Abstract
Background.
Risk for atherosclerotic cardiovascular disease (ASCVD) was a novel consideration for antihypertensive medication initiation in the 2017 ACC/AHA Blood Pressure (BP) guideline. Whether biomarkers of chronic myocardial injury [high-sensitivity cardiac troponin T (hs-cTnT) ≥ 6 ng/L] and stress [N-terminal pro-B-type natriuretic peptide (NT-proBNP) ≥ 100 pg/mL)] can inform cardiovascular (CV) risk stratification and treatment decisions among adults with elevated BP and hypertension is unclear.
Methods.
Participant-level data from 3 cohort studies (Atherosclerosis Risk in Communities Study, Dallas Heart Study, and Multiethnic Study of Atherosclerosis) were pooled, excluding individuals with prevalent CV disease and those taking antihypertensive medication at baseline. Participants were analyzed according to BP treatment group from the 2017 ACC/AHA BP guideline and those with high BP (BP:120–159/<100 mm Hg) were further stratified by biomarker status. Cumulative incidence rates for CV event (ASCVD or heart failure), and the corresponding 10-year number needed to treat to prevent one event (NNT10) with intensive BP lowering (to target systolic BP <120 mm Hg), were estimated for BP and biomarker-based subgroups.
Results.
The study included 12,987 participants (mean age:55 years, 55% women, 21.5% with elevated hs-cTnT, 17.7% with elevated NT-proBNP) with 825 incident CV events over 10-year follow-up. Participants with elevated BP or hypertension not recommended for antihypertensive medication with vs. without either elevated hs-cTnT or NT-proBNP had a 10-year CV incidence rate of 11.0% and 4.6%, with an NNT10 for intensive BP lowering of 36 and 85, respectively. Among participants with stage 1 or stage 2 hypertension recommended for antihypertensive medication with BP <160/100 mm Hg, those with vs. without an elevated biomarker had a 10-year CV incidence rate of 15.1% and 7.9%, with an NNT10 of 26 and 49, respectively.
Conclusions.
Elevations in hs-cTnT or NT-proBNP identify individuals with elevated BP or hypertension not currently recommended for antihypertensive medication who are at high risk for CV events. The presence of non-elevated biomarkers, even in the setting of stage 1 or stage 2 hypertension, was associated with lower risk. Incorporation of biomarkers into risk assessment algorithms may lead to more appropriate matching of intensive BP control with patient risk.
Keywords: Hypertension, biomarkers, risk stratification, heart failure, myocardial infarction
INTRODUCTION
Hypertension is common and associated with increased risk of atherosclerotic cardiovascular disease (ASCVD) and heart failure (HF).1–3 Recent evidence suggests that most incident cardiovascular (CV) events occur in adults with blood pressure (BP) <140/90 mm Hg, a level at which there are conflicting recommendations for initiation of antihypertensive medication across clinical practice guidelines.4–7 In the Systolic Blood Pressure Intervention Trial (SPRINT), intensive blood pressure (BP) control targeting a systolic BP <120 mm Hg reduced the risk of adverse CV events, particularly incident HF and CV mortality.8 Accordingly, the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults recommended a lower BP threshold for diagnosing hypertension and initiating antihypertensive medication.7 Implementation of the 2017 ACC/AHA BP guideline has led to a substantial increase in the prevalence of hypertension in the United States (US).9 A large proportion of individuals who are newly identified with elevated BP or hypertension are low-risk, younger adults with intermediate range BP (BP 120–139/<90 mm Hg) who are not currently recommended for antihypertensive medication based on the 2017 ACC/AHA BP guideline.9
The 2017 ACC/AHA BP guideline incorporates 10-year predicted ASCVD risk to determine which individuals with stage 1 hypertension (BP 130–139/80–89 mmHg) are likely to derive the most benefit from antihypertensive medication initiation. However, the ACC/AHA Pooled Cohort Equations (PCE), which are the recommended strategy for assessing ASCVD risk, only provide modest discrimination for ASCVD events, and have overestimated risk in contemporary cohorts.10–13 Importantly, these equations do not assess risk for HF, an important limitation of a tool used to guide initiation of BP lowering medications, given the benefits of intensive BP control on incident HF.8 Thus, there is a need for improved risk stratification strategies to identify individuals with intermediate range BP levels that are at the highest risk for ASCVD and HF events and may benefit from intensive BP control.
Cardiac troponin T (cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are biomarkers of chronic myocardial injury and stress, are detectable in the general population and are associated with adverse CV events.14–17 Mildly elevated levels of these biomarkers identify intermediate phenotypes along the progression from hypertension to clinical HF.18, 19 The utility of these biomarkers to identify individuals with elevated BP or hypertension who may benefit from intensive BP control is not well established. The purpose of this study was to evaluate whether cTnT measured by a high-sensitivity assay (hs-cTnT) and NT-proBNP can improve stratification of CV risk among adults with elevated BP or hypertension according to the 2017 ACC/AHA BP guideline. We hypothesized that elevated biomarker levels would identify individuals with elevated BP or hypertension not currently recommended for treatment who may benefit from antihypertensive medication.
METHODS
The data from the present study will not be made available by the authors for use by other investigators.
Study population
We pooled individual-level data from the following three prospective cohort studies: Atherosclerosis Risk in Communities (ARIC), Dallas Heart Study (DHS), and Multiethnic Study of Atherosclerosis (MESA). Study design, participant characteristics, and event adjudication have been reported previously for each of these cohort studies.20–22 Briefly, ARIC is a prospective, community-based cohort that enrolled 15,792 participants between 45 and 64 years of age from 1987 to 1989 in four communities across the US.20 For the present analysis, ARIC visit 2 (from 1990–1992) was considered the baseline visit as hs-cTnT and NT-proBNP were measured using samples obtained from this visit (n=14,348) 23. DHS is a multiethnic, probability-based cohort study of adults from Dallas County with deliberate oversampling of African Americans.14, 21 Data were collected at 3 separate visits from 2000 to 2002. At the second in-home visit, fasting blood was collected from 3,557 adults, 30 to 65 years of age. MESA is a multiethnic, community-based cohort study that enrolled 6,814 participants, 45 to 84 years of age, with no known CV disease (CVD) from 2000 to 2002 across six US communities.22, 24 The initial phases of DHS and MESA each occurred from 2000 to 2002 and are considered the baseline visits.
The present study included participants from all three cohorts who, at baseline for this study, were free of known CVD (coronary heart disease[CHD], stroke, and HF), not taking antihypertensive medication and had available biomarker (hs-cTnT and NT-proBNP) data and parameters needed to calculate estimated glomerular filtration rate (eGFR) as well as 10-year ASCVD risk according to the ACC/AHA PCE.10 The protocol for each study was approved by the Institutional Review Boards of each participating center. All study participants provided written informed consent. The present analysis was approved by the Institutional Review Board at UT Southwestern and the coordinating centers for each of the cohorts.
Assessment of baseline covariates in the study cohorts
Study participants from all three cohorts underwent comprehensive examinations that included self-reported and measured clinical characteristics using standardized protocols described previously.20–22 Age, sex, race, smoking status, history of hypertension, and medication use were self-reported. Body mass index (BMI), total cholesterol, and high-density lipoprotein cholesterol were measured. Diabetes mellitus and eGFR were defined as previously described.20–22 Left ventricular (LV) hypertrophy (LVH) by 12-lead electrocardiogram was determined according to the Sokolow-Lyon criteria across all three cohorts. In the cohorts with available cardiac MRI data (MESA and DHS), LVH was determined using sex-specific cut-offs for indexed LV mass as defined previously in the DHS (women: LV mass/BSA≥89 g/m2; men: LV mass/BSA≥112 g/m2)18 and MESA (women: LV mass/BSA ≥84.6 g/m2; men: LV mass/BSA ≥106.2 g/m2).25 Subclinical LV dysfunction was also determined in participants with available cardiac MRI data based on LV ejection fraction (EF) <50%. Antihypertensive medication use patterns during follow-up was assessed using data from subsequent visits after the baseline biomarker assessment across the study cohorts (visit 2 for DHS, visit 3 for MESA, and visit 4 for ARIC).
Blood pressure measurement at baseline
Study participants from all three cohorts had their BP measured in the seated position after five minutes of rest.26–28 In ARIC, a technician obtained three BP measurements using a random-zero sphygmomanometer and BP was defined as the mean of the last two BP readings 26. In DHS, a trained professional obtained five seated BP measurements with an automated oscillometric device (Series #52,000, Welch Allyn, Arden, North Carolina) and the mean of the last three BP recordings was used in these analyses.27 In MESA, an automated oscillometric device (Dinamap Monitor Pro100, GE Healthcare, Milwaukee, WI) was used at two-minute intervals to obtain three BP readings and BP was defined as the mean of the last two BP measurements.28
Assessment of biomarkers of interest (hs-cTnT & NT-proBNP)
Both hs-cTnT and NT-proBNP were measured using commercially available immunoassays, with Roche Elecsys 2010 analyzer used in ARIC and DHS, and the Roche Cobas e601 used in MESA (Roche Diagnostics, Indianapolis, IN).14, 15, 29 Based on clinical practice limits of quantification in the US, hs-cTnT ≥6 ng/L was defined as elevated. For NT-proBNP, a level ≥100 pg/mL was considered elevated based on prior studies.30, 31 Data-derived optimal cut points for these biomarker assays to predict incident CV events were also determined using the receiver operator characteristic analysis and the maximum value of Youden’s index and validated using bootstrapping analysis with 1,000 bootstraps.32
Study groups based on 2017 ACC/AHA BP guideline recommendations for treatment & biomarker status
The 2017 ACC/AHA BP guideline identifies three groups based on BP level and indication for antihypertensive medication initiation: 1) normal BP (BP <120/80 mm Hg); 2) elevated BP (BP 120–129/<80 mm Hg) or low-risk stage 1 hypertension (BP 130–139/80–89 mm Hg) not recommended for antihypertensive medication; and 3) high-risk stage 1 hypertension (BP 130–139/80–89 mm Hg) or stage 2 hypertension (BP ≥140/90 mm Hg) recommended for antihypertensive medication. In accordance with the 2017 ACC/AHA BP guideline, high-risk stage 1 hypertension was defined by the presence of any of the following: PCE-estimated 10-year ASCVD risk ≥10%, diabetes mellitus, estimated glomerular filtration rate <60 mL/min per 1.73 m2, or age ≥65 years with systolic BP ≥130 mm Hg.7 In the absence of all of these risk factors, individuals with stage 1 hypertension were classified as low-risk. In the current study, the PCE for non-Hispanic whites were used to estimate 10-year ASCVD risk for participants who identified as non-African American (white, Hispanic, and Asian).
Study participants were categorized into six groups based on a combination of the 2017 ACC/AHA BP guideline recommendation for antihypertensive medication initiation and categories of hs-cTnT or NT-proBNP: Group A: normal BP (<120/80 mm Hg); Group B: elevated BP (120–129/<80 mm Hg) or low-risk stage 1 hypertension (130–139/80–89 mm Hg) with non-elevated biomarkers; Group C: elevated BP (120–129/<80 mm Hg) or low-risk stage 1 hypertension (130–139/80–89 mm Hg) with elevated hs-cTnT and/or NT-proBNP; Group D: high-risk stage 1 hypertension (130–139/80–89 mm Hg) or stage 2 hypertension (140–159/90–99 mm Hg) with non-elevated biomarkers; Group E: high-risk stage 1 hypertension (130–139/80–89 mm Hg) or stage 2 hypertension (140–159/90–99 mm Hg) with elevated hs-cTnT and/or NT-proBNP; and Group F: stage 2 hypertension (≥160/100 mm Hg). As there is general consensus across various guideline recommendations regarding management of individuals with normal BP and those with BP ≥160/100 mm Hg, groups A and F were not stratified by biomarker status.5–7
Primary and secondary outcomes of interest
The primary outcome of interest was an incident CV event defined as a composite of incident ASCVD (non-fatal myocardial infarction [MI], non-fatal stroke, or CV death) or HF. The secondary outcome of interest was incident HF identified by the first HF hospitalization event. In each of the three cohort studies, an expert committee adjudicated all non-fatal CV events as described previously and detailed in the Supplemental Methods I.18, 30, 33, 34
Statistical analysis
Individual-level participant data were merged, and participants were categorized into Groups A through F based on the BP groups and biomarker status (elevated vs. non-elevated). Baseline characteristics were compared across the study groups using Chi-square test for categorical variables and Jonckheere-Terpstra test for continuous variables.
Unadjusted risks of the incident CV event (ASCVD or HF) and HF were assessed across the BP/ biomarker-based study groups using cumulative incidence plots and log-rank tests. The follow-up time was censored at 10 years in the pooled cohort to allow for comparable follow-up time across cohorts. The 10-year number needed to treat (NNT10) to prevent an incident CV event or HF was estimated assuming a 25% and 38% relative risk reduction (RRR) in these outcomes, respectively, based on the treatment effect of intensive BP lowering to target systolic BP <120 mm Hg in SPRINT.8 Given that one-third of the participants in SPRINT had systolic BP ≤132 mm Hg and intensive BP control led to similar reductions in CV event rates regardless of baseline BP level, we used the same RRR across all groups in the present analysis. Based on the 2017 ACC/AHA BP guideline systematic review, we also performed sensitivity analyses using RRRs of 19% and 25% for CV and HF, respectively, to calculate the NNT10 to prevent each outcome.35
Multivariable adjusted analysis was also performed to evaluate the association between the BP/biomarker-based study groups and risk of incident CV event and incident HF independent of other risk factors. Separate Cox models were constructed for each outcome with adjustment for the following potential confounders: demographics (age, sex, race), CV risk factors (BMI, diabetes mellitus status, smoking status), laboratory values (total cholesterol, HDL cholesterol, eGFR), medications (statin use), and study cohort. The contribution of the biomarker-based approach to risk stratification toward the prediction of incident CV and HF event above and beyond the traditional CV risk factors and BP treatment categories was assessed by calculating the Harrell C statistic for adjusted Cox models with the 2017 ACC/AHA BP guideline recommended treatment groups and the BP/biomarker-based study groups. The C statistic derived from the model with 2017 ACC/AHA BP guideline recommended treatment groups was compared with that calculated for the BP/biomarker-based study groups and were compared using bootstrapping method.
Several sensitivity analyses were also performed to assess the robustness of the observed associations between BP/biomarker-based study groups and risk of adverse cardiovascular events. First, to account for heterogeneity in study cohorts, a cohort-stratified pooled analysis was performed by pooling cohort-specific 10-year KM estimates for incident CV events associated with different BP/biomarker-based groups using random effect modeling technique as described previously 36. Second, sensitivity analyses were performed excluding individuals with LVH based on ECG at baseline. Furthermore, in the subset of participants with available cardiac MRI data, sensitivity analyses were also performed excluding individuals with LVH or subclinical LV dysfunction (LV EF <50%). Third, sensitivity analysis was also performed to evaluate the 10-year risk of incident CV event across different BP/biomarker-based groups among participants without any of the following high-risk features: prevalent diabetes mellitus, current smoker, eGFR <60 mL/min per 1.73 m2, and statin use. Finally, race- (black vs. non-black) and sex- (male vs. female) based subgroup analyses were performed examining risk of CV events across the BP/biomarker-based study groups. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA). Two-sided p-values < 0.05 were considered statistically significant without adjustment for multiple comparisons.
Alternative biomarkers of chronic myocardial injury and stress
A similar analytic approach was taken in the Jackson Heart Study (JHS) using the same BP level categories but different biomarkers for chronic myocardial injury and stress. Specifically, cardiac troponin I (cTnI) was assessed using a highly-sensitive assay (hs-cTnI, Roche Diagnostics) and B-type natriuretic peptide (BNP) was measured at the baseline visit 19. JHS design and study participants as well as details regarding assessment of baseline covariates (including BP, hs-cTnI, and BNP) and event adjudication are described in detail in Supplemental Methods II.
RESULTS
The present study included 12,987 participants (mean age 55 years, 55% women). ARIC contributed 50.2% of participants to the final study cohort while DHS and MESA contributed 17.3% and 32.5%, respectively (Supplemental Figure 1). In the pooled cohort, 54.2% of participants had normal BP and 22.8% had elevated BP or low-risk stage 1 hypertension (antihypertensive medication not recommended). The prevalence of high-risk stage 1 hypertension or stage 2 hypertension with BP<160/100 mm Hg (antihypertensive medication recommended) was 19.9% while 3.2% of study participants had stage 2 hypertension with BP ≥160/100 mm Hg in the present study.
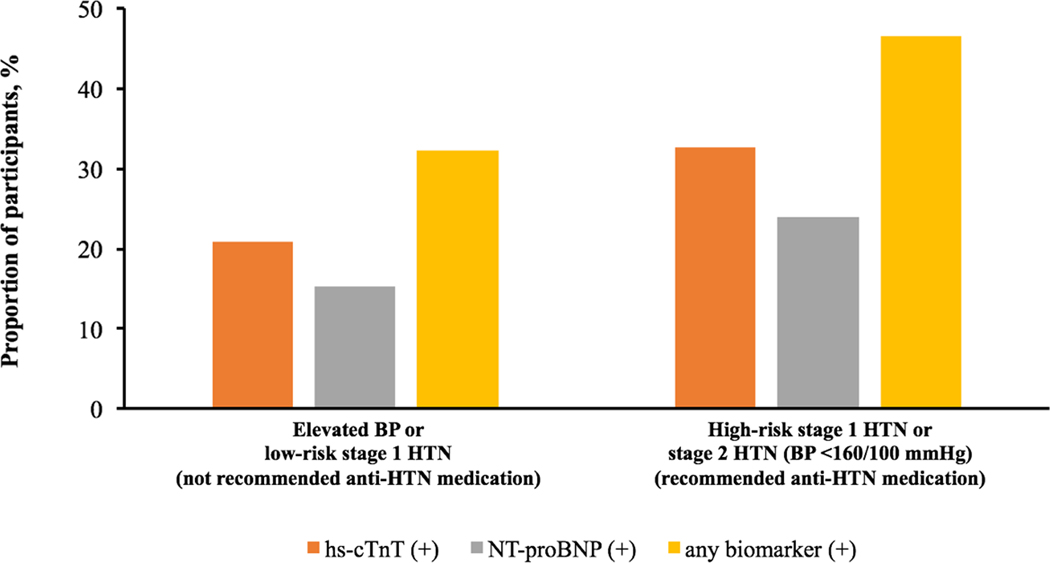
Among all study participants, elevated hs-cTnT and NT-proBNP was noted in 21.5% and 17.7%, respectively. The prevalence of elevated hs-cTnT or NT-proBNP was 32.3% among participants with elevated BP or low-risk stage 1 HTN (not recommended for antihypertensive medication) and 46.5% among those with high-risk stage 1 hypertension or stage 2 hypertension with BP <160/100 mm Hg (recommended for antihypertensive medication) (Figure 1).
Figure 1. Prevalence of elevated hs-cTnT or NT-proBNP across 2017 ACC/AHA BP guideline recommended treatment groups.
Elevated BP: 120–129/<80 mm Hg; stage 1 HTN: 130–139/80–89 mm Hg; stage 2 HTN: ≥140/90 mm Hg; high-risk stage 1 HTN was defined by the presence of any of the following: PCE-estimated 10-year ASCVD risk ≥10%, diabetes mellitus, estimated GFR <60 mL/min per 1.73 m2, or age ≥65 years with systolic BP ≥130 mmHg; in the absence of all of these risk factors, individuals with stage 1 HTN were classified as low-risk; hs-cTnT (+): hs-cTnT ≥6 ng/L; NT-proBNP (+): NT-proBNP ≥100 pg/mL; any biomarker (+): hs-cTnT ≥6 ng/L and/or NT-proBNP ≥100 pg/mL.
Abbreviations: anti-HTN= antihypertensive; ASCVD = atherosclerotic cardiovascular disease; BP = blood pressure; GFR = glomerular filtration rate; hs-cTnT = high-sensitivity cardiac troponin T; HTN = hypertension; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PCE = pooled cohort equation.
Baseline characteristics of the study population categorized by the 2017 ACC/AHA BP guideline recommended treatment groups and according to biomarker status are shown in Table 1. As expected, the burden of traditional CV risk factors was higher in participants with higher BP and those with indications for antihypertensive medication initiation. Participants with vs. without elevated biomarkers were older, more commonly men, and had higher burden of diabetes mellitus, smoking, and LVH. Among participants with any elevated biomarkers across both BP treatment groups, the median biomarker levels were only slightly above the pre-defined cut-offs and well below the clinical thresholds used for diagnosis of myocardial infarction (for hs-cTnT) or heart failure (for NT-proBNP) (Table 1).
Table 1.
Baseline characteristics across 2017 ACC/AHA BP guideline recommended treatment groups stratified by biomarker status
| Not recommended to receive anti-HTN medication | Recommended to receive anti-HTN medication | P value | |||||
|---|---|---|---|---|---|---|---|
| Normal BP (n = 7,038) | Elevated BP or low-risk stage 1 HTN, both biomarker (−) (n = 2,005) | Elevated BP or low-risk stage 1 HTN, any biomarker (+) (n = 955) | High-risk stage 1 HTN or stage 2 HTN (BP <160/100 mm Hg), both biomarker (−) (n = 1,380) | High-risk stage 1 HTN or stage 2 HTN (BP <160/100 mm Hg), any biomarker (+) (n = 1,199) | Stage 2 HTN (BP ≥ 160/100 mm Hg) (n = 410) | ||
| Age, years | 53 (49 – 59) | 52 (47 – 59) | 58 (52 – 64) | 57 (51 – 63) | 63 (57 – 69) | 61 (54 – 68) | <0.001 |
| Men, % | 2,885 (41.0) | 936 (46.7) | 498 (52.2) | 709 (51.4) | 640 (53.4) | 177 (43.2) | <0.001 |
| Black, % | 1,288 (18.3) | 690 (34.4) | 230 (24.1) | 367 (26.6) | 275 (22.9) | 168 (41.0) | <0.001 |
| BMI, kg/m2 | 25.9 (23.3 – 29.1) | 28.0 (25.1 – 31.9) | 27.6 (24.5 – 31.5) | 28.0 (25.1 – 31.4) | 27.4 (24.3 – 31.2) | 28.9 (25.3 – 33) | <0.001 |
| Systolic BP, mm Hg | 108 (102 – 114) | 125 (122 – 129) | 125 (122 – 128) | 138 (132 – 145) | 140 (133 – 147) | 168 (162 – 176) | <0.001 |
| Diastolic BP, mm Hg | 67 (62 – 72) | 77 (72 – 81) | 74 (69 – 78) | 82 (76 – 87) | 80 (73 – 85) | 89 (80 – 98) | <0.001 |
| Total cholesterol, mg/dL | 195 (173 – 222) | 198 (174 – 220) | 194 (174 – 223) | 204 (179 – 229) | 203 (180 – 228) | 196 (176 – 228) | <0.001 |
| HDL cholesterol, mg/dL | 49 (40 – 60) | 47 (40 – 57) | 47 (39 – 61) | 46 (38 – 57) | 47 (40 – 60) | 49 (40 – 58) | <0.001 |
| Estimated GFR, mL/min per 1.73 m2 | 68.9 (58.0 – 85.8) | 79.0 (64.2 – 95.4) | 68.8 (58.9 – 83.3) | 66.9 (57.0 – 85.9) | 67.0 (56.7 – 81.5) | 73.9 (61.4 – 90.4) | 0.25 |
| Diabetes, % | 309 (4.4) | 95 (4.7) | 66 (6.9) | 151 (10.9) | 160 (13.3) | 52 (12.7) | <0.001 |
| Current smoker, % | 1,450 (20.6) | 414 (20.7) | 160 (16.8) | 284 (20.6) | 185 (15.4) | 88 (21.5) | <0.001 |
| Biomarkers | |||||||
| hs-cTnT (+), % | 1,170 (16.6) | 0 (0) | 619 (64.8) | 0 (0) | 840 (70.1) | 167 (40.7) | <0.001 |
| NT-proBNP (+), % | 1,061 (15.1) | 0 (0) | 454 (47.5) | 0 (0) | 619 (51.6) | 162 (39.5) | <0.001 |
| both (+), % | 217 (3.1) | 0 (0) | 118 (12.4) | 0 (0) | 260 (21.7) | 82 (20.0) | <0.001 |
| hs-cTnT, ng/L | 3.0 (1.5 – 4.4) | 1.5 (1.5 – 3.6) | 6.8 (3.0 – 9.0) | 1.5 (1.5 – 4.0) | 7.0 (4.7 – 10.0) | 5.0 (1.5 – 8.1) | <0.001 |
| NT-proBNP, pg/mL | 40.9 (21.4 – 74.9) | 31.9 (15.4 – 53.9) | 90.9 (32.7 – 136.7) | 38.2 (20.8 – 62.0) | 102.0 (41.8 – 156.3) | 76.9 (41.1 – 152.3) | <0.001 |
| Left ventricular hypertrophy by ECG | 256 (3.7) | 153 (7.9) | 87 (9.3) | 120 (8.9) | 134 (11.4) | 96 (24.0) | <0.001 |
| Study group | |||||||
| ARIC | 3,780 (53.7) | 800 (39.9) | 488 (51.1) | 764 (55.4) | 558 (46.5) | 131 (32.0) | <0.001 |
| DHS | 1,124 (16.0) | 619 (30.9) | 126 (13.2) | 210 (15.2) | 74 (6.2) | 88 (21.5) | <0.001 |
| MESA | 2,134 (30.3) | 586 (29.2) | 341 (35.7) | 406 (29.4) | 567 (47.3) | 191 (46.6) | <0.001 |
Data presented as median (interquartile range) or n (percentile). Comparison across groups performed using Chi-square test for categorical variables and Jonckheere-Terpstra test for continuous variables. Normal BP: <120/80 mm Hg; elevated BP: 120–129/<80 mm Hg; stage 1 HTN: 130–139/80–89 mm Hg; stage 2 HTN: ≥140/90 mm Hg; high-risk stage 1 HTN was defined by the presence of any of the following: PCE-estimated 10-year ASCVD risk ≥10%, diabetes mellitus, estimated GFR <60 mL/min per 1.73 m2, or age ≥65 years with systolic BP ≥130 mmHg; in the absence of all of these risk factors, individuals with stage 1 HTN were classified as low-risk; both biomarker (−): hs-cTnT <6 ng/L and NT-proBNP <100 pg/mL; any biomarker (+): hs-cTnT ≥6 ng/L and/or NT-proBNP ≥100 pg/mL; hs-cTnT (+): hs-cTnT ≥6 ng/L; NT-proBNP (+): NT-proBNP ≥100 pg/mL; both biomarkers (+): hs-cTnT ≥6 ng/L and NT-proBNP ≥100 pg/mL. Number of participants with available ECG data used to estimate the proportion with left ventricular hypertrophy by ECG is slightly lower than the total number of participants in each group.
Abbreviations: ARIC = Atherosclerosis Risk in Communities Study; ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; BP = blood pressure; DHS = Dallas Heart Study; ECG: Electrocardiogram; GFR = glomerular filtration rate; HDL = high-density lipoprotein; hs-cTnT = high-sensitivity cardiac troponin T; HTN: hypertension; MESA = Multi-Ethnic Study of Atherosclerosis; NT-proBNP = N-terminal pro-B-type natriuretic peptide
Antihypertensive medication use pattern at the time of the follow-up visit (~5 years from baseline visit) across different BP/biomarker-based groups is shown in Supplemental Figure 2. Antihypertensive medication initiation during follow-up increased across higher baseline BP groups. However, antihypertensive medication use at the follow-up visit was comparable among individuals with vs. without biomarker elevation at baseline across both BP treatment groups.
Incident CV events among the 2017 ACC/AHA BP guideline recommended treatment groups
Overall, there were 825 incident CV events and 261 HF events over a maximum 10-year follow-up. The cumulative incidence of CV events and HF was lowest among participants with normal BP. Among those with elevated BP or hypertension, the incidence of CV events and HF were higher in the subgroup with vs. without guideline recommendations for antihypertensive medication initiation, as expected (Supplemental Figure 3).
Incident CV events across groups stratified by BP level and biomarker status
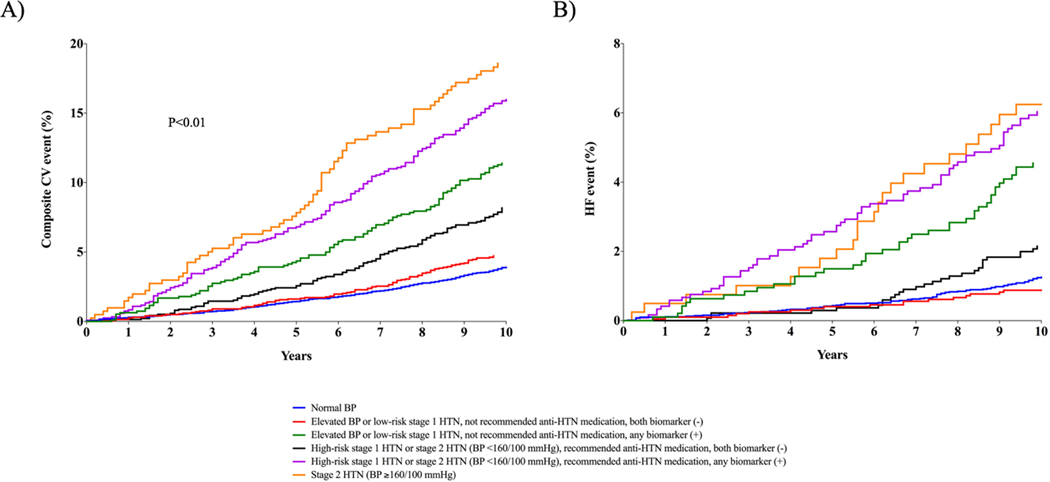
Among participants with elevated BP or hypertension not recommended for antihypertensive medication, the incidence of CV events in the subgroup with non-elevated biomarkers was low and comparable to the incidence observed in the group with normal BP (4.6% vs. 3.8%, respectively; p-value = 0.11) (Figure 2A, Table 2). In contrast, those with elevated (vs. non-elevated) hs-cTnT or NT-proBNP had more than a 2-fold higher incidence of CV events (11.0% vs. 4.6%, respectively). Among participants recommended for antihypertensive medication based on the 2017 ACC/AHA BP guideline with BP <160/100 mm Hg, the risk of a CV event was higher in those with (vs. without) an elevated biomarker (15.1% vs. 7.9%, respectively; p-value <0.01) and approached the risk for participants with BP ≥160/100 mm Hg (17.3%). A similar pattern of association was noted for incident HF, with consistently higher risk among participants with vs. without elevated biomarkers across both treatment recommendation groups (Figure 2B, Table 2). Associations with risk for incident CV events and HF were similar for hs-cTnT and NT-proBNP when each biomarker was considered individually (Supplemental Tables 1 & 2).
Figure 2. Cumulative incidence of composite CV event (ASCVD or HF, Panel A) and HF (Panel B) across 2017 ACC/AHA BP guideline recommended blood pressure treatment groups stratified by presence of elevated hs-cTnT or NT-proBNP.
Normal BP: <120/80 mm Hg; Elevated BP: 120–129/<80 mm Hg; stage 1 HTN: 130–139/80–89 mm Hg; stage 2 HTN: ≥140/90 mm Hg; high-risk stage 1 HTN was defined by the presence of any of the following: PCE-estimated 10-year ASCVD risk ≥10%, diabetes mellitus, estimated GFR <60 mL/min per 1.73 m2, or age ≥65 years with systolic BP ≥130 mmHg; in the absence of all of these risk factors, individuals with stage 1 HTN were classified as low-risk; both biomarker (−): hs-cTnT <6 ng/L and NT-proBNP <100 pg/mL; any biomarker (+): hs-cTnT ≥6 ng/L and/or NT-proBNP ≥100 pg/mL; Composite CV event = non-fatal MI, non-fatal stroke, HF, or CV death.
Abbreviations: anti-HTN= antihypertensive; ASCVD = atherosclerotic cardiovascular disease; BP = blood pressure; CV = cardiovascular; GFR = glomerular filtration rate; HF = heart failure; hs-cTnT = high-sensitivity cardiac troponin T; HTN = hypertension; MI = myocardial infarction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PCE = pooled cohort equation.
Table 2.
Proportion of participants with incident composite CV event (ASCVD or HF) and HF across 2017 ACC/AHA BP guideline recommended treatment groups stratified by biomarker status
| Participant Groups | Number of participants | Incident composite CV events (%) | Incident HF (%) | |
|---|---|---|---|---|
| Not recommended to receive anti-HTN medication | Normal BP | 7,038 | 267 (3.8) | 85 (1.2) |
| Elevated BP or low-risk stage 1 HTN, both biomarker (−) | 2,005 | 92 (4.6) | 17 (0.9) | |
| Elevated BP or low-risk stage 1 HTN, any biomarker (+) | 955 | 105 (11.0) | 41 (4.3) | |
| Recommended to receive anti-HTN medication | High-risk stage 1 HTN or stage 2 HTN (BP <160/100 mm Hg), both biomarker (−) | 1,380 | 109 (7.9) | 28 (2.0) |
| High-risk stage 1 HTN or stage 2 HTN (BP <160/100 mm Hg), any biomarker (+) | 1,199 | 181 (15.1) | 67 (5.6) | |
| Stage 2 HTN (BP ≥160/100 mm Hg) | 410 | 71 (17.3) | 23 (5.7) |
Normal BP: <120/80 mm Hg; elevated BP: 120–129/<80 mm Hg; stage 1 HTN: 130–139/80–89 mm Hg; stage 2 HTN: ≥140/90 mm Hg; high-risk stage 1 HTN was defined by the presence of any of the following: PCE-estimated 10-year ASCVD risk ≥10%, diabetes mellitus, estimated GFR <60 mL/min per 1.73 m2, or age ≥65 years with systolic BP ≥130 mmHg; in the absence of all of these risk factors, individuals with stage 1 HTN were classified as low-risk; both biomarker (−): hs-cTnT <6 ng/L and NT-proBNP <100 pg/mL; any biomarker (+): hs-cTnT ≥6 ng/L and/or NT-proBNP ≥100 pg/mL. Number at risk used to estimate the proportion of participants with incident HF is similar to or slightly lower than the total number of participants in each group.
Composite CV event = non-fatal MI, non-fatal stroke, HF, or CV death.
Abbreviations: anti-HTN = anti-hypertensive; ASCVD = atherosclerotic cardiovascular disease; BP = blood pressure; CV = cardiovascular; GFR: glomerular filtration rate; HF = heart failure; hs-cTnT = high-sensitivity cardiac troponin T; HTN = hypertension; MI = myocardial infarction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PCE = pooled cohort equation
In sensitivity analysis limited to the subset of individuals without LVH by ECG criteria as well as those without LVH or LV dysfunction by cardiac MRI, the patterns of association between BP/biomarker-based groups and risk of incident CV events and HF were consistent with those observed in the overall cohort (Supplemental Tables 3 & 4). In sensitivity analysis excluding individuals with prevalent risk factors including diabetes mellitus, current smoking, eGFR < 60 mL/min per 1.73 m2, and statin use, the pattern of association between BP/biomarker-based study groups and risk of CV events was similar to that observed in the overall cohort (Supplemental Table 5). In sex- and race-based subgroup analyses, elevated biomarkers were associated with higher risk of CV events and HF across both BP treatment groups for both men and women (Supplemental Table 6) as well as both blacks and non-blacks (Supplemental Table 7).
Number needed to treat to prevent a CV event
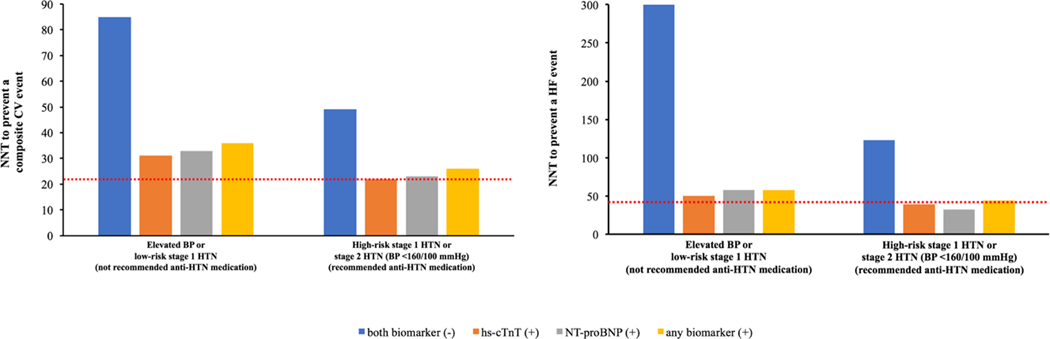
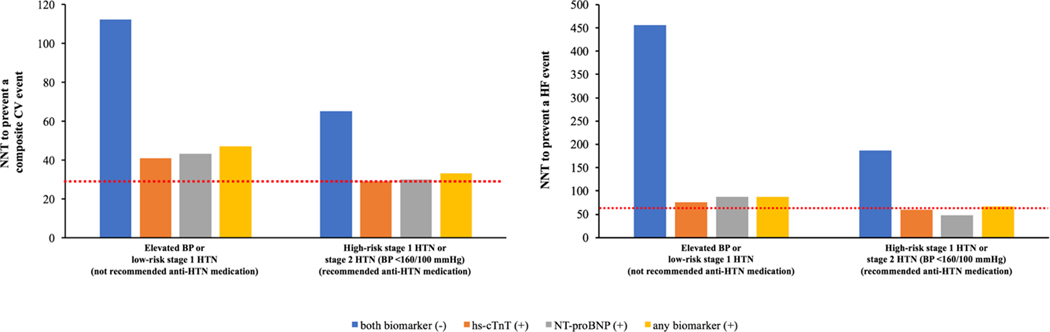
Among participants with elevated BP or hypertension not recommended for antihypertensive medication, the NNT10 for prevention of one CV event, using the SPRINT treatment effect estimates, in those with vs. without an elevated biomarker was 36 and 85, respectively (Figure 3A). Among participants recommended for antihypertensive medication initiation with BP <160/100 mm Hg, the NNT10 for prevention of a CV event in those with vs. without an elevated biomarker was 26 and 49, respectively. The NNT10 to prevent an incident CV event for individuals with BP ≥160/100 mm Hg was 22. The NNT10 to prevent an incident HF event was also lower in individuals with vs. without an elevated biomarker for both BP treatment groups (not recommended for antihypertensive medication: 58 vs. 300; recommended for antihypertensive medication: 44 vs. 123, respectively) (Figure 3B). A similar pattern of results was observed using the RRR from the 2017 ACC/AHA BP guideline systematic review for intensive BP control, with a lower NNT10 to prevent an incident CV event or HF among those with an elevated vs. non-elevated biomarker across both BP treatment groups (Figure 4).
Figure 3. 10-year number needed to treat for prevention of an incident composite CV event (ASCVD or HF, left panel) and HF (right panel) across 2017 ACC/AHA BP guideline recommended treatment groups stratified by biomarker status using the treatment effect of intensive BP control in SPRINT.
The red dotted line represents the 10-year NNT to prevent a composite CV event (left panel) and HF (right panel) for the group with stage 2 HTN (BP ≥160/100 mm Hg) (CV event = 22; HF = 43).
The 10-year number needed to treat (NNT10) to prevent an incident composite CV event and HF was estimated assuming a 25% and 38% relative risk reduction (RRR) in these outcomes, respectively, based on the treatment effect of intensive BP lowering to target systolic BP <120 mm Hg in SPRINT.
Elevated BP: 120–129/<80 mm Hg; stage 1 HTN: 130–139/80–89 mm Hg; stage 2 HTN: ≥140/90 mm Hg; high-risk stage 1 HTN was defined by the presence of any of the following: PCE-estimated 10-year ASCVD risk ≥10%, diabetes mellitus, estimated GFR <60 mL/min per 1.73 m2, or age ≥65 years with systolic BP ≥130 mmHg; in the absence of all of these risk factors, individuals with stage 1 HTN were classified as low-risk; both biomarker (−): hs-cTnT <6 ng/L and NT-proBNP <100 pg/mL; hs-cTnT (+): hs-cTnT ≥6 ng/L; NT-proBNP (+): NT-proBNP ≥100 pg/mL; any biomarker (+): hs-cTnT ≥6 ng/L and/or NT-proBNP ≥100 pg/mL; composite CV event = non-fatal MI, non-fatal stroke, HF, or CV death.
Abbreviations: anti-HTN= antihypertensive; ASCVD = atherosclerotic cardiovascular disease; BP = blood pressure; CV = cardiovascular; GFR = glomerular filtration rate; HF = heart failure; hs-cTnT = high-sensitivity cardiac troponin T; HTN = hypertension; MI = myocardial infarction; NNT: number needed to treat; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PCE = pooled cohort equation; SPRINT = Systolic Blood Pressure Intervention Trial.
Figure 4: 10-year number needed to treat for prevention of an incident composite CV event (ASCVD or HF, left panel) and HF (right panel) across 2017 ACC/AHA BP guideline recommended treatment groups stratified by biomarker status using the treatment effect of intensive BP control in the 2017 ACC/AHA BP guideline systematic review.
The red dotted line represents the 10-year NNT to prevent an incident composite CV event (left panel) and HF (right panel) for the group with stage 2 HTN (BP ≥160/100 mm Hg) (CV event = 29; HF = 65).
Based on the 2017 ACC/AHA BP guideline systematic review, a 19% and 25% relative risk reduction was used to calculate the NNT10 for prevention of an incident composite CV event and HF, respectively.
Elevated BP: 120–129/<80 mm Hg; stage 1 HTN: 130–139/80–89 mm Hg; stage 2 HTN: ≥140/90 mm Hg; high-risk stage 1 HTN was defined by the presence of any of the following: PCE-estimated 10-year ASCVD risk ≥10%, diabetes mellitus, estimated GFR <60 mL/min per 1.73 m2, or age ≥65 years with systolic BP ≥130 mmHg; in the absence of all of these risk factors, individuals with stage 1 HTN were classified as low-risk; both biomarker (−): hs-cTnT <6 ng/L and NT-proBNP <100 pg/mL; hs-cTnT (+): hs-cTnT ≥6 ng/L; NT-proBNP (+): NT-proBNP ≥100 pg/mL; any biomarker (+): hs-cTnT ≥6 ng/L and/or NT-proBNP ≥100 pg/mL; composite CV event = non-fatal MI, non-fatal stroke, HF, or CV death.
Abbreviations: anti-HTN= antihypertensive; ASCVD = atherosclerotic cardiovascular disease; BP = blood pressure; CV = cardiovascular; GFR = glomerular filtration rate; HF = heart failure; hs-cTnT = high-sensitivity cardiac troponin T; HTN = hypertension; MI = myocardial infarction; NNT = number needed to treat; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PCE = pooled cohort equation.
In sensitivity analysis pooling cohort-specific 10-year KM estimates, the NNT10 to prevent incident CV events across different BP/biomarker-based study groups were similar to that observed in the primary analysis (Supplemental Figure 4).
Optimal data-derived biomarker assay cut points for risk of CV events
Data-derived optimal hs-cTnT and NT-proBNP cut points for predicting incident CV events and HF as derived by Youden’s statistic and validated using 1000 bootstraps are shown in Supplemental Table 8. For predicting an incident CV event, the optimal cut points for hs-cTnT and NT-proBNP were 8.5 ng/L and 95.9 pg/mL respectively. The optimal cut points for predicting an incident HF event were 7.7 ng/L for hs-cTnT and 102.3 pg/mL for NT-proBNP.
Adjusted association between BP/biomarker groups and risk of CV event
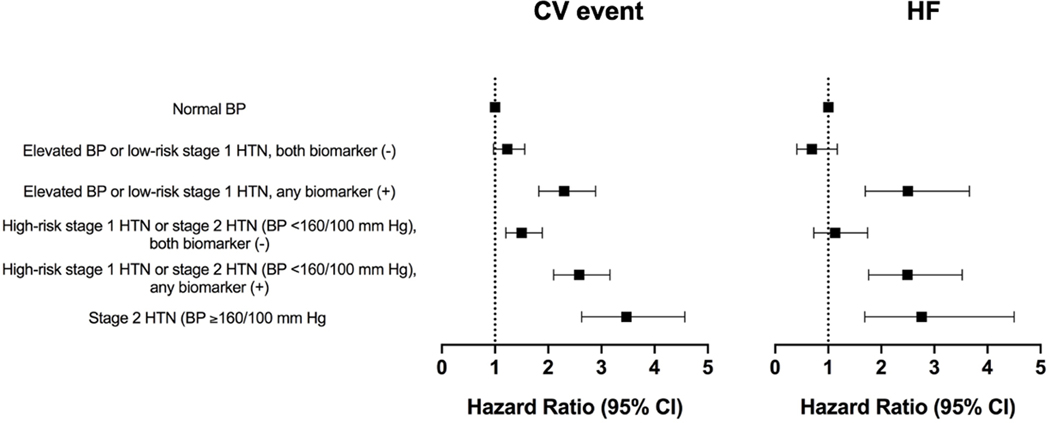
In adjusted analysis, compared with the normotensive group, the risk of incident CV event increased in a graded fashion across increasing BP groups, with a higher risk noted among individuals with (vs. without) elevated biomarkers across both BP treatment groups. The gradient in adjusted risk among individuals with (vs. without) biomarker elevation was even more pronounced for incident HF with a significantly higher risk noted only among those with biomarker elevation across both BP treatment groups (Figure 5). Compared with the 2017 ACC/AHA BP guideline recommended treatment groups, the BP/biomarker-based groups demonstrated a modest but statistically significant improvement in discrimination for incident CV events (C-index=0.732 vs. 0.745; p-value <0.001) and a more substantial improvement in discrimination for incident HF event (C-index=0.727 vs. 0.763; p-value <0.001).
Figure 5: Multivariable adjusted association between the 2017 ACC/AHA BP guideline recommended treatment groups stratified by biomarker status and incident composite CV event (ASCVD or HF) and HF.
Multivariable adjusted Cox models were constructed to evaluate the association between the BP/biomarker-based study groups and risk of outcome [composite CV event (non-fatal MI, non-fatal stroke, HF, or CV death) or HF (referent group = normal BP)] with adjustment for the following potential confounders: demographics (age, sex, race), CV risk factors (BMI, diabetes mellitus status, smoking status), laboratory values (total cholesterol, HDL cholesterol, estimated glomerular filtration rate), medications (statin use), and study cohort. Elevated BP: 120–129/<80 mm Hg; stage 1 HTN: 130–139/80–89 mm Hg; stage 2 HTN: ≥140/90 mm Hg; high-risk stage 1 HTN was defined by the presence of any of the following: PCE-estimated 10-year ASCVD risk ≥10%, diabetes mellitus, estimated GFR <60 mL/min per 1.73 m2, or age ≥65 years with systolic BP ≥130 mmHg; in the absence of all of these risk factors, individuals with stage 1 HTN were classified as low-risk; any biomarker (+): hs-cTnT ≥6 ng/L and/or NT-proBNP ≥100 pg/mL.
Abbreviations: ASCVD = atherosclerotic cardiovascular disease; BMI: body mass index; BP = blood pressure; CV = cardiovascular; GFR: glomerular filtration rate; HDL: high density lipoprotein; HF = heart failure; hs-cTnT = high-sensitivity cardiac troponin T; HTN = hypertension; MI = myocardial infarction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PCE: pooled cohort equation.
Alternative measures of chronic myocardial injury and stress
To further assess a biomarker-based approach to CV risk prediction in the context of the 2017 ACC/AHA BP guideline, we evaluated 1,626 JHS participants without baseline CVD who were not taking antihypertensive medication and had alternative markers of chronic myocardial injury and stress available (cTnI and BNP, respectively) (Supplemental Figure 5, Supplemental Table 9). There were 117 incident adverse CV events (non-fatal MI, non-fatal stroke, HF or all-cause death) over 10-year follow-up. Similar to the primary study population, among participants with high BP levels (BP 120–159/<100 mmHg), 10-year CV event rates were higher among individuals with elevated vs. non-elevated biomarkers, whether they were not recommended (4.6 vs. 7.5%) or recommended (10.9 vs. 20.6%) for antihypertensive medication based on the 2017 ACC/AHA BP guideline (Supplemental Table 10).
DISCUSSION
In this large, pooled analysis of 3 cohort studies, there was substantial heterogeneity in the burden of chronic myocardial injury or stress, as defined by elevated hs-cTnT or NT-proBNP, respectively, among participants with elevated BP or hypertension. For example, approximately one-third of individuals with elevated BP or stage 1 hypertension currently not recommended for antihypertensive medication had elevated levels of at least one biomarker, while more than half of individuals currently recommended for antihypertensive medication with BP <160/100 mm Hg had no elevated biomarkers. Elevated levels of these biomarkers identified individuals at notably higher risk for adverse CV events and who thus would be expected to benefit more from initiation of BP lowering medications or targeting of more intensive BP goals. This was observed both among participants with and those without current guideline recommended indications for initiating antihypertensive medication. The absence of elevated biomarker levels identified lower 10-year CV risk among participants with elevated BP or hypertension. Among participants with elevated BP or low-risk stage 1 hypertension, non-elevated biomarkers were seen in approximately two-thirds, and in this group CV risk was comparable with the group with normal BP. Taken together, the study findings highlight the potential usefulness of a biomarker-based strategy to assist with BP treatment decisions among patients with elevated BP or hypertension.
Prior studies have demonstrated that chronic myocardial injury is associated with a higher risk of CV events across all BP levels.33 The current findings extend prior observations regarding the prognostic utility of biomarkers in CV risk stratification, by considering the important context of the 2017 ACC/AHA BP guideline.33, 37 Specifically, a biomarker-based approach can improve risk stratification for CV events among individuals with elevated BP and hypertension with intermediate range BP levels. Based on the treatment effects derived from the SPRINT trial, the estimated NNT for prevention of a CV event among individuals with BP 120–159/<99 mm Hg and an elevated biomarker was comparable to that observed among individuals with BP ≥160/100 mm Hg. In contrast, the absence of elevated biomarkers identifies a lower-risk subset with a high NNT to prevent a CV event.
The results from the current study have important clinical implications. The current BP guideline has identified an additional 31.1 million individuals with hypertension in the US, of whom 4.2 million are newly recommended for antihypertensive medication.9 Our study findings suggest that supplementing the current guideline recommendations for antihypertensive medication initiation with a biomarker-based approach may help direct BP lowering medication to the highest risk individuals and thus maximize absolute CVD risk reduction (and lower NNT). An approach targeting medication initiation to those with the lowest NNT would have favorable risk- and cost-benefit implications. Along these lines, we observed that approximately one-third of the study participants with elevated BP or low-risk stage 1 HTN that are not currently recommended for antihypertensive medication had an elevated biomarker and higher CV risk. These individuals would be expected to benefit from initiation of antihypertensive pharmacotherapies based on the CV risk-based BP treatment approach recommended in the 2017 ACC/AHA BP guideline. Hs-cTnT and NT-proBNP testing are available in most countries worldwide at a relatively low cost. Considering the well-established cost-effectiveness of intensive BP control in individuals with high CV risk, similar to that observed in individuals with elevated biomarkers, it is anticipated that a biomarker driven approach to guide antihypertensive therapy initiation would be cost-effective in this group without current indications for therapy.38 We also found that among individuals with stage 1 or 2 hypertension with BP <160/100 mm Hg currently recommended for antihypertensive pharmacotherapies, the absence of elevated biomarkers identified a lower risk subgroup of participants. However, considering the well-established evidence in favor of BP treatment, particularly for individuals with systolic BP ≥140 mm Hg, 5–7, 39 an approach that continues to favor initiating antihypertensive therapies in the absence of any contraindications may be considered, with selective use of biomarker testing to better inform the shared-decision making process among individuals with any clinical or patient-reported concerns related to treatment.
Similar to the biomarker-based approach used in our study, recent studies have demonstrated that coronary artery calcium (CAC), a marker of subclinical atherosclerosis, may also be used to identify high-risk individuals with hypertension that may benefit from intensive BP control.40, 41 While the role of CAC in ASCVD risk prediction is well-established, there are limited studies that have examined the independent association between CAC and HF risk, and CAC testing is more difficult to apply broadly than blood tests.42 In contrast, our study findings suggest that simple, relatively inexpensive blood tests to identify chronic myocardial injury or stress may provide prognostic information regarding both ASCVD and HF risk. Future clinical trials are needed to confirm the clinical utility and cost-effectiveness of biomarker-based assessment strategies to guide BP management among individuals with hypertension and intermediate range BP. It is noteworthy that intensive BP control is but one possible intervention that may modify risk among individuals identified at higher risk based on elevated biomarker levels. Other strategies including, but not limited to, intensive lifestyle interventions, intensive lipid lowering, sodium glucose cotransporter-2 inhibitors for type 2 DM may also be considered to further modify the risk of adverse cardiovascular events in these high-risk individuals. The abundance of effective therapies for CV risk reduction, targeting distinct modifiable biological pathways, argues for a more personalized approach to primary prevention that focuses on appropriate matching of the available treatment strategies to the underlying burden and pathophysiological drivers of the CV risk.43
The primary strengths of our study include the large sample size, objective measurement of hs-cTnT and NT-proBNP in the primary analysis of the pooled cohorts, long follow-up duration, and adjudicated CV events. Additionally, our study cohort included nearly equal proportions of men and women with a large proportion of black participants. Furthermore, we validated the approach, using alternative biomarkers of chronic myocardial injury and stress (hs-cTnI and BNP) in a separate cohort, highlighting the robustness of the biomarker-based risk-stratification approach.
Several limitations to our study are noteworthy. First, participants included in this analysis were not taking antihypertensive medication prior to the baseline visit but were initiated on pharmacotherapies for BP treatment on follow-up. Antihypertensive medication initiation would be expected to lower incident CV event rates and may result in underestimation of CV risk in the present analysis. In the present study, the anti-hypertensive initiation during follow-up increased with higher baseline BP but was comparable among those with vs. without elevated biomarkers at baseline across both BP treatment groups. This suggests that the marked differences in the risks of adverse CV events among those with vs. without elevated biomarkers are unlikely to be related to the use of antihypertensive medications on follow-up. Second, BP assessment techniques varied slightly across different cohorts. Each study measured BP with a unique device, but BP was measured in the seated position after a rest period and the mean of multiple measurements was used in analyses. Third, we do not have data on risk of renal outcomes and safety endpoints (syncope, falls) that may also be influenced by BP levels, biomarkers, and intensive BP control. Fourth, we did not include albuminuria in the definition of chronic kidney disease in the present study due to non-uniform urine albumin assessment across cohorts. Fourth, the study cohort was relatively young, and these findings may not be generalizable to other populations that have distinct clinical characteristics from our cohort. Finally, owing to the observational nature of the study, there is a potential for residual confounding due to measured or unmeasured risk factors like subclinical coronary artery disease, silent ischemia or cardiomyopathy at baseline. However, the higher risk of adverse cardiovascular events among those with vs. without biomarker elevation across both BP treatment groups persisted in adjusted analysis accounting for other potential confounders and sensitivity analyses excluding participants with LVH and subclinical LV dysfunction. Furthermore, consistent patterns of associations were also observed in sensitivity analysis excluding higher risk individuals with comorbidities highlighting the robustness of our study findings.
In conclusion, elevated hs-cTnT or NT-proBNP identifies individuals with elevated BP and stage 1 hypertension not currently recommended for BP lowering medication who are at higher risk for CV events and may benefit from initiation of antihypertensive medication. In contrast, among individuals with intermediate BP levels and non-elevated biomarkers, event rates are comparable to those with normal BP. Incorporation of biomarkers into risk assessment algorithms may lead to more appropriate matching of intensive BP control with patient risk. Future clinical trials are needed to evaluate biomarker-based strategies for CV risk assessment to guide BP treatment decisions.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Approximately one-third of adults with elevated BP or stage 1 hypertension who are not recommended for antihypertensive medication initiation according to the 2017 ACC/AHA BP guideline had elevated hs-cTnT or NT-proBNP, and these individuals had more than 10% risk of ASCVD or HF over the 10 year follow-up period.
Among adults recommended antihypertensive medication with BP <160/100 mm Hg, more than one-half of individuals had non-elevated hs-cTnT and NT-proBNP, which was associated with less than 10% risk of an adverse CV event.
What are the clinical implications?
A biomarker-based approach to CV risk assessment may help identify high risk individuals with elevated BP or stage 1 HTN who are currently not recommended antihypertensive medication according to the 2017 ACC/AHA BP guideline, but who may benefit from BP lowering therapy.
Acknowledgements:
The authors thank the participants, staff, and investigators of the ARIC, DHS, JHS, and MESA studies for their important contributions.
Relevant disclosures of funding & conflicts of interest:
Dr. Ballantyne has grant/research support (all significant; all paid to institution, not individual) from Akcea, Amarin, Amgen, Esperion, Novartis, Regeneron, Sanofi-Synthelabo, National Institute of Health, American Heart Association, and American Diabetes Association and is a consultant for Akcea, Amarin, Amgen, Astra Zeneca (significant), Eli Lilly, Esperion, Matinas BioPharma Inc, Merck (significant), Novartis, Regeneron, and Sanofi-Synthelabo (significant). In addition, Dr. Ballantyne had a patent to patent 61721475 (“Biomarkers to Improve Prediction of Heart Failure Risk”) filed by Baylor College of Medicine and Roche pending. Dr. Berry reported grants from NIH (1RO1HL144112-01) Abbott and Roche (non-financial support for biomarker-related research activities) and serves as a National Coordinator for the STRENGTH trial sponsored by Astra Zeneca. Dr. Blaha is supported by U.S. National Institutes of Health/National Heart Lung and Blood Institute grant L30 HL110027. Dr. Butler has received research support from the National Institutes of Health, Patient-Centered Outcomes Research Institute, and the European Union; and serves as a consultant for Amgen, Array, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squib, CVRx, G3 Pharmaceutical, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Relypsa, StealthPeptide, SC Pharma, Vifor, ZS Pharma. Dr. deFilippi reported grants from Roche Diagnostics, Abbott Diagnostics, FujiRebio, and Siemens Healthcare Diagnostics and personal fees from Alere, Radiometer, Ortho Diagnostics, UpToDate, WebMD, Siemens Healthcare, Roche Diagnostics, and Metabolomics. In addition, Dr. deFilippi had a patent to US20170234888 issued. Dr. deFilippi and de Lemos had a patent to US20170234888 issued. Dr. de Lemos reported grants from Abbott Diagnostics and Roche Diagnostics; personal fees from Abbott Diagnostics, Roche Diagnostics, Ortho Clinical Diagnostics, Quidel, Siemen’s Health Care Diagnostics and Radiometer.
. Dr. Joshi reported grants from AHA, Novo Nordisk, AstraZeneca, GSK, Sanofi, Regeneron, Pfizer; personal fees from Bayer, Regeneron. Dr. McEvoy is the recipient of an American Heart Association award (17MCPRP33400031) and is supported by both the P.J. Schafer Cardiovascular Research Fund and the Johns Hopkins Magic That Matters Research Fund for Cardiovascular Research. Dr. Mentz reported grants and personal fees from Novartis, Amgen, and AstraZeneca; receives research support from the National Institutes of Health(grantsU01HL125511-01A1, U10HL110312, and R01AG045551-01A1), Akros, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, Luitpold, Medtronic, Merck, Novartis, Otsuka, and ResMed; receives honoraria from Abbott, Amgen, AstraZeneca, Bayer, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, and ResMed; and has served on an advisory board for Amgen, Luitpold, Merck and Boehringer Ingelheim. Dr. Muntner has received research support and honoraria from Amgen. Dr. Nambi serves as the site principle investigator on a study sponsored by Merck, holds a provisional patent on biomarkers for the prediction of heart failure along with Baylor College of Medicine and Roche, and is an event adjudicator on a study sponsored by Siemens. Dr. Pandey is supported by the Texas Health Resources Clinical Scholarship. Dr. Patel is supported by NHLBI T32 postdoctoral training grant (5T32HL125247-03). Dr. Seliger reported grants from Roche Diagnostics and personal fees from Abbvie Inc. In addition, Dr. Seliger had a patent to “Methods for Assessing Differential Risk of Developing Heart” pending. Dr. Muthiah Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), serves on advisory boards for Amgen, AstraZeneca, Bayer AG, and Baxter Healthcare, and participates in clinical endpoints committees for studies supported by Novartis and the NIH. Dr. Vongpatanasin is supported by R01HL133179, R01AG057571, and P30DK079328. Dr. Selvin was supported by NIH/NIDDK grants K24DK106414 and R01DK089174.
Study Funding
This study was supported by the Texas Health Resources Clinical Scholarship to Dr. Pandey.
The ARIC study is conducted as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN2 68201100006C, HHSN268201100007C, HHSN268201100008C, HHSN26820 1100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201 100012C). The Dallas Heart Study was funded by a grant from the Donald W. Reynolds Foundation. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105 to the University of Texas Southwestern Medical Center. The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities (NIMHD). A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The Multi-Ethnic Study of Atherosclerosis was supported by R01 HL071739 and contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, and N01HC 95169 from the National Heart, Lung, and Blood Institute. Reagents for the NT-proBNP and high sensitivity cardiac troponin T assays were donated by Roche Diagnostics
ABBRIVIATIONS
- ASCVD
Atherosclerotic cardiovascular disease (ASCVD)
- PCE
Pooled Cohort Equations
- cTnT
Cardiac troponin T
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- ARIC
Atherosclerosis Risk in Communities
- DHS
Dallas Heart Study (DHS)
- MESA
Multiethnic Study of Atherosclerosis (MESA)
- CVD
Cardiovascular disease
- eGFR
Estimated glomerular filtration rate
- LV
Left ventricle
- LVH
Left ventricular hypertrophy
- NNT10
10-year number needed to treat
- RRR
Relative risk reduction
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S . Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e66. [DOI] [PubMed] [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R and Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Larson MG, Vasan RS, Kannel WB and Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62. [PubMed] [Google Scholar]
- 4.Tajeu GS, Booth JN 3rd, Colantonio LD, Gottesman RF, Howard G, Lackland DT, O’Brien EC, Oparil S, Ravenell J, Safford MM, Seals SR, Shimbo D, Shea S, Spruill TM, Tanner RM and Muntner P. Incident Cardiovascular Disease Among Adults With Blood Pressure <140/90 mm Hg. Circulation. 2017;136:798–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr., Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr., Narva AS and Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20. [DOI] [PubMed] [Google Scholar]
- 6.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I and Group ESCSD. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 7.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/ PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 8.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK and Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr. and Whelton PK. Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation. 2018;137:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr., Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr., Tomaselli GF and American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. [DOI] [PubMed] [Google Scholar]
- 11.Kavousi M, Leening MJ, Nanchen D, Greenland P, Graham IM, Steyerberg EW, Ikram MA, Stricker BH, Hofman A and Franco OH. Comparison of application of the ACC/AHA guidelines, Adult Treatment Panel III guidelines, and European Society of Cardiology guidelines for cardiovascular disease prevention in a European cohort. JAMA. 2014;311:1416–23. [DOI] [PubMed] [Google Scholar]
- 12.DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K and Blaha MJ. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rana JS, Tabada GH, Solomon MD, Lo JC, Jaffe MG, Sung SH, Ballantyne CM and Go AS. Accuracy of the Atherosclerotic Cardiovascular Risk Equation in a Large Contemporary, Multiethnic Population. J Am Coll Cardiol. 2016;67:2118–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA and McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J and Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M and Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA and Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. [DOI] [PubMed] [Google Scholar]
- 18.Neeland IJ, Drazner MH, Berry JD, Ayers CR, deFilippi C, Seliger SL, Nambi V, McGuire DK, Omland T and de Lemos JA. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol. 2013;61:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey A, Keshvani N, Ayers C, Correa A, Drazner MH, Lewis A, Rodriguez CJ, Hall ME, Fox ER, Mentz RJ, deFilippi C, Seliger SL, Ballantyne CM, Neeland IJ, de Lemos JA and Berry JD. Association of Cardiac Injury and Malignant Left Ventricular Hypertrophy With Risk of Heart Failure in African Americans: The Jackson Heart Study. JAMA Cardiol. 2019;4:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 21.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH and Dallas Heart Study I. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. [DOI] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 23.McEvoy JW, Chen Y, Nambi V, Ballantyne CM, Sharrett AR, Appel LJ, Post WS, Blumenthal RS, Matsushita K and Selvin E. High-Sensitivity Cardiac Troponin T and Risk of Hypertension. Circulation. 2015;132:825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters MN, Seliger SL, Christenson RH, Hong-Zohlman SN, Daniels LB, Lima JAC, de Lemos JA, Neeland IJ and deFilippi CR. “Malignant” Left Ventricular Hypertrophy Identifies Subjects at High Risk for Progression to Asymptomatic Left Ventricular Dysfunction, Heart Failure, and Death: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7: e006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong AC, Gjesdal O, Almeida A, Nacif M, Wu C, Bluemke DA, Brumback L and Lima JA. Left ventricular mass and hypertrophy by echocardiography and cardiac magnetic resonance: the multi-ethnic study of atherosclerosis. Echocardiography. 2014;31:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yano Y, Griswold M, Wang W, Greenland P, Lloyd-Jones DM, Heiss G, Gottesman RF and Mosley TH. Long-Term Blood Pressure Level and Variability From Midlife to Later Life and Subsequent Cognitive Change: The ARIC Neurocognitive Study. J Am Heart Assoc. 2018;7:e009578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, Khera A, McGuire DK, de Lemos JA and Turer AT. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol. 2014;64:997–1002. [DOI] [PubMed] [Google Scholar]
- 28.Shimbo D, Shea S, McClelland RL, Viera AJ, Mann D, Newman J, Lima J, Polak JF, Psaty BM and Muntner P. Associations of aortic distensibility and arterial elasticity with long-term visit-to-visit blood pressure variability: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Hypertens. 2013;26:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seliger SL, Hong SN, Christenson RH, Kronmal R, Daniels LB, Lima JAC, de Lemos JA, Bertoni A and deFilippi CR. High-Sensitive Cardiac Troponin T as an Early Biochemical Signature for Clinical and Subclinical Heart Failure: MESA (Multi-Ethnic Study of Atherosclerosis). Circulation. 2017;135:1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Lemos JA, Ayers CR, Levine BD, deFilippi CR, Wang TJ, Hundley WG, Berry JD, Seliger SL, McGuire DK, Ouyang P, Drazner MH, Budoff M, Greenland P, Ballantyne CM and Khera A. Multimodality Strategy for Cardiovascular Risk Assessment: Performance in 2 Population-Based Cohorts. Circulation. 2017;135:2119–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Florido R, Kwak L, Lazo M, Nambi V, Ahmed HM, Hegde SM, Gerstenblith G, Blumenthal RS, Ballantyne CM, Selvin E, Folsom AR, Coresh J and Ndumele CE. Six-Year Changes in Physical Activity and the Risk of Incident Heart Failure: ARIC Study. Circulation. 2018;137:2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 33.Pokharel Y, Sun W, de Lemos JA, Taffet GE, Virani SS, Ndumele CE, Mosley TH, Hoogeveen RC, Coresh J, Wright JD, Heiss G, Boerwinkle EA, Bozkurt B, Solomon SD, Ballantyne CM and Nambi V. High-sensitivity troponin T and cardiovascular events in systolic blood pressure categories: atherosclerosis risk in communities study. Hypertension. 2015;65:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G and Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reboussin DM, Allen NB, Griswold ME, Guallar E, Hong Y, Lackland DT, Miller EPR 3rd, Polonsky T, Thompson-Paul AM and Vupputuri S. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:2176–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P and Berry JD. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J Am Coll Cardiol. 2017;69:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setsuta K, Kitahara Y, Arae M, Ohbayashi T, Seino Y and Mizuno K. Elevated cardiac troponin T predicts adverse outcomes in hypertensive patients. Int Heart J. 2011;52:164–9. [DOI] [PubMed] [Google Scholar]
- 38.Bress AP, Bellows BK, King JB, Hess R, Beddhu S, Zhang Z, Berlowitz DR, Conroy MB, Fine L, Oparil S, Morisky DE, Kazis LE, Ruiz-Negron N, Powell J, Tamariz L, Whittle J, Wright JT Jr., Supiano MA, Cheung AK, Weintraub WS, Moran AE and Group SR. Cost-Effectiveness of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2017;377:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, Reboldi G and Cardio-Sis i. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525–33. [DOI] [PubMed] [Google Scholar]
- 40.McEvoy JW Jr., Martin SS, Dardari ZA, Miedema MD, Sandfort V, Yeboah J, Budoff MJ, Goff DC, Psaty BM, Post WS, Nasir K, Blumenthal RS and Blaha MJ. Coronary Artery Calcium to Guide a Personalized Risk-Based Approach to Initiation and Intensification of Antihypertensive Therapy. Circulation. 2017;135:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uddin SMI, Mirbolouk M, Kianoush S, Orimoloye OA, Dardari Z, Whelton SP, Miedema MD, Nasir K, Rumberger JA, Shaw LJ, Berman DS, Budoff MJ, McEvoy JW, Matsushita K, Blaha MJ and Graham G. Role of Coronary Artery Calcium for Stratifying Cardiovascular Risk in Adults With Hypertension. Hypertension.2019;73:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latif MA and Budoff MJ. Coronary artery calcium scanning: a useful tool for refining heart failure risk prediction? Future Cardiol. 2013;9:1–3. [DOI] [PubMed] [Google Scholar]
- 43.Patel KV, Pandey A and de Lemos JA. Conceptual Framework for Addressing Residual Atherosclerotic Cardiovascular Disease Risk in the Era of Precision Medicine. Circulation. 2018;137:2551–2553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.