Abstract
Introduction
Melanoma is a malignant tumour and is the leading cause of death in patients with skin tumours.
Aim
Kaempferol belongs to a class of flavonoids, and is associated with many biological functions such as anti-inflammatory, anti-oxidation and anti-cancer. However, the inhibitory effect of kaempferol on melanoma still remains unclear.
Material and methods
The effect of kaempferol on melanoma was determined by conducting both in vitro and in vivo experiments using MTT assay and flow cytometry.
Results
The in vitro results revealed that kaempferol obviously inhibited cell viability of melanoma B16 cells, induced cell cycle arrest and cell apoptosis. The in vivo results showed that kaempferol effectively inhibited the growth of mice xenografts. More importantly, kaempferol down-regulated the number of MDSC cells and up-regulated the number of NKT cells and CD8 T cells in the spleen.
Conclusions
Taken together, these findings indicate that kaempferol might play an inhibitory role in the growth of melanoma by enhancing anti-tumour immunity of organisms.
Keywords: kaempferol, melanoma, inhibitory effect, proliferation, apoptosis, tumour immunity
Introduction
Malignant melanoma is a lethal and highly invasive form of skin cancer, with a 5-year survival rate of less than 10%. Its prognosis remains very poor and the morbidity and mortality rates are higher than in any other types of cancer [1]. Metastases to blood and lymphatic vessels occur early during tumour formation. Therefore, melanoma is a malignant tumour that poses a serious threat to human health [2]. However, the pathogenesis of melanoma has not been fully elucidated. But the current main opinion is that the incidence of melanin is mainly related to overexposure to ultraviolet radiation, solar radiation, and ionizing radiation and genetic mutations. Among them, ultraviolet radiation is considered as the main cause of melanoma [3], and this is because intense ultraviolet light damages the proteins in skin cells [4]. Previous studies have shown that race also is an important factor in the development of malignant melanoma. For example, Caucasians are more likely to develop malignant melanoma than Asians and Africans [5, 6]. In recent years, there have been several advancements in anti-cancer treatment, including targeted therapy and immunotherapy [7]. Although patients receiving targeted therapy and immunotherapy have higher response rates, their long-term survival rate remained low, and it might cause drug resistance in the later stages of treatment [8]. Development of more effective treatment strategies for melanoma remains a great challenge today [9]. Traditional medicinal plants have been used to treat a variety of cancers. Many Asian countries such as China, Thailand, Japan and other countries have been using traditional medicinal plants to treat cancers for many thousands of years [10]. Plants are the main source of new anticancer drugs, and these are associated with fewer side effects than chemical drugs [11]. Clinical anti-cancer drugs such as paclitaxel and vinblastine are derived from plant extracts. Therefore, more and more researchers are currently focusing on discovering natural compounds from medicinal plants to develop new anti-cancer drugs, which thereby increase the treatment response and long-term survival of cancer patients. Flavonoids are polyphenolic compounds that are abundant in a variety of plants [12], and are associated with a variety of health benefits primarily due to their antioxidant and anti-inflammatory properties. Many studies have shown that flavonoids inhibit proliferation and angiogenesis at various stages of cancer [13]. Kaempferol is also a well-known flavonoid that can prevent or reduce the risk of many types of tumours, such as lung cancer [14], pancreatic cancer [15], gastric cancer [16], and epithelial ovarian cancer [17]. Tea, apples, strawberries, beans and citrus fruits are rich sources of kaempferol [18]. Kaempferol regulates apoptosis [19, 20], cycle [21], and inflammation [22, 23] in cancer cells. In addition, kaempferol can inhibit the migration and invasion of medulloblastoma and breast cancer cells [24, 25]. However, its anti-tumour effects on melanoma cells are still unclear.
Material and methods
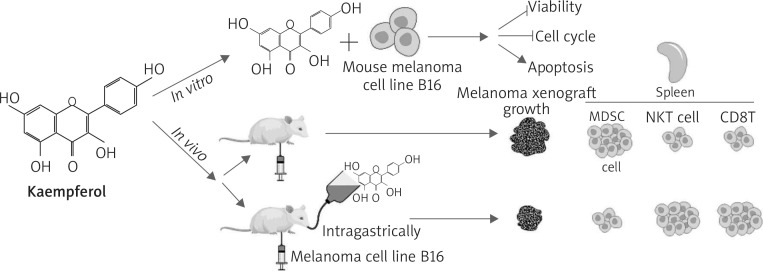
Graphical experiment steps were presented in Figure 1.
Figure 1.
Graphical experiment steps
Cell cultures and kaempferol preparation
Cells were maintained in a humidified incubator at 37°C in 5% CO2. B16 cells were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). B16 cells were cultured in 1640 medium (Gibco BRL, Grand Island, NY) supplemented with 10% foetal bovine serum (FBS; Hyclone, Logan, UT) and 1% antibiotics (penicillin and streptomycin). Kaempferol was dissolved using DMSO to prepare a 100 mM stock solution, which then was diluted with 1640 medium into different concentrations (6.25 μM, 12.5 μM, 25 μM, 50 μM, and 100 μM).
MTT assay
Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Beijing Baiao Lebo Technology co. LTD, China) according to the instructions of the manufacturer. B16 cells were plated in 96-well plates, and then were treated with different concentrations of kaempferol (6.25 μM, 12.5 μM, 25 μM, 50 μM, and 100 μM) when the cell density was 60%. 24 h later, the cells were incubated with MTT at a concentration of 0.5 mg/ml at 37°C for 4 h, followed by the addition of DMSO to dissolve the precipitates. Colorimetric analysis using a 96-well microplate reader (ELx800NB, Biotek, Winooski, VT, USA) was performed at a wavelength of 490 nm.
Cell cycle detection
B16 cells were treated with different doses of kaempferol (50 μM, 100 μM) when the cell density was 60%. 24 h later, the cells were collected and fixed in precooled 70% ethanol solution at 4°C overnight, then were centrifuged at 800×g at 4°C for 5 min. After removing the ethanol solution, the cells were washed twice with phosphate buffered saline (PBS). The cells were then suspended in 0.02 mg/ml propidium iodide (PI) and 500 μl phosphate buffer containing 0.1 mg/ml ribonuclease A. After incubation at 37°C in the dark for 30 min, the cells were analysed by flow cytometry (Beckman CytoFLEX).
Detection of apoptosis by flow cytometric analysis
B16 cells were treated with different doses of kaempferol (50 μM, 100 μM) when the cell density was 60%. 24 h later, the cells were collected and stained using Annexin V and PI using Annexin V-FITC Apoptosis Detection Kit according to the instructions. Beckman CytoFLEX was used to analyse the apoptotic rate of B16 cells.
Animal studies
C57BL/6 mice were purchased from the Experimental Animal Centre of Military Medical Sciences (Beijing, China). All animal experiments were approved by the Animal Ethics Committee of Integrated Traditional Chinese and Western Medicine, Tianjin Nankai Hospital. A single-cell suspension (1 × 106 cells/ml) of B16 cells was prepared and 50 μl suspension was subcutaneously injected into the back of the C57BL/6 mice. The mice were then randomly divided into two groups. The mice in the experimental group were intragastrically administered with kaempferol (20 mg/kg), and those in the control group were intragastrically administered with PBS. All mice were anesthetized and sacrificed 10 days after intragastric administration. The xenografts from the two groups were harvested, and then the volume and weight of the xenografts were measured.
Flow cytometric analysis of immune cells
The spleen was isolated from mice for detecting the immune cell subsets by flow cytometry. After the spleen was finely ground into a single cell suspension, direct immunofluorescence was performed with CD3 Percp, CD8PE, CD4 FITC (labelling CD4 and CD8 T cells); CD11b FITC, GR1 PE (labelling MDSCs cells); and CD3 Percp, NK1.1PE (labelling NK and NKT cells) (BioLegend, Germany). The cells were incubated with fluorochrome-labelled antibodies as above for 20 min at 4°C in the dark. Finally, the cells were washed twice with PBS and detected by flow cytometry (Beckman CytoFLEX).
Statistical analysis
The data were presented as means ± standard deviation (SD). Statistical analysis was performed using Student’s unpaired t-test and ANOVA. P-values of < 0.05 were considered to be statistically significant. GraphPad Prism 7 was used for data analysis.
Results
Kaempferol inhibits cell proliferation in melanoma B16 cells
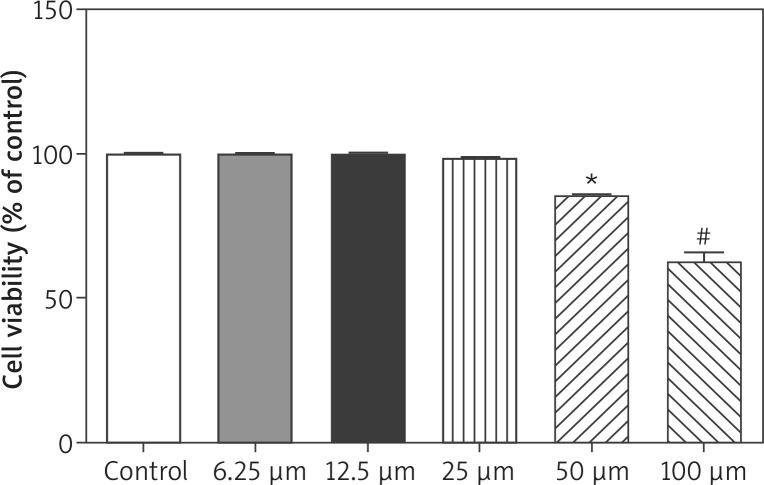
Kaempferol was used for the treatment of melanoma B16 cells, and then the cell viability was analysed by conducting MTT tests. As shown in Figure 2, kaempferol at doses of 50 μM and 100 μM, significantly inhibited the cell viability of B16 cells, suggesting that kaempferol doses of 50 uM and 100 uM, are effective against B16 cell, proliferation.
Figure 2.
Effects of kaempferol at different concentrations on the cell viability of melanoma B16 cells
*P < 0.05 vs. the control group; #p < 0.01 vs. the control group. The experiment was repeated three times.
Kaempferol induces cell cycle arrest in melanoma B16 cells
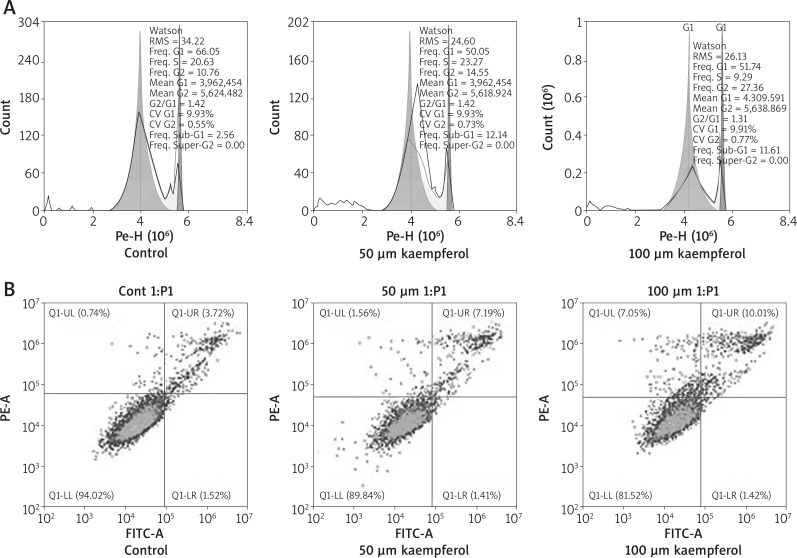
After treatment of B16 cells with kaempferol for 24 h, the cell cycle profile was analysed by flow cytometry. As shown in Figure 3 A, after treatment with 50, and 100 μM kaempferol, more cells were arrested in the G2-M phase (14.55% and 27.36%) when compared to the control group, indicating that kaempferol induces cell cycle arrest of melanoma B16 cells.
Figure 3.
A – Effects of kaempferol on melanoma B16 cell cycle arrest. After kaempferol treatment, cell cycle profiles of melanoma B16 cells were investigated using flow cytometry (FACS). The experiment was repeated three times. B – Effects of kaempferol on inducing apoptosis of melanoma B16 cells. The apoptotic cells are distributed in the quadrant labelled with Q1-UR. The experiment was repeated three times
Kaempferol induces apoptosis of melanoma B16 cells
The effect of kaempferol on cell apoptosis was detected by flow cytometry. After treatment with kaempferol of 50, and 100 μM for 24 h, the results showed that the apoptotic rates of both treatment groups were higher than the control group (Figure 3 B). These results indicated that kaempferol could induce apoptosis of melanoma B16 cells.
Kaempferol inhibits growth of melanoma B16 cell in vivo
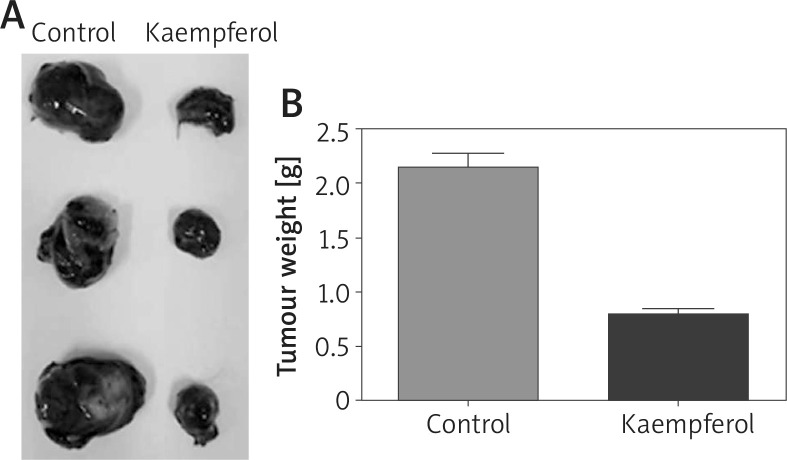
To assess the anti-tumour effects of kaempferol, an orthotopic melanoma tumour model was established using C57BL/6 mice. Xenograft formation assays showed that the volumes and weights of the xenografts in the treatment group were lower than those in the control group, indicating that kaempferol could inhibit the growth of B16 cells in vivo (Figure 4).
Figure 4.
Effects of kaempferol on melanoma xenograft growth in vivo. A – Kaempferol reduces the volume of melanoma. B –Kaempferol reduces the weight of melanoma
Effect of kaempferol on immune cells in the spleen
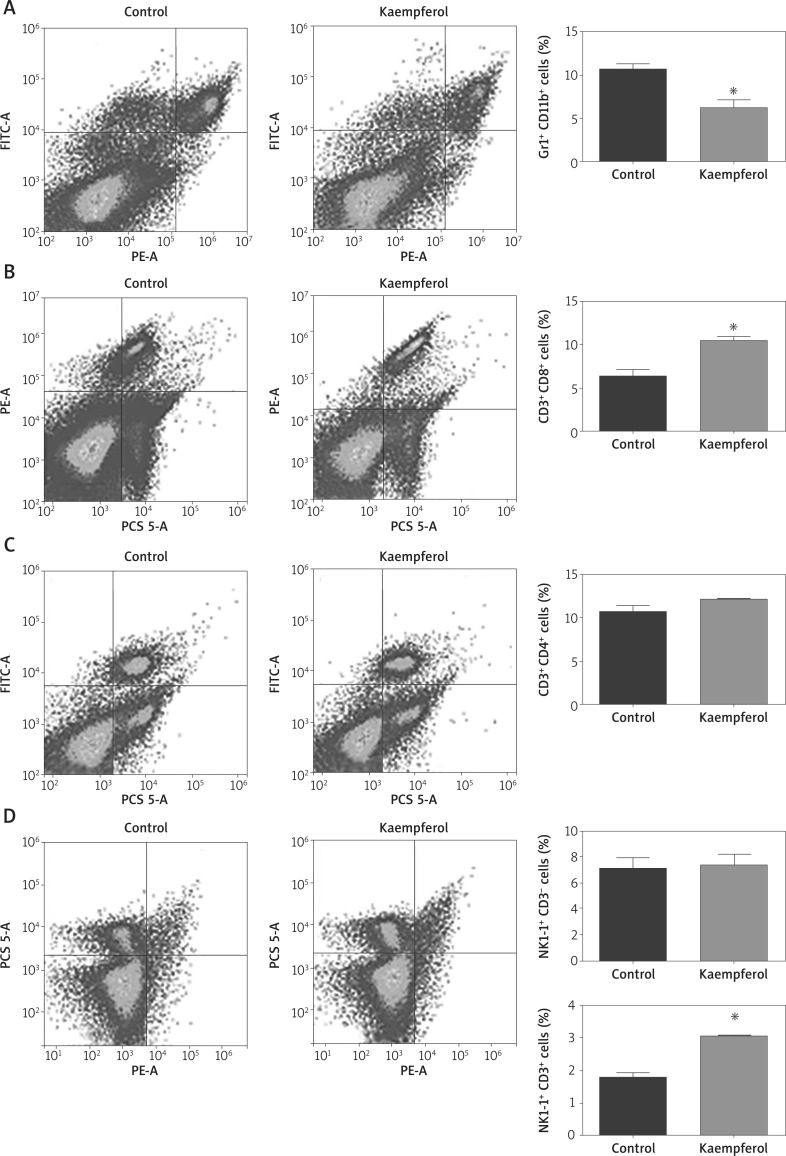
To study the effect of kaempferol on immune cells, a single cell suspension from the spleen was measured by flow cytometry analysis. The results revealed that after kaempferol treatment, the number of myeloid-derived suppressor cells (MDSCs) in the spleen was significantly decreased, while the number of CD8 T cells and NKT cells was obviously increased (Figure 5). These results suggest that kaempferol inhibits tumour growth by regulating immune cells in the spleen.
Figure 5.
Effects of kaempferol on immune cells. A – Kaempferol reduces the number of MDSCs. B – Kaempferol increases the number of CD8 T cells. C – Kaempferol has no effect on CD4 T cells. D – Kaempferol increases the number of NKT cells and has no effect on NK cells
Discussion
Kaempferol is associated with a variety of anti-tumour properties [13]. However, the anti-tumour activity of kaempferol against melanoma and its mechanism have not yet been elucidated. So, in this study, we found that kaempferol significantly inhibited the cell viability of B16 cells in a dose-dependent manner. Cancer is associated with uncontrolled cell proliferation, and most of the drugs achieve anti-tumour effects by inducing cell cycle arrest of tumour cells, inhibiting the uncontrolled cell proliferation [26]. The cell cycle has four regulatory points, G1, S, G2, and M phases for regulating cell cycle progression [27]. Previous studies have revealed that kaempferol inhibited tumour cell proliferation by blocking the cell cycle. Similarly, in this study, we also found that kaempferol can block cell cycle progression in B16 cells, leading to the inhibition of cell proliferation. Tumour development is not only associated with uncontrolled proliferation of cells, but also relates to abnormal apoptosis [28]. Therefore, enhanced apoptosis is considered as a potential method of cancer treatment. According to a previous study, kaempferol induced apoptosis of human bladder cancer cells [29]. In the present study, we hypothesized that kaempferol also played an effective role against B16 cells by inducing cell apoptosis. Taken together, these results indicate that the anticancer effect of kaempferol on B16 cells was achieved by inhibition of cell proliferation and induction of apoptosis.
As we know, spleen is the main lymphoid organ in the body that manages the organic immune function by hosting major immune cells, including lymphocytes, macrophages, dendritic cells (DCs), and monocytes [30]. Furthermore, multiple investigations have demonstrated that the immune system plays a vital role in preventing the occurrence and controlling the growth of tumours [31, 32]. In addition, kaempferol has been described to possess potent anti-inflammatory and regulating-immune functional properties [33, 34]. In the present study, the alterations of immune cells in the spleen after kaempferol treatment were measured to identify whether the cellular mechanisms of kaempferol against B16 cells occurs via immune system regulation. The results revealed that the amount of MDSCs was significantly decreased, while the number of CD8 T cells and NKT cells in the spleen was obviously increased.
MDSCs are a group of heterogeneous cells derived from bone marrow. These cells are considered to be the precursors of DCs, macrophages, and/or granulocytes, and have the ability to significantly suppress immune cell responses. During cancer, inflammation, infection, etc., MDSCs are the first cells to appear from the bone marrow to the periphery and are activated. MDSCs can inhibit the body’s natural anti-tumour immunity by a variety of ways, helping the tumour cells escape the body’s immune surveillance and attack, and promoting tumour development [35]. The present study showed that the amount of MDSCs was decreased in mice after kaempferol treatment, enhancing the anti-tumour immunity of mice and inhibiting the development of melanoma in vivo. CD8 T cells are the main effector cells for antigen-specific killing of tumour cells and also act as the major mediator of antitumor immunity. Several recent studies have shown that induction of CD8 T cells showed favourable clinical effects [36, 37]. NKT cells are new members of the immune community, and despite their small number, they have a profound impact on immune system and has a strong anti-cancer effect. NKT cells under the guidance of T cell receptor (TCR) and natural killer receptor (NKR) yield large amounts of IL-4 and INF-γ, which have a killing effect on tumour cells. Previous studies have shown that sequential activation of NKT cells provided effective innate immunotherapy in cancers, and was associated with beneficial clinical effects [38, 39]. In this study, the increased number of NKT cells and CD8 T cells in mice after kaempferol treatment showed enhancement in the anti-tumour immunity of mice.
Conclusions
Our study demonstrated that kaempferol could inhibit proliferation, and promote apoptosis of melanoma B16 cell, suggesting that kaempferol might play an important role in growth inhibition and melanoma development, and so is considered as a potential anti-tumour drug. Furthermore, it was firstly found that kaempferol treatment could down-regulate the number of MDSCs, and up-regulate the number of CD8 T cells and NKT cells, indicating that kaempferol inhibited melanoma by enhancing the anti-tumour immunity of organisms. A combination therapy of kaempferol treatment and MDSCs, and CD8 T and NKT cell-targeted adoptive immunotherapy against tumours might have better beneficial clinical effects, but further research is warranted.
Acknowledgments
This study was funded by the priority of research funds of Wannan Medical College (Grant No. WK2017ZF04), the teaching quality and teaching reform project of Wannan Medical College (Grant No. 2018jyxm58) and the Collegiate Major Natural Science Research Projects (Grant No. KJ2018ZD027).
We appreciate Xi Xu for the help in preparing Figure 1.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.O’Neill CH, Scoggins CR. Melanoma. J Surg Oncol. 2019 doi: 10.1002/jso.25604. [DOI] [PubMed] [Google Scholar]
- 2.Karimkhani C, Green AC, Nijsten T, et al. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol. 2017;177:134–40. doi: 10.1111/bjd.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varssano D, Mira F, Michaella G, et al. Association between cataract and keratinocytic skin cancers or melanoma: speculating on the common role of sun and ultraviolet radiation exposures. Ophthal Epidemiol. 2017;24:336–40. doi: 10.1080/09286586.2017.1291844. [DOI] [PubMed] [Google Scholar]
- 4.Tramutola A, Falcucci S, Brocco U, et al. Protein oxidative damage in uv-related skin cancer and dysplastic lesions contributes to neoplastic promotion and progression. Cancers. 2020;12:110. doi: 10.3390/cancers12010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrill SJ, Subramanian M, Godar DE. Worldwide cutaneous malignant melanoma incidences analyzed by sex, age, and skin type over time (1955-2007): is HPV infection of androgenic hair follicular melanocytes a risk factor for developing melanoma exclusively in people of European-ancestry? Dermatoendocrinology. 2016;8:e1215391. doi: 10.1080/19381980.2016.1215391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colebatch AJ, McArthur GA. Pathology of mlanoma. PET/CT in Melanoma. Springer; 2017. pp. 9–13. [DOI] [Google Scholar]
- 7.Gotwals P, Cameron S, Cipolletta D, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 8.Gladfelter P, Darwish NHE, Mousa SA. Current status and future direction in the management of malignant melanoma. Melanoma Research. 2017;27:403–10. doi: 10.1097/CMR.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 9.Millet A, Martin AR, Ronco C, et al. Metastatic melanoma: insights into the evolution of the treatments and future challenges. Med Res Rev. 2016;37:98–148. doi: 10.1002/med.21404. [DOI] [PubMed] [Google Scholar]
- 10.Yang XL, Yuan YL, Zhang DM, et al. A new quassinoid from the root bark of Ailanthus altissima. Nat Prod Res. 2014;28:1432–7. doi: 10.1080/14786419.2014.909418. [DOI] [PubMed] [Google Scholar]
- 11.Purushotham G, Padma Y, Nabiha Y, Venkata Raju RR. In vitro evaluation of anti-proliferative, anti-inflammatory and pro-apoptotic activities of the methanolic extracts of Andrographis nallamalayana Ellis on A375 and B16F10 melanoma cell lines. 3 Biotech. 2016:6–212. doi: 10.1007/s13205-016-0529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsa-bai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5:93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abotaleb M, Samuel S, Varghese E, et al. Flavonoids in cancer and apoptosis. Cancers. 2018;11:28. doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han X, Liu CF, Gao N, et al. Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. Biomed Pharmacother. 2018;108:809–16. doi: 10.1016/j.biopha.2018.09.087. [DOI] [PubMed] [Google Scholar]
- 15.Nöthlings U, Murphy SP, Wilkens LR, et al. Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;166:924–31. doi: 10.1093/aje/kwm172. [DOI] [PubMed] [Google Scholar]
- 16.Kim TW, Lee SY, Kim M, et al. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018;9:875. doi: 10.1038/s41419-018-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, Yin J, Rankin G, Chen Y. Kaempferol induces G2/M cell cycle arrest via checkpoint kinase 2 and promotes apoptosis via death receptors in human ovarian carcinoma A2780/CP70 cells. Molecules. 2018;23:1095. doi: 10.3390/molecules23051095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farooqui AA, Farooqui T. Importance of fruit and vegetable-derived flavonoids in the Mediterranean diet: molecular and pathological aspects. In: Farooqui T, Farooqui AA, editors. Role of the Mediterranean Diet in the Brain and Neurodegenerative Diseases. Elsevier Inc; 2018. pp. 417–27. [Google Scholar]
- 19.Demirel Sezer E, Oktay LM, Karadadaş E, et al. Assessing anticancer potential of blueberry flavonoids, quercetin, kaempferol, and gentisic acid, through oxidative stress and apoptosis parameters on HCT-116 cells. Jf Med Food. 2019;22:1118–26. doi: 10.1089/jmf.2019.0098. [DOI] [PubMed] [Google Scholar]
- 20.Kashafi E, Moradzadeh M, Mohamadkhani A, Erfanian S. Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed Pharmacother. 2017;89:573–7. doi: 10.1016/j.biopha.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L, Xue L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics. 2019;27:629–34. doi: 10.3727/096504018X15228018559434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeon MJ, Lee MH, Kim DH, et al. Anti-inflammatory effects of kaempferol on Helicobacter pylori-induced inflammation. Biosci Biotechnol Biochem. 2019;83:166–73. doi: 10.1080/09168451.2018.1528140. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Tang H, Zhang Z, et al. Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int Immunopharmacol. 2017;43:236–42. doi: 10.1016/j.intimp.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Labbé D, Provençal M, Lamy S, et al. The flavonols quercetin, kaempferol, and myricetin inhibit hepatocyte growth factor-induced medulloblastoma cell migration. J Nutr. 2009;139:646–52. doi: 10.3945/jn.108.102616. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Yan T, Deng R, et al. Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. OncoTargets Therapy. 2017;10:4809–19. doi: 10.2147/OTT.S140886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George VC, Kumar DR, Kumar RA. Relative in vitro potentials of parthenolide to induce apoptosis and cell cycle arrest in skin cancer cells. Curr Drug Discov Technol. 2016;13:34–40. doi: 10.2174/1570163813666160224124029. [DOI] [PubMed] [Google Scholar]
- 27.Uroz M, Wistorf S, Serra-Picamal X, et al. Regulation of cell cycle progression by cell-cell and cell-matrix forces. Nat Cell Biol. 2018;20:646–54. doi: 10.1038/s41556-018-0107-2. [DOI] [PubMed] [Google Scholar]
- 28.Letai A. Apoptosis and Cancer. Ann Rev Cancer Biol. 2017;1:275–94. [Google Scholar]
- 29.Wu P, Meng X, Zheng H, et al. Kaempferol attenuates ROS-induced hemolysis and the molecular mechanism of its induction of apoptosis on bladder cancer. Molecules. 2018;23:E2592. doi: 10.3390/molecules23102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci Immunol. 2019;4:eaau6085. doi: 10.1126/sciimmunol.aau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burlion A, Ramos RN, Pukar KC, et al. A novel combination of chemotherapy and immunotherapy controls tumor growth in mice with a human immune system. Oncoimmunology. 2019;8:e1596005. doi: 10.1080/2162402X.2019.1596005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Upadhyay S, Sharma N, Gupta KB, Dhiman M. Role of immune system in tumor progression and carcinogenesis. J Cell Biochem. 2018;119:5028–42. doi: 10.1002/jcb.26663. [DOI] [PubMed] [Google Scholar]
- 33.Guo P, Feng YY. Anti-inflammatory effects of kaempferol, myricetin, fisetin and ibuprofen in neonatal rats. Trop J Pharmaceut Res. 2017;16:1819. [Google Scholar]
- 34.Jia Z, Chen A, Wang C, et al. Amelioration effects of kaempferol on immune response following chronic intermittent cold-stress. ResVet Sci. 2019;125:390–6. doi: 10.1016/j.rvsc.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 35.de Haas N, de Koning C, Spilgies L, de Vries IJ, Hato SV. Improving cancer immunotherapy by targeting the STATe of MDSCs. Oncoimmunology. 2016;5:e1196312. doi: 10.1080/2162402X.2016.1196312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horton BL, Williams JB, Cabanov A, et al. Intratumoral CD8 + T-cell apoptosis is a major component of t-cell dysfunction and impedes antitumor immunity. Cancer Immunol Res. 2017;6:14–24. doi: 10.1158/2326-6066.CIR-17-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsukumo S, Yasutomo K. Regulation of CD8+ T cells and antitumor immunity by Notch signaling. Front Immunol. 2018;9:101. doi: 10.3389/fimmu.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bollino D, Webb TJ. Chimeric antigen receptor-engineered natural killer and natural killer T cells for cancer immunotherapy. Transl Res. 2017;187:32–43. doi: 10.1016/j.trsl.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Exley MA, Friedlander P, Alatrakchi N, et al. Adoptive transfer of invariant NKT cells as immunotherapy for advanced melanoma: a phase I clinical trial. Clin Cancer Res. 2017;23:3510–9. doi: 10.1158/1078-0432.CCR-16-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]