Abstract
Cyclodextrins (CDs) are biocompatible, cyclic oligosaccharides that are widely used in various industrial applications and have intriguing interfacial science properties. While CD molecules typically have low surface activity, they are capable of stabilizing emulsions by inclusion complexation of oil-phase components at the oil/water interface, which results in Pickering emulsion formation. Such surfactant-free formulations have gained considerable attention in recent years, owing to their enhanced physical stability, improved tolerability, and superior environmental compatibility compared to conventional, surfactant-based emulsions. In this review, we critically describe the latest insights into the molecular mechanisms involved in CD stabilization of Pickering emulsions, including covering practical aspects such as methods to prepare CD-based Pickering emulsions, lipid encapsulation, and relevant stability issues. In addition, the rheological and textural features of CD-based Pickering emulsions are discussed and particular attention is focused on promising examples for drug delivery, cosmetic, and nutraceutical applications. The functionality of currently developed CD-based Pickering emulsions is also summarised, including examples such as antifungal uses, and we close by discussing emerging possibilities to utilize the molecular encapsulation of CD-based emulsions for translational medicine applications in the antiviral and antibacterial spaces.
Keywords: Cyclodextrin, Pickering emulsion, Lipids, Stability, Drug delivery, Antifungals
Introduction
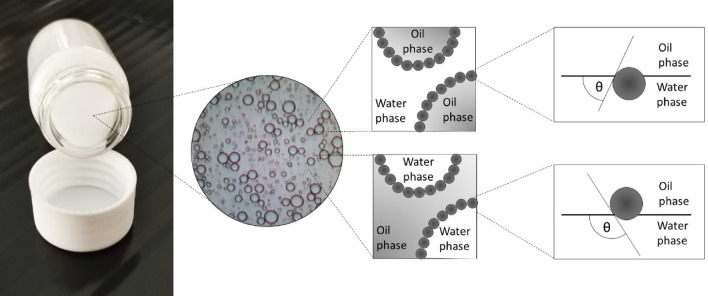
Emulsions are widely employed as dosage forms that provide parenteral nutrition and support administration of poorly soluble drugs, especially ones belonging to Class IV of the biopharmaceutical classification system. Furthermore, they are important formulations for the topical administration of such drugs to the skin and mucosal surfaces [1, 2]. As a disperse system consisting of two immiscible liquids, emulsions are thermodynamically unstable and require stabilization by surfactants. The list of surfactants suitable for pharmaceutical application is restricted, especially in the case of emulsions aimed for parenteral or ocular administration. Even under such restrictions, surfactants may give rise to various side effects, including acute hypersensitivity reactions, peripheral neurotoxicity, and membrane-damaging effects leading to haemolysis and tissue irritation [3–5]. Furthermore, some of the surfactants can be potentially hazardous for the environment [6]. This has prompted research aimed at replacing conventional surfactants with other materials that can act as emulsifiers. In this regard, Pickering emulsions, i.e. emulsions stabilised by insoluble solid particles (Fig. 1), have emerged as a possible substitute for the ones formulated with traditionally used surfactants [7]. Their increasing popularity lays in the fact that Pickering emulsions show higher resistance to coalescence and Oswald ripening, resulting in higher stability compared to that of conventional, surfactant-based emulsions. In addition, a high internal phase volume fraction (> 74 %) in Pickering emulsions can be prepared, which accounts for the enhanced cargo loading capacity [1, 7–9]. In the pharmaceutical field, Pickering emulsions are considered suitable for topical administration to the skin and mucosal surfaces of the body as well as for oral administration [2, 10] Different particles are used to stabilise Pickering emulsions, including those of inorganic origin like clay, hydroxyapatite, magnesium hydroxide, magnetic iron oxides, and silica. However, such inorganic particles are mostly of submicron dimension and may accumulate in an organism upon administration since they are not biodegradable, which raises potential safety issues [11]. In this context, biodegradable polysaccharide-based particles like chitosan, cellulose, and starch are receiving attention as more suitable options along with other nature-derived materials such as pollen grains and processed derivatives thereof [1, 12, 13].
Fig. 1.
Representative structure of a Pickering emulsion. Depending on the characteristics of the stabilising particles, O/W or W/O type emulsions can be formed. Particles dominantly wetted with the water phase (three-phase angle, θ < 90°) will form O/W type emulsions, while particles dominantly wetted by the oil phase (θ > 90°) will form W/O type emulsions
Another attractive option for the development of biocompatible Pickering emulsions is the use of cyclodextrins (CDs) as emulsion stabilizers. CDs are a group of structurally related, cyclic oligosaccharides that consist of 6, 7, or 8 glucopyranose units (αCD, βCD, and γCD, respectively), obtained by bacterial starch degradation. Chemical modification of naturally occurring CDs has resulted in numerous derivatives with enhanced functionality and improved safety profiles [14–16]. The structural features of CDs enable the spontaneous formation of supramolecular complexes in an aqueous environment by reversible inclusion of lipophilic drug molecules, or more often a sterically compatible lipophilic moiety, in the lipophilic central cavity of the CD molecule. The resulting inclusion complex acts as a carrier of the included drug in aqueous environments, thereby enhancing solubility, dissolution rate, and chemical stability, which contribute to drug bioavailability [17–19]. Furthermore, inclusion complexation can modify unfavourable organoleptic properties of drug molecules, converting liquids or even gases into technologically more acceptable solids and reducing tissue irritation at the drug administration site, which can improve patient compliance [20–22]. Besides serving as multifunctional pharmaceutical excipients, CDs are emerging as building blocks for advanced delivery systems and enabling a new class of active pharmaceutical ingredients [23–25].
The aim of this article is to discuss the application of CDs in the development of Pickering emulsions that are suitable for biomedical applications. In contrast to previously mentioned solid particles, CDs stabilise Pickering emulsions by inclusion complexation with lipids and resulting self-assembly of the complexes formed at the oil/water (O/W) interface [26–28]. The mechanism by which CDs stabilize such systems will be discussed and illustrated by drawing on examples from the literature. Although some other reviews on this topic are available [7, 10, 29], the aim of this paper is to provide an updated focus on emulsions containing oils that are approved for oral and topical administration, thus being suitable for development of pharmaceutical drug dosage forms, cosmetic formulations, and nutraceuticals. Functional properties of such CD-based Pickering emulsions will be discussed, including providing details on their preparation method, physical stability, rheological behaviour, drug release properties, and other functional characteristics that are of importance for applications such as drug delivery, cosmetics, and related fields.
Interaction of cyclodextrins with lipids
The hydrophobic central cavity of a CD molecule can encapsulate various lipophilic molecules by inclusion complex formation. Besides various low molecular-weight drugs that are the most common guests [30, 31], inclusion complexation can also occur with different lipids, including free fatty acids, their mono-, di-, and triglycerides, and phospholipids [29, 32–34]. Such interactions are dependent on the structure of both the CD and lipid. In general, one CD molecule can embed 5 to 6 CH2 moieties, as αCD, βCD and γCD all have the same torus depth of 7.9 Å [35]. However, some deviations have been observed that were caused by the different diameters of the CD central cavity. In the case of αCD and its derivatives, the narrower space of the central cavity (diameter 4.7–5.3 Å, volume 174 Å3) causes extension of the fatty acid chain, thus requiring a longer CD channel than the actual chain length of the fatty acids. The opposite case has been observed for βCD and its derivatives that have a larger central cavity (diameter 6.0–6.5 Å, volume 262 Å3) in which fatty acids are slightly twisted, leading to better intramolecular interactions than with αCD [29, 33]. The larger dimensions of the γCD central cavity (diameter 7.5–8.3 Å, volume 427 Å3 ) permit further coiling of the fatty acid hydrocarbon chain. By that, the whole hydrocarbon chain up to 18 C in length, as well as the carboxyl end of the fatty acid, may be included inside the γCD central cavity [36]. Consequently, a molar ratio of 3, 2, and 1.5 was observed for linoleic acid (C18:2) inclusion complexes with αCD, βCD, and γCD, respectively [32]. Due to steric reasons, αCD and derivatives have higher efficiency for interactions with short (≤ C8) and long-chain (≥ C12) fatty acids and their glycerides. On the other hand, βCD and derivatives are more suitable for the inclusion complexation of (poly)unsaturated fatty acids and their glycerides as well as for intermediate chain-length fatty acids (≈ C10) [29]. Besides fatty acids, CDs can interact with their glycerides, but the complexation affinity decreases in the following order: fatty acids > mono- >di- >triglycerides [29, 32]. αCD and βCD soluble complexes will be formed only with free fatty acids and monoglycerides, while triglycerides and phospholipids will form insoluble complexes. Chemically modified CDs, especially methylated derivatives, are very potent solubilizers of fatty acids. For example, randomly methylated αCD (RAMEA) and βCD (RAMEB) increased the aqueous solubility of C12–C18 fatty acids by 250–1660 and 240–910 times, respectively [32]. Furthermore, dimethyl βCD (DIMEB) shows an outstanding solubilising effect for phospholipids and can extract them from bilayers and even disrupt unilamellar liposomes at bulk concentrations above 50 mM [34, 37]. Beside solubilization and extraction of phospholipid molecules from lipid bilayers, CD complexation may significantly enhance the chemical stability of included fatty acids, which is of particular importance for polyunsaturated ω-fatty acids [38, 39]. βCD complexation enhances both thermal and oxidative stability of polyunsaturated ω-fatty acids in complex matrices like natural oil and oleogels [39–41]. Furthermore, complexation enhances their technological properties by converting them into compactible powders [41, 42]. Finally, the formation of CD/lipid inclusion complexes is of substantial importance for CD-based stabilization of Pickering emulsions [26–28] and will be discussed in following section.
The high affinity of βCD and its methylated derivatives for cholesterol complexation is well described in the literature and accounts for toxicological effects caused by parenteral administration [32, 43–46]. The insoluble βCD/cholesterol complexes aggregate in the kidney as intracellular needle-like crystals that cause kidney damage, while cholesterol depletion from erythrocyte membranes caused by methylated βCDs can result in haemolysis [16, 47]. According to currently available data, inclusion complexation of cholesterol with CDs has no relevance to the stabilization mechanism of Pickering emulsions, although such insoluble complexes may find application in topical formulations. Nevertheless, cholesterol complexation by CDs is currently under investigation as a new therapeutic approach to treat some inherited metabolic diseases like Nieman-Pick type C disease [48, 49] and atherosclerosis [50], and to enhance artificial insemination [51].
Mechanism of Pickering emulsion stabilization by CDs
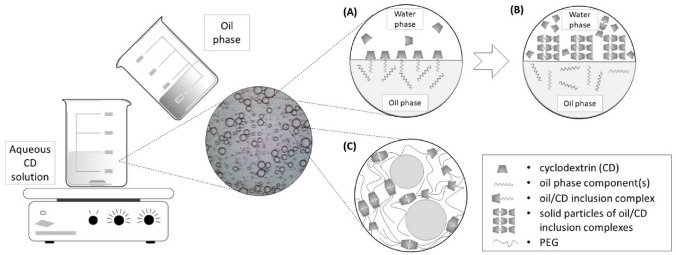
CDs alone, with the exception of methylated βCD derivatives, do not decrease the air/water surface tension of water [28]. The mechanism of Pickering emulsion stabilization by CD includes several steps (Fig. 2). The first step occurs immediately after mixing the oil and aqueous CD solution and involves inclusion complexation of lipophilic components of the oil phase by CDs. The formed complexes usually involve only partial inclusion of the lipophilic component in one cyclodextrin molecule, resulting in a structure resembling that of a surface-active molecule: CD, within the complex formed, corresponds to the hydrophilic head while the lipophilic chain protruding from the CD cavity behaves as the lipophilic tail. Such structures are aligned at the oil/water (O/W) interface like conventional surfactants where the CD part of the complex formed is immersed in the water phase of the emulsion, while the lipophilic tail is oriented toward the oil phase. This happens at low CD concentration and is believed to be the main mechanism of the emulsifying activity of highly soluble cyclodextrin derivatives [28]. At higher CD concentrations, especially in the presence of naturally occurring CDs, inclusion complexation continues resulting in poly(pseudo)rotaxane formation. Such structures, formed by the threading of more than one CD molecule along the length of the lipophilic chain molecule, are suggested to occur in several emulsion-containing organic solvents like tetradecane (TD). In this case, the molar stoichiometry of the complexes formed are approximately 6.8:1 and 5.7:1 for αCD/TD and βCD/TD, respectively, and remain as such even if the oil fraction increases [27, 28]. Similar structures may be presumed for inclusion complexes of liquid paraffin and isopropyl myristate [26]. As such, the complexes are generally poorly soluble and can precipitate at the O/W interface, resulting in Pickering emulsion formation. Typically, such crystalline structures formed at the O/W interface can be observed by optical microscopy and cause a significant decrease in O/W interphase tension [26, 52–55]. Leclercq [56] proposed the term “colloidal tectonics” to describe this assembling process occurring at the O/W interface, which is based on molecular recognition operating at the level of the complementary building blocks or tectons (CDs as hydrophilic and lipids as hydrophobic tectons), and provides an infinite variety of reversible (supra)colloidal systems with predictable, versatile and switchable properties. At higher CD concentrations, such solid particles are present in the aqueous phase of emulsions too, forming a three-dimensional network that contributes to the rheological properties and physical stability of the emulsion [27, 57]. The morphological properties of the formed solid inclusion complexes depend on the type of CDs employed. For example, αCD/tetradecane microrods formed at the O/W interface appear as microrods that are longer than 100 μm whereas the microcrystals of βCD/tetradecane inclusion complexes are typically shorter than 10 μm [27]. Smaller βCD microcrystals have denser packing at the O/W interface, in turn providing better stabilization of the emulsion formed. In general, the formation of such well-defined crystalline structures may be expected in systems containing a single type of lipid, like isopropyl myristate. However, in complex systems like natural oils, well-structured crystals are not observed as the simultaneous presence of various compounds does not allow the crystals to grow [58].
Fig. 2.
Mechanism of Pickering emulsion stabilisation with CDs: A formation of inclusion complexes at the O/W interface at low CD concentration and/or early stages of formation; B crystallisation of oil/CD complexes at the O/W interface occurring at high CD concentration and/or longer interaction time; and C emulsion stabilisation with CD/PEG hydrogel
In some cases, depending on the CD and oil type, inclusion complexation of the O/W interphase may lead to the formation of colloidosomes or CD beads, which are microparticles containing the oily core and a self-forming film containing partial inclusion complexes of CD and triglycerides. Such behaviour is typical for emulsions containing αCD and soybean oil [59] but may also occur in the case of both αCD and βCD with liquid paraffin and silicone oil [60]. The properties of such beads as a delivery system are reviewed elsewhere [61, 62].
Besides natural CDs, polymeric CDs may also stabilize the emulsions. The polymeric βCD prepared by polycondensation with epichlorohydrin under alkaline conditions (Mw of 105 g mol− 1 and polydispersity of 1.5) adsorbs at the O/W interface, resulting in an elastic layer that prevents coalescence and results in se emulsions [63]. The polymer prepared by crosslinking DMβCD with phenyl diisocyanate self-assembles in water to form nanoparticles with a diameter of 30–50 nm. By increasing the polymer concentration from 0.01% to 0.1 % (m/m), primary particles assemble into larger nanogel particles with a 120-nm diameter that show surface activity at the air/water interface. The observed surface activity of the CD nanogel is more pronounced than that of pure DIMEB. The CD nanogel absorbs at the O/W interface with effective saturation, forming a strongly interconnected network that results in Pickering emulsion formation, even when used at a low concentration of 0.1 % (m/m) [64].
The type of Pickering emulsion formed is governed by the wettability of the particles formed at the O/W interface. The particles must be partially wetted by both liquids to effectively stabilize the emulsion, or otherwise will be completely dispersed in a single phase and unable to obtain a stable emulsion. The wettability is usually characterised by the three-phase contact angle (θ, Fig. 1). For particles stabilizing the Pickering emulsion, θ is equivalent to the HLB (hydrophilic-lipophilic balance) value for surfactants [1, 2]. Hydrophilic particles with θ < 90° favour the formation of O/W emulsions because most of the particles are wetted by the aqueous phase of the emulsion. Such systems are usually formed when hydrophilic CD derivatives are employed as emulsion stabilizers [65]. For example, θ values for solid particles obtained by the inclusion complexation between soybean oil, squalene, isopropyl myristate, and liquid paraffin with βCD are 58°, 63°, 64°, and 68°, respectively [53, 54]. On the other hand, lipophilic particles with θ > 90° favour the formation of a W/O emulsion, like complexes of soybean oil, squalene, and liquid paraffin with lipophilic cyclodextrin derivatives such as triacetyl-βCD (TAβCD), tripropanoyl-βCD (TPβCD), and tributanoyl-βCD (TBβCD). Their θ values range from 104° to 142° [53, 54]. Here, it must be noted that the presented θ values are only valid for given cases, as the nature of the oil used directly affects the value of θ. Furthermore, the oil viscosity is a damping factor for stabilizing particles that anchor at the O/W interface as it slows down the particle’s diffusion and adsorption [1].
Another approach that may be used in the development of CD-based Pickering emulsions is the formation of stabilizing particles by inclusion complexation of some other compounds, besides the ones present in the oil-phase of the emulsion. Hu et al. prepared stabilizing nanoparticles of 60-nm diameter through inclusion complexation of short linear glucans (SLGs) with βCD [66]. The θ of the nanoparticles can be finely tuned in the 75–15° range by changing the CD to SLG ratio. Such particles facilitated preparation of Pickering emulsions of soybean oils with enhanced storage stability that have potential for applications in the food, cosmetic, and pharmaceutical fields [66]. Furthermore, inclusion complexes of αCD and sodium salts of n-alkyl sulphate or sulfonate surfactants result in films at the air/water interface of remarkable viscoelasticity [67]. The viscoelasticity of the films can be finely tuned by adjusting the bulk composition, temperature, alkyl chain length, and headgroup type, clearly demonstrating the versatility of such complexes. In addition, inclusion complexation of βCD with Tween 20 with consequent self-assembly driven by hydrogen bonding with excessive βCD resulted in nanoparticles that can produce stable Pickering emulsions of lavender essential oil. The optimal weight ratio of βCD to Tween 20 appears to be 1:0.3 and provides the possibility to incorporate up to around 44 % of lavender essential oil [68].
Nowadays, various novel CD derivatives with enhanced functionalities are being developed. The outer surface of CDs may be chemically modified, which provides them with amphiphilic properties. A series of amphiphilic CDs modified at their secondary face, known as skirt-shaped CDs, decreased the Miglyol/water interfacial tension [69]. A recent example is an octadecenyl succinic acid-βCD (ODSβCD) derivative that was obtained thorough βCD esterification with octadecenyl succinic anhydride in alkaline conditions [70]. The modified derivatives provide much more stable emulsions than those obtained with the parent βCD. Furthermore, they may act as a dual-function excipient by encapsulation of various antioxidants, including β-carotene and α-tocopherol, that can enhance the oxidative stability of the formed emulsions among numerous possibilities. Notably, the inclusion complexation of antioxidants does not impair their emulsion-forming capacity with this novel βCD derivative and, in fact, their physical stability is somewhat improved [71, 72].
Preparation of CD-based pickering emulsions
An overview of selected Pickering emulsions stabilised with CDs loaded with drugs or nutraceuticals is provided in Table 1. CD-based Pickering emulsions are usually prepared by a simple procedure. In most cases, the CDs are first dissolved/dispersed in the water phase of the emulsion, sometimes under moderate heating. Then, the inner phase of the emulsion is added, followed by sample homogenization, most often using rotor-stator type devices, operating in the 10,000–20,000 rpm range for 3 to 5 min [26, 53–55, 65]. Only in some cases, homogenization is obtained by applying lower shear stress, using vortex type devices operating at 3200 rpm [73] or an overhead stirrer operating at 6000 rpm [74]. In most cases, homogenization occurs at an ambient temperature.
Table 1.
Overview of Pickering emulsions stabilised with CDs that were developed for delivery of active pharmaceutical ingredients or nutraceuticals
| Active ingredient | Cyclodextrin | Emulsion | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Concentration | Aqueous phase | Oil phase | Type | Emulsification method | Droplet size | Consistency | Stability | ||
| - | βCD | 0–40% (w/w) |
Purified water (70–30%, w/w) |
Soybean oil (30–70%, w/w) |
O/W |
Rotor/stator homogenizer operating at 10,000 rpm for 5 min |
NA | NA | NA | [ 65 ] |
|
Squalene (30–70%, w/w) |
O/W | |||||||||
|
Liquid paraffin (30–70%, w/w) |
O/W | |||||||||
|
Camphor (0.25%, w/w) |
αCD βCD γCD |
0.1 mol/L |
Purified water (20%, w/w) |
O 1 – 30% (w/w) of soybean oil O 2 – 5% (w/w) of candelilla wax and 45% (w/w) of soybean oil |
O 1 /W/O 2 |
Rotor/stator homogenizer operating at 2,000 rpm for 1 min |
NA | NA | Temperature and drug dependent, blank emulsion with αCD is the most stable | [ 75 ] |
|
Econazole nitrate (1%, w/w) |
αCD βCD γCD |
13.93% (w/w) | Purified water (42.43%, w/w) containing 0.21% (w/w) of benzoic acid as preservative |
Liquid paraffin or isopropyl myristate (42.43%, w/w) |
O/W | Rotor/stator homogenizer operating at 11,500 rpm for 1 min | 9–16.1 μm, depending on the composition | Fluid or cream, depending on the composition | Better stability for emulsion formed with isopropyl myristate, γCD produced the most stable systems | [ 26 ] |
|
Bupivacaine hydrochloride (3%, w/w) |
αCD βCD |
5% (w/w) | Purified water |
Medium-chain triglycerides, castor oil, isopropyl myristate, diethyl sebaceate, limonene, or octyl salicylate (40%, w/w) |
O/W | Rotor/stator homogenizer operating at (20,000 rpm for 2 min) | 3.0–28.3 μm, depending on the composition | Liquid or cream | Dependent upon composition | [ 55 ] |
|
Captopril (5%, w/w) |
αCD βCD γCD |
15 – 25% (w/w) |
Purified water (42–46%, w/w) |
Isopropyl myristate, liquid paraffin, soybean oil (35 – 39%, w/w) |
O/W | Rotor/stator homogenizer operating at 10,000 rpm for 5 min | NA | NA | Stable formulation with 25% (w/w) of βCD | [ 54 ] |
| Tadalafil* |
αCD βCD |
2 – 6% (w/V) | Purified water (90 or 80%, v/v) | Sweet almond oil (10 or 20%, V/V) | O/W | Overhead stirrer operating at 6,000 rpm for 5 min followed by freeze-drying with addition of gelatine and mannitol to obtain tablets | 0.45 and 0.62 μm for drug free and drug loaded formulation | Solid upon freeze-drying | Emulsions formulated with βCD showed superior stability to those with αCD | [ 74 ] |
|
Miconazole nitrate or Miconazoctylium bromide (1%, w/w) |
βCD | 10% (w/w) |
Purified water (45%, w/w) |
Liquid paraffin, Carvacrol or terpinene-4-ol (45%, w/w) |
O/W | Vortex stirrer operating at 3200 rpm for 1.5 min |
35, 39 and 36 μm for Liquid paraffin, carvacrol or terpinene-4-ol, respectively |
Cream | Temperature and oil-type dependent, (liquid paraffin < terpinene-4-il < carvacrol) | [ 73 ] |
| Miconazoctylium bromide (1%, w/w) and undecylenic acid (10% w/w) | βCD | 10% (w/w) | Purified water (45% w/w) |
Liquid paraffin, carvacrol, or terpinene-4-ol (45%, w/w) |
O/W | Vortex stirrer operating at 3200 rpm for 1.5 min | 13 – 39 µm depending on the content, where drug loaded samples showed smaller droplet size (13–18 µm) |
Creams for liquid parafilm and carvacrol samples; Lotion for terpinene-4-ol based emulsion |
NA | [ 76 ] |
|
Miconazole, miconazole nitrate or econazole nitrate (1 or 2%, w/w) |
αCD/PEG hydrogel | 2.9/80.9/16.2 PEG 20,000 to water to αCD weight ratio | 49.33% (w/w) of αCD/PEG hydrogel preserved with 0.2% (w/w) of benzoic acid | Liquid paraffin (49.47%, w/w) | O/W | Rotor/stator homogenizer operating at 11,500 rpm for 3 min | 3.8–5.9 μm depending on the drug type | Cream | Excellent stability with less than 0.5% of the water phase separated after 12 months | [ 77 ] |
| Lavander essential oil | βCD/Tween 20 nanocrystals formed at 1:0.3 weight ration | ** | αCD/PEG 2 000 hydrogel (1:1) ** | Lavender essential oil ** | O/W | Nanocrystals were first prepared by moderate mixing at 300 rpm for 3 h, followed by oil addition and rotor/stator homogenization at 10,000 rpm for 5 min | 205 nm | NA | Stable over 60 days | [ 68 ] |
| Lactobacillus dellbrueckii | Span 80 and βCD |
Span 80 at 10% (V/V) in W 1 , βCD at 5% (w/V) in W 2 |
W 1 – L. dellbrueckii dispersion in sterile water (18%, V/V) W 2 – βCD solution (40%, V/V) |
Canola oil (36%, V/V) |
W 1 /O/W 2 |
W 1 /O emulsion was homogenised at 6,000 rpm for 4 min W 1 /O/W 2 emulsion was homogenised at 8,000 rpm for 4 min using rotating blade homogenizer |
4.43 – 13.04 μM | NA | Moderately stable | [ 78 ] |
| Lactobacillus casei | Span 80 and βCD |
Span 80 at 10% (V/V) in W 1 , βCD at 2.5% (w/V) in W 2 |
W 1 – L. dellbrueckii dispersion in sterile water (20–30%, V/V) W 2 βCD solution (40–60%, V/V |
Corn oil | W 1 /O/W 2 |
W 1 /O emulsion was homogenised at 3226 × g for 4 min, W 1 /O/W 2 emulsion was homogenised at 3226 × g for 4 min using rotating blade homogenizer |
2.9 – 3.3 μm, depending on the composition | NA | NA | [ 79 ] |
|
Lutein (0.5 mg/mL) |
βCD | 0.5 – 2.5% (w/V) | Purified water | Sunflower oil (60 – 80%, V/V) | High internal phase O/W emulsion | Rotor/stator homogenizer operating at 15.000 rpm for 3 min | 6.31 ± 0.61 at 2.5% (w/V) of βCD | NA | Dependent on βCD concentration | [ 9 ] |
NA Not analysed
*The drug was dissolved in an optimal Pickering emulsion formulation at 15 mg/mL. The emulsion was freeze-dried to yield tablets containing 10 mg drug per tablet
**The author did not provide data about the added quantity of water, so the exact composition of the samples cannot be deduced
When the emulsion contains a drug, it is usually dissolved in the oil phase [55, 73, 75, 77]. Only in some cases, the drug has been added to the aqueous phase or oil/water mixture before CD addition [26, 54]. With such parameters, the emulsion droplet size is usually broad, depending on the CD and oil type, their concentration, and the applied homogenization procedure.
An O/W Pickering high internal phase emulsion may be prepared using sunflower oil at an oil volume fraction of 75 % (v/v) and βCD in the concentration range of 0.5%–2.5 % through one-step high-speed shearing procedure achieved using a rotor-stator homogenizer operating at 15,000 rpm for 3 min [9].
When the Pickering emulsion is stabilised by nanoparticles formed between the CD and some other compound not originating from the oily phase, the nanoparticles are first prepared in the aqueous phase. Pertinent examples include nanoparticles obtained by inclusion complexation of Tween 20 or short linear glucans with βCD[66, 68]. The oil phase is then added to the aqueous dispersion of nanoparticles, followed by emulsification using a high-shear homogenizer.
It must be noted that currently used preparation methods do not take account into the mechanisms responsible for Pickering emulsion formation in the presence of CDs. Particle formation at the O/W interface is time-consuming, so short homogenisation procedures that are usually applied do not seem appropriate. They also cause high shear stress in the system that is necessary to disperse the oil and may oppose the formation of stabilising particles at the O/W interface. Perhaps, the preparation of Pickering emulsions stabilised with CDs should include two or more consecutive steps. The first step must provide the optimal conditions for stabilising particle formation at the O/W interface. The following step(s) should be directed toward the reduction of the size of emulsion droplets. In the case of Pickering emulsions and emulsions in general, this may be obtained by using rotor-stator, high pressure, ultrasonic, or microfluidic devices [1, 80]. However, the operating parameters should be adjusted to not disturb previously formed particles by application of excessive shearing stress. Also, an increase in the sample temperature caused by homogenisation may lead to the destruction of formed particles. Perhaps, membrane emulsification, where the oil phase is pressed through a microporous membrane into the continuous phase containing CDs, would be more beneficial [81]. For the moment, such approaches are not applied in the development of CD-based Pickering emulsions, representing an unexplored area requiring further research and offering new opportunities.
Stability issues of CD-based pickering emulsions
One of the most significant advantages of Pickering emulsions is their high physical stability. In general, particles with 0.01–10-µm diameters and an intermediate θ attach strongly to the O/W interface with high detachment energies. Such surface coatings prevent coalescence of the dispersed phase, resulting in Pickering emulsions with superior stability to emulsions stabilised by surfactants [26, 80]. However, such general performance claims cannot be directly related to Pickering emulsions stabilised by CDs because it must be considered that particles containing CDs are obtained by consecutive association phenomena, i.e. inclusion complexation and complex precipitation at the O/W interface. As both processes are reversible, several factors may affect the capacity of CDs to stabilize the emulsion and even result in complete phase separation.
Some early studies showed that temperatures higher than 50 °C negatively affect the inclusion complexation of liquid paraffin components with βCD, resulting in a lack of emulsification, while efficient emulsification can be obtained at a lower temperature [82]. Similarly, complete dissolution of tetradecane/αCD microcrystals occurs at temperatures higher than 80 °C, but upon cooling they recrystallise in the form of spherical structures [28]. In the case of particles stabilizing emulsions of olive oil and αCD, their dissolution started at temperatures above 30 °C when the oil content in the emulsion was greater than 25 % (m/m) [58]. Those examples show that the temperature at which Pickering emulsions based on CDs are prepared and stored should be carefully considered.
Regarding particle stabilizers of Pickering emulsions, parameters like wettability, size and shape, concentration, surface roughness, and charge are crucial considerations for emulsion formation and stability [1, 2, 80]. In general, the size of the stabilizing particles should be at least one order of magnitude smaller than the targeted size of emulsion droplets. The smaller size of stabilizing particles accounts for the formation of Pickering emulsions with smaller emulsion droplets, thus showing higher physical stability.
For a series of Pickering emulsions of octanol, decane, and toluene stabilised with 10 % of βCD, the diameter of emulsion droplets and the size of stabilizing particles were 15.14/1.35 μm, 7.22/1.32 and 12.58/2.98, respectively [83]. The most stable emulsion was the one with decane, showing a volume fraction of the emulsion phase that was greater than 90 % over a longer period. Besides particle size, the most pronounced decrease in interfacial tension and relatively high θ was observed for a βCD/toluene system, which accounted for the comparatively enhanced physical stability of this sample [83]. These findings motivate that a general design principle of CD-stabilised Pickering emulsions should be reducing the size of the formed solid particles, as particles smaller than 2 μm are not affected by gravity. Furthermore, nanoscale Pickering emulsions have also been reported [68, 84]. In the case of nanoparticles having θ in the range of 30–150°, the thermal energy of Brownian motion is several orders of magnitude lower than the desorption energy, which facilitates their irreversible adsorption at the O/W interface [85]. Currently, the exact parameters affecting the size of the forming particles at the O/W interface in systems containing oils and CDs are not known and should be further investigated. It may be presumed that the type and concentration of both the oil and CD, as well as the stirring rate, pH, and temperature at which the emulsion is prepared may be significant factors.
Regarding the shape of the particles, the first Pickering emulsions were constructed using spherical particles, but stabilization has also been obtained with different non-spherical particles, like rods, fibres, and cubes. It is recognised that deformable particles cover the O/W more efficiently, accounting for enhanced Pickering emulsion stability. In this sense, microgels are particularly efficient [1]. As already mentioned, polymeric CD derivatives stabilize emulsions more efficiently than parent derivatives, owing to their enhanced flexibility [63, 64].
Information about the shape of the formed particles in Pickering emulsions stabilised by CDs is scarce. Mathapa and Paunov demonstrated that different particle shapes occur during inclusion complexation of tetradecane with αCD and βCD [27, 28]. At the same concentration (10 mM), αCD formed microrods up to 100 μm long that were stacked into lamellar structures, while in the case of βCD, shorter crystals (up to 10 μm) were observed and did not form lamellar structures. The authors proposed that the microrods are formed by threading the CD molecule through the tetradecane chain. Such structures assemble into nano- and microrods over time. This process is guided by hydrogen bonding. The formation of αCD/tetradecane microrods was fast, resulting in the complete coverage of the oil droplet with microcrystals after 12 min, while the process was much slower in the case of βCD. Although αCD/tetradecane microrods produced a higher decrease of interfacial tension than those with βCD, higher emulsion stability was observed in the latter case, as smaller βCD/tetradecane microparticles adsorb and pack tightly on the O/W interface, which generates a steric barrier that prevents droplets coalescence and ensures effective emulsion stabilization [27]. By contrast, long and rigid αCD/tetradecane microrods cannot effectively pack when adsorbed at the O/W interface. This increases the likelihood of exposure of bare oil patches on the droplets, leading to coalescence and low emulsion stability. γCD does not form microcrystals at the O/W interface as its central cavity is probably too large to hold on to the tetradecane chain. Other factors affecting the formation and structure of CD/tetradecane microcrystals are temperature, pH, and the presence of chaotropic agents. As already discussed, elevated temperature promotes the degradation of CD/toluene microcrystals due to the breakdown of hydrogen bonds and de-threading produced by elevated kinetic energy in the system. The αCD/tetradecane microrods form preferably at neutral pH (7.16), in which condition the hydroxyl groups are not disturbed and fully participate in the cooperative hydrogen bonding that is necessary for enhanced threading and growth of the microrods. By contrast, βCD/tetradecane microrods are preferably formed at low pH (1.98), which is necessary to disrupt the intermolecular hydrogen bonds in the βCD. The free βCD hydroxyl groups can participate in inter-cooperative hydrogen bonding that results in an increased rate of threading and subsequent precipitation. Finally, the presence of a chaotropic agent like urea promotes the disruption of the microrods [28]. In the case of more pharmaceutically acceptable oils, like medium-chain triglycerides, castor oils, isopropyl myristate, diethyl sebacate, and octyl salicylate, rod-like crystals and large cubic crystals are observed in the case of complexes with αCD, while cubic crystals occur mainly in the case of complexes with βCD [55]. Conversely, Badr-Eldin et al. showed that the particles formed with sweet almond oil and αCD were smaller and shorter than those formed with βCD [74]. Moreover, emulsions containing 10 and 20 % of the oil phase stabilised with αCD contained smaller droplets, ranging from 0.37 to 0.97 μm, and were less stable than the corresponding samples, stabilised with the same concentration of βCD, containing larger droplets in the range from 0.68 up to 2.43 μm [74]. It seems that, besides the shape and size of the stabilising particles, their adsorption energy at the O/W interface has a significant impact on Pickering emulsion stability. The free energy of adsorption, ΔGd, represents the energy required to remove a spherical particle of radius r and three-phase contact angle θ from an O/W interface with an interfacial tension of γOW and is defined by the following Eq. [1]:
| 1 |
It may be concluded that the solid particles formed between the sweet almond oil and βCD cause a more significant reduction in the interface tension and/or have a higher θ than those with αCD and accordingly form Pickering emulsions with enhanced stability. However, as Badr-Eldin et al. [74] did not measure the O/W interfacial tension and θ, this assumption cannot be supported by the experimental data and requires future testing for validation.
The concentration of the stabilizing particles is another important parameter as it directly affects their coverage at the O/W interface. In general, a high concentration of the stabilizing particles is desired, as it increases the surface coverage of emulsion droplets and facilitates the formation of a network structure, which is advantageous for reducing the size and improving the stability of the emulsion [2, 85]. This, in general, requires the use of a relatively high CD concentration, at least in the case of parent CD derivatives. In many cases, the parent βCD is added to the emulsions in concentrations exceeding its aqueous solubility [26, 53–55, 73, 83]. Laurent et al. suggested that an excess of βCD in the aqueous phase of the emulsion acts as a reservoir during and after the emulsification process, providing the source of free βCD that is available for complexation with the lipid components of the oil phase [82]. This contributes to the formation of the stabilizing particles at the O/W interface, providing a sufficient coverage of the dispersed droplets. When the interface is fully saturated, the formed oil/CD particles increase the viscosity of the aqueous phase of the emulsion, thus further stabilising the system, as already discussed above in other examples.
The addition of surfactants may also have a wide range of effects on the CD stabilization ability of Pickering emulsions. Li et al. investigated the effect of Tween 80 and soybean lecithin (SL) on the stability of medium-chain triglyceride (MCT) emulsions stabilised with αCD [86]. In the absence of the surfactants, an emulsion containing a low oil fraction (φO = 0.03) was unstable while that containing a higher oil phase (φO = 0.2) appeared stable. As both formulations contained a similar concentration of αCD (i.e. 5 and 6 %, respectively) and had a comparable size of the emulsion droplets (mean spherical diameter of 18.6 and 22.0 μm, respectively), better stability of the emulsion containing the higher volume of the oil phase was attributed to its higher viscosity. The addition of 0.5 % Tween 80 enhanced the stability of the emulsion with a low oil fraction. This was attributed to the synergistic effect of αCD/MCT and Tween 80 and was explained by several phenomena. First, the interaction of αCD and Tween 80 and consequent assembly of the complexes into bilayers stabilised by hydrogen bonds increased the viscosity of the emulsion viscosity. Furthermore, the addition of Tween 80 decreased the zeta potential of the system (from − 32.2 mV to -40.7 mV), providing additional electrostatic stabilization. Finally, Tween 80 also caused a further reduction in the interfacial tension (from 29.62 to 16.47 mN m− 1). In the case of other surfactants, the effects are not straightforward. The effect of SL on CD-based emulsion stability is concentration-dependent. At 0.1–1.0 %, SL impaired the stability of emulsions containing a high oil fraction, which was caused by the replacement of αCD/MCT with αCD/SL particles at the interface. At high SL concentrations (3.0%–5.0 %), emulsions were stable against coalescence and phase separation, which was caused by a significant drop in the interfacial tension (from 23.09 to less than 2.00 mM m− 1) [86]. This example confirms that inclusion complexation between CDs and surfactants should be carefully considered in the case of CD-based Pickering emulsions, as it may provide a window of opportunity to enhance emulsion stability through changes in viscosity and interfacial tension, by using physiologically acceptable surfactants which alone cannot produce sufficiently stable emulsions. For example, βCD enhanced the stability of a sunflower oil emulsion (φO = 0.20) prepared with 4 % (w/w) citric acid esters of fatty acids as an emulsifier (Citrem® 3307). At a 1:1 (w:w) ratio with Citrem®, βCD decreased the droplet size of the resulting emulsion (from 4.3 ± 0.5 to 2.1 ± 0.8 μm) and simultaneously increased its viscosity (from 26.1 ± 0.9 to 33.8 ± 0.4 mPa s− 1). All of these modulations in turn enhanced storage stability while decreasing, to some extent, the oxidative stability of the emulsion, which required the addition of antioxidants as well [87].
Increasing the emulsion viscosity is another approach that may oppose the coalescence of dispersed oily droplets, thus enhancing stability [85]. The use of a αCD/PEG hydrogel appears particularly suitable for the development of stable Pickering emulsions [77]. This hydrogel is formed by heating PEG 20,000, water and αCD in a weight ratio of 2.9:80.9:16.2 at 80 °C. After cooling at 3 °C, the homogenised mixture results in a stable, transparent hydrogel. In this case, the formation and structure of the αCD/PEG hydrogel may also be explained by the colloidal tectonics concept [56, 88]. As shown in Fig. 2 C, the columnar CD domains (crystallites) act as physical cross-links between the polymer chains not included, leading to the formation of a supramolecular hydrogels that provides a good platform to obtain Pickering-like emulsions [56]. Simple addition of liquid paraffin (φO = 0.5) followed by homogenization at 11,500 rpm for 3 min yields a stable emulsion of the O/W type (Fig. 2). Even when loaded with azole antifungal drugs, at 1 and 2 % concentrations, the emulsions showed remarkable stability over 12 months of storage [77].
Nanoemulsions as systems with droplet sizes of the dispersed phase in the range of 20–500 nm, have evolved as a robust carrier for the delivery of a wide range of drugs with poor aqueous solubility and provide attractive features such as enhanced loading capacities, long-term stability, increased bioavailability, and controlled release of loaded drugs [89]. CDs can form robust Pickering nanoemulsions with high loading capacity in two different synergistic ways. For example, Tween 20/βCD nanoparticles, formed during nanoemulsion preparation by inclusion complexation of Tween 20 with βCD followed by hydrogen bonding driven assembly with an excessive amount of βCD, adsorbed at the O/W interface, which allowed Pickering nanoemulsion preparation with nanodroplets of 205-nm diameter and encapsulated up to 44.42 % of lavender essential oil. In addition, an αCD/PEG 20 kDa gel formed a three-dimensional network between nanodroplets, which helped to sustain system stability by resisting coalescence. With such an approach, the stability of Pickering nanoemulsions was extended from 15 to 60 days [68].
Effect of drug addition on CD-based Pickering emulsion formation and stability
The addition of a drug may have a destabilising effect on Pickering emulsions containing CDs due to competitive interactions with lipid components from the oil phase for inclusion complexation. Such effects were observed in the case of O/W/O multiple emulsions loaded with camphor [75]. The inner oily phase consisted of 30 % (w/w) of soybean oil dispersed in 20 % (w/w) of 0.1 M αCD, βCD, or γCD aqueous solution. The outer oily phase was prepared with 5 % (w/w) of candelilla wax dissolved in 45 % (w/w) of soybean oil. Camphor was loaded in the inner oil phase at 0.47 mM concentration. The stability of the as-prepared emulsions was affected by the type of CD used, storage temperature, and the presence of camphor. The αCD and βCD emulsions without camphor were considered stable, but their stability was disturbed by the addition of camphor. X-ray powder diffraction (XRPD) analysis showed different crystalline structures of solid particles in the emulsions with and without camphor. The authors assumed that the affinity of camphor for inclusion complexation with CDs (Ks are 80, 430, and 370 M− 1 for αCD, βCD, and γCD, respectively) was strong enough to induce the destabilization of emulsions by competing with the complexation of fatty acid residues of soybean triglycerides, which were insufficiently stable. As a possible approach to overcome such limitations, the authors suggested the use of αCD in the development of Pickering emulsions. αCD usually has a low affinity for inclusion complexation of high molecular-weight drugs [75].
However, some other authors demonstrated that stable emulsions may be obtained when the drug concentration in the oily phase is relatively low. In such systems, CD complexation of the components from the oil phase is dominant, as they are prevalent in the system with respect to the drug [26]. Leclercq and Nardello-Rataj prepared a series of Pickering emulsions containing isopropyl myristate of liquid paraffin as the oil phase (φO = 0,4243) that was stabilised with 13.39 % of αCD, βCD, or γCD. All formulations contained 0.21 % of benzoic acid as a preservative and 1 % of econazole nitrate as an active ingredient. Such emulsions showed optimal viscosity and were physically stable after storage at 60 °C. In general, the stability of emulsions was determined simultaneously by CD type and oil type. Emulsions formulated with isopropyl myristate were more stable than those with liquid paraffin and the most stable emulsions were obtained with γCD [26]. The authors did not prepare and evaluate emulsions without the drug, but all CD-stabilised emulsions with econazole were suitable for dermal applications.
Hu et al. prepared a series of emulsions containing 40 % (w/w) of the oil phase, 5 % (w/w) of CD, and 3 % (w/w) of bupivacaine using different oils and CDs [55]. MCTs and diethyl sebacate formed stable emulsions only with αCD as its central cavity size is appropriate to tightly fit their alkyl chains. Surprisingly, the emulsion formed with αCD isopropyl myristate, another linear chain compound, became phase-separated after only one day. The authors suggested that, as isopropyl myristate contains only one ester group, the hydrogen bonding was not sufficient to stabilize the complex at the O/W interface while, in the case of diethyl sebacate, a compound containing two ester groups, hydrogen bonding was more pronounced and thus suitable to stabilize the emulsion. Castor oil was efficiently emulsified by both αCD and βCD, which was attributed to the presence of the hydroxyl group on the alky tail of the castor oil. Hydrogen bonding of this hydroxyl group with those of the CD molecule reinforced the complexes formed at the O/W interface, resulting in stable Pickering emulsions. Limonene and octyl salicylate contain a hexa-carbon ring in their structure and should interact more preferably with βCD. However, a stable emulsion was only obtained with octyl salicylate and βCD while, in emulsions formulated with limonene, separation occurred after only one minute. Most of the emulsions formed showed mean particle sizes in the range of 1.9–5 μm and low polydispersity that may be attributed to the intense sample homogenization conditions that were used, i.e. 20,000 rpm for 5 min. The exception was the emulsion of diethyl sebacate and αCD, which was polydisperse with a mean particle size of 28.3 μm [55]. In these cases, the presence of the drug was not recognised as a destabilizing factor with respect to emulsion structure. In fact, it seems that the drug presence may aid in emulsion formation. Leclerq et al. used βCD to develop Pickering emulsions of liquid paraffin, carvacrol, and terpinene-4-ol as the base and aimed to increase the antimicrobial activity of miconazole nitrate and miconazoctylium bromide [73]. In all cases, the water to oil to CD weight ratio was 45/45/10 and resulted in a white emulsion of the O/W type with average droplet sizes of 35, 39, and 36 μm, respectively. SEM analysis revealed that the stabilizing particles in the case of liquid paraffin were granular solids, while those with carvacrol and terpinene-4-ol were of lamellar shape. The emulsions were stable at human body temperature of 37 °C for more than two weeks, while at an elevated temperature of 60 °C, the samples showed instability after only 6 h. The destabilization mechanism was studied by multiple light scattering. For liquid paraffin, a creaming process was detected that was coupled with a coalescence phenomenon, while for carvacrol and terpinene-4-ol, only coalescence was observed. The addition of a drug molecule at 1 % (w/w) had distinct, drug-specific effects on the emulsion droplet size. In the case of miconazole nitrate, the droplet size remained the same as in emulsions without the drug, while in the case of miconazoctylium bromide, a reduction in the droplet size down to the 17–26 μm range was observed, indicating that miconazoctylium bromide may interact with the O/W interface stabilised by βCD/oil particles. Drug addition did not affect the shear viscosity, regardless of its type [73]. However, it should be noted that inclusion complexation of miconazole nitrate with βCD is pH-dependent and the Ks of the complexes formed was relatively low, decreasing from 97 M− 1 at pH 6 down to 39 M− 1 at pH 9 [90]. On the other hand, miconazoctylium bromide alone can form emulsions, but they are of limited stability [73]. The presented examples clearly show that the drug concentration and, in particular the structural features of the drug, should be carefully evaluated when developing Pickering emulsions stabilised with CDs.
Rheological and textural features of CD-based Pickering emulsions
The rheological properties of CD-based Pickering emulsions are related to their microstructure and are crucial for understanding the physical stability of the emulsion during processing and storage. Furthermore, rheological features determine the emulsion quality and functionality, providing acceptable sensory characteristics and spreadability of the formulation during application at the skin or mucosal surfaces and controlling drug release, which determines the therapeutic potential of such dosage forms [91].
In general, Pickering emulsions stabilised by CDs show viscoelastic rheological behaviour, exhibiting high viscosity at low shear stress and low viscosity at high shear stress [26, 73, 77]. This is particularly important for topical drug administration, providing highly viscous formulations during storage (ensuring product stability) and low viscosity when applied to skin (contributing to comfort during product application). Moreover, CD-based Pickering emulsions are thixotropic, recovering their viscosity after the product is applied which enables controlled drug delivery. The thixotropy of these emulsions indicates the presence of a three-dimensional network structure of interconnected clusters that are formed between oil droplets and stabilising particles [26].
The consistency of CD-stabilised Pickering emulsions is dependent on both the CD and oil type and concentration. For example, αCD-stabilised Pickering emulsions of MCTs, castor oil, and dietyl sebacate (5 % w/w CD, 40 % w/w oil phase) appeared as liquid lotions, while those containing the same amount of octylsalicilate and βCD appeared as a cream [55]. The texture of Pickering emulsions containing liquid paraffin or isopropyl myristate as the oil phase (φO = 0.4243) and parent CDs (13.93 % w/w) varied from fluid to cream, depending on both the oil and CD type [26]. Pickering emulsions stabilised with αCD/PEG 20,000, containing around 50 % (w/w) of liquid paraffin as the oil phase, all appeared as creams, regardless of the type and concentration of the loaded drug [77]. The viscosity recorded in the high-shear region, ranging from 102 to 103 s− 1, may be used to predict the spreadability of the formulation on skin surfaces. In the case of Pickering emulsions stabilised by αCD/PEG 20,000 hydrogel, the viscosity remains below 2 Pa s, indicating its suitability for topical skin applications [77]. More precisely, the viscosities of such emulsions loaded with econazole are between the values obtained by commercially available products (Micatin® cream and Pevaryl® cream, respectively). However, previously mentioned CD-stabilised Pickering emulsions containing liquid paraphing or isopropyl myristate showed higher rubbing resistance than the Pevaryl® cream but remained in the suitable range for dermal administration [26]. The dependence of the shear viscosity on CD type is not straightforward and it seems to be primarily related to the droplet size of the formed Pickering emulsions. In general, low shear viscosity is observed for emulsions with very fine droplets. It is likely that the emulsions with large droplet sizes are more viscous as a lower concentration of the CD/oil inclusion complexes is needed to saturate the O/W interface. The remaining insoluble particles formed in the system create a complex three-dimensional structure that increases the shear viscosity of the samples. In the case of emulsions with less oily droplets, the situation is opposite [26], i.e. the presence of the drug does not seem to affect the shear viscosity of CD-based Pickering emulsions [73, 77].
In the case of Pickering emulsions stabilised with SLG/βCD nanoparticles, the apparent viscosity of the emulsions increased over the whole shear rate range (0.1–100.0 s− 1) as a function of increased βCD proportion in the stabilising nanoparticles, applied nanoparticle concentration, and oil phase fraction. Shear-thinning behaviour was also observed, indicating deflocculation of the oil droplets in the emulsions. The magnitudes of G′ (storage modulus) values were higher than those of G″ (loss modulus) values for all fabricated samples, suggesting a typical gel-like structure. Moreover, the G′ and G″ values were independent of the oscillatory shear frequency. The increasing trend of G′ and G″ with respect to the tested parameters corresponds to the enhanced gel strength, reflecting the superior physical stability of the emulsions formed [66]. Such rheological behaviour contrasts with that observed for Pickering emulsions formed by direct inclusion complexation of CDs and oil components [53, 57, 65, 92]. In the latter cases, variations in the G′ and G″ values as a function of the shear frequency were observed and the systems showed liquid-like behaviour (G′ < G″) in the low-frequency and solid-like behaviour (G′ > G″) in the high-frequency regions and such emulsions appeared less stable in general.
High internal-phase Pickering emulsions of sunflower oil stabilised with βCD presented somewhat different rheological behaviour. The rheological properties of emulsions are typically determined by the continuous phase, however, in the case of these emulsions, the distance between oil droplets was reduced and the stabilising particles had a significant influence on the corresponding rheological properties. In the strain sweep test, both the G′ and G″ values of the emulsions prepared at different βCD concentrations (0.5–2.5 %, w/w) were stable at first and then gradually decreased. In the linear viscoelastic region (0.1–100 Hz), the G′ values were superior to the corresponding G″ values, displaying elastic-like characteristics. Both G′ and G″ values were βCD concentration-dependent over the entire frequency range, indicating that the gel strength of the formed high internal-phase Pickering emulsions was positively related to the βCD concentration. In all cases, the apparent viscosities of the emulsions decreased with increasing shear rate and the formulation developed with 2.5 % (w/w) βCD exhibited the highest apparent viscosity over the entire shear rate range. This corresponds to the most compact microstructure and the strongest mechanical performance of such high internal-phase emulsions [9].
Functionality of CD-based Pickering emulsions
In most cases (Table 1), CD-stabilised Pickering emulsions have been developed as semisolid bases for topical administration of drugs, mainly azole antifungals, to the skin [26, 73, 77]. The emulsions formed are usually of the O/W type. Drug release from the Pickering emulsions, in general, is prolonged as the solid particle layer at the O/W interface forms a shell that controls the release of drugs entrapped in the oily particles [2]. Prolonged drug release has also been observed for CD-based Pickering emulsions loaded with bupivacaine, in which case less than 30 % of the loaded drug was released after 48 h [55]. In this example, the type of CD (αCD or βCD at 5 % w/w) or the oil type (MCTs, castor oil, diethyl sebacate, or octyl salicylate; φO = 0.4) did not significantly affect the observed drug release rate, which can be rationalized by two factors. First, stabilizing particles formed by CD complexation of oil-phase components formed a solid film consisting of tightly packed particles that covered the droplet surface. Second, the drug solubility in water (0.14 mg/mL) and selected oils (ranging from 41.9 mg/mL up to 99.4 mg/mL) was different, favouring drug partitioning into the oil phase of the emulsion. As such, a tight particulate film at the O/W interface and dominant partitioning of the drug into the oil phase of the emulsion synergistically reduced the bupivacaine release rate.
The cumulative amount of drug permeation across a porcine skin model showed the following order: octyl salicylate/βCD emulsion > diethyl sebacate/αCD emulsion = MCTs/αCD emulsion > castor oil/αCD emulsion = castor oil/βCD emulsion. Moreover, the cumulative extent of drug permeation in the case of the emulsion formulated with octyl salicylate was significantly higher than for the other formulations, indicating that octyl salicylate could substantially enhance skin penetration of bupivacaine. However, a rabbit skin irritation test showed that this formulation caused the most serious irritation of all formulations [55]. As topical bupivacaine is used for local anaesthesia, the formulation needs to target the nerves in the dermal layer without favouring systemic drug absorption. Hence, the drug amount retained in the skin is considered to be a more appropriate factor to assess the anaesthetic efficiency of CD-stabilised Pickering emulsions. From that point of view, emulsions formulated with diethyl sebacate and αCD appeared to be the most efficient. As oils are known skin permeation enhancers, the authors compared the skin permeability of the drug released from a plain oil solution and from CD-stabilised Pickering emulsions. It was demonstrated that the bupivacaine permeation was more efficient in the case of Pickering emulsions, suggesting a synergistic effect of CDs on skin permeability [55].
Leclercq and Nardello-Rataj demonstrated that, for Pickering emulsions formulated with liquid paraffin or isopropyl myristate as the oil phase (φO = 0.4243) and parent CDs (13.93 % w/w) as stabilisers, the release of econazole nitrate and its antimicrobial effect is highly dependent on the type of CD used. It seems that γCD impairs drug release from the resulting Pickering emulsions, regardless of the oil type. As such, the biocidal activity of such formulations against Candida albicans and Streptococcus aureus is absent. This effect is also pH dependent. On the contrary, emulsions formulated with αCD or βCD were as effective or even better than the commercially available econazole nitrate (Pevaryl® cream), stabilised with surfactants. The plain Pickering emulsion bases, without the loaded drug, showed no antimicrobial activity [26]. Pickering emulsion stabilised with αCD/PEG 20,000 hydrogel, loaded with econazole nitrate or miconazole nitrate, showed comparable efficiency against C. albicans as commercially available formulations of the same drug (Pevaryl® and Micatin® creams, respectively) [77]. With the use of phytochemicals like carvacrol and terpinene-4-ol as the oil phase of the Pickering emulsion (φO = 0.44), the antimicrobial activity of the loaded drug may be further enhanced [73]. Very stable emulsions without petro-sourced surfactants or modified silica nanoparticles were obtained with carvacrol and βCD (45 and 10 % w/w, respectively). Such a relatively simple formulation was shown to be 2-fold more effective on C. albicans and methicillin-resistant Staphylococcus aureus (MRSA) than the commercial cream (Monistat Derm®) containing the same dose of miconazole nitrate (1 % w/w). Furthermore, it showed high activity against Escherichia coli, whereas the commercial formulation was completely inactive. The CD-stabilised Pickering emulsion was found to provide a synergistic effect against C. albicans and has the potential to disrupt preformed methicillin-resistant Streptococcus aureus (MRSA) biofilms [73].
A recent report from the same group evaluated the addition of undecylenic acid (10 % w/w) to βCD stabilized Pickering emulsions containing the same phytoantimicrobial oils [76]. These very stable emulsions showed acceptable pH and viscosity properties for topical skin applications. The carvacrol/undecylenic acid emulsion containing 1 % (w/w) of miconazoctylium bromide appeared to be the most antimicrobial active formulation. In addition to notable activity against E. coli, a potent anti-staphylococcal activity was observed against MRSA, with up to 390 % greater inhibitory potency compared to commercially available formulations containing azole antifungal drugs (Monistat Derm™ Cream). Furthermore, this Pickering emulsion caused the eradication of MRSA biofilms. Such outstanding activities are attributed to the synergistic action of undecylenic acid (acting as an enzyme inhibitor) and carvacrol (acting as a membrane fluidizer) with the drug. Against C. albicans, this Pickering emulsion also showed comparable activity to commonly prescribed drugs. Nowadays, the pharmaceutical industry is focused on the development of more environmentally friendly pharmaceuticals that incorporate more sustainable processes and drugs. From this perspective, this highly efficient, surfactant-free, silica-free, and fully organic O/W Pickering emulsion can be considered a good example of how product candidates can be realized within a biobased economy and transition away from conventional development pathways [76]. Further development of such CD-based Pickering emulsions for clinical applications is currently underway as well.
Besides developing them for topical drug administration to the skin, CD-stabilised Pickering emulsions may be considered as dosage forms with the potential for transdermal drug delivery. Taguchi et al. demonstrated that the flux of captopril through hairless mouse skin from Pickering emulsions developed using 20 % (w/w) of βCD, 37 % (w/w) of isopropyl myristate, and 43 % (w/w) of water was approximately 6-times higher than that of the same emulsion stabilised with 20 % (w/w) of the surfactant, polyoxyethylene (60) hydrogenated castor oil [54]. Both formulations contained 5 % (w/w) captopril. Isopropyl myristate by itself is a well-known permeation enhancer, but this study demonstrated that emulsified isopropyl myristate alone is not sufficient to obtain maximum enhancement of captopril skin permeation, which occurs only when βCD is present in the system and indicates a degree of synergistic action between isopropyl myristate and βCD to facilitate transdermal delivery of captopril.
Badr-Eldin et al. employed CD-stabilised Pickering emulsions as a base to formulate oral tablets of tadalafil, which is a poorly soluble drug used for erectile dysfunction management [74]. In the first phase, the authors optimised the formulation of CD-based Pickering emulsions using full factorial design. An emulsion containing 20 mL of sweet almond oil, 6 g of αCD, and 75 mL of water, had an emulsified droplet size of 0.45 μm and was determined to be the most stable, while providing the highest drug solubility of 18.94 ± 0.55 mg/mL. However, the drug was loaded at a lower concentration of 15 mg/mL, to avoid drug precipitation during processing. In the second phase, gelatine and spray-dried mannitol were added to the aqueous phase of the emulsion in different weight ratios, ranging from 6:0 to 0:6. The emulsions were loaded into 13 mm blister packs and subjected to lyophilization, which resulted in tablets with friability and hardness ranging from 0.23 to 0.71 % and 3.30–6.27 kg, respectively. The disintegration time of the tablets was rapid, ranging from 21 up to 158 s. This approach resulted in a significant improvement in the in vitro release of tadalafil in 0.1 M HCl. In such conditions, the drug alone did not dissolve, while the drug release from the tablets was dependent on the gelatine to mannitol ratio. Tablets containing a higher ratio of mannitol (3:3, 2:4 and 1:5, respectively) showed faster drug release, which was completed within 15 to 20 min in the dissolution test. Tablets were stable during 6 months of storage at ambient temperature and 60 % relative humidity. After reconstitution in water, the tablets formed O/W emulsions with droplet sizes comparable to those before storage. Such a formulation has the potential to efficiently enhance the oral bioavailability of poorly soluble drugs, like that already demonstrated for self (micro)emulsifying drug delivery systems, without the risks of irritations connected with the use of conventional surfactants [74].
In addition to delivering active pharmaceutical ingredients, Pickering emulsions stabilised with CDs may be a suitable platform for the development of different nutraceutical formulations. For example, high internal-phase Pickering emulsion stabilised with βCD have been reported to be an effective delivery platform to protect lutein, a dietary supplement capable of reducing the progression of age-related macular degeneration, against UV-mediated degradation [9]. The observed protective capacity was related to the properties of the layer formed by insoluble complexes of βCD and sunflower oil triglycerides at the O/W interface that caused light scattering. Moreover, CDs can form multiple W1/O/W2 emulsions suitable for encapsulation of living cells, like probiotic bacteria. For example, Lactobacillus delbruckii was entrapped in the inner aqueous phase of a W1/O/W2 Pickering emulsion stabilised with βCD [78]. Multiple emulsions consisted of 60 % W1/O emulsion that contained 30 % (V/V) of the aqueous bacterial culture (108 CFU/mL) and 60 % (V/V) of canola oil emulsified with the aid of 10 % (V/V) Span 80. Such emulsions were then emulsified in 40 % (V/V) of an external aqueous phase (W2) containing 2.5 or 5 % (w/V) βCD. The stability of multiple emulsions stabilised with βCD was superior to those formulated with Tween 80 (1 or 2 %, V/V) as an emulsifier in the outer aqueous phase. Moreover, the viability of bacteria in the βCD-stabilised multiple emulsions was unaffected by the formulation components, while in those containing Tween 20, the viability decreased as functions of time and emulsifier concentration. Micron-sized multiple Pickering emulsions entrapping Lactobacillus casei were also prepared using βCD as the emulsifier in the outer aqueous phase [79]. The primary emulsion contained 45 % (V/V) sterile water containing bacterial culture, 45 % (V/V) corn oil, and 10 % (V/V) Span 80 and was dispersed in an outer aqueous phase containing 2.5 % (w/V) βCD, at different W1/O to W2 phase ratios. The formulation prepared at a 60:40 ratio provided effective protection of the entrapped L. casei against bile salts, acid environments, and elevated temperature. These findings suggest that edible CD-based multiple Pickering emulsions have great potential as a platform to support more efficient delivery of probiotic bacteria to the gut.
Concluding remarks and outlook
While CD-based emulsions have already demonstrated excellent possibilities for healthcare and biotechnology applications such as topical drug delivery and cellular encapsulation, there is excellent potential to continue developing CD-based emulsions for new translational medicine applications, especially related to infectious disease treatment. The colloidal tectonics architecture of such systems provides the possibility to design an infinite number of new, smart, and flexible formulations having high environmental compatibility. As discussed herein, CDs can interact with a variety of lipids and one promising direction involves developing CD-based emulsions that incorporate medium-chain fatty acids and monoglycerides (C6 to C12 chain length), which are among the most biologically potent lipids found in nature and exhibit anti-infective activity against membrane-enveloped viruses and bacteria through membrane-disruptive properties [93–96]. While antimicrobial lipids are highly active as components within supramolecular structures [e.g. in micelles that naturally form above the critical micelle concentration (CMC)], they tend to lose membrane-disruptive properties upon dilution in physiological environments due to micellar disaggregation and are typically inactive in monomeric form [97] below their corresponding CMC (see, e.g. biophysical and antiviral data in Refs. [98] and [99], respectively). To address this issue as well as to improve lipid solubility, a variety of liposomal, emulsion, and solid lipid nanoparticle strategies have been devised to encapsulate antimicrobial lipids with varying degrees of success for food and medical applications, including topical, oral, and parenteral administration uses [100]. Notably, the existing emulsion formulations have been limited to using conventional surfactants (e.g. Tween 20) and there is outstanding potential to explore developing CD-based emulsions to (1) create new types of supramolecular assemblies involving antimicrobial lipids (see, e.g. complexes of medium-chain fatty acids and peptide amphiphiles [101]with tuneable nanoscale morphologies and functionalities); (2) achieve controlled release of antimicrobial lipids; and (3) enable dilution-stable formulations to facilitate the membrane-disruptive activities of antimicrobial lipids in physiological environments upon therapeutic administration. Since certain medium-chain fatty acids and monoglycerides also exhibit immune-modulating activities [102], there are also additional healthcare application possibilities, especially in conjunction with tableting for oral administration associated with nutraceutical and preventative health uses.
Moreover, CDs alone may act as virucidal agents due to their interaction with viral membrane lipids [103]. Lipid rafts, enriched in sphingolipids, cholesterol, and associated proteins, are special plasma membrane microdomains involved in several processes in viral infections. Cholesterol-rich microdomains appear to be a general feature of the entry mechanism of medically important viruses, including several coronaviruses, and hence are an attractive target. For example, DIMEB is highly efficient in depleting cholesterol from biomembranes, owing to its prominent affinity for inclusion complexation with cholesterol [32, 104]. The extraction of cholesterol leads to the disorganization of lipid microdomains and the dissociation of proteins bound to lipid rafts. By that, DIMEB can potentially block downstream key molecules, such as receptors, involved in virus infectivity, thereby reducing the levels of proinflammatory molecules and/or affecting the autophagic process involved in both viral replication and clearance [105]. Furthermore, the combination of CDs with common disinfectants, like didecyldimethylammonium chloride and dodecyloctaglycol, presented synergistic effects against enveloped viruses (respiratory syncytial virus, herpes simplex virus type 1, vaccinia virus) and fungi (C. albicans), as well as additive responses against bacteria (P. aeruginosa) [103]. In this light, combining CDs and antimicrobial lipids into one formulation may boost antiviral effects of lipids against enveloped viruses, providing a novel approach to combat the coronavirus disease 2019 (COVID-19) pandemic [106]. Such possibilities are just one opportunity out of many that can emerge from future research efforts aimed at developing CD-based emulsions that incorporate bioactive lipids and other molecular components while highlighting how CDs – which are typically regarded as having low surface activity – are not only useful in terms of host-guest chemistry but also possess advantageous properties for interfacial science applications.
Author contributions
MJ and JAJ developed the idea for the article, MJ and BKY performed the literature search and data analysis, MJ drafted the manuscript and prepared illustrations while JAJ critically revised the work. All authors read and approved the final version of the manuscript.
Funding
This research was supported by the International Research & Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2020K1A3A1A39112724).
Data availability
All data generated or analysed during this study are included in this published article.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Albert C, Beladjine M, Tsapis N, Fattal E, Agnely F, Huang N. Pickering emulsions: preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control Release. 2019;309:302–332. doi: 10.1016/j.jconrel.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Tai Z, Huang Y, Zhu Q, Wu W, Yi T, Chen Z, Lu Y. Utility of Pickering emulsions in improved oral drug delivery. Drug Discov Today. 2020;25:2038–2045. doi: 10.1016/j.drudis.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Lémery E, Briançon S, Chevalier Y, Bordes C, Oddos T, Gohier A, Bolzinger MA. Skin toxicity of surfactants: structure/toxicity relationships. Colloids. Surf A. Physicochem. Eng. Asp. 2015;469:166–179. doi: 10.1016/j.colsurfa.2015.01.019. [DOI] [Google Scholar]
- 4.Seweryn A. Interactions between surfactants and the skin – theory and practice. Adv. Colloid. Interface Sci. 2018;256:242–255. doi: 10.1016/j.cis.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Söderberg L, Engblom J, Lanbeck P, Wahlgren M. Do surface active parenteral formulations cause inflammation? Int. J. Pharm. 2015;484:246–251. doi: 10.1016/j.ijpharm.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 6.Jardak K, Drogui P, Daghrir R. Surfactants in aquatic and terrestrial environment: occurrence, behavior, and treatment processes. Environ. Pollut. Res. 2016;23:3195–2316. doi: 10.1007/s11356-015-5803-x. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Fang Z, Chen X, Zhang W, Xie Y, Chen Y, Liu Z, Yuan W. An overview of Pickering emulsions: solid-particle materials, classification, morphology, and applications. Front. Pharmacol. 2017;8:1–20. doi: 10.3389/fphar.2017.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harman CLG, Patel MA, Guldin S, Davies GL. Recent developments in Pickering emulsions for biomedical applications. Curr. Opin. Colloid Interface Sci. 2019;39:173–189. doi: 10.1016/j.cocis.2019.01.017. [DOI] [Google Scholar]
- 9.Liu Z, Geng S, Jiang Z, Liu B. Fabrication and characterization of food-grade Pickering high internal emulsions stabilized with β-cyclodextrin. Lwt. 2020 doi: 10.1016/j.lwt.2020.110134. [DOI] [Google Scholar]
- 10.Marto J, Ascenso A, Simoes S, Almeida AJ, Ribeiro HM. Pickering emulsions: challenges and opportunities in topical delivery. Expert. Opin. Drug. Deliv. 2016;13:1093–1107. doi: 10.1080/17425247.2016.1182489. [DOI] [PubMed] [Google Scholar]
- 11.Choi SJ, Lee JK, Jeong J, Choy JH. Toxicity evaluation of inorganic nanoparticles: considerations and challenges. Mol. Cell. Toxicol. 2013;9:205–210. doi: 10.1007/s13273-013-0026-z. [DOI] [Google Scholar]
- 12.Tan EL, Potroz MG, Ferracci G, Wang L, Jackman JA, Cho NJ. Hydrophobic to superhydrophilic tuning of multifunctional sporopollenin for microcapsule and bio-composite applications. Appl. Mater. Today. 2020;18:100525. doi: 10.1016/j.apmt.2019.100525. [DOI] [Google Scholar]
- 13.Tan EL, Potroz MG, Ferracci G, Jackman JA, Jung H, Wang L, Cho NJ. Light-induced surface modification of natural plant microparticles: toward colloidal science and cellular adhesion applications. Adv. Funct. Mater. 2018;28:1707568. doi: 10.1002/adfm.201707568. [DOI] [Google Scholar]
- 14.Salem LB, Bosquillon C, Dailey LA, Delattre L, Martin GP, Evrard B, Forbes B. Sparing methylation of β-cyclodextrin mitigates cytotoxicity and permeability induction in respiratory epithelial cell layers in vitro. J. Control. Release. 2009;136:110–116. doi: 10.1016/j.jconrel.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Li P, Song J, Ni X, Guo Q, Wen H, Zhou Q, Shen Y, Huang Y, Qiu P, Lin S, Hu H. Comparison in toxicity and solubilizing capacity of hydroxypropyl-β-cyclodextrin with different degree of substitution. Int. J. Pharm. 2016;513:347–356. doi: 10.1016/j.ijpharm.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 16.Stella VJ, He Q. Cyclodextrins. Toxicol. Pathol. 2008;36:30–42. doi: 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 17.Jansook P, Loftsson T. CDs as solubilizers: effects of excipients and competing drugs. Int. J. Pharm. 2009;379:32–40. doi: 10.1016/j.ijpharm.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Jansook P, Ogawa N, Loftsson T. Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018;535:272–284. doi: 10.1016/j.ijpharm.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Popielec A, Loftsson T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017;531:532–542. doi: 10.1016/j.ijpharm.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Arima H, Higashi T, Motoyama K. Improvement of the bitter taste of drugs by complexation with cyclodextrins: applications, evaluations and mechanisms. Ther. Deliv. 2012;3:633–644. doi: 10.4155/tde.12.28. [DOI] [PubMed] [Google Scholar]
- 21.Anadolu R, Sen T, Tarimci N, Birol A, Erdem C. Improved efficacy and tolerability of retinoic acid in acne vulgaris: a new topical formulation with cyclodextrin complex psi. J. Eur. Acad. Dermatol. Venereol. . 2004;18:416–421. doi: 10.1111/j.1468-3083.2004.00929.x. [DOI] [PubMed] [Google Scholar]
- 22.Scavone C, Bonagura AC, Fiorentino S, Cimmaruta D, Cenami R, Torella M, Fossati T, Rossi F. Efficacy and safety profile of diclofenac/cyclodextrin and progesterone/cyclodextrin formulations: a review of the literature data. Drugs R&D. 2016;16:129–140. doi: 10.1007/s40268-016-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adeoye O, Cabral-Marques H. Cyclodextrin nanosystems in oral drug delivery: a mini review. Int. J. Pharm. 2017;531:521–531. doi: 10.1016/j.ijpharm.2017.04.050. [DOI] [PubMed] [Google Scholar]
- 24.Arima H, Motoyama K, Higashi T. Potential use of cyclodextrins as drug carriers and active pharmaceutical ingredients. Chem. Pharm. Bull. 2017;65:341–348. doi: 10.1248/cpb.c16-00779. [DOI] [PubMed] [Google Scholar]
- 25.Cada DJ, Levien TL, Baker DE. Sugammadex. Hosp Pharm. 2016;51:585–596. doi: 10.1310/hpj5107-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leclercq L, Nardello-Rataj V. Pickering emulsions based on cyclodextrins: a smart solution for antifungal azole derivatives topical delivery. Eur. J. Pharm. Sci. 2016;82:126–137. doi: 10.1016/j.ejps.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Mathapa BG, Paunov VN. Cyclodextrin stabilised emulsions and cyclodextrinosomes. Phys. Chem. Chem. Phys. 2013;15:17903–17914. doi: 10.1039/c3cp52116h. [DOI] [PubMed] [Google Scholar]
- 28.Mathapa BG, Paunov VN. Self-assembly of cyclodextrin-oil inclusion complexes at the oil-water interface: a route to surfactant-free emulsions. J. Mater. Chem. A. 2013;1:10836–10846. doi: 10.1039/c3ta12108a. [DOI] [Google Scholar]
- 29.Duchêne D, Bochot A, Yu SC, Pépin C, Seiller M. Cyclodextrins and emulsions. Int. J. Pharm. 2003;266:85–90. doi: 10.1016/S0378-5173(03)00384-3. [DOI] [PubMed] [Google Scholar]
- 30.Loftsson T. Drug solubilization by complexation. Int. J. Pharm. 2017;531:276–280. doi: 10.1016/j.ijpharm.2017.08.087. [DOI] [PubMed] [Google Scholar]
- 31.Dhiman P, Bhatia M. Pharmaceutical applications of cyclodextrins and their derivatives. J. Incl. Phenom. Macrocycl. Chem. 2020;98:171–186. doi: 10.1007/s10847-020-01029-3. [DOI] [Google Scholar]
- 32.Szente L, Fenyvesi É. Cyclodextrin-lipid complexes: cavity size matters. Struct. Chem. 2017;28:479–492. doi: 10.1007/s11224-016-0884-9. [DOI] [Google Scholar]
- 33.Szente L, Szejtli J, Szemán J, Kató L. Fatty acid-cyclodextrin complexes: properties and applications. J. Incl. Phenom. Macrocyclic Chem. 1993;16:339–354. doi: 10.1007/BF00708714. [DOI] [Google Scholar]
- 34.Denz M, Haralampiev I, Schiller S, Szente L, Herrmann A, Huster D, Müller P. Interaction of fluorescent phospholipids with cyclodextrins. Chem. Phys. Lipids. 2016;194:37–48. doi: 10.1016/j.chemphyslip.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Saokham P, Loftsson T. γ-Cyclodextrin. Int. J. Pharm. 2017;516:278–292. doi: 10.1016/j.ijpharm.2016.10.062. [DOI] [PubMed] [Google Scholar]
- 36.Singh J, Pronobesh N, Ashok C, Sharma K, Ram V. Preparation of gamma cyclodextrin stabilized solid lipid nanoparticles (SLNS) using stearic acid-γ-cyclodextrin inclusion complex. J. Incl. Phenom. Macrocycl. Chem. 2014;80:359–368. doi: 10.1007/s10847-014-0415-5. [DOI] [Google Scholar]
- 37.Hammoud Z, Khreich N, Auezova L, Fourmentin S, Elaissari A, Greige-Gerges H. Cyclodextrin-membrane interaction in drug delivery and membrane structure maintenance. Int. J. Pharm. 2019;564:59–76. doi: 10.1016/j.ijpharm.2019.03.063. [DOI] [PubMed] [Google Scholar]
- 38.Hǎdǎrugǎ NG, Hǎdǎrugǎ DI, Pǎunescu V, Tatu C, Ordodi VL, Bandur G, Lupea AX. Thermal stability of the linoleic acid/α- and β-cyclodextrin complexes. Food Chem. 2006;99:500–508. doi: 10.1016/j.foodchem.2005.08.012. [DOI] [Google Scholar]
- 39.Hǎdǎruga DI, Ünlüsayin M, Gruia AT, Birǎu C, Rusu G, Hǎdǎruga NG. Thermal and oxidative stability of Atlantic salmon oil (Salmo salar L.) and complexation with β-cyclodextrin. Beilstein J. Org. Chem. 2016;12:179–191. doi: 10.3762/bjoc.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vestland TL, Jacobsen Ø, Sande SA, Myrset AH, Klaveness J, Lee MC, Jiang X, Brenna JT, Abbaspourrad A, Hǎdǎruga DI, Ünlüsayin M, Gruia AT, Birǎu C, Rusu G, Hǎdǎruga NG, Hǎdǎrugǎ NG, Hǎdǎrugǎ DI, Pǎunescu V, Tatu C, Ordodi VL, Bandur G, Lupea AX. Oleogel-structured composite for the stabilization of ω3 fatty acids in fish oil. Food Chem. 2016;9:179–191. doi: 10.1016/j.foodchem.2005.08.012. [DOI] [Google Scholar]
- 41.David I, Orboi MD, Simandi MD, Chirilă CA, Megyesi CI, Rădulescu L, Drăghia LP, Lukinich-Gruia AT, Muntean C, Hădărugă DI, Hădărugă NG. Fatty acid profile of Romanian’s common bean (Phaseolus vulgaris L.) lipid fractions and their complexation ability by β-cyclodextrin. PLoS One. 2019;14:1–25. doi: 10.1371/journal.pone.0225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vestland TL, Jacobsen Ø, Sande SA, Myrset AH, Klaveness J. Compactible powders of omega-3 and β-cyclodextrin. Food Chem. 2015;185:151–158. doi: 10.1016/j.foodchem.2015.03.132. [DOI] [PubMed] [Google Scholar]
- 43.Szente L, Singhal A, Domokos A, Song B. Cyclodextrins: assessing the impact of cavity size, occupancy, and substitutions on cytotoxicity and cholesterol homeostasis. Molecules. 2018;23:1–15. doi: 10.3390/molecules23051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiss T, Fenyvesi F, Bácskay I, Váradi J, Fenyvesi É, Iványi R, Szente L, Tósaki Á, Vecsernyés M. Evaluation of the cytotoxicity of β-cyclodextrin derivatives: evidence for the role of cholesterol extraction. Eur. J. Pharm. Sci. 2010;40:376–380. doi: 10.1016/j.ejps.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Fenyvesi É, Szemán J, Csabai K, Malanga M, Szente L. Methyl-beta-cyclodextrins: the role of number and types of substituents in solubilizing power. J. Pharm. Sci. 2014;103:1443–1452. doi: 10.1002/jps.23917. [DOI] [PubMed] [Google Scholar]
- 46.Loftsson T. Cyclodextrins in parenteral formulations. J. Pharm. Sci. 2021;110:654–664. doi: 10.1016/j.xphs.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Kurkov SV, Loftsson T. Cyclodextrins. Int. J. Pharm. 2013;453:167–180. doi: 10.1016/j.ijpharm.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 48.Singhal A, Szente L, Hildreth JEKK, Song B. Hydroxypropyl-beta and -gamma cyclodextrins rescue cholesterol accumulation in Niemann–Pick C1 mutant cell via lysosome-associated membrane protein 1. Cell Death Dis. 2018;9:1–13. doi: 10.1038/s41419-018-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wheeler S, Sillence DJ. Niemann–Pick type C disease: cellular pathology and pharmacotherapy. J. Neurochem. 2020;153:674–692. doi: 10.1111/jnc.14895. [DOI] [PubMed] [Google Scholar]
- 50.Mahjoubin-Tehran M, Kovanen PT, Xu S, Jamialahmadi T, Sahebkar A. Cyclodextrins: potential therapeutics against atherosclerosis. Pharmacol. Ther. 2020;214:107620. doi: 10.1016/j.pharmthera.2020.107620. [DOI] [PubMed] [Google Scholar]
- 51.Lone SA. Possible mechanisms of cholesterol-loaded cyclodextrin action on sperm during cryopreservation. Anim. Reprod. Sci. 2018;192:1–5. doi: 10.1016/j.anireprosci.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Hashizaki K, Kageyama T, Inoue M, Taguchi H, Ueda H, Saito Y. Study on preparation and formation mechanism of n-alkanol/water emulsion using α-cyclodextrin. Chem. Pharm. Bull. 2007;55:1620–1625. doi: 10.1248/cpb.55.1620. [DOI] [PubMed] [Google Scholar]
- 53.Inoue M, Hashizaki K, Taguchi H, Saito Y. Emulsifying ability of β-cyclodextrins for common oils. J. Dispers. Sci. Technol. 2010;31:1648–1651. doi: 10.1080/01932690903297058. [DOI] [Google Scholar]
- 54.Taguchi H, Tanaka H, Hashizaki K, Saito Y, Fujii M. Application of Pickering emulsion with cyclodextrin as an emulsifier to a transdermal drug delivery vehicle. Biol. Pharm. Bull. 2019;42:116–122. doi: 10.1248/bpb.b18-00711. [DOI] [PubMed] [Google Scholar]
- 55.Hu J-WW, Yen M-WW, Wang A-JJ, Chu I-MM. Effect of oil structure on cyclodextrin-based Pickering emulsions for bupivacaine topical application. Colloids. Surf B. Biointerfaces. 2018;161:51–58. doi: 10.1016/j.colsurfb.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Leclercq L. Get beyond limits: from colloidal tectonics concept to the engineering of eco-friendly catalytic systems. Front. Chem. 2018;6:168. doi: 10.3389/fchem.2018.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashizaki K, Kageyama T, Inoue M, Taguchi H, Saito Y. Preparation and characterization of cycloalkanol/water emulsion using α-cyclodextrin as an emulsifier. J. Dispersion Sci. Technol. 2009;30:852–856. doi: 10.1080/01932690802644038. [DOI] [Google Scholar]
- 58.Diaz-Salmeron R, Chaab I, Carn F, Djabourov M, Bouchemal K. Pickering emulsions with α-cyclodextrin inclusions: Structure and thermal stability. J. Colloid. Interface. Sci. 2016;482:48–57. doi: 10.1016/j.jcis.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 59.Hamoudi MC, Bourasset F, Domergue-Dupont V, Gueutin C, Nicolas V, Fattal E, Bochot A. Formulations based on alpha cyclodextrin and soybean oil: An approach to modulate the oral release of lipophilic drugs. J. Control. Release. 2012;161:861–867. doi: 10.1016/j.jconrel.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 60.Trichard L, Fattal E, le Bas G, Duchêne D, Grossiord JL, Bochot A. Formulation and characterisation of beads prepared from natural cyclodextrins and vegetable, mineral or synthetic oils. Int. J. Pharm. 2008;354:88–94. doi: 10.1016/j.ijpharm.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 61.Aburahma MH. Insights on novel particulate self-assembled drug delivery beads based on partial inclusion complexes between triglycerides and cyclodextrins. Drug Deliv. 2016;23:2205–2219. doi: 10.3109/10717544.2014.956240. [DOI] [PubMed] [Google Scholar]
- 62.Hamoudi MC, Bochot A. Oil-cyclodextrin based beads for oral delivery of poorly-soluble drugs. Curr. Top. Med. Chem. 2014;14:510–517. doi: 10.2174/1568026613666131219124539. [DOI] [PubMed] [Google Scholar]
- 63.Laza-Knoerr A, Huang N, Grossiord JL, Couvreur P, Gref R. Interfacial rheology as a tool to study the potential of cyclodextrin polymers to stabilize oil-water interfaces. J. Incl. Phenom. Macrocycl. Chem. 2011;69:475–479. doi: 10.1007/s10847-010-9805-5. [DOI] [Google Scholar]
- 64.Kawano S, Kida T, Akashi M, Sato H, Shizuma M, Ono D. Preparation of Pickering emulsions through interfacial adsorption by soft cyclodextrin nanogels. Beilstein J. Org. Chem. 2015;11:2355–2364. doi: 10.3762/bjoc.11.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inoue M, Hashizaki K, Taguchi H, Saito Y. Emulsion preparation using β-cyclodextrin and its derivatives acting as an emulsifier. Chem. Pharm. Bull. 2008;56:1335–1337. doi: 10.1248/cpb.56.1335. [DOI] [PubMed] [Google Scholar]
- 66.Hu Y, Qiu C, Jin Z, Qin Y, Zhan C, Xu X, Wang J. Pickering emulsions with enhanced storage stabilities by using hybrid β-cyclodextrin/short linear glucan nanoparticles as stabilizers. Carbohydr. Polym. 2020;229:115418. doi: 10.1016/j.carbpol.2019.115418. [DOI] [PubMed] [Google Scholar]
- 67.Luviano AS, Hernández-Pascacio J, Ondo D, Campbell RA, Piñeiro Á, Campos-Terán J, Costas M. Highly viscoelastic films at the water/air interface: α-Cyclodextrin with anionic surfactants. J. Colloid Interface Sci. 2020;565:601–613. doi: 10.1016/j.jcis.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 68.Xiao Z, Liu Y, Niu Y, Kou X. Cyclodextrin supermolecules as excellent stabilizers for Pickering nanoemulsions. Colloids Surf. A Physicochem. Eng Asp. 2020;588:124367. doi: 10.1016/j.colsurfa.2019.124367. [DOI] [Google Scholar]
- 69.Ringard-Lefebvre C, Bochot A, Memisolu E, Charon D, Duchene D, Baszkin A. Effect of spread amphiphilic β-cyclodextrins on interfacial properties of the oil/water system. Colloids Surf. B Biointerfaces. 2002;25:109–117. doi: 10.1016/S0927-7765(01)00297-1. [DOI] [Google Scholar]
- 70.Xi Y, Luo Z, Lu X, Peng X. Modulation of cyclodextrin particle amphiphilic properties to stabilize Pickering emulsion. J. Agric. Food Chem. 2018;66:228–237. doi: 10.1021/acs.jafc.7b03940. [DOI] [PubMed] [Google Scholar]
- 71.Niu H, Chen W, Chen W, Yun Y, Zhong Q, Fu X, Chen H, Liu G. Preparation and characterization of a modified-β-Cyclodextrin/β-Carotene inclusion complex and its application in Pickering emulsions. J. Agric. Food Chem. 2019;67:12875–12884. doi: 10.1021/acs.jafc.9b05467. [DOI] [PubMed] [Google Scholar]
- 72.Ke D, Chen W, Chen W, Yun YH, Zhong Q, Su X, Chen H. Preparation and characterization of octenyl succinate β-cyclodextrin and Vitamin E inclusion complex and its application in emulsion. Molecules. 2020;25:654. doi: 10.3390/molecules25030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leclercq L, Tessier J, Douyère G, Nardello-Rataj V, Schmitzer AR. Phytochemical- and cyclodextrin-based Pickering Emulsions: natural potentiators of antibacterial, antifungal, and antibiofilm activity. Langmuir. 2020;36:4317–4323. doi: 10.1021/acs.langmuir.0c00314. [DOI] [PubMed] [Google Scholar]
- 74.Badr-Eldin SM, Labib GS, Aburahma MH. Eco-friendly tadalafil Surfactant-free dry emulsion tablets (SFDETs) stabilized by in situ self-assembled aggregates of natural oil and native cyclodextrins. AAPS Pharm. Sci. Tech. 2019;20:1–12. doi: 10.1208/s12249-019-1450-8. [DOI] [PubMed] [Google Scholar]
- 75.Yu SC, Bochot A, le Bas G, Chéron M, Mahuteau J, Grossiord JL, Seiller M, Duchêne D. Effect of camphor/cyclodextrin complexation on the stability of O/W/O multiple emulsions. Int. J. Pharm. 2003;261:1–8. doi: 10.1016/S0378-5173(03)00261-8. [DOI] [PubMed] [Google Scholar]
- 76.Leclercq L, Tessier J, Nardello-Rataj V, Schmitzer AR. Highly active, entirely biobased antimicrobial Pickering emulsions. Chem Med Chem. 2021;16:2223–2230. doi: 10.1002/cmdc.202100030. [DOI] [PubMed] [Google Scholar]
- 77.Leclercq L, Dechézelles JF, Rauwel G, Nardello-Rataj V. In vitro study of versatile drug formulations based on α-cyclodextrin and polyethylene glycol using colloidal tectonics. J. Drug Deliv. Sci. Technol. 2020;59:101913. doi: 10.1016/j.jddst.2020.101913. [DOI] [Google Scholar]
- 78.Eslami P, Davarpanah L, Vahabzadeh F. Encapsulating role of β-cyclodextrin in formation of Pickering water-in-oil-in-water (W1/O/W2) double emulsions containing Lactobacillus dellbrueckii. Food Hydrocoll. 2017;64:133–148. doi: 10.1016/j.foodhyd.2016.10.035. [DOI] [Google Scholar]
- 79.Eslami P, Forootan K, Davarpanh L, Vahabzadeh F. Incorporation of lactobacillus casei into the inner phase of the water-in-oil-in-water (W1/O/W2) emulsion prepared with β-cyclodextrin and bacterial survival in a model gastric environment. Appl. Food Biotechnol. 2020;7:171–182. doi: 10.22037/afb.v7i3.28877. [DOI] [Google Scholar]
- 80.Wu J, Ma GH. Recent studies of Pickering emulsions: particles make the difference. Small. 2016;12:4633–4648. doi: 10.1002/smll.201600877. [DOI] [PubMed] [Google Scholar]
- 81.Vladisavljević GT. Preparation of microemulsions and nanoemulsions by membrane emulsification. Colloids Surf. A Physicochem. Eng Asp. 2019;579:123709–123709. doi: 10.1016/j.colsurfa.2019.123709. [DOI] [Google Scholar]
- 82.Serge L, Serpelloni M, Piochurent D. A study of β-cyclodextrin-stabilized paraffin oil / water emulsions. J. Cosmet. Sci. 1999;50:15–22. [Google Scholar]
- 83.Davarpanah L, Vahabzadeh F. Formation of oil-in-water (O/W) Pickering emulsions via complexation between β-cyclodextrin and selected organic solvents. Starch/Staerke. 2012;64:898–913. doi: 10.1002/star.201200027. [DOI] [Google Scholar]
- 84.Saffarionpour S. Nanocellulose for stabilization of Pickering emulsions and delivery of nutraceuticals and its interfacial adsorption mechanism. Food. Bioproc Tech. 2020;13:1292–1328. doi: 10.1007/s11947-020-02481-2. [DOI] [Google Scholar]
- 85.Xia T, Xue C, Wei Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2021;107:1–15. doi: 10.1016/j.tifs.2020.11.019. [DOI] [Google Scholar]
- 86.Li X, Li H, Xiao Q, Wang L, Wang M, Lu X, York P, Shi S, Zhang J. Two-way effects of surfactants on Pickering emulsions stabilized by the self-assembled microcrystals of α-cyclodextrin and oil. Phys. Chem. Chem. Phys. 2014;16:14059–14069. doi: 10.1039/c4cp00807c. [DOI] [PubMed] [Google Scholar]
- 87.Kibici D, Kahveci D. Effect of emulsifier type, maltodextrin, and β-Cyclodextrin on physical and oxidative stability of oil-in-water emulsions. J. Food Sci. 2019;84:1273–1280. doi: 10.1111/1750-3841.14619. [DOI] [PubMed] [Google Scholar]
- 88.Potier J, Menuel S, Chambrier MH, Burylo L, Blach JF, Woisel P, Monflier E, Hapiot F. Pickering emulsions based on supramolecular hydrogels: application to higher olefins’ hydroformylation. ACS Catal. 2013;3:1618–1621. doi: 10.1021/cs4002282. [DOI] [Google Scholar]
- 89.Tayeb HH, Sainsbury F. Nanoemulsions in drug delivery: formulation to medical application. Nanomedicine. 2018;13:2507–2525. doi: 10.2217/nnm-2018-0088. [DOI] [PubMed] [Google Scholar]
- 90.Wang J, Cai Z. Investigation of inclusion complex of miconazole nitrate with β-cyclodextrin. Carbohydr. Polym. 2008;72:255–260. doi: 10.1016/j.carbpol.2007.08.009. [DOI] [Google Scholar]
- 91.Rawat A, Gupta SS, Kalluri H, Lowenborg M, Bhatia K, Warner K. Rheological characterization in the development of topical drug products. In: Perrie Y, editor. AAPS advances in the pharmaceutical sciences series. New York: Springer; 2019. pp. 3–45. [Google Scholar]
- 92.Inoue M, Hashizaki K, Taguchi H, Saito Y. Preparation and characterization of n-alkane/water emulsion stabilized by cyclodextrin. J. Oleo Sci. 2009;58:85–90. doi: 10.5650/jos.58.85. [DOI] [PubMed] [Google Scholar]
- 93.Casillas-Vargas G, Ocasio-Malavé C, Medina S, Morales-Guzmán C, del Valle RG, Carballeira NM, Sanabria-Ríos DJ. Antibacterial fatty acids: An update ofpossible mechanisms of action and implications in the development of thenext-generation of antibacterial agents. Prog Lipid. Res. 2021;82:101093. doi: 10.1016/j.plipres.2021.101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoon BK, Jeon WY, Sut TN, Cho NJ, Jackman JA. Stopping membrane-enveloped viruses with nanotechnology strategies: toward antiviral drug development and pandemic preparedness. ACS Nano. 2021;15:125–148. doi: 10.1021/acsnano.0c07489. [DOI] [PubMed] [Google Scholar]
- 95.Thormar H, Hilmarsson H. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chem. Phys. Lipids. 2007;150:1–11. doi: 10.1016/J.CHEMPHYSLIP.2007.06.220. [DOI] [PubMed] [Google Scholar]
- 96.Fischer CL. Antimicrobial activity of host-derived lipids. Antibiotics. 2020;9:75. doi: 10.3390/ANTIBIOTICS9020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoon, B.K., Jackman, J.A., Valle-González, E.R., Cho, N.J.: Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol.Sci. 19, 1114 (2018). 10.3390/ijms19041114 [DOI] [PMC free article] [PubMed]
- 98.Yoon BK, Jackman JA, Kim MC, Sut TN, Cho NJ. Correlating membrane morphological responses with micellar aggregation behavior of capric acid and monocaprin. Langmuir. 2017;33:2750–2759. doi: 10.1021/acs.langmuir.6b03944. [DOI] [PubMed] [Google Scholar]
- 99.Jackman JA, Hakobyan A, Zakaryan H, Elrod CC. Inhibition of African swine fever virus in liquid and feed by medium-chain fatty acids and glycerol monolaurate. J. Anim. Sci. Biotechnol. 2020;11:1–10. doi: 10.1186/s40104-020-00517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jackman JA, Yoon BK, Li D, Cho NJ. Nanotechnology formulations for antibacterial free fatty acids and monoglycerides. Molecules. 2016;21:305. doi: 10.3390/molecules21030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu Z, Erbas A, Tantakitti F, Palmer LC, Jackman JA, de La Cruz MO, Cho NJ, Stupp SI. Co-assembly of peptide amphiphiles and lipids into supramolecular nanostructures driven by anioni – π Interactions. J. Am. Chem. Soc. 2017;139:7823–7830. doi: 10.1021/jacs.7b02058. [DOI] [PubMed] [Google Scholar]
- 102.Zhang MS, Tran PM, Wolff AJ, Tremblay MM, Fosdick MG, Houtman JCD. Glycerol monolaurate induces filopodia formation by disrupting the association between LAT and SLP-76 microclusters. Sci. Signal. 2018;11:9095. doi: 10.1126/scisignal.aam9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leclercq L, Nardello-Rataj V. How to improve the chemical disinfection of contaminated surfaces by viruses, bacteria and fungus? Eur. J. Pharm. Sci. 2020;155:10559–10559. doi: 10.1016/j.ejps.2020.105559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mandal P, Noutsi P, Chaieb S. Cholesterol depletion from a ceramide/cholesterol mixed monolayer: a Brewster angle microscope study. Sci Rep. 2016;6:26907–26907. doi: 10.1038/srep26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sorice M, Misasi R, Riitano G, Manganelli V, Martellucci S, Longo A, Garofalo T, Mattei V. Targeting lipid rafts as a strategy against Coronavirus. Front. Cell Dev. Biol. 2021;8:1848–1848undefined. doi: 10.3389/fcell.2020.618296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carrouel F, Conte MP, Fisher J, Gonçalves LS, Dussart C, Llodra JC, Bourgeois D. COVID-19: A recommendation to examine the effect of Mouthrinses with β-Cyclodextrin combined with Citrox in preventing infection and progression. J. Clin. Med. 2020;9:1126. doi: 10.3390/jcm9041126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Not applicable.