Abstract
Opioid neurotransmission has been implicated in psychiatric disorders featuring impaired inhibitory control over appetitive motivation, such as addiction and binge-eating disorder. We have previously shown that infusions of the μ-opioid receptor (μOR) agonist DAMGO into the ventromedial prefrontal cortex (vmPFC) induced hyperphagia, increased motor activity, and augmented sucrose-reinforced responding in the task progressive ratio (PR) task, which assesses the motivational value of an incentive. These effects were not reproduced by intra-PFC infusion of a variety of dopamine (DA) agonists and antagonists, suggesting that manipulation of intra-PFC DA systems alone is not sufficient to reproduce μOR-like effects. Nevertheless, this does not rule out interactions between PFC DA and μ-opioid systems. Here we used intra-vmPFC drug cocktails containing DAMGO and SCH 23390 (a DA D1 receptor antagonist) to determine whether increases in appetitive motivation and motor activity elicited by intra-vmPFC μOR stimulation require intact signaling through vmPFC D1 receptors. Blockade of D1 receptors with SCH 23390 attenuated the enhancements in PR breakpoint, and increases in exploratorylike behavior and feeding initiation elicited by intra-vmPFC μOR stimulation. These results establish that intra-vmPFC D1 signaling is required for the expression of behavioral effects evoked by μOR stimulation within the PFC, and further suggest that D1 tone plays an enabling or permissive role in the expression of μOR –elicited effects. Simultaneous targeting of both μ-opioid and D1 systems may represent a more efficacious treatment strategy (compared to μOR blockade alone) for psychiatric disorders characterized by dysregulated appetitive motivation.
Keywords: prefrontal cortex, mu-opioid receptor, dopamine D1 receptor, feeding, food motivation, rat
1. INTRODUCTION
Multiple psychiatric disorders, including binge-eating disorder and substance use disorders, are characterized by dysregulated appetitive motivation (American Psychiatric Association, 2013). Neuroimaging studies of individuals with these disorders show abnormal function in frontal cortical areas that are responsive to reward-associated cues and that modulate reward-seeking behavior (Donnelly et al, 2014; Schienle et al, 2009). Nevertheless, the neurochemical basis for this putative frontal dysfunction is unclear. Previously, our lab demonstrated a role for μ-opioid signaling in the ventromedial prefrontal cortex (vmPFC) in the modulation of reward-seeking behavior (Selleck et al, 2015). Stimulation of vmPFC μ -opioid receptors (μORs) using the μ -selective agonist, DAMGO, increased feeding and lever-pressing for sucrose reward on a progressive ratio (PR) schedule (Selleck et al, 2015). When rats were shifted into a naturally motivated, impulsive behavioral state (prolonged food deprivation), blockade of vmPFC opioid receptors restored normal patterns of PR responding (Selleck et al, 2015). Hence, intra-vmPFC μOR signaling is necessary for hunger-induced alterations in food motivation, and, conversely, increased intra-vmPFC μOR signaling augments food-motivated behavior.
While the abovementioned findings reveal behaviorally relevant effects of intra-PFC μ-opioid signaling, the role of local interactions between μORs and other PFC neurochemical systems is less clear. Dopamine (DA) seems a particularly promising candidate. DA levels in the vmPFC are heightened during feeding after food deprivation (Hernandez and Hoebel, 1990) or after presentation of palatable foods and food-related cues to sated rats (Bassareo et al, 2002; Bassareo and Di Chiara, 1997), and dopamine depletion in the PFC alters food consumption and glucose preference (Galosi et al, 2015). Nevertheless, we showed previously that intra-vmPFC monoamine stimulation with d-amphetamine had no effect in PR, nor did intra-vmPFC administration of D1 or D2-selective DA antagonists alter food intake (Mena et al, 2011; Selleck et al, 2015), indicating that manipulating intravmPFC DA systems pharmacologically does not recapitulate μOR-like behavioral effects. The possibility remains, however, that opioid and dopaminergic systems within the vmPFC interact to mediate cortical influences over food-directed behavior. General support for DA/opioid interactions is provided by previous work in the nucleus accumbens, a prominent vmPFC projection target (Gabott et al, 2005; Taber et al, 1998; Cunningham and Kelley, 1992).
The goal of this study was to explore interactions between D1 receptors and μORs within the vmPFC. D1 signaling appears to be particularly important for PFC-based cognitive and behavioral functions, including working memory (Berridge and Arnsten, 2013; Vijayraghavan et al, 2007). Furthermore, a recent study showed that feeding activated D1 receptor-bearing PFC neurons, and optogenetic stimulation of D1-containing PFC neurons augmented feeding (Land et al, 2014). In the present study, drug ‘cocktails’ of DAMGO and the D1-selective antagonist, SCH 23390, were infused directly into the vmPFC, and resultant behavioral effects were assessed in two behavioral tests: sucrose reinforced PR, and a behavior-observation paradigm, in which food intake and spontaneous motor activity were measured. We hypothesized that antagonism of D1 DA receptors in the vmPFC would alter the behavioral effects of μOR stimulation, and our tasks were designed to detect either enhancement or attenuation of μOR-mediated effects. Use of the two tasks in parallel enabled the detection of possible differences in opioid/DA interactions among the interrelated, yet dissociable, μORmodulated processes of food-seeking, eating, and general motor activation.
2. METHODS
2.1. Subjects:
Subjects were male Sprague-Dawley rats (Harlan, Madison, WI) weighing 275–300 g upon arrival at the laboratory. Rats were housed in a light- and temperature-controlled vivarium, under a 12:12 hr light-dark cycle (lights on at 0700 hr). Food and water were available ad libitum, except as indicated for various experiments. Animals were handled daily to reduce stress. Testing occurred between 1100 and 1500 h, corresponding to the light phase, which facilitated low, stable baselines against which opioid-induced increases could be detected. All facilities and procedures were in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison.
2.2. Operant-Behavior Procedures:
Training and testing procedures were conducted in standard operant chambers, described in the Supplemental Materials.
Rats underwent an initial training period during which they were maintained at 90 ± 2% of free-feeding body weight using scheduled feedings. During this phase, rats were trained to lever press to obtain a sugar pellet reinforcer on a conjoint random-time 30 s/fixed ratio 1 schedule (RT-30 s/FR-1) and then a straight FR-1 schedule (Selleck et al, 2015).
Next, once responding was achieved on FR-1, rats advanced to FR-3, FR-5, and finally PR-2 schedules. The PR-2 schedule consisted of a constant increase in the number of lever presses required to obtain each successive reinforcer (+2 presses, so that one response was required for the first reinforcer, three for the second, etc.). PR sessions lasted 120 minutes. After 2–3 days at PR-2, rats were returned to ad libitum food access in their home cages, and food deprived for 2 h immediately preceding each testing session. This mild level of food restriction yielded stable levels of baseline responding, and allowed for the detection of either increases or decreases from baseline (as engendered by experimental manipulations). Rats were maintained on the PR-2 schedule until stable (<15% variability in the number of reinforcers earned in each of three sequential daily testing sessions). Note that it is unlikely that the mild levels food restriction schedules, necessary for operant training in the present study, influenced drug responses. The same patterns of drug effects and interactions were noted in the behavior-observation study described in the next section, which did not include food restriction (also, see Results and Discussion sections).”
2.3. Behavior-Observation Procedure:
Rats were habituated to clear polycarbonate cages (9.5 in width × 17 in length × 8 in height), identical to the home cages except with wire grid floors. Pre-measured chow pellets were placed on the cage floors.. One-hour long habituation sessions were carried out on two sequential days. Drug treatments commenced on the third day (see Experimental Design section). After infusion, rats were placed in the testing cages and videotaped with a digital camcorder for 60 min. Water was available during the testing sessions. An experimenter blind to treatment viewed the digital files and recorded spontaneous ambulation, rearing, eating bouts and bout duration using an event recorder interfaced to a PC-based desktop computer.
2.4. Surgical Procedures:
Stereotaxic surgery under Isoflurane anesthesia was carried out as described previously (Selleck et al, 2015). Stainless-steel guide cannulae (10-mm long; 25-gauge) were aimed at the vmPFC. Coordinates for cannulae placements were as follows: +3.0 mm anterior to bregma; ±2.2 mm from the midline; −2.7 mm from skull surface (2.5 mm above the final infusion site). For each group of rats, 1–2 rats of that group were used for “sham surgeries,” in which probes were lowered to the selected site, and the rats perfused immediately after surgery, and their brains processed histochemically for viewing of the placement site. This procedure was conducted in order to check that the stereotaxic coordinates chosen from the atlas would yield accurate placements (there can sometimes be subtle differences between groups of rats). Coordinates were adjusted accordingly, and the final coordinates used in the study were based on this ‘placement check. Additional details are provided in the Supplementary Materials.
2.5. Microinfusion Procedures and Drugs:
Intracerebral microinjections were carried out as previously described (Newman et al, 2013); details are provided in the Supplementary Materials. DAMGO ([DAla2, N-Me-Phe4, Gly5-ol]-enkephalin) was obtained from Bachem, and SCH 23390 (7-chloro-3methyl-1-phenyl-1,2,4,5-tetrahydro-3-benzazepin-8-ol; a D1-selective antagonist) was obtained from Sigma Aldrich. All drugs were dissolved in sterile 0.9% saline.
2.6. Experimental Design:
After a week of recovery from surgery, rats were re-baselined on their respective operant tasks (PR-2 or DRL-15 s) under 2-h food restriction, as described above. Upon exhibiting stable baseline responding (±15% variability over three consecutive testing days), rats were acclimated to microinjection procedures with sham infusions and intra-vmPFC saline injections on consecutive days. Rats were re-baselined after these injections, whereupon drug testing commenced.
Testing (either operant or behavior-observation) began with all rats receiving an intra-vmPFC DAMGO infusion (‘Pre-SCH 23390’; 0.25 μg/0.5 μL), followed by three testing days with intra-vmPFC SCH 23390 infusions (0, 1, or 2 μg/0.5 μL). Next, rats underwent three testing days where they received intra-vmPFC infusions of a 0.5-μL ‘cocktail’ containing both DAMGO (0.25 μg) and SCH 23390 (0, 1, or 2 μg). Finally, rats received one last intra-vmPFC DAMGO infusion (‘Post-SCH 23390’; 0.25 μg). The inclusion of three DAMGO-alone testing days (‘Pre-SCH 23390’, one counter-balanced among cocktail infusions, and ‘Post-SCH 23390) allowed for the detection of possible shifts in μOR sensitivity across the experiment. ‘Cocktail’ and ‘SCH-23390-alone’ treatment blocks were counterbalanced across subjects.
2.7. Histology:
At the end of each experiment, rats were deeply anesthetized and perfused with 10% formalin in phosphate buffer. Brains were stored in 10% formalin. Coronal sections (60-μm) were cut through the infusion site on a cryostat microtome, collected on slides, stained with cresyl violet, and subsequently reviewed to verify injection placements. Subjects with incorrect placements (2 out of 18 rats total), or with brain infections that were revealed in the histology (1 out of 18 rats) were not included in the analyses. A charting of the incorrect placement is included in the Supplementary Materials.
2.8. Statistical Analyses:
Data were analyzed using two-factor (DAMGO X SCH 23390) repeated measures ANOVAs. Multiple comparisons among means were conducted using Tukey’s test. The level of statistical significance was set at P < 0.05 for all experiments.
3. RESULTS
3.1. No Evidence of Shifts in μOR Sensitivity Across the Duration of the Study:
To assess whether the repeated intra-vmPFC DAMGO treatments of the within-subjects design led to either tolerance or sensitization, we compared responses from the three ‘DAMGO-alone’ challenges spanning the experiment (see Experimental Design section) for each individual study (PR, and the behavior-observation study). There were no detectable changes over the course of the experiment for PR measures (Fs = 0.18 – 1.13, not significant (NS)) or observation-paradigm measures (Fs = 0.015 – 0.293, NS). Hence, values from the three DAMGO treatments for each animal were averaged for subsequent analysis and presented in figures.
3.2. Intra-vmPFC D1 Receptor Antagonism Reversed the PR Facilitation Evoked by Intra-vmPFC μOR Stimulation:
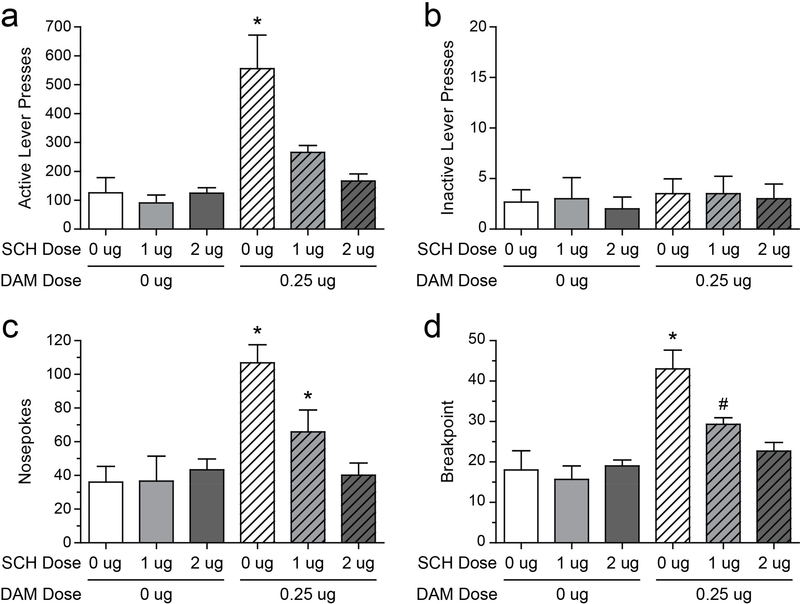
DAMGO produced an increase in responding that was reversed by SCH 23390, as evidenced by main effects of DAMGO (F(1,5) = 19.80, P = 0.0067 for active lever presses; F(1,5) = 19.43, P = 0.007 for breakpoint), SCH 23390 (F(2,5) = 8.00, P = 0.0084 for active lever presses; F(2,5) = 6.445, P = 0.0159 for breakpoint), and DAMGO × SCH 23390 interactions (F(2,10) = 5.88, P = 0.0205 for active lever presses; F(2,10) = 5.90, P = 0.0203 for breakpoint; Figs. 1a and d). A similar pattern of results was observed for nosepokes (DAMGO: F(1,5) = 87.94, P = 0.0002; SCH 23390: F(2,5) = 8.92, P = 0.006; DAMGO x SCH 23390 interaction: F(2,10) = 17.17, P = 0.0006; Figures 1c). Note that nosepokes (head entries into the food hopper) were recorded, but that this behavior had no programmed consequences. Inactive lever presses were unaffected (Fs = 0.093–0.527, NS; Figure 1b). Comparisons among means confirmed that increases in active lever presses, breakpoint, and nosepokes evoked by 0.25 μg DAMGO were significantly attenuated by antagonizing D1 DA receptors with SCH 23390 (for a summary of specific means comparisons, see Fig. 1 legend). Importantly, SCH 23390 had no statistically significant effects on PR responding in the absence of DAMGO.
Fig. 1:
Rats (N=6) treated with intra-vmPFC infusions of the selective D1 antagonist SCH-23390 showed attenuated responses to the increases in food motivation elicited by co-infusion of the μ-opioid agonist DAMGO in the progressive ratio task. Intra-vmPFC DAMGO increased responding on the active lever (a), resulting in increased breakpoint (i.e. the last completed ratio, d). Nose-poking was also increased (c). Co-administration of SCH-23390 attenuated increases in active lever pressing (a), breakpoint (d), and nose-poking (c) evoked by treatment with DAMGO alone. There were no effects of any treatment on responding on the inactive lever (b). *P < 0.05, different from all other treatments; #P < 0.05, different from 0.25 μg DAMGO/0 μg SCH and 0.25 μg DAMGO/2 μg SCH ‘cocktails’. Error bars depict one SEM.
3.3. Intra-vmPFC D1 Blockade Reversed the Motor Hyperactivity Evoked by Intra-vmPFC μOR
Stimulation:
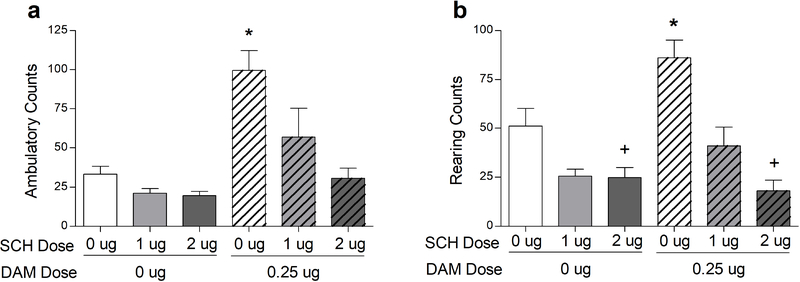
Intra-vmPFC infusion of 0.25 μg DAMGO significantly increased ambulation and rearing (Fig. 2). This effect was reversed by concomitant administration of SCH 23390, as evidenced by main effects of DAMGO on locomotion (F(1,8) = 13.998, P=0.0057) and rearing (F(1,8) = 11.602, P = 0.0093), main effects of SCH 23390 (F(2,16) = 15.143, P = 0.0002 for ambulation;; F(2,16) = 43.562, P < 0.0001 for rearing), and significant DAMGO × SCH 23390 interactions (F(2,16) = 6.941, P = 0.0068 for ambulation; F(2,16) = 4.211, P = 0.0339 for rearing; Figures 2a and b). Comparisons among means indicated that co-infusion of SCH 23390 reversed the increases in ambulation and rearing evoked by 0.25 μg DAMGO. Administration of SCH 23390 alone produced no effects on ambulation, but suppressed rearing below vehicle control levels at the 2-ug dose. However, both the 1-ug and 2-ug doses of SCH 23390 significantly reduced DAMGO-potentiated rearing. A summary of means comparisons is provided in Fig. 2. Together, these findings indicate that increases in spontaneous motor activity evoked by intra-vmPFC μOR stimulation require functional vmPFC D1 DA receptor signaling.
Fig. 2:
Intra-vmPFC DAMGO infusions in rats (N = 9) increased horizontal movement (ambulation, a) and vertical movement (rearing, b); these effects were reversed by co-infusion of SCH-23390. *P < 0.05, different from all other treatments (main effect of DAMGO). +P < 0.05, different from 0 μg SCH /0 μg DAMGO. Error bars depict one SEM.
3.4. Blockade of vmPFC-localized D1 Receptors Attenuated μOR-induced Effects on Feeding Behaviors:
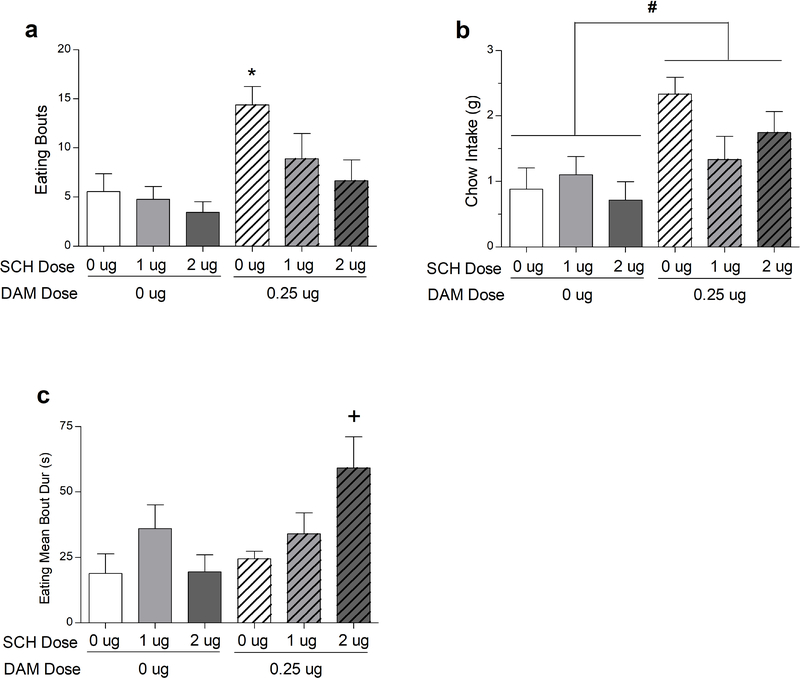
SCH 23390 reversed DAMGO-induced changes in feeding patterns and food intake (Fig. 3). First, intra-vmPFC DAMGO significantly increased the number of feeding bouts, an effect that was significantly attenuated by co-administration of SCH 23390 (main effect of DAMGO: F(1,8) = 13.097, P = 0.0068; main effect of SCH 23390: F(2,16) = 7.479, P = 0.0051; DAMGO X SCH 23390 interaction: (F(2, 16)= 1.606, NS). Given the presence of multiple, clear main effects and an experimental design strongly driven by our a priori hypothesis, we conducted Newman-Keuls post-hoc comparisons among means that were not collapsed across either main factor. These comparisons revealed that DAMGO elicited significantly more feeding bouts compared to saline or either dose of SCH 23390 alone, and that combining DAMGO with either dose of SCH-23390 produced significantly fewer feeding bouts compared to DAMGO alone (Fig. 3). Importantly, SCH 23390 alone did not significantly alter the number of feeding bouts, again supporting the hypothesis that the SCH 23390-mediated reversal of DAMGO’s effect was not an artifact of generalized performance suppression. In addition to feeding bouts, the average duration of each feeding bout was also significantly affected by drug treatments (main effect of DAMGO: F(1,8)= 5.904, P= 0.0412; DAMGO X SCH 23390 interaction: F(2,16)= 4.018, P= 0.0386). The main effect of SCH 23390 did not achieve statistical significance (F(1,8)= 3.388, NS). Comparisons among means indicated that the 2-ug dose of SCH 23390 combined with DAMGO significantly increased mean bout duration relative to saline control and both DAMGO-alone and SCH 23390 (2 ug)-alone values. SCH 23390 alone had no significant effect on mean feeding bout duration. Other comparisons are described in the legend for Fig. 3.
Fig. 3:
Administration of intra-vmPFC DAMGO to rats (N=9), augmented food-directed behaviors: total number of eating bouts (a), chow intake (b), and mean eating bout duration (c). Simultaneous treatment with SCH 23390 modified the effects of DAMGO on eating bouts and mean eating bout duration. *P < 0.05, different than all other treatments. #P < 0.05, main effect of DAMGO. +P < 0.05, different from 0 μg SCH /0 μg DAMGO, 2 μg SCH/0 μg DAMGO, and 0.25 μg DAMGO/0 μg SCH. Error bars depict one SEM.
Intra-vmPFC DAMGO also significantly increased food intake itself (main effect of DAMGO: (F(1,8) = 15.372, P = 0.0044); however, neither the main effect of SCH 23390, nor the DAMGO X SCH 23390 interaction, achieved statistical significance (Fs= 1.877–3.434, NS), although a strong trend was noted for the DAMGO X SCH 23390 interaction (P= 0.057).
3.5. Histological Analyses:
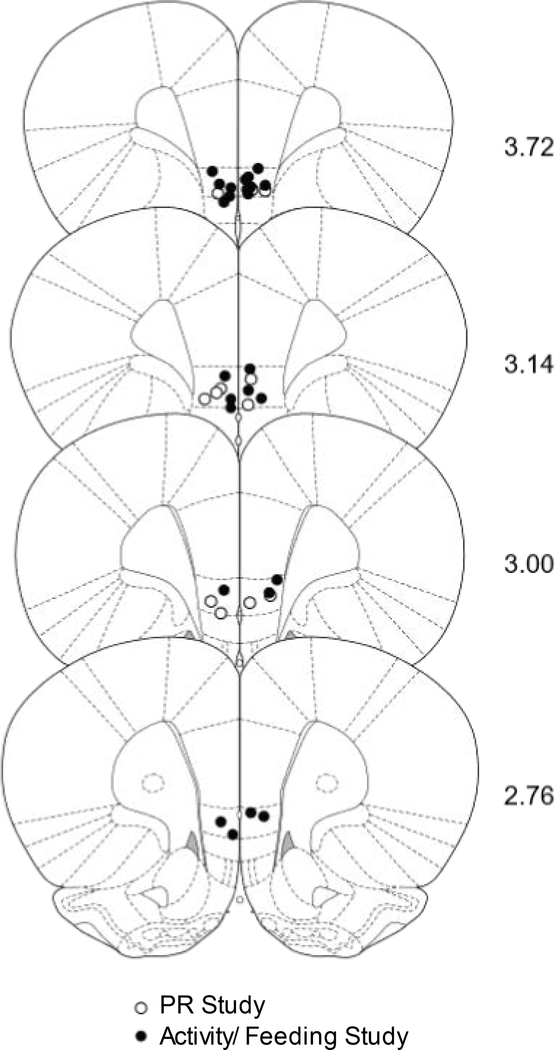
Chartings of injector placements into vmPFC reveal that placements fell mainly in the infralimbic area of ventromedial PFC (Fig. 4). Cannula and injector tracks were clearly visible with no unusual damage to the targeted area.
Fig. 4:
Chartings for injector placements in all experiments. Empty circles depict the placement of injector tips for the PR experiment, filled circles, for the activity/feeding study. Numbers indicate distance anterior to bregma in millimeters.
4. DISCUSSION
The present findings reveal a novel role for local D1 receptor signaling in modulating the behavioral effects of intra-vmPFC μOR stimulation. Intra-vmPFC administration of the D1 antagonist, SCH 23390, reversed the augmentation of food motivation and general motor activity induced by intramPFC DAMGO. This interaction did not appear to be an artifact of a putative D1 antagonist-mediated generalized motor impairment, as intra-vmPFC SCH 23390 alone failed to affect the majority of behavioral measures in this study (PR responding, feeding bouts, mean feeding bout duration, or spontaneous ambulation). SCH 23390 alone attenuated rearing; however, this effect occurred at a higher dose than that required to significantly reduce DAMGO-potentiated rearing. Furthermore, when given in conjunction with DAMGO, SCH 23390 never brought the DAMGO effect below baseline (vehicle-associated) levels. These findings indicate that that the dose range of SCH 23390 was below the threshold for affecting most behaviors in this study, supporting the interpretation that μOR/D1 receptor interactions result from a mechanism in which vmPFC D1 signaling mediates a “permissive” or “enabling” function that allows vmPFC μ-opioid-elicited effects to be expressed. It should be noted that the present study focused on infralimbic cortex, and it is possible that μOR and D1 receptors interact differently in other cortical regions. To our knowledge, however, these findings represent the first demonstration of behaviorally relevant cortical D1/ μOR interactions, and suggest that D1 receptor tone is necessary for the expression of μOR-elicited effects at least in certain sectors of prefrontal cortex.
The D1 antagonist produced complex and dissociable effects on DAMGO-driven food-seeking vs. feeding behaviors. Thus, whereas SCH 23390 robustly blocked intra-vmPFC DAMGO effects across behaviors associated with approaching or obtaining food reward (PR lever-pressing, initiation of feeding bouts), the D1 antagonist failed to significantly block DAMGO-driven food intake. Furthermore, the high dose of SCH 23390 increased the duration of individual feeding bouts when given with DAMGO. These results can be interpreted in the context of intra-vmPFC DAMGO’s complex effects on feeding microstructure. DAMGO elicits a behavioral pattern consisting of the initiation of numerous, brief feeding bouts that are then rapidly terminated. This behavioral pattern suggests intravmPFC μOR stimulation increases the incentive ‘pull’ of food, without augmenting the hedonic experience of eating (Spector et al, 1998; Ostlund et al, 2013), possibly because the sensory, motorperformance, and/or reward-valuation components of the feeding consummatory act are obstructed by competing response sets (Baldo, 2016). In general support of this hypothesis, a recent study showed that DAMGO infusion into the medial prefrontal cortex increased feeding while failing to enhance hedonic taste reactivity (‘wanting without liking’; Castro and Berridge, 2017). Because total food intake is a function of both feeding bout number and bout duration, the less-consistent effects shown here of SCH 23390 on DAMGO-induced intake could reflect the fact that D1-antagonist-associated lengthening of DAMGO-induced feeding bouts partly offset the reversal of DAMGO-mediated bout initiation. Relatedly, SCH 23390 robustly blocked DAMGO-driven motor behaviors (locomotion and rearing) that competed with the feeding consummatory act, which could have further contributed to the increased bout duration seen with the combination of DAMGO with the high dose of SCH 23390. Overall, these findings bolster the conclusion that D1 receptor antagonism reversed multiple, sometimes oppositional, behavioral effects engendered by vmPFC μOR stimulation. Furthermore, antagonizing D1 transmission in the PFC did not appear to degrade the rewarding properties of eating once the food had been contacted, consistent with dissociation between PFC substrates governing food-seeking vs. food reward during eating.
Currently, the mechanisms underlying cortical μOR/D1 receptor interactions are unknown. Considerable research has addressed the mechanisms of PFC D1 function at the cellular and circuit levels, especially in the context of working memory performance (Berridge et al, 2013; O’Donnell, 2003). PFC DA acts on D1 receptors on pyramidal cells and on primarily parvalbumin-containing GABAergic interneurons (Le Moine and Gaspar, 1998; Santana et al, 2009), where it exhibits concentration-dependent effects (Trantham-Davidson et al, 2004). At ‘optimal’ levels of cortical DA activity, D1 signaling increases firing of fast-spiking inhibitory interneurons (Gorelova et al, 2002). These same DA concentrations simultaneously activate D1 receptors on pyramidal neurons, resulting in depolarization of the membrane potential and heightened responsiveness to depolarizing inputs (Seamans et al, 2001; Seong and Carter, 2012). This conflux of actions results in pyramidal cells being held in an “up state”, a pattern of activity characterized by a sustained membrane depolarization coupled with interneuron-mediated suppression of spontaneous spiking (Lewis and O’Donnell, 2000; O’Donnell, 2003). During up states, strong inputs are able to overcome heightened tonic inhibition and evoke increases in pyramidal cell firing, which manifest as stable, active stimulus representations resistant to interfering inputs and noise (O’Donnell, 2003; Seamans et al, 2001). The DA concentration-dependent nature of PFC D1 actions gives rise to an inverted-U dose response function, with optimal D1 stimulation levels enhancing signal-to-noise ratio and maximizing performance on PFC-dependent tasks (Vijayraghavan et al, 2007).
In comparison to D1 receptors, the actions of cortical μORs are poorly understood. Evidence suggests that μOR signaling acts at several points within local cellular networks to modulate PFC responses to excitatory input (Baldo, 2016). Immunohistochemistry and single-cell PCR studies have shown that μORs are localized on cortical GABAergic interneurons primarily expressing vasoactive intestinal peptide (VIP), with little to no expression in pyramidal neurons or in D1-bearing parvalbumin interneurons (Ferezou et al, 2007; Taki et al, 2000). Intra-vmPFC μOR stimulation inhibits sodium currents in interneurons, decreasing interneuron spiking and reducing GABAergic miniature inhibitory postsynaptic currents (mIPSC) onto pyramidal cells (Ferezou et al, 2007; Witkowski and Szulczyk, 2006), possibly activating pyramidal cells via disinhibition (Tanaka and North, 1994). Given this understanding of receptor localization, pyramidal cell firing could be enhanced due to the combined effects of D1-elicited hyperexcitability and a loss of inhibitory tone arising from μOR-mediated suppression of VIP-expressing interneurons (Ferezou et al, 2007). In other words, D1 tone could maintain pyramidal neurons in a state through which μOR-elicited signals could be transmitted.
Recently, some studies have suggested that, in addition to interneurons, μORs may also be expressed on pyramidal cells (Juhasz et al, 2008; Olianas et al, 2012; Schmidt et al, 2003). For example, DAMGO application to freshly dispersed mPFC pyramidal neurons modulated high-threshold Ca2+ channel currents via protein kinase A (PKA)-mediated activity, an effect that was reversed by naloxone (Rola et al, 2008). Presumably, this effect on intracellular Ca2+ signaling could alter excitability and/or modulate Ca2+-dependent signaling pathways, reducing the threshold for activation either from stronger glutamatergic signaling or from disinhibition after μOR –mediated interneuron suppression (Carafoli, 2002). Additionally, PFC D1/μOR interactions could result from direct receptor cross-talk on individual pyramidal cells. Olianas and colleagues (2012) showed that some D1 receptor-immunoreactive cells in the mPFC with pyramidal neuron-like morphological features (e.g. “triangular cell bodies with apical and basal neurites”) also showed immunoreactivity for μOR. In transfected HEK 293 cells, μOR and D1 receptors form a functionally-active hetero-oligomer complex (Juhasz et al, 2008) and in cultured mouse mPFC neurons, μOR stimulation potentiated cyclic AMP increases evoked by D1-selective agonists (Olianas et al, 2012). Interestingly, the same study found that μOR stimulation in the absence of DA or D1 agonists had no effect, showing that functional D1 signaling was required for the expression of μOR-elicited effects.
Finally, application of μOR agonists to PFC slices suppresses 5-HT2A agonist-induced pyramidal cell excitatory postsynaptic currents (EPSCs), an effect which may be due to interactions between μOR and 5-HT2A receptors on presynaptic thalamocortical glutamate terminals (Marek and Aghajanian, 1998). Regarding this function, μORs could possibly reshape activation patterns by de-emphasizing thalamic input relative to other incoming signals, including those of limbic origin (Baldo, 2016). Clearly, D1 receptors and μORs could interact at multiple loci, and additional work is needed to identify the cellular mechanisms underlying the behavioral effects observed in the present study. Nevertheless, regardless of the precise mechanism, the results of the present study demonstrate that cortical D1 receptor signaling plays an enabling or permissive role that allows μOR-elicited effects to be expressed.
A growing body of literature suggests that aberrant activity in select frontal areas, including the vmPFC and anterior cingulate cortex (ACC), contributes to behavioral deficits in a number of psychiatric disorders (Dong et al, 2016; Schienle et al, 2009; Uher et al, 2004). Some evidence suggests that these deficits could result from supernormal opioid transmission (Blasio et al, 2014; Love et al, 2009; Morganstern et al, 2012; Selleck et al, 2015). Accordingly, opioid antagonists have some degree of clinical efficacy across several disorders characterized by dysfunctional appetitive motivation (Cambridge et al, 2013; Mitchell et al, 2007; Volpicelli et al, 1992). However, their use in treating binge eating has shown mixed or limited success (McElroy et al, 2013; Ziauddeen et al, 2013), suggesting that we do not fully understand the complexities of the clinical efficacy of opioid antagonists. The present results indicate that manipulating D1 receptors could modulate the cortical effects of excess opioid transmission, suggesting a poly-drug strategy targeting both D1 and mu-opioid receptors to treat dysregulated appetitive motivation across multiple psychiatric disorders.
Supplementary Material
Highlights:
Intra-vmPFC DAMGO increased sucrose-reinforced progressive-ratio responding
Intra-vmPFC DAMGO augmented free-feeding and locomotor activity.
Simultaneous intra-vmPFC D1 receptor antagonism reversed DAMGO’s effects.
Results indicate vmPFC-u-opioid effects are dependent on D1R signaling.
5. ACKNOWLEDGEMENTS
The authors wish to thank Dr. Matthew Andrzejewski for providing assistance with programming for behavioral testing equipment.
6. FUNDING AND DISCLOSURE
This work was funded by National Institutes of Health (R01 grant MH074723), a University of Wisconsin-Madison Vilas Life Cycle Award, and research funds from the Graduate School of the University of Wisconsin-Madison (PI on these awards: Dr. Brian A. Baldo). Mr. Ryan Selleck was supported by a training grant from the National Institutes of Health (T32 GM007507) to the Neuroscience Training Program of the University of Wisconsin-Madison.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders : DSM-5, 5th edn. American Psychiatric Association: Washington, D.C., xliv, 947 p.pp. [Google Scholar]
- Baldo BA, 2016. Prefrontal Cortical Opioids and Dysregulated Motivation: A Network Hypothesis. Trends Neurosci. 39, 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G, 2002. Differential Expression of Motivational Stimulus Properties by Dopamine in Nucleus Accumbens Shell versus Core and Prefrontal Cortex. J. Neurosci 22, 4709–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G, 1997. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J. Neurosci 17, 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Arnsten AF, 2013. Psychostimulants and motivated behavior: arousal and cognition. Neurosci. Biobehav. Rev 37, 1976–1984. [DOI] [PubMed] [Google Scholar]
- Blasio A, Steardo L, Sabino V, Cottone P, 2014. Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict. Biol 19, 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambridge VC, Ziauddeen H, Nathan PJ, Subramaniam N, Dodds C, Chamberlain SR, et al. , 2013. Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people. Biol. Psychiatry 73, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E, 2002. Calcium signaling: a tale for all seasons. Proc. Natl. Acad. Sci. U.S.A 99, 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Berridge KC, 2017. Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc. Natl. Acad. Sci. U.S.A 114, E9125–E0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ST, Kelley AE, 1992. Evidence for opiate-dopamine cross-sensitization in nucleus accumbens: studies of conditioned reward. Brain Res. Bull 29, 675–680. [DOI] [PubMed] [Google Scholar]
- Dong D, Wang Y, Jackson T, Chen S, Wang Y, Zhou F, et al. , 2016. Impulse control and restrained eating among young women: Evidence for compensatory cortical activation during a chocolate-specific delayed discounting task. Appetite 105, 477–86. [DOI] [PubMed] [Google Scholar]
- Donnelly NA, Holtzman T, Rich PD, Nevado-Holgado AJ, Fernando AB, Van Dijck G, et al. , 2014. Oscillatory activity in the medial prefrontal cortex and nucleus accumbens correlates with impulsivity and reward outcome. PLoS One 9, e111300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Hill EL, Cauli B, Gibelin N, Kaneko T, Rossier J, et al. , 2007. Extensive overlap of muopioid and nicotinic sensitivity in cortical interneurons. Cereb. Cortex 17, 1948–1957. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ, 2005. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol 492, 145–177. [DOI] [PubMed] [Google Scholar]
- Galosi R, Hajnal A, Petyko Z, Hartmann G, Karadi Z, Lenard L, 2015. The role of catecholamine innervation in the medial prefrontal cortex on the regulation of body weight and food intake. Behav. Brain Res 286, 318–327. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR, 2002. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J. Neurophysiol 88, 3150–3166. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG, 1990. Feeding can enhance dopamine turnover in the prefrontal cortex. Brain Res. Bull 25, 975–979. [DOI] [PubMed] [Google Scholar]
- Juhasz JR, Hasbi A, Rashid AJ, So CH, George SR, O’Dowd BF, 2008. Mu-opioid receptor heterooligomer formation with the dopamine D1 receptor as directly visualized in living cells. Eur. J. Pharmacol 581, 235–243. [DOI] [PubMed] [Google Scholar]
- Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, DiLeone RJ (2014). Medial prefrontal D1 dopamine neurons control food intake. Nat. Neurosci 17, 248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine C, Gaspar P, 1998. Subpopulations of cortical GABAergic interneurons differ by their expression of D1 and D2 dopamine receptor subtypes. Brain Res. Mol. Brain Res. 58, 231–236. [DOI] [PubMed] [Google Scholar]
- Lewis BL, O’Donnell P, 2000. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D(1) dopamine receptors. Cereb. Cortex 10, 1168–1175. [DOI] [PubMed] [Google Scholar]
- Love TM, Stohler CS, Zubieta JK, 2009. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch. Gen. Psychiatry 66, 1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK, 1998. 5-Hydroxytryptamine-induced excitatory postsynaptic currents in neocortical layer V pyramidal cells: suppression by mu-opiate receptor activation. Neuroscience 86, 485–497. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova AI, Blom TJ, Crow SJ, Memisoglu A, Silverman BL, et al. , 2013. A placebo-controlled pilot study of the novel opioid receptor antagonist ALKS-33 in binge eating disorder. Int. J. Eat. Disord 46, 239–245. [DOI] [PubMed] [Google Scholar]
- Mena JD, Sadeghian K, Baldo BA, 2011. Induction of hyperphagia and carbohydrate intake by muopioid receptor stimulation in circumscribed regions of frontal cortex. J. Neurosci 31, 3249–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Tavares VC, Fields HL, D’Esposito M, Boettiger CA, (2007). Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology 32, 439–449. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Liang S, Ye Z, Karatayev O, Leibowitz SF, 2012. Disturbances in behavior and cortical enkephalin gene expression during the anticipation of ethanol in rats characterized as high drinkers. Alcohol 46, 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S, Pascal L, Sadeghian K, Baldo BA, 2013. Sweetened-fat intake sensitizes gammaaminobutyric acid-mediated feeding responses elicited from the nucleus accumbens shell. Biol. Psychiatry 73, 843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, 2003. Dopamine gating of forebrain neural ensembles. Eur. J. Neurosci 17, 429–435. [DOI] [PubMed] [Google Scholar]
- Olianas MC, Dedoni S, Onali P, 2012. Potentiation of dopamine D1-like receptor signaling by concomitant activation of delta- and mu-opioid receptors in mouse medial prefrontal cortex. Neurochem. Int 61, 1404–1416. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Kosheleff A, Maidment NT, Murphy NP, 2013. Decreased consumption of sweet fluids in mu opioid receptor knockout mice: a microstructural analysis of licking behavior. Psychopharmacology 229, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Jarkiewicz M, Szulczyk P, 2008. Modulation of Ca2+ channel current by mu opioid receptors in prefrontal cortex pyramidal neurons in rats. Acta Neurobiol. Exp. (Wars.) 68, 10–25. [DOI] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F, 2009. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex 19, 849860. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Hermann A, Vaitl D, 2009. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol. Psychiatry 65, 654–661. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Schmolke C, Musshoff F, Menzen M, Prohaska C, Madea B, 2003. Area-specific increased density of mu-opioid receptor immunoreactive neurons in the cerebral cortex of drug-related fatalities. Forensic Sci. Int 133, 204–211. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ, 2001. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc. Natl. Acad. Sci. U.S.A 98, 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleck RA, Lake C, Estrada V, Riederer J, Andrzejewski M, Sadeghian K, et al. , 2015. Endogenous opioid signaling in the medial prefrontal cortex is required for the expression of hungerinduced impulsive action. Neuropsychopharmacology 40, 2464–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong HJ, Carter AG, 2012. D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. J. Neurosci 32, 10516–10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM, 1998. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav. Neurosci 112, 678–694. [DOI] [PubMed] [Google Scholar]
- Taber MT, Zernig G, Fibiger HC, 1998. Opioid receptor modulation of feeding-evoked dopamine release in the rat nucleus accumbens. Brain Res. 785, 24–30. [DOI] [PubMed] [Google Scholar]
- Taki K, Kaneko T, Mizuno N, 2000. A group of cortical interneurons expressing mu-opioid receptorlike immunoreactivity: a double immunofluorescence study in the rat cerebral cortex. Neuroscience 98, 221–231. [DOI] [PubMed] [Google Scholar]
- Tanaka E, North RA., 1994. Opioid actions on rat anterior cingulate cortex neurons in vitro. J. Neurosci 14, 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK, 2004. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J. Neurosci 24, 1065210659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, et al. , 2004. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am. J. Psychiatry 161, 1238–1246. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF, 2007. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci 10, 376–384. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP, 1992. Naltrexone in the treatment of alcohol dependence. Arch. Gen. Psychiatry 49, 876–880. [DOI] [PubMed] [Google Scholar]
- Witkowski G, Szulczyk P, 2006. Opioid mu receptor activation inhibits sodium currents in prefrontal cortical neurons via a protein kinase A- and C-dependent mechanism. Brain Res. 1094, 92–106. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Chamberlain SR, Nathan PJ, Koch A, Maltby K, Bush M, et al. , 2013. Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol. Psychiatry 18, 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.