Abstract
Background:
Midfoot and ankle movement dysfunction in people with diabetes mellitus and peripheral neuropathy (DMPN) is associated with midfoot deformity and increased plantar pressures during gait. If midfoot and ankle motion during heel rise and push-off of gait have similar mechanics, heel rise performance could be a clinically feasible way to identify abnormal midfoot and ankle function during gait.
Research question:
Is midfoot and ankle joint motion during a heel rise associated with midfoot and ankle motion at push-off during gait in people with DMPN?
Methods:
Sixty adults with DMPN completed double-limb heel rise, single-limb heel rise, and walking. A modified Oxford multi-segment foot model (forefoot, hindfoot, shank) was used to analyze midfoot (forefoot on hindfoot) and ankle (hindfoot on shank) sagittal angle during heel rise and gait. Pearson correlation was used to test the relationship between heel rise and gait kinematic variables (n=60). Additionally, we classified 60 participants into two subgroups based on midfoot and ankle position at peak heel rise: midfoot and ankle dorsiflexed (dorsiflexed; n=23) and midfoot and ankle plantarflexed (plantarflexed; n=20). Movement trajectories of midfoot and ankle motion during single-limb heel rise and gait of the subgroups were examined.
Results:
Peak double-limb heel rise and gait midfoot and ankle angles were significantly correlated (r=0.49 and r=0.40, respectively). Peak single-limb heel rise and gait midfoot and ankle angles were significantly correlated (r=0.63 and r=0.54, respectively). The dorsiflexed subgroup, identified by heel rise performance showed greater midfoot and ankle dorsiflexion during gait compared to the plantarflexed subgroup (mean difference between subgroups: midfoot 3°, ankle 3°).
Significance:
People with DMPN who fail to plantarflex the midfoot and ankle during heel rise have difficulty plantarflexing the midfoot and ankle during gait. Utilizing a heel rise task may help identify midfoot and ankle dysfunction associated with gait in people with DMPN.
Keywords: Kinematics, Plantarflexion, Walking, Forefoot, Hindfoot
1. Introduction
Midfoot and ankle movement dysfunction is often observed in people with diabetes mellitus and peripheral neuropathy (DMPN) [1-6] and is associated with medial column deformity and elevated plantar loading [2, 4]. The repetition of increased plantar pressure during walking accompanied with loss of sensation and deformity is a pathway to developing ulceration and amputation [7-10]. Identification of midfoot and ankle movement dysfunction during gait and provision of appropriate interventions (e.g. foot and ankle strengthening, off-loading footwear) to address the movement dysfunction is an important component in preventing the cascade of events in which abnormal plantar loading contributes to plantar ulceration and amputation in people with DMPN [11].
Midfoot and ankle plantarflexion during late stance phase of gait is a critical component of normal foot mechanics in which the foot becomes more rigid and allows efficient and safe transfer of plantarflexor force through the foot [12]. Previous studies have reported altered midfoot and ankle sagittal plane motions during gait in individuals with DMPN [4, 5]. DiLiberto et al. [5] observed a reduced sagittal excursion of the forefoot and hindfoot during stance phase of gait in people with DMPN compared to healthy matched-controls. Rao et al. [4] identified the relationship of reduced sagittal plane excursion of the first metatarsal and lateral forefoot and increased plantar loading during gait. Clinical assessment of gait often relies on visual assessment [13]. However, use of visual assessment to identify ankle and particularly midfoot movement dysfunction during gait is difficult because movements occur at the small joints, i.e. foot and ankle, within a short period of time [14, 15].
Heel rise performance is likely similar to the terminal stance phase of gait where the largest generation of the ankle plantarflexor power occurs at the timing of single-limb to double-limb transition between 40% and 60% of gait cycle [16]. Clinically, the heel rise task could be used to easily assess plantarflexion motion of midfoot and ankle, acting as a surrogate of midfoot and ankle motion during gait. Despite theoretical similarities of heel rise and gait midfoot and ankle mechanics, the relationships of the heel rise task to gait in people with DMPN have not been examined.
People with DMPN have shown reduced midfoot and ankle plantarflexion during double and single-limb heel rise compared to non-DMPN controls [2, 17]. Dorsiflexion of the midfoot or/and ankle during single-limb heel rise was observed in 67% of people with DMPN, whereas plantarflexion of the midfoot and ankle was consistently observed in non-DMPN controls [17]. If movement patterns of midfoot and ankle during heel rise and gait are related, individuals who fail to plantarflex during heel rise may also fail to plantarflex during late stance phase of gait.
By evaluating the association of midfoot and ankle motion between heel rise and gait, clinicians can benefit from using a simple heel rise task to aid the identification and clinical management of foot problems driven by poor midfoot and ankle mechanics during gait. The primary purpose of this study was to determine the relationship of heel rise performance to gait in people with DMPN. We hypothesized that measures of heel rise performance (midfoot and ankle plantarflexion angle at peak double- and single-limb heel rise) will be positively correlated with measures of gait performance (midfoot and ankle plantarflexion angle at peak ankle power during gait) in people with DMPN. The secondary purpose of this study was to characterize the trajectory of midfoot and ankle motion of single-limb heel rise and gait in people with DMPN by comparing subgroups of individuals who plantarflex the midfoot/ankle compared to individuals who dorsiflex the midfoot/ankle.
2. Methods
This study is a secondary analysis that used data at the baseline time point of a longitudinal study (Clinicaltrials.gov, NCT02616263). Thus, inclusion and exclusion criteria, sample size calculation, and selection of the foot for measurements were based on the parent study.
2.1. Participants
Sixty people with DMPN participated in this study. Inclusion criteria for this study were the presence of type 2 DM and PN. DM was diagnosed by the participant’s physician. At least one out of three positive findings on the following clinical tests verified PN: (1) inability to sense Semmes Weinstein 10 g monofilament on at least one of six plantar foot locations, (2) inability to sense vibration perception threshold of 25 Volts applied to the plantar surface of the great toe, and (3) Michigan Neuropathy Screening Instrument lower extremity screening exam score equal to or greater than two. Exclusion criteria were: people who were greater than 75 years old, on dialysis, pregnant, or had severe arterial disease (i.e., ankle-brachial index greater than 1.3 or less than 0.9), great toe extension less than 30°, had a current ulcer or lower extremity amputation, and unable to complete the testing for this study. The parent study included magnetic resonance imaging with the associated exclusion criteria of weight greater than 180 kg, metal or pacemaker implant. Data from 47 individuals without DMPN (non-DMPN) were retrospectively added to provide a reference of the linear relationship between heel rise and gait. The exclusion criteria for non-DMPN were: type 1 or type 2 DM, PN, current plantar ulcer, vascular disease, rheumatoid arthritis, on dialysis, pregnant, history of foot or ankle fracture or surgery, current injury that changes walking behavior, current foot and ankle pain, needed assistance during walking, or wearing prescribed shoes to accommodate foot problems. The study was approved by the Washington University Institutional Review Board (#201511090). All the participants read and signed a consent form to participate the study.
2.2. Sample size
The sample size of 60 participants was powered to evaluate the effects of foot interventions on intrinsic foot muscle deterioration in people with DMPN. Thus, a power analysis for this secondary analysis was not conducted a priori.
2.3. Kinematic and kinetic data acquisition
The foot measured for double-limb heel rise, single-limb heel rise, and gait was selected based on the criteria from the parent study which selected the foot with: (1) the fewest complications (i.e., history of surgery or traumatic injury); (2) metatarsophalangeal joint hyperextension angles needed to balance group assignment; and (3) the most consistent pattern of toe extension during active dorsiflexion, hypothesized to be a contributing factor to toe deformity in people with DMPN.
Kinematic data were collected from a 10-camera 100Hz Vicon motion analysis system (Vicon MX, Los Angeles, California, USA). A modified Oxford multi-segmental foot model was used to measure midfoot and ankle motion that showed good accuracy of between-trial variability [18]. Experienced examiners (HJJ, MKH, KLB) mounted twenty four reflective markers on the skin or thermoplastic plates for forefoot, hindfoot, and shank segments according to previously reported methods [2, 18]. Kinetic data were obtained using a Bertec force plate at a sampling rate at 1000 Hz (FP4060-10 model, Bertec Corporation, Columbus, OH, USA) [19]. Kinematic and kinetic data were analyzed from Visual3D software (C-Motion Inc. Germantown, MD, USA). Kinematic data was smoothed using a Butterworth filter with a cut-off frequency of 6 Hz. Kinetic data was filtered at a cut-off frequency of 25 Hz [20]. The kinetic data were used to select the trials analyzed for the heel rise and to set the event marker for extraction of gait variables. The neutral (0) position of the forefoot and hindfoot segments (midfoot) was set during the calibration trial in a relaxed standing position. The ankle position reflected the position of the shank segment relative to the neutral position of the hindfoot in relaxed standing. The kinematic variables of interest were: sagittal plane midfoot angle, defined as forefoot relative to hindfoot, and sagittal plane ankle angle, defined as hindfoot relative to shank, at peak heel rise and peak ankle power during gait.
Each participant performed five repetitions of both a double- and single-limb heel rise, going as high as possible. All the participants placed their hands on the extended forearm of the tester for balance. Among the five repetitions of the heel rise, the three trials that had the greatest plantarflexor power were selected and the kinematic variables of interest were averaged across the three trials [2].
All participants were instructed to walk barefoot at their self-selected speed (see Table 1 for average gait velocity) contacting two force plates with the involved-limb. Self-selected gait speed was chosen to capture the midfoot and ankle kinematics during the participants’ habitual walking pattern. Six individuals with DMPN (10% of DMPN group) walked with hand support of the tester for safety. None of the participants in non-DMPN group required hand support during walking. Two strides of the involved-limb were recorded during one walking trial. Trials were excluded if the participant’s foot did not completely step on a single force plate. Three valid walking trials were obtained for analysis. A total of six steps of midfoot and ankle angle at peak ankle power during gait were averaged.
Table 1.
Participant characteristics.a
| DMPN | Non-DMPN | |
|---|---|---|
| N, Sex (F/M) | 60 (34/26) | 47 (28/19) |
| Age (years) | 67 ± 6 [46, 75] | 43 ± 19 [22, 74] |
| Body mass index (kg/m2) | 35 ± 7 [22, 49] | 33 ± 7 [21, 54] |
| Hemoglobin A1c (%) | 7.1 ± 1.3 [5.1, 11.4] | - |
| Duration of DM (years) | 14 ± 10 [0.2, 49] | - |
| Gait velocity (m/s) | 0.81 ± 0.16 [0.30, 1.23] | 1.01 ± 0.15 [0.74, 1.38] |
Values are given as mean ± standard deviation [range], except for sex.
Abbreviation: DMPN, Diabetes mellitus and peripheral neuropathy.
2.4. Subgroups of midfoot and ankle motion
The classification of subgroups by midfoot and ankle motion during heel rise [17] was implemented in this study to illustrate two distinct heel rise performance patterns and its effect on the gait trajectory in individuals with DMPN. We defined subgroups based on the position of the midfoot and ankle at peak single-limb heel rise: (1) dorsiflexed subgroup: the midfoot and ankle were dorsiflexed and (2) plantarflexed subgroup: the midfoot and ankle plantarflexed. The midfoot and ankle angles of the two subgroups throughout a single-limb heel rise and stance phase of the gait were examined. Timing of heel rise and gait cycles was normalized to time of the entire task, and is reported as the percent of total task.
2.5. Statistical analysis
The data acquired from the participants were analyzed with R statistical package software version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). The Shapiro-Wilk normality test and Q-Q plots were used to test the assumptions for a Pearson correlation. Since assumptions for parametric testing were met, two-tailed Pearson correlations were used to determine the relationship of double-limb heel rise and single-limb heel rise to gait in people with DMPN (n=60) and non-DMPN individuals (n=47, for a reference line). An independent t-test was conducted to compare the characteristics and kinematic variables between dorsiflexed and plantarflexed subgroups. Significance level was set at p < 0.05.
3. Results
3.1. Participant characteristics
The characteristics of the DMPN and non-DMPN groups are provided in Table 1. The characteristics of dorsiflexed and plantarflexed subgroups are provided in supplementary file 1.
3.2. Midfoot and ankle angles of double-limb heel rise, single-limb heel rise, and gait in individuals with DMPN
The midfoot was plantarflexed at peak double-limb heel rise [mean (SD); −10° (7°)]. The midfoot was dorsiflexed at peak single-limb heel rise [1° (6°)] and at peak ankle power during gait [2° (4°); Fig. 1A]. The ankle was plantarflexed at peak double-limb heel rise [−10° (7°)]. The ankle was dorsiflexed at peak single-limb heel rise [0° (7°)] and at peak ankle power during gait [12° (4°); Fig. 1B].
Figure 1.
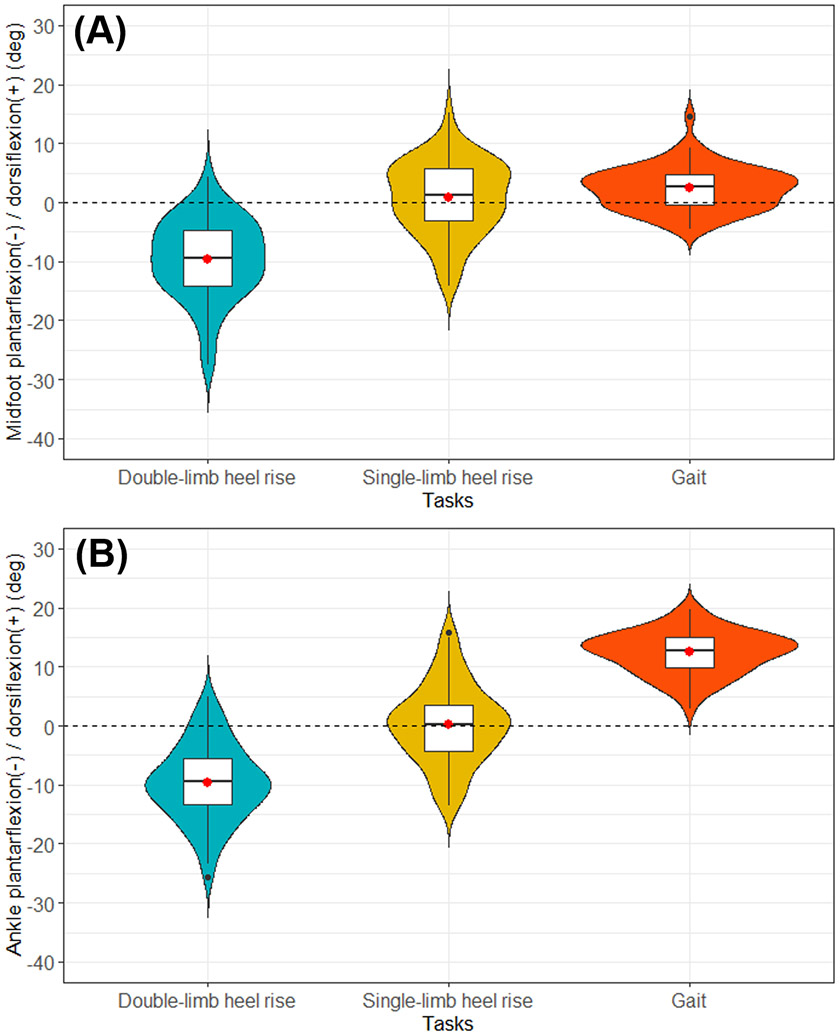
Violin and box plots of (A) midfoot and (B) ankle plantarflexion angles at peak double-limb heel rise, peak single-limb heel rise, and peak ankle power during gait. The width of the colored regions indicates the number of individuals with the measurement. A black line at the center of box plot is the median and the red dot is the mean of the group within the task. The box indicates the interquartile range. The vertical black lines from the box are third quartile + 1.5 interquartile range (upper bound) and first quartile - 1.5 interquartile range (lower bound). Black dot outside the vertical line is an outlier. A dashed line in the center indicates neutral position, defined as the midfoot or ankle at 0 degree.
3.3. Correlations of double-limb heel rise and single-limb heel rise to gait
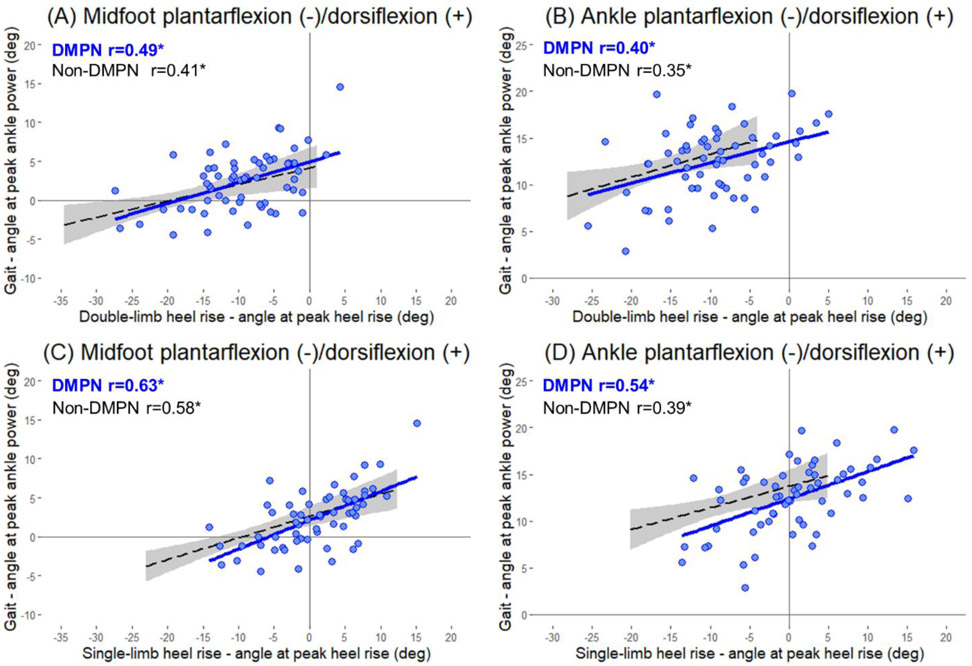
In this group of individuals with DMPN, midfoot and ankle angles at peak double-limb heel rise were both significantly correlated with midfoot (r=0.49, p<0.001) and ankle (r=0.40, p=0.002) angles at peak ankle power during walking (Fig. 2A and 2B). Midfoot and ankle angles at peak single-limb heel rise were both significantly correlated with midfoot (r=0.63, p<0.001) and ankle (r=0.54, p<0.001) angles at peak ankle power during walking (Fig. 2C and 2D).
Figure 2.
Relationship of the double-limb heel rise (A and B) and single-limb heel rise (C and D) to gait, of the midfoot and ankle sagittal angle in groups of DMPN (blue solid line) and non-DMPN (black dashed line). Shaded area represents 95% confidence interval for non-DMPN group. *significant Pearson correlation coefficient (p<0.05). Abbreviation: DMPN, diabetes mellitus and peripheral neuropathy.
3.4. Subgroup analysis of midfoot and ankle during the complete single-limb heel rise and gait cycle
As defined by subgroup assignment, the dorsiflexed subgroup’s midfoot dorsiflexed throughout single-limb heel rise, whereas the plantarflexed subgroup’s midfoot plantarflexed (Fig. 3C). Both subgroups plantarflexed the ankle throughout single-limb heel rise, although the dorsiflexed subgroup showed consistently less plantarflexion compared to the plantarflexed subgroup (Fig. 3D). Similar to single-limb heel rise performance, the dorsiflexed subgroup performed greater midfoot and ankle dorsiflexion throughout the stance phase of gait compared to the plantarflexed subgroup (Fig. 3E and 3F).
Figure 3.
Images of midfoot and ankle position at peak single-limb heel rise and gait in the (A) dorsiflexed and (B) plantarflexed subgroups. The participant in (A) show greater midfoot and ankle dorsiflexion at both single-limb heel rise and gait compared to that of the participant in (B). Midfoot and ankle motions (degrees) during single-limb heel rise (C and D) and stance phase of gait (E and F). The dorsiflexed subgroup is in red and the plantarflexed subgroup is in blue. Error bars represent standard deviation. The vertical line (gray) in (E) and (F) is the timing of peak ankle power during the stance phase of gait. In (E), note the dorsiflexed subgroup is in greater midfoot dorsiflexion throughout stance phase of gait and does not cross into plantarflexion until 94% of stance phase.
The dorsiflexed subgroup showed significantly less plantarflexion of midfoot and ankle at peak single-limb heel rise compared to plantarflexed subgroup (mean difference between subgroups in single-limb heel rise: midfoot 11°, p<0.001 and ankle 11°, p<0.001). The dorsiflexed subgroup showed significantly greater midfoot and ankle dorsiflexion at peak ankle power during gait compared to plantarflexed subgroup (mean difference between subgroups in gait: midfoot 3°, p=0.01 and ankle 3°, p=0.03; Table 2).
Table 2.
Kinematic variables in dorsiflexed and plantarflexed subgroups.a
| Task | Variables | Dorsiflexed subgroup (n=23) |
Plantarflexed subgroup (n=20) |
p-valueb |
|---|---|---|---|---|
| Single-limb heel rise (degree) | Midfoot | 6 ± 3 | −5 ± 4 | <0.001 |
| Ankle | 6 ± 5 | −5 ± 4 | <0.001 | |
| Gait (degree) | Midfoot | 4 ± 4 | 1 ± 3 | 0.01 |
| Ankle | 14 ± 3 | 11 ± 3 | 0.03 |
Values are given as mean ± standard deviation.
Significance of an independent t-test between dorsiflexed and plantarflexed subgroups.
4. Discussion
The key findings of our study indicate individuals demonstrating greater midfoot and ankle plantarflexion at peak double and single-limb heel rise also have greater midfoot and ankle plantarflexion at peak ankle power during gait. In addition, people with DMPN who dorsiflex their midfoot and ankle at peak single-limb heel rise have increased midfoot and ankle dorsiflexion through the entire stance phase of gait compared to people who plantarflexed midfoot and ankle. To our knowledge, this is the first study to report the midfoot and ankle kinematic relationship between heel rise and gait in individuals with DMPN. The stronger correlation of single-limb heel rise compared to double-limb heel rise suggests single-limb heel rise as the better surrogate for assessing midfoot and ankle movements during gait. These findings underscore the potential benefit of the heel rise as a simple, foot and ankle specific, clinically applicable test to screen for midfoot and ankle movement dysfunction that may be present during gait.
The significant relationship between midfoot motion during double and single-limb heel rise and gait indicates that people who have difficulty plantarflexing their midfoot during the heel rise tasks also have difficulty plantarflexing their midfoot during walking. When we examined the kinematic trajectories of midfoot motion during gait between dorsiflexed and plantarflexed subgroups, two primary differences are evident. First, at peak ankle power during gait, the dorsiflexed subgroup was in a more midfoot dorsiflexed position. Second, the dorsiflexed subgroup did not cross into midfoot plantarflexion at the instant of the peak ankle power during gait. The last portion of the stance phase of gait is a critical time during which midfoot plantarflexion, combined with inversion, assist the foot in becoming a more rigid lever allowing transfer of extremely high forces through the foot joints to avoid midfoot collapse [12].
Increased midfoot dorsiflexion into terminal stance (midfoot collapse), likely results in abnormal stress through the midfoot joints with every step. A repetitive, moderate stress is the most common cause of ulceration in people with DMPN [21]. People with DMPN take an average of 4909 to 8818 steps a day [22-24]. Given that midfoot collapse can occur with every step, the cumulative stress could be substantial. The heel rise task is a clinical test that could help identify individuals who may have midfoot collapse during walking and guide treatment programs aimed at reducing midfoot movement dysfunction and minimizing the risk of increasing mechanical plantar loading in people with DMPN.
People who perform less ankle plantarflexion during heel rise tasks also had reduced ankle plantarflexion at push-off during gait, in other words, they stayed in greater dorsiflexion throughout gait. Heel rise is a measure of plantarflexor strength [25] and a strengthening exercise for plantarflexor muscles [26]. The positive relationship between ankle motion during the heel rise task and gait suggests strengthening the plantarflexor muscles may improve gait performance. Further research is needed to test the effects of plantarflexor strengthening on heel rise and gait mechanics and plantar pressure in people with DMPN.
The difference between the dorsiflexed and plantarflexed subgroups for midfoot and ankle motion were larger during heel rise compared to gait. Small magnitude change of in midfoot and ankle motion during gait is difficult to observe for clinicians and results in unreliable assessment [14, 15]. The heel rise task is completed more slowly and magnifies the dysfunction making it easier to visually assess motion and suggests it could be a useful clinical examination item.
The plantarflexed subgroup had a lower body mass index and older age compared to dorsiflexed subgroup. Obesity, a modifiable factor, is known to directly increase midfoot pressure during walking [27] and increases the weight that must be lifted during daily tasks. Future work should examine the contribution of body mass index to foot function, so that we know targets and limits to improving foot function in patients with DMPN.
This study has a few limitations. First, the cause and effect relationship of movement dysfunction to deformity cannot be determined due to the cross-sectional study design. Future work should consider a longitudinal study design to understand the relationship of midfoot and ankle movement dysfunction to the development of midfoot deformity and ulceration in people with DMPN, and if heel rise tasks could be used to identify and treat movement dysfunction during gait. Second, the midfoot and ankle kinematic relationships between heel rise and walking may be uniquely altered by disease (e.g., DMPN) or pathology (e.g., foot and ankle tendon dysfunction, osteoarthritis, etc.). Further study is needed to expand our observations to confirm if the relationship of heel rise to gait is maintained in other people with foot and ankle pathology. Finally, gait kinematics were collected at a self-selected speed, thus, the results cannot be generalized to other walking speed. Despite these limitations, this study provides evidence of associations of midfoot and ankle movement dysfunction across tasks that may provide helpful information to clinical assessment of individuals with DMPN.
5. Conclusions
Our study showed a significant relationship of midfoot and ankle sagittal angles between heel rise and gait. In addition, those individuals with dorsiflexed midfoot and ankle at peak single-limb heel rise had greater dorsiflexion of the midfoot and ankle throughout the stance phase of gait compared to individuals with plantarflexed midfoot and ankle during heel rise. These results provide support for using the heel rise task to help identify movement dysfunction of midfoot and ankle during gait. Additional research is needed to clarify the proposed progression of midfoot movements to midfoot injury or deformity.
Supplementary Material
Acknowledgments
The authors acknowledge Kathryn Bohnert, Darrah Snozek, and Christopher Sorensen for their contributions to the subject recruitment and data collection and Haley Brogan, Jessica Stumpf, Kaitlyn Winter, Jadean Hoff, Hana Bernhardson, Nick Youmans, Mary Ellis, Whitney Korgan, and Nick Schroeder for their assistance with data processing. This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK107809, F32 DK123916) and the Research Division of the Program in Physical Therapy, Washington University School of Medicine, Saint Louis, Missouri.
6. References
- [1].Rao S, Saltzman C, Yack HJ, Segmental foot mobility in individuals with and without diabetes and neuropathy, Clin. Biomech 22(4) (2007) 464–471. 10.1016/j.clinbiomech.2006.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hastings MK, Woodburn J, Mueller MJ, Strube MJ, Johnson JE, Sinacore DR, Kinematics and kinetics of single-limb heel rise in diabetes related medial column foot deformity, Clin. Biomech 29(9) (2014) 1016–1022. 10.1016/j.clinbiomech.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hastings MK, Mueller MJ, Woodburn J, Strube MJ, Commean P, Johnson JE, et al. , Acquired midfoot deformity and function in individuals with diabetes and peripheral neuropathy, Clin. Biomech 32 (2016) 261–267. 10.1016/j.clinbiomech.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rao S, Saltzman CL, Yack HJ, Relationships between segmental foot mobility and plantar loading in individuals with and without diabetes and neuropathy, Gait Posture. 31(2) (2010) 251–255. 10.1016/j.gaitpost.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DiLiberto FE, Tome J, Baumhauer JF, Houck J, Nawoczenski DA, Individual metatarsal and forefoot kinematics during walking in people with diabetes mellitus and peripheral neuropathy, Gait Posture. 42(4) (2015) 435–441. 10.1016/j.gaitpost.2015.07.012. [DOI] [PubMed] [Google Scholar]
- [6].DiLiberto FE, Tome J, Baumhauer JF, Quinn JR, Houck J, Nawoczenski DA, Multi-joint foot kinetics during walking in people with diabetes mellitus and peripheral neuropathy, J. Biomech 48(13) (2015) 3679–3684. 10.1016/j.jbiomech.2015.08.020 [DOI] [PubMed] [Google Scholar]
- [7].Veves A, Murray H, Young M, Boulton A, The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study, Diabetologia. 35(7) (1992) 660–663. 10.1007/BF00400259 [DOI] [PubMed] [Google Scholar]
- [8].Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A, Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial, Diabetes Care. 23(5) (2000) 606–611. 10.2337/diacare.23.5.606. [DOI] [PubMed] [Google Scholar]
- [9].Pecoraro RE, Reiber GE, Burgess EM, Pathways to diabetic limb amputation: basis for prevention, Diabetes Care. 13(5) (1990) 513–521. 10.2337/diacare.13.5.513 [DOI] [PubMed] [Google Scholar]
- [10].Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ, Predictive value of foot pressure assessment as part of a population-based diabetes disease management program, Diabetes Care 26(4) (2003) 1069–1073. 10.2337/diacare.26.4.1069 [DOI] [PubMed] [Google Scholar]
- [11].Bus SA, Lavery L, Monteiro-Soares M, Rasmussen A, Raspovic A, Sacco I, IWGDF Guideline on the prevention of foot ulcers in persons with diabetes, Diabetes Metab. Res. Rev (2019). 10.1002/dmrr.3269 [DOI] [PubMed] [Google Scholar]
- [12].Angin S, Demirbüken İ, Chapter 23 - Ankle and foot complex, in: Angin S, Şimşek IE, Comparative Kinesiology of the Human Body, Academic Press; (2020), 411–439. 10.1016/B978-0-12-812162-7.00023-0 [DOI] [Google Scholar]
- [13].Toro B, Nester CJ, Farren PC, The status of gait assessment among physiotherapists in the United Kingdom, Arch. Phys. Med. Rehab 84(12) (2003) 1878–1884. 10.1016/S0003-9993(03)00482-9 [DOI] [PubMed] [Google Scholar]
- [14].Coutts F, Gait analysis in the therapeutic environment, Man. Ther 4(1) (1999) 2–10. 10.1016/S1356-689X(99)80003-4 [DOI] [PubMed] [Google Scholar]
- [15].Krebs DE, Edelstein JE, Fishman S, Reliability of observational kinematic gait analysis, Phys. Ther 65(7) (1985) 1027–1033. 10.1093/ptj/65.7.1027 [DOI] [PubMed] [Google Scholar]
- [16].Neumann D, Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation, second ed., Elsevier Health Sciences, 2013. [Google Scholar]
- [17].Jeong HJ, Mueller MJ, Zellers JA, Yan Y, Hastings MK, Heel rise and non-weight bearing tasks to assess foot and ankle function in people with diabetes mellitus and peripheral neuropathy, Under revision in Physical Therapy (PTJ) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carson M, Harrington M, Thompson N, O’connor J, Theologis T, Kinematic analysis of a multi-segment foot model for research and clinical applications: a repeatability analysis, J. Biomech 34(10) (2001) 1299–1307. 10.1016/S0021-9290(01)00101-4. [DOI] [PubMed] [Google Scholar]
- [19].Beckham G, Suchomel T, Mizuguchi S, Force plate use in performance monitoring and sport science testing, New Stud. Athl 29(3) (2014) 25–37. [Google Scholar]
- [20].Van den Bogert A, De Koning J, On optimal filtering for inverse dynamics analysis, Proceedings of the IXth Biennial Conference of the Canadian Society for Biomechanics, (1996) 214–215. [Google Scholar]
- [21].Bevans JS, Biomechanics and plantar ulcers in diabetes, Foot. 2(3) (1992) 166–172. 10.1016/0958-2592(92)90067-Y. [DOI] [Google Scholar]
- [22].Mueller MJ, Tuttle LJ, LeMaster JW, Strube MJ, McGill JB, Hastings MK, et al. , Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial, Arch. Phys. Med. Rehab 94(5) (2013) 829–838. 10.1016/j.apmr.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kanade R, Van Deursen RWM, Harding K, Price P, Walking performance in people with diabetic neuropathy: benefits and threats, Diabetologia. 49(8) (2006) 1747–1754. 10.1007/s00125-006-0309-1. [DOI] [PubMed] [Google Scholar]
- [24].Maluf KS, Mueller M, Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers, Clin. Biomech 18(7) (2003) 567–575. 10.1016/S0268-0033(03)00118-9. [DOI] [PubMed] [Google Scholar]
- [25].Hébert-Losier K, Newsham-West RJ, Schneiders AG, Sullivan SJ, Raising the standards of the calf-raise test: a systematic review, J. Sci. Med. Sport 12(6) (2009) 594–602. 10.1016/j.jsams.2008.12.628 [DOI] [PubMed] [Google Scholar]
- [26].Yang D, Vandongen Y, Stacey M, Effect of exercise on calf muscle pump function in patients with chronic venous disease, Brit. J. Surg 86(3) (1999) 338–341. 10.1046/j.1365-2168.1999.00993.x. [DOI] [PubMed] [Google Scholar]
- [27].Butterworth PA, Urquhart DM, Landorf KB, Wluka AE, Cicuttini FM, Menz HB, Foot posture, range of motion and plantar pressure characteristics in obese and non-obese individuals, Gait Posture 41(2) (2015) 465–469. 10.1016/j.gaitpost.2014.11.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.