Abstract
Disruptive or excessive repetitive motor patterns (stereotypies) are cardinal symptoms in numerous neuropsychiatric disorders. Stereotypies are also evoked by psychomotor stimulants such as amphetamine. The acquisition of motor sequences is paralleled by changes in activity patterns in the striatum, and stereotypies have been linked to abnormal plasticity in these reinforcement-related circuits. Here, we designed experiments in mice to identify transcriptomic changes that underlie striatal plasticity occurring alongside the development of drug-induced stereotypic behavior. We identified three schedules of amphetamine treatment inducing different degrees of stereotypy and used bulk RNAseq to compare striatal gene expression changes among groups of mice treated with the different drug-dose schedules and vehicle-treated, cage-mate controls. Mice were identified as naïve, sensitized, or tolerant to drug-induced stereotypy. All drug-treated groups exhibited expression changes in genes that encode members of the extracellular signal-regulated kinase (ERK) cascades known to regulate psychomotor stimulant responses. In the sensitized group with the most prolonged stereotypy, we found dysregulation of 20 genes that were not changed in other groups. Gene set enrichment analysis indicated highly significant overlap with genes regulated by neuregulin 1 (Nrg1). Nrg1 is known to be a schizophrenia and autism susceptibility gene that encodes a ligand for Erb-B receptors, which are involved in neuronal migration, myelination and cell survival, including that of dopamine-containing neurons. Stimulant abuse is a risk factor for schizophrenia onset, and these two disorders share behavioral stereotypy phenotypes. Our results raise the possibility that drug-induced sensitization of the Nrg1 signaling pathway might underlie these links.
Keywords: Amphetamine, Tolerance, Sensitization, Stereotypy, Restricted Repetitive Behavior
INTRODUCTION
All known drugs of abuse are thought to potentiate dopaminergic signaling (Sulzer, 2011) and to activate striatal projection neurons that express the D1 dopamine receptor (Bertran-Gonzalez et al., 2008). This activation is also dependent on expression of the D2 dopamine receptor (Solis et al., 2021), consistent with the involvement of both D1 and D2 dopamine receptor subtypes in normal behavioral responses to psychomotor stimulants (Capper-Loup et al., 2002). This dopamine signaling, normally evoked in relation to motivationally salient contexts and reinforcement-based learning, is associated with highly repetitive behaviors that are induced by drugs of abuse in humans and in other species. Locomotor behaviors that typically occur after low-dose exposure, and bouts of confined stereotypic behaviors that typically occur after higher dose exposure, have been recognized as potential signatures of ventral striatal activation (hyper-locomotion) and dorsal striatal activation (stereotypies), respectively (Schiorring, 1971; Dickson et al., 1994). The dorsal striatum, the input nucleus for major basal ganglia output pathways, is a key modulator of reward-based learning, action initiation and the automatization of motor routines (Besson et al., 1990; Gerfen et al., 1995; Yan et al., 1999; Barnes et al., 2005; Yano & Steiner, 2007; Graybiel, 2008; Yin et al., 2009; Desrochers et al., 2015). Hence the actions of amphetamine and related psychomotor stimulant drugs are thought to alter key balances in striatal signaling, leading to excessive, abnormal versions of the normal functions of these circuits in reinforcement learning, habit formation and motivational control.
Abnormally repetitive, stereotypic motor and cognitive behaviors are symptoms common to numerous neuropsychiatric disorders including schizophrenia, bipolar disorder, drug addiction, L-DOPA-induced dyskinesia, obsessive-compulsive disorder, Huntington’s disease and autism (Angrist et al., 1974; Canales & Graybiel, 2000; Yui et al., 2000; Geyer & Ellenbroek, 2003; Henry et al., 2003; Hollander et al., 2005; Volkow et al., 2006; Lewis et al., 2007; Langen et al., 2009). Amphetamine-induced stereotypies, the focus of the work reported here, have been extensively examined as potential models for repetitive behavior exhibited in some of these disorders, and as one model of the addictive potential of dopamine-receptor agonist drugs.
Amphetamine can increase extracellular dopamine by inhibiting dopamine transporter reuptake, by potentiating dopaminergic neuron activity and, at high concentrations, by directly driving release via reverse-transport (Fleckenstein et al., 2007). Drug-induced hyperactivity and stereotypies are blocked by antagonism of D1- and D2-type dopamine receptors (Capper-Loup et al., 2002), and D2-type dopamine receptor antagonists are prescribed as anti-psychotic and anti-manic medications (American Psychiatric Association, 2013; Tyler et al., 2017). These convergent findings support the view that habit-forming drugs overtake molecular mechanisms associated with reward-based learning and habit formation (Berke & Hyman, 2000; Hyman et al., 2006; Jedynak et al., 2007; Graybiel, 2008; Ostlund & Balleine, 2008; Redish et al., 2008). This view is in accord with evidence that with repeated drug exposure, the behavioral responses become stronger, a phenomenon known as sensitization, and others become weaker, referred to as tolerance to the drug.
Gene expression responses to drug exposure are also known to change with repeated treatment (Moratalla et al., 1996). However, most of these changes in drug-induced gene expression identified in the brains of drug-sensitized animals are not maintained as baseline differences for more than a few days after drug withdrawal, without further drug exposure. Thus, changes in transcription initiation and RNA stabilization may be key mechanisms underlying the plasticity of drug-induced gene induction. Accordingly, exposure to drugs of abuse has been shown to impact chromatin methylation state (Berke & Hyman, 2000; Yuferov et al., 2005; Nestler, 2014). Changes in early-gene transcription factor expression and other changes in gene expression following repeated amphetamine and related drug treatments have been documented to correlate highly with the development of confined stereotypical behaviors, and these have been shown to include imbalances in immediate early gene induction in striosomes, relative to the extrastriosomal matrix compartment of the striatum, (Besson et al., 1990; Steiner & Gerfen, 1995; Berke et al., 1998; Yan et al., 1999; Berke & Hyman, 2000; Canales & Graybiel, 2000; Vanderschuren et al., 2002; Saka et al., 2004; Schwendt & McGinty, 2010; Horner et al., 2012; Jedynak et al., 2012; Crittenden et al., 2016; Crittenden et al., 2017). A whole-transcriptome approach to identifying changes specifically associated with stereotypy has, however, been lacking.
To identify transcriptional changes that are specifically associated with stereotypy, we first defined amphetamine-treatment schedules that induced significantly different levels of stereotypy: minimal stereotypy (acute treatment), prolonged and confined stereotypy with orofacial features (7 daily treatments), and brief stereotypy that was curtailed by locomotion (21 daily treatments). To identify RNA transcripts that are poised immediately to respond to psychomotor stimulant treatment, and thus possibly to influence the intensity of the behavioral response to such treatments, we then harvested striatal tissue 20 min after D-amphetamine injection and compared transcriptomic data across the differently treated groups. Our results demonstrate plasticity in the overall pattern of the striatal transcriptomic response in the mice exposed to acute, intermediate or prolonged repeated treatments. We also demonstrate that among these changes are sets of genes whose expression is correlated with peak levels of stereotypy, including genes overlapping with Nrg1-regulated genes. Genes with expression changes that occurred specifically on the day with the most severe stereotypy, and that also were not observed upon acute or prolonged treatments associated with tolerance to the induction of stereotypies, are candidates for genetic susceptibility to pathological motor habits rendering animals susceptible to relapse. These candidate genes should be considered for the identification of gene networks underlying neuropsychiatric disorders with symptoms of extreme repetitive behaviors.
MATERIALS AND METHODS
Mice
All experimental procedures were performed in strict accordance with the Committee on Animal Care at the Massachusetts Institute of Technology (MIT), which is accredited by AAALAC International and followed the United States Public Health Service Guide for Care and Use of Laboratory Animals. Male 129Sv/Jae S4 mice between 8 and 10 months of age were used (n = 30 total). Mice were tested during the light phase and were maintained group-housed under a standard light/dark cycle (lights on at 7 am and off at 7 pm) with free access to food and water.
The locomotor and videotape/stereotypy data presented are from 12 sibling mice. For tissue extraction and RNAseq, 18 sibling mice were included, with 3 mice in each of 6 treatment groups: 1 day amphetamine or vehicle, 7 days amphetamine or vehicle, 21 days amphetamine or vehicle. A flowchart depicting the workflow is shown in Figure 1. Mice that showed signs of severe illness were excluded from the study; no mice were excluded for outlying data.
FIGURE 1.
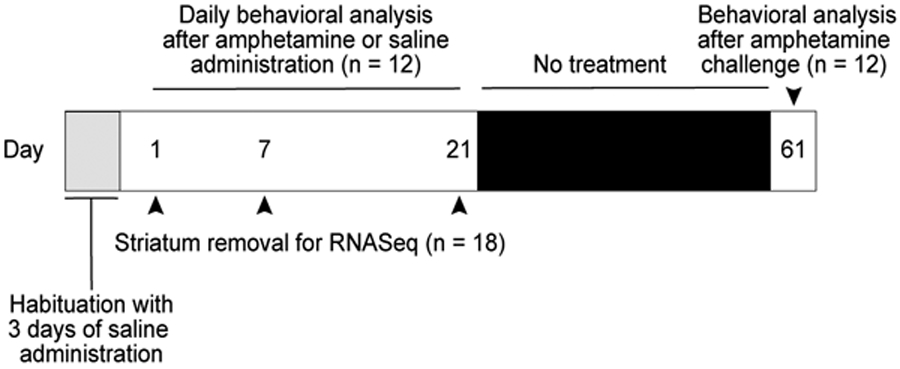
Timeline of drug treatments, behavioral assessments and tissue collection.
Drug preparation
D-amphetamine hemisulfate salt (Sigma) was dissolved in 0.9% saline just prior to use and injected, i.p., at 10 ml/kg for a total daily dose of 7 mg/kg. Saline (0.9%) was administered to controls.
Drug treatment and behavioral analyses
The timeline of drug treatments is shown in Figure 1. Drug injections and behavioral analyses were similar to previously described experiments (Crittenden et al., 2014). Behavior was assessed between 10 am and 5 pm in two sequentially treated groups of mice that were balanced for drug vs. vehicle treatment. Locomotor activity was measured in square chambers (25.4 x 25.4 cm) with transparent plastic walls and a solid plastic floor (TruScan monitor, Coulbourn Instruments). Photobeams were spaced at 1.52 cm intervals for a reported spatial resolution of 0.76 cm. Coordinate position data of the mice were recorded every 0.1 sec by the TruScan software. Distance traveled was automatically calculated by the TruScan software from floor-plate beam breaks. On test days, mice were placed into the monitors, were given 20 min to habituate, and then were injected with D-amphetamine or vehicle and left in the chamber for an additional 140 min. Owing to equipment failure, on D-amphetamine treatment day 16, data were missing for 6 of the 12 mice. All of the mice for all of the experiments were habituated to saline injections in the activity monitor chambers for three days prior to the beginning of behavioral measurements or of tissue collection.
For observational measurements of stereotypy and locomotion, mice were videotaped for 2 min beginning at 80 min after injection, and behaviors were subsequently rated by a rater blinded to genotype and treatment day with JWatcher™ v.1.0 (University of California, Los Angeles, and Macquarie University, Sidney, Australia) (http://www.jwatcher.ucla.edu/; Crittenden et al., 2014). The duration and frequency of each of the following behaviors were scored with a keypad in real time as the rater watched each videotape. Separate keys were assigned for: sniffing air, sniffing air whilst confined, sniffing floor, sniffing floor whilst confined, sniffing wall, sniffing wall while confined, any confined stereotypy, slow locomotion, medium locomotion, and fast locomotion. Measurements were compared across days by 2-tailed, paired t-tests. To analyze place preference, the coordinate position data, computed every 0.1 sec by TruScan software, were converted to radians with the center of the chamber as the origin. A histogram of angular location relative to the center of the chamber was made for the time before and after the D-amphetamine injection. These activity histograms were compared to the averaged histogram of either pre- or post-injection activity across the first 21 days of amphetamine treatment using Pearson’s correlation coefficient.
A random effects state-space model (Smith et al., 2005) was used to evaluate the distance traveled across days at the 80-85 min post-injection time bin. This pair-wise comparison method is more appropriate than the Student’s t-test because it takes into account that the distance traveled measurements across days are not independent variables. At a given time post injection, tpi , data were nm(tday), where m was the mouse number (m = 1,…,12) and tday was the day the measurement was taken (tday = 1,…,15,17,…,21 and 61). Distance traveled values on day 16 and days 22-60 were predicted, since there were no data. We assumed there was a common hidden state x(tday) which followed a random walk such that x(tday) ~ N(x(tday−1),τ1), where τ1 was the estimated precision. Each animal’s observations were related to the hidden state using nm(tday) ~ N(exp(βmd + x(tday)),τ), where βmd ~ N(β0d, τβd) (m = 1,…,12) and β0d = 0 (this is equivalent to using a lognormal distribution). The population state is N(exp(β0d + x(tday)), τ). Priors on precision were assumed to be inverse gamma with parameters 1 and 0.1. The WinBUGS code (Lunn et al., 2009) used is shown below.
Model{
for (t in 1:T) {
x1[t] ~ dnorm( mu1[t], tau1 ) ;
for (j1 in 1:J1) {
n1[j1, t] ~ dnorm(p1[j1, t], tau2) ;
log(p1[j1, t]) <- beta1[j1]+x1[t];
}
}
mu1[1] ~ dunif(0,100);
for (j1 in 1:J1) {
beta1[j1] ~ dnorm(beta01, taub1);
}
for (t in 2:T) {
mu1[t] <- x1[t-1]
}
for (t in 1:T) {
log(pPop1[t]) <- beta01+x1[t]
}
tau1 ~ dgamma(1,0.1)
tau2 ~ dgamma(1,0.1)
taub1 ~ dgamma(1,0.1)
beta01 <- 0 #~ dunif(−1000,1500)
}
Tissue isolation and RNA preparation
Groups of age-matched, sex-matched, and group-housed sibling mice were treated with saline or D-amphetamine in the activity monitors, exactly as described for the behavioral analyses except that only morning treatments were administered. The two groups of mice, one for behavioral and one for transcriptomic analyses, were necessarily separate groups because the behavioral analyses continued for 85 min after drug injection, whereas brain tissue for transcriptomics was harvested 20 min after the last saline or D-amphetamine injection. The mice were removed from the activity monitor and were given a lethal dose of Euthasol (pentobarbital sodium and phenytoin sodium; Virbac AH, Inc.). Within 5 min, the striatum of each hemisphere, dorsal to the anterior commissure, was dissected on a cold plate and frozen on dry ice. Frozen tissue samples were homogenized in Tri Reagent (Sigma), and RNA was precipitated using chloroform and isopropanol. RNA was purified with the RNEasy kit (Qiagen, Inc.) and DNAse-treated (Qiagen, Inc.), according to the manufacturer’s instructions. RNA integrity values based on Bioanalyzer data (Agilent Technologies Inc.) were between 7.8 and 9.0 for all samples. Total RNA from each mouse was submitted to MIT’s BioMicro Center for poly(A) RNA selection, fragmentation and cDNA generation with the TruSeq RNA Sample Preparation Kit (Illumina, Inc.), performed in parallel for all samples. Size selection and DNA preparation were completed on the SPRIworks system (Beckman Coulter, Inc.). The samples were then enriched with 16 cycles of PCR prior to loading on the flow cell for sequencing by the HiSeq2000 (Illumina, Inc.). Samples were multiplexed to run 9 samples on a single lane, and samples were then distinguished based upon their unique bar-code sequence. Each sequence read was single-ended and approximately 40 base pairs in length.
RNAseq analysis
RNAseq analysis was done according to the procedure described in Vashishtha et al. (Vashishtha et al., 2013). Reads were mapped to the mm9 version of UCSC known genes (http://genome.ucsc.edu/). To measure transcript abundance, the number of sequence reads in constitutive exons in the coding sequence of a gene were summed and then were normalized to account for gene length and depth of sequencing, according to the total reads per kilobase of transcript (based on mm9 model exons) per million mapped reads (RPKM). Thus, changes in constitutive exons, but not splice-form-specific changes, were identified. Raw counts were evaluated for differential expression by using R package DESeq with a 10% false discovery rate cutoff and log2 difference of 0.5 between amphetamine- and saline-injected mice. Outliers were further excluded by restricting the residual variance quotients to less than 10. Heatmaps representing gene expression were generated in Morpheus (Gould) from RPKM values.
For each of the 18 dorsal striatal samples submitted for RNA sequencing, 12–21 million base pairs were sequenced. For each sample, approximately 80% of the sequence reads were exonic and mapped to a unique site in the genome, approximately 1% mapped to intronic regions, and fewer than 0.1% mapped to intergenic regions. A total of 18,670 genes were queried and 11,200 had RPKM > 1 in at least one sample (GEO accession number GSE157913). Comparisons for genes with RPKM < 1 in all samples were considered unreliable (Conesa et al., 2016) and are not reported.
RESULTS
Sensitization to early-phase and late-phase locomotion induced by D-amphetamine
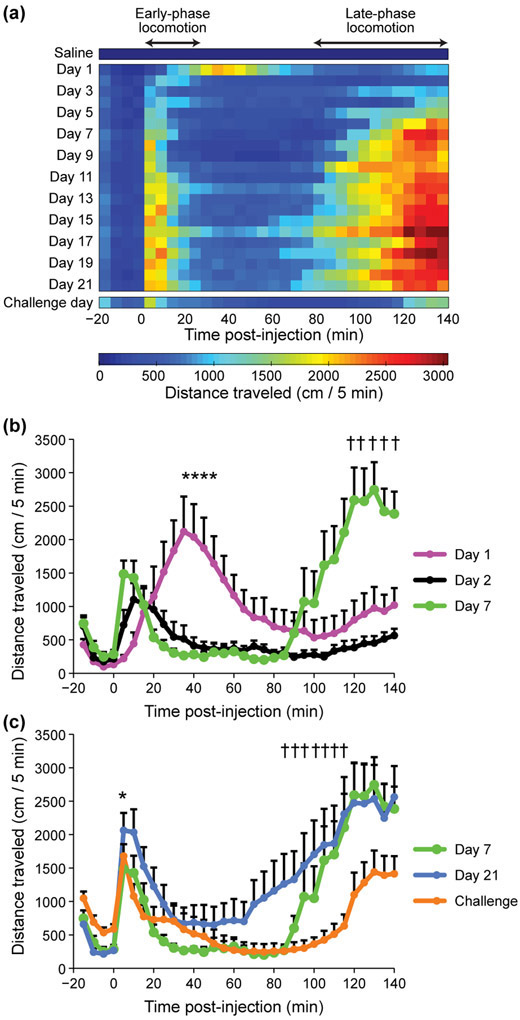
To measure how responses to amphetamine change with repeated exposures, we administered daily injections for 21 days and focused on day 1 (first time exposure), day 7 (sensitizing exposure) and day 21 (tolerance-inducing exposure) (Figure 1). All mice developed sensitization to repeated D-amphetamine injections and showed a classic triphasic motor response consisting of initial fast ambulation (early-phase locomotion) followed by confined stereotypic behaviors (stereotypy phase) that subsequently gave way to locomotion (late-phase locomotion) as the drug effect wore off. The average duration and intensity of these behaviors changed across days with repeated treatments (Figure 2a - c). On day 1 of D-amphetamine treatment, the average locomotor response to amphetamine consisted of a slow rise that peaked at about 40 min (Figure 2a, b). However, by the second day of treatment, most of the mice already exhibited sensitization relative to day 1 in that they began locomoting sooner after drug injection (Figure 2a, b), averaging 18 meters within the first 10 min after drug injection (Figure 2a) and an individual peak rate of 57 meters/10 min, compared to a peak locomotion rate of 20 meters/10 min on day 1 (Supporting Information File S1). The mice also exhibited strong sensitization of the late-phase locomotor effects (Figure 2a - c). By day 7, the mice, as a group, traveled significantly more during this late-phase period than on days 1 and 2 (Figure 2b). Inter-animal differences in the rates of sensitization were notable even in these age- and sex-matched sibling mice in an isogenic genetic background (Figure 3). Despite their differences, however, late-phase locomotion was evident only after the first week of treatment in most of the mice (Figure 3).
FIGURE 2.
Sensitization to amphetamine-induced early-phase and late-phase locomotion occurs at different rates. (a) Measurements of distance traveled across the last saline-treatment day and following amphetamine-treatment days. Each row shows one day with averaged distance traveled measurements in 5 min bins. Injection occurred at time = 0. The average duration of confined locomotion, between the early-phase and late-phase locomotion periods, diminishes with prolonged treatments but recurs on the challenge day. (b) Average distance traveled before and after D-amphetamine injection on day 1 (magenta), day 2 (black), and day 7 (green). There was significantly less mid-phase locomotion on treatment days 2 and 7 than on day 1 (*P < 0.005 at every point marked in the comparisons between day 1 and day 7 and between day 1 and day 2). By day 7, the mice showed increased late-phase locomotion, relative to day 1 (†P < 0.005 at every point marked in the comparison between day 1 and day 7). (c) On the challenge day (orange), mice maintained early-phase sensitization, based on their high levels of locomotion within 5 min of drug injection (*P = 8 x 10−6 by 2-tailed, paired t-test compared to day 1 data shown in panel b and File S1). During the late-phase locomotion period, however, the mice showed a diminished locomotor response on challenge day than on day 21 (blue) (†P < 0.03). Averages and +SEM across mice are shown (n = 12 mice).
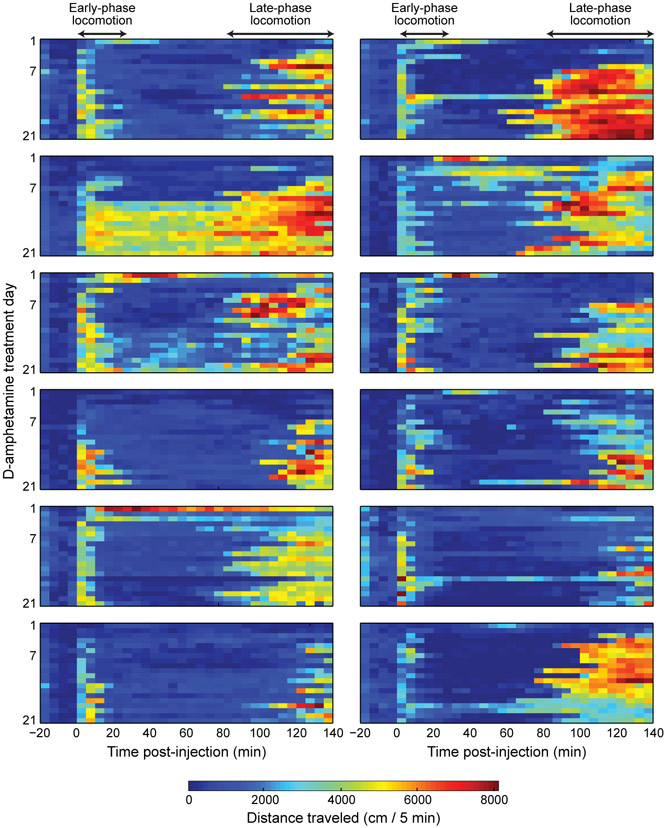
FIGURE 3.
Heat maps of distance traveled by each mouse, illustrating inter-animal variability in sensitization rate. D-amphetamine treatment days 1–21 are shown (day 16 data from some of the mice were lost).
After 21 days of daily D-amphetamine injections, the mice were maintained drug-free in their home cages for 40 days of withdrawal prior to receiving one last challenge dose (7 mg/kg). On the challenge day, the mice exhibited a sensitized early-phase locomotor response to amphetamine in that they locomoted significantly more within the first 5 min post-injection on challenge day than on acute treatment day 1 (Figure 2a - c). By contrast, on challenge day the late-phase locomotor peak was delayed and significantly lower than on day 21 (Figure 2c), indicating that sensitization to the late-phase locomotor response only became evident later in treatment and was not maintained during withdrawal.
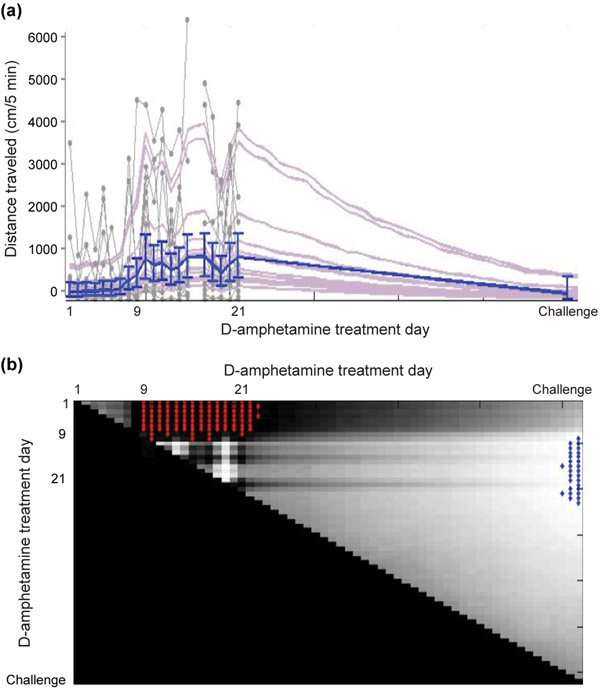
Heat maps of the distance traveled data illustrate the progressive earlier onset of the late-phase locomotion after the first week of treatment (Figure 2a). We tested on which treatment day the late-phase locomotion became significantly greater than previous days by comparing the distance traveled at the 80–85 min time bin, for which we also had videotape data. For this analysis, we designed a random effects state-space model (Smith et al., 2005) that accounts for co-variation of distance traveled across days (See Materials and Methods). The model indicated that, beginning on treatment day 9, there was a significant increase in distance traveled at the 80–85 min time bin after D-amphetamine injection, relative to all of the earlier treatment days (Figure 4a, b). The results were similar for times post-injection that flanked this time bin. This level of significantly increased late-phase locomotion was maintained throughout the repeated treatments to day 21. On challenge day, after the 40-day drug hiatus, the locomotion during this post-injection time bin was significantly lower than on treatment days 9–21 (Figure 4b). Altogether, our analyses demonstrate that early-phase locomotor sensitization to the drug emerged rapidly and remained stable during the daily treatments, and was retained across the withdrawal period. By contrast, the late-phase locomotor response emerged later and was not completely maintained across withdrawal.
FIGURE 4.
Model of distance traveled data shows a significant increase in late-phase locomotion beginning on D-amphetamine treatment day 9. (a) Distance traveled in the 80–85 min time bin across days. The raw data from each of the 12 mice are plotted as gray dots joined by lines, with estimates of individual fit to distance traveled (light purple) and group median estimate with 90% credible intervals (blue). (b) Day-by-day comparison of the state-space fit to the group estimates of distance traveled shown in A. Each point on the surface represents the probability that the group estimate on the day shown on the x-axis is higher than the day shown on the y-axis. Light colored surface indicates very low probability, and blue highlight indicates P < 0.005. Conversely, dark color shows that the day on the x-axis is higher than the day on the y-axis near P = 1, and red highlight means P > 0.995.
Sensitization and tolerance to the stereotypy-inducing effects of D-amphetamine
Our analysis of group-averaged distance traveled data, and previous behavioral rating experience (Crittenden et al., 2014), suggested that sensitized mice were engaged in confined stereotypy during the period between early- and late-phase locomotion. The distance traveled data allowed strict quantification of the timing of these phases, and suggested that the time spent in confined behaviors became shorter across treatment whereas the late-phase locomotion was initiated earlier (Figure 2a and Figure 4b). By the 21st day of treatment, the average distance traveled data indicated that mice were transitioning from confined stereotypy to resume locomotion at around 80 min after drug injection. We confirmed this pattern by observational scoring of videotapes of the mice.
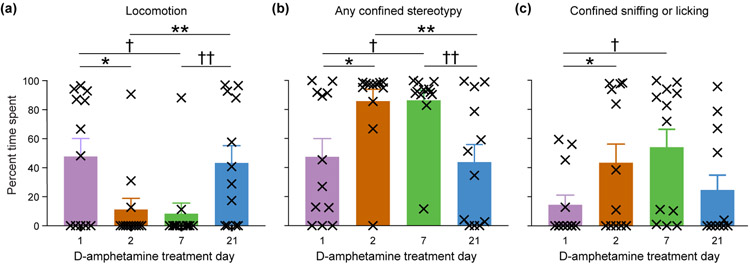
Mice typically appeared hyperactive on D-amphetamine treatment day 1 and engaged in a variety of behaviors. As treatment progressed, mice increasingly fell into behavioral patterns, including running around the perimeter of the cage and stopping in the corners, where they engaged in confined stereotypies, typically sniffing or licking the cage corner for prolonged periods in a reared position, before resuming locomotion. We scored videotapes of the mice on days 1, 2, 7 and 21, and computed the total time and frequency of each observed behavior at 80–82 min after drug-injection (Supporting Information File S2). Consistent with the automated distance traveled data, manual scoring showed that the mice were traveling less on day 2 and day 7 than they were on day 1 or day 21 (Figure 5a). The reduction in locomotion paralleled significant increases in any confined stereotypic behavior on both days 2 and 7, relative to days 1 and 21 of drug treatment (Figure 5b). We further subdivided the stereotypies by recording the time spent sniffing or licking the wall, the floor, or the air whilst in a confined location (Supporting Information File S2). Time spent sniffing the wall in a single spot was significantly longer on days 2 and 7, relative to day 1 (Figure 5c). In summary, our observational scoring of stereotypy and locomotion, along with the distance traveled data, are in accord: the stereotypic responses of the mice to successive doses of D-amphetamine during prolonged treatment underwent an early sensitization, as indicated by an increased response to each dose, followed by a tolerance to the doses, indicated by the waning stereotypic responses, and the distance traveled measures mirrored these changes over time.
FIGURE 5.
Mice show sensitization to confined stereotypy by day 2 of D-amphetamine injection and tolerance by day 21 of treatment. Plots show rating of behaviors during the 80–82 min post-injection time period for locomotion (a; *P = 0.02, †P = 0.008, **P = 0.01, ††P = 0.01, by 2-tailed, paired t-tests), any confined stereotypy (b; *P = 0.01, †P = 0.008, **P = 0.004, ††P = 0.005), and confined sniffing or licking at the wall (c; *P = 0.02, †P = 0.003). Averages and +SEM across mice are shown, with individual mice identified by the symbol X.
Cage-location preference for confined stereotypy
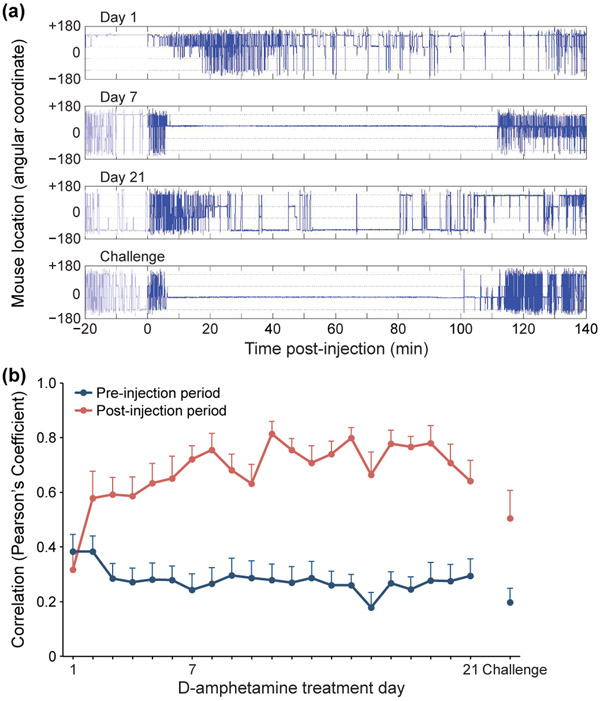
The amphetamine-treated mice tended to develop idiosyncratic stereotypies, with intermittent bursts of running around the cage to return to the same corner. To test whether a given mouse showed a preferred corner for engagement in stereotypy, or whether the location preferences changed across days, we computed the angular coordinate of the location of each mouse before and after D-amphetamine injection. An example of the results for one mouse (Figure 6a) illustrates a pattern of circular running on day 1 and day 21 of drug-treatment, with prolonged periods of confined behavior in two different corners on day 7 and on challenge day. To evaluate the group-averaged behavior of the mice, we defined the average coordinate locations across all days, before and after drug injection separately, as a mouse’s ‘preferred’ location. We then plotted how correlated a mouse’s position was on any given day, before and after injection, to his average preferred pre- or post-injection location across all 21 treatment periods (Figure 6b). This analysis showed that, across days, there was a higher correlation between the post-injection locations than between the pre-injection locations of the mice. In other words, mice under the influence of amphetamine exhibited an abnormal preference for one cage location. Thus, consistent with our observations during filming of behavior, each mouse had a preferred location for the emission of drug-induced stereotypy.
FIGURE 6.
Mice show a preferred location for stereotypy across continuous treatment. (a) Example of stationary vs. circling behavior of a single mouse on D-amphetamine treatment days 1, 7 and 21 and on challenge day. An angular coordinate deflection from −180° to +180° (vertical lines) represents a full revolution around the cage. (b) For each mouse, the coordinate position data, either before (pre) or after (post) drug injection was plotted against the average coordinate position during that time interval across all days and the average correlations were plotted. Beginning on the second day of drug treatment, there was a high correlation across days for the post-injection period, reflecting a relatively constant favored location. The correlation for the pre-injection period was low, indicating that the mice did not have a highly preferred location before drug injection. Averages and +SEM across mice are shown (n = 12 mice).
Patterns of striatal gene expression in mice that are naïve, sensitized or tolerant to D-amphetamine-induced stereotypy
The differences in behavioral response across D-amphetamine treatment days 1, 7 and 21 provided an opportunity to test for transcriptomic changes that were specifically associated with the treatment schedule that induced prolonged stereotypy. To identify gene expression changes that might be epigenetically primed to respond immediately after injection, and thus be in a position to be translated into proteins by the 80 min time-point at which we found sensitization and tolerance of stereotypy, we harvested tissue at 20 min after D-amphetamine or saline injection on days 1, 7 and 21 (n = 3 mice for each group, 18 mice total). Thus, transcriptomic changes reflected immediate response in transcription and RNA stabilization on all of the treatment days, as well as accumulated baseline changes on days 7 and 21.
For each gene, we compared RPKM-normalized sequence counts between the mice treated with D-amphetamine for 1, 7 or 21 days (n = 3 in each group) and the entire group of saline-treated mice (n = 9) (Supporting Information File S3). To identify changes specifically related to different drug treatment regimens, rather than to different numbers of restraint and injection experiences, we took a conservative approach by eliminating genes that changed in any pair-wise comparisons between the three groups of mice treated with saline alone (Table 1).
TABLE 1.
Genes with significant mRNA expression changes in pair-wise comparisons among habituated mice treated with daily saline injections for 1, 7 or 21 days. Genes with RPKM sequence counts fewer than 1 in all samples were removed.
| Ace | Clmp | Fxyd6 | Islr2 | Nr2f1 | Ptpn7 | Slc16a6 | Trhr |
| Agxt2l1 | Clspn | Gm11992 | Itga5 | Nr4a2 | Ptprv | Slc4a11 | Trim66 |
| Ak5 | Cnr1 | Gng4 | Itga9 | Nr4a3 | Rarg | Slc8a1 | Trpc3 |
| Atp2b4 | Cpne2 | Gpr137b | Kcna5 | Nrn1 | Rasl10a | Slit2 | Txnip |
| B3galt2 | Cpne6 | Gpr139 | Kctd4 | Olfm3 | Rgs16 | Socs2 | Upk1b |
| Bcl6 | Dlx2 | Gpr155 | Lin7a | Otof | Rgs4 | Stard13 | Wbscr17 |
| Btg2 | Egr2 | Gpr3 | Me2 | Pdyn | Rprm | Stat6 | Zbtb20 |
| C1ql3 | Epop | Greb1l | Medag | Per2 | Rtn4r | Stx1a | |
| Cadps2 | Fabp5 | Hap1 | Msr2 | Phex | Rtn4rl2 | Sulf1 | |
| Camk1g | Fam81a | Hmgn2 | Mycn | Pkp2 | Ryr1 | Sycp1 | |
| Cartpt | Fhdc1 | Htr1d | Mylk | Plxnc1 | S100a10 | Syt10 | |
| Ccdc72 | Fndc9 | Htr2a | Myo3b | Prima1 | Scn9a | Tmem163 | |
| Cckbr | Fos | Hydin | Npas4 | Prr36 | Sema7a | Tmem46 | |
| Cldn5 | Fosb | Irs2 | Nptx1 | Pter | Sez6l | Tppp3 |
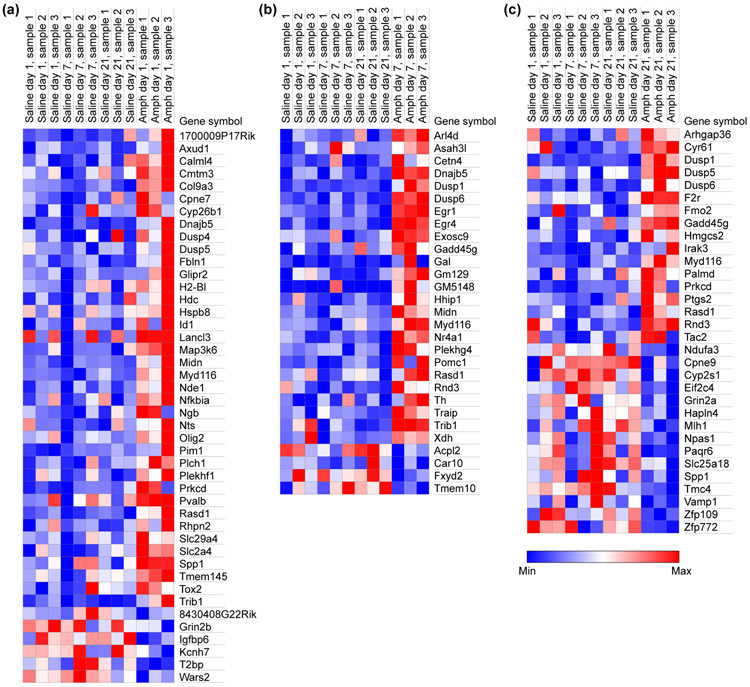
In response to the very first D-amphetamine exposure, we found significant expression changes for 44 genes, relative to the group of all saline-injected controls, that did not change in any saline group comparisons (Figure 7a and Table 2). By the 7th day of D-amphetamine treatment, the number of genes with significant expression changes had dropped to 29 and now included immediate early genes (Egr1, Egr4 and Nr4a1; Figure 7b and Table 3). On the 1st and 7th days of D-amphetamine treatment, the majority of significant changes were increases in expression (86% of the 44 genes that were changed on day 1 and 86% of the 29 genes that were changed on day 7). However, by the 21st day of D-amphetamine treatment, only 53% of the 32 significantly changed gene transcripts were up-regulated (Figure 7c and Table 4).
FIGURE 7.
The heat maps for gene expression changes show the sharpest differences on high-stereotypy day 7, and progressively lower proportions of upregulated genes across all days. Each column shows results from one mouse for genes that were significantly different in group comparisons between saline-treated mice and mice treated with D-amphetamine for 1 day (a; modest behavioral response), 7 days (b; prolonged stereotypy response) and 21 days (c; strong behavioral response with curtailed stereotypy period) . Genes with RPKM < 1 in all samples, or that were changed in any pair-wise comparisons among saline-treatment groups, were removed.
TABLE 2.
Genes with significant mRNA expression changes in pair-wise comparisons between habituated mice treated with saline and habituated mice treated with 1 injection of D-amphetamine prior to striatal collection. Genes in bold font were downregulated and genes in regular font were upregulated in the amphetamine-treated group. Genes with RPKM sequence counts fewer than 1 in all samples were removed.
| Agxt2l1 | Cpne7 | Ecrg4 | Gbx1 | Igfbp6 | Nde1 | Prkcd | T2bp |
| Axud1 | Cyp26b1 | Fabp5 | Glipr2 | Kcnh7 | Nfkbia | Pvalb | Tmem145 |
| Calml4 | Cyyr1 | Fam216b | Gm11992 | Lancl3 | Ngb | Rasd1 | Tox2 |
| Camk1g | Depp1 | Fbln1 | Grin2b | Map3k6 | Nts | Rhpn2 | Trib1 |
| Cdsn | Dnajb5 | Fhdc1 | H2-Bl | Midn | Olig2 | S100a10 | Wars2 |
| Cfap126 | Dnali1 | Fndc9 | Hdc | Msr2 | Pim1 | Slc29a4 | |
| Cmtm3 | Dusp4 | Foxj1 | Hspb8 | Myd116 | Plch1 | Slc2a4 | |
| Col9a3 | Dusp5 | Gabrr2 | Id1 | Myo3b | Plekhf1 | Spp1 |
TABLE 3.
Genes with significant mRNA expression changes in pair-wise comparisons of striatal transcriptomes between habituated mice treated with saline and habituated mice treated with 7 daily injections of D-amphetamine. Genes in bold font were downregulated and genes in regular font were upregulated in the amphetamine-treated group. Genes with RPKM sequence counts fewer than 1 in all samples were removed.
| Acpl2 | Dusp1 | Fos | Gm5148 | Msr2 | Plekhg4 | Rxfp1 | Traip |
| Arl4d | Dusp6 | Fosb | Gpr3 | Myd116 | Pomc1 | S100a10 | Trib1 |
| Asah3l | Egr1 | Fxyd2 | Hhip | Npas4 | Prss12 | Sulf1 | Xdh |
| Car10 | Egr2 | Gadd45g | Medag | Nr4a1 | Pter | Th | |
| Cetn4 | Egr4 | Gal | Midn | Nr4a3 | Rasd1 | Tmem10 | |
| Dnajb5 | Exosc9 | Gm129 | Mpzl2 | Per2 | Rnd3 | Tmem46 |
TABLE 4.
Genes with significant mRNA expression changes in pair-wise comparisons of striatal transcriptomes between habituated mice treated with saline and habituated mice treated with 21 daily injections of D-amphetamine. Genes in bold font were downregulated and genes in regular font were upregulated in the amphetamine-treated group. Genes with RPKM sequence counts fewer than 1 in all samples were removed.
| Arghap36 | Cyp2s1 | Dusp6 | Gpr139 | Lrrc74b | Palmd | Rnd3 | Tmem46 |
| Btg2 | Cyr61 | Eif2c4 | Grin2a | Mlh1 | Paqr6 | Ryr1 | Vamp1 |
| Cartpt | Dlx2 | F2r | Hapln4 | Myd116 | Pmaip1 | Slc25a18 | Zfp109 |
| Clmp | Dnali1 | Fmo2 | Hmgcs2 | Ndufa3 | Prkcd | Spp1 | Zfp772 |
| Cpne2 | Dusp1 | Fosb | Hspa1a | Npas1 | Ptgs2 | Tac2 | |
| Cpne9 | Dusp5 | Gadd45g | Irak3 | Oscar | Rasd1 | Tmc4 |
Out of 80 genes that were upregulated on any D-amphetamine day vs. saline treatment comparison, 12 were changed on more than one drug-treatment day (Table 5). Eleven of these genes were upregulated on multiple days and one gene, Spp1 (secreted phosphoprotein 1, aka osteopontin), was up on day 1, unchanged on day 7 and down on day 21 of drug treatment. Of the 11 upregulated changes in common across days, 6 are involved in the extracellular-signal regulated kinases (ERKs) cascade pathway that is known to respond to treatments that elevate dopamine signaling, including D-amphetamine (Bertran-Gonzalez et al., 2008; Ding et al., 2011; Shi & McGinty, 2011; Cirnaru et al., 2019): [Dusp1, Dusp5, Dusp6 (Dual Specificity Phosphatases), Prkcd (Protein Kinase C Delta) (Limnander et al., 2011), Rasd1 (Ras Related Dexamethasone Induced 1, aka Ags1) (Graham et al., 2002) and Trib1 (Kiss-Toth et al., 2004)]. Members of other MAP kinase cascades and dephosphorylation cascades [Gadd45g, Myd116 (aka Ppp1r15a)] were also changed on multiple drug treatment days. Altogether, the majority of the changes that were shared across multiple schedules of drug-treatment, representing early responses to acute D-amphetamine treatment and responses to repeated treatments, were involved in deactivation of MAP kinase phosphorylation cascades.
TABLE 5.
Matrix of genes that showed expression changes on multiple days of D-amphetamine treatment.
| D-amphetamine treatment days | |||
|---|---|---|---|
| Days 1 and 7 | Days 1 and 21 | Days 7 and 21 | Days 1, 7 and 21 |
| Dnajb5 | Spp1 | Dusp6 | Myd116 |
| Midn | Rasd1 | Dusp1 | Rasd1 |
| Rasd1 | Myd116 | Gadd45g | |
| Myd116 | Dusp5 | Rnd3 | |
| Trib1 | Prkcd | Myd116 | |
| Rasd1 | |||
Gene set enrichment analysis to identify pathways related to stereotypy
We evaluated all gene expression changes unique to each drug treatment day (but without eliminating genes found to change among saline treatment comparisons) by GSEA comparisons to curated gene sets (Subramanian et al., 2005). For day 1, the most significant overlap was with genes that were identified, in mouse brain tissue, to have high-CpG-density promoters (HCP) bearing histone H3 dimethylation at K4 (H3K4me2) and trimethylation at K27 (H3K27me3), a methylation state common to genes expressed in differentiated cells (Meissner et al., 2008). The nine overlapping genes in this set were Glipr2, Dusp4, Olig2, Plekhf1, Slc2A4, Slc29A4, Map3k6, Cpne7 and T2bp (aka Tifa). Evaluation of the genes that were changed only on D-amphetamine treatment day 21 indicated that there were very few significant overlaps with curated gene sets, the clearest being 3 upregulated genes (Ptgs2, Cyr61 and F2r) that overlap with those in a gene set that is downregulated during differentiation of cultured fibroblasts (Burton et al., 2004). Genes with consistent and significant changes in the mice that were drug-treated for 21 days included upregulation of Arhgap36 (Rho GTPase activating protein 36), which is known to dampen cellular responses to cAMP (Eccles et al., 2016), immune system markers [Irak3 (interleukin-1 receptor-associated kinase 3)] and platelet activators [F2r (coagulation factor II thrombin receptor) and Ptgs2 (prostaglandin-endoperoxide synthase 2 aka Cox2)]. Notably downregulated genes on day 21 included functional neuronal markers [Grin2a (NMDA glutamate receptor) and Npas1 (neuronal PAS domain protein 1)] and Eif2c4 (Ago4), which encodes a member of the RNA-induced silencing complex.
Considering that the most severe and prolonged stereotypy was observed on D-amphetamine treatment day 7, relative to days 1 and 21 for which we had comparator RNAseq data, we focused on changes that were specific to this gene set. Notable changes that were unique to treatment day 7 included upregulation of Th, which encodes tyrosine hydroxylase, a synthetic enzyme for dopamine and noradrenalin that is expressed in the terminals of dopamine-containing neurons of the substantia nigra, as well as in striatal interneurons. Hhip (Hedgehog-interacting protein) was also upregulated uniquely on day 7 and, according to transgene-reporter expression, appears to be enriched in the forebrain and in fibers enriched in the striatal striosomes (Gong et al., 2003), but is relatively understudied. The associated protein Hedgehog is essential for signaling between neurons in the nigrostriatal circuit, and conditional deletion of Hedgehog in dopamine-containing neurons results in severe neurodegeneration of dopamine-containing neurons in the substantia nigra and interneurons of the striatum along with bradykinesia and other motor symptoms (Gonzalez-Reyes et al., 2012). Nr4a1, which is induced in D1-positive striosomal neurons upon methamphetamine treatment (Davis & Puhl, 2011) and mediates cocaine-induced ERK activation (Cirnaru et al., 2019), was also upregulated most on day 7 in our study. Upregulation specific to day 7 was also identified for Exosc9, which encodes a scaffold protein for RNA degradation (Vickers & Crooke, 2012).
Notably, by GSEA, the most significant overlap for the genes that were upregulated on high-stereotypy day 7, but not on low-stereotypy day 1 or 21 of amphetamine treatment, was with the Nrg1-responsive gene set (Nagashima et al., 2007) (false discovery rate corrected P = 6 x 10−4 for day 7 set only and P = 6 x 10−17 for days 1, 7 and 21 gene sets combined). There were 12 gene overlaps between the Nrg1-responsive list and those changed on day 7: Egr1, Egr2, Egr4 (early growth response 1, 2, 4), Myd116/Ppp1r15a (protein phosphatase 1, regulatory subunit 15A), Trib1 (tribbles homolog 1), Per2 (period homolog 2), Dusp1 (dual specificity phosphatase 1), Gal (galanin prepropeptide), and the immediate early genes Fos, FosB, Nr4a1 and Nr4a3. Overlap of the drug-treatment day 7 upregulated gene set was also significant for genes upregulated by ErbB2/HER2 (false discovery rate corrected P = 2 x 10−7) in a heterologous cell type (Pedersen et al., 2009) — the overlapping genes were Egr1, Egr4, Dusp6, Trib1, Gal and Rasd1.
Identification of candidate stress-related genes
Changes related to pathways that are common to both stressful experiences (e.g., restraint and saline injection in the activity monitor) and drug-responses might be discovered by identifying overlaps in the genes that were changed in both the drug- and saline-treatment groups. In the 3 groups of mice treated only with saline, we identified 105 significant gene expression differences by pairwise comparisons between 1-day, 7-day and 21-day saline-treated mice (Table 6). Of these genes, 26 genes were also found to be changed on an amphetamine treatment day, including genes previously reported to change in response to psychomotor stimulant treatment [Egr2, Fos, FosB, Npas4 and Cartpt (cocaine- and amphetamine-regulated transcript protein)]. The most significant changes identified in comparisons among saline-treatment groups were in transcripts for Cnr1 (cannabinoid receptor 1), Sema7a (semaphorin 7A), Gpr155 (G protein-coupled receptor 155), Nr4a2 (nuclear receptor subfamily 4, group A, member 2), Nptx1 (neuronal pentraxin 1), Rtn4r (reticulon 4 receptor), Cckbr (cholecystokinin B receptor), Dnahc9 (dynein, axonemal, heavy chain 9 containing), Islr2 (immunoglobulin superfamily) and Sez6l (seizure related 6 homolog).
TABLE 6.
Genes that were changed in amphetamine versus saline comparisons and also in pair-wise comparisons between groups of control mice treated with saline only. Genes with RPKM sequence counts fewer than 1 in all samples were removed.
| Agxt2l1 | Cpne2 | Fos | Gpr3 | Nr4a3 | S100a10 | Clmp |
| Btg2 | Dlx2 | Fosb | Msr2 | Per2 | Sulf1 | Fndc9 |
| Camk1g | Egr2 | Gm1192 | Myo3b | Pter | Tmem46 | |
| Cartpt | Fabp5 | Gpr139 | Npas4 | Ryr1 | Fhdc1 |
In addition to identifying gene expression changes common to both drug exposure and physical stress, we considered transcripts that were changed in disease models with stress to striatal cells. Having previously used the same gene expression pipeline to identify differentially expressed genes in mouse models of Huntington’s disease (Vashishtha et al., 2013), a genetic disorder that causes striatal cell dysfunction and death, we directly compared the data. Many of the expression level changes that we report here are opposite to those changes that we had previously found in mouse models of Huntington’s disease relative to their wildtype controls. There was a significant overlap between genes that were changed in the amphetamine-treatment groups and those changed in striatal samples from untreated R6/2 Huntington’s disease model mice at 8 weeks of age (hypergeometric P value, P = 2.3 x 10−3) and 12 weeks of age (hypergeometric P value, P = 2.7 x 10−4). In the comparison to 8-week-old R6/2 mice (a moderately severe disease time-point), we found 4 gene overlaps (Gal, Nr4a1, Nts, and Traip), all of which were decreased in the R6/2 samples and increased in the samples from mice on day 7 of D-amphetamine treatment. In the comparison to 12-week-old R6/2 mice (a very severe disease time-point), we found 10 gene overlaps [Arl4d (down in R6/2, up in amphetamine day 7), Car10 (up in R6/2, down in amphetamine day 7), Cpne7 (up in R6/2, up in amphetamine day 1), Cpne9 (down in R6/2, down in amphetamine day 21), Egr1 (down in R6/2, up in amphetamine day 7), Gadd45g (down in R6/2, up in amphetamine day 7 and day 21), Gm129 (down in R6/2, up in amphetamine day 7), Ngb (up in R6/2, up in amphetamine day 1), Nr4a1 (down in R6/2, up in amphetamine day 7), and Plch1 (up in R6/2, up in amphetamine day 1)]. In total, we found 13 gene overlaps, and 77% of these genes were changed, relative to controls, in the opposite direction in the amphetamine-treated mice and untreated R6/2 mouse model of Huntington’s disease. These findings point to subsets of genes with dual vulnerability to drug-induced, physical stress-induced and disease-induced changes in the striatum.
DISCUSSION
To survey gene-induction plasticity associated with drug-induced repetitive behaviors, we defined amphetamine treatment schedules that induce different levels of stereotypy in mice (naïve, sensitized, and tolerant). We found that early gene expression changes in the striatum were strikingly different across these behavioral response phases. A multitude of studies have been done to identify transcriptional changes in the striatum that are correlated to psychomotor stimulant sensitization (Yuferov et al., 2005), but our study design is unique, as far as we know, for querying transcriptome-wide changes associated with confined repetitive behavior. The drug-induced stereotypy phenotype is viewed as a model for the stereotypies observed in neuropsychiatric disorders such as schizophrenia (Angrist et al., 1974; Moran et al., 2019). The findings that we report here point toward plausible shared transcriptomic mechanisms underlying this cardinal phenotype.
Measurements of confined stereotypy in naïve, sensitized and tolerant states, and after a month-long hiatus followed by challenge
Clear patterns in the behavioral responses to drug and drug withdrawal were evident. In the averaged responses, we observed sensitization of stereotypy on day 2 of D-amphetamine treatment, consistent with the hypothesis that ‘priming’ of some behaviors occurs after a single exposure (Kuczenski & Segal, 1999; Boileau et al., 2006; Chinen et al., 2006). Stereotypy was still severe and prolonged on day 7 of drug treatment, but by day 21, the duration of the heightened stereotypy was significantly shorter, supplanted by the emergence of what has been termed late-phase locomotion, based on studies in rats (Segal et al., 1980; Kuczenski & Segal, 1997). The same mice, when challenged after 40 days off treatment, maintained and even augmented their sensitized stereotypy responses, flanked by early- and late-phase locomotor responses. Thus, the mechanisms that underlie the sensitization to amphetamine-induced stereotypy appear to be only temporarily masked, not reversed, by prolonged drug treatment inducing tolerance. The challenge, a further exposure to the drug, thus reinstates much of the sensitized behavior, as experienced by human drug-users in relapse. In humans, monkeys, rats and mice, even after many months of drug-abstinence, various sensitized responses to a further drug exposure are still present, supporting the notion that sensitization is related to the enduring risk of relapse (Segal et al., 1980; Vanderschuren et al., 1999; Berke & Hyman, 2000; Boileau et al., 2006; Vezina & Leyton, 2009; Valjent et al., 2010; Zhou et al., 2010). Sensitization mechanisms related to the relapse of drug habits might be the same as those that underlie the re-emergence of extreme repetitive behaviors such as those we have worked to identify here by using both automated and observational measures of confined stereotypy.
A general pattern of transcriptomic changes emerged in mice with treatments to induce stereotypy sensitization and tolerance
Changes in epigenetic status, transcription initiation and completion, and mRNA stability, localization, folding and translation, all impact changes in gene expression (Cheadle et al., 2005; Rabani et al., 2011). We found changes in transcripts that encode proteins involved in mRNA degradation, translation, and microRNA-mediated regulation. Our sequence data should reflect polyadenylated RNAs present at ~20 min after D-amphetamine or saline injection. For the first amphetamine exposure, the transcript changes relative to saline controls should include acute responses to drug injection whereas sequence data from mice treated repeatedly with drug would also include steady-state changes from the previous drug exposures. Nevertheless, there were fewer significantly changed transcripts on day 7 and day 21 of treatment than there were on day 1. This pattern is consistent with a progressive decrease in overall gene induction across treatments and fits with our finding that most gene expression changes on day 1 and day 7 were upregulations, whereas by day 21 nearly half of the changed genes were downregulated. One candidate for the downregulation of transcripts by day 21 is Exosc9, which is upregulated on day 7 and encodes an exosome protein involved in RNA degradation via destabilization of the target mRNAs’ polyA tail and facilitation of RNA-induced silencing (Vickers & Crooke, 2012). Eif2c4 (ago4, argonaute RISC catalytic subunit 4) was downregulated on day 21, and itself encodes a key component of the RNA-induced silencing complex (Quevillon Huberdeau et al., 2017), further highlighting RNA regulatory mechanisms in the transcriptomic changes that we found to be evident at only 20 min after drug injection. Amphetamine treatments have been reported previously to increase phosphorylation of the translation factor eIF2α, which in turn reduces striatal mRNA translation overall, but spares a subset of transcripts, including Myd116 (aka Ppp1r15a, Gadd34) (Biever et al., 2016). We found increases in Myd116 on all amphetamine treatment days, supporting involvement of this cascade in our study as well.
Our RNAseq results across treatment paint a picture of varied gene upregulation on the first day of amphetamine treatment that becomes more specific for upregulation of abundant and dynamically regulated immediate early genes by day 7. Several immediate early genes have been shown to have increased striosome-to-matrix ratios of expression that are correlated to the expression of stereotypy (Graybiel et al., 1990; Moratalla et al., 1996; Canales & Graybiel, 2000; Tan et al., 2000; Saka et al., 2004; Crittenden & Graybiel, 2017). This pattern of overall downregulation across the large matrix compartment of the striatum and strong upregulation of immediate early genes in striosomes is similar to our whole striatum RNAseq data in which there was a progressive reduction in overall gene activation across days of repeated treatment but very strong upregulation of immediate early genes such as Egr1, Egr4 and Nr4a1, in mice with stereotypy-inducing treatments.
The ERK phosphorylation cascade is central to numerous cellular responses throughout the body. In the striatum, ERK undergoes dynamic regulation (as measured by its phosphorylation status) upon treatment with psychomotor stimulants (Valjent et al., 2005; Shi & McGinty, 2011). We found increased expression of multiple genes associated with the ERK cascade, relative to controls, in all three groups of amphetamine-treated mice. Most of these genes encode proteins that deactivate the ERK cascade, including two in the DUSP family of phosphatases, Prkcd and Rasd1 that were both upregulated in multiple amphetamine treatment groups. In transcriptomics studies of naïve mice (not drug treated), Prkcd and Rasd1 mRNAs have been found to be lowly expressed in striatal projection neurons of the dorsal striatum (Gokce et al., 2016; Saunders et al., 2018; Puighermanal et al., 2020). However, Puighermanal and colleagues did find Rasd1 mRNA in D2 dopamine receptor-positive projection neurons in the nucleus accumbens, a region ventral to the dorsal striatum that is strongly activated by drugs of abuse (Valjent et al., 2005). Our findings raise the possibility that Rasd1 is expressed de novo in the dorsal striatal projection neurons upon exposure to amphetamine.
ERK is also dynamically regulated (as measured by its phosphorylation status) in the striatum of mouse models of L-DOPA-induced dyskinesia, in which dopamine-depleted animals exhibit a sensitized response to dopamine receptor agonists (Pavon et al., 2006; Darmopil et al., 2009;Ding et al., 2011). Moreover, in striatal projection neurons of this mouse model, ERK activation is high on day 1, lower on day 7 and lowest after more prolonged L-DOPA treatment (Ding et al., 2011). A potential parallel finding in our study is that Spp1, a transcriptional reporter of ERK activity status (Lai et al., 2001), was increased on amphetamine treatment day 1, unchanged on day 7 and downregulated on day 21. In bone development, Spp1 is upregulated by ERK activation to mediate cell adhesion upon differentiation (Lai et al., 2001), but its function in the striatum, where it has been found in fibroblast-like cells (Saunders et al., 2018), remains unknown.
Not all of our results, however, are consistent with a progressive downregulation of ERK cascade members. For example, Prkcd, which was upregulated in our samples, encodes a kinase that has been reported, in B cells, to work together with the striosome-enriched molecule CalDAG-GEFII (aka RasGRP) (Kawasaki et al., 1998) to activate ERK (Limnander et al., 2011). CalDAG-GEFII is upregulated in striosomes of a rat model of L-DOPA-induced dyskinesias where it has been proposed as an ERK regulator (Crittenden et al., 2009). Thus, reporters for the ERK cascade changed dynamically across acute and repeated D-amphetamine treatments and likely involve multiple cell types like in models of L-DOPA induced dyskinesia (Pavon et al., 2006; Darmopil et al., 2009; Ding et al., 2011).
Identification of genes that were selectively dysregulated on a day of peak stereotypic behavior
Two of the upregulated transcripts that were changed in mice on a day of high stereotypy (day 7 of treatment), but not in mice that had not yet become sensitized (day 1) or that had developed tolerance (day 21), were Egr1 and Th. Egr1 is highly expressed in striatal projection neurons, whereas Th is expressed in interneurons (Saunders et al., 2018). L-DOPA replacement therapy in parkinsonian mice has been shown to increase the number of striatal interneurons that express tyrosine hydroxylase (TH) (Espadas et al., 2012). This is of special interest because the pattern of striosome-enriched immediate early gene induction that is correlated to amphetamine-induced stereotypy is similar to that observed in dopamine agonist-induced dyskinesia in parkinsonian rats (Saka et al., 1999). Whether the number of TH-positive striatal interneurons is augmented by amphetamine treatment has not been examined to our knowledge. Microdialysis measurements in behaving rats show increased dopamine levels in the dorsal striatum of rats exhibiting methamphetamine-induced repetitive behaviors, whereas rats subjected to prolonged treatment that induces tolerance to stereotypy have diminished dopamine levels (Kuczenski & Segal, 1997). Our results suggest that increased Th expression specific to stereotypy sensitization might be responsible for these fluctuating dopamine levels (but see Xenias et al., 2015). In adrenal medulla of rats that experience immobilization stress, it has been shown that Th and the transcription factor Egr1 are upregulated and that Th expression is regulated by Egr1 binding to the promoter sequence (Papanikolaou & Sabban, 1999). Considering the strong link between extreme stress, stereotypy, and drug relapse, it is possible that our finding of increased Egr1 and Th in drug-treated mice is directly related to the ability of Egr1 to drive Th transcription in animals exposed to stress.
One of the most notable findings from our study was the over-representation of genes responsive to Nrg1 signaling in the striatal samples from the day 7 group mice that exhibited prolonged stereotypy. Nrg1, which is expressed in striatal projection neurons (Saunders et al., 2018), is a strong candidate gene for schizophrenia (Stefansson et al., 2002; Boileau et al., 2006) and encodes an extracellular signaling molecule for ErbB4 (Erb-B2 Receptor Tyrosine Kinase 4), a receptor that is expressed, among other places, in dopamine-containing neurons of the substantia nigra that project to the striatum (Steiner et al., 1999; Abe et al., 2009). Transgenic reporters for Nrg1 expression (Gong et al., 2003) show enrichment in striosomal neurons, which are known to send direct fiber projections to dopamine-containing nigral neurons (Watabe-Uchida et al., 2012; Crittenden et al., 2016; Matsushima & Graybiel, 2020) and Nrg1 stimulation of ErbB4 drives dopamine release (Yurek et al., 2004; Skirzewski et al., 2018). Together, these data bring up the highly interesting possibility that the upregulation of Nrg1-responsive genes under conditions of intense stereotypy drive excess dopamine release. If true, this driving function would have direct implications for stereotypy in schizophrenia, in which dopamine release in the striosome-rich anterior striatum (Kegeles et al., 2010) and Nrg1 signaling to ErbB4 are reported to be abnormally high (Petryshen et al., 2005; Hahn et al., 2006).
Supplementary Material
Distance traveled data. Average distance traveled, in 5 min bins, for each mouse on each day.
Measurements of stereotypy and locomotion. Observational scores of the bouts and total time engaged in each behavior for each mouse, on each day that was scored.
Expression measurements for all genes that showed a significant difference on an amphetamine day relative to the average expression across saline days. Normalized sequence read counts are given for all samples on all days.
ACKNOWLEDGEMENTS
We thank Dr. Daniel J. Gibson for generating the angular coordinate plots, Dr. Charlie Whittaker for assistance with bioinformatics analyses, and Dr. Yasuo Kubota and Cynthia Schofield for help with manuscript preparation. This work was funded by the National Institute of Child Health and Human Development (R37-HD028341, A.M.G.), the Saks Kavanaugh Foundation (A.M.G.), Broderick Fund for Phytocannabinoid Research at MIT (A.M.G.), the National Institute of Mental Health (R01-MH060379, A.M.G.), the James and Pat Poitras Research Fund (A.M.G.), The Simons Foundation (A.M.G. and J.R.C), the National Institute on Aging (R01-AG050548, A.C.S.) and The Stanley Center for Psychiatric Research at the Broad Institute, via a grant to Edward Scolnick from the Stanley Medical Research Institute (A.M.G. and J.R.C.).
Footnotes
CONFLICT OF INTEREST
The authors report no competing interests.
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author on request.
REFERENCES
- Abe Y, Namba H, Zheng Y & Nawa H (2009) In situ hybridization reveals developmental regulation of ErbB1-4 mRNA expression in mouse midbrain: implication of ErbB receptors for dopaminergic neurons. Neuroscience, 161, 95–110. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, D.-V. (ed) (2013) American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition., Washington, DC. [Google Scholar]
- Angrist B, Sathananthan G, Wilk S & Gershon S (1974) Amphetamine psychosis: behavioral and biochemical aspects. Journal of Psychiatric Research, 11, 13–23. [DOI] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ & Graybiel AM (2005) Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature, 437, 1158–1161. [DOI] [PubMed] [Google Scholar]
- Berke JD & Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron, 25, 515–532. [DOI] [PubMed] [Google Scholar]
- Berke JD, Paletzki RF, Aronson GJ, Hyman SE & Gerfen CR (1998) A complex program of striatal gene expression induced by dopaminergic stimulation. Journal of Neuroscience, 18, 5301–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E & Girault JA (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. Journal of Neuroscience, 28, 5671–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson MJ, Graybiel AM & Quinn B (1990) Co-expression of neuropeptides in the cat's striatum: an immunohistochemical study of substance P, dynorphin B and enkephalin. Neuroscience, 39, 33–58. [DOI] [PubMed] [Google Scholar]
- Biever A, Boubaker-Vitre J, Cutando L, Gracia-Rubio I, Costa-Mattioli M, Puighermanal E & Valjent E (2016) Repeated exposure to d-amphetamine decreases global protein synthesis and regulates the translation of a subset of mRNAs in the striatum. Frontiers in Molecular Neuroscience, 9, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M & Benkelfat C (2006) Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Archives of General Psychiatry, 63, 1386–1395. [DOI] [PubMed] [Google Scholar]
- Burton GR, Nagarajan R, Peterson CA & McGehee RE Jr. (2004) Microarray analysis of differentiation-specific gene expression during 3T3-L1 adipogenesis. Gene, 329, 167–185. [DOI] [PubMed] [Google Scholar]
- Canales JJ & Graybiel AM (2000) A measure of striatal function predicts motor stereotypy. Nature Neuroscience, 3, 377–383. [DOI] [PubMed] [Google Scholar]
- Capper-Loup C, Canales JJ, Kadaba N & Graybiel AM (2002) Concurrent activation of dopamine D1 and D2 receptors is required to evoke neural and behavioral phenotypes of cocaine sensitization. Journal of Neuroscience, 22, 6218–6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle C, Fan J, Cho-Chung YS, Werner T, Ray J, Do L, Gorospe M & Becker KG (2005) Control of gene expression during T cell activation: alternate regulation of mRNA transcription and mRNA stability. BMC Genomics, 6, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinen CC, Faria RR & Frussa-Filho R (2006) Characterization of the rapid-onset type of behavioral sensitization to amphetamine in mice: role of drug-environment conditioning. Neuropsychopharmacology, 31, 151–159. [DOI] [PubMed] [Google Scholar]
- Cirnaru MD, Melis C, Fanutza T, Naphade S, Tshilenge KT, Muntean BS, Martemyanov KA, Plotkin JL, Ellerby LM & Ehrlich ME (2019) Nuclear receptor Nr4a1 regulates striatal striosome development and dopamine D1 receptor signaling. eNeuro, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Madrigal P, Tarazona S, Gomez-Cabrero D, Cervera A, McPherson A, Szczesniak MW, Gaffney DJ, Elo LL, Zhang X & Mortazavi A (2016) A survey of best practices for RNA-seq data analysis. Genome Biology, 17, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Cantuti-Castelvetri I, Saka E, Keller-McGandy CE, Hernandez LF, Kett LR, Young AB, Standaert DG & Graybiel AM (2009). Dysregulation of CalDAG-GEFI and CalDAG-GEFII predicts the severity of motor side-effects induced by anti-parkinsonian therapy. Proceedings of the National Academy of Sciences of the United States of America, 106, 2892–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR & Graybiel AM (2017) Disease-associated changes in the striosome and matrix compartments of the dorsal striatum. In Steiner H, Tseng K (ed) Handbook of Basal Ganglia Structure and Function, Second Edition. Elsevier. [Google Scholar]
- Crittenden JR, Lacey CJ, Lee T, Bowden HA & Graybiel AM (2014) Severe drug-induced repetitive behaviors and striatal overexpression of VAChT in ChAT-ChR2-EYFP BAC transgenic mice. Frontiers in Neural Circuits, 8, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Lacey CJ, Weng FJ, Garrison CE, Gibson DJ, Lin Y & Graybiel AM (2017) Striatal cholinergic interneurons modulate spike-timing in striosomes and matrix by an amphetamine-sensitive mechanism. Frontiers in Neuroanatomy, 11, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Tillberg PW, Riad MH, Shima Y, Gerfen CR, Curry J, Housman DE, Nelson SB, Boyden ES & Graybiel AM (2016) Striosome-dendron bouquets highlight a unique striatonigral circuit targeting dopamine-containing neurons. Proceedings of the National Academy of Sciences of the United States of America, 113, 11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmopil S, Martín AB, De Diego IR, Ares S & Moratalla R (2009) Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biological Psychiatry, 66, 603–13. [DOI] [PubMed] [Google Scholar]
- Davis MI & Puhl HL 3rd (2011) Nr4a1-eGFP is a marker of striosome-matrix architecture, development and activity in the extended striatum. PLoS One, 6, e16619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers TM, Amemori K & Graybiel AM (2015) Habit learning by naive macaques is marked by response sharpening of striatal neurons representing the cost and outcome of acquired action sequences. Neuron, 87, 853–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson PR, Lang CG, Hinton SC & Kelley AE (1994) Oral stereotypy induced by amphetamine microinjection into striatum: an anatomical mapping study. Neuroscience, 61, 81–91. [DOI] [PubMed] [Google Scholar]
- Ding Y, Won L, Britt JP, Lim SA, McGehee DS & Kang UJ (2011) Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proceedings of the National Academy of Sciences of the United States of America, 108, 840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles RL, Czajkowski MT, Barth C, Muller PM, McShane E, Grunwald S, Beaudette P, Mecklenburg N, Volkmer R, Zuhlke K, Dittmar G, Selbach M, Hammes A, Daumke O, Klussmann E, Urbe S & Rocks O (2016) Bimodal antagonism of PKA signalling by ARHGAP36. Nature Communications, 7, 12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espadas I, Darmopil S, Vergano-Vera E, Ortiz O, Oliva I, Vicario-Abejon C, Martin ED & Moratalla R (2012) L-DOPA-induced increase in TH-immunoreactive striatal neurons in parkinsonian mice: insights into regulation and function. Neurobiology of Disease, 48, 271–281. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW & Hanson GR (2007) New insights into the mechanism of action of amphetamines. Annual Review of Pharmacology and Toxicology, 47, 681–698. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Keefe KA & Gauda EB (1995) D1 and D2 dopamine receptor function in the striatum: coactivation of D1- and D2-dopamine receptors on separate populations of neurons results in potentiated immediate early gene response in D1-containing neurons. Journal of Neuroscience, 15, 8167–8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA & Ellenbroek B (2003) Animal behavior models of the mechanisms underlying antipsychotic atypicality. Progress in Neuropsychopharmacology & Biological Psychiatry, 27, 1071–1079. [DOI] [PubMed] [Google Scholar]
- Gokce O, Stanley GM, Treutlein B, Neff NF, Camp JG, Malenka RC, Rothwell PE, Fuccillo MV, Südhof TC, & Quake SR (2016) Cellular taxonomy of the mouse striatum as revealed by single-cell RNA-seq. Cell Reports, 16, 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME & Heintz N (2003) A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature, 425, 917–925. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes LE, Verbitsky M, Blesa J, Jackson-Lewis V, Paredes D, Tillack K, Phani S, Kramer ER, Przedborski S & Kottmann AH (2012) Sonic hedgehog maintains cellular and neurochemical homeostasis in the adult nigrostriatal circuit. Neuron, 75, 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J. https://software.broadinstitute.org/GENE-E/.
- Graham TE, Prossnitz ER & Dorin RI (2002) Dexras1/AGS-1 inhibits signal transduction from the Gi-coupled formyl peptide receptor to Erk-1/2 MAP kinases. Journal of Biological Chemistry, 277, 10876–10882. [DOI] [PubMed] [Google Scholar]
- Graybiel AM (2008) Habits, rituals, and the evaluative brain. Annual Review of Neuroscience, 31, 359–387. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R & Robertson HA (1990) Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proceedings of the National Academy of Sciences of the United States of America, 87, 6912–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ & Arnold SE (2006) Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nature Medicine, 12, 824–828. [DOI] [PubMed] [Google Scholar]
- Henry B, Duty S, Fox SH, Crossman AR & Brotchie JM (2003) Increased striatal pre-proenkephalin B expression is associated with dyskinesia in Parkinson's disease. Experimental Neurology, 183, 458–468. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, Wasserman S, Soorya L & Buchsbaum M (2005) Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biological Psychiatry, 58, 226–232. [DOI] [PubMed] [Google Scholar]
- Horner KA, Hebbard JC, Logan AS, Vanchipurakel GA & Gilbert YE (2012) Activation of mu opioid receptors in the striatum differentially augments methamphetamine-induced gene expression and enhances stereotypic behavior. Journal of Neurochemistry, 120, 779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.jwatcher.ucla.edu/.
- Hyman SE, Malenka RC & Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Review of Neuroscience, 29, 565–598. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Cameron CM & Robinson TE (2012) Repeated methamphetamine administration differentially alters fos expression in caudate-putamen patch and matrix compartments and nucleus accumbens. PLoS One, 7, e34227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA & Robinson TE (2007) Methamphetamine-induced structural plasticity in the dorsal striatum. European Journal of Neuroscience, 25, 847–853. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Toki S, Canales JJ, Harlan P, Blumenstiel JP, Chen EJ, Bany IA, Mochizuki N, Ashbacher A, Matsuda M, Housman DE & Graybiel AM (1998). A Rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proceedings of the National Academy of Sciences of the United States of America, 95, 13278–13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Hwang DR, Huang Y, Haber SN & Laruelle M (2010) Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Archives of General Psychiatry, 67, 231–239. [DOI] [PubMed] [Google Scholar]
- Kiss-Toth E, Bagstaff SM, Sung HY, Jozsa V, Dempsey C, Caunt JC, Oxley KM, Wyllie DH, Polgar T, Harte M, O'Neill L A, Qwarnstrom EE & Dower SK (2004) Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. Journal of Biological Chemistry, 279, 42703–42708. [DOI] [PubMed] [Google Scholar]
- Kuczenski R & Segal DS (1997) An escalating dose/multiple high-dose binge pattern of amphetamine administration results in differential changes in the extracellular dopamine response profiles in caudate-putamen and nucleus accumbens. Journal of Neuroscience, 17, 4441–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R & Segal DS (1999) Sensitization of amphetamine-induced stereotyped behaviors during the acute response: role of D1 and D2 dopamine receptors. Brain Research, 822, 164–174. [DOI] [PubMed] [Google Scholar]
- Lai CF, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV & Cheng SL (2001) Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. Journal of Biological Chemistry, 276, 14443–14450. [DOI] [PubMed] [Google Scholar]
- Langen M, Schnack HG, Nederveen H, Bos D, Lahuis BE, de Jonge MV, van Engeland H & Durston S (2009) Changes in the developmental trajectories of striatum in autism. Biological Psychiatry, 66, 327–333. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Tanimura Y, Lee LW & Bodfish JW (2007) Animal models of restricted repetitive behavior in autism. Behavioural Brain Research, 176, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limnander A, Depeille P, Freedman TS, Liou J, Leitges M, Kurosaki T, Roose JP & Weiss A (2011) STIM1, PKC-delta and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nature Immunology, 12, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn D, Spiegelhalter D, Thomas A & Best N (2009) The BUGS project: Evolution, critique and future directions. Statistics in Medicine, 28, 3049–3067. [DOI] [PubMed] [Google Scholar]
- Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R & Lander ES (2008) Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature, 454, 766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima A & Graybiel AM Combinatorial developmental controls on striatonigral circuits. Cell Reports, 31(11):107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran SP, Maksymetz J & Conn PJ (2019) Targeting muscarinic acetylcholine receptors for the treatment of psychiatric and neurological disorders. Trends in Pharmacological Sciences, 40, 1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Elibol B, Vallejo M & Graybiel AM (1996) Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron, 17, 147–156. [DOI] [PubMed] [Google Scholar]
- Nagashima T, Shimodaira H, Ide K, Nakakuki T, Tani Y, Takahashi K, Yumoto N & Hatakeyama M (2007) Quantitative transcriptional control of ErbB receptor signaling undergoes graded to biphasic response for cell differentiation. Journal of Biological Chemistry, 282, 4045–4056. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2014) Epigenetic mechanisms of drug addiction. Neuropharmacology, 76 Pt B, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB & Balleine BW (2008) On habits and addiction: An associative analysis of compulsive drug seeking. Drug Discovery Today: Disease Models, 5, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou NA & Sabban EL (1999) Sp1/Egr1 motif: a new candidate in the regulation of rat tyrosine hydroxylase gene transcription by immobilization stress. Journal of Neurochemistry, 73, 433–436. [DOI] [PubMed] [Google Scholar]
- Pavón N, Martín AB, Mendialdua A & Moratalla R (2006) ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol. Psychiatry 59, 64–74. [DOI] [PubMed] [Google Scholar]
- Pedersen K, Angelini PD, Laos S, Bach-Faig A, Cunningham MP, Ferrer-Ramon C, Luque-Garcia A, Garcia-Castillo J, Parra-Palau JL, Scaltriti M, Ramon y Cajal S, Baselga J & Arribas J (2009) A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Molecular and Cellular Biology, 29, 3319–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshen TL, Middleton FA, Kirby A, Aldinger KA, Purcell S, Tahl AR, Morley CP, McGann L, Gentile KL, Rockwell GN, Medeiros HM, Carvalho C, Macedo A, Dourado A, Valente J, Ferreira CP, Patterson NJ, Azevedo MH, Daly MJ, Pato CN, Pato MT & Sklar P (2005) Support for involvement of neuregulin 1 in schizophrenia pathophysiology. Molecular Psychiatry, 10, 366–374. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Castell L, Esteve-Codina A, Melser S, Kaganovsky K, Zussy C, Boubaker-Vitre J, Gut M, Rialle S, Kellendonk C, Sanz E, Quintana A, Marsicano G, Martin M, Rubinstein M, Girault J, Ding JB & Valjent E (2020) Functional and molecular heterogeneity of D2R neurons along dorsal ventral axis in the striatum. Nature Communications, 11, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevillon Huberdeau M, Zeitler DM, Hauptmann J, Bruckmann A, Fressigne L, Danner J, Piquet S, Strieder N, Engelmann JC, Jannot G, Deutzmann R, Simard MJ & Meister G (2017) Phosphorylation of Argonaute proteins affects mRNA binding and is essential for microRNA-guided gene silencing in vivo. EMBO Journal, 36, 2088–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabani M, Levin JZ, Fan L, Adiconis X, Raychowdhury R, Garber M, Gnirke A, Nusbaum C, Hacohen N, Friedman N, Amit I & Regev A (2011) Metabolic labeling of RNA uncovers principles of RNA production and degradation dynamics in mammalian cells. Nature Biotechnology, 29, 436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, Jensen S & Johnson A (2008) A unified framework for addiction: vulnerabilities in the decision process. Behavioral and Brain Sciences, 31, 415–437; discussion 437-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka E, Elibol B, Erdem S & Dalkara T (1999) Compartmental changes in expression of c-Fos and FosB proteins in intact and dopamine-depleted striatum after chronic apomorphine treatment. Brain Research, 825, 104–114. [DOI] [PubMed] [Google Scholar]
- Saka E, Goodrich C, Harlan P, Madras BK & Graybiel AM (2004) Repetitive behaviors in monkeys are linked to specific striatal activation patterns. Journal of Neuroscience, 24, 7557–7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A*, Macosko EZ*, Wysoker A, Goldman M, Krienen F, de Rivera H, Bien E, Baum M, Wang S, Bortolin L, Goeva A, Nemesh J, Kamitaki N, Brumbaugh S, Kulp D & McCarroll SA (2018) Molecular diversity and specializations among the cells of the adult mouse brain. Cell, 174(4) P1015–1030.E16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiorring E (1971) Amphetamine induced selective stimulation of certain behaviour items with concurrent inhibition of others in an open-field test with rats. Behaviour, 39, 1–17. [PubMed] [Google Scholar]
- Schwendt M & McGinty JF (2010) Amphetamine up-regulates activator of G-protein signaling 1 mRNA and protein levels in rat frontal cortex: the role of dopamine and glucocorticoid receptors. Neuroscience, 168, 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Weinberger SB, Cahill J & McCunney SJ (1980) Multiple daily amphetamine administration: behavioral and neurochemical alterations. Science, 207, 905–907. [DOI] [PubMed] [Google Scholar]
- Shi X & McGinty JF (2011) D1 and D2 dopamine receptors differentially mediate the activation of phosphoproteins in the striatum of amphetamine-sensitized rats. Psychopharmacology (Berl), 214, 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirzewski M, Karavanova I, Shamir A, Erben L, Garcia-Olivares J, Shin JH, Vullhorst D, Alvarez VA, Amara SG & Buonanno A (2018) ErbB4 signaling in dopaminergic axonal projections increases extracellular dopamine levels and regulates spatial/working memory behaviors. Molecular Psychiatry, 23, 2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Stefani MR, Moghaddam B & Brown EN (2005) Analysis and design of behavioral experiments to characterize population learning. Journal of Neurophysiology, 93, 1776–1792. [DOI] [PubMed] [Google Scholar]
- Solís O, García-Sanz P, Martín AB, Granado N, Sanz-Magro A, Podlesniy P, Trullas R, Murer MG, Maldonado R & Moratalla R (2021) Behavioral sensitization and cellular responses to psychostimulants are reduced in D2R knockout mice. Addiction Biology, 26, e12840. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H & Stefansson K (2002) Neuregulin 1 and susceptibility to schizophrenia. American Journal of Human Genetics, 71, 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Blum M, Kitai ST & Fedi P (1999) Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Experimental Neurology, 159, 494–503. [DOI] [PubMed] [Google Scholar]
- Steiner H & Gerfen CR (1995) Dynorphin opioid inhibition of cocaine-induced, D1 dopamine receptor-mediated immediate-early gene expression in the striatum. Journal of Comparative Neurology, 353, 200–212. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES & Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America, 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron, 69, 628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, Moratalla R, Lyford GL, Worley P & Graybiel AM (2000) The activity-regulated cytoskeletal-associated protein arc is expressed in different striosome-matrix patterns following exposure to amphetamine and cocaine. Journal of Neurochemistry, 74, 2074–2078. [DOI] [PubMed] [Google Scholar]
- Tyler MW, Zaldivar-Diez J & Haggarty SJ (2017) Classics in Chemical Neuroscience: Haloperidol. ACS Chemical Neuroscience, 8, 444–453. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Herve D & Girault JA (2010) Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology, 35, 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D & Girault JA (2005) Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proceedings of the National Academy of Sciences of the United States of America, 102, 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schmidt ED, De Vries TJ, Van Moorsel CA, Tilders FJ & Schoffelmeer AN (1999) A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. Journal of Neuroscience, 19, 9579–9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schoffelmeer AN, Van Leeuwen SD, Hof L, Jonker AJ & Voorn P (2002) Compartment-specific changes in striatal neuronal activity during expression of amphetamine sensitization are the result of drug hypersensitivity. European Journal of Neuroscience, 16, 2462–2468. [DOI] [PubMed] [Google Scholar]
- Vashishtha M, Ng CW, Yildirim F, Gipson TA, Kratter IH, Bodai L, Song W, Lau A, Labadorf A, Vogel-Ciernia A, Troncosco J, Ross CA, Bates GP, Krainc D, Sadri-Vakili G, Finkbeiner S, Marsh JL, Housman DE, Fraenkel E & Thompson LM (2013) Targeting H3K4 trimethylation in Huntington disease. Proceedings of the National Academy of Sciences of the United States of America, 110, E3027–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P & Leyton M (2009) Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology, 56 Suppl 1, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers TA & Crooke ST (2012) siRNAs targeted to certain polyadenylation sites promote specific, RISC-independent degradation of messenger RNAs. Nucleic Acids Research, 40, 6223–6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y & Wong C (2006) Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. Journal of Neuroscience, 26, 6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A & Uchida N (2012) Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron, 74, 858–873. [DOI] [PubMed] [Google Scholar]
- Xenias HS, Ibáñez-Sandoval O, Koós T & Tepper JM (2015). Are striatal tyrosine hydroxylase interneurons dopaminergic? Journal of Neuroscience, 35, 6584–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Feng J, Fienberg AA & Greengard P (1999) D(2) dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proceedings of the National Academy of Sciences of the United States of America, 96, 11607–11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M & Steiner H (2007) Methylphenidate and cocaine: the same effects on gene regulation? Trends in Pharmacological Sciences, 28, 588–596. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM & Costa RM (2009) Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature Neuroscience, 12, 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Nielsen D, Butelman E & Kreek MJ (2005) Microarray studies of psychostimulant-induced changes in gene expression. Addiction Biology, 10, 101–118. [DOI] [PubMed] [Google Scholar]
- Yui K, Ikemoto S, Ishiguro T & Goto K (2000) Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Annals of the New York Academy of Sciences, 914, 1–12. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Zhang L, Fletcher-Turner A & Seroogy KB (2004) Supranigral injection of neuregulin1-beta induces striatal dopamine overflow. Brain Research, 1028, 116–119. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Proudnikov D, Yuferov V & Kreek MJ (2010) Drug-induced and genetic alterations in stress-responsive systems: Implications for specific addictive diseases. Brain Research, 1314, 235–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distance traveled data. Average distance traveled, in 5 min bins, for each mouse on each day.
Measurements of stereotypy and locomotion. Observational scores of the bouts and total time engaged in each behavior for each mouse, on each day that was scored.
Expression measurements for all genes that showed a significant difference on an amphetamine day relative to the average expression across saline days. Normalized sequence read counts are given for all samples on all days.
Data Availability Statement
Data are available from the corresponding author on request.