Abstract
Background:
The purpose of this study was to determine the utility and diagnostic performance of strain elastography (SE) in differentiating benign from malignant lesions of the breast.
Methods:
In this prospective study, 50 palpable breast masses in 50 patients were examined by mammography, B-mode ultrasound (US) and SE. Lesions were categorized using Breast Imaging Reporting and Data System (BIRADS) scoring based on mammographic and sonographic features. Elasticity scores were assessed on a five-point scale based on the distribution of strain, and the lesion size on SE imaging and B-mode (elasticity imaging/B mode [EI/B] ratio) was compared. Findings were correlated with the BIRADS assessment and diagnostic performance of sonoelastography was evaluated taking histopathology as reference standard.
Results:
Histopathology revealed 29 (58%) malignant and 21 (42%) benign lesions. Infiltrative ductal carcinoma and fibroadenoma were the most common malignant and benign lesions, respectively. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of SE was 100%, 76.1%, 85.2%, 100%, and 90%, respectively. Higher elasticity score was significantly associated with malignant histopathology (P < 0.00001). The mean EI/B ratio for malignant lesions was 1.36 ± 0.24 while that of benign lesions was 1.03 ± 0.30 (P = 0.000).
Conclusion:
Real-time SE of the breast, with its superior sensitivity and specificity, could provide improved characterization of benign and malignant breast masses compared with mammography and conventional US. Due to greater diagnostic accuracy, SE can be an effective adjunctive tool to B-mode US in predicting malignancy of breast, as well as in reducing the need for biopsies in benign breast lesions.
Keywords: Breast, elasticity imaging technique, sonoelastography
INTRODUCTION
Breast cancer is the most common malignancy in women all over the world and continues to remain a leading cause of cancer-related death in females. The incidence of breast cancer has been increasing steadily in both developing and developed countries.[1] Increased life expectancy, urbanization, changes in lifestyle, and delay in diagnosis contribute to the increasing incidence as well as mortality in the low- and middle-income countries. Early detection is critical to improve the breast cancer outcome and survival.
Current imaging techniques available for screening of breast cancer include mammography, ultrasonography, and magnetic resonance imaging (MRI). Although mammography is the current standard breast screening technique, its sensitivity is reduced in high-density breast tissue, in smaller tumors, and in young women.[2] Breast ultrasonography is widely used in the evaluation and categorization of breast lesions, owing to its high sensitivity. However, inability to image deeper lesions, limited ability to distinguish isoechoic lesions from surrounding fat, low specificity and increased biopsy rates resulting in patient discomfort and anxiety are some potential limitations of breast ultrasound (US).[3] Even though MRI is useful for screening of subjects with high breast cancer risk, its use for routine screening is limited because of high cost, time consumption and lack of widespread availability as well as need for experienced radiologists.
US elastography (USE) is a noninvasive imaging modality complimentary to US that detects tissue elasticity by the application of an external stress. As malignant tissues are firmer than benign ones, the estimate of tissue stiffness by USE can aid in the differentiation between benign and malignant lesions. Recent studies have reported the utility of USE in the characterization of lesions in various tissues, such as thyroid, liver, prostate, lymph nodes, and breast.[4,5,6,7,8] It has been suggested that current elastography systems can evaluate histological information by depicting the distribution of tissue stiffness or strain which might have the potential to predict the therapeutic effect of treatment with anticancer agents.[9]
Published literature on the usefulness of strain elastography (SE) in the evaluation of breast lesions and its correlation with histopathology is limited, especially from India. The purpose of this study was to determine the utility and diagnostic performance of SE in differentiating benign from malignant lesions of the breast with histopathology as the reference standard.
MATERIAL AND METHODS
This prospective study was conducted at a tertiary care center in South India from December 2014 to December 2017. The institutional review board approved the study protocol (IRB approval no. 12/010), and the protocol complied with the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants. Fifty consecutive female patients aged 40 years and above referred to the department of radiology for the evaluation of palpable breast lump and sonography-guided biopsy were included in the study. Exclusion criteria consisted of those with cystic lesions of breast, history of prior biopsy, postsurgical status, prosthetic breast implants, and subcentimeter lesions. All patients underwent mammography, B-mode US, and SE followed by US-guided biopsy in the same session. All examinations and biopsy were performed by a single expert radiologist with over 10 years of experience in sonography.
Mammography was performed with SIEMENS MAMMOMAT 3000. Mediolateral oblique and craniocaudal views were taken in all patients, and in addition, coned-down or magnification views were used when necessary.
Ultrasonography of the breast was performed with SIEMENS ACUSON S3000 using high-frequency probe 18 L6. Sonographic features studied included size of the lesion, shape, margins, orientation, internal echogenicity, vascularity, calcification, and posterior acoustic shadowing. The lesions were categorized using the Breast Imaging Reporting and Data System (BIRADS) scoring based on mammographic and sonographic features as the following categories – category 2: benign; category 3: probably benign; category 4: suspicious for malignancy; and category 5: highly suggestive of malignancy.[10] BIRADS category 1 lesion was excluded from the study. Categories 2 and 3 were considered negative for malignancy and categories 4 and 5 were labeled as malignant.
SE of the breast was performed using the same probe in the supine position. The probe was placed over the region such that the lesion was at the center of the image. Holding the scan plane perpendicular to the skin surface, a slight compression–decompression movement was applied. The appropriate pressure and frequency of compression/decompression were optimized with the help of real-time display of quality index. Elastograms with quality index equal to or greater than 60 were included. The region of interest (ROI) included the lesion with subcutaneous layers and pectoralis muscle. It was ensured that the target tissue occupied no more than 25% of the total area of ROI. At least 5 mm thickness of normal adjacent tissue was included to assess the lesion stiffness in relation with average elasticity of surrounding tissue. Color map settings with blue coded as hard area and green coded as soft area were used. The images were displayed on a split screen mode with B-mode images on the left and elastographic images on the right. The best fit B-mode elastographic image pairs were selected for analysis. [Figures 1-4] The longest dimension of the lesion on elastographic image was compared with that of the corresponding B-mode image, and elasticity imaging/B mode ratio (EI/B ratio) was calculated.
Figure 1.
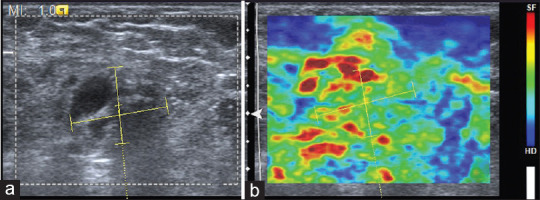
(a) B-mode ultrasound showing ill-defined hypoechoic lesion which was categorized as Breast Imaging Reporting and Data System category 4. (b) With strain elastography, entire hypoechoic lesion is deformable and evenly shaded green as surrounding breast tissue, corresponding to elasticity score 1. Histopathology was suggestive of nonspecific mastitis
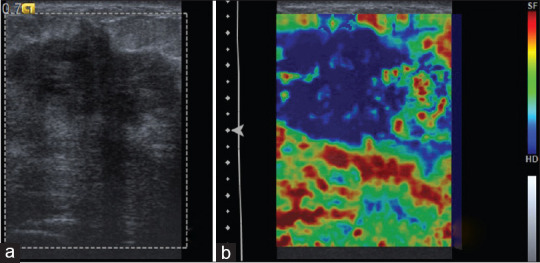
Figure 4.
(a) B-mode ultrasound showing ill-defined hypoechoic lesion categorized as Breast Imaging Reporting and Data System 5. (b) With strain elastography, entire lesion and surrounding area are stiff, corresponding to elasticity score 5. Histopathology was suggestive of infiltrative ductal carcinoma
Figure 2.
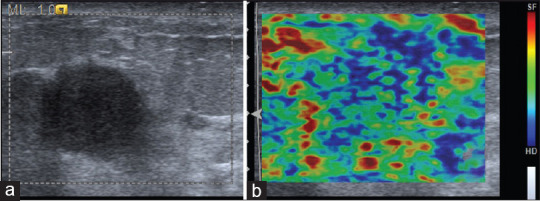
(a) B-mode ultrasound showing poorly marginated round hypoechoic lesion categorized as Breast Imaging Reporting and Data System category 3. (b) Strain elastographic image showing deformability of the peripheral portion of the lesion with stiff tissue in the center corresponding to elasticity score 3. Histopathology was suggestive of fibroadenoma
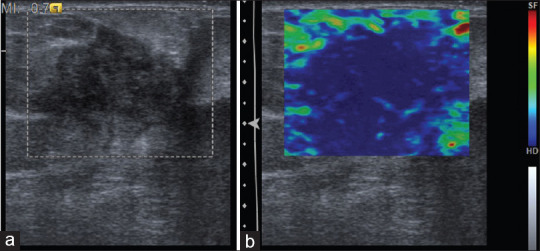
Figure 3.
(a) B-mode ultrasound showing ill-defined hypoechoic lesion categorized as Breast Imaging Reporting and Data System 4. (b) With strain elastography, entire lesion is stiff, corresponding to elasticity score 4. Histopathology was suggestive of calcified fibroadenoma
The elasticity patterns were scored using the TSUKUBA scoring system [Table 1] described by Itoh et al.[11] The elasticity scores 1, 2, and 3 were considered benign, while scores 4 and 5 were labeled as malignant.
Table 1.
TSUKUBA elasticity score
| Elasticity score | Description |
|---|---|
| 1 | Even strain in the entire lesion |
| 2 | Parts of the hypoechoic lesion does not show strain |
| 3 | Periphery of the hypoechoic lesion shows strain, no strain at the center |
| 4 | Entire lesion shows no strain |
| 5 | Entire lesion and surrounding area shows no strain |
Biopsy of the lesion was performed under US guidance using an 18G tru-cut needle. Histopathology results were taken as the reference standard.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS, SPSS Inc., Chicago, IL, USA) version 24. Descriptive statistics was presented as mean ± standard deviation. Qualitative variables were presented in the form of frequency and percentages. Chi-square test was used to know the association between categorical variables. The diagnostic sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated using 2 × 2 tables with histopathological analysis as the reference standard. Statistical significance was set at P < 0.05.
RESULTS
Patients were aged between 40 and 72 years (mean: 48.96 ± 7.52 years). The mean age of the patients with benign and malignant lesions was 45.4 ± 5.47 and 51.5 ± 7.84 years, respectively (P = 0.001). On histopathological evaluation, 29 (58%) were found to be malignant and 21 (42%) were benign. Infiltrative ductal carcinoma and fibroadenoma were the most common malignant and benign lesions, respectively. Of the 29 malignant lesions, 17 (59%) were infiltrative ductal carcinoma, 11 (38%) were invasive lobular carcinoma and 1 (3%) was mucinous carcinoma. Among the 21 benign lesions, 7 (33%) were fibroadenoma, 4 (19%) were atypical ductal hyperplasia, 3 (15%) were breast abscess, 2 (9%) were sclerosing adenosis, and 2 (9%) were nonspecific mastitis. Granulomatous mastitis, complex cyst, and benign phyllodes tumor accounted for 1 (5%) each.
Mammography of 14 patients (28%) did not show any demonstrable lesion due to dense breast with predominantly fibroglandular tissue. 41% of the malignant lesions (12/29) had spiculated appearance on mammography, and microcalcification was encountered in 34% (10/29). 14% (4/29) showed architectural distortion.
Based on the BIRADS assessment, three were assigned BIRADS category 3, 18 were assigned category 4, and 29 were assigned category 5. The sensitivity, specificity, PPV, NPV, and accuracy of BIRADS assessment were 96.5%, 9.5%, 59.5%, 66.6%, and 60%, respectively, with histopathology as the reference standard.
Qualitative SE using elasticity scores had a sensitivity of 100% and specificity of 76.1% in detecting malignancy. The PPV and NPV were 85.2% and 100%, respectively, with diagnostic accuracy of 90%. Higher elasticity score was significantly associated with malignant histopathology (P < 0.00001). The mean EI/B ratio for malignant lesions was 1.36 ± 0.24 while that of benign lesions was 1.03 ± 0.30 (P = 0.000). Table 2 shows the correlation between histopathology, BIRADS assessment, and elasticity scoring among the lesions.
Table 2.
Correlation between histopathology, Breast Imaging Reporting and Data System assessment and elasticity scoring among the lesions
| Histopathology | BIRADS category | Elasticity score | Total (n=50) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Benign | 3 | 2 | 2 | ||||
| 4 | 1 | 1 | 8 | 1 | 2 | 13 | |
| 5 | 1 | 1 | 2 | 2 | 6 | ||
| Malignant | 3 | 1 | 1 | ||||
| 4 | 5 | 5 | |||||
| 5 | 23 | 23 | |||||
BIRADS: Breast Imaging Reporting and Data System
DISCUSSION
In this prospective study, we evaluated the diagnostic performance of SE in predicting malignancy in lesions of breast. Qualitative SE with elasticity scoring was found to have a superior sensitivity, specificity, and diagnostic accuracy compared to conventional BIRADS assessment.
USE estimates the stiffness of tissues based on the degree of deformation of a tissue in response to an externally applied force. SE is a qualitative technique that uses either manual compression or an acoustic radiation force impulse push pulse to deform tissues.
Several authors have reported the additional value of SE to improve the specificity of conventional US in the differentiation of benign and malignant breast masses and decrease the need for biopsies. While few have used EI/B ratio,[12] others have used five-point color scale and strain ratio[13,14] in the interpretation of SE images of the breast. However, the superior diagnostic performance of SE over B-mode US is controversial and factors such as operator experience, use of varied cutoffs, tumor size, and histologic subtype have been shown to influence the outcome.[15,16] Elasticity scoring had a sensitivity of 100% and specificity of 76.1%% in detecting malignancy among breast lesions in our study. The mean size of benign and malignant lesions was 17.06 ± 3.7 and 16.77 ± 3.1 mm, respectively. No significant difference in size was noted between benign and malignant lesions (P = 0.767). The superior diagnostic performance observed in our study can be attributed to the fact that most of the lesions (40/50) were smaller than 2 cm in size. Giuseppetti et al. have reported a better diagnostic performance of elastosonography in lesions smaller than 2 cm.[15] This is because a larger lesion has more chances of having hemorrhage or necrosis which might alter the tissue stiffness inside the lesion. Parajuly et al., based on their experience, have observed that lesions more than 5 cm should not be recruited in a study.[17]
Our results are comparable to that of Schaefer et al. and Atabey et al., who evaluated the performance of SE in breast lesions using a five-point scoring system.[18,19] With a cutoff between elasticity scores 3 and 4, elastography provided higher sensitivity and NPV. The authors have concluded that elastography can be an effective adjunct imaging modality in differential diagnosis of breast lesions, before the decision to biopsy a lesion in certain cases.[19]
In our study, 34 lesions were categorized as malignant on SE. Among these, 85% (29/34) were confirmed as malignancy on histopathology. Five benign lesions which had been categorized as malignant on SE were fibroadenoma (1), atypical ductal hyperplasia (3), and sclerosing adenosis (1) associated with calcification, which might have increased the stiffness of lesion. The false-positive rate was 23.8% in our study. In contrast, Zhi et al. have noted a false-positivity rate of 4.3% with SE in their study. Calcification and organized hemorrhage in benign lesions were responsible for false-positive diagnoses on SE.[3]
All the lesions with elasticity score less than or equal to 3 turned out to be negative for malignancy in our study. There were 13 benign lesions that presented as BIRADS category 4 necessitating biopsy. With a cutoff between 3 and 4 on SE, 10 of these 13 (76.9%) were true negatives on SE. Our observations are in line with recent literature, suggesting that SE can be useful in downgrading BIRADS category 4 lesions and reduce benign biopsy rates.[20]
Seven out of 18 BIRADS category 4 lesions and 25/29 BIRADS category 5 lesions had elasticity score 5. Among these, 87.8% turned out to be malignant, suggesting that elasticity scoring can be a useful predictor of malignancy. Satake et al. evaluated the ability of sonoelastography in predicting malignancy in breast masses classified as BIRADS category 4 or 5. Elasticity score was found to be a significant predictor of malignancy in BIRADS 4 masses (P = 0.002). However, elasticity score was not a significant predictor of malignancy in BIRADS category 5 lesions.[21]
A recent meta-analysis by Gong et al. on real-time elastography (RTE) in the differentiation of benign and malignant lesions of the breast that included 22 studies evaluating 4713 breast nodules reported an overall mean sensitivity and specificity of 0.834 and 0.842, respectively for elasticity scoring.[22] The authors have stated that RTE has high sensitivity and specificity in differentiating benign from malignant lesions and therefore can potentially reduce unnecessary breast biopsies. The mean EI/B ratio for malignant lesions was 1.36 ± 0.24 while that of benign lesions was 1.03 ± 0.30 (P = 0.000, P < 0.00001) in our study. Several studies have shown that tumors appear larger on elastography than on B-mode US.[23,24] A cutoff value of less than 1 for benign lesions and greater than or equal to 1 for malignant lesions has been shown to have high sensitivity and specificity for the characterization of lesions.[12] The variable dimension in elastography has been attributed to the desmoplastic reaction occurring in many cancers of the breast. Malignant lesions have been reported to appear significantly larger on strain imaging presumably because strain is uniquely sensitive to desmoplasia surrounding the lesion.[25]
Our study has few limitations. The sample size was limited and a single experienced radiologist performed all the examinations. SE is an operator-dependent technique and steady compression is needed for optimal images. However, in our study only images with optimal compression as displayed by the quality index were included. Intra- or inter-observer variability was not taken into account.
To the best of our knowledge, this is the first study on the utility of SE among the Indian population. The cancer projection data from India show that the number of breast cancer cases will become almost double by 2020.[26] Owing to the fact that the density of Indian breasts is different from that of the Western population[27] and density of breast parenchyma can significantly influence the diagnostic performance of elastography, there is a need for more studies from this part of the world.
CONCLUSION
In this initial study from India, we conclude that real-time SE of the breast, with its superior sensitivity and specificity, could provide improved characterization of benign and malignant breast masses compared with mammography and conventional US. Due to greater diagnostic accuracy, SE can be an effective adjunctive tool to B-mode US in predicting malignancy of breast as well as decreasing unnecessary biopsies in clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lin L, Yan L, Liu Y, Yuan F, Li H, Ni J. Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J Hematol Oncol. 2019;12:96. doi: 10.1186/s13045-019-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao H, Zou L, Geng X, Zheng S. Limitations of mammography in the diagnosis of breast diseases compared with ultrasonography: A single-center retrospective analysis of 274 cases. Eur J Med Res. 2015;20:49. doi: 10.1186/s40001-015-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhi H, Ou B, Luo BM, Feng X, Wen YL, Yang HY. Comparison of ultrasound elastography, mammography, and sonography in the diagnosis of solid breast lesions. J Ultrasound Med. 2007;26:807–15. doi: 10.7863/jum.2007.26.6.807. [DOI] [PubMed] [Google Scholar]
- 4.Asteria C, Giovanardi A, Pizzocaro A, Cozzaglio L, Morabito A, Somalvico F, et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid. 2008;18:523–31. doi: 10.1089/thy.2007.0323. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, et al. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758–64. doi: 10.2214/AJR.06.0322. [DOI] [PubMed] [Google Scholar]
- 6.Kamoi K, Okihara K, Ochiai A, Ukimura O, Mizutani Y, Kawauchi A, et al. The utility of transrectal real-time elastography in the diagnosis of prostate cancer. Ultrasound Med Biol. 2008;34:1025–32. doi: 10.1016/j.ultrasmedbio.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Turgut E, Celenk C, Tanrivermis Sayit A, Bekci T, Gunbey HP, Aslan K. Efficiency of B-mode ultrasound and strain elastography in differentiating between benign and malignant cervical lymph nodes. Ultrasound Q. 2017;33:201–7. doi: 10.1097/RUQ.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 8.Thomas A, Fischer T, Frey H, Ohlinger R, Grunwald S, Blohmer JU, et al. Real-time elastography--an advanced method of ultrasound: First results in 108 patients with breast lesions. Ultrasound Obstet Gynecol. 2006;28:335–40. doi: 10.1002/uog.2823. [DOI] [PubMed] [Google Scholar]
- 9.Barr RG, Nakashima K, Amy D, Cosgrove D, Farrokh A, Schafer F, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 2: Breast. Ultrasound Med Biol. 2015;41:1148–60. doi: 10.1016/j.ultrasmedbio.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus E, Mainiero MB, Schepps B, Koelliker SL, Livingston LS. BI-RADS lexicon for US and mammography: Interobserver variability and positive predictive value. Radiology. 2006;239:385–91. doi: 10.1148/radiol.2392042127. [DOI] [PubMed] [Google Scholar]
- 11.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: Clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 12.Barr RG, Destounis S, Lackey LB, 2nd, Svensson WE, Balleyguier C, Smith C. Evaluation of breast lesions using sonographic elasticity imaging: A multicenter trial. J Ultrasound Med. 2012;31:281–7. doi: 10.7863/jum.2012.31.2.281. [DOI] [PubMed] [Google Scholar]
- 13.Stachs A, Hartmann S, Stubert J, Dieterich M, Martin A, Kundt G, et al. Differentiating between malignant and benign breast masses: Factors limiting sonoelastographic strain ratio. Ultraschall Med. 2013;34:131–6. doi: 10.1055/s-0032-1313168. [DOI] [PubMed] [Google Scholar]
- 14.Zhao QL, Ruan LT, Zhang H, Yin YM, Duan SX. Diagnosis of solid breast lesions by elastography 5-point score and strain ratio method. Eur J Radiol. 2012;81:3245–9. doi: 10.1016/j.ejrad.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Giuseppetti GM, Martegani A, Di Cioccio B, Baldassarre S. Elastosonography in the diagnosis of the nodular breast lesions: Preliminary report. Radiol Med. 2005;110:69–76. [PubMed] [Google Scholar]
- 16.Chang JM, Won JK, Lee KB, Park IA, Yi A, Moon WK. Comparison of shear-wave and strain ultrasound elastography in the differentiation of benign and malignant breast lesions. AJR Am J Roentgenol. 2013;201:W347–56. doi: 10.2214/AJR.12.10416. [DOI] [PubMed] [Google Scholar]
- 17.Parajuly SS, Lan PY, Yun MB, Gang YZ, Hua Z. Diagnostic potential of strain ratio measurement and a 5 point scoring method for detection of breast cancer: Chinese experience. Asian Pac J Cancer Prev. 2012;13:1447–52. doi: 10.7314/apjcp.2012.13.4.1447. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer FK, Heer I, Schaefer PJ, Mundhenke C, Osterholz S, Order BM, et al. Breast ultrasound elastography – Results of 193 breast lesions in a prospective study with histopathologic correlation. Eur J Radiol. 2011;77:450–6. doi: 10.1016/j.ejrad.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Atabey AO, Arıbal E, Ergelen R, Kaya H. Value of strain elastography ultrasound in differentiation of breast masses and histopathologic correlation. J Breast Health. 2014;10:234–8. doi: 10.5152/tjbh.2014.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho N, Moon WK, Park JS, Cha JH, Jang M, Seong MH. Nonpalpable breast masses: Evaluation by US elastography. Korean J Radiol. 2008;9:111–8. doi: 10.3348/kjr.2008.9.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satake H, Nishio A, Ikeda M, Ishigaki S, Shimamoto K, Hirano M, et al. Predictive value for malignancy of suspicious breast masses of BI-RADS categories 4 and 5 using ultrasound elastography and MR diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:202–9. doi: 10.2214/AJR.09.4108. [DOI] [PubMed] [Google Scholar]
- 22.Gong X, Xu Q, Xu Z, Xiong P, Yan W, Chen Y. Real-time elastography for the differentiation of benign and malignant breast lesions: A meta-analysis. Breast Cancer Res Treat. 2011;130:11–8. doi: 10.1007/s10549-011-1745-2. [DOI] [PubMed] [Google Scholar]
- 23.Isermann R, Grunwald S, Hatzung G, Könsgen-Mustea D, Behrndt PO, Geaid AA, et al. Breast lesion sizing by B-mode imaging and sonoelastography in comparison to histopathological sizing – A prospective study. Ultraschall Med. 2011;32(Suppl 1):S21–6. doi: 10.1055/s-0029-1245297. [DOI] [PubMed] [Google Scholar]
- 24.Stachs A, Pandjaitan A, Martin A, Stubert J, Hartmann S, Gerber B, et al. Accuracy of tumor sizing in breast cancer: A comparison of strain elastography, 3-D ultrasound and conventional B-mode ultrasound with and without compound imaging. Ultrasound Med Biol. 2016;42:2758–65. doi: 10.1016/j.ultrasmedbio.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Insana MF, Pellot-Barakat C, Sridhar M, Lindfors KK. Viscoelastic imaging of breast tumor microenvironment with ultrasound. J Mammary Gland Biol Neoplasia. 2004;9:393–404. doi: 10.1007/s10911-004-1409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malvia S, Bagadi SA, Dubey US, Saxena S. Epidemiology of breast cancer in Indian women. Asia Pac J Clin Oncol. 2017;13:289–95. doi: 10.1111/ajco.12661. [DOI] [PubMed] [Google Scholar]
- 27.Singh T, Khandelwal N, Singla V, Kumar D, Gupta M, Singh G, et al. Breast density in screening mammography in Indian population – Is it different from western population? Breast J. 2018;24:365–8. doi: 10.1111/tbj.12949. [DOI] [PubMed] [Google Scholar]


