Abstract
Purpose:
Real-time tumor tracking through active correction by the multileaf collimator or treatment couch offers a promising strategy to mitigate delivery uncertainty due to intrafractional tumor motion. This study evaluated the performance of MLC and couch tracking using the prototype iTools Tracking system in TrueBeam Developer Mode and the application for abdominal cancer treatments.
Methods:
Experiments were carried out using a phantom with embedded Calypso transponders and a motion simulation platform. Geometric evaluations were performed using a circular conformal field with sinusoidal traces and pancreatic tumor motion traces. Geometric tracking accuracy was retrospectively calculated by comparing the compensational MLC or couch motion extracted from machine log files to the target motion reconstructed from real-time MV and kV images. Dosimetric tracking accuracy was measured with radiochromic films using clinical abdominal VMAT plans and pancreatic tumor traces.
Results:
Geometrically, the root-mean-square errors for MLC tracking were 0.5 mm and 1.8 mm parallel and perpendicular to leaf travel direction, respectively. Couch tracking on the other hand showed an average of 0.8 mm or less geometric error in all directions. Dosimetrically, both MLC and couch tracking reduced motion-induced local dose errors compared to no tracking. Evaluated with five pancreatic tumor motion traces, the average 2%/2mm global gamma pass rate of eight clinical abdominal VMAT plans was 67.4% (range: 26.4% – 92.7%) without tracking, which was improved to 86.0% (range: 67.9% – 95.6%) with MLC tracking, and 98.1% (range: 94.9% – 100.0%) with couch tracking. In sixteen out of forty deliveries with different plans and motion traces, MLC tracking did not achieve clinically acceptable dosimetric accuracy with 3%/3mm gamma pass rate below 95%.
Conclusions:
This study demonstrated the capability of MLC and couch tracking to reduce motion-induced dose errors in abdominal cases using a prototype tracking system. Clinically significant dose errors were observed with MLC tracking for certain plans which could be attributed to the inferior MLC tracking accuracy in the direction perpendicular to leaf travel, as well as the interplay between motion tracking and plan delivery for highly modulated plans. Couch tracking outperformed MLC tracking with consistently high dosimetric accuracy in all plans evaluated, indicating its clinical potential in the treatment of abdominal cancers.
Keywords: Motion management, real-time tumor tracking, MLC tracking, couch tracking, abdominal cancers
I. Introduction
Intrafractional target motion is common during radiation therapy and affects most cancer sites. In particular, tumors in abdominal and thoracic cavities are shown to suffer greatly from respiration induced motion with variable motion characteristics1, 2. Depending on its amplitude, direction, and frequency, if not addressed, motion can cause significant uncertainty in dose delivery and compromise the treatment effectiveness. Clinically, the simplest and widely adopted method is to expand the treatment volume by incorporating a margin to encompass the tumor motion trajectory. However, the nearby healthy tissue and critical organs are subsequently exposed to higher doses of radiation, which may be counterproductive in hypofractionated and stereotactic treatments, as the steep dose gradients and dose escalation require tight dose conformity. Alternatively, continuous target detection and active motion compensation during treatment delivery can be used to minimize the doses to normal tissue and further improve dosimetric accuracy. Various methods are available for intrafraction target detection such as continuous radiographic imaging3–10. Non-radiographical motion monitoring includes using optical imaging systems to localize an external surrogate11–13, patient surface14, 15, or electromagnetic detection of implanted radiofrequency beacons16–18. Motion compensation can be achieved with several different techniques. A number of active motion management strategies during treatment delivery are used clinically, including the respiratory-gating method19–22 and the breath-hold techniques such as deep-inspiration-breath-hold (DIBH)23–25 and forced shallow-breathing26, 27. These techniques manage to reduce, but not eliminate, target motion during dose delivery by controlling the radiation beam on and off based on the patient’s respiration. They typically result in lower duty cycle, which lengthens the treatment time. While they are partially effective for targets with large respiration induced oscillations, motions with continuous baseline drift caused by changes in the filling status of gastrointestinal organs or by small shift in patient position due to muscle relaxation are difficult to compensate with the above techniques. Studies have shown that substantial amount of tumor motion could still be present during breath-hold such as for patients with pancreatic cancer28, 29. Furthermore, DIBH and forced shallow breathing require patient’s cooperation and may not be tolerated by some patients.
Different from gating and breath-hold techniques, real-time tumor tracking is a more desirable way to minimize motion effect in external beam radiotherapy30, 31. It aims to actively align radiation beam with the target at all times during delivery and does not involve patient’s cooperation. Currently in the clinic, this is achieved on dedicated systems such as the Cyberknife® (Accuracy Inc., Sunnyvale, CA)32–35 and the Vero gimbal tracking system (BrainLAB AG, Feldkirchen, Germany, Mitsubishi Heavy Industries, Ltd., Tokyo, Japan)36–41. However, both systems are used mostly for stereotactic radiosurgery (SRS) or stereotactic body radiation therapy (SBRT) treatments with relatively small field sizes. On a conventional linear accelerator, real-time motion compensation, i.e. realignment of the target with the radiation beam, can be achieved by adjusting the treatment beams with the MLC chasing the target as it moves, or by introducing counteracting couch motion. Compared to the aforementioned techniques, tracking could potentially offer higher delivery efficiency and less residual target motion, and it is applicable for a wider patient population with various cancer sites affected by target motion. MLC tracking and couch tracking with a conventional linear accelerator have been investigated42–52 but are not yet commercially available.
A recent multi-institutional study was published by Colvill et al comparing the dosimetric accuracy of non-adaptive radiotherapy with that of adaptive treatment for prostate and lung cases via various tracking modalities53. The study was conducted on various tracking systems including dedicated tracking units (i.e. Cyberknife and Vero), an in-house system, as well as a research prototype system. MLC and couch tracking performed similarly, both presenting significant dosimetric advantage compared to standard non-adaptive treatment. Hansen et al evaluated and compared the MLC and couch tracking performance using the Varian prototype tracking system on prostate and lung cases54. Similar to the findings from Colvill et al, they concluded the overall comparable dosimetric accuracy of MLC and couch tracking in lung cases, while MLC tracking slightly underperformed compared to couch tracking for prostate cases. A similar comparison study for prostate SBRT using the same system was reported by Ehrbar et al. where couch tracking showed generally better performance compared to MLC tracking55.
The vast majority of publications of MLC and couch tracking focus on lung and prostate cases, but limited studies have been reported for abdominal cancers56, which are also significantly affected by respiratory motion and other physiological motion in the gastrointestinal track. In this study, we evaluated the accuracy of both MLC and couch tracking for pancreas and liver diseases using Varian iTools Tracking.
II. Materials and methods
II.A. System description
The study was carried out on a TrueBeam accelerator (version 2.5) with Millenium 120-leaf MLC and a 6-degree-of-freedom treatment couch with Qfix kVue couchtop (Qfix, Avondale, PA). The Varian iTools Tracking is a research prototype software module available on TrueBeam (Varian Medical Systems Inc., Palo Alto, CA) in Developer Mode. It allows MLC tracking (with jaw tracking) and couch tracking. Both modes consider the target as a rigid body and correct for translational displacement of the target during treatment. Target rotation or deformation is not compensated in the current version. The tracking system acquires target position from either RPM (Varian Medical Systems, Palo Alto, CA) or Calypso (Varian Medical Systems, Palo Alto, CA) at a frequency of 24 Hz and uses a linear Kalman filter for motion prediction to compensate any system latency57, 58. Continuous MV or kV verification images can be collected during tracking delivery.
Several user-defined parameters are available in iTools Tracking, including the conformity index and capability ratio. The conformity index limits the total area (cm2) of over-exposure or under-exposure relative to the planned aperture at each moment of delivery and triggers beam-holds if the preset value is exceeded. The capability ratio, which applies to dynamic treatment with MLC tracking (i.e. MLC tracking for sliding window IMRT/VMAT treatments), specifies the percentage of maximum leaf speed reserved for the original plan delivery versus for compensatory tracking motion. For example, for a capability ratio of 100%, the plan delivery is prioritized with maximum possible leaf speed if needed. If a leaf is traveling at maximum speed for planned motion, target motion in the same direction would not be compensated and would result in tracking error. The capability ratio can be set <100% to reserve leaf speed for compensatory tracking motion but the treatment delivery time would be lengthened. For all motion traces and treatment plans used in this study, these control parameters were selected such that the amount of beam holdoff was minimized.
II.B. Experimental setup
Phantom experiments were carried out using the HexaMotion platform (ScandiDos Inc., Uppsala, Sweden), which allows programmable motion simulation with 5-degrees-of-freedom (3D translation and 2D rotation). A customized acrylic plank was fabricated to replace the Delta4 detector that is designed to be used in parallel with the HexaMotion. This customized plank provides phantom support in our experiments as shown in Figure 1. The phantom consists of two 15 cm × 15 cm × 5 cm Solid Water slabs, with two acrylic layers (16 cm × 16 cm × 2.54 cm) sandwiched between them. Three Calypso beacon transponders were embedded in the bottom acrylic slab. The embedded transponders served two purposes, i.e. tracking input for iTools Tracking and target identification for verification imaging. Calypso was operated under research mode and the output smoothing filter was disabled in this study to avoid additional latency.
Figure 1:
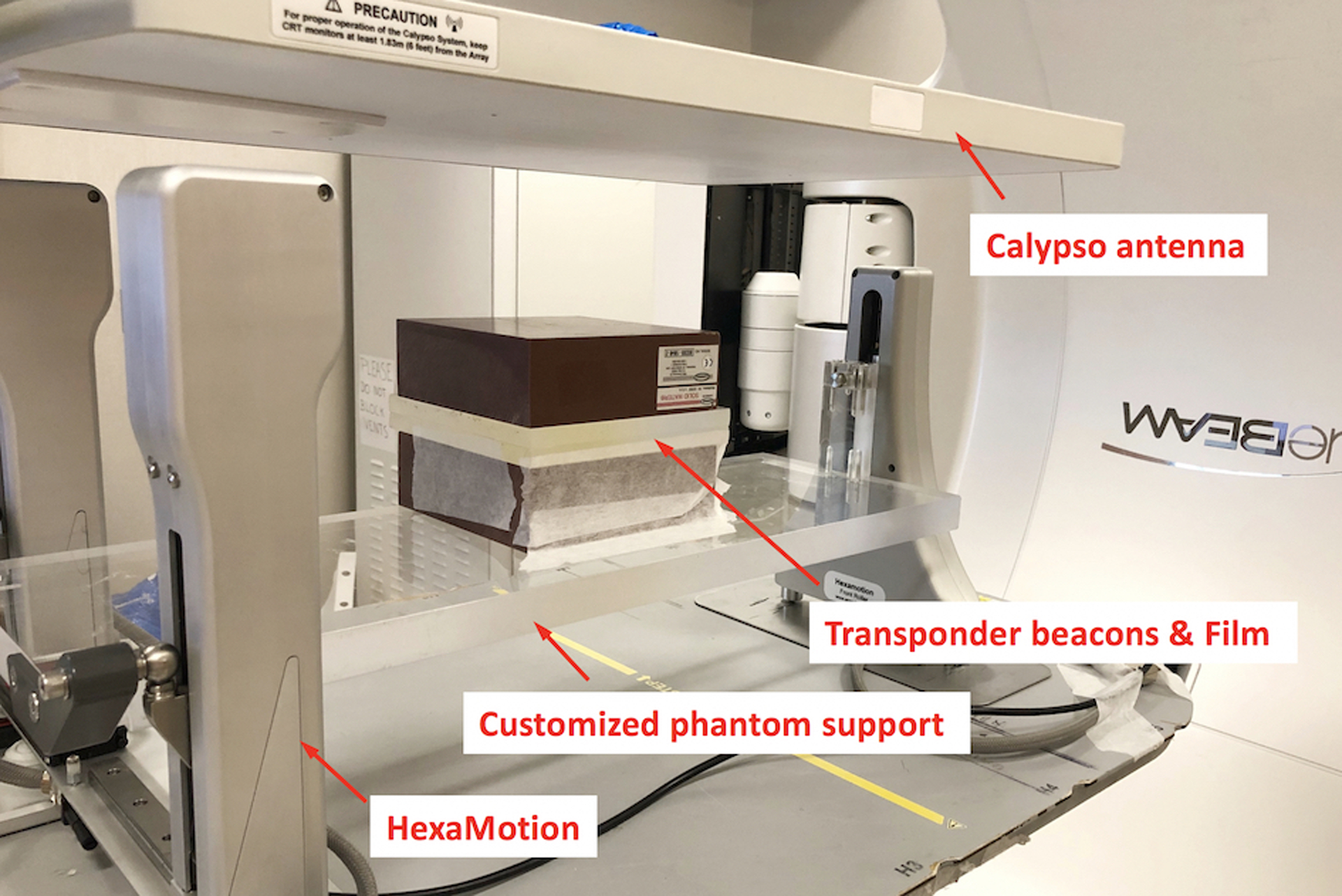
Experimental setup with HexaMotion and customized phantom support. The phantom was constructed with two acrylic plates and two slabs of Solid Water. Three Calypso transponder beacons were embedded in the bottom acrylic plate to guide tracking. Films were sandwiched between the acrylic layers for dose measurement.
II.C. System evaluation
The performances of MLC and couch tracking using iTools Tracking were evaluated in terms of geometric accuracy, dosimetric accuracy and system latency. These investigations required the knowledge of the accurate target position during the tracking deliveries. Since the Calypso log files were out of synchronization with TrueBeam machine time, in this study, target motion traces were reconstructed using verification images taken during deliveries. The workflow is illustrated in Figure 2. Reference template images of the beams-eye-view (BEV) were generated using the phantom CT scan10. MV and kV images were acquired during tracking deliveries using Varian iTools Capture. MV images were acquired in HiQual mode at 10 Hz with the 6X treatment beam and ~ 1 MU per image, whereas kV images were triggered at 15 Hz acquired with 100 kVp and 0.5 mAs. Using an in-house software10, the target motion was reconstructed retrospectively through image registration with the template images. Since the kV images showed better image quality and were acquired at a higher frequency compared to MV images, they were used whenever possible to provide a more accurate motion reconstruction. The acquired MV and kV images were in the Varian proprietary XIM format. The delivered MU at the instance of image acquisition (MVStartDose) was extracted from the image header. This information allowed us to map the reconstructed target motion to the time frame of treatment delivery recorded in trajectory log files (sampling rate: 50 Hz) and establish synchronized timestamps with the MLC or couch motion.
Figure 2:
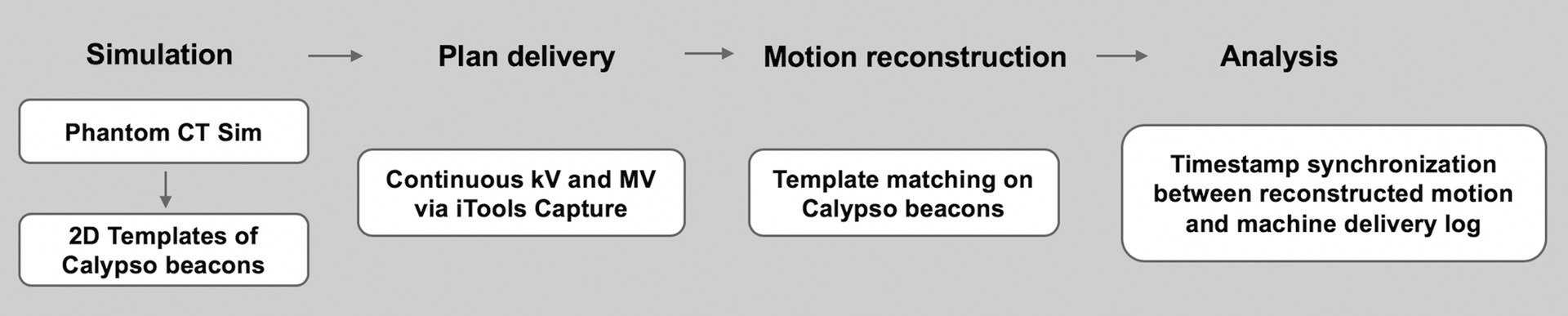
Process of geometric evaluation using MV and kV images acquired during deliveries
II.C.i. Geometric tracking accuracy
The geometric accuracies of MLC and couch tracking were evaluated using 1D sinusoidal motion traces along all three axes (period range: 3 s to 8 s, peak-to-peak amplitude: 1.5 cm), and two 3D pancreatic tumor motion traces collected from pancreatic cancer patients with implanted Calypso transponders previously treated at our institution. The maximum magnitudes of the tumor motion in these traces were 14 mm (S-I), 4.6 mm (L-R), and 5.2 mm (A-P), which were within the typical ranges reported previously2. For each trace a conformal plan with MLC-defined circular aperture of 10 cm in diameter (fixed gantry angle 0°, and collimator angle 90°) was delivered with MLC and couch tracking while the phantom was driven by the HexaMotion platform following the planned target motion. Jaw tracking was enabled during MLC tracking. The conformity index was set at 8 cm2 for both MLC and couch tracking (~10% of field size), and capability ratio of 100% for MLC tracking.
To compare the compensating MLC/couch motion with the target motion, three steps were taken in the retrospective analysis. 1) Target motion relative to the isocenter was reconstructed from the MV/kV images through template matching as described above. For deliveries with MLC tracking, the reconstructed motion was the original motion driven by the motion platform, whereas for deliveries with couch tracking the motion extracted from images was the residual motion after couch compensation, i.e., the geometric error of couch tracking; 2) MLC or couch positions (i.e. compensating motion during tracking) were extracted from the linac trajectory log files (sampling rate: 50 Hz); 3) Target motion and the compensating motion were compared after synchronizing the timestamps of the two.
The tracking performance was evaluated separately in each motion axis. The geometric tracking accuracy was quantified for MLC tracking and couch tracking in each direction, generalized as:
where GeoErrori is the geometric tracking error at any time instance t; TMi is the target motion reconstructed from MV/kV images, CMi is the compensating motion of the couch or MLC, and i represents the specific tracking direction. For MLC tracking, i is either parallel or perpendicular to leaf travel direction; for couch tracking, i is longitudinal, lateral or vertical direction.
For target that moved parallel to leaf travel, leaves in each bank in the conformal plan moved collectively to compensate for the target motion. The MLC compensating motion in this direction was directly extracted from the leaf motion in the log files subtracting the originally planned leaf positions. In the case where the target motion was perpendicular to leaf travel, MLC tracking was discretized with an intrinsic delay. MLC compensation would not occur until the target motion reached a threshold which was half the leaf width of the outermost leaf pair in the target motion direction. As illustrated in Figure 3, the entire field was adjusted along the target motion direction which involved opening leaf pairs adjacent to the original field (leaf pairs on the bottom of the field in frame 2, 3, and 4 in Figure 3) and closing leaf pairs on the other end (first three leaf pairs on top of the field in frame 1). This eventually resulted in a shift of the field, i.e. motion of the centroid of the aperture, perpendicular to the leaf travel direction. To calculate aperture shift, MLC apertures were reconstructed using the trajectory log files at 50 Hz for the entire tracking delivery. For each of these reconstructed apertures, the centroid was located and traced continuously throughout the delivery to determine the compensating motion as illustrated in Figure 3. For couch tracking, the target displacement reconstructed from the MV and kV images was the residual target displacement relative to the isocenter after couch correction at each instance, i.e. TMi − CMi. The magnitude of the residual motion was equal to that of the geometric error along each axis.
Figure 3:
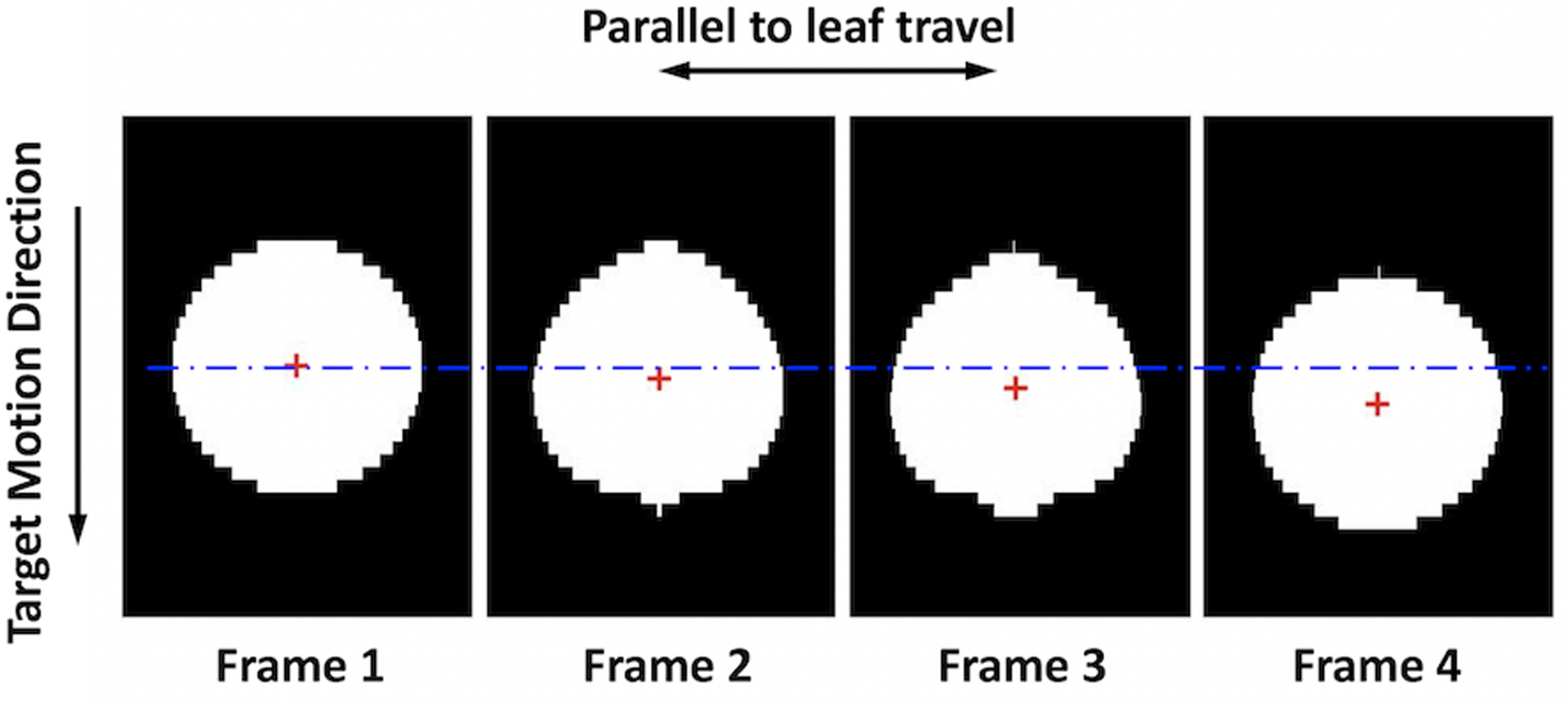
Illustration of the MLC aperture reconstructed from the trajectory log files. MLC tracks the target by shifting the field in the direction perpendicular to leaf travel. The compensation is quantified by the deviation of the aperture centroid (red crosses) from its original position (blue dash line) in the target motion direction.
The geometric tracking accuracy for each delivery was quantified by the root-mean-square (RMS) of GeoError. For deliveries without tracking, i.e. CM = 0, the geometric tracking accuracy was the RMS of the excursion from initial position, i.e. motion amplitude relative to zero, based on target motion reconstructed from MV/kV images.
II.C.ii. Tracking system latency
The tracking system latency usually refers to the time delay between target motion and MLC or couch correction, which includes the latency associated with the target localization using Calypso, the time required to re-fit the MLC to adapt for motion, and the time it takes for MLC or couch to reach the anticipated positions. In this study, the tracking latency was evaluated using the same sinusoidal traces and the circular conformal field and with the Kalman filter disabled. After synchronizing the timestamps of the reconstructed target motion and the compensating motion of MLC or couch, the latency was obtained by applying cross-correlation function of the two for each delivery (i.e. for each motion trace). Each compensating axis was evaluated separately, and three measurements were taken for each motion trace and each compensation mode. MLC tracking perpendicular to leaf travel direction was not included in this part of evaluation due to the intrinsic latency, i.e. half leaf width motion threshold to trigger tracking.
II.C.iii. Dosimetric evaluation
Dosimetric accuracy of the tracking system was investigated with eight clinical VMAT plans for abdominal cancer treatment including four pancreas cases and four liver cases. Details of the treatment plans are listed in Table 1. These clinical plans were previously treated and generated based on departmental guidelines at our institution. Each plan consisted of either two full or two partial arcs and the collimator angles for the arcs were 0° and 90°. All pancreas plans were SBRT with 3 to 5 fractions. The liver plans were moderate-hypofractionated, and two of them had simultaneous integrated boost with two to three prescribed dose levels. Target volumes ranged from 22 cc to 284 cc. Five 3D tumor traces from patients previously treated for pancreas cancer with implanted Calypso beacons were used in the dosimetric evaluation, as shown in Figure 4 (more details of the motion characteristics for each trace can be found in Table S1 in the supplementary document). Dose measurements were performed using GafChromic EBT3 films (Ashland Advanced Materials LLC, NJ). Each treatment plan was delivered four times with different motion management strategies: 1) reference delivery without target motion; 2) delivery with target motion and no tracking; 3) delivery with target motion and MLC tracking; and 4) delivery with target motion and couch tracking. In each delivery, one EBT3 film was sandwiched between the two acrylic slabs of the phantom setup described in section II B, capturing the 2D dose distribution of the coronal plane. The position of isocenter was marked on the film before each delivery and was later used for registration and dose comparison. Capability ratio was 100% for MLC tracking; the conformity index was 8 or 10 cm2 for MLC tracking, and 10 cm2 for couch tracking. The conformity indexes were chosen to minimize beam holdoff. Film calibration was carried out for each box of films used in this study on the day of experiments. Films were scanned using the Epson Expression 11000XL flatbed scanner (Epson Seiko Corporation, Japan) at least 24 hours after exposure, and the calibration curve was obtained using the software FilmQA Pro (Ashland Advanced Materials LLC, NJ). Dose analysis was performed using an in-house software where dose distributions were compared between deliveries with different tracking strategies and the reference delivery, i.e. delivery without target motion. Global gamma was calculated with 2%/2mm and 3%/3mm criteria and 20% threshold. Average gamma passing rate of 95% (3%/3mm) for each individual plan was deemed clinically acceptable.
Table 1.
Details of the patient plans used in the dosimetric evaluation. Modulation factor is defined as the ratio of total monitor units and prescribed dose.
| Plan ID | Target Size | Modulation Factor (MU/cGy) | Dose/Fx (cGy) | # Fx |
|---|---|---|---|---|
| Pancreas 1 | 60.5 cc | 3.4 | 660 | 5 |
| Pancreas 2 | 46.2 cc | 2.2 | 900 | 3 |
| Pancreas 3 | 74.3 cc | 3.0 | 660 | 5 |
| Pancreas 4 | 37.4 cc | 3.0 | 660 | 5 |
| Liver 1 | 283.8 cc | 3.3 | 300/450/500 | 15 |
| Liver 2 | 162.98 cc | 2.8 | 450 | 15 |
| Liver 3 | 80.34 cc | 2.8 | 450/600 | 15 |
| Liver 4 | 22.32 cc | 4.0 | 450 | 15 |
Figure 4:
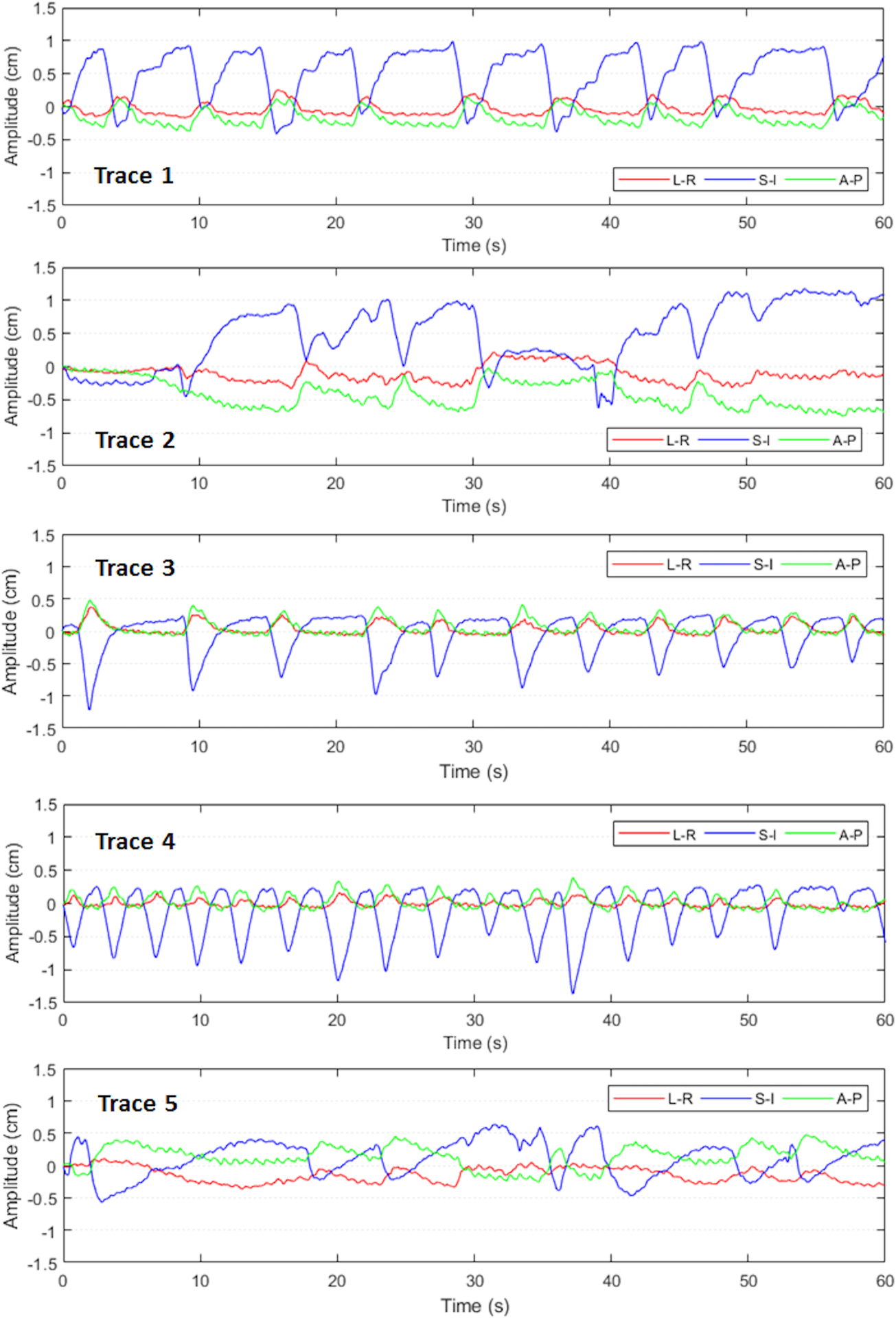
Patient traces used in dosimetric evaluation representing different motion characteristics in abdominal cancers.
III. Results
III.A. Geometric tracking accuracy
The geometric tracking accuracy for both tracking modalities based on the conformal plan with MLC-defined circular aperture and 1D sinusoidal motion traces are summarized in Table 2. Tracking performance was highly reproducible with multiple deliveries but varied among different axes for both MLC and couch tracking. Couch tracking achieved better than 1.6 mm accuracy with all motion periods, although the three couch axes performed differently; this may be related to the difference in couch dynamics among the three motion axes. MLC tracking performance was inferior for motion perpendicular to leaf travel direction (geometric error ranges from 1.5 – 3.2 mm), where it involved constant opening and closing adjacent leaf pairs outside the original plan. Additional leaf pairs were called upon only if the excursion exceeded half leaf width of the corresponding MLC leaf. Hence, tracking in this direction was discretized as illustrated in Figure 5. Tracking error increased as the motion period decreased for each direction of both MLC and couch tracking. This was caused by the MLC or couch overshoot, as the target experienced large acceleration or deceleration, i.e. the peak and valley regions of the sine waveforms (see Figure 6). The greater the acceleration, the larger was the overshoot. This overshoot was result from both errors in target position prediction from the Kalman filter and the inertial limitation of the MLC and couch.
Table 2.
Root-Mean-Square of geometric tracking errors (unit: mm) for MLC and couch tracking with sinusoidal motion traces and patient traces. All sinusoidal motion traces have the same peak-to-peak amplitude of 1.5 cm.
| Motion Trace | No Tracking | Couch Tracking | MLC Tracking | |||||
|---|---|---|---|---|---|---|---|---|
| Longitudinal | Lateral | Vertical | Longitudinal | Lateral | Vertical | Parallel to Leaf Travel | Perpendicular to Leaf Travel | |
| Sine 1 (Ts = 3 s) | 5.3 | 5.3 | 5.3 | 1.0 | 1.3 | 1.6 | 0.8 | 3.2 |
| Sine 2 (Ts = 4 s) | 5.3 | 5.3 | 5.3 | 0.6 | 0.9 | 0.8 | 0.5 | 2.3 |
| Sine 3 (Ts = 5 s) | 5.3 | 5.3 | 5.3 | 0.4 | 0.6 | 0.5 | 0.4 | 1.8 |
| Sine 4 (Ts = 6 s) | 5.3 | 5.3 | 5.3 | 0.4 | 0.7 | 0.5 | 0.2 | 1.6 |
| Sine 5 (Ts = 7 s) | 5.3 | 5.3 | 5.3 | 0.3 | 0.7 | 0.4 | 0.2 | 1.6 |
| Sine 6 (Ts = 8 s) | 5.3 | 5.3 | 5.3 | 0.3 | 0.6 | 0.3 | 0.2 | 1.5 |
| Patient trace 1 | 4.0 | 1.2 | 1.4 | 1.0 | 0.7 | 0.7 | 0.7 | 1.2 |
| Patient trace 2 | 6.3 | 0.9 | 2.1 | 0.8 | 0.7 | 0.7 | 0.6 | 0.9 |
Figure 5:
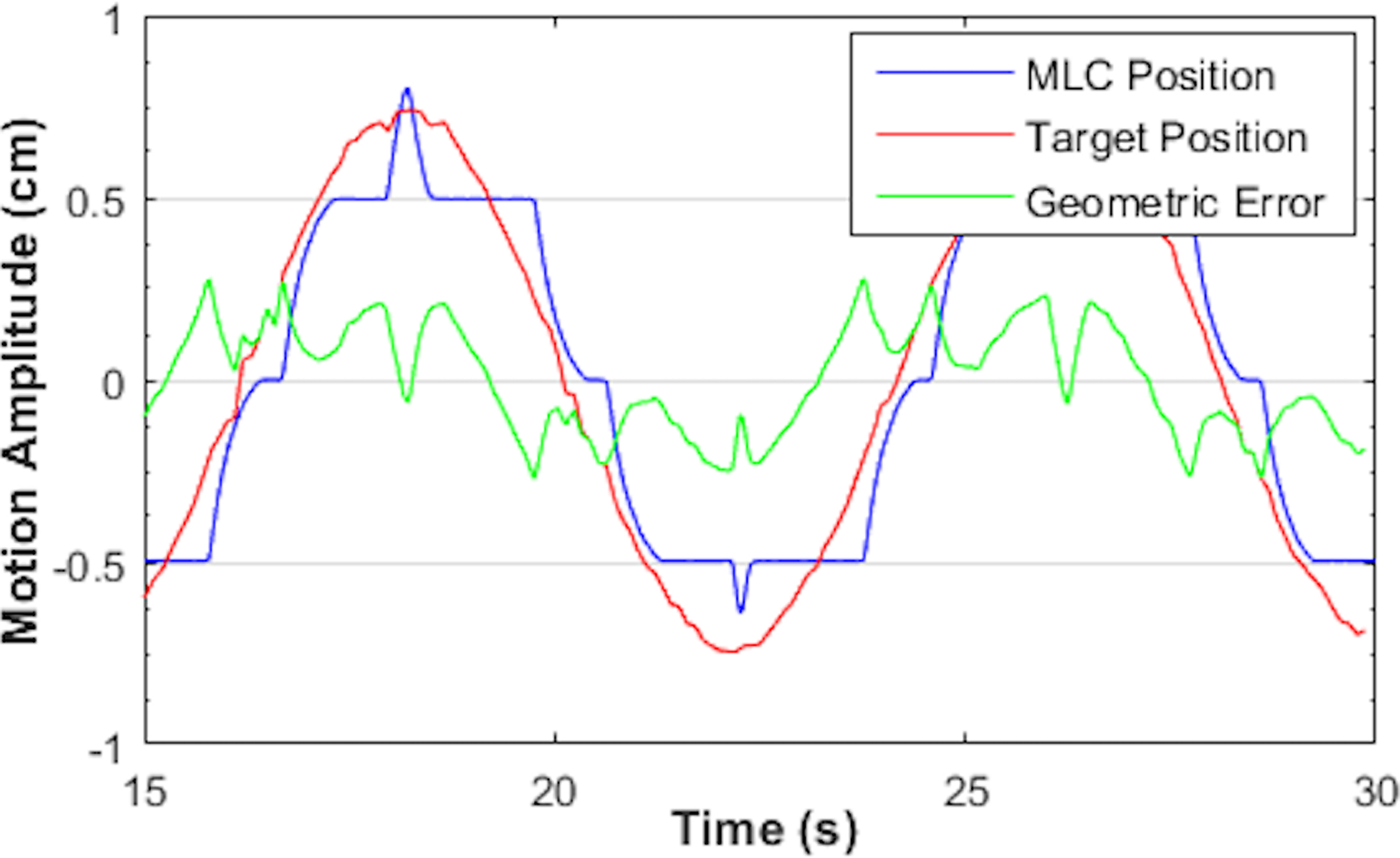
MLC tracking with sinusoidal motion perpendicular to leaf travel direction. Motion period Ts = 8 s. The aperture was defined by MLC leaf pairs located near the central axis with leaf width of 5 mm.
Figure 6:
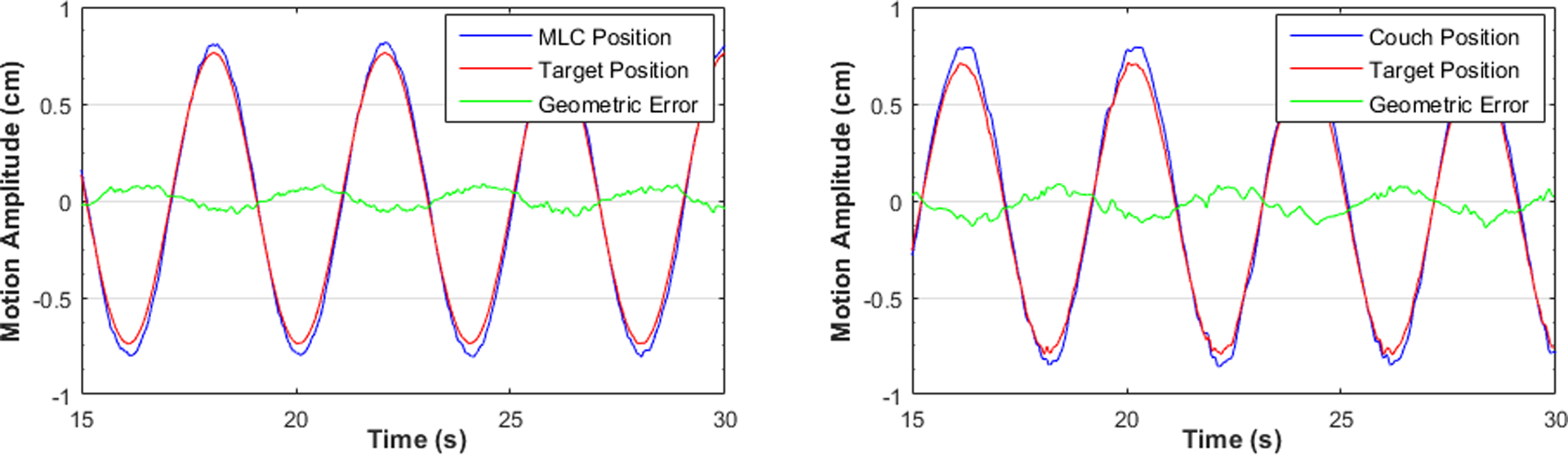
MLC tracking (left) and couch tracking (right) with sinusoidal motion parallel to leaf travel direction and the longitudinal axis of the couch. Motion period Ts = 4 s.
With 3D pancreatic tumor traces, the RMS geometric errors for MLC tracking were 0.7 mm and 1.1 mm, parallel and perpendicular to leaf travel direction, respectively. Couch tracking yielded comparable results, with 0.9 mm along longitudinal axis, 0.7 mm along lateral axis, and 0.7 mm along vertical axis. Figure 7 and Figure 8 illustrate the compensatory motion of MLC and couch along each axis when tracking a pancreatic tumor motion trace as well as the tracking errors over the delivery of 60 seconds.
Figure 7:
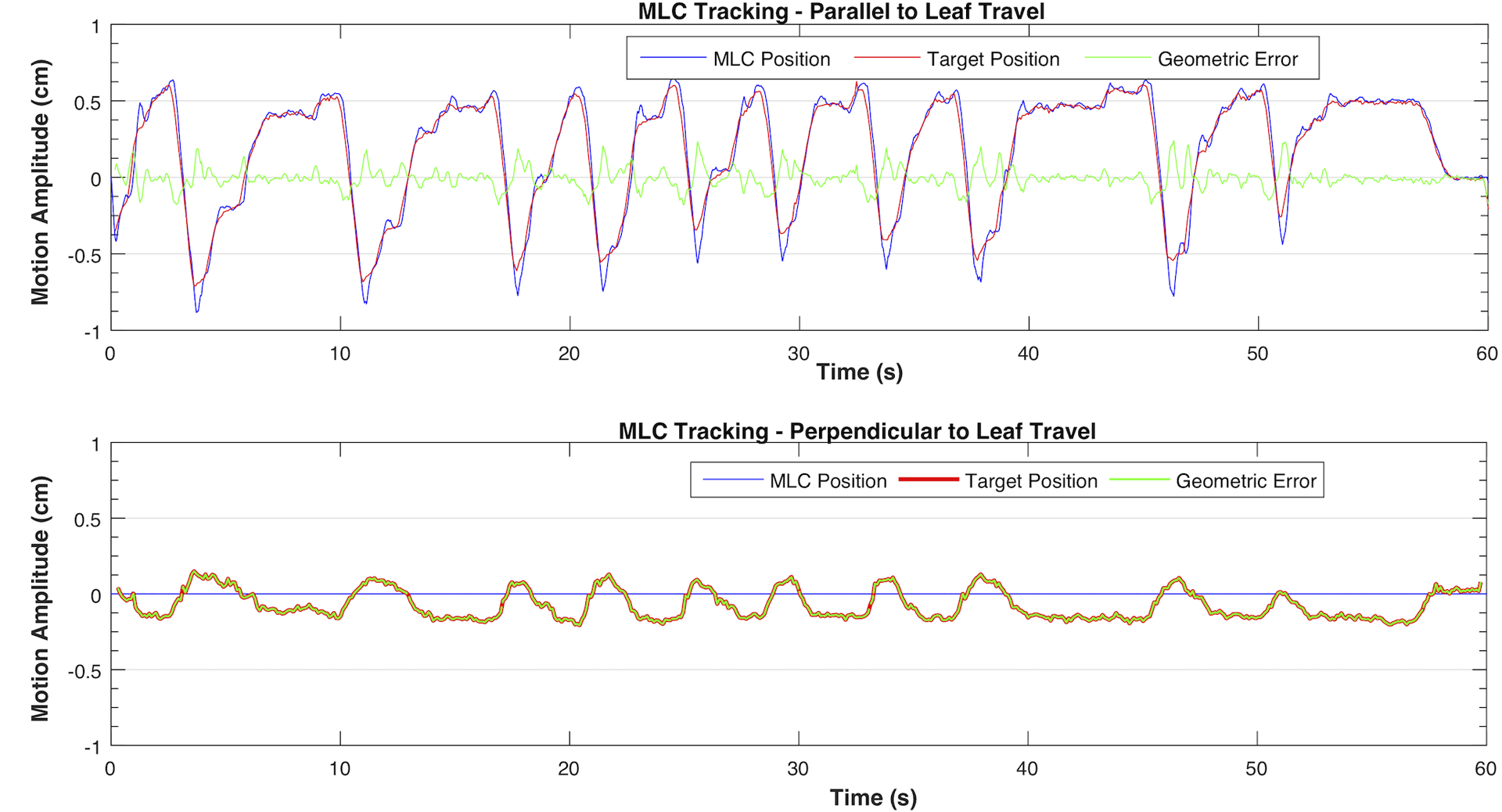
MLC tracking of the pancreatic patient trace 1 in parallel (top) and perpendicular to leaf travel (bottom) directions. The motion in this direction was below the threshold (2.5 mm) therefore tracking was not triggered.
Figure 8:
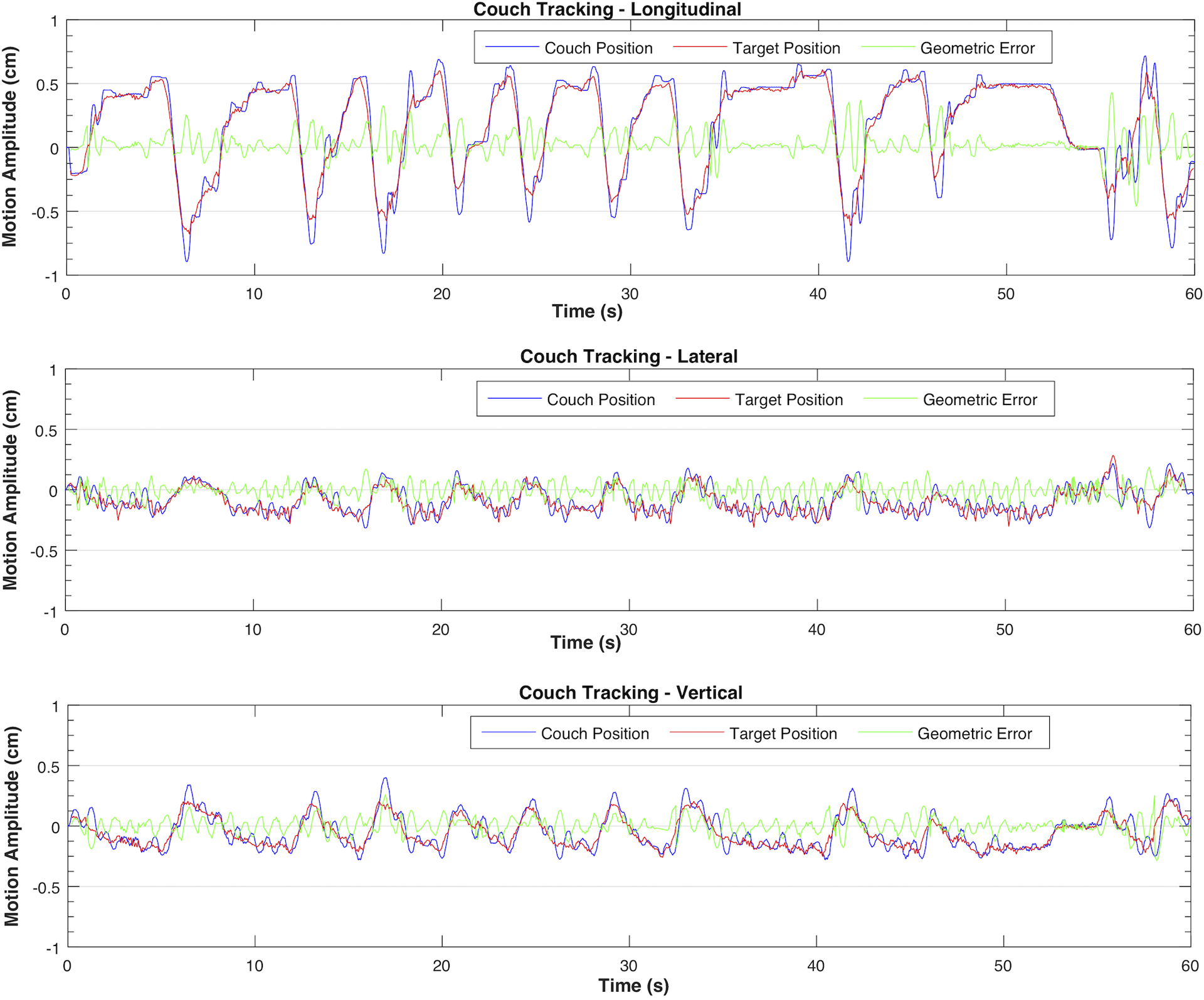
Couch tracking of pancreatic patient trace 1 in three axes
III.B. Tracking system latency
With the prediction filter disabled, the measured latency of MLC and couch tracking is shown in Figure 9. Except for the shortest period (i.e. Ts = 3 s), the tracking latency appeared relatively constant with a slight increase with increasing target speed, averaging 108 ms for MLC tracking and 182 ms for couch tracking. For the shortest period of Ts = 3 s, the maximum target acceleration and deceleration (~ 3.3 cm/s2) was likely approaching the limit of the couch acceleration especially on the vertical axis, which might have resulted in the slightly larger latency compared to those seen with motion periods Ts > 3 s. The predictive filter markedly improved the geometric tracking accuracy for both MLC and couch tracking as shown in the examples in Figure 10.
Figure 9:
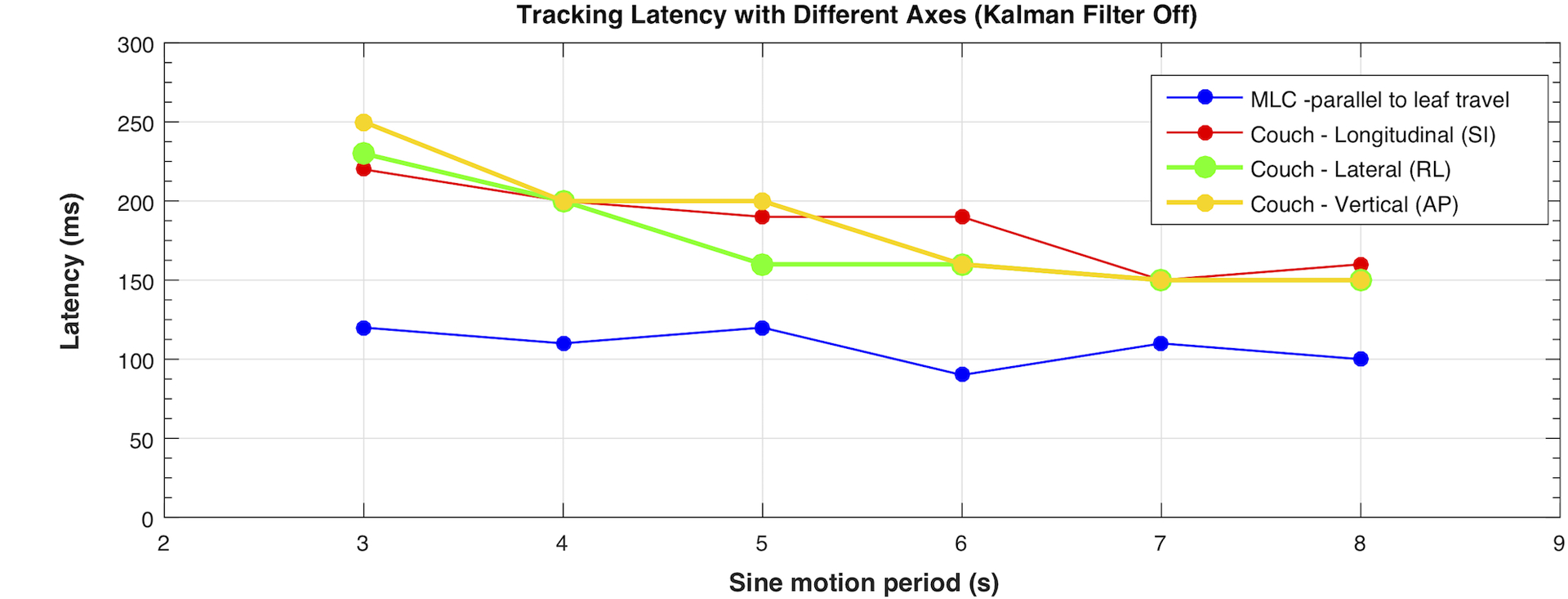
Tracking latency of different compensation axes evaluated with Kalman filter turned off. The error bar for each motion trace and tracking mode is equal to or less than 20 ms (from repeated measurements) and is not shown in the plot.
Figure 10:
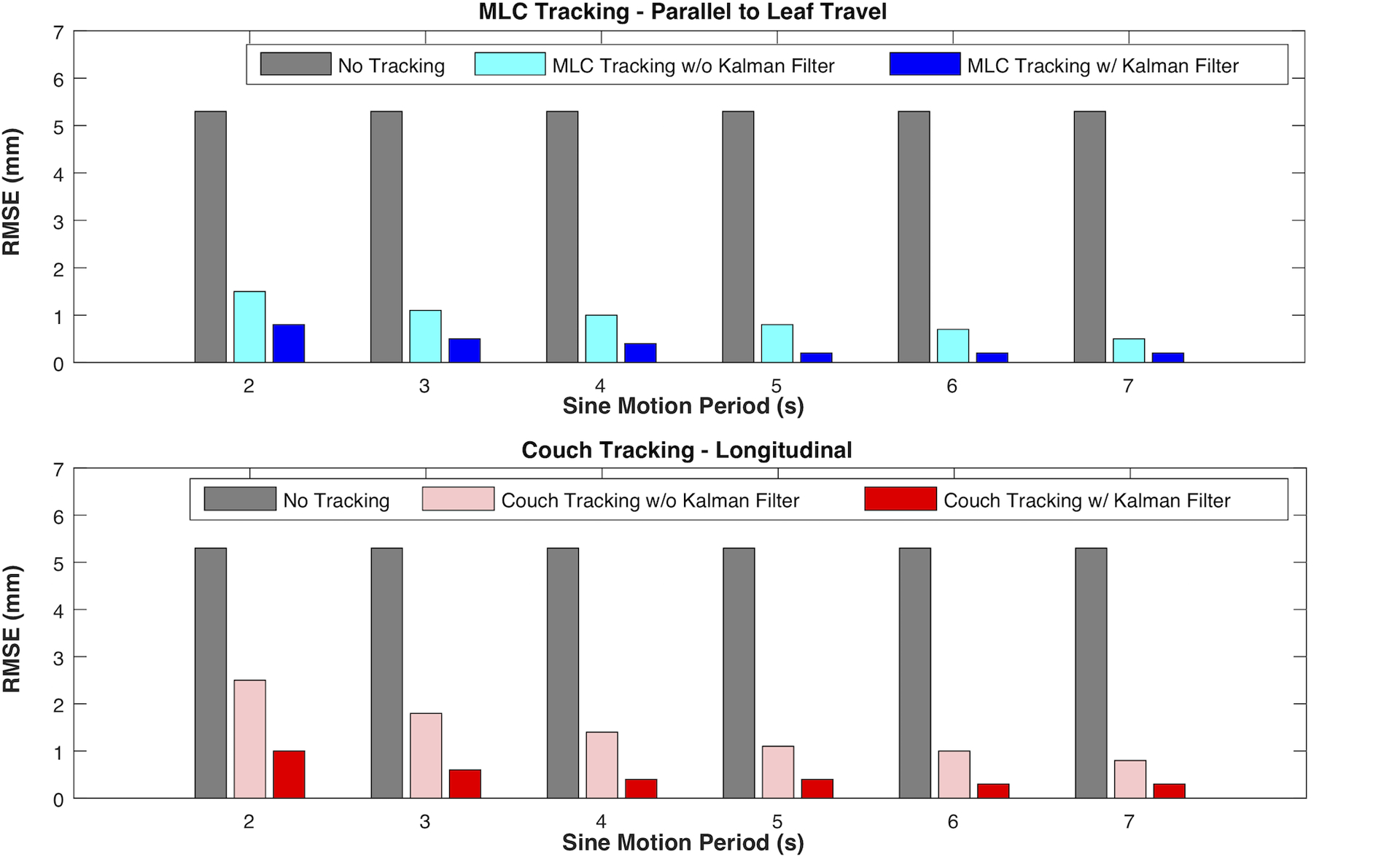
Comparison of geometric tracking accuracy with and without predictive filter for MLC tracking along the leaf travel direction (top) and for couch tracking in the longitudinal direction (bottom) with sinusoidal motion traces of different periods.
III.C. Dosimetric evaluations
Without tracking, 3D tumor motion caused large dose errors in all patient plans. Local dose errors were as high as 50% and were mostly located at the two distal edges of the fields along the major target motion axis, i.e. sup-inf direction for the particular motion trace used in this study. With MLC and couch tracking, the distribution and the magnitude of the errors were reduced significantly. The plan-specific and motion trace specific gamma analysis are summarized in Figure 11 and Figure 12. Overall, couch tracking showed consistently high dosimetric accuracy with clinically acceptable dose errors, where the average 2%/2mm gamma pass rate over eight plans was 98.1% (range: 94.9% – 100.0%). No significant difference in couch tracking accuracy was observed among plans and traces. Performance of MLC tracking varied from plan to plan and with different traces as well. The average gamma pass rate for MLC tracking was 86.0% (range of 67.9% – 95.6%) using 2%/2mm criteria and 94.6% (range 83.1% – 99.7%) with 3%/3mm criteria. In sixteen out of the forty deliveries (combination of different plans and motion traces), MLC tracking did not achieve clinically acceptable accuracy (clinical criteria: 3%/3mm gamma ≥ 95%).
Figure 11:
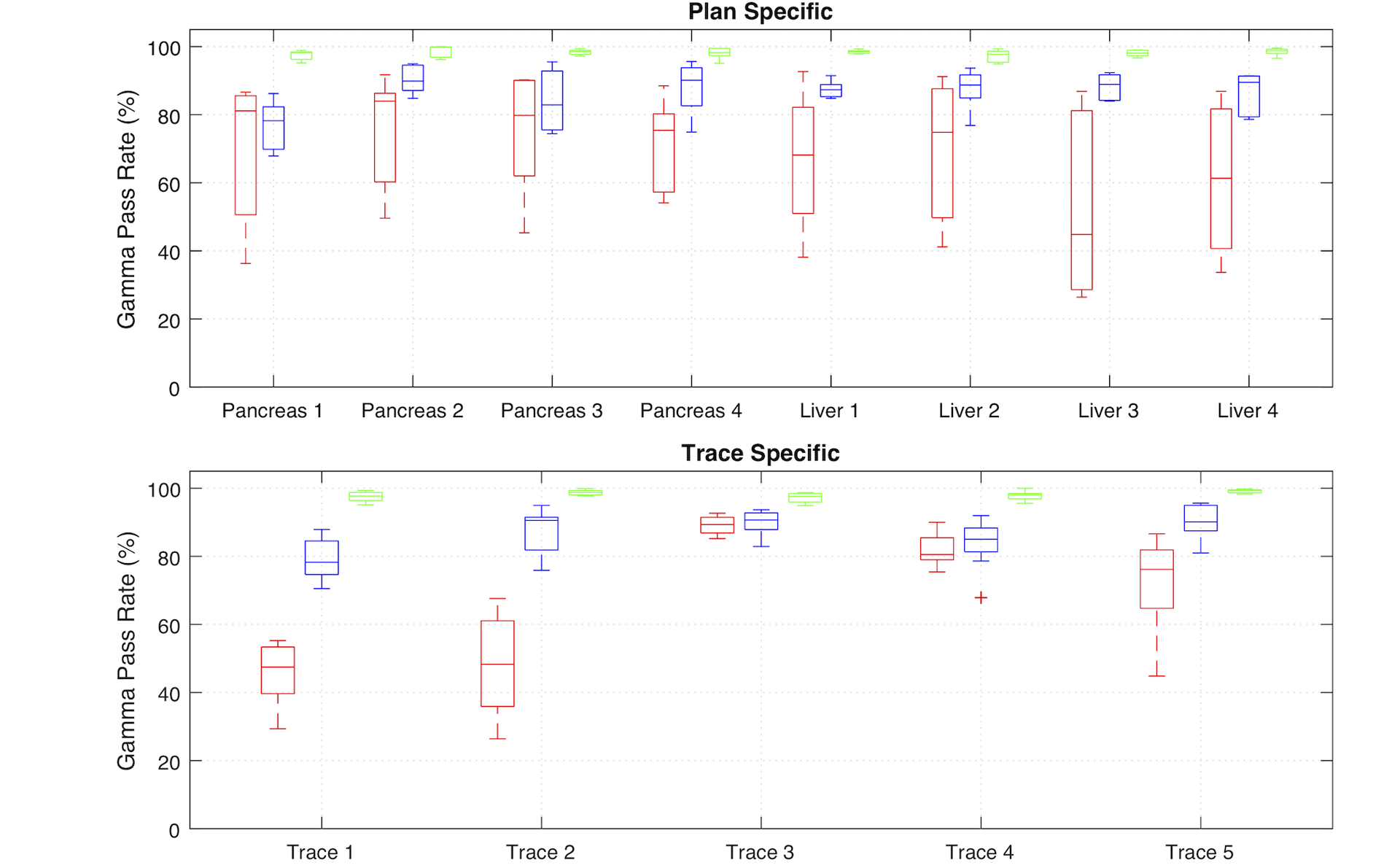
Plan specific (top) and motion trace specific (bottom) comparison of 2%/2mm gamma pass rate for deliveries without tracking, with MLC tracking, and with couch tracking. Results of each modality is shown as median and 25th and 75th percentiles, as well as the range of all data points. Data points outside ± 3 standard deviation were shown as outliers.
Figure 12:
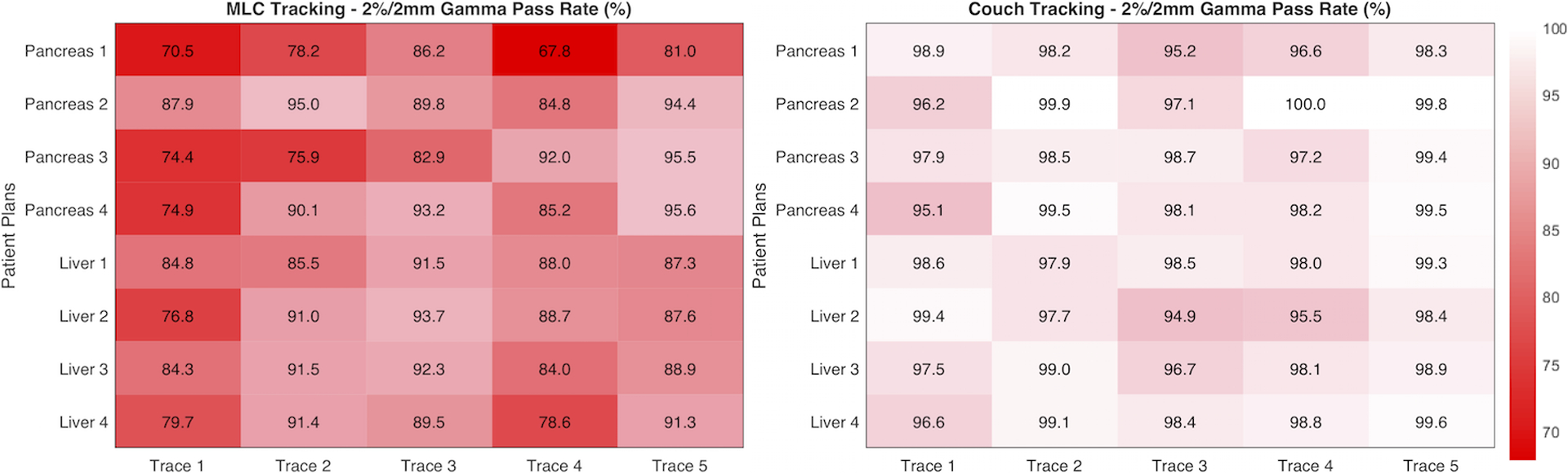
Summary of dosimetric tracking accuracy of MLC (left) and couch tracking (right) for each plan and motion trace. Results are shown in percentage gamma pass rate with 2%/2mm criteria within an ROI defined by the 20% isodose line.
MLC tracking performance showed dependency on treatment plans and motion traces. In general, MLC tracking was less accurate with pancreas SBRT plans compared to that with liver plans and was found positively correlated with the plan complexity as indicated by the modulation factor. On the other hand, motion trace 1 and trace 4 appeared to be challenging for MLC tracking due to either large motion amplitude or rapid motion, resulting in an average gamma pass rate (2%/2mm) among all plans of less than 80% and 84%, respectively. The average speed in motion trace 4 is 0.6 cm/s and goes up to 2.4 cm/s in S-I direction. Liver plan 4 has the highest modulation factor with the smallest PTV. MLC tracking accuracy was inferior especially with trace 1 and trace 4.
Collimator angle has significant impact on MLC tracking, as summarized in the arc specific gamma analysis in Table 3. Tracking accuracy was considerably higher in arcs with collimator angle of 90° compared to those with 0°. This is due to the fact that the dominant motion axis with the largest motion amplitude is in the superior-inferior direction for all traces used in the dosimetric evaluation. With collimator 90°, MLC leaf travel direction was aligned with the dominant motion, which to a certain extent avoided the intrinsic latencies as well as the low resolution in the direction perpendicular to leaf travel.
Table 3.
Collimator angle specific MLC tracking accuracy. Results are shown as average and one standard deviation among all plans and all traces. Gamma is calculated within an ROI defined by 20% isodose line.
| 3%/3mm Gamma Pass Rate: Average (range) |
2%/2mm Gamma Pass Rate: Average (range) |
|
|---|---|---|
| Arc 1 (coll = 0°) | 92.9% (74.6% – 99.5%) |
81.7% (59.7% – 97.0%) |
| Arc 2 (coll = 90°) | 98.0% (89.4% – 100.0%) |
91.8% (73.1% – 99.7%) |
| Plan (Arc1 + 2) | 94.6% (83.1% – 99.7%) |
86.0% (67.9% – 95.6%) |
Figure 13 and Figure 14 compare the results from tracking the same motion trace (trace 1) with two of the pancreas cases, each with two full arcs at collimator angles of 0° and 90°. Figure 13 was a SBRT plan (pancreas 2 in Figure 12) with single prescription dose level and a relatively small modulation factor of 2.2 MU/cGy. The MLC tracking accuracy for this plan was the highest among the eight plans evaluated with this motion trace. Figure 14 illustrates another SBRT plan (pancreas 1 in Figure 12) with a modulation factor of 3.4 MU/cGy. While the performance of couch tracking was similar to that in Figure 13, MLC tracking was significantly inferior for this plan, with 3%/3mm and 2%/2mm global gamma pass rates being 83.7% and 70.5%, respectively.
Figure 13:
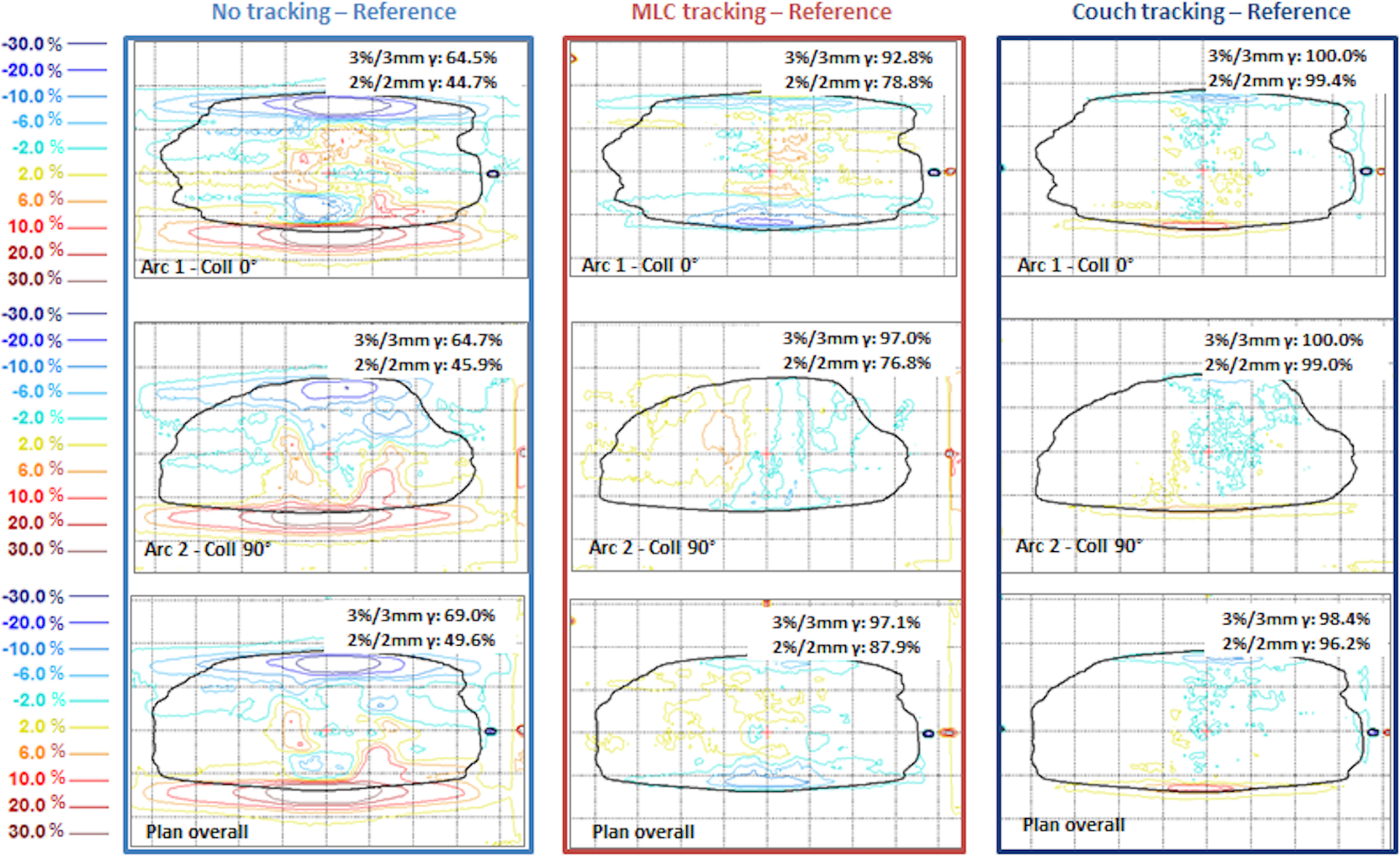
Dose difference maps from pancreas plan 2 and patient trace 1, a single prescription dose level SBRT plan for pancreas. Reference dose refers to the delivery without target motion. Results are shown in percentage dose difference relative to the dose maximum on the plane. Solid black line indicates the ROI defined as 20% of maximum dose.
Figure 14:
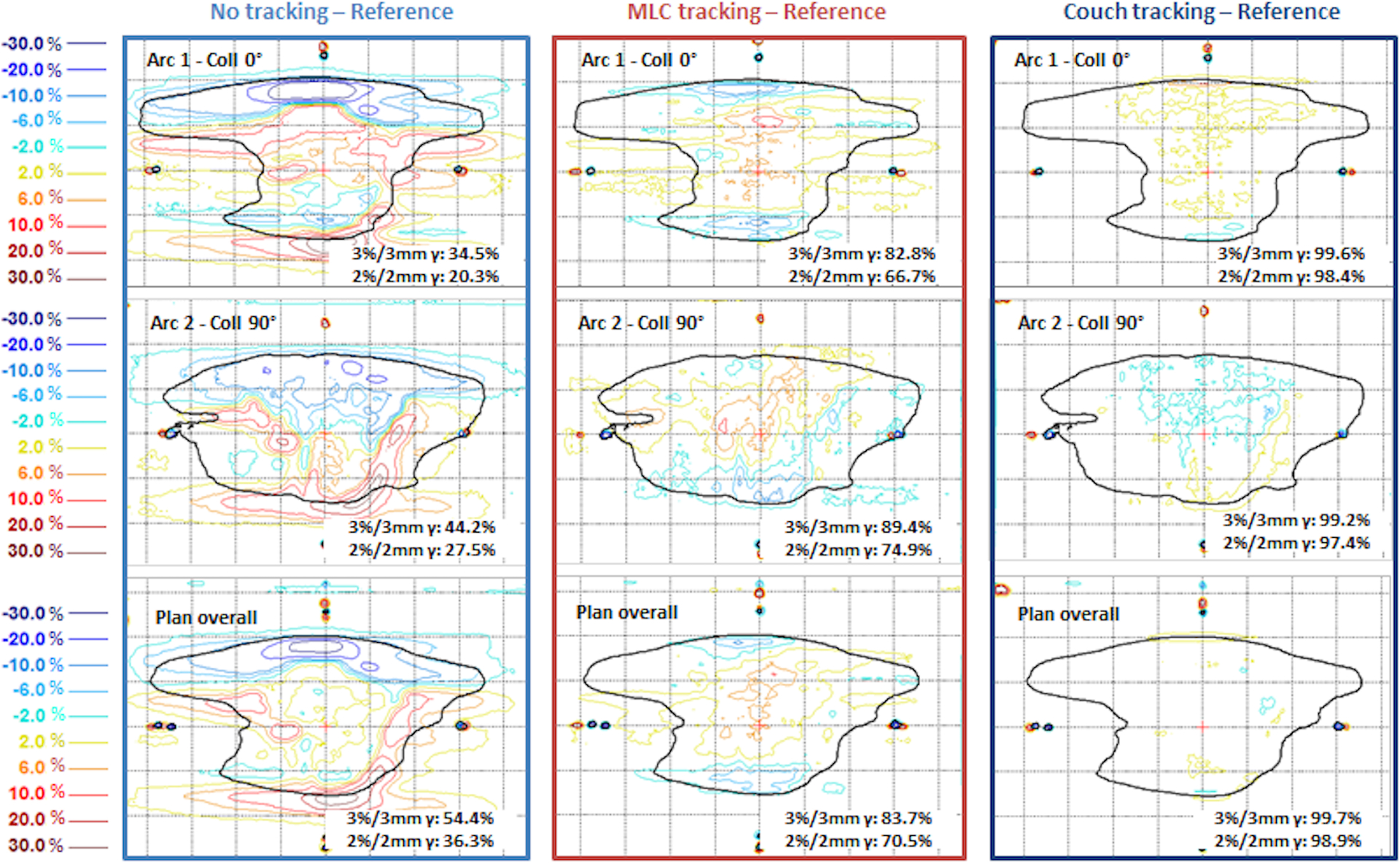
Dose difference maps for pancreas SBRT plan (plan 1) and patient trace 1. Percentage dose difference was relative to the dose maximum on the plane. Solid black line indicates the ROI defined as 20% of maximum dose.
IV. Discussion
In this study we evaluated and compared the performance of MLC and couch tracking and the clinical feasibility during external beam radiotherapy of abdominal cancers using the prototype iTools Tracking in TrueBeam Developer Mode. Geometrically, MLC tracking along the leaf travel direction showed the highest tracking accuracy, slightly better than couch tracking when evaluated with sinusoidal traces for each axis separately. The geometric tracking errors were shown to be more pronounced perpendicular to leaf travel direction with MLC tracking compared to MLC tracking along leaf travel direction and couch tracking in all directions. Although the 3D geometric accuracies were comparable between MLC and couch tracking as demonstrated with circular conformal plan and pancreatic tumor motion, film dosimetry showed that couch tracking outperformed MLC tracking with consistently higher dosimetric accuracy evaluated with abdominal plans and pancreas tumor motion traces.
Couch tracking in all directions and MLC tracking along the leaf travel direction achieved better than 1.6 mm in geometric accuracy for 1D sinusoidal and 3D pancreatic tumor motions with circular conformal field. Given the limitations of MLC leaf width and traveling speed, MLC tracking perpendicular to leaf travel was discretized with intrinsic time delay that resulted in relatively large geometric error. The magnitude of error in this direction also depended on the speed and range of target motion as well as the shape and size of the aperture in the original plan. For couch tracking in the lateral and vertical directions relatively large errors were observed given the small motion amplitude in these two directions, as shown in Figure 8. A major component degrading the tracking accuracy in these two axes was the high-frequency noise which arose from the interference between Calypso and the metallic structures of the HexaMotion platform. Occasionally, a fluctuation of ± 0.5 mm was observed in Calypso signals, which introduced further positional errors. These fluctuations appeared as high-frequency noise, which subsequently degraded the performance of the Kalman filter and the resultant couch tracking accuracy. Noise with amplitude as much as 2 mm resulted from these fluctuations was shown in the middle and bottom plots in Figure 8. Couch vibration also caused fluctuations in target position and contributed to tracking errors.
Other sources might have contributed to measurement uncertainties in the geometric evaluation. The template-based automatic registration in our in-house software has an uncertainty of 0.17 ± 0.08 mm (mean ± standard deviation) when using kV images as previously reported9. Since MV and kV images were acquired at relatively low frequencies (10 and 15 Hz, respectively) compared to the sampling rate in the tracking log files (50 Hz), linear interpolation was applied when reconstructing target motion trace which resulted in uncertainty up to 0.3 mm given the maximum speed in the traces used in this study.
The results from latency evaluation are consistent with those from a previously published study54. The geometric tracking accuracy was significantly improved with the Kalman filter, overcoming the system latency and the low sampling rate of the target localization with the imaging systems. With predictive filter, tracking errors mainly occurred as the MLC or couch overshot at prompted target acceleration or deceleration points. Customizing the predictive filter may improve prediction accuracy and the performance of the tracking system59. Sharp et al showed that for a 200 ms system latency and reduced sampling rates (e.g. 3 – 4 Hz), linear prediction and artificial neural networks algorithms outperformed the Kalman filter57. Fine-tuning of the prediction algorithms based on the characteristics of the target motion of a particular site may provide additional improvement to account for irregular motion patterns or persistent drift during treatment. Note that the default Kalman filter settings in current version of iTools Tracking is not customizable by users.
Compared to previous studies at other institutions using the same tracking system54, 55, the dosimetric performance of MLC tracking presented in this paper is inferior. The difference could be attributed mainly to two reasons, differences in motion characteristics and treatment plans. The aforementioned two reports compared MLC and couch tracking for lung and prostate motion where the motion amplitude and oscillation frequency was relatively small compared to the pancreatic tumor motion used in this study. For MLC tracking in lung54, the target motion used by Hansen et al is somewhat similar to the pancreas motion trace used in this study. However other plan characteristics such as collimator angle, target size and dose prescription may affect leaf modulation and therefore tracking performance, making a direct comparison challenging. The study by Ehrbar et al. reported MLC tracking in prostate plans with both collimator 0 and 90 degrees. Compared to their results of 2%/2mm global gamma agreement, our studies showed much larger dosimetric errors and lower gamma pass rate in pancreas and liver plans with MLC tracking with both collimator 0 and 90 degrees. This is likely due to the inherent complexity of abdominal cases compared to prostate plans.
MLC tracking performance depends on various parameters, including the characteristics of the MLC, the leaf refitting algorithms, plan complexity, collimator angle, motion prediction algorithm, as well as target motion characteristics. In this study with abdominal VMAT plans and pancreatic tumor motion, it was found that MLC tracking was not able to achieve clinically acceptable dosimetric accuracy in certain cases. Two main factors contributed to the inferior performance of MLC tracking, i.e. plan complexity and inferior tracking performance in the direction perpendicular to leaf travel. First of all, the GI plans in this study were either SBRT or used dose painting with multiple Rx dose levels up to a factor of 1.7. Meanwhile, the target in GI tract are surrounded by multiple organs at risk (OARs) such as stomach, the bowels, and liver. To achieve a high dose conformity and heterogeneity within the PTV while meeting the critical organ dose constraints, the plans became highly modulated involving complex leaf sequence and isolated beamlets. The interplay between complex leaf sequence and target motion compensation compromised the dosimetric accuracy with MLC tracking. Using the modulation factor as an indicator of the plan complexity, we observed positive correlation between the MLC tracking accuracy and the modulation factor. Secondly, these plans were clinical plans where the collimator angles followed our institutional convention, i.e. 0° and 90° for two arcs. As mentioned earlier, for arcs with collimator angle 0°, the dominant motion axis was perpendicular to leaf travel direction. Given the limitations of the half-leaf threshold and the maximum leaf speed (2.5 cm/s), MLC tracking was hampered by constant and rapid re-fitting of the leaves to compensate the motion, which would ultimately result in delivery errors. Similarly, for arcs with collimator angle 90°, the motion perpendicular to leaf travel, though less in magnitude, was affected as well. Even with the leaf travel direction aligned with the dominant target motion axis, we observed large local dose errors and less than 85% gamma pass rate in certain cases. This could be an indication that the desired delivery was beyond the mechanical limit of MLC to achieve the planned leaf sequence while tracking target motion. Employing MLC that allows for higher leaf speed in such sense would certainly improve the overall tracking performance, especially when MLC has to compensate for target motion perpendicular to leaf travel direction. In addition, the intrinsic lag in the direction perpendicular to leaf travel could have resulted errors when MLC attempted to compensate very swift but minimal motion in this direction.
Further optimization of tracking parameters could improve MLC tracking accuracy. For example, a lower capability ratio (<100%) would reserve more leaf speed for tracking at the cost of reduced dose rate and extended delivery time. A lower conformity index may limit the tracking errors by introducing beam holds, again at the expense of beam-on time. Furthermore, treatment plans should be individually planned with collimator angles that are optimized to all three target motion axes. For example, in a preliminary simulation it was found out that the dominant motion axis for the projected motion in BEV for trace 1 (used in figure 13 and 14) was aligned with collimator angle between 80° to 100° (assuming a constant gantry speed of 6 degree/s). Pancreas plan 1 in Figure 14 was then re-optimized using collimator angles 80° and 100°, while keeping the same optimization/dose constraints as the original plan. The dosimetric accuracy of MLC tracking was improved compared to that with the original collimator angles (Figure S1 in supplementary data). These preliminary results demonstrate a possible solution to improve MLC tracking accuracy and further investigation is underway.
Couch tracking offered consistently accurate motion correction in all three dimensions with the pancreatic tumor trace, and the dosimetric accuracy was independent of the complexity of the treatment plan. However, the rapid couch motion during couch tracking remains to be a concern. Couch acceleration and deceleration could also introduce relative motion between the target or the critical organs and the couchtop, leading to tracking errors60. Couch motion during treatment may also increase the respiration frequency61 or breathing amplitude62, both of which may potentially lead to increased compensation errors, although results from several trials indicate that the couch motion was tolerated among healthy volunteers62–64.
In the current version of iTools Tracking, target and normal tissue rotation or deformation is not considered. Since the target and critical organs may not maintain a rigid configuration, real-time tumor tracking, with either MLC or couch compensation, should also consider the changes in their relative location. If the target moves towards a critical structure, the OAR dose tolerance may be exceeded. Therefore, evaluation is needed regarding the anatomic changes between the target and surrounding organs during planning and during the motion-compensated delivery to avoid over exposure to nearby structures.
V. Conclusion
MLC and couch tracking have been demonstrated to reduce undesired dosimetric perturbation caused by intrafractional tumor motion presented in abdominal cases. While couch tracking was able to deliver with high dosimetric accuracy, there are remaining challenges in MLC tracking for abdominal target motion and highly modulated plans. Further investigation is warranted in terms of improving MLC tracking efficiency and accuracy with further plan optimization taking into the account of the target motion characteristics.
Supplementary Material
Figure S1 Re-optimized plan 1 (same plan as that in Figure 14) with different collimator angles. Percentage dose difference was calculated relative to the maximum dose on the plane. Solid black lines indicated the ROI for gamma calculation which was the isodose line of the 20% of the maximum dose.
Table S1 Characteristics of the five patient motion traces (values are shown in mean and one standard deviation). Note the range of the motion is quantified as the tumor position relative to the planned position, i.e. position 0.
Disclosure and Acknowledgment
This project was funded by a research grant from Varian Medical Systems and NIH/NCI Cancer Center Support Grant (P30 CA008748). The authors would like to thank Kristijan Macek, Marius Koehl, Thanos Etmektzoglou, Jay Peterson, and Hong Wan from Varian for their technical assistance on the iTools Tracking and the Calypso systems. The authors are also grateful for the kind support from Ping Wang and Hai Pham of the MSKCC Department of Medical Physics regarding the in-house software used in this project. The use of patient record was approved by IRB at MSKCC.
References
- 1.Korreman SS, “Motion in radiotherapy: photon therapy,” Phys Med Biol 57, R161–191 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Abbas H, Chang B, Chen ZJ, “Motion management in gastrointestinal cancers,” Journal of gastrointestinal oncology 5, 223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso-Arrizabalaga S, Brualla Gonzalez L, Rosello Ferrando JV, Pastor Peidro J, Lopez Torrecilla J, Planes Meseguer D, Garcia Hernandez T, “Prostate planning treatment volume margin calculation based on the ExacTrac X-Ray 6D image-guided system: margins for various clinical implementations,” International journal of radiation oncology, biology, physics 69, 936–943 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Cho B, Poulsen PR, Sloutsky A, Sawant A, Keall PJ, “First demonstration of combined kV/MV image-guided real-time dynamic multileaf-collimator target tracking,” International journal of radiation oncology, biology, physics 74, 859–867 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao W, Riaz N, Lee L, Wiersma R, Xing L, “A fiducial detection algorithm for real-time image guided IMRT based on simultaneous MV and kV imaging,” Med Phys 35, 3554–3564 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao W, Hsu A, Riaz N, Lee L, Wiersma R, Luxton G, King C, Xing L, Solberg T, “Image-guided radiotherapy in near real time with intensity-modulated radiotherapy megavoltage treatment beam imaging,” International journal of radiation oncology, biology, physics 75, 603–610 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Azcona JD, Li RJ, Mok E, Hancock S, Xing L, “Development and clinical evaluation of automatic fiducial detection for tumor tracking in cine megavoltage images during volumetric modulated arc therapy,” Medical Physics 40, 031708 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin W-Y, Lin S-F, Yang S-C, Liou S-C, Nath R, Liu W, “Real-time automatic fiducial marker tracking in low contrast cine-MV images,” Medical Physics 40, 011715 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Regmi R, Lovelock DM, Hunt M, Zhang P, Pham H, Xiong J, Yorke ED, Goodman KA, Rimner A, Mostafavi H, Mageras GS, “Automatic tracking of arbitrarily shaped implanted markers in kilovoltage projection images: A feasibility study,” Medical Physics 41, 071906 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt MA, Sonnick M, Pham H, Regmi R, Xiong JP, Morf D, Mageras GS, Zelefsky M, Zhang P, “Simultaneous MV-kV imaging for intrafractional motion management during volumetric-modulated arc therapy delivery,” J Appl Clin Med Phys 17, 473–486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsey CR, Scaperoth D, Arwood D, “Clinical experience with a commercial respiratory gating system,” International Journal of Radiation Oncology • Biology • Physics 48, 164–165 (2000). [Google Scholar]
- 12.Mageras GS, Yorke E, Rosenzweig K, Braban L, Keatley E, Ford E, Leibel SA, Ling CC, “Fluoroscopic evaluation of diaphragmatic motion reduction with a respiratory gated radiotherapy system,” Journal of applied clinical medical physics 2, 191–200 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford EC, Mageras GS, Yorke E, Rosenzweig KE, Wagman R, Ling CC, “Evaluation of respiratory movement during gated radiotherapy using film and electronic portal imaging,” International Journal of Radiation Oncology*Biology*Physics 52, 522–531 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Alderliesten T, Sonke J-J, Betgen A, Honnef J, van Vliet-Vroegindeweij C, Remeijer P, “Accuracy Evaluation of a 3-Dimensional Surface Imaging System for Guidance in Deep-Inspiration Breath-Hold Radiation Therapy,” International Journal of Radiation Oncology*Biology*Physics 85, 536–542 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Betgen A, Alderliesten T, Sonke J-J, van Vliet-Vroegindeweij C, Bartelink H, Remeijer P, “Assessment of set-up variability during deep inspiration breath hold radiotherapy for breast cancer patients by 3D-surface imaging,” Radiotherapy and Oncology 106, 225–230 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Willoughby TR, Kupelian PA, Pouliot J, Shinohara K, Aubin M, Roach M, Skrumeda LL, Balter JM, Litzenberg DW, Hadley SW, Wei JT, Sandler HM, “Target localization and real-time tracking using the Calypso 4D localization system in patients with localized prostate cancer,” International Journal of Radiation Oncology*Biology*Physics 65, 528–534 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Kupelian P, Willoughby T, Mahadevan A, Djemil T, Weinstein G, Jani S, Enke C, Solberg T, Flores N, Liu D, Beyer D, Levine L, “Multi-institutional clinical experience with the Calypso System in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy,” International Journal of Radiation Oncology • Biology • Physics 67, 1088–1098 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Langen KM, Willoughby TR, Meeks SL, Santhanam A, Cunningham A, Levine L, Kupelian PA, “Observations on Real-Time Prostate Gland Motion Using Electromagnetic Tracking,” International Journal of Radiation Oncology • Biology • Physics 71, 1084–1090 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Kubo HD, Len PM, Minohara S, Mostafavi H, “Breathing-synchronized radiotherapy program at the University of California Davis Cancer Center,” Med Phys 27, 346–353 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Wagman R, Yorke E, Giraud P, Ford E, Sidhu K, Mageras G, Minsky B, Rosenzweig K, “Reproducibility of organ position with respiratory gating for liver tumors: use in dose-escalation,” International Journal of Radiation Oncology • Biology • Physics 51, 28 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Hara R, Itami J, Kondo T, Aruga T, Abe Y, Ito M, Fuse M, Shinohara D, Nagaoka T, Kobiki T, “Stereotactic single high dose irradiation of lung tumors under respiratory gating,” Radiotherapy and Oncology 63, 159–163 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Mageras GS, Yorke E, “Deep inspiration breath hold and respiratory gating strategies for reducing organ motion in radiation treatment,” Seminars in Radiation Oncology 14, 65–75 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Hanley J, Debois MM, Mah D, Mageras GS, Raben A, Rosenzweig K, Mychalczak B, Schwartz LH, Gloeggler PJ, Lutz W, Ling CC, Leibel SA, Fuks Z, Kutcher GJ, “Deep inspiration breath-hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation,” International Journal of Radiation Oncology*Biology*Physics 45, 603–611 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Mah D, Hanley J, Rosenzweig KE, Yorke E, Braban L, Ling CC, Leibel SA, Mageras G, “Technical aspects of the deep inspiration breath-hold technique in the treatment of thoracic cancer,” International journal of radiation oncology, biology, physics 48, 1175–1185 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Rosenzweig KE, Hanley J, Mah D, Mageras G, Hunt M, Toner S, Burman C, Ling CC, Mychalczak B, Fuks Z, Leibel SA, “The deep inspiration breath-hold technique in the treatment of inoperable non-small-cell lung cancer,” International journal of radiation oncology, biology, physics 48, 81–87 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Wong JW, Sharpe MB, Jaffray DA, Kini VR, Robertson JM, Stromberg JS, Martinez AA, “The use of active breathing control (ABC) to reduce margin for breathing motion,” International Journal of Radiation Oncology*Biology*Physics 44, 911–919 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Stromberg JS, Sharpe MB, Kim LH, Kini VR, Jaffray DA, Martinez AA, Wong JW, “Active breathing control (ABC) for Hodgkin’s disease: reduction in normal tissue irradiation with deep inspiration and implications for treatment,” International journal of radiation oncology, biology, physics 48, 797–806 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Lens E, van der Horst A, Versteijne E, Bel A, van Tienhoven G, “Considerable pancreatic tumor motion during breath-holding,” Acta Oncol 55, 1360–1368 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Lens E, Gurney-Champion OJ, Tekelenburg DR, van Kesteren Z, Parkes MJ, van Tienhoven G, Nederveen AJ, van der Horst A, Bel A, “Abdominal organ motion during inhalation and exhalation breath-holds: pancreatic motion at different lung volumes compared,” Radiotherapy and Oncology 121, 268–275 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Murphy MJ, “Tracking moving organs in real time,” Seminars in Radiation Oncology 14, 91–100 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Keall PJ, Mageras GS, Balter JM, Emery RS, Forster KM, Jiang SB, Kapatoes JM, Low DA, Murphy MJ, Murray BR, Ramsey CR, Van Herk MB, Vedam SS, Wong JW, Yorke E, “The management of respiratory motion in radiation oncology report of AAPM Task Group 76,” Med Phys 33, 3874–3900 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Adler JR Jr, Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL, “The Cyberknife: A Frameless Robotic System for Radiosurgery,” Stereotactic and Functional Neurosurgery 69, 124–128 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Gerszten PC, Ozhasoglu C, Burton SA, Vogel WJ, Atkins BA, Kalnicki S, Welch WC, “CyberKnife Frameless Stereotactic Radiosurgery for Spinal Lesions: Clinical Experience in 125 Cases,” Neurosurgery 55, 89–99 (2004). [PubMed] [Google Scholar]
- 34.Hoogeman M, Prévost J-B, Nuyttens J, Pöll J, Levendag P, Heijmen B, “Clinical Accuracy of the Respiratory Tumor Tracking System of the CyberKnife: Assessment by Analysis of Log Files,” International Journal of Radiation Oncology*Biology*Physics 74, 297–303 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Kilby W, Dooley JR, Kuduvalli G, Sayeh S, Maurer CR, “The CyberKnife® Robotic Radiosurgery System in 2010,” Technology in Cancer Research & Treatment 9, 433–452 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Kamino Y, Takayama K, Kokubo M, Narita Y, Hirai E, Kawawda N, Mizowaki T, Nagata Y, Nishidai T, Hiraoka M, “Development of a four-dimensional image-guided radiotherapy system with a gimbaled X-ray head,” International journal of radiation oncology, biology, physics 66, 271–278 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Depuydt T, Verellen D, Haas O, Gevaert T, Linthout N, Duchateau M, Tournel K, Reynders T, Leysen K, Hoogeman M, Storme G, Ridder MD, “Geometric accuracy of a novel gimbals based radiation therapy tumor tracking system,” Radiotherapy and Oncology 98, 365–372 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Depuydt T, Poels K, Verellen D, Engels B, Collen C, Buleteanu M, Van den Begin R, Boussaer M, Duchateau M, Gevaert T, Storme G, De Ridder M, “Treating patients with real-time tumor tracking using the Vero gimbaled linac system: Implementation and first review,” Radiotherapy and Oncology 112, 343–351 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Iizuka Y, Matsuo Y, Ishihara Y, Akimoto M, Tanabe H, Takayama K, Ueki N, Yokota K, Mizowaki T, Kokubo M, Hiraoka M, “Dynamic tumor-tracking radiotherapy with real-time monitoring for liver tumors using a gimbal mounted linac,” Radiotherapy and Oncology 117, 496–500 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Ono T, Miyabe Y, Takahashi K, Akimoto M, Mukumoto N, Ishihara Y, Nakamura M, Mizowaki T, Hiraoka M, “Geometric and dosimetric accuracy of dynamic tumor tracking during volumetric-modulated arc therapy using a gimbal mounted linac,” Radiotherapy and Oncology 2017). [DOI] [PubMed] [Google Scholar]
- 41.Nakamura A, Hiraoka M, Itasaka S, Nakamura M, Akimoto M, Ishihara Y, Mukumoto N, Goto Y, Kishi T, Yoshimura M, Matsuo Y, Yano S, Mizowaki T, “Evaluation of Dynamic Tumor-tracking Intensity-modulated Radiotherapy for Locally Advanced Pancreatic Cancer,” Sci Rep 8, 17096 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keall P, Kini V, Vedam S, Mohan R, “Motion adaptive x-ray therapy: a feasibility study,” Physics in medicine and biology 46, 1 (2001). [DOI] [PubMed] [Google Scholar]
- 43.McQuaid D, Webb S, “IMRT delivery to a moving target by dynamic MLC tracking: delivery for targets moving in two dimensions in the beam’s eye view,” Physics in medicine and biology 51, 4819 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Giraud P, Yorke E, Jiang S, Simon L, Rosenzweig K, Mageras G, “Reduction of organ motion effects in IMRT and conformal 3D radiation delivery by using gating and tracking techniques,” Cancer Radiother 10, 269–282 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman J, Korreman S, Persson G, Cattell H, Svatos M, Sawant A, Venkat R, Carlson D, Keall P, “DMLC motion tracking of moving targets for intensity modulated arc therapy treatment: a feasibility study,” Acta Oncol 48, 245–250 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Keall PJ, Sawant A, Cho B, Ruan D, Wu J, Poulsen P, Petersen J, Newell LJ, Cattell H, Korreman S, “Electromagnetic-guided dynamic multileaf collimator tracking enables motion management for intensity-modulated arc therapy,” International journal of radiation oncology, biology, physics 79, 312–320 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krauss A, Nill S, Tacke M, Oelfke U, “Electromagnetic Real-Time Tumor Position Monitoring and Dynamic Multileaf Collimator Tracking Using a Siemens 160 MLC: Geometric and Dosimetric Accuracy of an Integrated System,” International Journal of Radiation Oncology*Biology*Physics 79, 579–587 (2011). [DOI] [PubMed] [Google Scholar]
- 48.Poulsen PR, Fledelius W, Cho B, Keall P, “Image-based dynamic multileaf collimator tracking of moving targets during intensity-modulated arc therapy,” International Journal of Radiation Oncology* Biology* Physics 83, e265–e271 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fast MF, Nill S, Bedford JL, Oelfke U, “Dynamic tumor tracking using the Elekta Agility MLC,” Med Phys 41, 111719 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Colvill E, Booth JT, O’Brien RT, Eade TN, Kneebone AB, Poulsen PR, Keall PJ, “Multileaf Collimator Tracking Improves Dose Delivery for Prostate Cancer Radiation Therapy: Results of the First Clinical Trial,” International journal of radiation oncology, biology, physics 92, 1141–1147 (2015). [DOI] [PubMed] [Google Scholar]
- 51.D D’Souza W, Naqvi SA, Cedric XY, “Real-time intra-fraction-motion tracking using the treatment couch: a feasibility study,” Physics in medicine and biology 50, 4021 (2005). [DOI] [PubMed] [Google Scholar]
- 52.McNamara JE, Regmi R, Michael Lovelock D, Yorke ED, Goodman KA, Rimner A, Mostafavi H, Mageras GS, “Toward correcting drift in target position during radiotherapy via computer-controlled couch adjustments on a programmable Linac,” Med Phys 40, 051719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colvill E, Booth J, Nill S, Fast M, Bedford J, Oelfke U, Nakamura M, Poulsen P, Worm E, Hansen R, Ravkilde T, Scherman Rydhög J, Pommer T, Munck af Rosenschold P, Lang S, Guckenberger M, Groh C, Herrmann C, Verellen D, Poels K, Wang L, Hadsell M, Sothmann T, Blanck O, Keall P, “A dosimetric comparison of real-time adaptive and non-adaptive radiotherapy: A multi-institutional study encompassing robotic, gimbaled, multileaf collimator and couch tracking,” Radiotherapy and Oncology 119, 159–165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen R, Ravkilde T, Worm ES, Toftegaard J, Grau C, Macek K, Poulsen PR, “Electromagnetic guided couch and multileaf collimator tracking on a TrueBeam accelerator,” Medical physics 43, 2387–2398 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Ehrbar S, Schmid S, Jöhl A, Klöck S, Guckenberger M, Riesterer O, Tanadini-Lang S, “Comparison of multi-leaf collimator tracking and treatment-couch tracking during stereotactic body radiation therapy of prostate cancer,” Radiotherapy and Oncology 125, 445–452 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Nankali S, Worm ES, Hansen R, Weber B, Hoyer M, Zirak A, Poulsen PR, “Geometric and dosimetric comparison of four intrafraction motion adaptation strategies for stereotactic liver radiotherapy,” Phys Med Biol 63, 145010 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Sharp GC, Jiang SB, Shimizu S, Shirato H, “Prediction of respiratory tumour motion for real-time image-guided radiotherapy,” Physics in medicine and biology 49, 425 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Bar-Shalom Y, Li XR, Kirubarajan T, Estimation with applications to tracking and navigation: theory algorithms and software. (John Wiley & Sons, 2004). [Google Scholar]
- 59.Toftegaard J, Keall PJ, O’Brien R, Ruan D, Ernst F, Homma N, Ichiji K, Poulsen PR, “Potential improvements of lung and prostate MLC tracking investigated by treatment simulations,” Med Phys 45, 2218–2229 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Ehrbar S, Schmid S, Jöhl A, Klöck S, Guckenberger M, Riesterer O, Tanadini-Lang S, presented at the BMTMedPhys Dresden, Biomedical Engineering / Biomedizinische Technik Vol 62 (s1), 2017. (unpublished). [Google Scholar]
- 61.Jöhl A, Bogowicz M, Ehrbar S, Guckenberger M, Klöck S, Meboldt M, Riesterer O, Zeilinger M, Schmid Daners M, Tanadini-Lang S, “Unconscious physiological response of healthy volunteers to dynamic respiration-synchronized couch motion,” Radiation Oncology 12, 189 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilbert J, Baier K, Richter A, Herrmann C, Ma L, Flentje M, Guckenberger M, “Influence of Continuous Table Motion on Patient Breathing Patterns,” International Journal of Radiation Oncology*Biology*Physics 77, 622–629 (2010). [DOI] [PubMed] [Google Scholar]
- 63.D’Souza WD, Malinowski KT, Van Liew S, D’Souza G, Asbury K, McAvoy TJ, Suntharalingam M, Regine WF, “Investigation of motion sickness and inertial stability on a moving couch for intra-fraction motion compensation,” Acta Oncologica 48, 1198–1203 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sweeney RA, Arnold W, Steixner E, Nevinny-Stickel M, Lukas P, “Compensating for Tumor Motion by a 6-Degree-of-Freedom Treatment Couch: Is Patient Tolerance an Issue?,” International Journal of Radiation Oncology*Biology*Physics 74, 168–171 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Re-optimized plan 1 (same plan as that in Figure 14) with different collimator angles. Percentage dose difference was calculated relative to the maximum dose on the plane. Solid black lines indicated the ROI for gamma calculation which was the isodose line of the 20% of the maximum dose.
Table S1 Characteristics of the five patient motion traces (values are shown in mean and one standard deviation). Note the range of the motion is quantified as the tumor position relative to the planned position, i.e. position 0.


