Abstract
Human amniotic fluid stem cells (hAFSCs) harbor high proliferative capacity and high differentiation potential and do not raise the ethical concerns associated with human embryonic stem cells. The formation of three-dimensional aggregates known as embryoid bodies (EBs) is the principal step in the differentiation of pluripotent embryonic stem cells. Using c-Kit-positive hAFSC lines, we show here that these stem cells harbor the potential to form EBs. As part of the two kinase complexes, mTORC1 and mTORC2, mammalian target of rapamycin (mTOR) is the key component of an important signaling pathway, which is involved in the regulation of cell proliferation, growth, tumor development and differentiation. Blocking intracellular mTOR activity through the inhibitor rapamycin or through specific small interfering RNA approaches revealed hAFSC EB formation to depend on mTORC1 and mTORC2. These findings demonstrate hAFSCs to be a new and powerful biological system to recapitulate the three-dimensional and tissue level contexts of in vivo development and identify the mTOR pathway to be essential for this process.
Keywords: amniotic fluid, stem cell, EB, mTOR
Introduction
It was always assumed that both the differentiation potential and proliferative capacity of adult stem cells are limited compared with embryonic stem cells. However, adult stem cells do not raise ethical concerns and harbor a low risk of tumor development. Many different studies have been initiated to search for alternative human sources for stem cells harboring the potential to differentiate into specific lineages, including the induction of pluripotency directly in somatic cells (Pennings, 2007; Aboushwareb and Atala, 2008; Maherali and Hochedlinger, 2008).
Since the description of cells within the human amniotic fluid expressing the transcription factor Oct4, a marker for pluripotent human stem cells (Prusa et al., 2003), a variety of different stem cell populations have been described to exist in human amniotic fluid. AFSCs have been shown to harbor the potential for osteogenic, chondrogenic, adipogenic, renal, hematopoietic or neurogenic differentiation (in ‘t Anker et al., 2003; Prusa et al., 2004; Tsai et al., 2004, 2006; Karlmark et al., 2005; Bossolasco et al., 2006; Kim et al., 2007; Kolambkar et al., 2007; Perin et al., 2007; Ditadi et al., 2009). Importantly, using immunoselected CD117 (c-Kit)-positive hAFSCs it has been shown that, descending from a single cell, differentiation along adipogenic, osteogenic, myogenic, endothelial, neurogenic and hepatic lineages could be induced. These hAFSCs can be expanded in culture as stable lines with high proliferative capacity and do not induce tumor formation in mice (De Coppi et al., 2007). hAFSCs might have advantages (such as a higher proliferation rate and a higher differentiation potential) over other adult stem cell types. They even might have advantages (such as a lower risk for tumor development and no ethical concerns) over embryonic stem cells. In addition, hAFSCs might allow to study specific differentiation processes under normal and pathological conditions with the aim to clarify how the underlying molecular mechanisms are deregulated in cells harboring natural occurring molecular alterations being causatively involved in the development of specific human genetic diseases (reviewed in Siegel et al., 2007; Perin et al., 2008; Cananzi et al., 2009). However, embryonic stem cells, when cultured without antidifferentiation factors, can spontaneously form three-dimensional multicellular aggregates called embryoid bodies (EBs). This cell biological process allows the recapitulation and investigation of the three-dimensional and tissue level contexts of many cell differentiation phenomena during early mammalian embryogenesis (Itskovitz-Eldor et al., 2000; Koike et al., 2007; Ungrin et al., 2008). The question whether hAFSCs harbor the potential to form EBs remained elusive so far.
The mammalian target of rapamycin (mTOR) kinase has a central role in the regulation of cell proliferation, growth, tumor development and many cell differentiation processes. In mammalian cells two different mTOR containing complexes, namely mTORC1 and mTORC2, have been identified. mTORC1 contains the mTOR protein, raptor and mLST8. mTORC2 consists of mTOR, mLST8, rictor and sin1. mTORC1 is involved in the regulation of mRNA translation, for example, through its potential to phosphorylate and activate one of its major targets, the kinase p70S6K, at T389 to activate the ribosomal protein S6 through phosphorylation at S240/244. mTORC2 phosphorylates Akt at S473, which, in conjunction with the PDK1-mediated phosphorylation at T308, drives full activation of Akt. Upstream of mTOR, activated receptor tyrosine kinases activate the kinase PI3K through, for example, IRS1, regulating the potential of PDK1 to phosphorylate Akt. Akt-mediated phosphorylation downregulates the GTPase-activating potential of tuberin (TSC2) toward Rheb, which is a potent regulator of mTORC1. The tuberin/hamartin complex can also associate with and positively regulate mTORC2 (Wullschleger et al., 2006; Dann et al., 2007; Guertin and Sabatini, 2007; Yang and Guan, 2007).
Here we demonstrate hAFSCs to harbor the potential to form EBs and we identify mTOR as a major regulator of this cell biological process. Our findings provide important additional evidence that hAFSCs indeed represent a powerful tool for human developmental biology and allow new insights into the underlying molecular mechanism of EB formation.
Results
Characterization of CD117-positive hAFSC lines
In addition to the earlier established clonal hAFSC line, Q1, (De Coppi et al., 2007) we generated two new CD117-positive hAFSC lines, namely CB3 and CD117/2, from native human amniotic fluid cell samples using the previously described protocol for immunoselection through magnetic cell sorting.
The morphological appearance and cell size of CD117/2 cells, established without prior minimal dilution, was very comparable with Q1 and CB3 hAFSCs, descending from one single cell on minimal dilution. All three cell lines grow without the need of feeder layers, without evidence for spontaneous differentiation, with stable normal karyo-types, with slight differences in the amount of cells in the different cell cycle phases and without significant appearance of apoptotic sub-G1 cells (Figure 1). In summary, these data again prove that the described magnetic cell sorting approach is a reproducible method to generate genomically stable CD117-positive hAFSC lines.
Figure 1.
Biological characterization of the used human amniotic fluid stem cell (hAFSC) lines. (a) Cytogenetical analyses revealed normal and stable karyotypes for the used hAFSC lines, CD117/2, Q1 and CB3 (data not shown). Logarithmically growing cells were microscopically analysed for cell morphology and cytofluorometrically analysed for DNA content. For the latter, the percentage ( ± s.d.) of cells in G0/G1, S and G2/M phases of the cell cycle and the percentage ( ± s.d.) of apoptotic cells with sub-G1 DNA is presented. (b) The cell size of the three hAFSC lines was compared with the cell size of nonimmortalized nontransformed primary human IMR-90 fibroblasts through forward scatter analyses. (c) Logarithmically growing CD117/2, Q1 and CB3 cells were analysed for cell size in femtoliter on the Casy Cell Counter.
EB formation of hAFSCs
To answer the question whether hAFSCs can form EBs, we decided to use two different growth media, one that has already been used earlier to cultivate hAFSCs (medium 1; De Coppi et al., 2007) and another one, which is commonly used for embryonic stem cell growth (medium 2; Narita et al., 1996; Kurosawa, 2007) (Table 1). In this study two standard commonly used approaches, the hanging drop method and cultivation in suspension in low-adherence 96-well plates, were used to induce EB formation of hAFSCs.
Table 1.
The cell culture media used
| Medium 1 |
Medium 2 |
||
|---|---|---|---|
| α-MEM (Gibco-41061; Invitrogen, Carlsbad, CA, USA) | DMEM (Gibco-11960z) | ||
| Penicillin | 0.03 mg/ml | Penicillin | 0.03 mg/ml |
| Streptomycin | 0.05 mg/ml | Streptomycin | 0.05 mg/ml |
| Chang B | 18% | 2-mercaptoethanol | 0.1 mm |
| Chang C | 2% | Glutamine | 2 mm |
| Fetal bovine serum (HyClone-30070.03; Waltham, MA, USA) | 15% | Fetal bovine serum (Sigma-F7524) | 15% |
Abbreviations: DMEM, Dulbecco’s modified Eagle’s medium; α-MEM, α-Minimal Essential Medium.
We have cultivated CD117/2, Q1 and CB3 cells, resuspended in medium1 or medium 2 for 4 days in hanging drop cultures. Thereafter, the formed EBs were transferred on 0.1% gelatin-coated tissue culture dishes (Figure 2). The incidence of EB formation (given as percentage of the number of attached EBs recovered from 15 hanging drops seeded) was investigated in three independent experiments. Each independent experiment was carried out by seeding 15 hanging drops in triplicate. Compared with the other two cell lines, CD117/2 cells exhibited a lower EB formation incidence, which might be due to the fact that these hAFSCs do not necessarily represent a clonal cell population (Table 2). In summary, EB formation was observed in every experiment using three different hAFSC lines and two different cultivation media.
Figure 2.
Embryoid body (EB) formation of CD117/2, Q1 and CB3 cells was induced using the hanging drop method as described in the Materials and methods section. For the two different media used in these experiments compare Table 1. Pictures of representative of EBs detected on day 5 (4 days in hanging drop and 1 day after attachment in cell culture) in medium 1 (a–c), on day 15 (4 days in hanging drop and 11 days after attachment in cell culture) in medium 1 (d–f), on day 5 in medium 2 (g–i) and on day 15 in medium 2 (j–l) are presented. Scale bar: 100 µm.
Table 2.
Incidence of EB formation in the different hAFSC lines
| Medium | CD117/2 | Q1 | CB3 | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | |
| EB formation (average ± s.d.) | 38% ± 10 | 18% ± 10 | 69% ± 3 | 40% ±13 | 76% ± 10 | 82% ± 4 |
Abbreviations: EB, embryoid body; hAFSC, human amniotic fluid stem cell.
Marker expression in EBs derived from hAFSCs
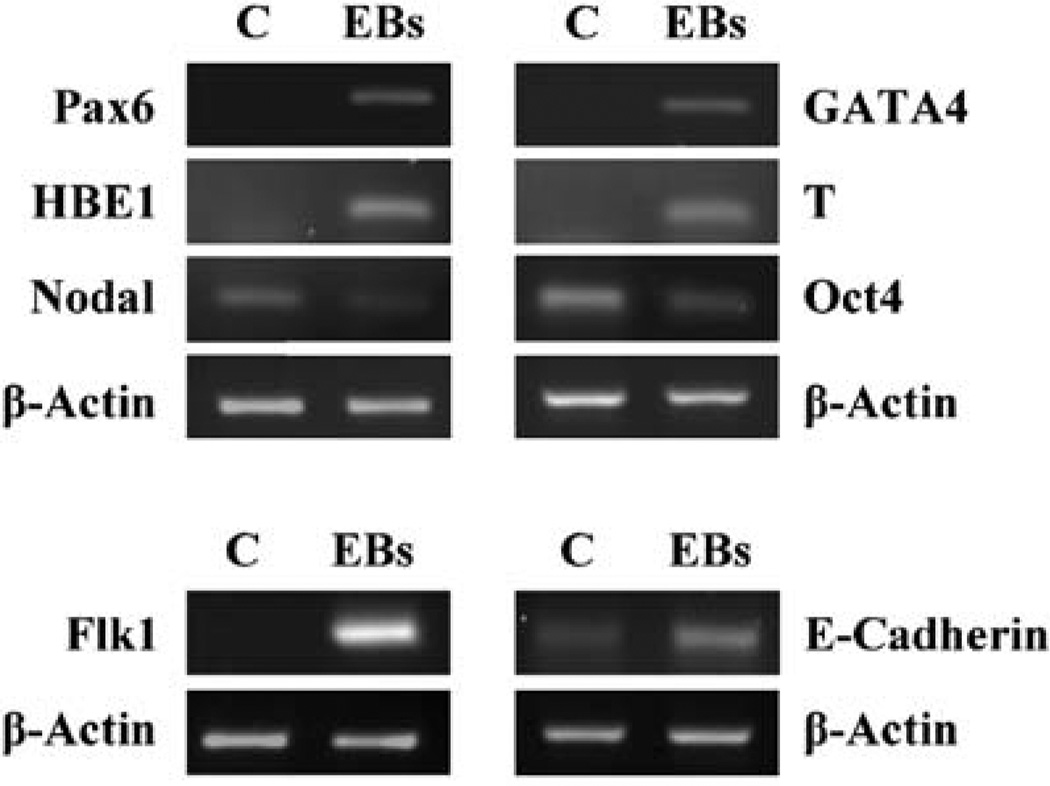
The Oct4 transcription factor is a well-known stem cell marker for pluripotency (Pesce and Schöler, 2001). Oct4-positive cells were detected in human amniotic fluid (Prusa et al., 2003) and the CD117-positive hAFSC lines have been shown to also express Oct4 (De Coppi et al., 2007). Performing reverse transcriptase–PCR experiments, we have confirmed hAFSCs to be Oct4-positive. We found hAFSCs to also express the self-renewal (human embryonic) stem cell marker nodal (Besser, 2004) (Figure 3). We observed EB formation of hAFSCs to be accompanied by a decrease of nodal and Oct-4 expression and by induction of the differentiation markers Pax 6 (ectodermal), Flk1 (endothelial), E-cadherin (epithelial), GATA4 (endodermal), T (brachyury; mesodermal) and HBE1 (mesodermal) (Figure 3), which is in agreement with the observations on EB formation of embryonic stem cells (Narita et al., 1996; Itskovitz-Eldor et al., 2000; Yamashita et al., 2000; Besser, 2004; Ng et al., 2005; Koike et al., 2007; Kurosawa, 2007; Gualandris et al., 2008; Kim et al., 2008; Lim et al., 2008; Ungrin et al., 2008).
Figure 3.
Marker expression before and after embryoid body (EB) formation of human amniotic fluid stem cells (hAFSCs). Logarithmically growing CB3 cells (C, control) have been induced to form EBs (day 15). The expression of Pax6 mRNA, HBE1 mRNA, nodal mRNA, Flk1 mRNA, GATA4 mRNA, T (brachyury) mRNA, Oct4 mRNA and E-cadherin mRNA was analysed using reverse transcriptase–PCR. β-actin mRNA expression was co-analysed as a control. All primers used have been shown to be specific earlier (see Materials and methods).
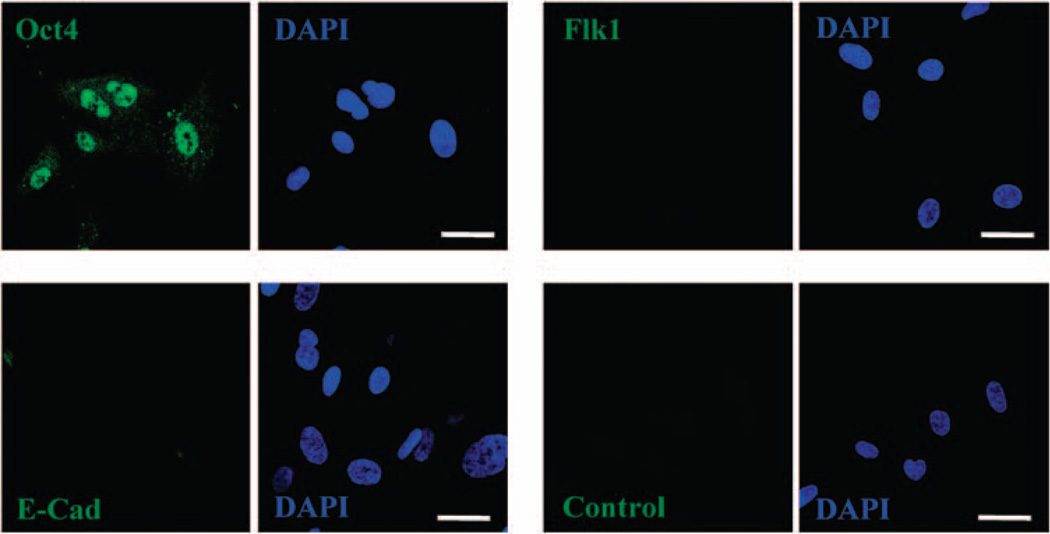
Confocal microscopy of immunocytochemical investigations further proved hAFSCs to be positive for Oct-4 and negative for Flk1. In case of E-cadherin, the expression level was between extremely low and undetectable through immunocytochemistry (Figure 4). On induction of EB formation in suspension, we detected the characteristic formation of a distinct peripheral layer on the outer surface of the EBs, including cells that express laminin or α-fetoprotein. In addition, these confocal analyses showed specific cells within the EBs to express the endothelial marker Flk1, the epithelial marker E-cadherin or the ectodermal marker nestin (Figure 5). Here it is important to note that the vascular endothelial growth factor receptor 2, Flk1, is known to be localized to both the cytoplasm and nucleus, depending on its phosphorylation status (Yamashita et al., 2000; Blazquez et al., 2006).
Figure 4.
Marker expression in CB3 human amniotic fluid stem cells (hAFSCs). CB3 cells were grown on slides, fixed, permeabilized and incubated with antibodies against the indicated proteins (green). Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) (blue). A control experiment, in which the primary antibody has been omitted, is presented. Scale bars: 20 µm.
Figure 5.
Marker analyses in CB3 human amniotic fluid stem cells (hAFSCs)-derived embryoid bodies (EBs). CB3 cells were induced to form EBs in suspension using low-adherence 96-well plates, fixed after 6 days of culture, permeabilized and stained for several differentiation markers (green). Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI) (blue). (a) α-Fetoprotein (aFP) and laminin (Lam) expression in EBs. EBs solely stained with the fluorescent-labelled secondary antibody served as negative control (Control). A phase-contrast microscopic image is included (right). (b) Laminin is expressed in the most outer layer of cells (white arrows). Cells beneath the outer structures are negative for laminin (asterisks). A distinct outer layer was also seen using phase-contrast microscopy (right, black arrows). (c) Flk1 expression was detectable in a subset of cells (white arrows). Asterisks indicate cells that are negative for Flk1. (d) E-cadherin (E-cad) staining and corresponding phase contrast image. (e) Nestin staining and corresponding phase contrast image. Scale bars: 20 µm.
The conclusion that different cells with different differentiation status are part of hAFSC-derived EBs is supported by our observation that, within one EB, cells positive and negative for specific differentiation markers exist (Figure 5).
EB formation of hAFSCs depends on mTOR
Conflicting results from studies in murine models have been published regarding the role of mTOR for the development of embryonic and extraembryonic tissues (Gangloff et al., 2004; Shiota et al., 2006; Sampath et al., 2008). To test the role of mTOR for EB formation of hAFSCs, we made use of the widely used mTOR inhibitor rapamycin. First, we found that blocking mTOR activity significantly affected the cell cycle regulation and proliferation rate of hAFSCs without inducing apoptosis (Figure 6).
Figure 6.
Rapamycin-mediated effects on cell cycle progression and proliferation of human amniotic fluid stem cell (hAFSC) lines. (a) Logarithmically growing CB3 and Q1 cells were treated with the mammalian target of rapamycin (mTOR) inhibitor rapamycin dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 100 nM for 24 h or with DMSO as a control, stained with propidiumiodide and cytofluorometrically analysed for DNA distribution. Representative DNA profiles are presented. (b) In addition, the percentage ( ± s.d.) of so treated cells in G0/G1, S and G2/M phase of the cell cycle is indicated. Nontransformed, nonimmortalized IMR-90 lung fibroblasts were analysed in parallel. (c) Cells as described above were treated with rapamycin for a total of 5 days and analysed for proliferation rates by counting cells at indicated time points (d1, day 1, represents cells collected after 24 h rapamycin treatment). Cell numbers are given as a percentage ( ± s. d.) relative to the number of cells seeded, set as 100%. At the final day 5, cells were additionally investigated for the phosphorylation status of the mTOR downstream effector ribosomal protein S6 by immunoblotting (upper right panel). (d) Besides, CB3, Q1 and IMR-90 cells analysed in (a) were cytofluorometrically investigated for the percentage ( ± s.d.) of apoptotic cells with sub-G1 DNA content.
Western blot analyses revealed that rapamycin treatment triggered a block of mTOR-regulated S6 phosphorylation in hAFSCs (Figures 6c, 7a). In addition, FSC analyses of cells of the induced EBs showed that rapamycin induced a downregulation of hAFSC size (Figure 7b), which is in perfect agreement with previously reported effects of mTOR on cell size regulation (compare, for example, Sarbassov et al., 2006; Rosner and Hengstschläger, 2008).
Figure 7.
Embryoid body (EB) formation of human amniotic fluid stem cell (hAFSC) depends on mammalian target of rapamycin (mTOR). (a) CB3 stem cells logarithmically growing in medium 1 (Table 1) were incubated with and without the mTOR inhibitor rapamycin at a final concentration of 100 nM and total protein lysates were prepared. These protein extracts were used to study the amount of phosphorylated S6 S240/244, which is a substrate of the mTOR-regulated p70S6 Kinase, and total S6 through western blot analyses. Detection of α-tubulin was included to prove equal loading of lysates. (b) CB3 cells with and without incubation with rapamycin were induced to form EBs in medium 1. EB cells were analysed for cell size through forward scatter determination of the flow cytometer. (c) The incidence of EB formation of CB3 cells on day 15 was investigated in three independent experiments. Each independent experiment was carried out by seeding 15 hanging drops in triplicate. The incidence is given as percentage ( ± s.d.) of the number of attached EBs recovered from 15 hanging drops seeded. (d) Representative pictures of CB3-derived EBs on day 15 in medium 1 with and without treatment with rapamycin. (e) The size of the EBs obtained in the experiments described in (c) and (d) was determined using the cell’D software from Olympus and is given in µm2 ( ± s.d.).
The incidence of EB formation of hAFSCs (given as percentage of the number of attached EBs recovered from 15 hanging drops seeded) was significantly diminished on negatively regulating mTOR through rapamycin (Figure 7c; P < 0.05). In addition, rapamycin treatment caused a pronounced effect on EB size (Figure 7d and e; P < 0.05). Taken together, these findings show for the first time that mTOR has a major role in the EB formation process of hAFSCs.
The role of mTORC1 and mTORC2 for EB formation of hAFSCs
In mammalian cells, two mTOR-containing complexes, namely mTORC1 and mTORC2, with different substrate specificities have been described. Rapamycin bound to the protein FKBP12 generates a drug–receptor complex that binds and inhibits mTORC1. As FKBP12-rapamycin does not bind to preformed mTORC2, rapamycin was originally thought to only inhibit mTORC1 (Wullschleger et al., 2006; Guertin and Sabatini, 2007; Yang and Guan, 2007). However, recently it was shown that long-term rapamycin treatment also suppresses the function of mTORC2 and that this property is important for the effects of this drug (Sarbassov et al., 2006; Rosner and Hengstschläger, 2008). Accordingly, the fact that rapamycin affects the EB formation process of hAFSCs described above made it interesting to further investigate the specific roles of mTORC1 and mTORC2. We carried out small interfering RNA (siRNA) experiments to downregulate the endogenous protein levels of either the mTORC1-specific component raptor or of the mTORC2-specific component rictor in hAFSCs. Western blot analyses revealed that raptor-specific siRNA treatment caused a pronounced downregulation of endogenous raptor protein levels without effects on rictor and vice versa (Figure 8a). Here it is important to note that the siRNA-mediated downregulation of these proteins in hAFSCs remained stable over the time period of the EB formation experiments performed here (data not shown). Raptor-specific siRNA treatment triggered a downregulation of the amount of phosphorylated S6 S240/244, which is a substrate of the mTORC1-regulated p70S6 Kinase, without negative effects on the amount of phosphorylated Akt S473, which is a substrate of mTORC2. However, although, as expected, rictor-specific siRNA treatment caused a strong reduction of endogenous phospho-Akt S473 levels, it also seemed to affect the S6 S240/244 phosphorylation levels (Figure 8a).
Figure 8.
Embryoid body (EB) formation of human amniotic fluid stem cell (hAFSC) depends on mTORC1 and mTORC2. (a) CB3 stem cells were transfected with unspecific control small interfering RNAs (siRNAs), or siRNAs specific for raptor or rictor, respectively. Total protein lysates were used to prove the downregulation of endogenous raptor and rictor protein through western blot analyses. In addition, the amount of phosphorylated S6 S240/244, which is a substrate of the mTORC1-regulated p70S6 Kinase, total S6 protein, phosphorylated Akt S473, which is a substrate of mTORC2, and total Akt protein has been analysed. Detection of α-tubulin was included to prove equal loading of lysates. (b) The cells described in (a) were induced to form EBs in medium 1 as described in the Materials and methods section. The incidence of EB formation on day 15 was investigated in two independent experiments. Each independent experiment was carried out by seeding 15 hanging drops in triplicate. The incidence is given as percentage ( ± s.d.) of the number of attached EBs recovered from 15 hanging drops seeded. (c) Representative pictures of CB3-derived EBs on day 15 in medium 1 obtained in the experiments described in (a) and (b). (d) The size of the EBs obtained in the experiments described in (a–c) was determined using the cell’D software from Olympus and is given in µm2 ( ± s.d.).
The incidence of EB formation of hAFSCs (given as percentage of the number of attached EBs recovered from 15 hanging drops seeded) was significantly diminished on treatment with raptor-specific siRNAs (Figure 8b; P < 0.05). Interestingly, rictor-specific siR-NAs did not affect the incidence of EB formation with statistical significance (Figure 8b). However, both raptor-specific and rictor-specific siRNA treatment caused pronounced effects on EB size (Figure 8c, d; P < 0.05). These data confirm that mTOR is a major regulator of EB formation of hAFSCs. However, these results also highlight that there might be differences in the role of mTORC1 and mTORC2 for this process.
Discussion
Human amniotic fluid stem cells are on the way to become an important source for both basic science and regenerative medicine. Pluripotentiality and a high proliferation rate are their advantages compared with adult stem cells. Accessibility, no ethical concerns and the fact that they do not produce teratomas when transplanted to animals could be their advantages when compared with embryonic stem cells (Trounson, 2007; Perin et al., 2008; Cananzi et al., 2009). In addition, comparable with induced pluripotent stem cells, hAFSCs could be a useful source for the generation of disease specific human stem cell lines providing an optimal biological model to recapitulate normal and pathological differentiation processes in vitro. In the past, tumor cell lines or transformed derivatives of native tissues from diseased patients represented the most commonly used biological systems for such research studies. Nowadays, it has already been shown to be possible to establish specific induced pluripotent stem cells from patients diagnosed with different diseases (Dimos et al., 2008; Maherali and Hochedlinger, 2008; Park et al., 2008). Worldwide, amniocenteses are performed for prenatal genetic diagnosis of mono-genetic diseases. It could be a promising strategy of highest value for basic research to establish hAFSCs derived from a wide variety of pregnancies with specific genetic aberrations. And compared with induced pluripotent stem cells, hAFSCs do not need to undergo prior processes of inducing stem cell properties (Siegel et al., 2007). However, the question whether induced pluripotent stem cells and hAFSCs harbor pluripotentiality of the same level as embryonic stem cells is still under investigation. In addition, it is of importance to answer the question whether these stem cell types merely allow to investigate different specific differentiation processes of individual cells, or are also usable for studies of the according three-dimensional and tissue-level contexts. Especially, so far, the potential to form EBs has not been proven for hAFSCs.
When cultured in suspension, under conditions in which they are unable to attach to the surface of culture dishes, without antidifferentiation factors and without contact with feeder cells, embryonic stem cells harbor the potential to form such three-dimensional aggregates, named EBs (Narita et al., 1996; Itskovitz-Eldor et al., 2000; Kurosawa, 2007; Ungrin et al., 2008). Here it is important to note that compared with mouse EBs, human EB formation and the accompanied differentiation is known to be much more chaotic and disorganized with wide variability both between and within individual EBs. However, three criteria must be fulfilled to define the potential of human stem cells to form EBs (Narita et al., 1996; Itskovitz-Eldor et al., 2000; Koike et al., 2007; Kurosawa, 2007; Kim et al., 2008; Ungrin et al., 2008): (1) It must be possible to reproducibly induce EB formation right under the growth conditions described above. Performing many independent experiments using different approaches, we demonstrate in this report that hAFSCs grown under such conditions can indeed form EBs. (2) EB formation does not only represent a change in growth characteristics, but must rather be accompanied by specific differentiation processes. As well described for EBs derived from human or mouse embryonic stem cells, EB formation of hAFSCs is accompanied by a decrease of nodal and Oct-4 expression and by induction of the differentiation markers Pax 6 (ectodermal), nestin (ectodermal), Flk1 (endothelial), E-cadherin (epithelial), GATA4 (endodermal), T (brachyury; mesodermal) and HBE1 (mesodermal). We additionally detected the characteristic formation of a distinct peripheral layer on the outer surface of the EBs, including cells that express laminin or α-fetoprotein. (3) Finally, it was important to prove that different cells with different differentiation status are part of the generated EBs formed from one clonal hAFSC type. In summary, to our knowledge this is the first demonstration that hAFSCs can form EBs and therefore can be used for investigations of the three-dimensional and tissue-level contexts of cell differentiation processes.
The mTOR kinase is well known to have a central role in the regulation of many cell differentiation processes. However, conflicting results from studies in murine models have been published regarding the role of mTOR for the development of embryonic and extraembryonic tissues (Gangloff et al., 2004; Shiota et al., 2006; Wullschleger et al., 2006; Guertin and Sabatini, 2007; Yang and Guan, 2007; Rosner et al., 2008). Very recently, integrating transcriptome analysis with global assessment of ribosome loading, the gene expression during differentiation of murine embryonic stem cells into EBs has been profiled. It was reported that a variety of translational regulators, including mTOR, control global and selective protein synthesis during this process and that translational regulation may be an important quality control for self-renewal and choice of fate in murine embryonic stem cells (Sampath et al., 2008). However, until now, the question whether mTOR function is essential for the development of murine or human EBs remained elusive. In this study, we show such a role of mTOR for EB formation of hAFSCs. We found the mTOR inhibitor rapamycin to significantly diminish both the incidence of EB formation of hAFSCs and the size of these aggregates.
In mammalian cells, two different mTOR complexes with different substrate specificities exist. mTORC1-containing raptor is involved in the regulation of mRNA translation through its potential to phosphorylate the p70S6 Kinase to activate the ribosomal protein S6 by phosphorylation at S240/244. mTORC2 contains rictor and phosphorylates Akt at S473, which, in conjunction with the PDK1-mediated phosphorylation at T308, drives full activation of Akt (Wullschleger et al., 2006; Dann et al., 2007; Guertin and Sabatini, 2007; Yang and Guan, 2007). Recently it was shown that rapamycin suppresses the function of both mTORC1 and mTORC2 (Sarbassov et al., 2006; Rosner and Hengstschläger, 2008). Accordingly, our demonstration that rapamycin affects the EB formation process of hAFSCs did not allow conclusions concerning the specific roles of mTORC1 and mTORC2 in this process. To investigate this issues in more detail, we carried out experiments using raptor (mTORC1)-specific siRNA and rictor (mTORC2)-specific siRNA. Specifically downregulating endogenous raptor protein negatively affected the incidence of formation and the size of hAFSC-derived EBs to an extent that was very comparable with the effects of rapamycin. Treatment with rictor-specific siRNA also triggered very comparable effects on EB size, but was without significant consequences on the incidence of EB formation. These data provide evidence that mTORC2 is involved in the quality rather than in the quantity of hAFSC-derived EB development and show that mTORC1 and mTORC2 differently affect this process. The latter is not surprising considering the different biological functions and targets of the two mTOR-containing complexes (see above). Still, it was interesting to see that under the experimental conditions used here, modulating raptor indeed specifically affected only the mTORC1-regulated S6 phosphorylation, whereas modulating endogenous rictor had effects on both the mTORC2-mediated Akt phosphorylation and mTORC1-regulated S6 phosphorylation. The latter could be due to the fact that mTORC2 also functions upstream of mTORC1. As described, mTORC2 is responsible for the full activation of Akt. And Akt-mediated phosphorylation downregulates the GTPase-activating potential of tuberin toward Rheb, which is a potent regulator of mTORC1 (Wullschleger et al., 2006; Guertin and Sabatini, 2007; Yang and Guan, 2007). However, our obtained data strongly suggest that the mTORC2-mediated effects on the process of EB formation cannot exclusively be attributed to its potential to regulate mTORC1, as we observed pronounced differences between the effects of modulating mTORC2 activity and modulating mTORC1 on the incidence of EB formation.
In the last years, evidence has been provided for a role of mTOR in a wide variety of different human diseases (Dann et al., 2007; Rosner et al., 2008). Our findings reported here that they can form EBs and that mTOR has a major role in this process identify hAFSCs as a human stem cell model, which can be used to investigate the role of alterations of the mTOR pathway in studies of the three-dimensional and tissue-level contexts of a variety of differentiation processes, thereby enabling more detailed investigations of mTOR-associated diseases and drug development.
Materials and methods
Cells, cell culture, flow cytometry and cytogenetics
The stem cell lines, CB3 and CD117/2, used here were established by isolation of hAFSCs through magnetic cell sorting using the CD117 MicroBead Kit (Miltenyi Biotec, Bergisch Gladbach, Germany, 130–091–332) (De Coppi et al., 2007). This project has been reviewed and accepted by the ethics commission of the Medical University of Vienna, Austria (project number: 036/2002). The clonal Q1 hAFSC line has already been established earlier (De Coppi et al., 2007). IMR-90 fibroblasts were obtained from the American Type Culture Collection (Rosner and Hengstschläger, 2008). Cells were harvested and fixed by rapid submersion in ice-cold 85% ethanol, DNA was stained with 0.25 mg/ml propidium iodide, 0.05 mg/ml RNase, 0.1% Triton X-100 in citrate buffer, pH 7.8, and analysed on a Beckton Dickinson FACScan (Becton Dickinson, San Jose, CA, USA) (Rosner et al., 2003). Cell numbers and cell size were determined on a hemocytometer or on a Casy Cell Counter (Schärfe System, Innovatis, Reutlingen, Germany). Chromosome banding was produced by means of a conventional 550-band trypsin–Giemsa analysis (Hengstschläger et al., 2005).
EB formation
Human amniotic fluid stem cells were harvested and resuspended in either medium 1 or medium 2 (Table 1), and two standard methods were used to induce EB formation: the hanging drop method (Narita et al., 1996; Koike et al., 2007) and cultivation in suspension in low-adherence 96-well plate (Ng et al., 2005). For the hanging drop method, 20 µl drops containing 4000 cells in suspension were placed on the lid of a 100-mm Petri dish. The lid was inverted and placed over the bottom of a Petri dish filled with phosphate-buffered saline. On day 4, the EBs formed were transferred on 0.1% gelatin-coated 100-mm tissue culture dish. Growth media were renewed every 3 days and EBs were kept in culture up to 15 days. EBs were also formed in suspension using low-adherence 96-well plates. hAFSCs were seeded (2000 cells per well) into nonadhesive-surface 96-well plates (Sterilin, Caerphilly, UK, 612V96) using medium 1 containing 0.3% methylcellulose. Aggregation was promoted by centrifugation and EBs were collected on day 6 after inducing the aggregation.
Reverse transcriptase–PCR
RNA was prepared using the Cell-to-cDNA-II kit (Ambion, Austin, TX, USA). RNA was checked for DNA contamination using GAPDH primers specific for DNA recognition and transcribed into cDNA following the Cell-to-cDNA-II kit manufacturer’s instructions. The PCR reactions were prepared to contain 0.2 mM dNTPs and 1.25U GoTaq-Flexi-DNA polymerase in the according reaction buffer provided by Promega (Promega, Madison, WI, USA). To the reaction, 2 µM of each primer was added (Prusa et al., 2003). The used primers, published to be specific earlier, are listed below with the expected product sizes in parentheses (Chunhui et al., 2001; Sato et al., 2004; Ng et al., 2005; Takahashi et al., 2007; Ungrin et al., 2008):
| Oct4 (169bp) | Forward: 5′-CTTGCTGCAGAAGTGGGTGGAGGAA-3′ |
| Reverse: 5′ -CTGCAGTGTGGGTTTCGGGCA-3′ | |
| GATA4 (194bp) | Forward: 5′-TCCCTCTTCCCTCCTCAAATTC-3′ |
| Reverse: 5′ -TCAGCGTGTAAAGGCATCTG-3′ | |
| Flk1 (400 bp) | Forward: 5′-CATATCTGTCCTGATGTGATATGTC-3′ |
| Reverse: 5′ -CATAGCATGTCTTATAGTCATTGTTC-3′ | |
| HBE1 (126 bp) | Forward: 5′-TGCATGTGGATCCTGAGAAC-3′ |
| Reverse: 5′ -CGACAGCAGACACCAGCTT-3′ | |
| Pax6 (317 bp) | Forward: 5′-ACCCATTATCCA-GATGTGTTTGCCCGAG-3′ |
| Reverse: 5′ -ATGGTGAAGCTGGGCATAGGCGGCAG-3′ | |
| T (274 bp) | Forward: 5′-GCCCTCTCCCTCCCCTCCACGCACAG-3′ |
| Reverse: 5′ -CGGCGCCGTTGCTCACAGACCACAGG-3′ | |
| E-cadherin (129 bp) | Forward: 5′-CCTGGTTCAGATCAAATCCAAC-3′ |
| Reverse: 5′ -GTCACCTTCAGCCATCCTG-3′ | |
| β-Actin (401 bp) | Forward: 5′-ACAGCAGTCGGTTGGAGCGAGCATC-3′ |
| Reverse: 5′ -CAAGTCAGTGTACAGGTAAGCCCTG-3′ | |
| Nodal (126 bp) | Forward: 5′-ACCGAGTCCCTTCCACTTGTTG-3′ |
| reverse: 5′-AGAGGCACCCACATTCTTCCAC-3′ |
Immunocytochemistry
Cells or EBs were fixed in 4% paraformaldehyde for 10 min at 4 °C, treated with 0.5% Tween 20 and blocked for nonspecific binding with phosphate-buffered saline containing 1% bovine serum albumin and 0.5% Tween 20 for 30 min at room temperature. Overnight incubation at 4 °C was performed with anti-Oct4 antibody (Chemicon, Billerica, MA, USA, MAB4305), anti-Flk1 antibody (Abcam, Cambridge, UK, ab39256), anti-nestin antibody (Neuromics, Edina, MN, USA, MO15012), anti-laminin antibody (Sigma-Aldrich, St Louis, MO, USA, L9393), anti-α-fetoprotein antibody (R&D System, Minneapolis, MN, USA, MAB1369) or anti-E-cadherin antibody (BD biosciences, San Jose, CA, USA, 610181). After washing with phosphate-buffered saline, 0.5% Tween 20 cells and EBs were incubated with secondary antibodies (anti-mouse IgG Alexa Fluor 488, Invitrogen, Carlsbad, CA, USA, or anti-rabbit IgG Alexa Fluor 488, Invitrogen) for 4 h at room temperature. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (1 µg/ml).
Microscopy
Immunofluorescence analysis of cells and EBs was carried out with a Zeiss LSM5 Exciter confocal microscope (Zeiss, Oberkochen, Germany). The size of the EBs was determined using the cell’D software from Olympus (Olympus Austria, Vienna, Austria).
siRNA treatment
RNA silencing was achieved using human raptor and rictor siRNA (Dharmacon, Lafayette, CO, USA) at a final concentration of 50 nM. siRNA was delivered to the cells using RNAiMAX reagent (Invitrogen) following the transfection protocol provided by the manufacturers. A pool of four nontargeting siRNAs was used as a control for nonsequence-specific effects.
Protein extraction and immunoblotting
Protein samples were extracted as described earlier (Rosner et al., 2007) and run on an SDS–polyacrylamide gel and transferred to nitrocellulose. For immunodetection, antibodies specific for the following proteins were used: phospho-S6 ribosomal protein S240/244 (Cell Signaling, Danvers, MA, USA, #2215), S6 (Cell Signaling, #2317), rictor (Cell Signaling, #2114), raptor (Cell Signaling, #2280), phospo-Akt S473 (Cell Signaling, #4060), Akt (Cell Signaling, #9272) and α-tubulin (Calbiochem, La Jolla, CA, USA, #CP06). Rabbit polyclonal and monoclonal antibodies were detected using anti-rabbit IgG, a horseradish peroxidase (HRP)-linked heavy and light chain antibody from goat (Bethyl Laboratories, Montgomery, TX, USA, A120-101P); mouse monoclonal antibodies were detected using anti-mouse IgG, an HRP-linked heavy and light chain antibody from goat (A90–116P, Bethyl Laboratories). Signals were detected using the enhanced chemiluminescence method (Pierce, Rockland, IL, USA) (Rosner and Hengstschläger, 2008).
Statistical analyses
Flow cytometry data, Casy Cell Counter (Schärfe System, Innovatis, Reutlingen, Germany) results, incidence of EB formation and EB size data are presented as average ± s.d. Comparisons between groups were carried out by using Student’s t-test (paired, two-tailed) using Graph-Pad INSTAT software (GraphPad Software Inc, La Jolla, CA, USA) with P-values <0.05 indicating statistical significance.
Acknowledgements
Research in our laboratory is supported by the FWF Austrian Science Fund (P18894-B12), by the Herzfelder’sche Familien-stiftung and by the Research Training Network ‘Developing a stem cell based therapy to replace nephrons lost through reflux nephropathy’ (http://www.kidstem.org) funded by the European Community as part of the Framework program 6 (FP6 036097–2).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Aboushwareb T, Atala A. Stem cells in urology. Nat Clin Pract Urol. 2008;5:621–631. doi: 10.1038/ncpuro1228. [DOI] [PubMed] [Google Scholar]
- Besser D. Expression of nodal, lefty-A, and lefty-B in undifferentiated human embryonic stem cells requires activation of smad2/3. J Biol Chem. 2004;279:45076–45084. doi: 10.1074/jbc.M404979200. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Cook N, Micklem K, Harris AL, Gatter KC, Pezzella F. Phopshorylated KDR can be located in the nucleus of neoplastic cells. Cell Res. 2006;16:93–98. doi: 10.1038/sj.cr.7310012. [DOI] [PubMed] [Google Scholar]
- Bossolasco P, Montemurro T, Cova L, Zangrossi S, Calzarossa C, Miatiotis S, et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006;16:329–336. doi: 10.1038/sj.cr.7310043. [DOI] [PubMed] [Google Scholar]
- Cananzi M, Atala A, DeCoppi P. Stem cells derived from amniotic fluid: new potentials in regenerative medicine. Reprod Biomed Online. 2009;18:17–27. doi: 10.1016/s1472-6483(10)60111-3. [DOI] [PubMed] [Google Scholar]
- Chunhui X, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotech. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A, Thomas G. mTOR complex 1-S6K1 signaling: at the cross roads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos TX, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotech. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Ditadi A, de Coppi P, Picone O, Gautreau L, Smati R, Six E, et al. Human and murine amniotic fluid c-Kit+ cells display hematopoietic activity. Blood. 2009;113:3953–3960. doi: 10.1182/blood-2008-10-182105. e-pub ahead of print 12 February 2009. [DOI] [PubMed] [Google Scholar]
- Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualandris A, Noghero A, Geuna M, Arese M, Valdembri D, Serini G, et al. Microenvironment drives the endothelial or neural fate of differentiating embryonic stem cells coexpressing neuropilin-1 and Flk-1. FASEB J. 2008;23:1–11. doi: 10.1096/fj.08-112847. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Hengstschläger M, Prusa A, Repa C, Deutinger J, Pollak A, Bernaschek G. Subtelomeric rearrangements as neutral genomic polymorphisms. Am J Med Genet. 2005;133:48–52. doi: 10.1002/ajmg.a.30520. [DOI] [PubMed] [Google Scholar]
- in ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548–1549. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies comprising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- Karlmark KR, Freilinger A, Marton E, Rosner M, Lubec G, Hengstschläger M. Activation of ectopic Oct4 and Rex-1 promoters in human amniotic fluid cells. Int J Mol Med. 2005;16:987–992. [PubMed] [Google Scholar]
- Kim J, Lee Y, Kim H, Hwang KJ, Kwon HC, Kim SK, et al. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GD, Kim GJ, Seok JH, Chung H-M, Chee K-M, Rhee G-S. Differentiation of endothelial cells derived from mouse embryoid bodies: a possible in vitro vasculogenesis model. Toxic Lett. 2008;180:166–173. doi: 10.1016/j.toxlet.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Koike M, Sakaki S, Amano Y, Kurosawa H. Characterization of embryoid bodies of mouse embryonic stem cells formed under various culture conditions and estimation of differentiation status of such bodies. J Biosci Bioeng. 2007;104:294–299. doi: 10.1263/jbb.104.294. [DOI] [PubMed] [Google Scholar]
- Kolambkar YM, Peister A, Soker S, Atala A, Guldberg RE. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol. 2007;38:405–413. doi: 10.1007/s10735-007-9118-1. [DOI] [PubMed] [Google Scholar]
- Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- Lim SM, Pereira L, Wong MS, Hirst CE, VanVranken BE, Pick M, et al. Enforced expression of Mix1 during mouse ES cell differentiation suppresses hematopoietic mesoderm and promotes endoderm formation. Stem Cells. 2008;27:363–374. doi: 10.1634/stemcells.2008-1008. [DOI] [PubMed] [Google Scholar]
- Maherali N, Hochedlinger K. Induced pluripotency of mouse and human somatic cells. Cold Spring Harb Symp Quant Biol. 2008;73:157–162. doi: 10.1101/sqb.2008.73.017. [DOI] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson DB. Cardiomyocyte differentiation by GATA-4 deficient embryonic stem cells. Development. 1996;122:3755–3764. doi: 10.1242/dev.124.19.3755. [DOI] [PubMed] [Google Scholar]
- Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106:1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- Park I-H, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings G. The ethics of using embryos in research. Reprod Biomed Online. 2007;14:92–97. [Google Scholar]
- Perin L, Giuliani S, Jin D, Sedrakyan S, Carraro G, Habibian R, et al. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007;40:936–948. doi: 10.1111/j.1365-2184.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin L, Sedrakyan S, Da Sacco S, De Filippo R. Characterization of human amniotic fluid stem cells and their pluripotential capability. Methods Cell Biol. 2008;86:85–99. doi: 10.1016/S0091-679X(08)00005-8. [DOI] [PubMed] [Google Scholar]
- Pesce M, Schöler HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschläger M. Oct4 expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod. 2003;18:1489–1493. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollak A, et al. Neurogenic cell in human amniotic fluid. Am J Obstet Gyn. 2004;191:309–314. doi: 10.1016/j.ajog.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Rosner M, Freilinger A, Hanneder M, Fujita N, Lubec G, Tsuruo T, et al. p27 localization depends on the tumor suppressor protein tuberin. Hum Mol Genet. 2007;16:1541–1556. doi: 10.1093/hmg/ddm103. [DOI] [PubMed] [Google Scholar]
- Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, Hengstschläger M. The mTOR pathway and its role in human genetic diseases. Rev Mutat Res. 2008;659:284–292. doi: 10.1016/j.mrrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Rosner M, Hengstschläger M. Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet. 2008;17:2934–2948. doi: 10.1093/hmg/ddn192. [DOI] [PubMed] [Google Scholar]
- Rosner M, Hofer K, Kubista M, Hengstschläger M. Cell size regulation by the human TSC tumor suppressor proteins depends on PI3 K and FKBP38. Oncogene. 2003;22:4786–4798. doi: 10.1038/sj.onc.1206776. [DOI] [PubMed] [Google Scholar]
- Sampath P, Pritchard DK, Pabon L, Reinecke H, Schwartz SM, Morris DR, et al. A hierarchical network controls protein translation during murine embryonic stem cell self-renewal and differentiation. Cell Stem Cell. 2008;5:448–460. doi: 10.1016/j.stem.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen J-H, Hus PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Shiota C, Woo JT, Lindner J, Sleton KD, Magnuson MA. Multi-allelic disruption of the rector gene in mice revelas that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Siegel N, Rosner M, Hanneder M, Valli A, Hengstschläger M. Stem cells in amniotic fluid as new tools to study human genetic diseases. Stem Cell Rev. 2007;3:256–264. doi: 10.1007/s12015-007-9003-z. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Trounson A. A fluid means of stem cell generation. Nature Biotech. 2007;25:62–63. doi: 10.1038/nbt0107-62. [DOI] [PubMed] [Google Scholar]
- Tsai M-S, Hwang S-M, Tsai Y-L, Lee J-L, Chang Y-L. Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol Reprod. 2006;74:545–551. doi: 10.1095/biolreprod.105.046029. [DOI] [PubMed] [Google Scholar]
- Tsai M-S, Lee J-L, Chang Y-J, Hwang S-M. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450–1456. doi: 10.1093/humrep/deh279. [DOI] [PubMed] [Google Scholar]
- Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS ONE. 2008;3:e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall M. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Yang Q, Guan K-L. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]