Abstract
Leukaemia cutis (LC) describes infiltration of the skin by leukaemia cells, resulting in clinically identifiable cutaneous lesions. LC has a wide range of clinical manifestations, which can make it difficult to distinguish LC from other skin changes. In a group of patients, LC can be the first manifestation of leukaemia, therefore skin biopsy is crucial for the diagnosis. In this mini review, we discuss various types of leukaemia most frequently represented in leukaemia cutis, in both children and adults and skin changes in multiple myeloma, focusing on the clinical presentation of LC and prognosis in patients.
Keywords: leukaemia cutis, clinical presentation, chronic lymphocytic leukaemia, acute myeloid leukaemia, multiple myeloma
Definition
Leukaemia cutis (LC) describes the infiltration of the epidermis, dermis, or subcutis by neoplastic leukocytes, myeloid or lymphoid, resulting in clinically identifiable cutaneous lesions [1, 2].
Leukaemia cutis has been described in patients with acute myeloid leukaemia, chronic myeloproliferative disease, including chronic myelogenous leukaemia (CML), myelodysplastic syndromes, and myelodysplastic/lym- phoproliferative diseases including chronic myelomono- cytic leukaemia (CMML). LC may also occur in lymphocytic leukaemia such as acute lymphoblastic leukaemia B or T (B-ALL, T-ALL), precursor B- or T-cell lymphoblastic leukaemia/lymphoma (pre-B ALL, pre-T ALL) and chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL) [3, 4].
Clinical presentation of LC
Cutaneous manifestations of leukaemia can be classified as specific (infiltrates of leukemic cells) or nonspecific (inflammatory, related to marrow failure). The specific lesions called LC are considered as malignant lesions associated with localized or disseminated infiltrations of the skin by leukemic cells [4]. The time between the diagnosis of leukaemia and the development of leukaemia cutis varies. In the study of Kang et al., the vast majority (95%) of LC lesions developed after the diagnosis of leukaemia or showed concurrent involvement [5].
The clinical findings of LC often involve asymptomatic nodular lesions, tumours, and plaques. The nodules and papules are usually dome-shaped, firm in consistency and erythematous [6, 7]. In a retrospective study of 75 cases of LC, nodules (33%), papules (30%), and plaques (17%) were the three most common types of LC lesions [5]. The skin lesions were usually multiple (84%). These findings are consistent with the data presented by Li et al, who demonstrated that multiple nodules and papules were most commonly seen clinical features in patients with myeloid LC [8]. Leukaemia cutis may rarely present as other clinical manifestations like erythema, macules, blisters, and ulcers [9].
These rare clinical manifestations may occur simultaneously. There is no apparent site of predilection for LC. Skin lesions usually appear in the area of the trunk, extremities and head [10]. On the face, the transition from erythema to nodular and plaque stage may resemble leonine facies [10]. LC rarely affects palmoplantar surface and oral mucosa. Individual lesions’ morphology and distribution are not characteristic of specific forms of leukaemia; however, the different growth dynamics of cutaneous lesions may indicate acute or chronic forms of the disease. Rapid and disseminated growth is usually seen in the course of acute leukaemia while gradual, stepwise dynamics is more characteristic in chronic forms [9]. The cutaneous lesions produced by different leukaemia subtypes have remarkable uniformity; however, a patient may develop different morphologies over the course of the disease [11, 12]. Localized in the sites of the scars, herpetic lesions, trauma, and recent surgical procedures [13].
Nonspecific cutaneous lesions in the course of leukaemia occur in 30-40% of patients suffering from the disease [14]. The most common nonspecific signs include haemorrhagic skin lesions such as petechiae or purpura. Patients with leukaemia cutis are at higher risk of developing infectious diseases due to bone marrow failure. The list of conditions associated with infections include varicella-zoster virus, herpes simplex virus, or cutaneous mycoses. There is also an increased incidence of reactive and paraneoplastic lesions: generalized pruritus, Sweet’s syndrome, or pyoderma gangrenosum [15]. In the course of other malignant conditions, such as lymphoma, me- tastases of visceral tumours, skin cancers or Kaposi sarcoma, skin lesions in the form of papules and nodules on the trunk and extremities may also appear [14, 16]. Those entities should be taken into consideration in differential diagnoses. Sudden, exanthematous spread of skin lesions may occur in the course of papular drug eruptions, leukocytoclastic vasculitis or infectious diseases [9, 17].
The frequency of leukaemia cutis seems to be higher among children than adults, 25% to 30% of infants with congenital leukaemia develop skin involvement [7, 9, 18]. Acute lymphoblastic leukaemia/lymphoblastic lymphoma (ALL/LBL) accounts for approximately one-quarter of all childhood malignancies and is the most common form of cancer in children; ALL/LBL is five times more common in children than acute myeloid leukaemia (AML) [19]. Similar to the adult population, LC in children may manifest as papules, macules, patches, plaques and purpura. Leukaemia cutis has been described in children with both ALL/LBL and AML.
Andriescu et al. investigated clinical presentations and outcomes in thirty-one paediatric patients with leukaemia cutis [20]. The most common type of leukaemia associated with LC was AML (74%), followed by ALL (16%). The authors of the study did not note any significant differences in the clinical manifestation between two subtypes.
The head and lower extremities were the two main sites of predilection. The skin lesions usually presented as erythematosus and/or violaceous nodules and papules. In more than half of the patients, more than one morphology of skin lesions was present. In the majority of cases, LC presented concomitantly with systemic leukaemia.
In another retrospective observational study, the incidence rate of LC in children diagnosed with AML was 5.5% (24/438). The authors demonstrated that children presenting LC associated with AML were usually younger and had worse overall survival rates than the ones without skin involvement. In the light of these findings leukaemia cutis could be a negative prognostic factor in childhood AML [21].
Leukaemia cutis is reported to be the initial presenting sign in 50% of neonates with leukaemia [22]. The most common clinical manifestation present at birth are multiple randomly distributed bluish subcutaneous nodules with a predilection to the trunk and face, described in the literature as blueberry muffin rash. Other causes of blueberry muffin rash include congenital infections, congenital vascular lesions and other malignancies [22].
Myelogenous leukaemia
LC occurs in about 4% of patients with AML and less frequently in CML [2]. Certain subtypes of AML are more commonly associated with skin infiltrations. The most frequent association occurs with acute myelomonocytic and monocytic differentiation [3], (former M4 and M5 according to FAB classification) [23], with skin involvement in up to 50% of patients [1, 19–22]. Those who were treated previously with chemotherapy are prone to develop secondary leukaemia cutis [24, 25].
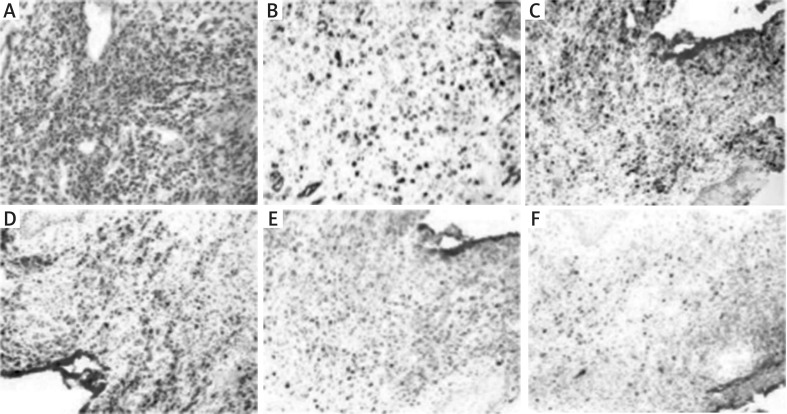
Leukemic skin infiltrates usually show diffuse involvement of the dermis and subcutis, sparing the upper papillary dermis. Perivascular and periadnexal infiltrations are common. The nature of neoplastic cells depends on the type of leukaemia. Neoplasms can be composed of myeloblasts with or without features of promyelocytic or neutrophilic maturation, cells with monocytic or monoblastic morphology. Erythroid precursors or megakaryoblasts are rare. Immunohistochemistry is essential for the diagnosis. In Figure 1, we present a case from the author’s practice: skin with acute myelomonocytic leukaemia infiltrations (Figures 1 A–F). Figure 2 shows the clinical presentation of LC in monocytic leukaemia (Figure 2 A).
Figure 1.
Skin with acute myelomonocytic leukaemia infiltration. A – H&E (200x magnification). B – Positive CD34 stain in blasts (200x magnification). C – Positive CD117 stain in blasts (100x magnification). D – Positive CD56 stain in neoplastic cells of monocytic differentiation (100x magnification). E – Positive CD68 stain in neoplastic cells of monocytic differentiation (100x magnification). F – Positive MPO stain in neoplastic cells ofgranulocytic differentiation (100x magnification)
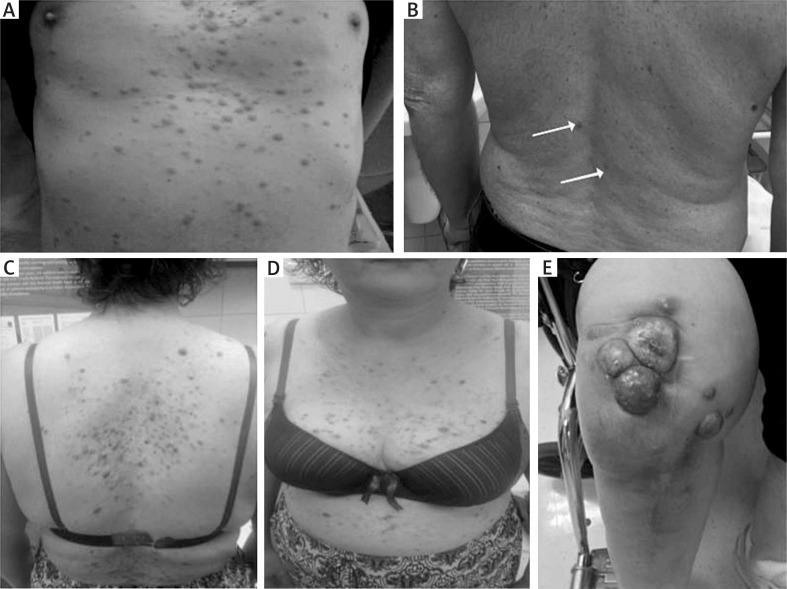
Figure 2.
Clinical presentation ofleukaemia cutis. A – Diffusederythematosus infiltrated papules often coalescing into small plaques on the trunk; LC inmonocyticleukaemia (M5 according to FAB classification) in a 30-year-old male patient. B – Single infiltratederythematosus papules and nodules in the lumbar area, confirmed as skin infiltrations of chronic lymphocyticleukaemia (CLL/SLL) in a 65-year-old male patient (arrows) C-E – Skin involvement in multiple myeloma (MM). Diffuseerythematosus papules and nodules on the trunk (C, D). E – Groupederythematosus firmtumours on the right thigh, certain with central necrosis, appearing in proximity of a scar afterorthopaedic surgery of the knee in the same MM patient
Besides LC in acute myeloid leukaemia, we can distinguish another form of extramedullary leukaemia (EML), namely myeloid sarcoma, also known as granulocytic sarcoma or chloroma. It is a rare EML tumour of immature myeloid cells, reported in 2.5–9.1% of patients with AML [11]. It was historically named “chloroma” because of its green colour caused by the presence of myeloperoxidase (MPO) [6]. The site of EML may be the central nervous system, skin, ovary, orbits, gums, lymph nodes, soft tissues and other organs [7]. The prognosis in the case of the presence of EML has been suggested to be a marker of an aggressive disease, difficult to control and patients prone to extramedullary relapses after an intensive chemotherapy regimen [21, 22].
Extramedullary disease has been also reported in acute promyelocytic leukaemia (APL), a distinct subtype of acute myeloblastic leukaemia with specific clinical, morphologic and genetic features. Acute promyelocytic leukaemia is characterized by a 15;17 chromosome translocation, associated with the PML/RAR-a gene rearrangement [24, 25].
EML is affecting around 3% of APL patients, mostly at the time of relapse, and has poor prognosis [26, 27]. The largest number of reports regarding EML in APL was associated with previous treatment with all-trans retinoic acid (ATRA) [28, 29]. The most common site of EML was the central nervous system and skin [27, 29–32].
A special form of LC is aleukemic leukaemia cutis. This term describes cases when skin infiltrations by leukaemia cells occurs before bone marrow or peripheral blood involvement and in the absence of systemic symptoms. It is uncommon and occurs predominantly in patients with AML [33–36].
Lymphocytic leukaemia
Chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL) is the most common leukaemia in Western countries with an incidence of 4.2 : 100,000 per year [37]. LC in CLL is relatively rare and occurs in less than 5% of affected patients [4]. It is significantly less common than skin cancers complicating the course of CLL with an incidence up to 20% [38], and non-specific skin lesions.
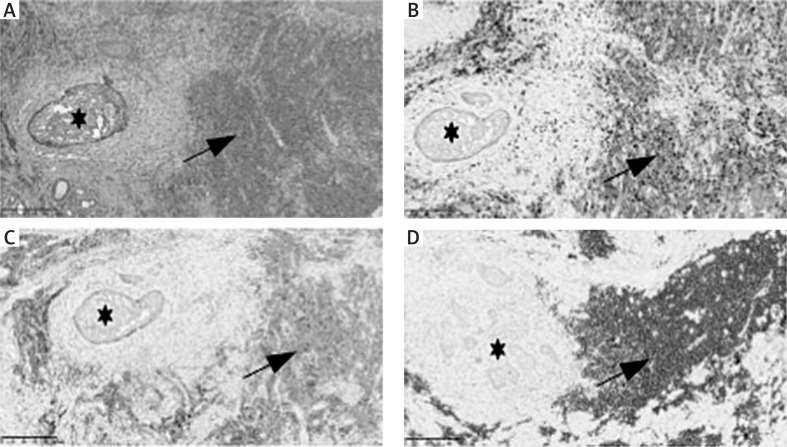
There are three main histopathological types of skin involvement of LC in CLL, (1) perivascular and periadnexal pattern with lymphocytic infiltration around the vessels and skin appendages, (2) nodular and diffuse pattern, and (3) band-like pattern [39]. In tissue sections, small B lymphocytes with scant cytoplasm are seen, usually with a round nucleus with clumped chromatin, occasionally a small nucleus is present. Monoclonal B lymphocytes most often coexpress CD20, CD5, CD23, CD43 and LEF1 molecules (Figure 3).
Figure 3.
Skin with basal cell carcinoma (BCC) (asterisk) and chronic lymphocyticleukaemia (CLL/SLL) (arrow) coexistence.A- H&E (100x magnification).B- Positive CD5 stain in CLL/SLL cells (100x magnification).C- Positive CD23 stain in CLL/ SLL cells (100x magnification).D- Positive PAX-5/BSAP stain in CLL/SLL cells (100x magnification)
In patients with CLL, the incidence of non-melanoma skin cancers (NMSC) is significantly higher compared to controls [38]. The most frequent NMSC was squamous cell carcinoma (63%), followed by basal cell carcinoma (55%), and Merkel cell carcinoma (1%). The authors suggest that screening for NMSC is important in CLL patients particularly among patients with aggressive CLL receiving T-cell immunosuppressive treatments as well as those who have a prior history of NMSC, or history of severe sunburn [38]. Therefore, differential diagnosis of skin cancers in patients with CLL should always be taken into consideration. In the study of Thiesen et al., it was shown that nearly half (33/70) of LC lesions were located in close proximity or overlapped with other skin lesions observed in non-melanoma skin tumours, precancerousstates (actinic keratosis, Bowen’s disease) and reactive inflammatory dermatoses, such as arthropod bites. It is hypothesized that damage to the epidermal barrier may be a factor provoking the occurrence of LC [40]. In the author’s experience, LC may coexist with skin cancers, such as basal cell carcinoma as it is presented in our case (Figures 3 A–D).
The prognosis of LC in the course of CLL remains controversial. According to Raufi et al., LC did not worsen the prognosis of patients with CLL [41]. Other authors suggested better prognosis in the case of LC in Richter’s syndrome and in CLL patients [42, 43].
In the case of LC, various therapeutic options seem to be beneficial in symptomatic treatment. Authors of individual clinical case reports described therapeutic successes after using locoregional treatment [44], but systemic treatment of the underlying disease is crucial [44]. Figure 2 shows the clinical presentation of LC in CLL (Figure 2B).
In patients with acute lymphoblastic leukaemia (ALL), LC is rarely reported and affects 1–3% of ALL patients [45, 46].
In patients with precursor-B-cell ALL (Pre-B-ALL) only few cases of LC were described worldwide [46], presenting as asymptomatic firm erythematous nodules, but a case of pre-B-ALL associated LC presenting as soft, lipoma-like mounds was also reported [46].
Microscopically neoplastic cells diffusely infiltrate the dermis, surrounding a blood vessel but sparing the epidermis. The morphological features of B-ALL/LBL and T-ALL/LBL are indistinguishable [47], Specific immunohis- tochemistry must be applied. The B- and T-lymphoblasts are almost always positive for terminal deoxynucleotidyl transferase (TdT).
In a study performed by Kata et al, and analysing patients with relapse after allogeneic hematopoietic stem cell transplantation (alloHSCT), 62 of 324 patients relapsed at any side. 11.3% of patients had extramedullary relapse, including leukaemia cutis (2 ALL and 2 pre-B-ALL, 3 AML patients). Despite the treatment, all the patients died after a median time of 10 months due to resistant systemic relapse. According to the author’s experience and the literature, EM relapse following alloHSCT is associated with poor prognosis and the optimal therapy remains a challenge [48].
Multiple myeloma
Cutaneous involvement of multiple myeloma (MM) is uncommon, typically occurs in the late stage of the disease, and is a poor prognostic indicator. In the study performed by Jurczyszyn et al, [49], there is an overrepresentation of MM with immunoglobulin class A (IgA) and light chain in skin involvement. Patients with skin MM and skin involvement presented in all MM International Staging System (ISS) stages, from I to III, and there was no preferential cytogenetic abnormality. Those patients carry a very poor prognosis with a median overall survival (OS) of 8.5 months [49, 50]. Clinical presentation of cutaneous involvement of MM can be erythematosus papules and nodules or erythematosus firm tumours (Figure 2 C–E).
Molecular pathogenesis
The molecular background responsible for the invasion of leukemic cells into the skin is not fully understood. Homing to specific tissues is controlled by expression of different chemokine receptors and adhesion molecules. Blast neural cell adhesion molecule (CD56) has long been implicated in EM pathogenesis [35, 50, 51]. In a study performed by Kuwabara, AML expressing CD56 molecule showed a significantly frequent cutaneous involvement compared to CD56-negative cases [52].
Concerning cytogenetic abnormalities in AML, trisomy and tetrasomy of chromosome 8 are more common in patients with AML with leukaemia cutis than in patients with AML without leukaemia cutis [53, 54]. The 8;21 chromosome translocation is common in patients with myeloid sarcoma [55].
Conclusions
The diagnosis of leukaemia cutis is based on the morphologic pattern of skin infiltration, cytologic features, and the immunophenotype of the tumour cells. Patients presenting with leukaemia, or a history of leukaemia, especially AML patients with skin infiltrations, should undergo a skin biopsy.
Histopathological examination of the tissue sample should include: haematoxylin and eosin staining, immu- nohistochemistry and, when feasible, flow cytometry,fluorescence in situ hybridization and molecular analysis [4]. If a diagnosis of leukaemia is not already established, a bone marrow biopsy should be performed [6]. The correlation of clinical data, bone marrow and peripheral blood findings is often helpful to confirm the diagnosis.
Clinically, the presence of LC varies and is not specific for leukaemia subtype and has been suggested to be a marker of an aggressive disease, difficult to control and patients are prone to relapse after an intensive chemotherapy regimen.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Agis H, Weltermann A, Fonatsch C, et al. A comparative study on demographic, hematological and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol. 2002;81:90–5. doi: 10.1007/s00277-001-0412-9. [DOI] [PubMed] [Google Scholar]
- 2.Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130–42. doi: 10.1309/WYACYWF6NGM3WBRT. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weedon D. Weedon’s Skin Pathology. 3rd ed. London: Churchill Livingstone Elsevier, 2010. Dermatol Pract Concept. 2012;2:1118–1120. [Google Scholar]
- 5.Kang YS, Kim HS, Park HJ, et al. Clinical characteristics of 75 patients with leukemia cutis. J Korean Med Sci. 2013;28:614–9. doi: 10.3346/jkms.2013.28.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakst RL, Tallman MS, Douer D, et al. How I treat extramed- ullary acute myeloid leukemia. Blood. 2011;118:3785–93. doi: 10.1182/blood-2011-04-347229. [DOI] [PubMed] [Google Scholar]
- 7.Almond LM, Charalampakis M, Ford SJ, et al. Myeloid sarcoma: presentation, diagnosis, and treatment. Clin Lymphoma Myeloma Leuk. 2017;17:263–7. doi: 10.1016/j.clml.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Lian CG, Jin H, et al. Clinical and pathological features of myeloid leukemia cutis. An Bras Dermatol. 2018;93:216–21. doi: 10.1590/abd1806-4841.20186327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner G, Fenchel K, Back W, et al. Leukemia cutis - epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges. 2012;10:27–37. doi: 10.1111/j.1610-0387.2011.07842.x. [DOI] [PubMed] [Google Scholar]
- 10.Su WP, Buechner SA, Li CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11:121–8. doi: 10.1016/s0190-9622(84)70145-9. [DOI] [PubMed] [Google Scholar]
- 11.Watson KMT, Mufti G, Salisbury JR, et al. Spectrum of clinical presentation, treatment and prognosis in a series of eight patients with leukaemia cutis. Clin Exp Dermatol. 2006;31:218–21. doi: 10.1111/j.1365-2230.2005.02022.x. [DOI] [PubMed] [Google Scholar]
- 12.Ratnam KV, Khor CJL, Su WP. Leukemia cutis. Dermatol Clin. 1994;12:419–31. [PubMed] [Google Scholar]
- 13.Lee JI, Park HJ, Oh ST, et al. A case of leukemia cutis at the site of a prior catheter insertion. Ann Dermatol. 2009;21:193–6. doi: 10.5021/ad.2009.21.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunwald MR, McDonnell MH, Induru R, et al. Cutaneous manifestations in leukemia patients. Semin Oncol. 2016;43:359–65. doi: 10.1053/j.seminoncol.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Biswal S, Godnaik C. Incidence and management of infections in patients with acute leukemia following chemotherapy in general wards. Ecancermedicalscience. 2013;7:310. doi: 10.3332/ecancer.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsi M, Go MS, Ahmed A. Cancer, Leukemia Cutis. Stat- Pearls Publishing; 2019. [PubMed] [Google Scholar]
- 17.Martinez-Escaname M, Zuriel D, Tee SI, et al. Cutaneous infiltrates of acute myelogenous leukemia simulating inflammatory dermatoses. Am J Dermatopathol. 2013;35:419–24. doi: 10.1097/DAD.0b013e31826ffe6f. [DOI] [PubMed] [Google Scholar]
- 18.Resnik KS, Brod BB. Leukemia cutis in congenital leukemia: analysis and review of the world literature with report of an additional case. Arch Dermatol. 1993;129:1301–6. doi: 10.1001/archderm.129.10.1301. [DOI] [PubMed] [Google Scholar]
- 19.Bhojwani D, Yang JJ, Pui CH. Biology of childhood acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62:47–60. doi: 10.1016/j.pcl.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andriescu EC, Coughlin CC, Cheng CE, et al. Pediatric leukemia cutis: a case series. Pediatr Dermatol. 2019;36:658–63. doi: 10.1111/pde.13864. [DOI] [PubMed] [Google Scholar]
- 21.Gouache E, Greze V, Strullu M, et al. Leukemia cutis in childhood acute myeloid leukemia. HemaSphere. 2018;2:e141. doi: 10.1097/HS9.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondi V, Piersigilli F, Salvatori G, et al. Darlenski R, editor. The skin as an early expression of malignancies in the neonatal age: a review of the literature and a case series. Biomed Res Int. 2015;2015:809406. doi: 10.1155/2015/809406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias French-American-Brit- ish (FAB) co operative group. Br J Haematol. 1976;33:451–8. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966–78. doi: 10.1016/s0190-9622(99)70086-1. [DOI] [PubMed] [Google Scholar]
- 25.Weinel S, Malone J, Jain D, et al. Therapy-related leukaemia cutis: a review. Australas J Dermatol. 2008;49:187–90. doi: 10.1111/j.1440-0960.2008.00466.x. [DOI] [PubMed] [Google Scholar]
- 26.Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223–30. [PubMed] [Google Scholar]
- 27.Michel G, Boulad F, Small TN, et al. Risk of extramedullary relapse following allogeneic bone marrow transplantation for acute myelogenous leukemia with leukemia cutis. Bone Marrow Transplant. 1997;20:107–12. doi: 10.1038/sj.bmt.1700857. [DOI] [PubMed] [Google Scholar]
- 28.Grignani F, Ferrucci PF, Testa U, et al. The acute promyelo- cytic leukemia-specific PML-RAR fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell. 1993;74:423–31. doi: 10.1016/0092-8674(93)80044-f. [DOI] [PubMed] [Google Scholar]
- 29.Li YP, Andersen J, Zelent A, et al. RAR alpha1/RAR alpha2- PML mRNA expression in acute promyelocytic leukemia cells: a molecular and laboratory-clinical correlative study. Blood. 1997;90:306–12. [PubMed] [Google Scholar]
- 30.Vega-Ruiz A, Faderl S, Estrov Z, et al. Incidence of extramedullary disease in patients with acute promyelocytic leukemia: a single-institution experience. Int J Hematol. 2009;89:489–96. doi: 10.1007/s12185-009-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montesinos P, Diaz-Mediavilla J, Deben G, et al. Central nervous system involvement at first relapse in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline monochemotherapy without intrathecal prophylaxis. Haematologica. 2009;94:1242–9. doi: 10.3324/haematol.2009.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang H, Brandwein J, Yi OL, et al. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004;28:1007–11. doi: 10.1016/j.leukres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Giralt S, O’brien S, Weeks E, et al. Leukemia cutis in acute promyelocytic leukemia: report of three cases after treatment with all-trans retinoic acid. Leuk Lymphoma. 1994;14:453–6. doi: 10.3109/10428199409049703. [DOI] [PubMed] [Google Scholar]
- 34.Araujo NS, Dos Santos Junior CJ, Gomes VM, da S, et al. A rare case of relapsed pediatric acute promyelocytic leukemia with skin involvement by myeloid sarcoma. Am J Case Rep. 2018;19:438–41. doi: 10.12659/AJCR.907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrd JC, Edenfield WJ, Shields DJ, et al. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol. 1995;13:1800–16. doi: 10.1200/JCO.1995.13.7.1800. [DOI] [PubMed] [Google Scholar]
- 36.Annessi G, Signoretti S, Simoni R. Minimally differentiated acute myeloid leukaemia revealed by specific cutaneous lesions. Br J Dermatol. 1996;135:119–23. [PubMed] [Google Scholar]
- 37.Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v78–84. doi: 10.1093/annonc/mdv303. [DOI] [PubMed] [Google Scholar]
- 38.Kleinstern G, Rishi A, Achenbach SJ, et al. Factors associated with non-melanoma skin cancer (NMSC) among chronic lymphocytic leukemia (CLL) patients. Blood. 2017;130(Suppl 1):2158. [Google Scholar]
- 39.Cerroni L, Zenahlik P, Hofler G, et al. Specific cutaneous infiltrates of B-cell chronic lymphocytic leukemia: a clinicopatho- logic and prognostic study of 42 patients. Am J Surg Pathol. 1996;20:1000–10. doi: 10.1097/00000478-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Thiesen I, Wehkamp U, Bruggemann M, et al. Skin involvement by chronic lymphocytic leukaemia is frequently associated with unrelated neoplastic or inflammatory cutaneous disease and is not indicative of general disease progression. Br J Dermatol. 2019;180:227–8. doi: 10.1111/bjd.17135. [DOI] [PubMed] [Google Scholar]
- 41.Raufi A, Alsharedi M, Khelfa Y, et al. Leukemia cutis in a patient with chronic lymphocytic leukemia presenting as bilateral helical nodules. SAGE Open Med Case Reports. 2016;4:2050313X1668362. doi: 10.1177/2050313X16683624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kluk J, Moonim M, Duran A, et al. Cutaneous Richter syndrome: a better place to transform? Br J Dermatol. 2015;172:513–21. doi: 10.1111/bjd.13193. [DOI] [PubMed] [Google Scholar]
- 43.Colburn DE, Welch MA, Giles FJ. Skin infiltration with chronic lymphocytic leukemia is consistent with a good prognosis. Hematology. 2002;7:187–8. doi: 10.1080/1024533021000008164. [DOI] [PubMed] [Google Scholar]
- 44.Osmola M, Gierej B, Waszczuk-Gajda A, et al. A rare manifestation of chronic lymphocytic leukaemia - leukaemia cutis treated with ibrutinib. Palliat Med. 2019;11:180–3. [Google Scholar]
- 45.Obiozor C, Ganguly S, Fraga GR. Leukemia cutis with lym- phoglandular bodies: a clue to acute lymphoblastic leukemia cutis. Dermatol Online J. 2015;21:13030/qt6m18g35f. [PubMed] [Google Scholar]
- 46.Huang YYM, Liu M, Ruth JS, et al. Precursor-B-cell-ALL leukemia cutis resembling lipomas: an atypical presentation of a rare entity and a review of the literature. Dermatol Online J. 2017;23:13030/qt6bs2d542. [PubMed] [Google Scholar]
- 47.Mansoori P, Taheri A, O’Neill SS, et al. T-lymphoblastic leukemia/lymphoma with annular skin rash and epidermotro- pism. Am J Dermatopathol. 2018;40:676–8. doi: 10.1097/DAD.0000000000001113. [DOI] [PubMed] [Google Scholar]
- 48.Kata D, Kyrcz-Krzemien S, Czerw T, et al. Incidence, treatment and outcome of isolated extramedullary relapses after allogeneic hematopoietic stem cell transplantation for acute lymphoblastic and myeloid leukemias: single-center experience with 324 patients. Blood. 2008;112:4302. [Google Scholar]
- 49.Jurczyszyn A, Olszewska-Szopa M, Hungria V, et al. Cutaneous involvement in multiple myeloma: a multi-institutional retrospective study of 53 patients. Leuk Lymphoma. 2016;57:2071–6. doi: 10.3109/10428194.2015.1128542. [DOI] [PubMed] [Google Scholar]
- 50.Jurczyszyn A, Olszewska-Szopa M. Nietypowe objawy klini- czne szpiczaka plazmocytowego. Acta Haematol Pol. 2017;48:189–94. [Google Scholar]
- 51.Chang H, Brandwein J, Yi OL, et al. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004;28:1007–11. doi: 10.1016/j.leukres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Kuwabara H, Nagai M, Yamaoka G, et al. Specific skin manifestations in CD56 positive acute myeloid leukemia. J Cutan Pathol. 1999;26:1–5. doi: 10.1111/j.1600-0560.1999.tb01782.x. [DOI] [PubMed] [Google Scholar]
- 53.Jen F, Zhang XX, Prieto VG, et al. Increased incidence of trisomy 8 in acute myeloid leukemia with skin infiltration (leukemia cutis) Diagnostic Mol Pathol. 2000;9:190–4. doi: 10.1097/00019606-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Ferrara F, Cancemi D, Friso P, et al. Tetrasomy 8 and t(1;11) (p32;q24) in acute myelo-monocytic leukemia with extensive leukemic cutaneous involvement. Leuk Lymphoma. 1996;20:513–5. doi: 10.3109/10428199609052439. [DOI] [PubMed] [Google Scholar]
- 55.Tallman MS, Hakimian D, Shaw JM, et al. Granulocytic sarcoma is associated with the 8;21 translocation in acute myeloid leukemia. J Clin Oncol. 1993;11:690–7. doi: 10.1200/JCO.1993.11.4.690. [DOI] [PubMed] [Google Scholar]