Abstract
Introduction
Mastocytosis is a rare disease characterized by abnormal growth and accumulation of tissue mast cells (MC) in one or more organ systems and is classified as being either cutaneous mastocytosis (CM) or systemic mastocytosis (SM). In the pioneer studies of Slominski's group, a fully functional hypothalamic-pituitary-adrenal axis equivalent has been discovered in various tissues, including skin.
Aim
In the present study we investigated potential involvement of hypothalamus-pituitary-adrenal (HPA) cutaneous equivalent in ongoing mastocytosis.
Material and methods
The expression of HPA elements: CRH, UCN1, UCN2, UCN3, CRHR1, POMC, MC1R, MC2R and NR3C1 was assessed for their mRNA level in skin biopsies of adult patients with mastocytosis and healthy donors (n = 16 and 19, respectively), while CRH, UCN1, CRHR1, ACTH and MC1R were selected for immunostaining assay (n = 13 and 7, respectively). The expression of CRH receptor 1 (CRHR1) isomers was investigated by RT-PCR. The ELISA was used for detection of cortisol, CRH, UCN and ACTH in the serum.
Results
The decrease in the expression of HPA element of skin equivalent was observed on both mRNA and protein levels, however quantification of immunohistochemical data was impeded due to melanin in epidermis. Furthermore, we observed infiltration of dermis with HPA elements overexpressing mononuclear cells, which is in the agreement with an in vitro study showing a high expression of HPA elements by mast cells.
Conclusions
Taken together, it was confirmed that the expression elements of HPA was modulated in mastocytosis, thus the potential involvement of general and local stress responses in its pathogenesis should be postulated and further investigated.
Keywords: skin hypothalamic-pituitary-adrenal axis, mastocytosis, mast cells, corticotrophin-releasing hormone, adrenocorticotropic hormone, corticotrophin-releasing hormone receptor
Introduction
Mastocytosis (MS) is a heterogeneous disease resulting from the abnormal proliferation and activation of mast cells (MCs). MCs are bone marrow-derived cells residing in the connective tissue or mucous membranes. Tissue inflammation activates mastocytes to infiltrate the tissues, where they take part in the immediate immune response by secreting prostaglandins, cytokines, heparin and histamine [1], In mastocytosis, mast cells infiltrate one or more organs; mainly the skin, liver, spleen and bone marrow leading to their dysfunctions [2, 3]. This rare condition may affect a specific organ or the whole body [4]. Hence, based on the site of mast cell accumulation, the disease can be classified as cutaneous (CM) or systemic (SM). The latter is characterised by a more aggressive course of the disease but occurs with a lower rate. CM usually manifests early in the patient’s life but is more prone to remission [5, 6]. The main feature of CM is the occurrence of hyperpigmented skin lesions (plaques or wheals) termed urticaria pigmentosa and an increased mast cell count in the skin [7].
Skin pigmentation is a visible manifestation of melanin production by epidermal melanocytes. Melanogenesis is stimulated by ultraviolet radiation and directly regulated by multiple factors including neuropeptides or nitric oxide [8-10]. The major role in the process is played by POMC-derived neuropeptides: a-MSH and adrenocorticotropic hormone (ACTH), which are also the key elements of the hypothalamic-pituitary-adrenal axis (HPA) – the systemic stress response mechanism. Stressors trigger the hypothalamus to secrete corticotrophin-releasing hormone (CRH) [11]. Then CRH, through interaction with its receptor CRHR1 in the pituitary, stimulates the secretion of ACTH [12] into circulation, which in turn increases the production of glucocorticoids by adrenal cortex. The secretion of cortisol results in down-regulation of CRH and POMC production, and leads to adaptation to stress [13, 14]. Studies in recent years showed that the skin and its appendages express a fully functional analog of the HPA axis (sHPA) [9, 15, 16]. It was shown in an in vitro study that CRH induces POMC expression in human dermal fibroblasts [17]. CRH was also shown to be involved in the differentiation of keratinocytes [18,19] and regulation of the hair cycle [20]. Moreover, an alternative splicing of CRHR1 was suggested to regulate the response of keratinocytes and mouse pituitary ATT-20 cells to CRH [21, 22]. CRH, ACTH and a-MSH affect melanocyte proliferation and melanin production [23, 24]. Interestingly, a correlation between melanoma tumour progression and an increase in POMC peptide synthesis was observed [25, 26]. Mast cells also produce elements of HPA [27, 28] and respond to CRH with selective degranulation [29]. In an animal study, acute psychosomatic stress caused an increased skin CRH level which resulted in mast cell-dependent vascular permeability [30]. Furthermore, an elevated level of CRHR1 was found to coincide with an increased mast cell content in chronic urticaria [31]. Our recent studies revealed that keratinocyte differentiation and vitamin D modulate the expression of elements of sHPA [32-34]. Thus, it seems that several factors may trigger the hyperpigmentation (urticaria pigmentosa) observed in CM, however the expression of the elements of the sHPA axis was not investigated in MS.
Aim
Therefore, in order to assess the potential involvement of the HPA axis, the aim of this study was to investigate the expression of the elements of the HPA axis in mast cells and mastocytosis.
Material and methods
All chemicals were purchased from Sigma-Aldrich (Saint Louis, Missouri, USA) or as indicated.
Patients
The presence of the elements of the HPA axis was investigated in the serum of 30 adult MS patients (15 with CM and 15 with SM) and 15 healthy adult individuals by ELISA. Skin biopsies for gene expression study were taken from 16 MS patients and 19 unaffected individuals (control group – free from any immune-related diseases). Table 1 summarizes the groups’ demographic data. Patients were diagnosed in the Gdansk Mastocytosis Center – the mastocytosis reference site of the European Competence Network on Mastocytosis in Poland. Four micrometre needle biopsies of the affected skin (urticaria pigmentosa) and matching biopsies of potentially disease-free skin were collected from MS patients (16 biopsies). In addition, full thickness skin biopsies were acquired from healthy individuals (19 biopsies). All participants gave written consent to take part in the study and the study was approved by the local bioethical committee. The bioptates were snap frozen in liquid nitrogen and stored at -80°C. The frozen samples were cut into 5 to 8 urn sections and divided into two parts: the first was used for total mRNA extraction, the second was mounted on a glass microscope slide and used for immunochemistry studies.
Table 1.
qPCR study group
| Diagnosis | Average age | Sex ratio | Tryptase | SCORMA1 |
|---|---|---|---|---|
| Mastocytosis skin – 16 patients: | ||||
| CM (6) | 44 ±9.2 | F/M = 83/17% | 16.1 ±11.5 | 44 ±9.2 |
| SM (ISM/SSM) (10) | 52 ±7.9 | F/M = 70/30% | 82.2 ±44.5 | 52 ±7.9 |
| Unaffected skin – 19 individuals: | ||||
| Healthy control (19) | 45 ±25 | F/M = 62/38% | NA | NA |
NA-not applicable,1 Scoring Mastocytosis index, according to Lange et al. [34].
Measurement of the serum level of HPA neuropeptides in CM/SM patients
An independent assessment of the serum concentrations of CRH, UCN1, ACTH and cortisol was determined in a group of 30 CM/SM patients and 15 healthy subjects. The tests were performed with ELISA kits according to the manufacturer’s protocol.
Cell culture
Immortalised human keratinocytes – HaCaT (obtained from the Histology Department cell bank at the Medical University of Gdansk, Gdansk, Poland), mast cells – HMC-1 (thanks to the courtesy of DH Butterfield) [35] and human fibroblasts – HDF (thanks to the courtesy of R Sadej, IFB, UG&MUG, Gdansk, Poland).
cDNA preparation and qPCR
The skin bioptates were pulverized with a mortar under liquid nitrogen. The epidermis was enzymatically separated from the dermis of selected skin bioptates. Obtained samples were submerged in Fenozol (A&A Biotechnology, Gdynia, Poland). The cells were collected through lysis in Fenozol. All described samples were stored at -80°C until further preparation.
The RNA from the samples was purified using a Total Mini RNA isolation kit (A&A Biotechnology) and subsequently used for cDNA preparation with a First Strand cDNA Preparation kit (Thermo Scientific, Waltham, Massachusetts, USA) – according to the manufacturer’s protocols.
The estimation of relative mRNA levels for selected genes was carried out using a Real-Time HS 2x PCR Master Mix SYBR B kit (A&A Biotechnology) and StepOnePlus Real-Time PCR System (Applied Biosystems, Grand Island, NY, USA). The primers (Sigma-Aldrich) are listed in Table 2. The collected data were normalised by the comparative AA-Ct method (housekeeping gene: RPL37A). The normalised data were related to the average result of the control group (set as = 1) and presented as a relative fold-change.
Table 2.
List of primers
| Gene name | Sequence | Annealing temp [°C] |
|---|---|---|
| qPCR: | ||
| CRH | Forward:CACCCTCAGCCCTTGGATTTC | 57 |
| Reverse: GCCCTGGCCATTTCCAAGAC | ||
| POMC | Forward: GAGGGCAAGCGCTCCTACTCC | 63 |
| reverse: GGGGCCCTCGTCCTTCTTCTC | ||
| MC1R | Forward: ACTCACCCATGTACTGCTTC | 63 |
| Reverse: TACAGCACGGCCATGAGCAC | ||
| MC4R | Forward: CAGCATGGTGAAGAACATGG | 63 |
| Reverse: ATGACAGTTAAGCGGGTTGG | ||
| UCN1 | Forward: CAGGCGAGCGGCCGCG | 65 |
| Reverse: CTTGCCCACCGAGTCGAAT | ||
| UCN2 | Forward: GTGTCGGCCACTGCTGAGCCTGAGAGA | 65 |
| Reverse:ATCTGATATGACCTGCATGACAGTGGCT | ||
| UCN3 | Forward: TGCTGCTCCTGCTGCTGCTC | 58 |
| Reverse: GTGTCCTGGCGTGGCTTTCCC | ||
| NR3C1 | Forward: GAGACCAGATGTAAGCTCTCCT | 55 |
| Reverse: GCAATCATTCCTTCCAGCAC | ||
| RPL37A | Forward: TTCTGATGGCGGACTTTACC | 55 |
| Reverse: CACTTGCTCTTTCTGTGGCA | ||
| Nested PCR: | ||
| CRHR1 exon 2-7 round 1 | Forward:TCCGTCTCGTCAAGGCCCTTC | 60 |
| Reverse: AGTGGATGATGTTTCGCAGGCAC | ||
| CRHR1 exon 2-7 round 2 | Forward:TGTCCCTGGCCAGCAACATCTC | 60 |
| Reverse: GGCTCATGGTTAGCTGGACCAC | ||
The expression of CRHR1 splicing variants was evaluated using the nested PCR method as previously described [21].
Immunohistochemistry
Immunohistochemical labelling was performed on 8 um thick skin sections embedded in paraffin (13 CM/SM and 7 control bioptates) according to standard ABC protocol with modifications (IHC labelling kits listed in Table 3).
Table 3.
List of antibodies used in the study
| Primary immunoglobulin | Manufacturer | Catalogue No. | Dilution | Secondary immunoglobulin/detection kit |
|---|---|---|---|---|
| Anti-CRH (Gt) | Santa Cruz | sc-21675 | 1 : 100 | VECTASTAIN® ABC HRP Kit (Peroxidase, Goat IgG) |
| Anti-CRHRl (Gt) | Santa Cruz | ac-12381 | 1 : 100 | VECTASTAIN® ABC HRP Kit (Peroxidase, Goat IgG) |
| Anti-ACTH (Rb) | courtesy of dr. Parlow, | 1 : 100 | ImmPRESS™ HRPAnti-Rabbit IgG (Peroxidase) Polymer Detection Kit | |
| Harbor-UCLA Medical Center | 1 : 100 | |||
| Anti-UCN1 (Rb) | Abcam | ab58459 | 1 : 100 | ImmPRESS™ HRPAnti-Rabbit IgG (Peroxidase) Polymer Detection Kit |
| Anti-MCIR (Rb) | Abcam | ab125031 | 1 : 100 | ImmPRESS™ HRPAnti-Rabbit IgG (Peroxidase) Polymer Detection Kit |
Gt – host organism: goat, Rb – host organism: rabbit.
For immunoreactive score (IRS) assessment, the semi-quantitative scale of Jablonska et al. [36] was utilised with our modifications. The following algorithm was applied: colour intensity x labelling area. The values for intensity assessment ranged from 0.5 to 3 (gradation: 0.5); the area assessment ranged from 0 – no signal to 3 – signal in all epidermal layers (gradation: 0.5). The presented data show the average scores obtained by 3 independent observers.
Neoplastic mast cells (NMCs) have been classified in this study according to the criteria proposed by Sperr et al. [37] for mast cells common for mastocytosis ("atypical mast cells type I”). In short, two of the three following criteria had to be fulfilled: (i) cytoplasmic extensions (special shapes: spindles or fusiform shapes), (ii) oval nuclei with decentralised position, and (iii) hypogranulated cytoplasm with focal accumulations of granules with or without granule fusions. The NMCs were identified in the dermis at 40x magnification and quantified in 5 random areas per each IHC specimen, within which the IRS for the selected sHPA antigenlabelling was then assessed. The IRS for dermal NMCs was non-gradable (0/1) and reflected the percentage of signal-positive NMCs in the whole NMC content in a given area.
Statistical analysis
Statistical analysis of the results obtained for the compared groups was performed using an unpaired twotailed f-test with Welch’s correction, or otherwhere stated, with GraphPad Prism ver. 6.01 for Windows (Graph-Pad Software, San Diego California USA). P-values < 0.05 were considered statistically significant. For quantitative PCR, the statistical evaluation of the data was performed with the Mann-Whitney U two-tailed test due to the negative result on normality in each group.
The numerical results are presented in the form (mean ± SEM).
Results
Level of HPA elements in blood serum of CM/SM patients
The serum levels of the selected elements of the cutaneous HPA axis (CRH, UCN1, ACTH) were studied by ELISA. A significant difference between the CM/SM and control groups was shown only for ACTH (Table 4). Interestingly, even though the results of cortisol measurement lacked significance, its mean level was increased over 2-fold in CM patients, but within the group, high variability was observed.
Table 4.
Serum level of HPA elements in CM/SM patients
| Variable | CM/SM n = 30 | CM n = 15 | SM n = 15 | Control n = 15 |
|---|---|---|---|---|
| CRH | 6.8 ±2.3 | 6.8 ±2.7 | 6.9 ±1.9 | 6.7 ±1.5 |
| UCN1 | 5.3 ±1.4 | 5.2 ±1.7 | 5.4 ±1.2 | 4.8 ±2.3 |
| ACTH | 1.13 ±0.46** | 1.1 ±0.5* | 1.2 ±0.3** | 0.64 ±0.3 |
| Cortisol | 13.63 ±6.3 | 12.29 ±5.31 | 15.07 ±7.29 | 12.4 ±4.7 |
p = 0.02
p = O.OOl, CM/SM vs. control controls [ng/ml].
Expression of genes coding HPA neuropeptides and their receptors in the whole skin (alternative splicing)
The relative mRNA levels of the CRH, CRHR1, POMC, MC1R, MC4R, UCN1, UCN2, UCN3 and NR3C1 genes were analysed by qPCR, while CRHR1 mRNA level was investigated by nested PCR analysis (Table 5).
Table 5.
Nested PCR CRHR1 expression summary
| Healthy control | CRHR1 isoform | CM/SM patients | CRHR1 isoform |
|---|---|---|---|
| 1 | ND | 1 | a, c, e, h |
| 2 | A | 2 | E |
| 3 | ND | 3 | a, e |
| 4 | ND | 4 | ND |
| 5 | ND | 5 | c, e, h |
| 6 | a, c | 6 | a, c, e, h |
| 7 | ND | 7 | E |
| 8 | ND | 8 | a, c, e, h |
| 9 | ND | 9 | E |
| 10 | ND | 10 | A |
ND – not detected.
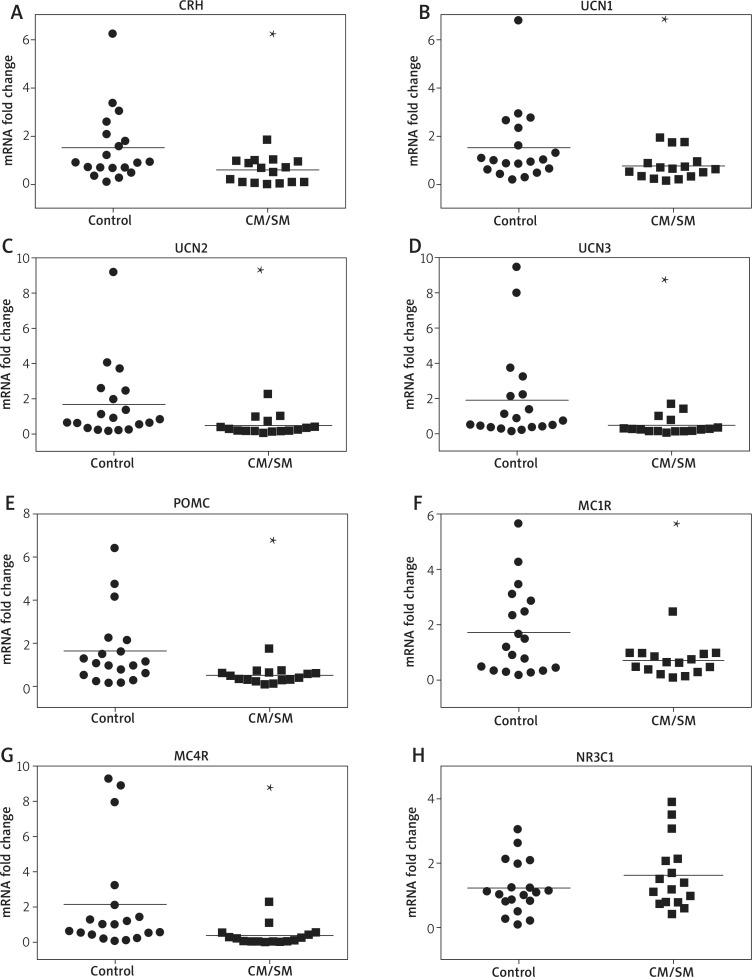
A nearly 3-fold downregulation of the CRH mRNA level was observed in the CM/SM skin biopsies in comparison to the non-affected skin samples of healthy donors (Fig-Figure 1. Differential expression of elements of sHPA axis in the urticaria pigmentosa (CM/SM patients) in comparison to control skin samples. The relative levels of mRNA for the chosen genes (as indicated on panels: A-G) were assessed in full thickness skin bioptates of MS patients (squares) and non-affected donors (dots). The relative mRNA levels for each individual in both groups were shown in relation to the average results for the control group (set as 1). Fold changes were shown in a logarithmic scale. The horizontal line represents the mean result for each group. Student's t-test analysis was used to analyse the data. *p < 0.05; **p < 0.01; ***p < 0.001ure 1 A). The mRNA level of urocortins was decreased in the CM/SM skin, by 2-fold for UCN1 (no statistical significance), nearly 4-fold for UCN2 and 4-fold for UCN3 in comparison to the healthy control (Figures 1 B-D). The relative level of POMC mRNA was also decreased in the CM/SM biopsies (3-fold, Figure 1 E). The MC1R mRNA level was decreased in the CM/SM biopsies by over 2-fold, while for MC4R a 6-fold decrease was observed (Figures 1 F, G). Only the mRNA level of glucocorticoid receptor NR3C1 was not altered in the skin biopsies of the MS patients (Figure 1 H).
Figure 1.
Differential expression of elements of sHPA axis in the urticaria pigmentosa (CM/SM patients) in comparison to control skin samples. The relative levels of mRNA for the chosen genes (as indicated on panels: A-G) were assessed in full thickness skin bioptates of MS patients (squares) and non-affected donors (dots). The relative mRNA levels for each individual in both groups were shown in relation to the average results for the control group (set as 1). Fold changes were shown in a logarithmic scale. The horizontal line represents the mean result for each group. Student's t-test analysis was used to analyse the data. *p < 0.05; **p < 0.01; ***p < 0.001
In addition, alternative splicing of CRHR1 receptor mRNA was investigated by nested PCR with two sets of primers spanning exons 2-7, as described previously [21]. The experiment was based on mRNA isolated from disease-affected skin of 10 CM/SM patients and compared to 10 healthy control samples (Table 5). The distinctive mRNA fragments for CRHR1 mRNA isoforms were detected in 9 of 10 patients, while only isoforms a, or a and c were detected in only two control samples. Interestingly, the expression of mRNA coding short isoform CRHR1e was detected in 8 patients, while four PCR fragments typical for a, c, e and h CRHR1 isoforms were detected in 3 patients.
Expression of genes coding HPA neuropeptides in selected cell lines
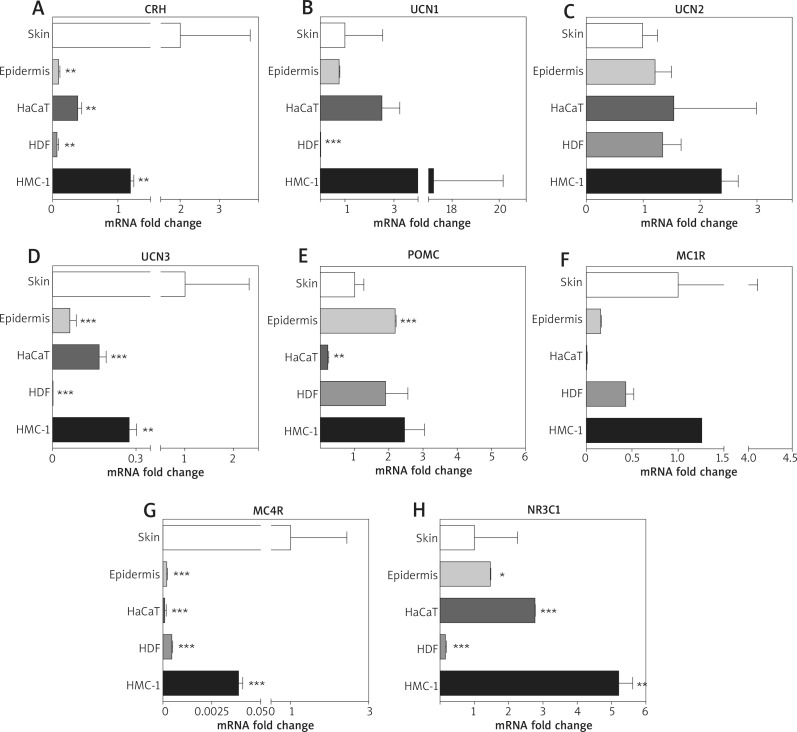
In order to investigate the contribution of specific cell types in sHPA neuropeptide production in the whole skin, the level of mRNA was evaluated for selected neuropeptides and their receptors in isolated epidermis of healthy donors, immortalised human keratinocytes (HaCaT), human fibroblasts (HDF) and mast cells line (HMC-1) (Figure 2). The collected data were processed and related to the control group average results analogously to the whole skin qPCR results described earlier.
Figure 2.
The relative level of mRNA for HPA elements in the selected skin cell lines. The relative levels of the mRNA selected genes were studied in full thickness biopsies of (skin) or separated epidermis (epidermis) of human skin collected from healthy donors and compared to immortalised human epidermal keratinocytes (HACAT), human dermal fibroblasts (HDF) and human mast cell line – HMC-1. The results are presented as fold changes vs. control (epidermis; set as 1). Student's f-test analysis was used to analyse the data. *p< 0.05; **p < 0.01; ***p < 0.001
Overall, the whole skin (control) showed the highest mRNA levels for CRH, UCN3 and MC4R (Figures 2 A, D, G). Out of the compared cell types, HMC-1 mast cells presented the highest expression of each assayed gene, frequently exceeding the skin control as well (Figures 2 B, C, E, F, H; Table 6). Interestingly, the lowest average expression of neuropeptides was found in HDF fibroblasts (i.e. Figures 2 A, B, D, H; Table 6). The cells of the epidermis (mainly keratinocytes) ranked midway, while the HaCaT immortal keratinocytes presented average expression of neuropeptides close to the whole skin control (Table 6). The mRNA level of POMC was the lowest in the HaCaT keratinocytes, while maintaining a similar level among the remaining cell lines (Figure 2 E). Finally, NR3C1 (with the abovementioned CRH) was expressed exceedingly high by HMC-1 mastocytes (Figure 2 H), while in contrast, of all cell types, the HDF cells presented the distinctively lowest NR3C1 expression.
Table 6.
The relative level of the mRNA of sHPA elements in the dermis, epidermis and representative skin cell lines. The fold changes were converted into percentages and the results for the full thickness skin were used as a reference (100%)
| Variable | CRH | UCN1 | UCN2 | UCN3 | POMC | MC1R | MC4R | NR3C1 | Average |
|---|---|---|---|---|---|---|---|---|---|
| Whole skin (control) | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% |
| Epidermis | 0.01% | 80.00% | 100.00% | 6.00% | 200.00% | 20.00% | 0.20% | 100.00% | 63.28% |
| HaCaT | 0.04% | 300.00% | 200.00% | 20.00% | 20.00% | 1.00% | 0.10% | 300.00% | 105.14% |
| HDF | 0.01% | 0.20% | 100.00% | 0.08% | 200.00% | 40.00% | 0.50% | 20.00% | 45.10%* |
| HMC-1 | 0.10% | 2000.00% | 200.00% | 30.00% | 200.00% | 100.00% | 4.00% | 500.00% | 379.26%* |
| Average | 20.03% | 496.04% | 140.00% | 31.22% | 144.00% | 52.20% | 20.96% | 204.00% |
p < 0.05.
Level of HPA antigens in the whole skin
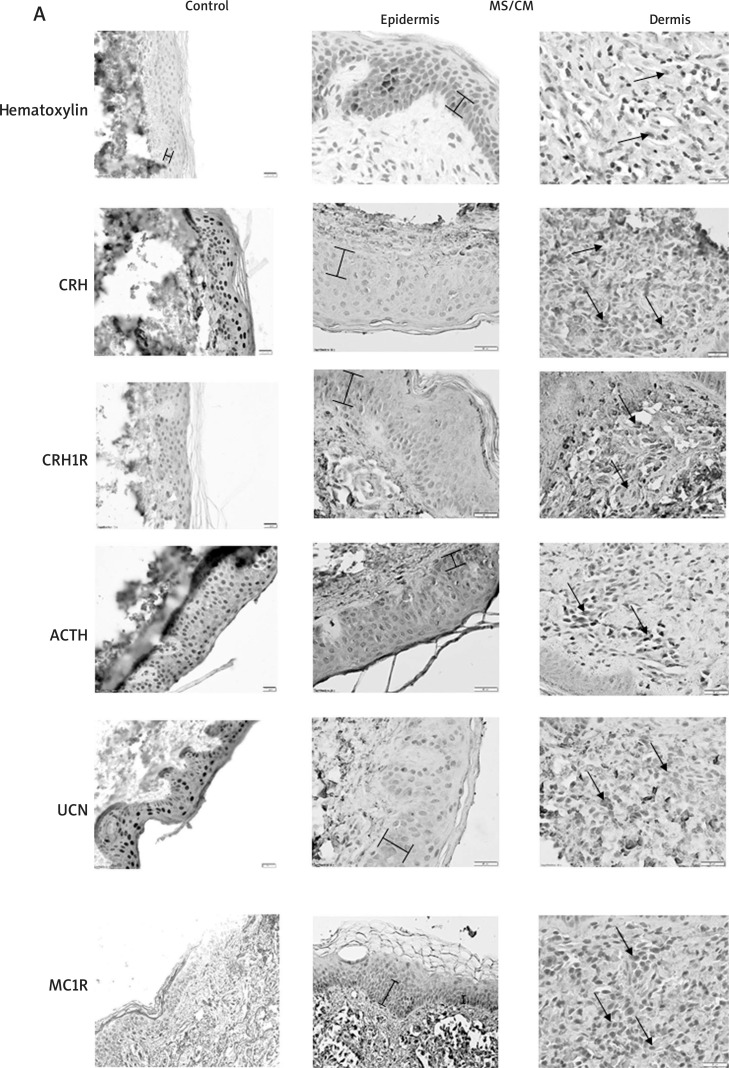
The level of immunoreactivity for five antigens – CRH, UCN1, CRHR1, ACTH and MC1R – were assessed in biopsies derived from 13 patients (CM/SM) and compared to the control group (7 samples). The relative intensity for each marker was calculated according to the previously published scale [36] with some modifications (see Material and methods). Figure 3 A shows representative stainings. Unfortunately, we were not able to observe any statistically significant alteration in immunoreactivities characteristic for the selected skin HPA elements in the SM/CM group in comparison to the control.
Figure 3 A.
Immunodetection of selected neuropeptides and their receptors in positive IHC labelling is represented in brown. The left panel represents healthy controls, middle – representative epidermis of SM/CM patients, right – representative dermis of SM/CM patients. Braces mark the range of pigment; arrows indicate positive NMC cells. Haematoxylin stained samples, where primary antibodies omitted, serve as negative controls. See Tables 3 or 4 for details.
It has to be noted that urticaria pigmentosa is characterised by hyperpigmentation, with elevated production of dark pigment – melanin and its deposition in keratinocytes of the stratum basale and stratum spinosum (Figure 3 A, marked on haematoxylin stained controls). The presence of the melanin pigment in MS-affected skin may hinder the assessment of the intensity of classic labelling (DAB) in the epidermis. However, it could be relatively easily distinguished from DAB labelling due to its localisation (it forms characteristic “caps” over keratinocyte nuclei). Nevertheless, the observed low levels of expression of the studied antigens in the epidermis and its high variability in the CM/SM group did not allow us to report a statistical difference between the groups (CM/ SM vs. controls).
In order to assess whether neoplastic mast cells (NMCs) could be a source or target for HPA-elements in the skin of CM/SM patients, the dermal components of full skin sections were analysed. The immunoreactive score (IRS) of HPA antigen labelling was estimated for mastocyte-like cells (NMCs) across the papillary and reticular dermis (Table 7, Figure 3 B). A one-tailed t-test with Welch’s correction was employed (IRS consequently higher in CM/SM NMCs).
Table 7.
Immunoreactivity score (IRS) for immunochemical labelling of HPA peptides in NMCs found in CM/SM and control skin specimens
| Bioptate type (group) | Average NMCs number | Percentage of antigen-positive NMCs | Mean (group)1 | SD (group)1 | ||||
|---|---|---|---|---|---|---|---|---|
| CRH | UCN1 | CRHR1 | ACTH | MC1R | ||||
| CM/SM patients | 215 | 0.8 ±0.2 | 5.2 ±3.2 | 0.9 ±0.4 | 14.1 ±4.3* | 6.2 ±3.4 | 5.4 ±1.4** | 11 |
| Control skin | 58 | 0.3 ±0.2 | 1.0 ±0.4 | 0.5 ±0.2 | 4.2 ±1.6 | 1.4 ±0.5 | 1.5 ±0.4 | 2.4 |
ased on all IRS results obtainedfor a given group (group members * antigens).
p < 0.05
p < 0.005, CM/SM vs. control.
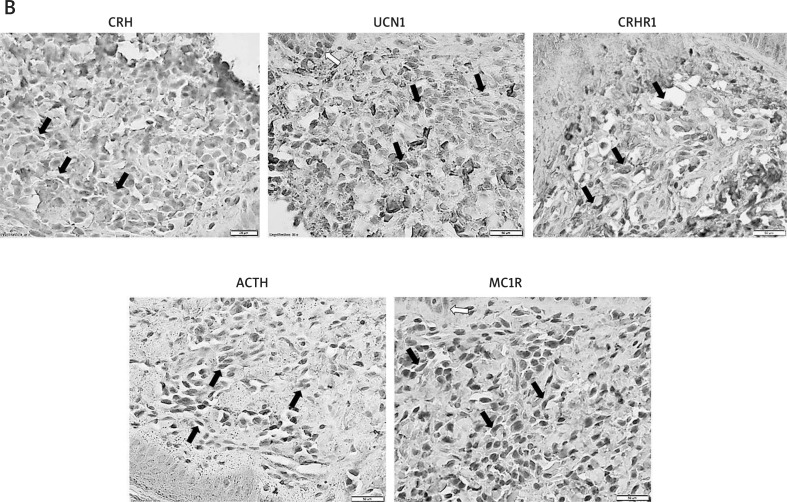
Figure 3. B.
Immunodetection of selected neuropeptides and their receptors in the dermal mononuclear cells of SM/CM patients in positive IHC labelling is represented in brown, arrows indicate positive NMC cells
The average density of dermal NMCs was higher in CM/SM skin (nearly 4-fold, p < 0.0001). Relative to nonaffected skin, the average IRS for CRH, UCN1, and ACTH in NMCs were nearly 3-, over 5- and 3-fold higher in mastocytosis, respectively. Similarly, the IRS for CRHR1 and MC1R receptors were higher in patients’ skin NMCs by nearly 2- and over 4-fold, respectively. Due to the high standard deviation of IRS results in the CM/SM group (over 5-fold higher than in the control group), statistical significance was reached only in the case of ACTH. Nonetheless, the average of total IRS values in NMCs differed highly between the groups (Table 7). As shown in Figure 3 B, a prominent expression of CRH, UCN1 and ACTH was identified in the NMC of CM/SM skin. On the other hand, the expression of CRHR1 and MC1R receptors was observed only in a fraction of NMCs, which might suggest that NMCs in MS are more likely a source than a target for neuropeptides.
Discussion
The presence of the hallmark of cutaneous mastocytosis – urticaria pigmentosa lesions [34, 38, 39] suggests the involvement of a-MSH and/or ACTH, as well as melanocortin receptors in its pathogenesis [23, 25, 40, 41]. It has to be noted that skin cells, including mast cells, were not only shown to express a fully functional analog of the HPA axis [9, 14, 28, 29, 42], but mast cells in particular are also readily regulated by CRH [28, 43]. However, the association of sHPA with CM has not been investigated so far.
In this study, we reveal dysregulation of sHPA axis expression in urticaria pigmentosa biopsies derived from MS patients. Despite considerable variations of the expression of HPA elements between donors (SM/CM patients and controls), the average CRH, UCN2, UCN3, POMC, MC1R and MC4R mRNA level was found to be significantly lower in the MS group. Our study showed an upregulation of CRHR1 splicing in CM/SM skin bioptates, with a high prevalence of shorter transcripts (c, e, h) which could result in potential attenuation of the CRH signalling in urticaria pigmentosa, due to deregulation of HPA signalling [44]. Interestingly, the element responsible for HPA self-attenuation – glucocorticoid receptor coding gene (NR3C1) – was shown to be the sole non-down-regulated HPA element in the MS group. However, these results were not confirmed by immunohistochemical detection of the selected elements of the HPA axis in the epidermis of urticaria pigmentosa lesions. On the other hand, high variations in the expression of HPA elements was observed within both groups. This could be caused by various factors, including recent skin exposure to ultraviolet radiation [8, 9, 14] or infection [14, 45], but also, as was recently shown, calcium level and/or vitamin D levels may affect the expression of sHPA elements [32, 33]. In addition, a modulation of the HPA central element – CRH – was reported in other stress-related inflammatory skin diseases, such as psoriasis [46-49]. In comparison to healthy skin, Tagen et al. [49] and independently Zhou et al. [50] found a decreased CRHR1 mRNA level and decreased CRH/CRHR1 IRS in psoriatic lesions, respectively. In contrast, Kim et al. and separately Cemil et al. found increased IRS for CRH and CRHR1, respectively [47, 48]. Interestingly, Kim et al. demonstrated, similarly to our study, clear overexpression of ACTH and MSH in only a subset of patients.
In spite of the decrease and highly variable expression of sHPA elements in the epidermis, mast cells in vitro as well as NMCs infiltrating the dermis seem to be a significant source of neuropeptides including UCN1 and ACTH, and to a lesser extent, CRH. Interestingly, our study showed that in comparison to other sHPA elements in MS, a low expression of CRH in NMCs correlated with a low expression of its receptor CRHR1. Mast cells are known to produce and respond to CRH by degranulation [28], however it was also shown that CRH inhibits the proliferation of keratinocytes [9]. Thus, the observed increase in the expression of CRH and its receptor on the mast cells of urticaria pigmentosa may affect the proliferation of cells and stress response, in both dermis and epidermis, despite the observed decrease in expression in keratinocytes. In fact, similar findings to ours were reported in psoriasis, where the CRH serum level was found to be increased while skin expression of CRH and CRHR1 was decreased [50, 51]. Since it is well established that MS results from the proliferation of transformed, constitutively activated MCs [52, 53], a significant contribution of hyperactive MCs to the pool of neuropeptides in the skin could be expected [28, 54, 55]. In addition, the elevated level of ACTH in the serum of CM/SM patients and its overproduction by NMCs could result in the development of urticaria pigmentosa. However, in our study, the mRNA levels for all chosen HPA genes, including POMC, were found to be downregulated. This potentially contradictory observation could be explained by increased POMC translation, processing and/or protein stability in comparison in MS as it was suggested previously for obesity induced hypothalamic stress [56]. Fickel et al. found intriguing upregulation of ACTH secretion in response to CRH treatment despite relative downregulation of POMC expression in serum-depleted growth conditions [57]. Moreover, a recent study of Yamamoto on melanogenesis presents a novel mechanism of POMC processing in response to UV-irradiation (melanin production) which interestingly involves ACTH processed by mast cell tryptase [58]. When combined, these results suggest the involvement of dysregulated HPA axis equivalent in urticaria pigmentosa development.
Conclusions
In the present study, we have presented data suggesting increased mast cell activity, increased involvement of POMC-derived neuropeptides (ACTH and presumably MSH) with potential downregulation of CRH-driven signalling in MS skin lesions, for the first time. An interplay between the systemic HPA axis and its cutaneous equivalent is indicated to play a central role in pathogenesis of cutaneous MS. It could be postulated that an increased serum level of ACTH and increased production of neuropeptides by dermal mast cells, at least partially, attenuates the expression of epidermal elements of the sHPA axis in the epidermis, but may also induce pigmentation as well as immunomodulation. Thus, further investigation of the role of the skin equivalent of the HPA axis in the development of MS skin lesion is highly recommended.
Acknowledgments
The study was supported by a grant of the Polish Ministry of Science and Higher Education, Project No. N N402 424939 to BN, MN and ML, 02-0066/07/253 to BN, RJN and ML, and Polpharma Scientific Foundation to JA.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Golkar L, Bernhard JD. Mastocytosis. Lancet. 1997;349:137985. doi: 10.1016/S0140-6736(96)07056-0. [DOI] [PubMed] [Google Scholar]
- 2.Lennert K, Parwaresch MR. Mast-cells and mast-cell neoplasia – review. Histopathology. 1979;3:349–65. doi: 10.1111/j.1365-2559.1979.tb03017.x. [DOI] [PubMed] [Google Scholar]
- 3.Parwaresch MR, Horny HP, Lennert K. Tissue mast-cells in health and disease. Pathol Res Pract. 1985;179:439–61. doi: 10.1016/s0344-0338(85)80184-9. [DOI] [PubMed] [Google Scholar]
- 4.Valent P, Arock M, Bischoff SC, et al. The European competence network on mastocytosis (ECNM) Wien Klin Wochenschr. 2004;116:647–51. doi: 10.1007/s00508-004-0253-3. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann K, Metcalfe DD. Pediatric mastocytosis. Hematology-Oncology Clin N Am. 2000;14:625–40. doi: 10.1016/s0889-8588(05)70299-9. [DOI] [PubMed] [Google Scholar]
- 6.Deren-Wagemann I, Kuliszkiewicz-Janus M, Kuliczkowski K. Mastocytosis – diagnostic criteria and treatment. Postep Hig Med Dosw. 2009;63:564–76. [PubMed] [Google Scholar]
- 7.Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children practical recommendations. Am J Clin Dermatol. 2011;12:259–70. doi: 10.2165/11588890-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski AT, Zmijewski MA, Plonka PM, et al. How UV light touches the brain and endocrine system through skin, and why. Endocrinology. 2018;159:1992–2007. doi: 10.1210/en.2017-03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slominski AT, Zmijewski MA, Zbytek B, et al. Key role of CRF in the skin stress response system. Endocr Rev. 2013;34:827–84. doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobin DJ. Biochemistry of human skIn: our brain on the outside. Chem Soc Rev. 2006;35:52–67. doi: 10.1039/b505793k. [DOI] [PubMed] [Google Scholar]
- 11.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 12.Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature. 1983;305:325–7. doi: 10.1038/305325a0. [DOI] [PubMed] [Google Scholar]
- 13.Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17:245–61. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- 14.Slominski AT, Zmijewski MA, Skobowiat C, et al. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slominski A, Zbytek B, Szczesniewski A, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metabol. 2005;288:E701–6. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 16.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol Cell Endocrinol. 2007;265-266:143–9. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zapletal E, Kraus 0, Cupic B, Gabrilovac J. Differential expression of proopiomelanocortin (POMC) transcriptional variants in human skin cells. Neuropeptides. 2013;47:99–107. doi: 10.1016/j.npep.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Zbytek B, Slominski AT. Corticotropin-releasing hormone induces keratinocyte differentiation in the adult human epidermis. J Cell Physiol. 2005;203:118–26. doi: 10.1002/jcp.20209. [DOI] [PubMed] [Google Scholar]
- 19.Zbytek B, Pikula M, Slominski RM, et al. Corticotropin-releasing hormone triggers differentiation in HaCaT keratinocytes. Br J Dermatol. 2005;152:474–80. doi: 10.1111/J.1365-2133.2005.06217.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitaryadrenal (HPA) axis and synthesize cortisol. FASEB J. 2005;19:1332–4. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 21.Zmijewski MA, Slominski AT. CRF1 receptor splicing in epidermal keratinocytes: potential biological role and environmental regulations. J Cell Physiol. 2009;218:593–602. doi: 10.1002/jcp.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zmijewski MA, Slominski AT. Modulation of corticotropin releasing factor (CRF) signaling through receptor splicing in mouse pituitary cell line ATT-20-emerging role of soluble isoforms. J Physiol Pharmacol. 2009;60:39–46. [PMC free article] [PubMed] [Google Scholar]
- 23.Paus R. A neuroendocrinological perspective on human hair follicle pigmentation. Pigment Cell Melanoma Res. 2011;24:89–106. doi: 10.1111/j.1755-148X.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- 24.Jozic I, Stojadinovic O, Kirsner RS, Tomic-Canic M. Skin under the (spot)-light: cross-talk with the central hypothalamicpituitary-adrenal (HPA) axis. J Invest Dermatol. 2015;135:1469–71. doi: 10.1038/jid.2015.56. [DOI] [PubMed] [Google Scholar]
- 25.Slominski RM, Zmijewski MA, Slominski AT. The role of melanin pigment in melanoma. Exp Dermatol. 2015;24:258–9. doi: 10.1111/exd.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Funasaka Y, Sato H, Chakraborty AK, et al. Expression of proopiomelanocortin, corticotropin-releasing hormone (CRH), and CRH receptor in melanoma cells, nevus cells, and normal human melanocytes. J Investig Dermatol Symp Proc. 1999;4:105–9. doi: 10.1038/sj.jidsp.5640192. [DOI] [PubMed] [Google Scholar]
- 27.Artuc M, Bohm M, Grutzkau A, et al. Human mast cells in the neurohormonal network: expression of POMC, detection of precursor proteases, and evidence for IgE-dependent secretion of alpha-MSH. J Invest Dermatol. 2006;126:1976–81. doi: 10.1038/sj.jid.5700318. [DOI] [PubMed] [Google Scholar]
- 28.Theoharides TC. Neuroendocrinology of mast cells: challenges and controversies. Exp Dermatol. 2017;26:751–9. doi: 10.1111/exd.13288. [DOI] [PubMed] [Google Scholar]
- 29.Cao J, Papadopoulou N, Kempuraj D, et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–75. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 30.Lytinas M, Kempuraj D, Huang M, et al. Acute stress results in skin corticotropin-releasing hormone secretion, mast cell activation and vascular permeability, an effect mimicked by intradermal corticotropin-releasing hormone and inhibited by histamine-1 receptor antagonists. Int Arch Allergy Immunol. 2003;130:224–31. doi: 10.1159/000069516. [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulou N, Kalogeromitros D, Staurianeas NG, et al. Corticotropin-releasing hormone receptor-1 and histidine decarboxylase expression in chronic urticaria. J Investig Dermatol. 2005;125:952–5. doi: 10.1111/j.0022-202X.2005.23913.x. [DOI] [PubMed] [Google Scholar]
- 32.Wierzbicka JM, Zmijewski MA, Antoniewicz J, et al. Differentiation of keratinocytes modulates skin HPA analog. J Cell Physiol. 2017;232:154–66. doi: 10.1002/jcp.25400. [DOI] [PubMed] [Google Scholar]
- 33.Wierzbicka JM, Zmijewski MA, Piotrowska A, et al. Bioactive forms of vitamin D selectively stimulate the skin analog of the hypothalamus-pituitary-adrenal axis in human epidermal keratinocytes. Mol Cell Endocrinol. 2016;437:312–22. doi: 10.1016/j.mce.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange M, Nedoszytko B, Gorska A, et al. Mastocytosis in children and adults: clinical disease heterogeneity. Arch Med Sci. 2012;8:533–41. doi: 10.5114/aoms.2012.29409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–55. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 36.Jablonska K, Pula B, Zemla A, et al. Expression of melatonin receptor MT1 in cells of human invasive ductal breast carcinoma. J Pineal Res. 2013;54:334–45. doi: 10.1111/jpi.12032. [DOI] [PubMed] [Google Scholar]
- 37.Sperr WR, Escribano L, Jordan JH, et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leukemia Res. 2001;25:529–36. doi: 10.1016/s0145-2126(01)00041-8. [DOI] [PubMed] [Google Scholar]
- 38.Sperr WR, Kundi M, Alvarez-Twose I, et al. International prognostic scoring system for mastocytosis (IPSM): a retrospective cohort study. Lancet Haematol. 2019;6:e638–49. doi: 10.1016/S2352-3026(19)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emergingtreatment concepts. Blood. 2017;129:1420–7. doi: 10.1182/blood-2016-09-731893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 41.Abdel-Malek ZA, Knittel J, Kadekaro AL, et al. The melanocortin 1 receptor and the UV response of human melanocytes: a shift in paradigm. Photochem Photobiol. 2008;84:501–8. doi: 10.1111/j.1751-1097.2008.00294.x. [DOI] [PubMed] [Google Scholar]
- 42.Arck PC, Slominski A, Theoharides TC, et al. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697–704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theoharides TC, Donelan JM, Papadopoulou N, et al. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–8. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Zmijewski MA, Slominski AT. Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim Pol. 2010;57:1–13. [PMC free article] [PubMed] [Google Scholar]
- 45.Slominski AT, Zmijewski MA. Glucocorticoids inhibit wound healing: novel mechanism of action. J Invest Dermatol. 2017;137:1012–4. doi: 10.1016/j.jid.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou C, Yu X, Cai D, et al. Role of corticotropin-releasing hormone and receptor in the pathogenesis of psoriasis. Med Hypotheses. 2009;73:513–5. doi: 10.1016/j.mehy.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 47.Kim JE, Cho DH, Kim HS, et al. Expression of the corticotropin-releasing hormone-proopiomelanocortin axis in the various clinical types of psoriasis. Exp Dermatol. 2007;16:104–9. doi: 10.1111/j.1600-0625.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 48.Cemil BC, Canpolat F, Yilmazer D, et al. The association of PASI scores with CRH-R1 expression in patients with psoriasis. Arch Dermatol Res. 2012;304:127–32. doi: 10.1007/s00403-012-1218-4. [DOI] [PubMed] [Google Scholar]
- 49.Tagen M, Stiles L, Kalogeromitros D, et al. Skin corticotropin-releasing hormone receptor expression in psoriasis. J Investig Dermatol. 2007;127:1789–91. doi: 10.1038/sj.jid.5700757. [DOI] [PubMed] [Google Scholar]
- 50.Zhou C, Yu X, Cai D, et al. Role of corticotropin-releasing hormone and receptor in the pathogenesis of psoriasis. Med Hypotheses. 2009;73:513–5. doi: 10.1016/j.mehy.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 51.Vasiadi M, Therianou A, Sideri K, et al. Increased serum CRH levels with decreased skin CRHR-1 gene expression in psoriasis and atopic dermatitis. J Allergy Clin Immunol. 2012;129:1410–3. doi: 10.1016/j.jaci.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berezowska S, Flaig MJ, Rueff F, et al. Adult-onset mastocytosis in the skin is highly suggestive of systemic mastocytosis. Mod Pathol. 2014;27:19–29. doi: 10.1038/modpathol.2013.117. [DOI] [PubMed] [Google Scholar]
- 53.Lange M, tugowska-Umer H, Niedoszytko M, et al. Diagnosis of mastocytosis in children and adults in daily clinical practice. Acta Derm Venereol. 2016;96:292–7. doi: 10.2340/00015555-2210. [DOI] [PubMed] [Google Scholar]
- 54.Kempuraj D, Papadopoulou NG, Lytinas M, et al. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–8. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 55.Maintz L, Wardelmann E, Walgenbach K, et al. Neuropeptide blood levels correlate with mast cell load in patients with mastocytosis. Allergy. 2011;66:862–9. doi: 10.1111/j.1398-9995.2011.02550.x. [DOI] [PubMed] [Google Scholar]
- 56.Cakir I, Cyr NE, Perello M, et al. Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing. J Biol Chem. 2013;288:17675–88. doi: 10.1074/jbc.M113.475343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fickel J, Savoly S, Vogel U, et al. The proopiomelanocortin (POMC) gene-expression of ATT20 mouse pituitary-cells is dependent on cell-culture conditions. Cell Mol Biol. 1994;40:201–9. [PubMed] [Google Scholar]
- 58.Yamamoto H, Yamane T, Iguchi K, et al. Melanin production through novel processing of proopiomelanocortin in the extracellular compartment of the auricular skin of C57BL/6 mice after UV-irradiation. Sci Rep. 2015;5:14579. doi: 10.1038/srep14579. [DOI] [PMC free article] [PubMed] [Google Scholar]