Abstract
PURPOSE
The purpose of this phase II study was to evaluate hyperthermic intraperitoneal chemotherapy (HIPEC) with carboplatin for recurrent ovarian cancer during secondary cytoreductive surgery.
MATERIALS AND METHODS
Patients were intraoperatively randomly assigned to carboplatin HIPEC (800 mg/m2 for 90 minutes) or no HIPEC, followed by five or six cycles of postoperative IV carboplatin-based chemotherapy, respectively. Based on a binomial single-stage pick-the-winner design, an arm was considered winner if ≥ 17 of 49 patients were without disease progression at 24 months post-surgery. Secondary objectives included postoperative toxicity and HIPEC pharmacokinetics.
RESULTS
Of 98 patients, 49 (50%) received HIPEC. Complete gross resection was achieved in 82% of the HIPEC patients and 94% of the standard-arm patients. Bowel resection was performed in 37% of patients in the HIPEC arm compared with 65% in the standard (P = .008). There was no perioperative mortality and no difference in use of ostomies, length of stay, or postoperative toxicity. At 24 months, eight patients (16.3%; 1-sided 90% CI, 9.7 to 100) were without progression or death in the HIPEC arm and 12 (24.5%; 1-sided 90% CI, 16.5 to 100) in the standard arm. With a medium follow-up of 39.5 months, 82 patients progressed and 37 died. The median progression-free survival in the HIPEC and standard arms were 12.3 and 15.7 months, respectively (hazard ratio, 1.54; 95% CI, 1 to 2.37; P = .05). There was no significant difference in median overall survival (52.5 v 59.7 months, respectively; hazard ratio, 1.39; 95% CI, 0.73 to 2.67; P = .31). These analyses were exploratory.
CONCLUSION
HIPEC with carboplatin was well tolerated but did not result in superior clinical outcomes. This study does not support the use of HIPEC with carboplatin during secondary cytoreductive surgery for platinum-sensitive recurrent ovarian cancer.
INTRODUCTION
In 2021, an estimated 21,410 women will be diagnosed with ovarian cancer in the United States, and approximately 13,770 women will die from this disease.1 Peritoneal metastases are characteristic of ovarian cancer, and recurrence after initial response is almost inevitable. Intraperitoneal (IP) chemotherapy with cisplatin and paclitaxel after primary cytoreductive surgery resulted in improved progression-free survival (PFS) and overall survival (OS) compared with intravenous (IV) chemotherapy in patients with optimally resected stage III ovarian cancer.2,3 Despite the survival benefit, IP treatment remains underused in comprehensive cancer centers secondary to toxicity and the difficulty of administering IP therapy.4 Furthermore, in the recent GOG252 study, two postoperative IP-containing regimens with cisplatin or carboplatin did not result in improved outcomes compared with IV treatment alone when combined with bevacizumab.5 Quality of life during chemotherapy was statistically worse in the IP cisplatin arm. Hyperthermic intraperitoneal chemotherapy (HIPEC) differs from postoperative IP chemotherapy as it is a single administration delivered intraperitoneally in a hyperthermic state upon completion of cytoreduction. Hyperthermia has direct cytotoxic effect and enhances tumor penetration and DNA-adduct formation of platinum compounds.6-9 HIPEC with cisplatin at 100 mg/m2 has been shown to be safe and cost effective and results in superior PFS and OS in patients with stage III ovarian cancer undergoing interval debulking surgery after neoadjuvant chemotherapy.10,11 Another recent study suggested a survival benefit with HIPEC at the time of primary cytoreductive surgery.12 The role of HIPEC at secondary cytoreductive surgery for recurrent ovarian cancer is not established.
CONTEXT
Key Objective
Is the addition of hyperthermic intraperitoneal chemotherapy (HIPEC) with carboplatin during secondary cytoreductive surgery beneficial in the treatment of recurrent ovarian cancer?
Knowledge Generated
The median progression-free survival was 12.3 months for patients who received HIPEC during secondary cytoreduction and 15.7 months for those who did not receive HIPEC (P = .05). Median overall survival was 52.5 months and 59.7 months, respectively (P = .31).
Relevance
The findings of this phase II study do not support the use of HIPEC with carboplatin during secondary cytoreductive surgery for platinum-sensitive recurrent ovarian cancer.
Outcomes with recurrent ovarian cancer depend on various factors, such as time from last treatment with platinum-based chemotherapy, mutational status (eg, BRCA), residual disease at the time of surgery, and general performance status.13-17 Multiple retrospective but few prospective studies have evaluated HIPEC in patients with platinum-sensitive ovarian cancer.18-21 Because of concerns of cisplatin-induced grade 3 and 4 adverse events (AEs) seen in IP-containing studies, carboplatin has emerged as an alternative drug, with potentially less treatment-related toxicities when administered intraperitoneally.2,5,6,8,22,23 The aim of this phase II study was to evaluate the efficacy of HIPEC with carboplatin at 800 mg/m2 in patients undergoing secondary cytoreduction for first recurrence of platinum-sensitive ovarian cancer.
MATERIALS AND METHODS
This is an investigator-initiated, multicenter, open-label, randomized phase II study in patients with platinum-sensitive epithelial ovarian cancer undergoing secondary cytoreduction. The study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC #12-275) and registered at (ClinicalTrials.gov identifier: NCT01767675; Protocol, online only). Potentially eligible patients were identified and consented at outpatient gynecologic oncology practices.
End Points
The primary objective was proportion of patients without evidence of disease progression at 24 months following secondary cytoreduction in the two arms—one with and one without HIPEC with carboplatin, followed by standard carboplatin-based postoperative IV chemotherapy. Secondary end points included 30-day postoperative morbidity, ability to complete assigned postoperative chemotherapy, pharmacokinetics, and OS. Data were censored at the date of last clinical assessment for patients alive and without disease progression. Disease progression was assessed using RECIST version 1.1.24 OS, defined as the time from random assignment to death from any cause, was a secondary end point.
Eligibility Criteria
Eligible patients were 21 years of age or older with first recurrence of high-grade epithelial ovarian cancer confirmed radiographically 6-30 months after completion of first-line platinum-based chemotherapy and deemed resectable based on our previously reported selection criteria for secondary cytoreduction.15,25 No prior chemotherapy or surgery for recurrent epithelial ovarian cancer was allowed. Other key eligibility criteria included a Karnofsky performance score of ≥ 70%, and adequate bone marrow, coagulation, renal, and hepatic function. Patients were ineligible if they had pre-existing neuropathy (National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.0) grade > 1; known platinum allergy; low-grade serous or borderline histology; and other serious disabling conditions that would contraindicate secondary cytoreductive surgery.
Study Design
After informed consent, patients were taken to the operating room for planned secondary cytoreduction. Random assignment occurred intraoperatively, after the surgeon confirmed resectability to ≤ 0.5 cm residual disease. Patients were stratified by platinum-free interval (6-12 months v > 12-30 months) and number of disease sites (single v multiple). Patients with > 0.5 cm residual disease were not randomly assigned and replaced. Carboplatin was administered as HIPEC at 800 mg/m2 for 90 minutes at 41°C-43°C. The dose selected was based on studies using carboplatin as IP or HIPEC in patients with ovarian carcinoma.5,26-28 Patients received five additional cycles of postoperative IV carboplatin-based chemotherapy in the HIPEC arm and six in the standard arm (carboplatin and paclitaxel or carboplatin and gemcitabine or carboplatin and liposomal doxorubicin, at the treating physician's discretion). Maintenance treatment (bevacizumab, polyadenosine diphosphate-ribose polymerase inhibition, or endocrine therapy) was not permitted.
Treatment
Secondary cytoreductive surgery was performed as per institutional standards. All patients randomly assigned to HIPEC received intraoperative IV antiemetics, including a serotonin 5-HT3 receptor antagonist, a neurokinin 1 antagonist, and dexamethasone before initiation of HIPEC. After surgical cytoreduction via laparotomy, percutaneous inflow and outflow catheters and IP temperature probes were placed. The skin was closed temporarily. The catheters were connected to the perfusion system (ThermoChemTM HT-1000 System, IN, PA), and 3,000 mL of normal saline was heated to 41°C-43°C and circulated through the abdomen. Subsequently, carboplatin was added to the perfusate. HIPEC was administered for 90 minutes, during which the perfusion rate, perfusion volume, and inflow and outflow temperatures were monitored and adjusted, if necessary. After completion of perfusion, the perfusate was drained and the abdomen was opened and irrigated with 3,000 mL of normal saline. Fascia and skin were closed in standard fashion. Postoperatively, all patients were observed and transferred to the floor as per standard institutional guidelines.
Pharmacokinetics and Pharmacodynamics
In 15 consecutive patients randomly assigned to HIPEC, peritoneal samples were collected at 0, 5, 10, 15, 30, 45, 60, 75, and 90 minutes of HIPEC. Blood samples were collected at 0, 15, 30, 45, 60, 75, and 90 minutes, and at 3, 6, 12, 18, 24, 48, and 72 hours. Ultrafiltrable carboplatin was separated from plasma by using the CentrisartVR ultrafiltration system (Sartorius AG, Göttingen, Germany). Platinum concentrations were measured by flameless atomic absorption spectrometry, as previously described.29 Platinum pharmacokinetics were assessed using a standard noncompartmental approach with estimation of the area under the concentration-time curve (AUC) by use of the trapezoidal rule.
Safety Assessment
Safety analyses included 30-day surgical morbidity and mortality and treatment-related AEs according to the Memorial Sloan Kettering Cancer Center surgical complication grading system.30,31 All patients underwent a baseline assessment within 28 days before surgery. After secondary cytoreduction, patients were assessed daily during hospitalization. After hospital discharge toxicities, AEs, hematology, and chemistries were tracked systematically. Patients were followed during standard postoperative IV platinum-based chemotherapy.
Disease Assessment
Postoperative chest and abdominopelvic computed tomography or magnetic resonance imaging was to be completed after secondary cytoreduction during a 28-day window. Disease was also assessed with imaging every 6 months for 2 years or if there was an unplanned imaging for suspected recurrence. CA-125 levels were measured every 3 months after secondary cytoreduction for 2 years but were not used to assess response or progression. Progression was determined by RECIST version 1.1. After 2 years, institutional standard follow-up guidelines were followed. The study radiologist who reviewed all scans was blinded to the treatment arm.
Statistical Considerations
We chose a pick-the-winner design by using a single-stage design in a phase II randomized trial to determine the winner arm. Each arm was powered to show an improvement over a historical control estimate of 24-month PFS rate of 25.5%.15,32 Each arm used the null hypothesis of 24-month PFS rate of 25.5% and an alternative hypothesis of 40%. With a type I error rate of 10% and type II error rate of 20%, a target accrual of 49 patients per arm was planned. Based on an exact binomial single-stage design, each regimen was considered efficacious if at least 17 of 49 patients were progression-free at 24 months. Early stopping rules were incorporated to halt the study if the overall rate of major postoperative complications was twice the acceptable rate (> 50%) following accrual of the first 20 patients and again after the first 49 patients. Secondary objectives included OS, postoperative complication rates, and completion rates of IV standard platinum-based chemotherapy in both arms. The primary objective of PFS rate at 24 months was reported as well as its exact one-sided 90% CI for each arm separately assuming binomial proportions. All except two patients had 24 months of follow-up. Both patients were in the standard arm with a follow-up of 22 months. Patients with < 24 months of follow-up were considered failures. The study was not powered for a head-to-head comparison of the two arms as in a phase III trial; each arm was compared to a historical control estimate.
Patient characteristics and other clinical factors were compared between the two arms using the Fisher exact test for categorical variables and Wilcoxon-rank sum test for continuous variables. Grade ≥ 3 postoperational AEs were summarized using descriptive statistics. PFS was calculated from the date of random assignment to the date of progression, death, or last clinical assessment, whichever occurred first. OS was calculated from the date of random assignment to the date of death or last follow-up. Survival was calculated using the Kaplan-Meier method. Log-rank test and Wald test based on the Cox proportional hazards (PH) model were used to obtain P values for categorical variables or continuous variables in the univariate setting. Multivariate PFS and OS models were built by using variables that were univariately significant (P < .05). The effect of HIPEC on survival among certain clinical factors' strata was modeled in a Cox PH model with the treatment arm, the clinical factor, and the interaction term. The hazard ratio (HR) of HIPEC versus no HIPEC for specific subgroups is presented in a forest plot. These analyses were exploratory; the study was not powered to detect interaction effects.
RESULTS
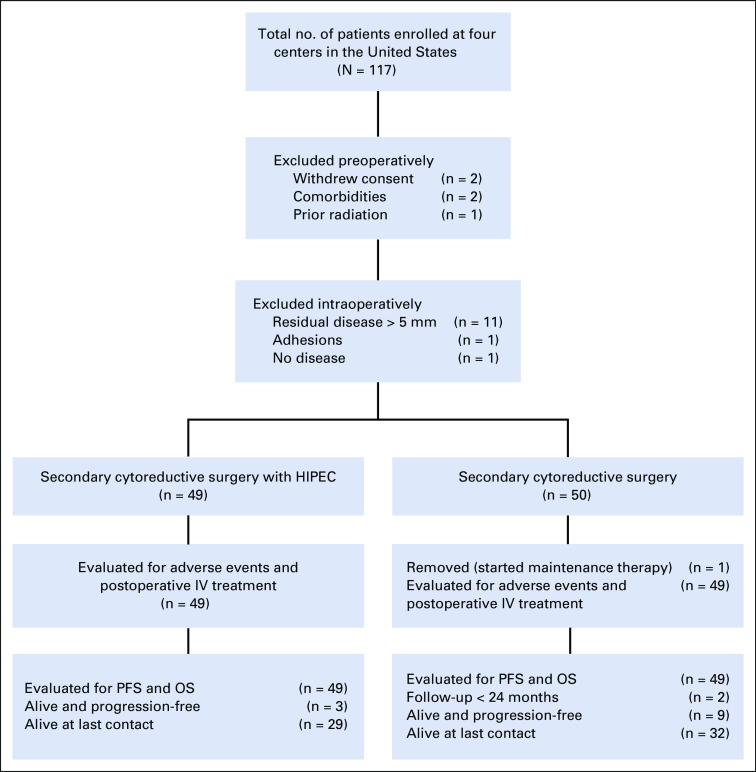
A total of 117 patients were consented from February 2014-November 2019 (Fig 1, CONSORT diagram). Of these patients, 99 were randomly assigned to secondary cytoreduction followed by six cycles or secondary cytoreduction with HIPEC with carboplatin at 800 mg/m2 followed by five cycles of IV carboplatin-based chemotherapy. Five patients withdrew or were found ineligible before planned surgery. Thirteen patients were not randomly assigned because of intraoperative findings (11 because of extent of disease, one because of extensive adhesions, and one because of absence of any evidence of disease). One patient was excluded from the study because of initiation of maintenance treatment before progression of disease.
FIG 1.
CONSORT diagram. HIPEC, hyperthermic intraperitoneal chemotherapy; IV, intravenous; OS, overall survival; PFS, progression-free survival.
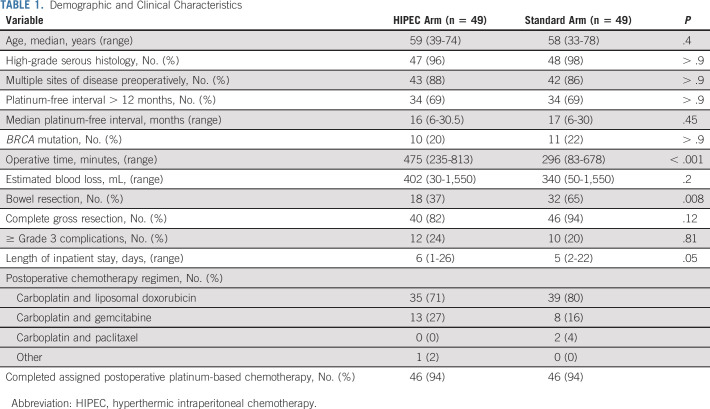
Demographic and clinical characteristics and specific intraoperative characteristics are presented in Table 1. Most variables were balanced between the groups. The median platinum-free interval for the entire cohort before study enrollment was 16 months; 69% had a platinum-free interval of 12-30 months. A complete gross resection (CGR) was achieved in 40 patients (82%) in the HIPEC arm and 46 (94%) in the standard arm (P = .12). Bowel resections were performed in 50 patients (51%). Significantly fewer patients in the HIPEC arm underwent a bowel resection. As expected, the median operative time was significantly longer in patients randomly assigned to HIPEC (474 minutes v 292 minutes; P < .001).
TABLE 1.
Demographic and Clinical Characteristics
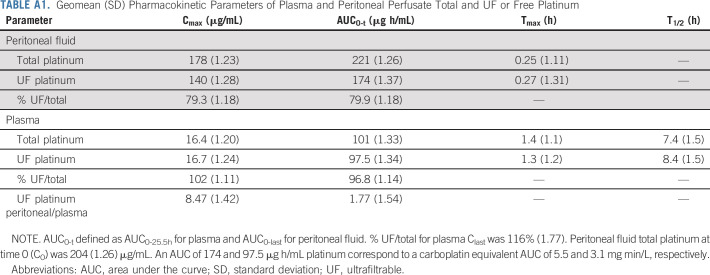
Platinum pharmacokinetics after carboplatin administered as HIPEC are shown in Figure 2. Figure 2A depicts the first 24 hours and Figure 2B the first 6 hours post HIPEC. The extrapolated time = 0 platinum concentration of 204 µg/mL corresponded with the 242 µg/mL theoretical starting concentration of the infusate based on the 800 mg/m2 carboplatin dose, typical body surface area, and the 3 L infusate volume (800 mg/m2·1.73 m2·(195/371)/3 L). Plasma platinum increased during the perfusion procedure and appeared to reach a steady state toward the end of the perfusion. The geometric mean (geometric standard deviation) maximum ultrafiltrable platinum concentrations in the peritoneum and plasma were 140 (1.28) μg/mL and 16.7 (1.24) μg/mL, respectively, with a peritoneum-to-plasma ratio of 8.47 (Appendix Table A1, online only). Corresponding platinum AUC values in the peritoneum and plasma were 174 (1.37) μg·h/mL and 97.5 (1.34) μg·h/mL, respectively, with a peritoneum-to-plasma ratio of 1.77. Post-treatment tumor tissue (1 cm3) was available from three patients and yielded a geometric mean (geometric standard deviation) platinum tissue concentration of 3.38 (1.62) µg/g. The majority of peritoneal and all of plasma total platinum exposure was accounted for by the respective ultrafiltrable portions.
FIG 2.
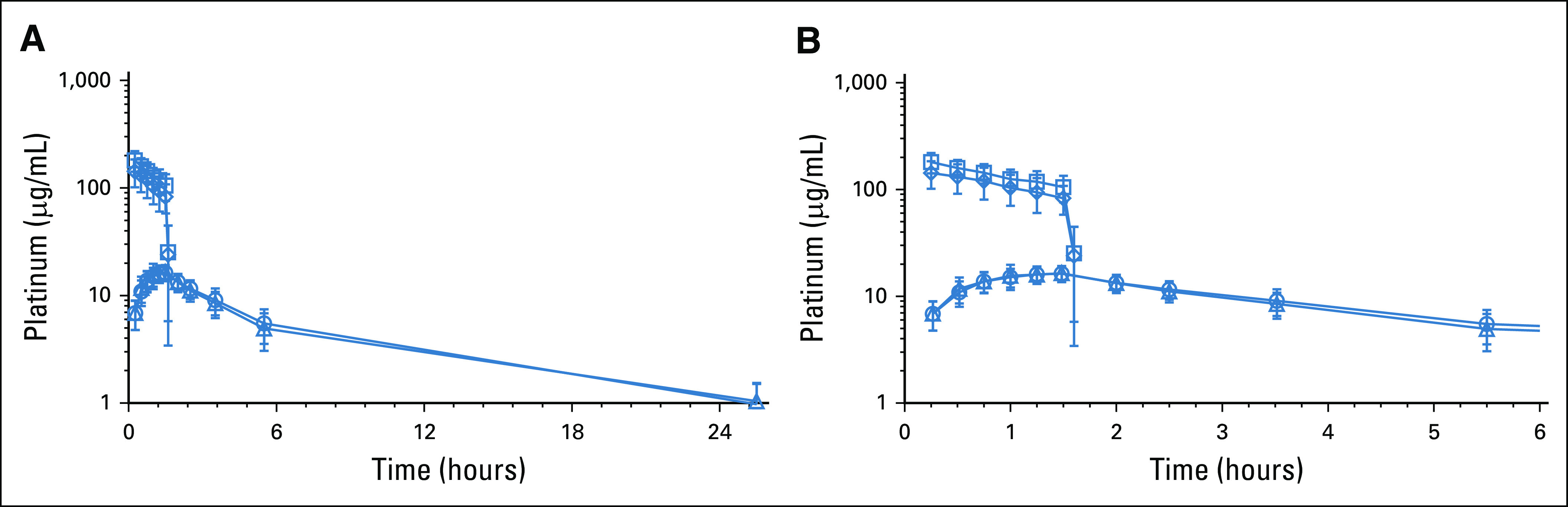
HIPEC carboplatin pharmacokinetics at (A) 24 hours and (B) 6 hours post HIPEC with carboplatin. HIPEC, hyperthermic intraperitoneal chemotherapy.
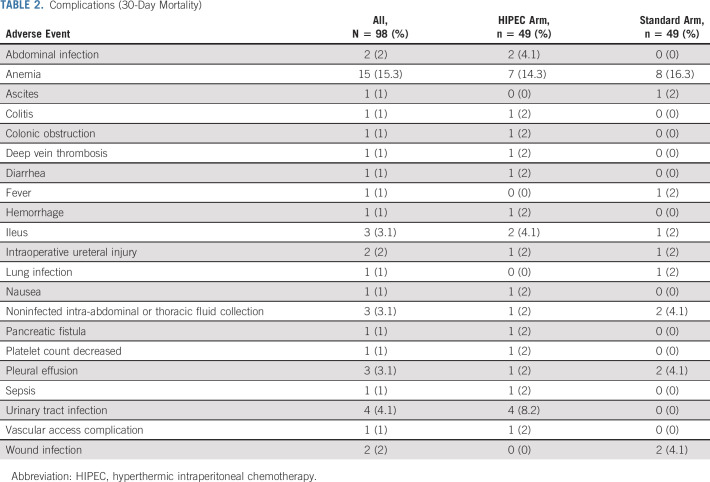
No 30-day mortality was observed. There was no difference in 30-day morbidity (Table 2); 12 patients (24%) in the HIPEC arm and 10 (20%) in the standard arm experienced a grade ≥ 3 AE (P = .81). The toxicity rate was 45% (9 patients) in the first 20 patients and 35% (17 patients) in the first 49 patients. The stopping threshold of > 50% toxicity rate was not observed. Overall, 92 patients (94%) completed all assigned postoperative IV chemotherapy treatment, with no difference between groups. The most common postoperative chemotherapy regimen used was carboplatin and liposomal doxorubicin in 74 patients (76%). The median time from surgery to initiation of postoperative IV chemotherapy was 32 days (18-91 days), without difference between arms.
TABLE 2.
Complications (30-Day Mortality)
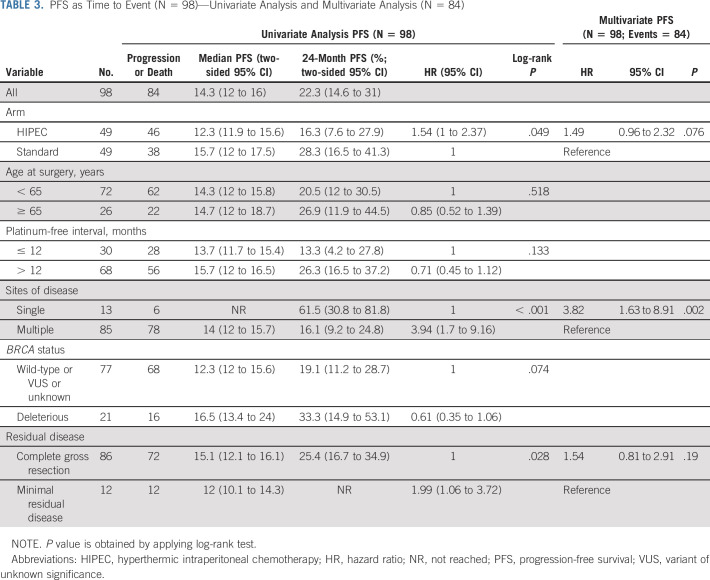
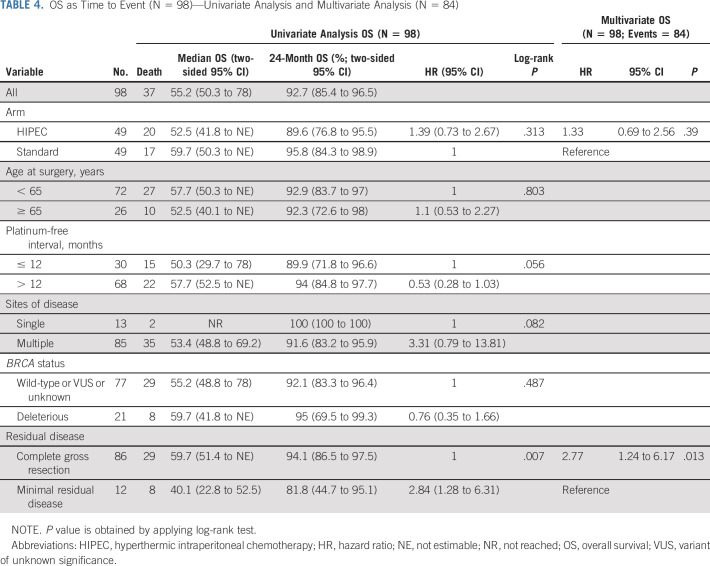
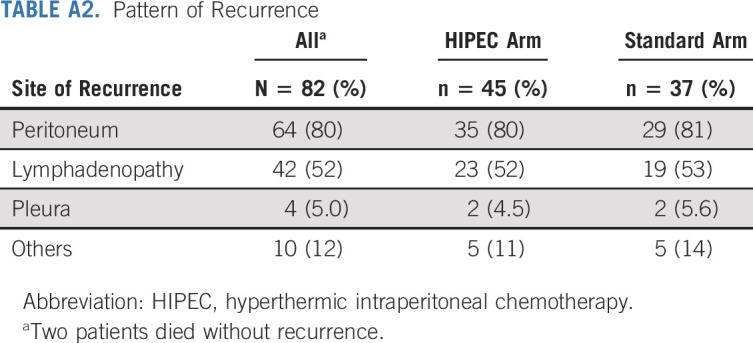
The last follow-up date was February 15, 2021. At that time, 82 patients had progressed and 37 had died. At 24 months, eight patients (16.3%; 1-sided 90% CI, 9.7 to 100) in the HIPEC arm and 12 (24.5%; 1-sided 90% CI, 16.5 to 100) in the standard arm were without progression or death. The median follow-up for the 14 progression-free survivors was 52.8 months (range, 51.4-88.9 months). The median follow-up for the 61 survivors was 39.5 months (range, 19.8-88.9 months). The median PFS and OS for the entire cohort were 14.3 months (95% CI, 12 to 16) and 55.2 months (95% CI, 50.3 to 78), respectively. The median PFS and OS for patients who achieved a CGR were 15.1 and 59.7 months, respectively. Survival curves are shown in Figures 3A and 3B. Patients randomly assigned to HIPEC had a median PFS of 12.3 months, compared with 15.7 months for patients randomly assigned to the standard arm (HR, 1.54; 95% CI, 1 to 2.37). Patients randomly assigned to HIPEC had a median OS of 52.5 months, compared with 59.7 months for patients randomly assigned to the standard arm (HR, 1.39; 95% CI, 0.73 to 2.67). Univariate analysis and multivariate analysis for PFS and OS are shown in Tables 3 and 4. Single-site disease was independently associated with improved PFS, whereas CGR was independently associated with OS. In a post-hoc blinded radiologic review of all recurrences, the two groups did not demonstrate differences in pattern of recurrence (Appendix Table A2, online only). An unplanned Cox PH model for PFS and OS was created to evaluate the effect of HIPEC on other patient variables (Appendix Figs A1 and A2, online only). In this analysis, patients with a long preceding platinum-free interval and patients with a known deleterious BRCA mutation appeared to have no PFS benefit with HIPEC.
FIG 3.
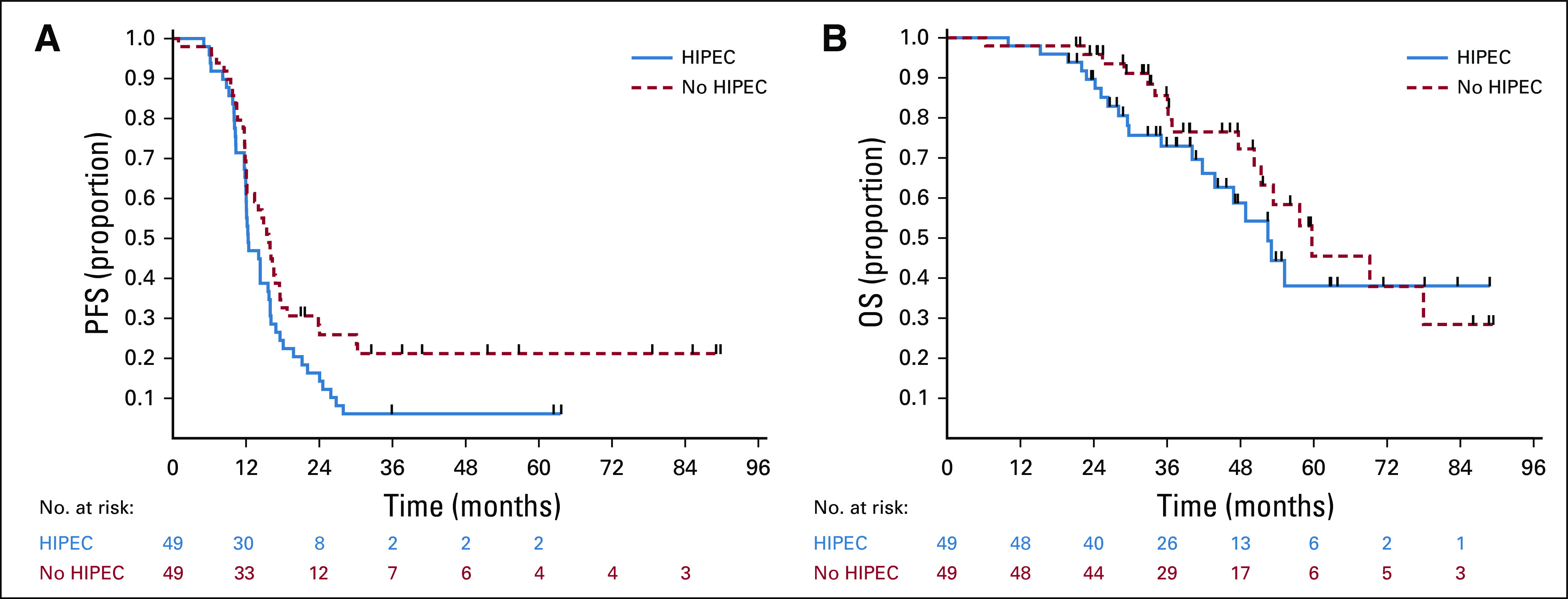
(A) PFS by treatment arm. Kaplan-Meier survival plots of PFS. (B) OS by treatment arm. Kaplan-Meier survival plots of OS. HIPEC, hyperthermic intraperitoneal chemotherapy; OS, overall survival; PFS, progression-free survival.
TABLE 3.
PFS as Time to Event (N = 98)—Univariate Analysis and Multivariate Analysis (N = 84)
TABLE 4.
OS as Time to Event (N = 98)—Univariate Analysis and Multivariate Analysis (N = 84)
DISCUSSION
This randomized, multicenter phase II study evaluated the safety and efficacy of HIPEC with carboplatin in patients undergoing secondary cytoreduction for platinum-sensitive recurrent ovarian cancer. Carboplatin administered as HIPEC was well tolerated. No perioperative mortality or increased perioperative morbidity or toxicity was observed with HIPEC. A winner was not determined because no treatment arm achieved our prespecified end point of 17 disease-free patients at 24 months. This prespecified end point was based on a retrospective analysis of select patients undergoing secondary cytoreduction at our center.15 Secondary cytoreductive surgery with 800 mg/m2 carboplatin HIPEC followed by five cycles of IV carboplatin-based chemotherapy was not superior to surgery without HIPEC followed by six cycles of IV carboplatin-based chemotherapy. Further evaluation of this regimen is not warranted at this time.
Our trial design does not permit direct arm-to-arm comparison. The comparisons of survival estimates and subgroup analyses are hypothesis-generating and should be interpreted with caution. The secondary post-hoc analyses, however, support the primary conclusion. Furthermore, at the time of study design, carboplatin HIPEC was chosen because of concerns of cisplatin-induced nephrotoxicity.8 The dose of 800 mg/m2 was based on previous studies in patients with ovarian cancer. In a phase I study, the recommended dose of carboplatin used as HIPEC was 1,000 mg/m2, with dose-limiting toxicity observed at 1,200 mg/m2.27 In GOG252, carboplatin was administered intraperitoneally at a dose of AUC 6, in a volume of up to 2 L, without retrieval of infusate.5 The dose of carboplatin at AUC 6 corresponds to approximately 400 mg/m2, depending on kidney function.33 Peritoneal exposure was 174 µg·h/mL ultrafiltrable platinum, which corresponds to a conventional ultrafiltrable carboplatin AUC of 5.5 mg·min/L. Similarly, the plasma exposure corresponds to an ultrafiltrable carboplatin AUC of 3.1 mg·min/L. However, the latter plasma exposure is possibly not biologically active. Although after IV carboplatin dosing, ultrafiltrable platinum corresponds to free and still biologically active carboplatin,34 the same may not be true of plasma ultrafiltrable platinum after peritoneal cavity dosing. Since total plasma platinum is accounted for by ultrafiltrable platinum, especially at the late 24-hour time point, this suggests that plasma platinum, although having low molecular weight and being ultrafiltrable, is no longer capable of reaction and consequently is inactive. If the ultrafiltrable (low-molecular-weight) platinum had been reactive, total platinum should have exceeded ultrafiltrable platinum at the late time point, the difference being the reaction product of reactive platinum and macromolecules such as albumin.35 Therefore, the 1.77-fold AUC ratio of peritoneum to plasma is likely a large underestimate of the true exposure advantage to reactive platinum that IP administration conveys. The tumor platinum tissue concentration of 3.38 (1.62) µg/g compared relatively favorable to the approximately 0.7 µg/g observed in preclinical rat models.8,36 Furthermore, carboplatin exposure was truncated because the carboplatin perfusate was removed immediately following HIPEC. It is unclear if a higher carboplatin dose, leaving the carboplatin in the peritoneum, or the use of cisplatin would have resulted in superior outcomes.
Furthermore, patients randomly assigned to no HIPEC received one additional cycle of standard postoperative IV chemotherapy. When the study was designed, the intraoperative administration of carboplatin was counted toward the first of six cycles of chemotherapy to avoid an overestimate of the HIPEC effect.
Although stratification was not necessary in this noncomparative randomized phase II trial, differences in prognostic factors between arms should be considered when interpreting the survival estimates. We did not stratify for residual disease after surgery, which is a strong prognostic factor. Although not statistically significant, 40 patients (82%) achieved a CGR in the HIPEC arm compared with 46 (94%) in the standard arm, favoring the standard arm. Patients were randomly assigned after cytoreduction to < 0.5-cm residual disease. Despite protocol-specific intraoperative random assignment, bowel resections were performed less frequently in the HIPEC arm. Morbidity concerns possibly resulted in an underuse of bowel resections in the HIPEC arm. In patients randomly assigned to HIPEC, surgeons potentially accepted minimal residual disease when a CGR was achievable with a bowel resection. Future head-to-head comparison studies should stratify by residual status to reduce bias following intraoperative random assignment. We did not, however, observe a higher rate of ostomies in the HIPEC arm. This was observed in a previous randomized trial, which could also be a result of intraoperative bias.10
The obvious negative aspect of intraoperative random assignment is the longer operative time. The logistical challenges of this clinical trial with preparation of the drug, transportation to the operative room, and the setup of the perfusion machine after random assignment and the HIPEC on average resulted in a 3-hour longer procedure, likely significantly overestimating the additional time needed for HIPEC in a nonexperimental setting.
Although no maintenance treatment was approved for patients with platinum-sensitive recurrent ovarian cancer at the time of study design, the FDA granted approval to both bevacizumab and polyadenosine diphosphate-ribose polymerase inhibitors while the study was ongoing.37-40 To avoid potential treatment imbalance between arms, we did not permit the use of any maintenance treatment. This was carefully discussed with the patients during the informed consent process. Furthermore, patients with a platinum-free interval of > 30 months were ineligible, resulting in a median platinum-free interval of 16 months, which is substantially shorter than what was reported in recent studies evaluating the role of secondary cytoreduction in patients with platinum-sensitive ovarian cancer.13,41 In addition, a bowel resection was necessary in 51% of patients as part of cytoreductive surgery. This is significantly more than reported in GOG213 (28%), suggesting that patients enrolled in this trial had higher disease burden. The omission of maintenance treatment, the shorter platinum-free interval, and the inclusion of higher-risk patients explains the shorter median PFS in our study. Despite this, the OS was comparable to GOG213, suggesting that maintenance strategies were successfully administered in later lines of therapy.
Secondary cytoreduction and HIPEC with carboplatin was well tolerated but did not result in superior outcomes compared with standard of care as estimated by a historical control estimate. Our study does not support the use of HIPEC with carboplatin at 800 mg/m2 during secondary cytoreduction. Further studies are needed to address how to best incorporate HIPEC with optimization of the intraoperative random assignment process to minimize surgeon bias as well as careful consideration for relevant stratification factors, such as residual disease and BRCA status.
APPENDIX
FIG A1.
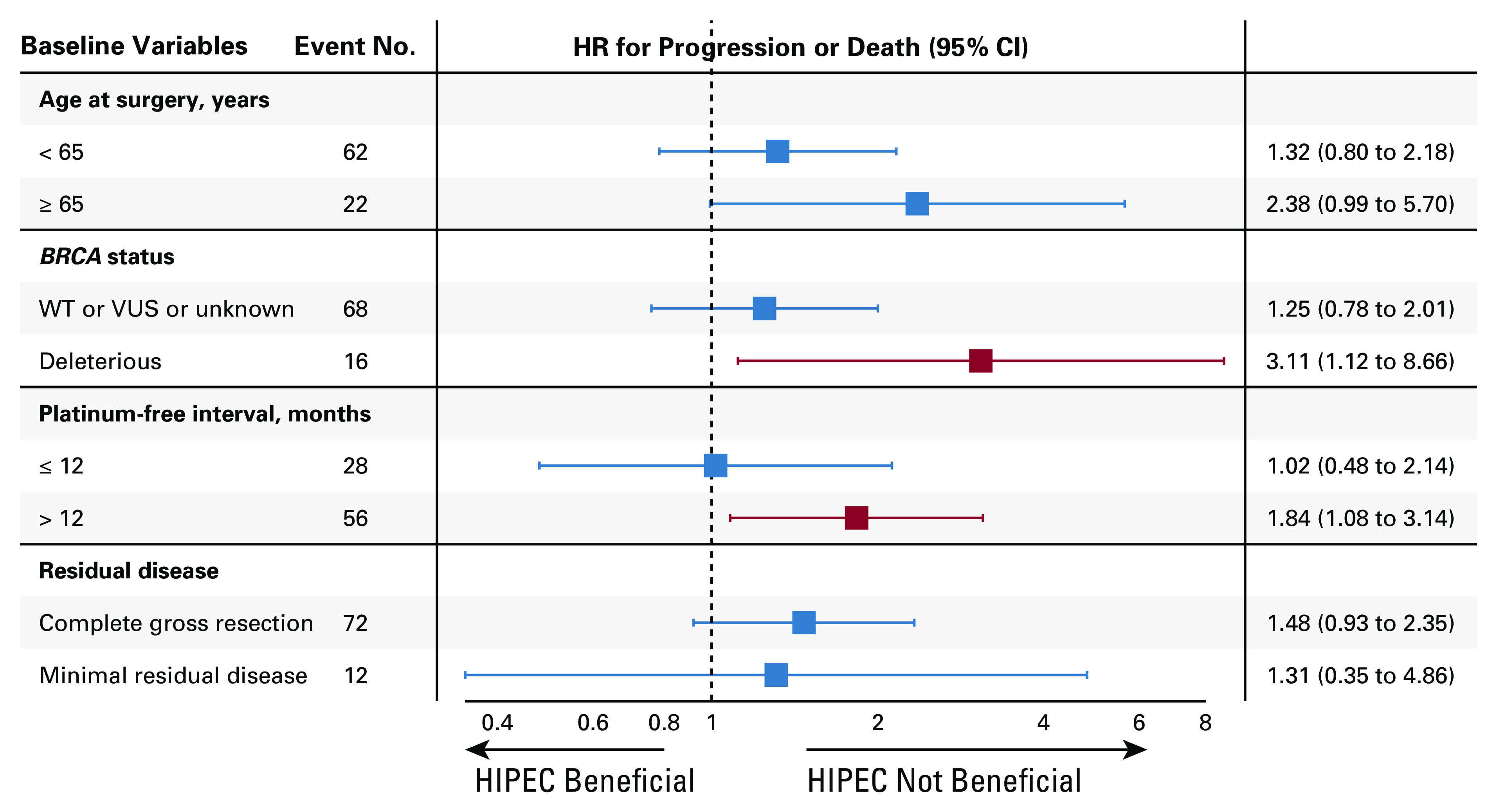
Forest plot for baseline variables and HR for progression or death. HIPEC, hyperthermic intraperitoneal chemotherapy; HR, hazard ratio; VUS, variant of unknown significance; WT, wild type.
FIG A2.
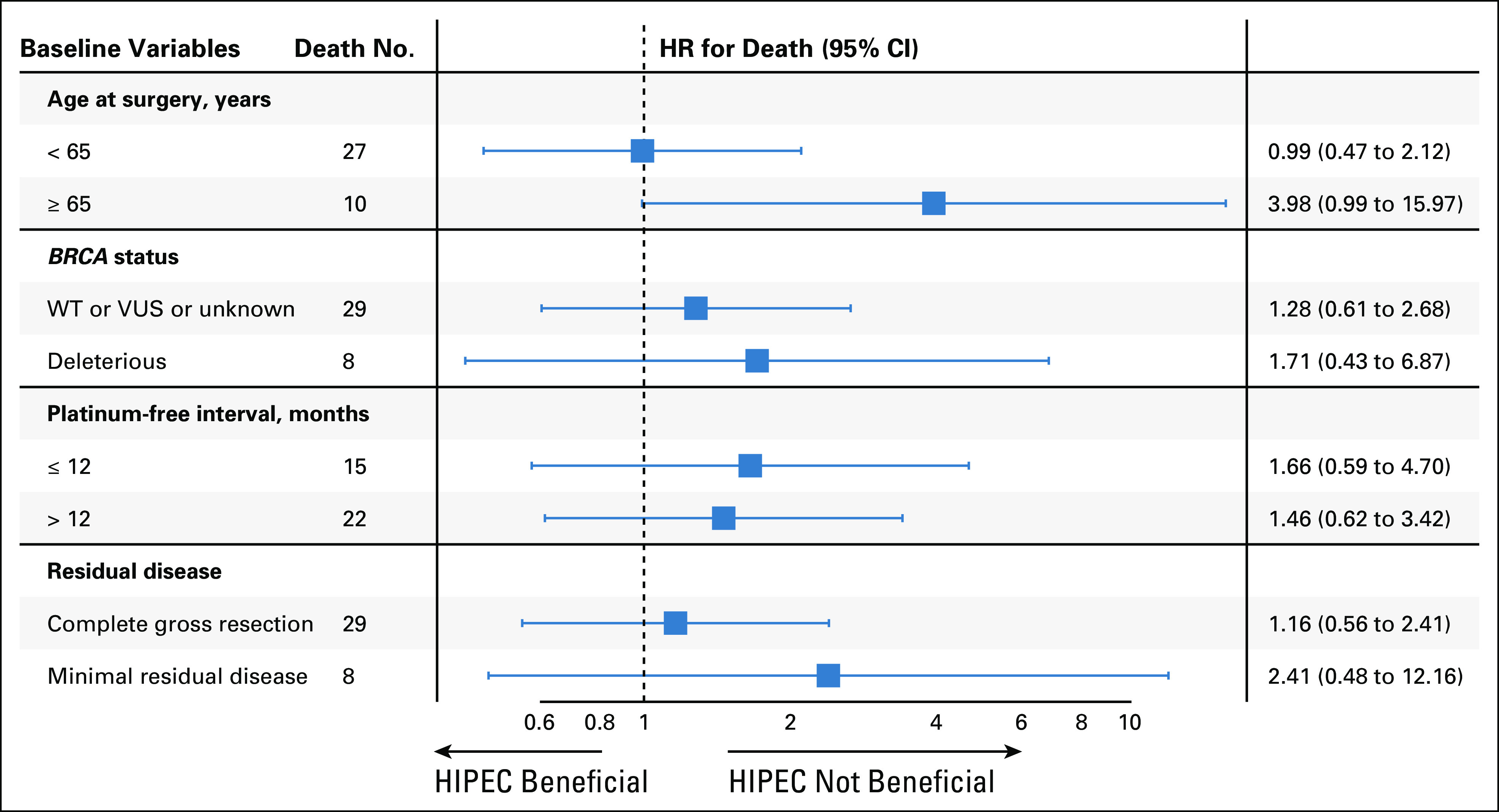
Forest plot for baseline variables and HR for death. HIPEC, hyperthermic intraperitoneal chemotherapy; HR, hazard ratio; VUS, variant of unknown significance; WT, wild type.
TABLE A1.
Geomean (SD) Pharmacokinetic Parameters of Plasma and Peritoneal Perfusate Total and UF or Free Platinum
TABLE A2.
Pattern of Recurrence
Dennis S. Chi
Leadership: CSurgeries
Stock and Other Ownership Interests: Bovie Medical, Verthermia, Intuitive Surgical, Transenterix
Consulting or Advisory Role: Bovie Medical, Verthermia, Biom'up
Alexia Iasonos
Stock and Other Ownership Interests: Bristol Myers Squibb/Sanofi
Consulting or Advisory Role: Mylan, BrightPath Biotheraputics, Intelligencia
Jason A. Konner
Consulting or Advisory Role: Immunogen, AstraZeneca, Tesaro, AbbVie
Research Funding: Genentech/Roche
Travel, Accommodations, Expenses: AstraZeneca
Vicky Makker
Honoraria: Eisai, Merck, Karyopharm Therapeutics, IBM Watson Health
Consulting or Advisory Role: Eisai, Merck, Karyopharm Therapeutics, Takeda, ArQule, IBM Watson Health, GlaxoSmithKline, Clovis Oncology, Faeth
Research Funding: Lilly, AstraZeneca, Eisai, Merck, Bristol Myers Squibb, Karyopharm Therapeutics, Takeda, Clovis Oncology, Bayer, Zymeworks
Travel, Accommodations, Expenses: Eisai, Merck, Karyopharm Therapeutics
Other Relationship: IBM
Rachel N. Grisham
Consulting or Advisory Role: Mateon Therapeutics, Clovis Oncology, Regeneron, GlaxoSmithKline
Research Funding: Context Therapeutics
Travel, Accommodations, Expenses: EMD Serono
Other Relationship: Prime Oncology, MCM Education, OncLive, Aptitude Health
Uncompensated Relationships: Verastem
Amy K. Brown
Speakers' Bureau: Tesaro
Research Funding: Tesaro, AstraZeneca
John P. Diaz
Speakers' Bureau: AstraZeneca
Carrie L. Langstraat
Other Relationship: Eight Medical, Covidien/Medtronic, ConMed
Yulia Lakhman
Stock and Other Ownership Interests: Y-mAbs Therapeutics
Krysten Soldan
Consulting or Advisory Role: Eisai
Travel, Accommodations, Expenses: Clovis Oncology
Ginger J. Gardner
Honoraria: BioAscent
Travel, Accommodations, Expenses: BioAscent
Elizabeth L. Jewell
Honoraria: Survivornet
Consulting or Advisory Role: Intuitive Surgical, Covidien/Medtronic
Research Funding: Summit Biomedical
Tiffany Troso-Sandoval
Honoraria: Bio Ascend
Travel, Accommodations, Expenses: Bio Ascend
Stuart M. Lichtman
Consulting or Advisory Role: Remedy One, Magellan Health
Nadeem R. Abu-Rustum
Honoraria: Prime Oncology
Research Funding: Stryker/Novadaq, Grail
Travel, Accommodations, Expenses: Prime Oncology
Carol Aghajanian
Consulting or Advisory Role: Mersana, Eisai, Roche, AbbVie, Eisai, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche, AbbVie, Clovis Oncology, AstraZeneca
Jan Beumer
Employment: Voisin Consulting
Stock and Other Ownership Interests: GlaxoSmithKline
Consulting or Advisory Role: GNS Healthcare
Research Funding: AbbVie, Spectrum Pharmaceuticals, TriSalus Life Sciences
Patents, Royalties, Other Intellectual Property: Sulphoraphane for Melanoma Chemoprevention
Expert Testimony: Pfizer
Uncompensated Relationships: Qrono
Yukio Sonoda
Patents, Royalties, Other Intellectual Property: Pending Patent for a surgical instrument (uterine manipulator)
Roisin E. O'Cearbhaill
Honoraria: GlaxoSmithKline
Consulting or Advisory Role: GlaxoSmithKline
Research Funding: Juno Therapeutics, Sellas Life Sciences, Ludwig Institute for Cancer Research, Stem CentRx, TapImmune Inc, TCR2 Therapeutics, Regeneron, Genmab, Atara Biotherapeutics, GlaxoSmithKline, AstraZeneca/Merck, Syndax, Genentech, Kite/Gilead, Gynecologic Oncology Group Foundation
Travel, Accommodations, Expenses: AstraZeneca
Open Payments Link: https://openpaymentsdata.cms.gov/physician/785539
No other potential conflicts of interest were reported.
SUPPORT
Supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. This project used the UPMC Hillman Cancer Center's Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by Award P30 CA47904. The study was also funded in part by Cycle for Survival, the Baird Family Foundation, and the Weickart Ovarian Cancer Postdoctoral Fellowship.
CLINICAL TRIAL INFORMATION
O.Z. and R.E.O. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Oliver Zivanovic, Dennis S. Chi, Alexia Iasonos, John P. Diaz, Vaagn Andikyan, Kara Long Roche, Carol Aghajanian, William P. Tew, Yukio Sonoda, Roisin E. O'Cearbhaill
Administrative support: Katy Su
Provision of study materials or patients: Oliver Zivanovic, Dennis S. Chi, Jason A. Konner, Amy K. Brown, Stacy Nerenstone, John P. Diaz, Krysten Soldan, Ginger J. Gardner, Kara Long Roche, Tiffany Troso-Sandoval, Stuart M. Lichtman, Carol Aghajanian, Yukio Sonoda, Roisin E. O'Cearbhaill
Collection and assembly of data: Oliver Zivanovic, Dennis S. Chi, Jason A. Konner, Vicky Makker, Rachel N. Grisham, Amy K. Brown, Stacy Nerenstone, John P. Diaz, Eric D. Schroeder, Carrie L. Langstraat, Viktoriya Paroder, Krysten Soldan, Katy Su, Ginger J. Gardner, Jianxia Guo, Elizabeth L. Jewell, Kara Long Roche, Tiffany Troso-Sandoval, Stuart M. Lichtman, Kimberly Dessources, William P. Tew, Jan Beumer, Yukio Sonoda, Roisin E. O'Cearbhaill
Data analysis and interpretation: Oliver Zivanovic, Qin Zhou, Alexia Iasonos, Jason A. Konner, Rachel N. Grisham, Carrie L. Langstraat, Viktoriya Paroder, Yulia Lakhman, Katy Su, Jianxia Guo, Kara Long Roche, Lea A. Moukarzel, Nadeem R. Abu-Rustum, Carol Aghajanian, William P. Tew, Jan Beumer, Yukio Sonoda, Roisin E. O'Cearbhaill
Manuscript writing: Oliver Zivanovic, Qin Zhou, Alexia Iasonos, Jan Beumer, Roisin E. O'Cearbhaill
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Secondary Cytoreduction and Carboplatin Hyperthermic Intraperitoneal Chemotherapy for Platinum-Sensitive Recurrent Ovarian Cancer: An MSK Team Ovary Phase II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Dennis S. Chi
Leadership: CSurgeries
Stock and Other Ownership Interests: Bovie Medical, Verthermia, Intuitive Surgical, Transenterix
Consulting or Advisory Role: Bovie Medical, Verthermia, Biom'up
Alexia Iasonos
Stock and Other Ownership Interests: Bristol Myers Squibb/Sanofi
Consulting or Advisory Role: Mylan, BrightPath Biotheraputics, Intelligencia
Jason A. Konner
Consulting or Advisory Role: Immunogen, AstraZeneca, Tesaro, AbbVie
Research Funding: Genentech/Roche
Travel, Accommodations, Expenses: AstraZeneca
Vicky Makker
Honoraria: Eisai, Merck, Karyopharm Therapeutics, IBM Watson Health
Consulting or Advisory Role: Eisai, Merck, Karyopharm Therapeutics, Takeda, ArQule, IBM Watson Health, GlaxoSmithKline, Clovis Oncology, Faeth
Research Funding: Lilly, AstraZeneca, Eisai, Merck, Bristol Myers Squibb, Karyopharm Therapeutics, Takeda, Clovis Oncology, Bayer, Zymeworks
Travel, Accommodations, Expenses: Eisai, Merck, Karyopharm Therapeutics
Other Relationship: IBM
Rachel N. Grisham
Consulting or Advisory Role: Mateon Therapeutics, Clovis Oncology, Regeneron, GlaxoSmithKline
Research Funding: Context Therapeutics
Travel, Accommodations, Expenses: EMD Serono
Other Relationship: Prime Oncology, MCM Education, OncLive, Aptitude Health
Uncompensated Relationships: Verastem
Amy K. Brown
Speakers' Bureau: Tesaro
Research Funding: Tesaro, AstraZeneca
John P. Diaz
Speakers' Bureau: AstraZeneca
Carrie L. Langstraat
Other Relationship: Eight Medical, Covidien/Medtronic, ConMed
Yulia Lakhman
Stock and Other Ownership Interests: Y-mAbs Therapeutics
Krysten Soldan
Consulting or Advisory Role: Eisai
Travel, Accommodations, Expenses: Clovis Oncology
Ginger J. Gardner
Honoraria: BioAscent
Travel, Accommodations, Expenses: BioAscent
Elizabeth L. Jewell
Honoraria: Survivornet
Consulting or Advisory Role: Intuitive Surgical, Covidien/Medtronic
Research Funding: Summit Biomedical
Tiffany Troso-Sandoval
Honoraria: Bio Ascend
Travel, Accommodations, Expenses: Bio Ascend
Stuart M. Lichtman
Consulting or Advisory Role: Remedy One, Magellan Health
Nadeem R. Abu-Rustum
Honoraria: Prime Oncology
Research Funding: Stryker/Novadaq, Grail
Travel, Accommodations, Expenses: Prime Oncology
Carol Aghajanian
Consulting or Advisory Role: Mersana, Eisai, Roche, AbbVie, Eisai, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche, AbbVie, Clovis Oncology, AstraZeneca
Jan Beumer
Employment: Voisin Consulting
Stock and Other Ownership Interests: GlaxoSmithKline
Consulting or Advisory Role: GNS Healthcare
Research Funding: AbbVie, Spectrum Pharmaceuticals, TriSalus Life Sciences
Patents, Royalties, Other Intellectual Property: Sulphoraphane for Melanoma Chemoprevention
Expert Testimony: Pfizer
Uncompensated Relationships: Qrono
Yukio Sonoda
Patents, Royalties, Other Intellectual Property: Pending Patent for a surgical instrument (uterine manipulator)
Roisin E. O'Cearbhaill
Honoraria: GlaxoSmithKline
Consulting or Advisory Role: GlaxoSmithKline
Research Funding: Juno Therapeutics, Sellas Life Sciences, Ludwig Institute for Cancer Research, Stem CentRx, TapImmune Inc, TCR2 Therapeutics, Regeneron, Genmab, Atara Biotherapeutics, GlaxoSmithKline, AstraZeneca/Merck, Syndax, Genentech, Kite/Gilead, Gynecologic Oncology Group Foundation
Travel, Accommodations, Expenses: AstraZeneca
Open Payments Link: https://openpaymentsdata.cms.gov/physician/785539
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021 CA Cancer J Clin 717–332021 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer N Engl J Med 35434–432006 [DOI] [PubMed] [Google Scholar]
- 3.Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: A Gynecologic Oncology Group study J Clin Oncol 331460–14662015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright AA, Cronin A, Milne DE, et al. Use and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancer J Clin Oncol 332841–28472015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker JL, Brady MF, Wenzel L, et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: An NRG Oncology/Gynecologic Oncology Group study J Clin Oncol 371380–13902019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zivanovic O, Abramian A, Kullmann M, et al. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer Int J Cancer 136699–7082015 [DOI] [PubMed] [Google Scholar]
- 7.Los G, van Vugt MJ, den Engelse L, et al. Effects of temperature on the interaction of cisplatin and carboplatin with cellular DNA Biochem Pharmacol 461229–12371993 [DOI] [PubMed] [Google Scholar]
- 8.Los G, van Vugt MJ, Pinedo HM.Response of peritoneal solid tumours after intraperitoneal chemohyperthermia treatment with cisplatin or carboplatin Br J Cancer 69235–2411994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Los G, Verdegaal E, Noteborn HPJM, et al. Cellular pharmacokinetics of carboplatin and cisplatin in relation to their cytotoxic action Biochem Pharmacol 42357–3631991 [DOI] [PubMed] [Google Scholar]
- 10.van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer N Engl J Med 378230–2402018 [DOI] [PubMed] [Google Scholar]
- 11.Koole SN, van Lieshout C, van Driel WJ, et al. Cost effectiveness of interval cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in stage III ovarian cancer on the basis of a randomized phase III trial J Clin Oncol 372041–20502019 [DOI] [PubMed] [Google Scholar]
- 12.Lei Z, Wang Y, Wong J, et al. Evaluation of cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for stage III epithelial ovarian cancer. JAMA Netw Open. 2020;3:e2013940. doi: 10.1001/jamanetworkopen.2020.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer N Engl J Med 3811929–19392019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bristow RE, Puri I, Chi DS.Cytoreductive surgery for recurrent ovarian cancer: A meta-analysis Gynecol Oncol 112265–2742009 [DOI] [PubMed] [Google Scholar]
- 15.Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma Cancer 1061933–19392006 [DOI] [PubMed] [Google Scholar]
- 16.Harter P, du Bois A, Hahmann M, et al. Surgery in recurrent ovarian cancer: The Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial Ann Surg Oncol 131702–17102006 [DOI] [PubMed] [Google Scholar]
- 17.Harter P, Hahmann M, Leuck HJ, et al. Surgery for recurrent ovarian cancer: Role of peritoneal carcinomatosis: Exploratory analysis of the DESKTOP I trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis Ann Surg Oncol 161324–13302009 [DOI] [PubMed] [Google Scholar]
- 18.Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: A prospective randomized phase III study Ann Surg Oncol 221570–15752015 [DOI] [PubMed] [Google Scholar]
- 19.Konigsrainer I, Beckert S, Becker S, et al. Cytoreductive surgery and HIPEC in peritoneal recurrent ovarian cancer: Experience and lessons learned Langenbecks Arch Surg 3961077–10812011 [DOI] [PubMed] [Google Scholar]
- 20.Fagotti A, Costantini B, Petrillo M, et al. Cytoreductive surgery plus HIPEC in platinum-sensitive recurrent ovarian cancer patients: A case-control study on survival in patients with two year follow-up Gynecol Oncol 127502–5052012 [DOI] [PubMed] [Google Scholar]
- 21.Bakrin N, Cotte E, Golfier F, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: A multicenter, prospective study of 246 patients Ann Surg Oncol 194052–40582012 [DOI] [PubMed] [Google Scholar]
- 22.Gould N, Sill MW, Mannel RS, et al. A phase I study with an expanded cohort to assess feasibility of intravenous docetaxel, intraperitoneal carboplatin and intraperitoneal paclitaxel in patients with previously untreated ovarian, fallopian tube or primary peritoneal carcinoma: A Gynecologic Oncology Group study Gynecol Oncol 127506–5102012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan MA, Sill MW, Fujiwara K, et al. A phase I study with an expanded cohort to assess the feasibility of intraperitoneal carboplatin and intravenous paclitaxel in untreated ovarian, fallopian tube, and primary peritoneal carcinoma: A Gynecologic Oncology Group study Gynecol Oncol 121264–2682011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer 45228–2472009 [DOI] [PubMed] [Google Scholar]
- 25.Cowan RA, Eriksson AGZ, Jaber SM, et al. A comparative analysis of prediction models for complete gross resection in secondary cytoreductive surgery for ovarian cancer Gynecol Oncol 145230–2352017 [DOI] [PubMed] [Google Scholar]
- 26.Steller MA, Egorin MJ, Trimble EL, et al. A pilot phase I trial of continuous hyperthermic peritoneal perfusion with high-dose carboplatin as primary treatment of patients with small-volume residual ovarian cancer Cancer Chemother Pharmacol 43106–1141999 [DOI] [PubMed] [Google Scholar]
- 27.Lentz SS, Miller BE, Kucera GL, et al. Intraperitoneal hyperthermic chemotherapy using carboplatin: a phase I analysis in ovarian carcinoma Gynecol Oncol 106207–2102007 [DOI] [PubMed] [Google Scholar]
- 28.Argenta PA, Sueblinvong T, Geller MA, et al. Hyperthermic intraperitoneal chemotherapy with carboplatin for optimally-cytoreduced, recurrent, platinum-sensitive ovarian carcinoma: A pilot study Gynecol Oncol 12981–852013 [DOI] [PubMed] [Google Scholar]
- 29.Joshi A, Guo J, Holleran JL, et al. Evaluation of the pharmacokinetic drug-drug interaction potential of iohexol, a renal filtration marker Cancer Chemother Pharmacol 86535–5452020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strong VE, Selby LV, Sovel M, et al. Development and assessment of Memorial Sloan Kettering Cancer Center's Surgical Secondary Events grading system Ann Surg Oncol 221061–10672015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grobmyer SR, Pieracci FM, Allen PJ, et al. Defining morbidity after pancreaticoduodenectomy: Use of a prospective complication grading system J Am Coll Surg 204356–3642007 [DOI] [PubMed] [Google Scholar]
- 32.Simon R, Wittes RE, Ellenberg SS.Randomized phase II clinical trials Cancer Treat Rep 691375–13811985 [PubMed] [Google Scholar]
- 33.Bookman MA, McGuire WP, Kilpatrick D, et al. Carboplatin and paclitaxel in ovarian carcinoma: a phase I study of the Gynecologic Oncology Group J Clin Oncol 141895–19021996 [DOI] [PubMed] [Google Scholar]
- 34.Harland SJ, Newell DR, Siddik ZH, et al. Pharmacokinetics of cis-diammine-1,1-cyclobutane dicarboxylate platinum(II) in patients with normal and impaired renal function Cancer Res 441693–16971984 [PubMed] [Google Scholar]
- 35.Eads JR, Beumer JH, Negrea L, et al. A pharmacokinetic analysis of cisplatin and 5-fluorouracil in a patient with esophageal cancer on peritoneal dialysis Cancer Chemother Pharmacol 77333–3382016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Los G, Smals OA, van Vugt MJ, et al. A rationale for carboplatin treatment and abdominal hyperthermia in cancers restricted to the peritoneal cavity Cancer Res 521252–12581992 [PubMed] [Google Scholar]
- 37.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer J Clin Oncol 302039–20452012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer N Engl J Med 3752154–21642016 [DOI] [PubMed] [Google Scholar]
- 39.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial Lancet Oncol 181274–12842017 [DOI] [PubMed] [Google Scholar]
- 40.Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial Lancet Oncol 18779–7912017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi T, Zhu J, Feng Y, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): A multicentre, open-label, randomised, phase 3 trial Lancet Oncol 22439–4492021 [DOI] [PubMed] [Google Scholar]