (83%) | 157 (17%) | Aspirin 100 mg | At index PCI | 18 months | Target lesion failure. |
| 12 | 920 | 744 (81%) | 176 (19%) | Aspirin 100 mg and clopidogrel 75 mg | ||||
| ISAR-SAFE (Schulz-Schupke et al43 2015) | 6 | 1997 | 794 (40%) | 1203 (60%) | Aspirin 81–162 mg | Six months after index PCI | 9 months | Composite of death, MI, stent thrombosis (definite or probable), stroke or thrombolysis in myocardial infarction (TIMI) major bleeding. |
| 12 | 2003 | 807 (40%) | 1196 (60%) | Aspirin 81–162 mg combined with clopidogrel 75 mg or ticlopidine 200 mg | ||||
| ITALIC (Didier et al44 2017) | 6 | 926 | 400 (43%) | 526 (57%) | Aspirin 75 mg | Six months following index PCI | 24 months | Composite of all-cause mortality, MI, target vessel revascularisation, stroke, major bleeding, stent thrombosis. |
| 24 | 924 | 406 (44%) | 518 (56%) | Aspirin 75 mg and clopidogrel 75 mg or prasugrel 60 mg or ticagrelor 90 mg | ||||
| IVUS-XPL (Hong et al45 2016) | 6 | 699 | 343 (49%) | 356 (51%) | Aspirin 100 mg | At index PCI | 12 months | Composite of cardiac death, MI, stroke or TIMI major bleeding. |
| 12 | 701 | 343 (49%) | 358 (51%) | Aspirin 100 mg plus clopidogrel 75 mg | ||||
| NIPPON (Nakamura et al46 2017) | 6 | 1654 | 527 (32%) | 1127 (68%) | Aspirin 81–162 mg | At index PCI | 18 months | Net adverse clinical and cerebrovascular events defined as all cause death, Q-wave or non-Q-wave MI, cerebrovascular events, and major bleeding events. |
| 18 | 1653 | 552 (33%) | 1101 (67%) | Aspirin 81–162 mg combined with clopidogrel 75 mg or ticlopidine 200 mg | ||||
| OPTIMA-C (Lee et al47 2018) | 6 | 684 | 348 (51%) | 336 (49%) | Aspirin 100 mg | At index PCI | 12 months | Composite of major adverse cardiovascular events (MACCE; cardiac death, target vessel-related MI, ischaemia driven target lesion revascularisation. |
| 12 | 683 | 344 (50%) | 339 (50%) | Aspirin 100 mg plus clopidogrel 75 mg | ||||
| OPTIMIZE* (Feres et al48 2013) | 3 | 1563 | 494 (32%) | 1069 (68%) | Aspirin 100–200 mg | At index PCI | 12 months | Composite of death from all causes, MI, stroke or major bleeding. |
| 12 | 1556 | 502 (32%) | 1054 (68%) | Aspirin 100–200 mg plus clopidogrel 75 mg | ||||
| PRODIGY (Valgimigli et al49 2012) | 6 | 983 | 733 (75%) | 250 (25%) | Aspirin 80–160 mg | One month after index PCI | 24 months | Composite of death of any cause, nonfatal MI or cerebrovascular accident; cardiovascular death, the incidence of stent thrombosis and bleeding outcomes. |
| 24 | 987 | 732 (74%) | 255 (26%) | Aspirin 80–160 mg plus clopidogrel 75 mg | ||||
| REDUCE* (De Luca et al50 2019) | 3 | 733 | 733 (100%) | 0 (0%) | Aspirin | At index PCI | 24 months | Composite of all-cause death, MI, stent thrombosis, stroke, target vessel revascularisation, bleeding. |
| 12 | 727 | 727 (100%) | 0 (0%) | Aspirin and P2Y12 inhibitor (prasugrel, ticagrelor or clopidogrel) | ||||
| RESET* (Kim et al51 2012) | 3 | 1059 | 588 (56%) | 471 (44%) | Aspirin 100 mg | At index PCI | 12 months | Composite of death from cardiovascular cause, MI, stent thrombosis, ischaemia driven target-vessel revascularisation or bleeding. |
| 12 | 1058 | 568 (54%) | 490 (46%) | Aspirin 100 mg and clopidogrel 75 mg | ||||
| SECURITY* (Colombo et al52 2014) | 6 | 682 | 213 (31%) | 469 (69%) | Aspirin | At index PCI | 24 months | Composite of cardiac death, MI, stroke, definite or probable stent thrombosis, BARC 3 or 5 bleeding, target vessel revascularisation, all-cause mortality. |
| 12 | 717 | 229 (32%) | 488 (68%) | Aspirin plus clopidogrel 75 mg | ||||
| SMART-DATE (Hahn et al9 2018) | 6 | 1357 | 1357 (100%) | 0 (0%) | Aspirin 100 mg | At index PCI | 18 months | Composite of all-cause mortality, MI or stroke. |
| 12 | 1355 | 1355 (100%) | 0 (0%) | Aspirin 100 mg and clopidogrel 75 mg, prasugrel 10 mg or ticagrelor 90 mg | ||||
| STOPDAPT-2* (Watanabe et al53 2019) |
1 | 1500 | 565 (38%) | 935 (62%) | Clopidogrel 75 mg | At index PCI | 12 months | Composite of cardiovascular and bleeding events (cardiovascular death, MI, definite stent thrombosis, ischaemic or haemorrhagic strokeor TIMI major or minor bleeding. |
| 12 | 1509 | 583 (39%) | 926 (61%) | Aspirin 81–200 mg and clopidogrel 75 mg or prasugrel 3.75 mg | ||||
| TICO* (Kim et al25 2020) |
3 | 1527 | 1527 (100%) | 0 (0%) | Ticagrelor 90 mg | At index PCI | 12 months | Net adverse clinical events (TIMI major bleeding and MACCE). |
| 12 | 1529 | 1529 (100%) | 0 (0%) | Ticagrelor 90 mg and aspirin | ||||
| TWILIGHT* (Mehran et al8 2019) | 3 | 3555 | 2273 (56%) | 1282 (44%) | Ticagrelor 90 mg plus placebo | At index PCI | 12 months | The first occurrence of BARC type 2, 3 or 5 bleeding between randomisation and 1 year in a time-to-event analysis. |
| 12 | 3564 | 2341 (66%) | 1223 (34%) | Ticagrelor 90 mg and aspirin |
*Trials included in the sensitivity analysis (compared ≤3 months of DAPT with 12 months of DAPT).
ACS, acute coronary syndrome; BARC, Bleeding Academic Research Consortium; CCS, chronic coronary syndrome; DAPT, dual antiplatelet therapy; MACCE, major adverse cardiac and cerebrovascular events; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
The study quality was assessed using the Cochrane Collaboration tool for assessment of risk of bias, which includes random sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective reporting and other sources of bias.16 Disagreements were resolved by consensus.
Definition of outcomes
The primary outcome was all-cause mortality and secondary efficacy outcomes were cardiac death, myocardial infarction, stent thrombosis, coronary revascularisation and stroke. Secondary safety endpoints were any bleeding and major bleeding. Stent thrombosis included definite or probable thrombosis according to individual trial definitions and criteria from the Academic Research Consortium.17 Trial definitions for major and any bleeding were applied, and these included the Thrombolysis in Myocardial Infarction or Bleeding Academic Research Consortium criteria (online supplemental S4 table).18 19 Randomised controlled trials which did not report event rates or risk estimates for the prespecified endpoints were not included in the overall meta-analysis estimates.
Statistical analysis
In this pairwise meta-analysis, risk estimates and event rates for each outcome of interest were extracted from the randomised controlled trials. Risk ratios and 95% CI were used as summary statistics to evaluate the effect of DAPT duration on the outcomes of interest. Pooled meta-analysis risk estimates were computed using a random-effects model. Risk ratios greater than one represented benefit associated with the longer DAPT duration arm (control), and less than one was associated with benefit favouring the shorter duration arm (intervention).
Between study heterogeneity was assessed using the statistical inconsistency test (I2=100% × (Q−df)/Q, where Q= χ2 (Cochran’s heterogeneity statistic) and df=its degrees of freedom), where I2 ≤25% signifies low heterogeneity, I2 ≤50% is moderate heterogeneity and I2 >50% is considered high heterogeneity.20 Small study effects and potential publication bias were examined by constructing funnel plots for the clinical outcomes in which the SE of the log of the risk ratio was plotted against the risk ratio (central estimate).21
Sensitivity analyses restricted to trials evaluating shorter durations of DAPT (≤3 months) were conducted to explore the primary outcome of all-cause mortality and the secondary efficacy and safety outcomes. A further sensitivity analysis was conducted to explore the effect of the type of P2Y12 inhibitor on study outcomes, restricting analysis to trials that used clopidogrel only or to studies that used any type of P2Y12 inhibitor (clopidogrel or prasugrel or ticagrelor). Subgroup analysis evaluating the effect of clinical presentation was also performed from data in trials that reported risk ratios stratified by clinical presentation. ACS was defined as patients suspected of acute myocardial infarction/ischaemia, and chronic coronary syndromes was defined as patients with stable symptoms of coronary artery disease.
Analysis was performed using R V.3.5.0 (R Foundation for Statistical Computing, Vienna, Austria) using the meta, metafor and metaviz packages.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Our systematic search identified 44 424 articles and 28 863 underwent title and abstract screening after duplicates were removed (online supplemental S1 figure). Of these, 46 potentially eligible articles underwent full-text review, and a further 27 articles were excluded based on pre-specified criteria. A total of 19 randomised controlled trials from 2001 to 2018 with an overall population of 60 111 patients (ranging from 870 to 15 968 in individual studies) were included. Of the total population, 33 952 (56%) were ACS and 26 159 (44%) were chronic coronary syndromes. Four randomised controlled trials evaluated duration of DAPT in ACS exclusively (n=8098), while 15 trials included both acute and chronic presentations. No randomised controlled trial investigating duration of DAPT in patients with ACS managed medically or undergoing coronary artery bypass graft surgery were identified.
The duration of DAPT across trials ranged from 1 month to 24 months. Duration of follow-up also varied between trials ranging from 9 months to 24 months. Of the 19 included randomised controlled trials, eight trials compared shorter-term DAPT (≤3 months) with long-term DAPT (>12 months) with an overall population of 38 036 patients and two of these studies included ACS presentations only (Table 1 and online supplemental S3 table).
Risk of bias and publication bias
The risk of bias assessment was performed for each randomised controlled trial (online supplemental S4 table). All studies were assessed as having low risk of bias for random sequence allocation (19/19, 100%) with majority of studies being low risk for allocation concealment (15/19, 79%), blinding of outcome assessment (15/19, 79%), incomplete outcome data (18/19, 95%), selective reporting (17/19, 90%) and other bias (18/19, 95%). The majority of studies were identified to be at risk of bias due to inadequate blinding of participants and personnel (16/19, 84%). Allocation concealment was unclear in 3/19 (16%) studies. Evaluation of the funnel plots suggests a degree of publication bias when considering the safety outcomes of any bleeding and major bleeding (online supplemental S2 figure).
Short-term (≤6 months) versus long-term (≥12 months) dual antiplatelet therapy
All 19 randomised trials reported the primary outcome of all-cause mortality. Short-term DAPT was associated with an apparent decrease in all-cause mortality (RR: 0.90, 95% CI: 0.81 to 1.01) (figure 1). There was no significant heterogeneity between studies when considering all-cause mortality (I2=0%). Individual trial data are presented in online supplemental S3 figure. A similar trend towards reduced all-cause mortality was observed with short-term DAPT in trials (n=8) which used different P2Y12 receptor antagonists including clopidogrel, ticagrelor or prasugrel (RR: 0.87, 95% CI: 0.76 to 1.00). While in an analysis restricted to studies (n=11) that used clopidogrel only as the P2Y12 receptor antagonist, the pooled risk estimates for all-cause mortality were equivalent (RR: 0.97, 95% CI: 0.80 to 1.18) when considering DAPT duration online supplemental S4 figure.
Figure 1.
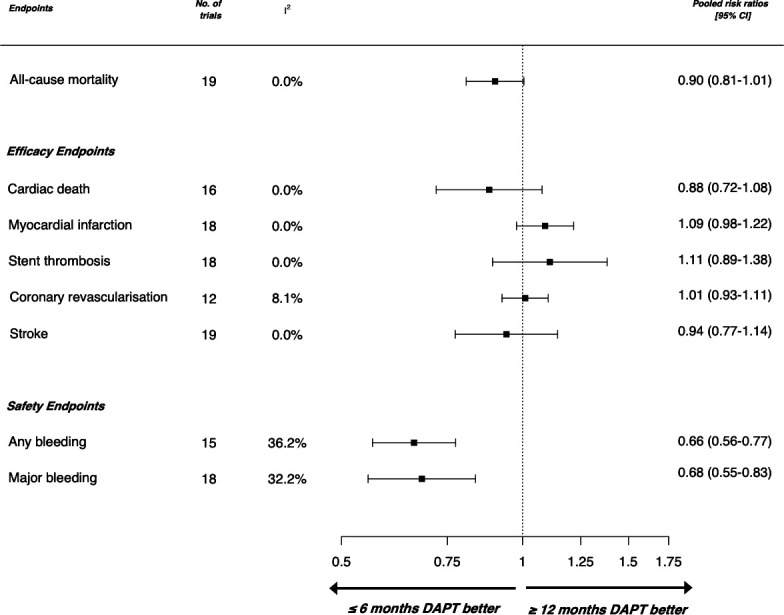
Forest plot showing overall pooled risk estimates according to outcomes of interest from all randomised controlled trials comparing short duration of dual-antiplatelet therapy (≤6 months) and long duration (≥12 months) included in this meta-analysis (n=60 111). DAPT, dual antiplatelet therapy.
All studies reported the efficacy end point of stroke, 18 studies evaluated the secondary endpoints of myocardial infarction and stent thrombosis, 16 and 12 studies reported cardiac mortality and coronary revascularisation, respectively. A trend towards increased risk of myocardial infarction (RR: 1.09, 95% CI: 0.98 to 1.22) and equivalent risk of stent thrombosis (RR: 1.11, 95% CI: 0.89 to 1.38) and coronary revascularisation (RR: 1.01, 95% CI: 0.93 to 1.11) was observed with short-term DAPT when compared with long-term DAPT (≥12 months). Short-term DAPT was associated with similar risk of cardiac mortality (RR: 0.88, 95% CI: 0.72 to 1.08) and stroke (RR: 0.94, CI: 95% 0.77 to 1.14). There was no significant heterogeneity between studies when considering these efficacy outcomes (I2 <25%). Individual trial data are presented in online supplemental S3 figure.
Of the 19 studies, 18 reported the safety endpoint of major bleeding and 15 reported ‘any bleeding events’. Study-specific definitions are summarised in online supplemental S4 table. Short-term DAPT was associated with a reduction in bleeding when compared with long-term DAPT, with RR of 0.68 (95% CI: 0.55 to 0.83) for major bleeding and RR: 0.66 (95% CI: 0.56 to 0.77) for any bleeding. Modest heterogeneity (I2=32.2%) was observed across the studies when assessing these safety outcomes. Individual trial data are presented in online supplemental S3 figure.
Shorter duration (≤3 months) versus long-term (≥12 months) dual antiplatelet therapy
Meta-estimates were consistent in sensitivity analysis restricted to the eight trials comparing shorter durations of DAPT with long-term DAPT. The trend towards a reduction in all-cause mortality was maintained with shorter duration DAPT (RR: 0.91, 95% CI: 0.79 to 1.05) with no significant heterogeneity across the studies (I2=0%) (figure 2).
Figure 2.
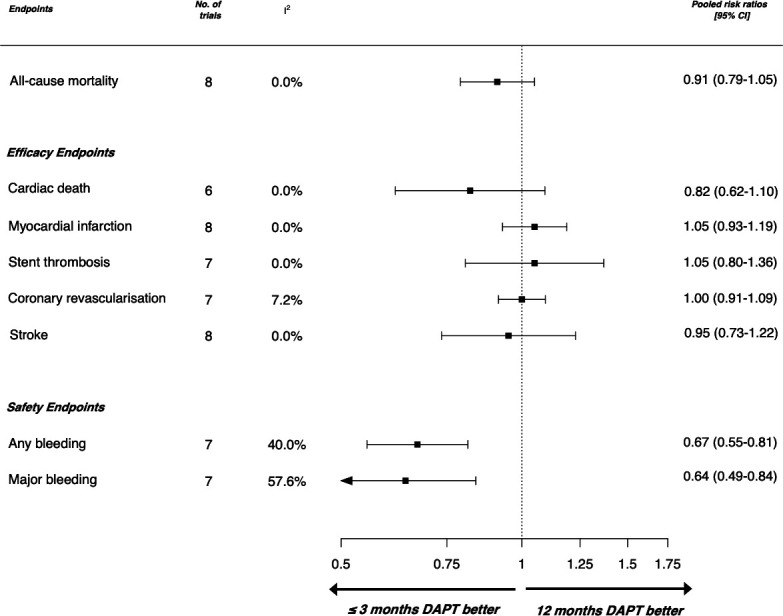
Forest plot showing pooled risk estimates according to outcomes of interest restricted to randomised controlled trials comparing even shorter duration of dual-antiplatelet therapy (≤3 months) and standard duration (12 months) included in this meta-analysis (n=38 036) DAPT, dual antiplatelet therapy.
The pooled risk meta-estimates were equivalent for myocardial infarction (RR: 1.05, 95% CI: 0.93 to 1.19), stent thrombosis (RR: 1.05, 95% CI: 0.8 to 1.36), repeat revascularisation (RR: 1.0, 95% CI: 0.91 to 1.09) and stroke (RR: 0.95, 95% CI: 0.73 to 1.22). In shorter duration DAPT, estimates appeared to suggest a lower risk for cardiac death (RR: 0.82, 95% CI: 0.62 to 1.1). There was no significant heterogeneity between studies when considering these efficacy outcomes (I2 <10%).
Of the eight trials, seven reported results on major bleeding and any bleeding events. The observed reduction in major bleeding was maintained with shorter duration DAPT when compared with long-term DAPT (RR: 0.64, 95% CI: 0.49 to 0.84). There was however high heterogeneity observed across these studies (I2=57.6%).
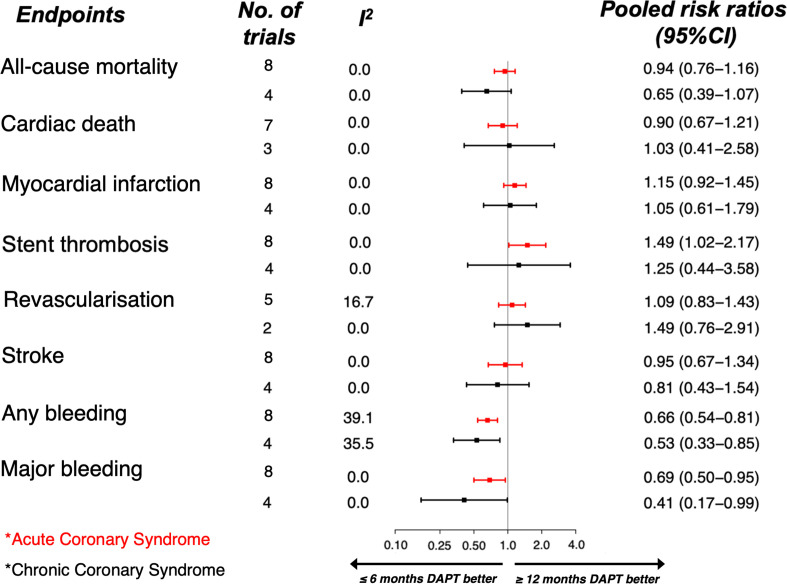
Duration of DAPT in acute or chronic coronary syndromes
Subgroup meta-analyses revealed a trend towards reduced risk of all-cause mortality with shorter duration DAPT in patients in whom the index presentation was ACS (RR: 0.94, 95% CI: 0.76 to 1.16) and towards further reduced risk in those with chronic coronary syndrome (RR: 0.65, 95% CI: 0.39 to 1.07). Risk estimates did not differ across the majority of efficacy outcomes with cardiac death, myocardial infarction, repeat revascularisation and stroke demonstrating equivalent risk ratios regardless of presentation (figure 3). There was an apparent increased risk of stent thrombosis in patients on shorter durations of DAPT presenting with ACS (RR: 1.49, 95% CI: 1.02 to 2.17 for ACS and RR: 1.25, 95% CI: 0.44 to 3.58 for chronic coronary syndromes). There was no significant heterogeneity between studies when considering these outcomes (I2 <25%). Short duration DAPT was associated with a reduction in bleeding across subgroups, both for major bleeding (ACS RR: 0.69, 95% CI: 0.5 to 0.95, and chronic coronary syndrome RR: 0.41, 95% CI: 0.17 to 0.99) and any bleeding (ACS RR: 0.66, 95% CI: 0.54 to 0.81, and chronic coronary syndromes RR: 0.53, 95% CI: 0.33 to 0.65).
Figure 3.
Forest plot showing pooled risk estimates according to outcomes of interest in subgroups of patients with acute coronary syndrome (red) (n=13 466) and chronic coronary syndrome (black) (n=4281) comparing short duration of dual-antiplatelet therapy (≤6 months) and long duration (12 months); (DAPT, dual antiplatelet therapy).
Discussion
We here report a systematic review and meta-analysis of 19 randomised controlled trials evaluating the efficacy and safety of short-term DAPT compared with long-term DAPT. Our principal finding suggests a trend towards a reduced risk of all-cause mortality in patients who had short-term DAPT. This was true even when duration of therapy was reduced from ≤6 months to ≤3 months of DAPT, with no apparent increase in atherothrombotic events. Moreover, these observations were consistent when comparing patients who presented with acute or chronic coronary syndromes, with the exception of stent thrombosis where an increased risk was noted in those on shorter durations of DAPT for patients presenting acutely. These findings highlight the uncertainty regarding current guideline recommendations for a default strategy of 12 months of DAPT in patients with ACS.3 4
In a meta-analysis of 10 trials of patients with chronic coronary syndrome undergoing percutaneous coronary intervention, Palmerini and colleagues suggested that, while 6 months of DAPT resulted in increased rates of myocardial infarction and stent thrombosis, this did not translate into a reduction in cardiovascular death when compared with 12 months of therapy.22 However, they observed lower all-cause mortality with the use of short-term DAPT driven by a lower risk of major bleeding and significant reduction in non-cardiovascular death. As a result of this meta-analysis, the European Society of Cardiology and the American Heart Association/American College of Cardiology guidance changed for patients with chronic coronary syndromes who underwent percutaneous coronary intervention to make 6 months of DAPT the standard of care. These observations did not influence recommendations on the duration of DAPT in ACS where these guidelines continue to recommend a 12-month duration of therapy as standard of care.3 4
Yin and colleagues recently published a network meta-analysis comparing short-term (<6 months) with standard term (12 months) and longer-term (≥18 months) DAPT.6 Their analysis included 17 studies and also reported a reduction all-cause mortality and fewer bleeding events in patients on short-term DAPT, despite including more studies that had enrolled patients with ACS. Their sensitivity analysis comparing patients by acute or chronic presentation, demonstrated short-term DAPT had equivalent safety and efficacy outcomes when compared with longer durations. Khan and colleagues conducted a network meta-analysis of 24 trials on patients requiring DAPT following percutaneous coronary intervention, which additionally compared outcomes in those on mid-term DAPT (6–12 months). They reported equivalent outcomes for all-cause mortality across groups, though a trend towards reduced risk in patients on short-term DAPT was noted. While risk ratios for myocardial infarction were reduced in long-term DAPT, this was again counter-balanced by an increase in bleeding events.23 Even in high-risk patients with diabetes mellitus, meta-analyses suggest equivalent rates of all-cause mortality, cardiac death and adverse cardiac events regardless of duration of DAPT.24 Our report is consistent with these recent meta-analyses. However, we have here included newer trials such as Kim et al and Mehran et al, which assessed shorter term DAPT (<3 months vs ≥12 months).8 25 In our analysis, shorter duration of DAPT (≤3 months) was associated with a trend towards lower all-cause mortality, remained similarly effective in key efficacy outcomes, but had substantially lower rates of bleeding when compared with long-term DAPT (≥12 months). While majority of trials evaluating duration of DAPT used clopidogrel, more recent trials have evaluated potent P2Y12 receptor antagonists.8 25 Similar to findings from Navarese et al, we observed a reduction in all-cause mortality with shorter durations of therapy in studies including potent P2Y12 receptor antagonists compared with those that used clopidogrel only.26
Why should we consider 3 months of DAPT to be any different to 6 months of DAPT? Multiple trials in the patients with ACS have demonstrated high initial ischaemic event rates which revert to lower linear rates from 3 months onwards.1 2 27 28 Consequently, the largest absolute reductions in cardiovascular events are driven by the use of DAPT in the first 3 months after an ACS. Indeed, in the CURE trial, DAPT caused the majority of the reductions in recurrent myocardial infarction within the first 3 months with only modest benefits thereafter.29 In contrast, there was a persistent and continuous bleeding hazard that was not time dependent, suggesting that the prevention of myocardial infarction may become counterbalanced by the hazards of bleeding beyond 3 months.29
Withdrawal of P2Y12 receptor antagonists from DAPT is associated with a rebound prothrombotic effect and is associated with an increase in rates of stent thrombosis.30 We observed this phenomenon, especially in those with ACS randomised to a shorter duration of DAPT. Stent thrombosis does however occur irrespective of the timing of withdrawal as demonstrated in the DAPT trial where rebound stent thrombosis was seen after DAPT cessation at both 12 and 30 months.13 This perhaps emphasises the importance of procedural variables, such as optimal stent deployment especially in patients with ACS when deciding on the duration of DAPT. As such, a small but persistent risk of stent-thrombosis will persist when transitioning from dual therapy to monotherapy whenever this occurs.
It should be noted that stent thrombosis occurs infrequently and did not correlate with increased mortality. Advances in stent technologies have reduced rates of stent thrombosis.31 Bleeding events on the other hand occur much more often, and the subsequent risk of all-cause mortality has been demonstrated in a wide variety of trials in patients with coronary artery disease regardless of trial intervention. For example, in trials of anticoagulant therapy use in ACS, those therapies with a lower bleeding hazard have a lower all-cause mortality despite having similar efficacy in preventing atherothrombotic events.32 Moreover, trials of arterial access sites for percutaneous coronary intervention in ACS also demonstrate a mortality benefit that is attributable to lower rates of bleeding with radial artery access.33 34 This supports the notion that bleeding events are an important determinant of all-cause mortality in patients receiving treatment for coronary artery disease and consequently, therapeutic approaches that minimise the risk of bleeding have the potential to reduce mortality in these patients.
Our systemic review and meta-analysis highlight the paucity of randomised controlled trial evidence to guide DAPT in patients with ACS who are managed without percutaneous coronary intervention such as those receiving medical therapy only or those undergoing coronary artery bypass grafting. Patients with ACS who are managed with medical therapy only are often at the extremes of risk with either an event attributable to minor coronary artery disease or multiple comorbidity and a contraindication to invasive coronary angiography. Registry data suggest between 20% and 40% of all admissions for non-ST segment elevation myocardial infarctions are managed medically and recurrent events can be as much as three times more likely to occur in this population.35 36 The balance of bleeding and ischaemic risk is clearly challenging in these situations. For patients who are treated with coronary artery bypass graft surgery, DAPT is only offered to those with ACS, and following a brief interruption for surgery, are usually maintained on therapy for 12 months. Bleeding and ischaemic risk in these patients are likely to be affected by the surgical procedure itself and therefore they represent a group that is distinct from other patients with acute or chronic coronary syndromes. While meta-analyses show DAPT prevents graft occlusion, none have robustly assessed the optimum duration of therapy.37
It is important to acknowledge that randomised controlled trials rightly have strict entry and exclusion criteria for their study participants. Patients with bleeding risks have been systematically excluded from these randomised controlled trials which report lower rates of bleeding and non-cardiovascular mortality than the general population.38 However, in real-world practice, clinicians make individual decisions with their patients on whether to initiate DAPT and this may include those who would otherwise not have been entered into clinical trials because of a history of bleeding. There is, therefore, a real concern that bleeding risk may be under appreciated and bleeding events may be disproportionately greater with the wider use of DAPT in clinical practice. As such, we believe that there is a clear and pressing need to address what the optimum duration of DAPT is in a broad and unselected cohort of patients suffering ACS. Major randomised controlled trials, such as Duration of Dual Antiplatelet Therapy in Acute Coronary Syndrome (DUAL-ACS2), may help answer this question (NCT03252249).
We should acknowledge the limitations of our meta-analysis. First, the data were gathered, and conclusions drawn from study-level data, and the majority of trials included were designed to test for non-inferiority. Individual patient-level data may have added further insights particularly when considering clinical presentations. Time to randomisation varied across the trials, as did duration of follow-up, which may affect the robustness of overall results. Different antiplatelet combinations were used, some with more potent P2Y12 receptor antagonists than others, and some discontinuing aspirin rather than P2Y12 receptor antagonists at the end of the DAPT treatment period. The data gathered for our analysis of DAPT in ACS are derived mostly from subgroup analyses and may not be reflective of ‘real-world higher risk’ populations. As such, care should be taken when interpreting the results. Additionally, the majority of trials included were deemed to be at risk of bias due to inadequate blinding of participants and personnel. Finally, endpoint definitions varied across the studies leading to increased heterogeneity particularly when considering bleeding outcomes.
In conclusion, our systematic review and meta-analysis suggest that short-term (≤6 months) and shorter durations (≤3 months) of DAPT are associated with lower risk of bleeding, equivalent efficacy and a trend towards lower all-cause mortality. There remains major uncertainty about the optimal duration of DAPT that requires to be resolved in future trials, particularly for patients with ACS, and those managed without percutaneous coronary intervention.
Footnotes
AB and MNM contributed equally.
Contributors: AB and MNM: conceptualisation, screening, data extraction, data analysis, data interpretation, writing—original draft; DD: data analysis, writing—review and editing; ASVS and NLM: writing—review and editing; DEN: conceptualisation, writing—review and editing; KL: conceptualisation, data analysis, data interpretation, writing—review and editing.
Funding: This work was supported by a British Heart Foundation (BHF) Research Excellence Award to the University of Edinburgh (RE/18/5/34216) and DUAL-ACS trial funding (SP/17/12/32960). AB is supported by a clinical research training fellowship (MR/V007254/1). MNM is supported by the British Heart Foundation (FS/19/46/34445). DEN and NLM are supported by the BHF through a Chair Award (CH/09/002), and Senior Clinical Research Fellowship (FS/16/14/32023), respectively. DEN is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA). KL is supported by the British Heart Foundation (FS/18/25/33454).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Data tables and analysis code can be made available upon reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–17. 10.1056/NEJMoa060989 [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. 10.1056/NEJMoa010746 [DOI] [PubMed] [Google Scholar]
- 3.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of cardiology (ESC) and of the European association for cardio-thoracic surgery (EACTS). Eur Heart J 2018;39:213–60. 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 4.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 2016;134:e123–55. 10.1161/CIR.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 5.Navarese EP, Andreotti F, Schulze V, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ 2015;350:h1618. 10.1136/bmj.h1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin S-H-L, Xu P, Wang B, et al. Duration of dual antiplatelet therapy after percutaneous coronary intervention with drug-eluting stent: systematic review and network meta-analysis. BMJ 2019;365:l2222. 10.1136/bmj.l2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vranckx P, Valgimigli M, Jüni P, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 2018;392:940–9. 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 8.Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med 2019;381:2032–42. 10.1056/NEJMoa1908419 [DOI] [PubMed] [Google Scholar]
- 9.Hahn J-Y, Song YB, Oh J-H, et al. 6-Month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet 2018;391:1274–84. 10.1016/S0140-6736(18)30493-8 [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions version 6.1. Cochrane: Cochrane, 2020. www.training.cochrane.org/handbook [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collet J-P, Silvain J, Barthélémy O, et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014;384:1577–85. 10.1016/S0140-6736(14)60612-7 [DOI] [PubMed] [Google Scholar]
- 13.Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–66. 10.1056/NEJMoa1409312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CW, Ahn J-M, Park D-W, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014;129:304–12. 10.1161/CIRCULATIONAHA.113.003303 [DOI] [PubMed] [Google Scholar]
- 15.Helft G, Steg PG, Le Feuvre C, et al. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J 2016;37:365–74. 10.1093/eurheartj/ehv481 [DOI] [PubMed] [Google Scholar]
- 16.Savović J, Weeks L, Sterne JAC, et al. Evaluation of the Cochrane collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev 2014;3:37. 10.1186/2046-4053-3-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–51. 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 18.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in myocardial infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. clinical findings through hospital discharge. Circulation 1987;76:142-54. 10.1161/01.cir.76.1.142 [DOI] [PubMed] [Google Scholar]
- 19.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research Consortium. Circulation 2011;123:2736–47. 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 2006;25:3443–57. 10.1002/sim.2380 [DOI] [PubMed] [Google Scholar]
- 22.Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet 2015;385:2371–82. 10.1016/S0140-6736(15)60263-X [DOI] [PubMed] [Google Scholar]
- 23.Khan SU, Singh M, Valavoor S, et al. Dual antiplatelet therapy after percutaneous coronary intervention and drug-eluting stents: a systematic review and network meta-analysis. Circulation 2020;142:1425–36. 10.1161/CIRCULATIONAHA.120.046308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma A, Garg A, Elmariah S, et al. Duration of dual antiplatelet therapy following drug-eluting stent implantation in diabetic and non-diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Prog Cardiovasc Dis 2018;60:500–7. 10.1016/j.pcad.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 25.Kim B-K, Hong S-J, Cho Y-H, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA 2020;323:2407–16. 10.1001/jama.2020.7580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navarese EP, Khan SU, Kołodziejczak M, et al. Comparative efficacy and safety of oral P2Y12 inhibitors in acute coronary syndrome: network meta-analysis of 52 816 Patients from 12 randomized trials. Circulation 2020;142:150–60. 10.1161/CIRCULATIONAHA.120.046786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox KAA, Anderson FA, Goodman SG, et al. Time course of events in acute coronary syndromes: implications for clinical practice from the grace registry. Nat Clin Pract Cardiovasc Med 2008;5:580–9. 10.1038/ncpcardio1302 [DOI] [PubMed] [Google Scholar]
- 28.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57. 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 29.Yusuf S, Mehta SR, Zhao F, et al. Early and late effects of clopidogrel in patients with acute coronary syndromes. Circulation 2003;107:966–72. 10.1161/01.cir.0000051362.96946.15 [DOI] [PubMed] [Google Scholar]
- 30.Ford I. Coming safely to a stop: a review of platelet activity after cessation of antiplatelet drugs. Ther Adv Drug Saf 2015;6:141–50. 10.1177/2042098615588085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bangalore S, Toklu B, Patel N, et al. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease. Circulation 2018;138:2216–26. 10.1161/CIRCULATIONAHA.118.034456 [DOI] [PubMed] [Google Scholar]
- 32., Yusuf S, Mehta SR, et al. , Fifth Organization to Assess Strategies in Acute Ischemic Syndromes Investigators . Comparison of fondaparinux and enoxaparin in acute coronary syndromes. N Engl J Med 2006;354:1464–76. 10.1056/NEJMoa055443 [DOI] [PubMed] [Google Scholar]
- 33.Valgimigli M, Frigoli E, Leonardi S, et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet 2018;392:835–48. 10.1016/S0140-6736(18)31714-8 [DOI] [PubMed] [Google Scholar]
- 34.Ferrante G, Rao SV, Jüni P, et al. Radial versus femoral access for coronary interventions across the entire spectrum of patients with coronary artery disease: a meta-analysis of randomized trials. JACC Cardiovasc Interv 2016;9:1419–34. 10.1016/j.jcin.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 35.Gurbel PA, Tantry US. Antithrombotic therapy in medically managed patients with non-ST-segment elevation acute coronary syndromes. Heart 2016;102:882–92. 10.1136/heartjnl-2014-306695 [DOI] [PubMed] [Google Scholar]
- 36.Candela E, Marín F, Rivera-Caravaca JM, et al. Conservatively managed patients with non-ST-segment elevation acute coronary syndrome are undertreated with indicated medicines. PLoS One 2018;13:e0208069. 10.1371/journal.pone.0208069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solo K, Lavi S, Kabali C, et al. Antithrombotic treatment after coronary artery bypass graft surgery: systematic review and network meta-analysis. BMJ 2019;367:l5476. 10.1136/bmj.l5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camm AJ, Amarenco P, Haas S, et al. Real-World vs. randomized trial outcomes in similar populations of rivaroxaban-treated patients with non-valvular atrial fibrillation in rocket AF and XANTUS. Europace 2019;21:421–7. 10.1093/europace/euy160 [DOI] [PubMed] [Google Scholar]
- 39.Steinhubl SR, Berger PB, Mann JT. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;288:2411–20. 10.1001/jama.288.19.2411 [DOI] [PubMed] [Google Scholar]
- 40.Kedhi E, Fabris E, van der Ent M, et al. Six months versus 12 months dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): randomised, multicentre, non-inferiority trial. BMJ 2018;363:k3793. 10.1136/bmj.k3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gwon H-C, Hahn J-Y, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the efficacy of Xience/Promus versus Cypher to reduce late loss after stenting (excellent) randomized, multicenter study. Circulation 2012;125:505–13. 10.1161/CIRCULATIONAHA.111.059022 [DOI] [PubMed] [Google Scholar]
- 42.Han Y, Xu B, Xu K, et al. Six versus 12 months of dual antiplatelet therapy after implantation of biodegradable polymer sirolimus-eluting stent: randomized substudy of the I-LOVE-IT 2 trial. Circ Cardiovasc Interv 2016;9:e003145. 10.1161/CIRCINTERVENTIONS.115.003145 [DOI] [PubMed] [Google Scholar]
- 43.Schulz-Schüpke S, Byrne RA, Ten Berg JM, et al. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015;36:1252–63. 10.1093/eurheartj/ehu523 [DOI] [PubMed] [Google Scholar]
- 44.Didier R, Morice MC, Barragan P, et al. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: final results of the ITALIC trial (Is there a life for DES After discontinuation of clopidogrel). JACC Cardiovasc Interv 2017;10:1202–10. 10.1016/j.jcin.2017.03.049 [DOI] [PubMed] [Google Scholar]
- 45.Hong S-J, Shin D-H, Kim J-S, et al. 6-month versus 12-, month dual-antiplatelet therapy following long everolimus-eluting stent implantation: The IVUS-XPL Randomized Clinical Trial. JACC Cardiovasc Interv 2016;9:1438–46. 10.1016/j.jcin.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 46.Nakamura M, Iijima R, Ako J, et al. Dual antiplatelet therapy for 6 versus 18 months after biodegradable polymer drug-eluting stent implantation. JACC Cardiovasc Interv 2017;10:1189–98. 10.1016/j.jcin.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 47.Lee B-K, Kim J-S, Lee O-H, et al. Safety of six-month dual antiplatelet therapy after second-generation drug-eluting stent implantation: OPTIMA-C randomised clinical trial and OCT substudy. EuroIntervention 2018;13:1923–30. 10.4244/EIJ-D-17-00792 [DOI] [PubMed] [Google Scholar]
- 48.Feres F, Costa RA, Abizaid A, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the optimize randomized trial. JAMA 2013;310:2510–22. 10.1001/jama.2013.282183 [DOI] [PubMed] [Google Scholar]
- 49.Valgimigli M, Campo G, Monti M, et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012;125:2015–26. 10.1161/CIRCULATIONAHA.111.071589 [DOI] [PubMed] [Google Scholar]
- 50.De Luca G, Damen SA, Camaro C, et al. Final results of the randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with a new-generation stent (reduce trial). EuroIntervention 2019;15:e990–8. 10.4244/EIJ-D-19-00539 [DOI] [PubMed] [Google Scholar]
- 51.Kim B-K, Hong M-K, Shin D-H, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET trial (real safety and efficacy of 3-month dual antiplatelet therapy following endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012;60:1340–8. 10.1016/j.jacc.2012.06.043 [DOI] [PubMed] [Google Scholar]
- 52.Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the security randomized clinical trial. J Am Coll Cardiol 2014;64:2086–97. 10.1016/j.jacc.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 53.Watanabe H, Domei T, Morimoto T, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA 2019;321:2414–27. 10.1001/jama.2019.8145 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2021-001707supp001.pdf (11.5MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Data tables and analysis code can be made available upon reasonable request to the corresponding author.