Supplemental Digital Content is available in the text.
Abstract
Coronavirus disease 2019 (COVID-19) has a high incidence of cardiac involvement, commonly described as a new-onset cardiomyopathy. In this report, we describe a patient with a new manifestation of cardiac involvement in the setting of a COVID-19 diagnosis: that of takotsubo cardiomyopathy.
Cardiac involvement is a prominent feature of coronavirus disease 2019 (COVID-19) and is associated with increased mortality risk. New-onset cardiac disease may be a worse prognosticator of poor outcome than advanced age, diabetes, chronic pulmonary disease, or previous cardiovascular disease. Cardiac involvement in this population typically involves acute systolic dysfunction. This case demonstrates that COVID-19 may result in uncommon patterns of heart failure.
This manuscript adheres to the CAse REport (CARE) guidelines of the Enhancing the QUAlity and Transparency Of health Research (EQUATOR) network.
This manuscript was prepared in compliance with Health Insurance Portability and Accountability Act (HIPAA) privacy regulations and contained no protected health information.
Institutional HIPAA authorization was obtained from the patient’s next of kin.
Institutional review board (IRB) exemption was obtained for this manuscript.
CASE DESCRIPTION
We present a 59-year-old woman with a history of hypertension, previous 70 pack-year smoking history, and severe chronic obstructive pulmonary disease (COPD) on prophylactic azithromycin who presented to our emergency department with 5 days of progressive dyspnea, cough, wheezing, fever, chills, myalgias, poor oral intake, and diarrhea. On presentation, she was normotensive, afebrile, with normal pulse oximetry (Spo2). She was mildly tachypneic with an otherwise unremarkable examination.
Her admission laboratory data work was notable for hyponatremia, metabolic acidosis, and elevated inflammatory markers (Table 1), as is commonly found in COVID-19 patients. Viral respiratory panel was negative. Portable chest x-ray showed right middle and lower lung infiltrates with unremarkable cardiomediastinal silhouette. A COVID-19 polymerase chain reaction (PCR) was sent.
Table 1.
Clinical Laboratory Results From Morning Laboratories
| Measure | Reference Range | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Ferritin | 13–150 ng/mL | 295 | 638 | 800 | 1037 | 1237 | 1865 | 2558 | 1682 | |
| CRP | ≤5.0 mg/L | 60.8 | 90.3 | 85.9 | 108.9 | 193.6 | 239.9 | 182.3 | 74.9 | 36.7 |
| D-dimer | ≤0.59 mg/L FEU | 0.80 | 1.35 | 1.08 | 1.54 | 1.96 | 11.65 | 2.73 | 1.55 | |
| Interleukin-6 | ≤1.8 pg/L | 13.4 | 41.0 | 346 | ||||||
| Troponin T Gen 5 | ≤14 ng/L for females | 8 | 8 | 9 | 9 | 10 | 10 | 690 | 631 | 114 |
| NT-proBNP | <900 pg/mL | 110 | 81 | 124 | 448 | 28,425 |
Abbreviations: CRP, c-reactive protein; FEU, fibrinogen equivalent units; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
The patient was admitted with suspected COVID-19 viral pneumonia, continued on her routine COPD medications, and started on maintenance intravenous fluids (IVF), given hyponatremia and hypovolemia. On hospital day 3, the patient’s COVID-19 testing resulted positive, and she was started on hydroxychloroquine. On hospital day 5, she developed worsening hypoxia, increased work of breathing, tachypnea, and wheezing. A portable chest x-ray showed worsening bibasilar opacities. She was started on methylprednisolone, albuterol/ipratropium inhaler, ceftriaxone, and doxycycline, was transferred to the intensive care unit (ICU), and given 1 dose of tocilizumab.
Shortly after ICU transfer, she experienced progressive respiratory failure and hypotension requiring urgent intubation, epinephrine, norepinephrine, and vasopressin. Repeat chest x-ray showed diffuse pulmonary infiltrates. Antibiotics were broadened to vancomycin and piperacillin/tazobactam, and she was continued on doxycycline and hydroxychloroquine. On hospital day 7, the patient’s daily troponin levels, obtained as part of our institution’s COVID-19 laboratory protocol, increased from 10 to 690 ng/L, and she was started on empiric anticoagulation. Electrocardiogram (EKG) at this time showed sinus tachycardia and nonspecific T-wave abnormality in the lateral leads. At this time, she was hypotensive on inotropic and vasopressor support with highest doses: epinephrine 0.05 µg/kg/min, norepinephrine 0.2 µg/kg/min, and vasopressin 0.04 U/min. The patient also had worsening oliguric renal failure, and she was started on continuous renal replacement therapy (CRRT) with citrate anticoagulation.
On hospital day 8, transthoracic echocardiogram demonstrated normal right ventricular function, left ventricular ejection fraction (LVEF) 26% with preserved basal function, and apical ballooning (Figure) consistent with takotsubo cardiomyopathy. This can be seen in the parasternal long, apical 2-chamber, 3-chamber, and 4-chamber views in Supplemental Digital Content, Videos 1–4, http://links.lww.com/AACR/A364, http://links.lww.com/AACR/A365, http://links.lww.com/AACR/A366, and http://links.lww.com/AACR/A367. A pulmonary artery catheter was placed at this time to measure invasive hemodynamics (Table 2). She had elevated ventricular filling pressures with a borderline-low cardiac index (CI) with elevated systemic vascular resistance (SVR). Given the elevated SVR and concern for continued myocardial stress with high-dose epinephrine, attempts to wean vasopressors and inotropes were attempted. However, she subsequently became increasingly hypotensive, requiring reinitiation and escalation of pressors overnight. Repeat hemodynamics on hospital day 9 revealed more significantly elevated SVR, which led to a lower CI.
Table 2.
Invasive Hemodynamics
| Hospital Day | MAP (mm Hg) | RAP (mm Hg) | PAP (mm Hg) | CO (L/min) | CI (L/min/m2) | SVR (dynes·s·cm−5) |
|---|---|---|---|---|---|---|
| 8 | 100 | 18 | 43/27/33 | 3.9 | 2.2 | 1682 |
| 9 | 74 | 9 | 43/19/27 | 2.2 | 1.3 | 2300 |
Abbreviations: CI, cardiac index; CO, cardiac output; MAP, mean arterial pressure; PAP, pulmonary arterial pressure (systolic/diastolic/mean); RAP, right atrial pressure; SVR, systemic vascular resistance.
Figure.
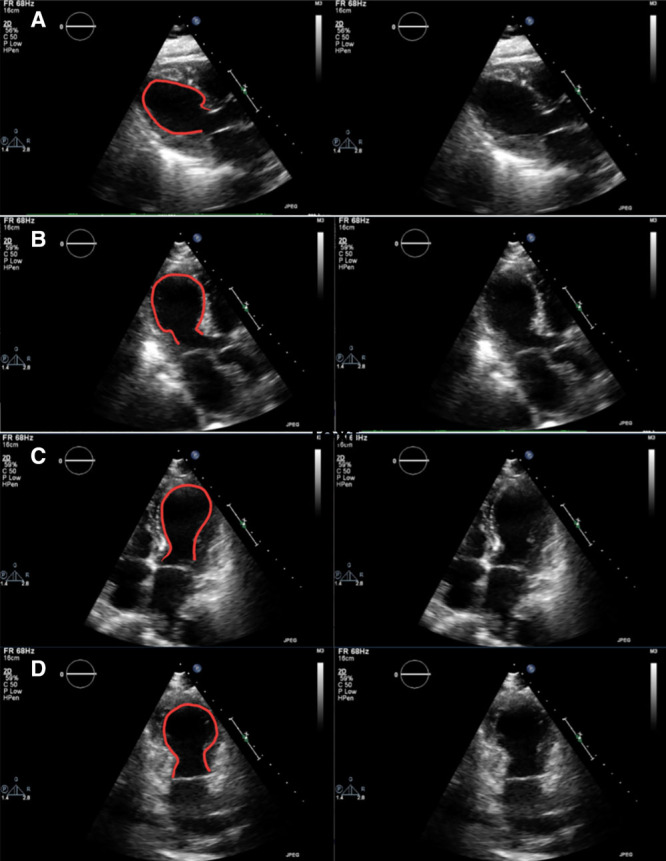
Apical ballooning. Transthoracic echo showing parasternal long (A), apical 3-chamber (B), apical 4-chamber (C), and apical 2-chamber (D) views in systole notable for apical ballooning typical of takotsubo cardiomyopathy.
Vasopressin was stopped with a plan to wean norepinephrine and epinephrine to decrease SVR, decrease myocardial stress, and improve cardiac output. It was believed the low CI was driven by the significantly elevated SVR; therefore, mechanical circulatory support was not pursued. Her pulmonary status was improving, as demonstrated by decreasing ventilator requirements. As her troponin levels were decreasing, systemic anticoagulation was stopped.
Later that day, the patient experienced clotting within the CRRT circuit followed by worsening hypotension requiring a rapid increase in norepinephrine infusion and then had pulseless electrical activity (PEA) arrest. This was suspected to be secondary to clot formation and blood loss within the clotted dialysis circuit and likely pulmonary embolism. She underwent 9 rounds of advanced cardiac life support (ACLS) with chest compressions, epinephrine, sodium bicarbonate, calcium chloride, rapid transfusion of 2 U of blood due to blood loss into her CRRT circuit and tissue plasminogen activator (tPA) given concern for thrombus and potential pulmonary embolism. The patient was found to be asystolic after 29 minutes of ACLS and the decision was made to halt further efforts. Given the COVID-19 pandemic, the policy of our institution, as well as the state Medical Examiner, was to defer postmortem examinations.
DISCUSSION
Takotsubo cardiomyopathy was originally described by Sato et al.1 The classic description involves acute and transient left ventricular (LV) dysfunction, with regional wall motion abnormalities (RWMA) extending beyond a single coronary artery territory, frequently triggered by a stressor (emotional or physical). The typical pattern of LV RWMA is apical hypokinesis/akinesis with basal hyperkinesis, leading to the characteristic “ballooning” of the LV. Takotsubo cardiomyopathy is named after the Japanese term for octopus pot, due to its similar appearance with its rounded and inflated bottom and narrow neck.
Patients presenting with takotsubo cardiomyopathy typically present with findings suggestive of acute coronary syndrome (ACS), such as angina, syncope, pulmonary edema, dysrhythmias, or cardiogenic shock.2 Often these findings are preceded by a significant psychological, emotional, or physical stressor. Takotsubo cardiomyopathy is thought to account for approximately 7% of all nonischemic cardiomyopathies, with postmenopausal women being the most affected, comprising 90% of all cases.3 Typical EKG findings include ST-elevations, QTc prolongation, T-wave inversions, and left bundle branch block (LBBB). The duration and severity of EKG abnormalities may not correlate with RWMA in takotsubo cardiomyopathy, and symptoms typically resolve within several days of presentation.4
The exact pathophysiology of takotsubo cardiomyopathy is not known, although several mechanisms have been proposed.5 Stress-related neuropeptides and norepinephrine may be released by the brain during stress. The increase in circulating catecholamines or adrenergic may have a direct myocardial toxic effect or may lead to epicardial or microvascular dysfunction or vasospasm.
Treatment of takotsubo cardiomyopathy is mainly supportive. For those who present with cardiogenic shock, supportive therapy with inotropic agents or temporary mechanical circulatory support may be necessary. Acute heart failure is managed with diuretics and vasodilators to correct elevated filling pressures and provide afterload reduction. Withdrawal of beta-agonists as soon as hemodynamics allow may help reduce myocardial stress. In hemodynamically stable patients, treatment with standard medical therapy for heart failure with beta-blockers and renin-angiotensin system blockade is reasonable. As this is a transient process, with recovery of systolic function typically within 1–4 weeks, the appropriate duration of therapy is not known. As takotsubo cardiomyopathy may recur with repeat stressors, it is not unreasonable to continue adrenergic blockade indefinitely.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the virus responsible for the recent outbreak of the disease known as COVID-19. It is a positive-sense, single-stranded RNA virus from the larger family of Coronaviridae. This subfamily contains 4 groups: α, β, γ, and δ. The α and β viruses are known to cause infections in humans.6 This group also includes SARS-CoV, which was responsible for the SARS outbreak of 2002; the Middle East respiratory syndrome–related coronavirus (MERS-CoV), which caused the MERS epidemics of 2012, 2015, and 2018; and several strains that cause the common cold (Human Coronaviruses OC43, HKU1, 229E, and NL63).7 SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as the receptor for entry into the host cell. Systemic manifestations of the disease may be related to the distribution of ACE2 in multiple organ systems. Nausea, vomiting, and diarrhea may be secondary to ACE2 in the gastrointestinal tract, while cardiac damage and myocarditis may be related to ACE2 in the myocardium.8
Current data put the incidence of acute cardiac injury at 8%–12% in patients with COVID-19.9 The exact pathways of cardiac injury in COVID-19 are incompletely understood, but there are several likely mechanisms. Respiratory failure is a common feature in COVID-19. Early studies have reported that computed tomography (CT) abnormalities are present in as much as 85% of patients, with 75% showing bilateral involvement with subpleural and peripheral areas of ground-glass opacities. Autopsy studies have shown evidence of direct cardiac tissue damage and inflammatory infiltrates composed of macrophages and CD4+ T cells, as well as regions of cardiomyocyte necrosis. COVID-19 patients have also been found to have a cytokine profile comprised of elevated levels of interleukin (IL)-2, IL-7, interferon-γ inducible protein 10, macrophage inflammatory protein 1-α, tumor necrosis factor-α, granulocyte colony-stimulating factor, and monocyte chemoattractant protein 1. This cytokine profile is similar to other hyperinflammatory conditions marked by multiorgan failure.10 Taken together, these data show that myocardial injury as classically seen in COVID-19 may be a result of hypoxemia, direct myocardial infection, myocardial damage from systemic inflammation, or a combination thereof. Herein, we describe another mechanism of cardiac involvement with COVID-19: development of takotsubo cardiomyopathy, most likely driven by catecholamine excess either due to the physiologic stressor of infection with COVID-19 or by exogenous catecholamines given due to hypotension leading to a direct myocardial toxic effect or epicardial or microvascular dysfunction or vasospasm, which can lead to the development of takotsubo cardiomyopathy.
Most cases of takotsubo cardiomyopathy are noted to resolve spontaneously within a matter of days to weeks; however, this patient unfortunately did not survive her hospital course. While cardiac injury is a known complication of the COVID-19 process, this patient’s findings may represent a rare manifestation of cardiac involvement in the COVID-19 population and confirms the findings of previously published case reports of takotsubo cardiomyopathy in this population.11–13
DISCLOSURES
Name: Siddharth Dave, MD.
Contribution: This author helped draft, review, and revise significant portions of this manuscript.
Name: Jennifer T. Thibodeau, MD, MSCS.
Contribution: This author helped review and revise significant portions of this manuscript.
Name: Kim Styrvoky, MD.
Contribution: This author helped draft, review, and revise significant portions of this manuscript.
Name: Shyam H. Bhatt, MPH.
Contribution: This author helped draft significant portions of the case discussion.
This manuscript was handled by: Kent H. Rehfeldt, MD.
Supplementary Material
GLOSSARY
- ACE2 =
- angiotensin-converting enzyme 2
- ACLS =
- Advanced Cardiac Life Support
- ACS =
- acute coronary syndrome
- CARE =
- CAse REport
- CI =
- cardiac index
- CO =
- cardiac output
- COPD =
- chronic obstructive pulmonary disease
- COVID-19 =
- coronavirus disease 2019
- CRP =
- c-reactive protein
- CRRT =
- continuous renal replacement therapy
- CT =
- computed tomography
- EKG =
- electrocardiogram
- EQUATOR =
- Enhancing the QUAlity and Transparency Of health Research
- FEU =
- fibrinogen equivalent units
- HIPAA =
- Health Insurance Portability and Accountability Act
- ICU =
- intensive care unit
- IL =
- interleukin
- IRB =
- institutional review board
- IVF =
- intravenous fluids
- LBBB =
- left bundle branch block
- LV =
- left ventricle
- LVEF =
- left ventricular ejection fraction
- MAP =
- mean arterial pressure
- MERS-CoV =
- Middle East respiratory syndrome-related coronavirus
- NT-proBNP =
- N-terminal pro-B-type natriuretic peptide
- PAP =
- pulmonary arterial pressure (systolic/diastolic/mean)
- PCR =
- polymerase chain reaction
- PEA =
- pulseless electrical activity
- RAP =
- right atrial pressure
- RWMA =
- regional wall motion abnormality
- SARS-CoV-2 =
- severe acute respiratory syndrome coronavirus-2
- Spo2 =
- pulse oximetry
- SVR =
- systemic vascular resistance
- tPA =
- tissue plasminogen activator
Funding: None.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website.
REFERENCES
- 1.Sato H, Tateishi H, Dote K, et al. Kodama K, Haze K, Hori M. Tako-tsubo like left ventricular dysfunction due to multivessel coronary spasm. Clinical Aspects of Myocardial Injury: From Ischemia to Heart Failure. 1990, Tokyo, Japan: Tokio Kagakuhyoronsha Publ Co, 56–64 [Google Scholar]
- 2.Dawson DK. Acute stress-induced (takotsubo) cardiomyopathy. Heart. 2018; 104:96–102 [DOI] [PubMed] [Google Scholar]
- 3.Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of takotsubo syndrome. Circulation. 2017; 135:2426–2441 [DOI] [PubMed] [Google Scholar]
- 4.Kurisu S, Kihara Y. Clinical management of takotsubo cardiomyopathy. Circ J. 2014; 78:1559–1566 [PubMed] [Google Scholar]
- 5.Medina de Chazal H, Del Buono MG, Keyser-Marcus L, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. 2018; 72:1955–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang SF, Tuo JL, Huang XB, et al. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV-OC43 during 2010-2015 in Guangzhou. PLoS One. 2018; 13:e0191789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieto-Torres JL, DeDiego ML, Verdiá-Báguena C, et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014; 10:e1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz. 2020; 45:230–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020; 58:1131–1134 [DOI] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roca E, Lombardi C, Campana M, et al. Takotsubo syndrome associated with COVID-19. Eur J Case Rep Intern Med. 2020; 7:001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen D, Nguyen T, De Bels D, Castro Rodriguez J. A case of takotsubo cardiomyopathy with COVID 19. Eur Heart J Cardiovasc Imaging. 2020; 21:jeaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minhas AS, Scheel P, Garibaldi B, et al. Takotsubo syndrome in the setting of COVID-19 infection. JACC Case Rep. 2020; 2:1321–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


