BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection elicits a humoral immune response characterized by neutralizing antibodies against the spike protein. While prior studies have demonstrated detectable antibodies against SARS-CoV-2 for few months post infection, there is no consistent data regarding the long-term persistence of protective antibody titers.
OBJECTIVE
We investigated the longevity of antibody responses to SARS-CoV-2 in a cohort of patients who received care for COVID-19 in the outpatient, emergency department (ED), or hospital settings.
METHODS
The study cohort consisted of post-COVID-19 patients enrolled in a prospective research registry at the Mount Sinai Health System in New York City from July 20, 2020, to April 13, 2021. Eligible participants were ≥ 18 years old, spoke English or Spanish, and had laboratory-confirmed COVID-19 infection and had not received COVID-19 vaccination before enrollment. Potentially eligible participants were contacted by mail followed by a phone call or approached in person while receiving acute or post-COVID-19 care. None of the participants received COVID-19 vaccination during the period of follow-up.
We used an enzyme-linked immunosorbent assay (ELISA) based on the stabilized full-length spike protein to assess SARS-CoV-2 antibodies from at least 30 days to up to 13 months after acute infection. ELISA results were characterized in discrete titers of 1:80, 1:160, 1:320, 1:960, or ≥ 1:2880. Starting in December 2020, the laboratory started using arbitrary units (AU/mL, 0–125) to report ELISA results. A titer ≥ 1:320 (≥ 16 AU/mL) has been correlated with ≥ 90% neutralizing activity in a microneutralization assay and has been used as a criterion for convalescent plasma donation [1].
We calculated the unadjusted rates of an immunoglobulin G titer ≥ 1:320 and used a generalized estimating equation model to estimate the probability of a positive test over time according to the site of acute COVID-19 care after adjusting for age, sex, race, and ethnicity (SAS 9.4, Cary, NC). The study was approved by our Institutional Review Board.
RESULTS
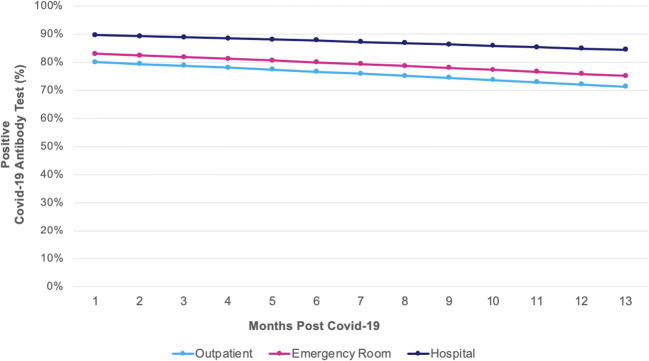
Of 758 participants enrolled in the cohort, 35 (5%) were missing the date of COVID-19 diagnosis, 75 (10%) received COVID-19 vaccination before enrollment, and 28 (4%) did not undergo antibody testing, leaving a cohort of 620 participants who contributed 1195 antibody tests. The mean age was 49 years, 64% were female, self-identified as 53% white, 16% Black, and 20% Latinx (Table 1). Overall, 90% (95% CI: 84–94%) and 83% (95% CI: 67–92%) of patients had positive antibody tests at 6 and 13 months post-COVID-19, respectively. Antibody titers were relatively stable over 13 months post infection (odds ratio [OR]: 0.95, 95% CI: 0.90–1.01 for every 30 days post-COVID-19) (Fig. 1). The adjusted probability of having a positive antibody test at 13 months was 71% (95 CI: 75–91%) for outpatients, 75% (95% CI: 62–85%) for ER patients, and 84% (95% CI: 74–91%) for hospitalized patients. Positive tests were more likely among inpatients (OR: 2.18, 95% CI: 1.23–3.86), but not ER patients (OR: 1.21, 95% CI: 0.75–1.97) compared to outpatients.
Table 1.
Characteristics of COVID-19 Patients (N = 620)
| Characteristic | Value |
|---|---|
| Time from diagnosis to antibody test, months, mean (SD) | 7.0 (2.8) |
| Age, years, mean (SD) | 49.0 (13.5) |
| Female, no. (%) | 397 (64) |
| Race/ethnicity, no. (%) | |
| White | 327 (53) |
| Black | 97 (16) |
| Hispanic | 123 (20) |
| Multiracial/other | 73 (12) |
| Education, no. (%) | |
| ≤ 12 years | 75 (12) |
| > 12 years | 531 (88) |
| Income, no. (%) | |
| < $25,000 | 85 (15) |
| $25,000–$60,000 | 100 (18) |
| $60,000–$150,000 | 198 (36) |
| > $150,000 | 168 (30) |
| Former smoker, no. (%) | 184 (31) |
| Comorbidities, no. (%) | |
| Hypertension | 160 (26) |
| Diabetes | 57 (9) |
| Asthma | 155 (25) |
| Cancer | 60 (10) |
| Body mass index, no. (%) | |
| Normal weight | 212 (37) |
| Overweight | 176 (28) |
| Obese | 214 (35) |
| Site of COVID-19 care, no. (%) | |
| Outpatient | 261 (42) |
| Emergency room | 160 (26) |
| Hospital | 199 (32) |
Figure 1.
Adjusted predicted probability of positive neutralizing antibody test over time according to site of acute care. Rates of positive antibody test against SARS-CoV-2 remained high up to 13 months post-COVID-19. Patients treated in the hospital had significant higher rates of positive results compared to those who were managed in the outpatient setting. There were no significant differences among patients treated in the emergency department or managed as outpatients for acute COVID-19.
Discussion
We found that neutralizing titers against the spike protein of SARS-CoV-2 remained relatively high with modest declines for up to 13 months post infection. Patients with more severe disease showed higher rates of positivity over time suggesting a more robust antibody response.
Understanding immunological memory post-COVID-19 can provide insights into the risk for reinfection and the potential durability of vaccines. While early reports suggested that antibody titers may rapidly decrease [2], other studies found high antibody titers several months after acute COVID-19 infection [3–6]. However, many of these studies included relatively small samples and reported overall rates of positivity at titers that may not confer neutralizing activity. We extend these results by showing persistent rates of a positive antibody response (at potentially neutralizing titers) for up to 13 months after acute infection. It is important to note that while titers > 1:320 have been correlated with high neutralizing activity in vitro, it is unclear if they provide protection against reinfection. We did not have information on severity of outpatient illness; thus, we were not able to compare the immune response in asymptomatic vs. mild or moderate symptomatic patients. Additionally, we cannot exclude that severity of acute COVID-19 was associated with the likelihood of participating in the institutional registry.
Declarations
Conflict of Interest
Dr. Juan Wisnivesky has received consulting honoraria from Sanofi, GSK, Banook, and Atea and research grants from Sanofi, Regeneron, and Arnold Consultants. Other authors report no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lee WT, Girardin RC, Dupuis AP, et al. Neutralizing Antibody Responses in COVID-19 Convalescent Sera. J Infect Dis. Published online October 26, 2020. doi:10.1093/infdis/jiaa673 [DOI] [PMC free article] [PubMed]
- 2.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ripperger TJ, Uhrlaub JL, Watanabe M, et al. Orthogonal SARS-CoV-2 Serological Assays Enable Surveillance of Low-Prevalence Communities and Reveal Durable Humoral Immunity. Immunity. 2020;53(5):925–933.e4. doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequalae of COVID-19. Nature. Published online April 22, 2021:1-8. doi:10.1038/s41586-021-03553-9 [DOI] [PubMed]
- 6.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529). 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed]