Supplemental Digital Content is available in the text.
Keywords: intensive care, risk factors, sepsis, trauma
Abstract
OBJECTIVES:
Overall outcomes for trauma patients have improved over time. However, mortality for postinjury sepsis has been reported to be unchanged. Estimate incidence of and risk factors for sepsis in ICU patients after major trauma and the association between sepsis, mortality, and clinical course.
DESIGN, SETTING, AND PATIENTS:
ICU in a large urban trauma center in Sweden with a well-developed trauma system. Retrospective cohort study of trauma patients admitted to the ICU for more than 24 hours were included.
MEASUREMENTS AND MAIN RESULTS:
Primary outcome measure was 30-day mortality. Secondary outcomes were 1-year mortality and impact on clinical course. In total, 722 patients with a median Injury Severity Score of 26 (interquartile range, 18–38) were included. Incidence of sepsis was 22%. Septic patients had a four-fold increase in length of stay and need for organ supportive therapy. The overall 30-day mortality rate was 9.3%. After exclusion of early trauma-related deaths in the first 48 hours, the 30-day mortality rate was 6.7%. There was an association between sepsis and this adjusted 30-day mortality (day 3 odds ratio, 2.1 [95% CI, 1.1–3.9]; day 4 odds ratio, 3.1 [95% CI, 1.5–6.1]; day 5 odds ratio, 3.0 [95% CI, 1.4–6.2]). Septic patients had a 1-year mortality of 17.7% (nonseptic 11.0%). Development of sepsis was independently associated with age, spine and chest injury, shock, red cell transfusion, and positive blood alcohol concentration at admission. The risk of sepsis increased, in a dose-dependent manner, with the number of transfusions.
CONCLUSIONS:
Postinjury sepsis was associated with a complicated clinical course and with mortality after exclusion of early, trauma-related deaths.
With improved trauma care systems, more severely injured patients survive the initial insult (1, 2). These improvements are mainly due to decisive management of severe bleedings reducing hemorrhagic deaths, whereas mortality due to organ failure and sepsis seems unchanged or even increased (2, 3). As more severely injured patients survive the initial insult, the medical challenge is transferred to the later phase of care in the ICU. In this context, postinjury sepsis constitutes a serious complication that significantly contributes to morbidity and mortality. Although overall outcomes improve for trauma victims, mortality is reported to be unchanged for septic trauma patients (4, 5).
In this single-center study at a large urban trauma center study, we wanted to analyze the incidence, impact on outcomes, and risk factors for postinjury sepsis according to the new sepsis definition, Sepsis-3. We hypothesized that postinjury sepsis is associated with mortality and a complicated clinical course. The aim of this study was to estimate the incidence of postinjury sepsis in ICU patients and the association between postinjury sepsis and mortality and a complicated clinical course for these patients treated in a high-income country with a well-developed trauma system. We also wanted to evaluate potential risk factors for development of postinjury sepsis.
MATERIALS AND METHODS
Ethics
This study was approved by the regional ethical review board in Stockholm, Sweden (approval number 2008/249-31/3, amendment approval number 2009/862-32). The study adhered to the Streng thening the Reporting of Observational Studies in Epidemiology guidelines (6).
Setting
This retrospective cohort study of severely injured trauma patients was conducted at a mixed 13-bed ICU in the trauma center at the Karolinska University Hospital, Stockholm, Sweden. This is the referral center for severe trauma cases covering the largest urban region in Sweden with over 2 million inhabitants. The center is equivalent to a level-1 trauma center. Annually, approximately 1,500 adult patients are admitted to the trauma center. Of these patients, around 80% have indications for full trauma team activation, based on prespecified physiologic variables, anatomical injuries, and mechanism of injury. Approximately 300 admissions annually have an Injury Severity Score (ISS) greater than 15.
Study Population and Data Collection
The study cohort consisted of trauma patients admitted to the trauma ICU following initial resuscitation and, where indicated, interventional surgery. Patients 15 years old or older with an expected ICU length of stay (LOS) of more than 24 hours were included between February 2007 and November 2016. First, all data were entered into a database, by research nurses. Second, data were cross-checked and validated against the data sources (medical records) in retrospect by the researchers. If indicated, data were corrected in accordance with the source records. Trauma data such as ISS, mechanism of injury, admission blood pressure, and admission Glasgow Coma Scale (GCS) were retrieved from the hospital trauma register. Data on comorbidity were collected from the patient charts.
Outcomes
Patient data were collected up until ICU day 30, discharge or death, whichever occurred first. The primary outcome measure was overall 30-day mortality, including a censoring analysis for early deaths. Secondary outcome was long-term mortality, defined as 1-year mortality and impact on clinical course.
Definitions
Comorbidity data were classified according to the Charlson Comorbidity Index as adapted by Gabbe et al (7). Injury severity was defined with ISS, using the Abbreviated Injury Scale (AIS) Version 2005. Shock on arrival was defined as systolic arterial blood pressure of less than 90 mm Hg, and massive transfusion was defined as 10 or more units of packed RBCs (PRBCs) transfused in the first 24 hours. Trauma-induced coagulopathy (TIC) was defined as international normalised ratio greater than 1.2 at admission. Surgery within 24 hours was defined as surgical intervention involving a surgical team; thus, chest drains and suturing of minor injuries were not included. Sepsis was defined according to the new definition (Sepsis-3) as an increase in Sequential Organ Failure Assessment (SOFA) score of 2 or more in conjunction with an infection during the stay at the trauma ICU (8). Since trauma patients may have an inherently elevated SOFA score due to trauma, an increase in SOFA with greater than or equal to 2 points from the previous day was used in the Sepsis-3 definition. Infection was defined according to the international sepsis forum classification (9). Culture-positive sepsis was defined as a positive culture retrieved on the day of onset of sepsis. If the patient’s SOFA score returned to the offset level or if the infection resolved, the patient was no longer regarded as septic. SOFA total maximum was defined as the sum of the highest SOFA score seen in each domain during the ICU stay. Multiple organ dysfunction syndrome (MODS) was defined as greater than or equal to 6 SOFA points.
Statistics
Categorical data are presented as proportions and percentages. Continuous data are presented with median and interquartile ranges. Comparisons of proportions were performed using chi-square tests. Comparisons of continuous variables were performed using the Mann-Whitney U test.
Analyses of potential risk factors for sepsis were performed using univariate logistic regression. Variables in the model included age, sex, comorbidity, severe injury in eight AIS categories, penetrating trauma, shock on arrival, admission creatinine, blood alcohol level, PRBC transfusion, and surgery during the first 24 hours. Variables with a p value of less than 0.2 and sex were carried forward to the multivariable model. Data are presented as odds ratios (ORs) with corresponding 95% CIs.
Kaplan-Meier survival curves were plotted for 30-day postinjury survival for septic and nonseptic patients. Analysis of 30-day mortality was performed with univariate logistic regression and presented as OR with corresponding 95% CIs. In order to account for the competing risk of early trauma-related deaths before being at risk for sepsis, a temporal analysis was made by consecutive censoring of patients dying on day 1 and forward. Analyses of risk of death were made for each censoring step. A logistic regression analysis of the risk of postinjury sepsis in relation to the number of PRBCs during the first 24 hours was also performed.
All reported p values are two-sided, and p values less than 0.05 were considered statistically significant. Stata/MP 14.2 (StataCorp, College Station, TX) was used for all analyses.
RESULTS
Patients
The study population consisted of 722 trauma patients admitted to the ICU (for flowchart of included study patients, see Supplemental Fig. 1, http://links.lww.com/CCX/A727).
Admission Data
Admission characteristics in all, nonsepsis, and sepsis patients are shown in Table 1. Septic patients, nonseptic patients, and patients dying within 24 hours are depicted separately in Supplemental Table 1 (http://links.lww.com/CCX/A728). The cohort of ICU admitted trauma victims had a median age of 41 with a male dominance (78%). A quarter of the patients had pre-existing medical conditions as defined by Charlson Comorbidity Index. Road traffic accidents and falls dominated the injury mechanisms. The study patients were severely injured with a median ISS of 26 and over 80% with an ISS greater than 15. One sixth of patients was in shock on arrival, 17% received massive transfusion. The proportion of patients with penetrating injury was 12%, and TIC at admission was seen in 16% of the patients. About half of the cohort had surgery within the first 24 hours.
TABLE 1.
Admission Data
| Factor | Level | All | Missing (n) | Nonsepsis | Sepsis |
|---|---|---|---|---|---|
| N, n (%) | 722 | 564 (78) | 158 (22) | ||
| Age, median (interquartile range) | 41 (28–58) | 0 | 39 (27–56) | 47 (31–63) | |
| Sex, n (%) | Female | 161 (22) | 0 | 129 (23) | 32 (20) |
| CCI > 0, n (%) | 166 (23) | 0 | 122 (22) | 44 (28) | |
| CCI, points, median (interquartile range) | 0 (0–0) | 0 | 0 (0–0) | 0 (0–1) | |
| Injury mechanisms, n (%) | 0 | ||||
| Traffic | 302 (42) | 0 | 229 (41) | 73 (46) | |
| Fall | 123 (17) | 0 | 96 (17) | 27 (17) | |
| Self-inflicted | 120 (17) | 0 | 89 (16) | 31 (20) | |
| Assault | 86 (12) | 0 | 75 (13) | 11 (7) | |
| Others | 91 (13) | 0 | 75 (13) | 16 (10) | |
| Intubated at scene, n (%) | 140 (19) | 0 | 103 (18) | 37 (23) | |
| ISS, median (interquartile range) | 26 (18–38) | 2 | 24 (17–35) | 34 (24–43) | |
| ISS > 15, n (%) | 605 (84) | 2 | 460 (82) | 145 (92) | |
| AIS head > 2, n (%) | 294 (41) | 0 | 223 (40) | 71 (45) | |
| AIS face > 2, n (%) | 20 (3) | 0 | 14 (3) | 6 (4) | |
| AIS neck > 2, n (%) | 42 (6) | 0 | 32 (6) | 10 (6) | |
| AIS spine > 2, n (%) | 175 (24) | 0 | 115 (20) | 60 (38) | |
| AIS upper extremity > 2, n (%) | 36 (5) | 0 | 25 (4) | 11 (7) | |
| AIS thorax > 2, n (%) | 421 (58) | 0 | 311 (55) | 110 (70) | |
| AIS abdomen > 2, n (%) | 178 (25) | 0 | 125 (22) | 53 (34) | |
| AIS lower extremity > 2, n (%) | 233 (32) | 0 | 166 (29) | 67 (42) | |
| Penetrating trauma, n (%) | 88 (12) | 0 | 75 (13) | 13 (8) | |
| Shock on arrival, n (%) | 115 (16) | 9 | 67 (12) | 48 (30) | |
| Admission systolic arterial blood pressure, median (interquartile range) | 122 (103–148) |
9 | 126 (109–150) | 110 (84–135) | |
| Admission Glasgow Coma Scale, median (interquartile range) | 13 (8–15) | 59 | 14 (8–15) | 11 (8–15) | |
| blood alcohol concentration > 0 mM, n (%) | 184 (27) | 33 | 138 (26) | 46 (31) | |
| Admission creatinine, median (interquartile range) | 92 (77–112) | 34 | 91 (75–110) | 99 (84–119) | |
| Admission trauma-induced coagulopathy, n (%) | 105 (16) | 76 | 72 (14) | 33 (23) | |
| Massive transfusion, n (%) | 125 (17) | 0 | 76 (14) | 49 (31) | |
| Number of packed RBCs 24 hr | 2 (0–7) | 0 | 1 (0–5) | 5 (0–11) | |
| Total fluid load 24 hr, median (interquartile range) | 5,550 (3,400–8,800) | 0 | 5,086 (3,084–8,219) | 7,810 (4,750–12,100) | |
| Surgery first 24 hr, n (%) | 378 (52) | 0 | 286 (51) | 92 (58) | |
| Acute and Chronic Health Evaluation II, median (interquartile range) | 15 (11–21) | 0 | 14 (10–20) | 18 (14–23) | |
| Admission Sequential Organ Failure Assessment, median (interquartile range) | 5 (3–7) | 0 | 5 (3–7) | 7 (5–9) |
AIS = Abbreviated Injury Scale, CCI = Charlson Comorbidity Index, ISS = Injury Severity Score.
Missing data depicted as (n). Admission refers to the admission to the trauma unit.
Postinjury Sepsis
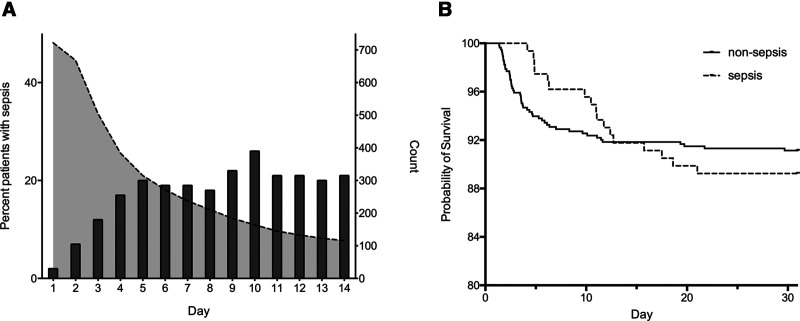
Sepsis was seen in 178 patients (22%) during their ICU stay. The daily prevalence of sepsis increased continuously during the first 5 days (Fig. 1A). These septic patients were older, more severely injured with an ISS over 15 observed in more than 90%. Severe chest, abdominal, spine, and lower extremity injuries were more common as were shock on arrival and massive transfusion. Patients later developing sepsis also had a higher admission creatinine and a two-fold higher incidence of TIC. No differences were seen in sex, injury mechanisms, intubation at scene, severe head injury, or surgery within 24 hours (Table 1). Information infectious localization and bacterial identification are depicted in Supplementary Table 2 (http://links.lww.com/CCX/A729).
Figure 1.
A, Daily relative prevalence of sepsis. Relative daily prevalence of sepsis among ICU admitted patients (bars, left y-axis) and number of ICU admitted patients (dotted line, right y-axis). B, Thirty-day postinjury survival. Kaplan-Meier curves displaying 30-d postinjury survival for nonsepsis (n = 564, solid line) and sepsis patients (n = 158, dotted line).
Risk Factors for Postinjury Sepsis
In the univariate logistic regression, age; severe spine, chest, abdominal, and lower extremity injuries; shock on arrival; admission creatinine; and PRBC transfusion were associated with postinjury sepsis development (Table 2). All variables with a p value of less than 0.2 as well as sex were used in the multivariable analysis. In the adjusted analysis, age, spine and chest injury, shock on arrival, positive blood alcohol, and PRBC transfusion were associated with later sepsis development. A trend to a lower risk from penetrating injury was also noted.
TABLE 2.
Univariate and Multivariable Analysis of Risk Factors for Postinjury Sepsis
| Factor | Univariate OR (95% CI) | p | Multivariable OR (95% CI) | p |
|---|---|---|---|---|
| Age (continuous) | 1.01 (1.01–1.02) | 0.002 | 1.02 (1.01–1.03) | 0.002 |
| Male sex | 1.2 (0.8–1.8) | 0.485 | 1.3 (0.7–2.1) | 0.390 |
| Charlson Comorbidity Index > 0 | 1.4 (0.9–2.1) | 0.102 | 1.1 (0.7–1.8) | 0.676 |
| AIS head > 2 | 1.2 (0.9–1.8) | 0.223 | ||
| AIS face > 2 | 1.6 (0.6–4.1) | 0.377 | ||
| AIS neck > 2 | 1.1 (0.5–2.3) | 0.756 | ||
| AIS spine > 2 | 2.4 (1.6–3.5) | < 0.001 | 2.0 (1.3–3.2) | 0.002 |
| AIS upper extremity > 2 | 1.6 (0.8–3.4) | 0.200 | ||
| AIS chest > 2 | 1.9 (1.3–2.7) | 0.001 | 1.6 (1.0–2.4) | 0.047 |
| AIS abdomen > 2 | 1.8 (1.2–2.6) | 0.004 | 1.4 (0.9–2.3) | 0.139 |
| AIS lower extremity > 2 | 1.8 (1.2–2.5) | 0.002 | 1.5 (0.9–2.3) | 0.088 |
| Penetrating trauma | 0.6 (0.3–1.1) | 0.088 | 0.5 (0.2–1.1) | 0.087 |
| Shock on arrival | 3.2 (2.1–4.9) | < 0.001 | 2.0 (1.2–3.3) | 0.011 |
| Admission creatinine > 100 mM | 1.8 (1.2–2.5) | 0.003 | 1.4 (0.9–2.2) | 0.109 |
| Blood alcohol concentration > 0 mM | 1.3 (0.9–2.0) | 0.175 | 1.8 (1.2–2.9) | 0.010 |
| Packed RBC first 24 hr (continuous) | 1.06 (1.04–1.08) | < 0.001 | 1.04 (1.01–1.06) | 0.005 |
| Surgery first 24 hr | 1.4 (0.95–1.9) | 0.095 | 1.0 (0.6–1.5) | 0.924 |
AIS = Abbreviated Injury Scale, OR = odds ratio.
Admission refers to the admission to the trauma unit. Variables with a p < 0.2 in the univariate analysis and sex forwarded to the multivariable analysis.
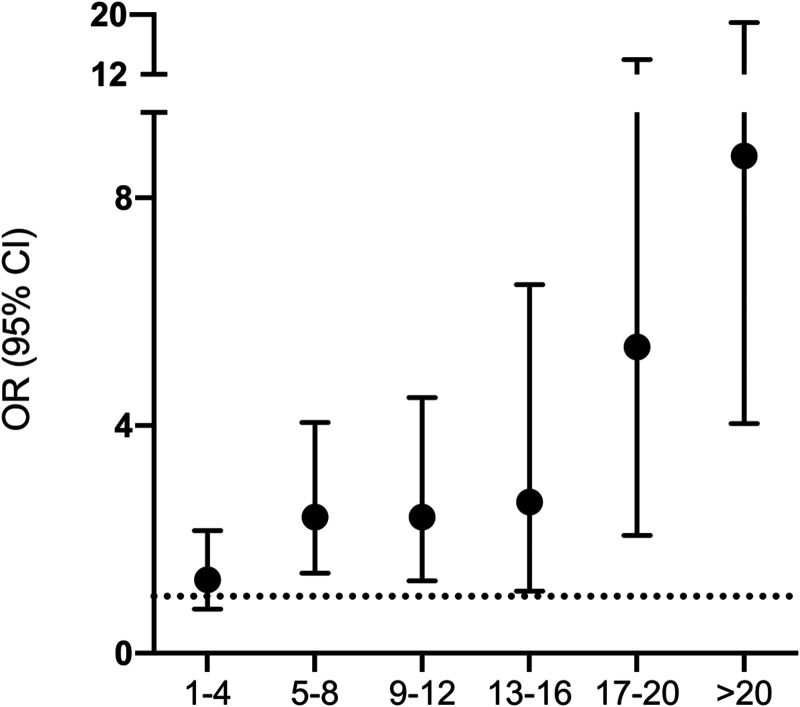
There was an association between transfusions and later development of sepsis. The median number of PRBCs during the first 24 hours was five-fold higher in septic patients, and massive transfusion was almost three times more common (Table 1). In a separate analysis, the risk of sepsis increased in a dose-dependent manner with the number of transfused PRBCs (Fig. 2).
Figure 2.
Analyses of odds ratio for postinjury sepsis. Logistic regression analyses exploring odds ratio (OR) and 95% CIs for postinjury sepsis in relation to packed RBC (PRBC) unit transfusions. The x-axis depicts the number of PRBCs transfused during the first 24 hr of admission.
Clinical Course
Developing sepsis was associated with a notable difference in clinical course. Sepsis patients had more organ failure measured as SOFA total maximum and days with MODS (Table 3). Furthermore, the proportion of patients with MODS greater than or equal to 2 consecutive days was two-fold higher in the sepsis group. The number of days on vasopressor treatment, mechanical ventilation, and continuous renal replacement therapy were increased several-fold. The median ICU LOS was 13 days compared with less than 3 days for nonseptic patients (Table 3).
TABLE 3.
Clinical Course and Outcomes
| Factor | Nonsepsis | Sepsis | p Nonsepsis vs Sepsis |
|---|---|---|---|
| N | 564 | 158 | |
| Sequential Organ Failure Assessment total maximum, median (interquartile range) | 6 (4–9) | 11 (9–14) | < 0.001 |
| MODS days, median (interquartile range) | 1 (0–3) | 8 (4–13) | < 0.001 |
| MODS ≥ consecutive 2 d, n (%) | 248 (44) | 138 (87) | < 0.001 |
| ICU days on vasopressor, median (interquartile range) | 1 (0–3) | 7 (4–12) | < 0.001 |
| ICU days on mechanical ventilation, median (interquartile range) | 2 (0–4) | 11 (7–19) | < 0.001 |
| ICU days on continuous renal replacement therapy, median (interquartile range) | 0 (0–0) | 0 (0–1) | < 0.001 |
| ICU LOS, median (interquartile range) | 2.8 (1.8–4.9) | 13 (8.0–20) | < 0.001 |
| Hospital LOS, median (interquartile range) | 14 (8–25) | 28 (17–57) | < 0.001 |
| ICU mortality, n (%) | 38 (6.7) | 12 (7.6) | 0.71 |
| Hospital mortality, n (%) | 47 (8.3) | 21 (13.3) | 0.059 |
| 30-d mortality, n (%) | 50 (8.9) | 17 (10.8) | 0.47 |
| 1-yr mortality, n (%) | 62 (11.0) | 28 (17.7) | 0.025 |
LOS = length of stay, MODS = multiple organ dysfunction syndrome (≥ 6 Sequential Organ Failure Assessment points).
One-yr follow-up was missing for one nonsepsis patient.
Mortality
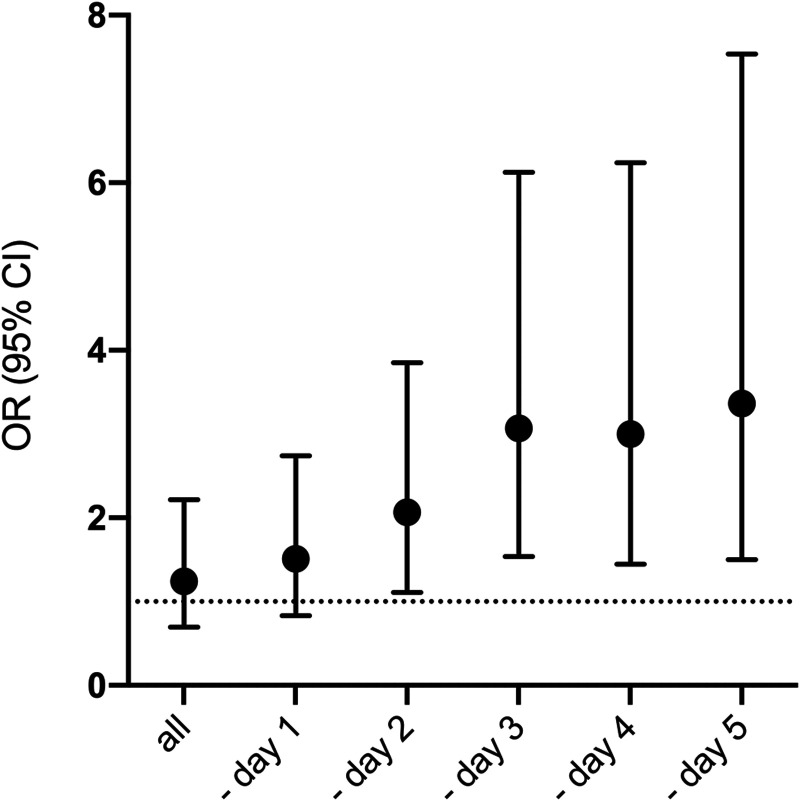
No significant difference was seen in short-term mortalities between septic and nonseptic patients (see Table 3 and Fig. 1B) even though a trend toward higher hospital mortality was observed in septic patients. However, when the competing risk of early trauma-related deaths before being at risk for sepsis was considered, a temporal analysis censoring patients who died in the early phase showed that sepsis was associated with death beyond day 2 (Fig. 3). Furthermore, 1-year mortality was higher in septic patients.
Figure 3.
Analyses of odds ratio for 30-d mortality. Logistic regression analyses exploring 30-d mortality consecutively censoring patients dying at the early stages. Odds ratio (OR) and 95% CIs for 30-d mortality. The x-axis depicts all patients and subsequently censoring patients dying on day 1 and on, up until day 5.
DISCUSSION
Key Findings
In this retrospective observational single-center study, we showed that postinjury sepsis was a common complication with notable effects on the clinical course. Development of sepsis was independently associated with age, spine and chest injury, shock, PRBC transfusion, and positive blood alcohol at admission. The risk of sepsis increased in a dose-dependent manner with the number of transfusions. Furthermore, sepsis was associated with 30-day mortality beyond day 2 as well as 1-year mortality. Septic patients had a significantly different clinical course with a several-fold increase in LOS, number of days on vasopressor treatment, mechanical ventilation, and renal replacement therapy.
Relationship to Previous Studies and Study Implications
A classical trimodal pattern of trauma death where septic death was seen in the late peak was described by Trunkey (10) in the 1980s. In ICU admitted trauma patients, a tetramodal pattern has been described (11). Later data suggest that the third peak, as described by Trunkey (10), is largely attenuated by improved trauma systems and critical care (12–15). However, more recent data suggest that improvements in outcomes over the last decade are largely attributed to a reduction in hemorrhagic deaths, whereas later death due to sepsis seems to be unchanged (2, 4, 5).
Some reports even indicate a relative increase in deaths due to organ failure and sepsis (3). Clearly, these changes are linked to some extent in that improved survival of exsanguinated patients may generate survivors that are more prone to develop later complications such as sepsis (16). These aspects were somewhat reflected in the current study where patients who became septic were significantly more likely to have been in shock and subject to massive transfusion during admission.
Sepsis is a well-recognized complication after major trauma. Apart from obvious causes such as bowel perforation and tissue contamination, there are several other suggested mechanisms that may promote sepsis. Emerging data point to the importance of damage-associated molecular patterns (DAMPs) released from injured and hypoperfused tissues affecting the immunologic balance (17, 18). Although purposeful in the short perspective for local tissue immune defense and wound healing, overwhelming levels of DAMPs may lead to immunosuppression (19). High plasma levels of DAMPs are associated with adverse outcomes in trauma (20, 21). Injury severity, shock, and massive transfusions, all risk factors for sepsis in the current and other studies, could arguably be related to high DAMP levels (4). We also noted a dose-dependent risk of sepsis with increased number of PRBCs. Transfusions have been shown to be associated with several postinjury complications (22, 23), including infections (24), a finding that raises the question of their role in the pathophysiology of subsequent sepsis development. There are several plausible mechanisms, including transfusion-induced DAMP release, that could explain such a link (17). To randomize patients to less transfusions, considering the present literature, is not a clinical option; thus, the questions on causality will remain highly difficult to address.
A third of the patient cohort had detectable blood alcohol levels at admission, this proved to be one of the stronger risk factors for sepsis development. This finding is somewhat in contrast to a recent large U.S. retrospective analysis in trauma patients showing an association between alcohol and pneumonia but not sepsis (25). This study, however, was not limited to ICU patients, and the mean ISS was only 13. Chronic alcohol abuse has been associated with an increased risk of acute respiratory distress syndrome and organ dysfunction where reduced levels of endogenous scavengers have been suggested as a potential mechanism (26, 27). There are no data on the extent of chronic alcohol abuse in our study.
The incidence of sepsis was 22% in the current study. Relating this figure to other studies should be done with some caution as the denominator varies considerably between studies. Various degrees of injury and different definitions of sepsis have been used. Incidences ranging from 2% to over 30% are found in the literature (23, 28, 29). Our cohort was severely injured and therefore expected to be prone to complications such as sepsis. On the other hand, we used the new Sepsis-3 definition which seems to generate fewer patients as compared with the earlier Sepsis-2 definition (30). About a third of the sepsis patients had a positive culture on day of onset of sepsis, where Staphylococcus aureus was the most common pathogen. The low rate of culture-positive sepsis is in part explained by the fact that only cultures obtained on day of onset of sepsis were accounted in order to keep a strict time association.
In contrast to some previous studies, sex proved not be associated with sepsis in the adjusted analysis. Several reports indicate that male gender is associated with worse outcomes in trauma in general and also a risk factor for postinjury sepsis (4, 31). It has been suggested that this may be due to protective effects from female sex hormones (31). However, data on this topic are far from conclusive in that several studies have failed to show any significant gender differences (32, 33). In our cohort, largely dominated by male gender, this finding is not surprising.
Although a trend toward increased grade of comorbidity was seen in septic patients, it was not a risk factor in the adjusted analysis. This finding is unexpected as pre-existing medical conditions are commonly associated with adverse outcomes in trauma (29). It might be explained by a significant mortality at the early stage of ICU care before these patients were at risk of becoming septic, and increased comorbidity may have contributed to these early deaths. The same circumstances may apply to severe head injury, not a risk factor for sepsis but possibly linked to early deaths.
The injury patterns associated with sepsis included spine and chest injuries. Severe spine injury, indicating advanced fractures or cord injury, had a fairly strong association with postinjury sepsis. Sepsis is a known complication among patients with spinal cord injuries (34); however, we have not found any previous data on the risk of sepsis in patients with spine injury in the ICU phase after trauma. Immobilization and prolonged mechanical ventilation may have contributed to the association for both spine and chest injuries in the current study. Surgery during the first 24 hours was not a risk factor for sepsis. This contrasts somewhat to previous studies showing that laparotomy is associated with sepsis after trauma (4). We have no data on the specific risk from laparotomy in our study.
The clinical course was markedly affected by sepsis with a several-fold increase in LOS and organ supportive therapy. This is well in line with previous studies showing that sepsis may increase LOS with more than a week (29, 35). Postinjury sepsis has been shown to increase mortality in several studies (4, 29) but was not associated with 30-day mortality in our study. However, in this severely injured cohort, there was a high initial mortality largely occurring before these patients had the possibility to develop sepsis. When censoring the analysis for early deaths, sepsis was significantly associated with mortality beyond day 2. In addition, 1-year mortality was higher in the sepsis subgroup.
Postinjury sepsis proved to be a serious complication with significant effects on outcomes. With more elaborated trauma care systems and improved initial survival of severely injured patients, this complication can be anticipated to increase in the future. Enhanced preventive measures and better tools for early identification are necessary to address this medical challenge.
Strengths
Strengths include data extraction from a high-resolution ICU trauma research database where all data were retrospectively validated by the researchers. Missing data were minimal for the used variables. There was no loss to follow-up regarding the endpoints, and all data were collected prospectively.
Limitations
First, this is a single-center study in a mature trauma system with patients admitted to ICU. Therefore, no generalization is possible. Second, not all eligible patients were included due to limited access to research staff. Thus, a potential selection bias cannot be excluded. The inclusion rate was, however, evenly distributed over time, possibly reducing bias. Third, the study was performed over a long time period, and a potential time bias due to unidentified changes of the trauma system over the time cannot be excluded. Fourth, this study reported on associations between sepsis and mortality only and not on causality; the association was identified retrospectively based on register data. Fifth, potential imprecision of certain complex variables such as ISS and GCS cannot be completely ruled out. Last but not least, the outcome mortality only partially describes the burden after trauma and therefore underestimates the impact on the patients and their relatives.
CONCLUSIONS
Postinjury sepsis was associated with a complicated clinical course, 1-year mortality, and the primary outcome, 30-day mortality, after censoring patients dying within the first 2 days.
ACKNOWLEDGMENTS
We are grateful to the research nurse Åsa Bengtsson and nurse Helena Nilsson as well as to Lena A. Jansson, Lisbet Bergendal, Katarina Ramsberg Enegren, and Tina Friberg at Trauma Registry Karolinska.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by The Swedish Carnegie Hero Funds and Karolinska Institute. Supported, in part, also through the regional agreement on medical and clinical research (ALF) between Stockholm County Council and Karolinska Institute.
The authors have disclosed that they do not have any conflicts of interest.
REFERENCES
- 1.DiMaggio C, Ayoung-Chee P, Shinseki M, et al. : Traumatic injury in the United States: In-patient epidemiology 2000-2011. Injury. 2016; 47:1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oyeniyi BT, Fox EE, Scerbo M, et al. : Trends in 1029 trauma deaths at a level 1 trauma center: Impact of a bleeding control bundle of care. Injury. 2017; 48:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Saverio S, Gambale G, Coccolini F, et al. : Changes in the outcomes of severe trauma patients from 15-year experience in a Western European trauma ICU of Emilia Romagna region (1996-2010). A population cross-sectional survey study. Langenbecks Arch Surg. 2014; 399:109–126 [DOI] [PubMed] [Google Scholar]
- 4.Wafaisade A, Lefering R, Bouillon B, et al. ; Trauma Registry of the German Society for Trauma Surgery: Epidemiology and risk factors of sepsis after multiple trauma: An analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery. Crit Care Med. 2011; 39:621–628 [DOI] [PubMed] [Google Scholar]
- 5.Eguia E, Bunn C, Kulshrestha S, et al. : Trends, cost, and mortality from sepsis after trauma in the United States: An evaluation of the national inpatient sample of hospitalizations, 2012-2016. Crit Care Med. 2020; 48:1296–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative: The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007; 370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 7.Gabbe BJ, Magtengaard K, Hannaford AP, et al. : Is the Charlson comorbidity index useful for predicting trauma outcomes? Acad Emerg Med. 2005; 12:318–321 [DOI] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calandra T, Cohen J; International Sepsis Forum Definition of Infection in the ICU Consensus Conference: The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005; 33:1538–1548 [DOI] [PubMed] [Google Scholar]
- 10.Trunkey DD: Trauma. Accidental and intentional injuries account for more years of life lost in the U.S. than cancer and heart disease. Among the prescribed remedies are improved preventive efforts, speedier surgery and further research. Sci Am. 1983; 249:28–35 [PubMed] [Google Scholar]
- 11.Gomes E, Araújo R, Carneiro A, et al. : Mortality distribution in a trauma system: From data to health policy recommendations. Eur J Trauma Emerg Surg. 2008; 34:561–569 [DOI] [PubMed] [Google Scholar]
- 12.Sobrino J, Shafi S: Timing and causes of death after injuries. Proc (Bayl Univ Med Cent). 2013; 26:120–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lansink KW, Gunning AC, Leenen LP: Cause of death and time of death distribution of trauma patients in a level I trauma centre in the Netherlands. Eur J Trauma Emerg Surg. 2013; 39:375–383 [DOI] [PubMed] [Google Scholar]
- 14.Gunst M, Ghaemmaghami V, Gruszecki A, et al. : Changing epidemiology of trauma deaths leads to a bimodal distribution. Proc (Bayl Univ Med Cent). 2010; 23:349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardes JM, Inaba K, Schellenberg M, et al. : The contemporary timing of trauma deaths. J Trauma Acute Care Surg. 2018; 84:893–899 [DOI] [PubMed] [Google Scholar]
- 16.Lord JM, Midwinter MJ, Chen YF, et al. : The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet. 2014; 384:1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vourc’h M, Roquilly A, Asehnoune K: Trauma-induced damage-associated molecular patterns-mediated remote organ injury and immunosuppression in the acutely ill patient. Front Immunol. 2018; 9:1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma SK, Naidu G: The role of danger-associated molecular patterns (DAMPs) in trauma and infections. J Thorac Dis. 2016; 8:1406–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timmermans K, Kox M, Vaneker M, et al. : Plasma levels of danger-associated molecular patterns are associated with immune suppression in trauma patients. Intensive Care Med. 2016; 42:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simmons JD, Lee YL, Mulekar S, et al. : Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg. 2013; 258:591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrington JS, Huh JW, Schenck EJ, et al. : Circulating mitochondrial DNA as predictor of mortality in critically ill patients: A systematic review of clinical studies. Chest. 2019; 156:1120–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fröhlich M, Lefering R, Probst C, et al. ; Committee on Emergency Medicine, Intensive Care and Trauma Management of the German Trauma Society Sektion NIS: Epidemiology and risk factors of multiple-organ failure after multiple trauma: An analysis of 31,154 patients from the TraumaRegister DGU. J Trauma Acute Care Surg. 2014; 76:921–927 [DOI] [PubMed] [Google Scholar]
- 23.Brattström O, Granath F, Rossi P, et al. : Early predictors of morbidity and mortality in trauma patients treated in the intensive care unit. Acta Anaesthesiol Scand. 2010; 54:1007–1017 [DOI] [PubMed] [Google Scholar]
- 24.Nederpelt CJ, El Hechi M, Parks J, et al. : The dose-dependent relationship between blood transfusions and infections after trauma: A population-based study. J Trauma Acute Care Surg. 2020; 89:51–57 [DOI] [PubMed] [Google Scholar]
- 25.Ahmed N, Greenberg P: Examining the influence of blood alcohol level on the incidence of pneumonia & sepsis complications following traumatic injury. Alcohol. 2019; 76:111–115 [DOI] [PubMed] [Google Scholar]
- 26.Moss M, Burnham EL: Chronic alcohol abuse, acute respiratory distress syndrome, and multiple organ dysfunction. Crit Care Med. 2003; 31:S207–S212 [DOI] [PubMed] [Google Scholar]
- 27.von Heymann C, Langenkamp J, Dubisz N, et al. : Posttraumatic immune modulation in chronic alcoholics is associated with multiple organ dysfunction syndrome. J Trauma. 2002; 52:95–103 [DOI] [PubMed] [Google Scholar]
- 28.Shalhub S, Junker CE, Imahara SD, et al. : Variation in the TLR4 gene influences the risk of organ failure and shock posttrauma: A cohort study. J Trauma. 2009; 66:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborn TM, Tracy JK, Dunne JR, et al. : Epidemiology of sepsis in patients with traumatic injury. Crit Care Med. 2004; 32:2234–2240 [DOI] [PubMed] [Google Scholar]
- 30.Eriksson J, Eriksson M, Brattström O, et al. : Comparison of the sepsis-2 and sepsis-3 definitions in severely injured trauma patients. J Crit Care. 2019; 54:125–129 [DOI] [PubMed] [Google Scholar]
- 31.Trentzsch H, Nienaber U, Behnke M, et al. : Female sex protects from organ failure and sepsis after major trauma haemorrhage. Injury. 2014; 45 Suppl 3:S20–S28 [DOI] [PubMed] [Google Scholar]
- 32.Magnotti LJ, Fischer PE, Zarzaur BL, et al. : Impact of gender on outcomes after blunt injury: A definitive analysis of more than 36,000 trauma patients. J Am Coll Surg. 2008; 206:984–991 [DOI] [PubMed] [Google Scholar]
- 33.Mushkudiani NA, Engel DC, Steyerberg EW, et al. : Prognostic value of demographic characteristics in traumatic brain injury: Results from the IMPACT study. J Neurotrauma. 2007; 24:259–269 [DOI] [PubMed] [Google Scholar]
- 34.Jaja BNR, Jiang F, Badhiwala JH, et al. : Association of pneumonia, wound infection, and sepsis with clinical outcomes after acute traumatic spinal cord injury. J Neurotrauma. 2019; 36:3044–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Böhmer AB, Just KS, Lefering R, et al. : Factors influencing lengths of stay in the intensive care unit for surviving trauma patients: A retrospective analysis of 30,157 cases. Crit Care. 2014; 18:R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.