Abstract
Purpose: Physiotherapists have an important role to play in the early detection and treatment of lymphedema, a chronic inflammatory condition characterized by excess interstitial protein-rich fluid, which is estimated to affect more than one million Canadians. Obesity has been identified both as an important cause of and as a risk factor for developing lymphedema of various aetiologies. Little is currently known about obesity in Canadians affected by lymphedema. The objective of this study was to report on the prevalence of overweight and obesity in a Canadian lymphedema clinic population and the relationships among BMI; demographic, medical, and lymphedema characteristics; and cellulitis history. Method: We conducted a retrospective electronic record review of the clinical data collected from new patients evaluated for suspected lymphedema at a specialized Canadian hospital-based clinic over a 2-year period. We used descriptive analyses to characterize the sample and one-way analysis of variance and χ2 tests for comparative analyses. Results: Of the 178 patients whose records were reviewed, 36.5% were classified as overweight and 39.3% as obese. Patients with non-cancer diagnoses had a higher mean BMI than those with cancer-related diagnoses (p < 0.001). A higher BMI was associated with a longer time since lymphedema onset (p < 0.001), bilateral lymphedema (p = 0.010), and history of cellulitis (p < 0.001). Conclusions:Obesity is prevalent in the Canadian population with lymphedema and is associated with delayed referral and increased cellulitis rates. Early detection and tailored management strategies are needed to address obesity in patients with lymphedema and the complexities associated with these two related conditions.
Key Words: cellulitis, edema, lymphedema, neoplasms, obesity
Abstract
Objectif : les physiothérapeutes ont un rôle important à jouer pour favoriser le dépistage précoce et le traitement du lymphœdème, un trouble inflammatoire chronique caractérisé par un excès de liquide interstitiel riche en protéines qui toucherait plus d’un million de Canadiens. L’obésité est considérée à la fois comme une cause importante et un facteur de risque de lymphœdème de diverses étiologies. On ne sait pas grand-chose sur l’obésité des Canadiens atteints de lymphœdème. La présente étude visait à rendre compte de la prévalence de surpoids et d’obésité dans la population de la clinique canadienne de lymphœdème des chercheurs et des liens entre l’indice de masse corporelle (IMC), les données démographiques, les caractéristiques médicales et lymphœdémateuses et les antécédents de cellulite. Méthodologie : les chercheurs ont procédé à une analyse rétrospective des données cliniques tirées de dossiers électroniques de nouveaux patients évalués à cause d’une présomption de lymphœdème dans une clinique hospitalière canadienne spécialisée, et ce, sur une période de deux ans. Ils ont utilisé des analyses descriptives pour caractériser l’échantillon et procédé aux évaluations comparatives à l’aide des analyses de variance unidirectionnelle et des tests du chi carré. Résultats : des 178 patients dont le dossier a été examiné, 36,5 % étaient classés comme en surpoids et 39,3 % comme obèses. Les patients dont le diagnostic n’était pas lié au cancer présentaient un IMC moyen plus élevé que ceux dont le diagnostic était lié au cancer (p < 0,001). Un IMC plus élevé chez les patients s’associait à une période plus longue depuis l’apparition de lymphœdème (p < 0,001), à un lymphœdème bilatéral (p = 0,010) et à des antécédents de cellulite (p < 0,001). Conclusion :L’obésité est prévalente dans la population canadienne atteinte de lymphœdème et s’associe à un délai avant l’envoi en consultation et à une augmentation des cellulites. Un dépistage précoce et des stratégies de prise en charge adaptées s’imposent pour agir sur l’obésité chez les patients atteints de lymphœdème et sur les complexités liées à ces deux troubles connexes.
Mots-clés : : cellulite, lymphœdème, néoplasmes, obésité, œdème
Lymphedema is a chronic, inflammatory condition characterized by excess protein-rich fluid accumulation in the interstitium as a result of lymphatic abnormality (primary) or damage (secondary).1 Lymphedema is estimated to affect at least one million people in Canada, and the vast majority of these cases are not associated with cancer.2,3 Nevertheless, despite its increasing prevalence in Canada and around the world, non-cancer-related lymphedema remains little understood.3,4 In addition to genetic or congenital factors involved in primary lymphedema, non-cancer-related risk factors for developing lymphedema include venous disease, immobility, obesity, and lymphatic filariasis.1,5 Risk factors related to cancer treatment include lymph node dissection and radiotherapy.6 Recent studies have suggested the possibility of an underlying predisposition among patients with cancer who ultimately develop lymphedema.7–13
Because of the irreversible nature of lymphedema, its long-term management is challenging and requires multiple physical therapeutic modalities, including compression therapy, exercise, lymphatic drainage, and skin care, collectively known as complex decongestive therapy.1 Inadequate control results in a progressive worsening of the edema and an associated chronic inflammatory process as well as a risk of recurrent cellulitis infections, chronic wounds, and frequent hospitalizations.2 Lymphedema is associated with pain and impaired mobility in affected patients. It negatively affects psychosocial, sexual, and physical functioning, including occupation-related and leisure activities.14–17 In addition to its consequences for quality of life, the financial burden of lymphedema for patients and the health care system is significant.18–20
Obesity and Lymphedema
The dynamic interaction between obesity and lymphedema is a focus of growing research and clinical interest.21–24 Affecting one-quarter of Canadian adults,25 obesity is a major health concern around the world, and it has been identified as an important risk factor for lymphedema and an independent cause of non-cancer-related lymphedema.23,26–28 People with severe obesity who have chronic leg edema demonstrate functional and medical concerns, such as cellulitis and venous thromboembolism.29 The mechanisms linking obesity and lymphedema are not yet well delineated but are thought to involve a cascade of pathological events characterized by lymphatic vessel dysfunction, adipocyte hypertrophy and dysfunction, and chronic inflammatory processes.30
Rationale for Our Study
An important link between obesity and lymphedema has been recognized, highlighting a potentially common coexistence of these two conditions. However, there is a paucity of Canadian research on this topic. Our objectives were to report on the prevalence of overweight and obesity in patients referred to our Canadian lymphedema clinic and to investigate the relationships between BMI and demographic, medical, and lymphedema characteristics and cellulitis history.
Methods
Design and setting
This retrospective cohort study was an electronic record review of data collected from the initial patient assessments conducted at a Canadian hospital-based lymphedema clinic over a 2-year period (April 2012–March 2014). A descriptive, exploratory analysis of this dataset has previously been published.31 For this review, we cross-referenced the dataset with another clinical database developed more recently to obtain data on BMI. We obtained approval for this study from the McGill University Health Centre (MUHC) Research Ethics Board.
The assessments occurred at the MUHC Lymphedema Clinic, a hospital-based programme located in Montreal, Quebec. A specialized, multidisciplinary team, including a physician, a physiotherapist, and an exercise specialist, is responsible for conducting comprehensive assessments, developing tailored treatment plans, providing education on individualized self-care techniques (e.g., skin care, exercise, self-bandaging, self-drainage), and referring patients to private lymphedema therapists for complex decongestive therapy, to medical suppliers for custom compression garments, and to other specialized services (e.g., nutrition, dermatology, and rehabilitation).
Population
Patients with suspected lymphedema are referred to the clinic by physicians in Quebec and eastern Ontario. Although the primary mandate of the clinic is to assess cancer-related lymphedema, referrals are also accepted for non-cancer diagnoses. The study sample included all patients with available data on BMI evaluated at the clinic over the 2-year period.
Data collection
Chart review, medical history taking, and specialized lymphedema assessments are conducted with the patients primarily by the physician and physiotherapist during visits to the clinic. Height and weight are measured by administrative staff at each visit. For this review, we extracted the following information for each patient’s initial visit:
Demographic characteristics and medical history: age, gender, diagnosis, surgical history, and comorbidities (from medical chart and patient report)
Lymphedema characteristics: Time since initial lymphedema onset and lymphedema location and laterality (from medical chart, patient report, and physical assessment)
Cellulitis history: Number of bacterial skin infections (from medical chart and patient report; missing data for cellulitis history were recorded as unknown)
BMI: calculated on the basis of measured height and weight (from physical assessment).
Data analysis
We performed the statistical analyses with JMP data analysis software, version 13.2 (SAS Institute Inc., Cary, NC). We used descriptive analyses to characterize the study sample according to demographics, medical and lymphedema characteristics, cellulitis history, and BMI. To verify whether the subgroup with available BMI data represented all the patients with suspected lymphedema in our dataset, we performed one-way analysis of variance (ANOVA) and Pearson’s c2 tests to compare patients with available BMI data with those without. We followed the Canadian Guidelines for Body Weight Classification in Adults (categories: underweight; normal weight; overweight; and obese classes I, II and III).32 For comparative analyses, underweight and normal BMI were combined into one category (normal), and all obese classes were amalgamated into the obese category.
We performed comparisons of the study variables between the BMI categories using one-way ANOVA and Pearson’s χ2 tests. To further explore significant findings from these analyses, we used continuous data to calculate BMI means and SDs and compared them among the subgroups according to diagnosis, time since onset, lymphedema location and laterality, and cellulitis history.
Results
Demographic, medical, and lymphedema characteristics
During the study period, BMI data were available for 178 new patients assessed at the clinic for suspected lymphedema. Comparing these patients with those for whom there were no BMI data (n = 251), we found no differences in age, gender, diagnosis, surgical history, time since lymphedema onset, or cellulitis history (p > 0.05). The only significant difference was the date of assessment: 98.1% of the patients with BMI data were assessed after February 1, 2013, compared with 8.4% of the patients with no BMI data (p < 0.001).
In the dataset on patients with BMI data (n = 178), 144 patients had cancer diagnoses, most commonly breast (82; 56.9%) and gynecological (30; 20.8%). Among the 34 patients with non-cancer diagnoses, 8 (23.5%) had primary lymphedema. Other diagnoses were related to obesity (n = 6; 17.6%), surgical complications (n = 4; 11.8%), chronic venous insufficiency (n = 1; 2.9%) and mixed (or other) aetiologies (n = 15; 44.1%).
BMI categories
Of the 178 patients with BMI data, 1 (0.6%) was classified as underweight, 42 (23.6%) as normal weight, 65 (36.5%) as overweight, and 70 (39.3%) as obese; 39 (21.9%) were considered obese class I, 12 (6.7%) as class II, and 19 (10.7%) as class III.
Descriptive characteristics are presented in Table 1. Age, gender, and surgical history were not associated with BMI category (p > 0.05) in patients with suspected lymphedema. There were associations between BMI category and diagnosis (p < 0.001), time since lymphedema onset (p = 0.043), lymphedema laterality (p = 0.015), and cellulitis history (p = 0.048). There was also a trend towards significance between BMI category and lymphedema location (p = 0.08).
Table 1.
Descriptive Characteristics of Patients with Suspected Lymphedema According to BMI Category
| No. (%)* |
||||
|---|---|---|---|---|
| Characteristic | Normal† (BMI < 25.0);n = 43 | Overweight (BMI 25.0–29.9);n = 65 | Obese† (BMI > 30.0);n = 70 | All n = 178 |
| Age, y, mean (SD) | 59.9 (17.1) | 62.7 (13.4) | 60.0 (13.6) | 61.0 (14.4) |
| Gender | ||||
| Female | 36 (83.7) | 53 (81.5) | 61 (87.1) | 150 (84.3) |
| Male | 7 (16.3) | 12 (18.5) | 9 (12.9) | 28 (15.7) |
| Diagnosis‡ | ||||
| Cancer | 40 (93.0) | 58 (89.2) | 46 (65.7) | 144 (80.9) |
| Non-cancer | 3 (7.0) | 7 (10.8) | 24 (34.3) | 34 (19.1) |
| Surgical history | ||||
| No | 1 (2.3) | 5 (7.7) | 3 (4.3) | 9 (5.1) |
| Yes | 42 (97.7) | 60 (92.3) | 67 (95.7) | 169 (94.9) |
| Time since lymphedema onset,‡ y, mean (SD) | 3.7 (6.9) | 3.0 (6.7) | 6.7 (10.9) | 4.6 (8.7) |
| Lymphedema location | ||||
| Upper body | 16 (37.2) | 40 (61.5) | 33 (47.1) | 89 (50.0) |
| Lower body | 23 (53.5) | 22 (33.8) | 34 (48.6) | 79 (44.4) |
| Head and neck | 2 (4.7) | 1 (1.5) | 0 (0.0) | 3 (1.7) |
| Both upper and lower | 1 (2.3) | 2 (3.1) | 0 (0.0) | 3 (1.7) |
| None | 1 (2.3) | 0 (0.0) | 3 (4.3) | 4 (2.2) |
| Lymphedema laterality‡ | ||||
| Unilateral | 30 (69.8) | 56 (86.2) | 42 (60.0) | 128 (71.9) |
| Bilateral | 12 (27.9) | 9 (13.8) | 25 (35.7) | 46 (25.8) |
| None | 1 (2.3) | 0 (0.0) | 3 (4.3) | 4 (2.3) |
| Cellulitis history‡ | ||||
| No | 36 (83.7) | 57 (87.7) | 47 (67.1) | 140 (78.7) |
| Yes | 6 (14.0) | 7 (10.8) | 21 (30.0) | 34 (19.1) |
| Missing data | 1 (2.3) | 1 (1.5) | 2 (2.9) | 4 (2.2) |
Unless otherwise specified.
Underweight (BMI <18.5) and normal weight (BMI 18.5–24.9) were classified as normal, and obese classes I (BMI 30.0–34.9), II (BMI 35.0–39.9), and III (BMI ≥40) were classified as obese.
Significant between-groups differences on comparative analyses (p < 0.05).
Mean BMI and subgroup comparisons
Overall, the patients with suspected lymphedema had a mean BMI of 27.9 (SD = 7.3). It was higher in patients with non-cancer diagnoses than in those with cancer (36.1 [9.4] vs. 28.2 [5.8]; p < 0.001). We found no differences in BMI among patients with breast, gynecological, or other types of cancer (p = 0.62), whereas people with primary lymphedema had a lower BMI than those with other non-cancer diagnoses (p = 0.046; see Figure 1).
Figure 1.
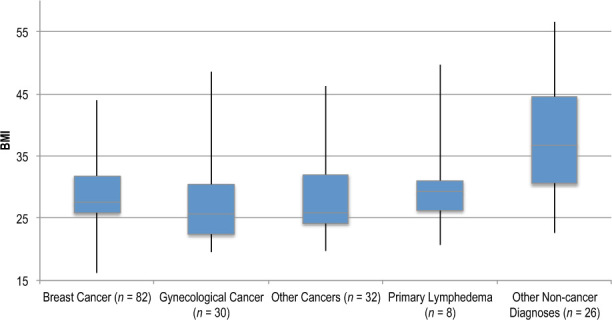
Box plot showing mean BMI of patients with suspected lymphedema, according to diagnosis.
Patients reporting a shorter duration (< 1 y) since lymphedema onset had a lower BMI than those with a longer duration (>1 y; 27.3 [5.6] vs. 31.8 [8.1]; p < 0.001). We found higher BMI among patients with bilateral lymphedema than among those with unilateral lymphedema (32.1 [9.3] vs. 28.9 [6.3]; p = 0.010). We found no difference in BMI between patients with lower body and upper body lymphedema (30.7 [8.6] vs. 29.3 [6.1]; p = 0.20). The mean BMI of patients with a history of cellulitis was higher than among those without such a history (33.7 [8.8] vs. 28.8 [6.7]; p < 0.001). Mean BMI according to number of cellulitis episodes reported at initial assessment is presented in Figure 2.
Figure 2.
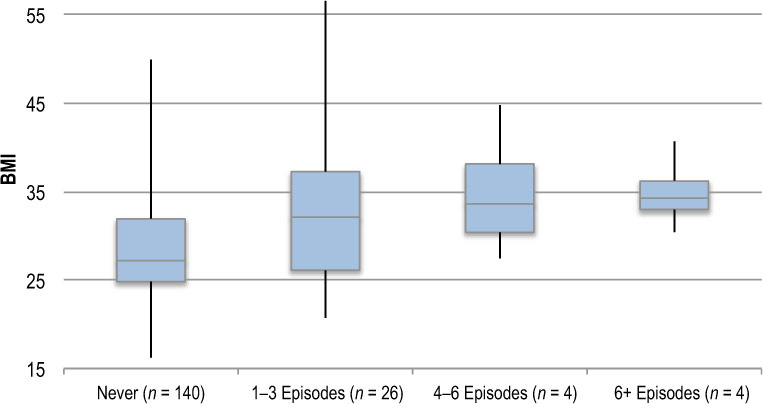
Box plot showing mean BMI of patients according to number of cellulitis episodes reported at initial assessment.
Discussion
Overweight and obesity in lymphedema
To our knowledge, this is the first published study exploring obesity in a Canadian population with lymphedema. In our sample of referrals for suspected lymphedema, more than three-quarters of the patients were considered overweight or obese, and more than 10% were classified as extremely obese. Overall, the proportion of patients who were overweight was similar to that of the Canadian population (34.9%).25 However, the rates of obesity and morbid obesity in our sample were higher than those reported in the Canadian population of 24.4% and 2.5%, respectively. The mean BMI of our sample fell in the overweight category and was similar to that reported by Vignes and colleagues (2017) in their study of 254 patients referred to a hospital-based lymphedema clinic in France.33
Given that our sample consisted mostly of patients with cancer-related lymphedema because of the nature of our clinic, these figures, although high, may still underestimate the overall prevalence of obesity among patients with lymphedema.3 Nonetheless, these results corroborate how common and important obesity is among patients with lymphedema, and they uniquely highlight the extent and scope of this concern among Canadians affected by lymphedema.
Our findings add to the growing body of research exploring the relationship between obesity and lymphedema. Because each problem influences the other, it is hard to determine where the vicious cycle begins. In women after breast cancer treatment, BMI has been identified as a predictor for the development of lymphedema.23,26 Ridner and colleagues found that women with a BMI more than 30.0 were 3.6 times more likely to develop lymphedema than those with a BMI less than30.0.23 Among 51 patients with non-cancer-related leg lymphedema, Greene and colleagues found that a higher BMI predicted abnormal lymphatic dysfunction on lymphoscintigraphy.27
Medical characteristics
With respect to diagnosis, patients in our dataset with non-cancer-related lymphedema had a higher BMI than those with cancer-related lymphedema. Although mean BMI did not differ according to cancer diagnosis, people with primary lymphedema were less overweight than those with secondary causes of lymphedema in the non-cancer subgroup. These results are consistent with those reported by Vignes and colleagues.33 Our findings support the notion that obesity plays an important role in the development of secondary non-cancer-related lymphedema.
Although the pathophysiological mechanisms behind this connection are not well understood, it has been hypothesized that the development of lymphedema involves pathological changes in lymphatic transport and adipocyte function that manifest as chronic edema, inflammation, abnormal fat deposition, fibrosis, and skin changes in affected patients.30 The last steps of this process are thought to further induce lymphatic and adipocyte dysfunction, leading to a vicious self-maintaining cycle of lymphedema. The initial trigger for this process may result from lymphatic damage (e.g., lymph node dissection) or metabolic dysfunction (e.g., obesity).
Lymphedema characteristics
In our review, patients with obesity had mean durations of lymphedema before being referred that were about twice as long as people with normal and overweight BMIs. These differences may be related to a general lack of awareness among medical practitioners of non-cancer-related lymphedema and the scarcity of appropriate, specialized assessment and treatment services for this subgroup, resulting in delays in and challenges with timely diagnosis and management.2,31,34–37 Patients with lymphedema affecting bilateral extremities also had a higher mean BMI than those with unilateral presentations. Bilateral lymphedema has previously been reported to be associated with a heavier symptom burden and increased history of cellulitis in patients compared with unilateral lymphedema.38 Delays in referring patients and diagnosing lymphedema are worrisome because it can eventually progress to an irreversible state and potentially result in associated complications.2,16
Cellulitis
Lymphedema is associated with regional immune deficiency and greatly increased susceptibility to bacterial soft tissue infections.39,40 In our study, patients with suspected lymphedema presenting with a history of cellulitis at the initial assessment had a higher BMI than those with no history. About one-third of those in the obesity category presented with a history of cellulitis. Similarly, O’Malley and colleagues reported that 36% of obese patients with chronic swelling had a previous history of cellulitis.29 Although antibiotics are the standard treatment for cellulitis, the need for enhanced guidelines on dosing for primary care physicians has been observed.41 Another study concluded that the preventive effects of antibiotics for recurrent cellulitis and erysipelas might not last once the medications are discontinued.42 For patients with recurrent cellulitis, particularly when obesity and lymphedema are co-existing, a multifaceted and long-term approach may be necessary to address these complex health concerns.
Implications for physiotherapists
With the rates of obesity in Canada steadily increasing,43 there is a pressing need for health professionals to be cognizant of the important complications associated with lymphedema and chronic edema. The findings of delayed diagnosis and higher cellulitis infection rates in this Canadian population further emphasize the importance of appropriate, timely referrals for specialized lymphatic therapy interventions. Physiotherapists, family physicians, vascular specialists, and other health professionals play a shared role in the early recognition of obesity-related lymphedema and in timely referrals to appropriate comprehensive therapy programmes. Moreover, physiotherapists specialized in lymphedema management can greatly contribute in an interdisciplinary care approach to delivering tailored treatment strategies, such as compression therapy, exercise, and skin care, to address the complex needs of this population.
Practice guidelines are limited in providing specific recommendations for evaluating and managing obesity in lymphedema.1,44–47 The 2006 International Lymphoedema Framework document, Best Practice for the Management of Lymphoedema, recommended including measurements of BMI and waist–waist-to-hip ratio as part of regular nutritional assessments to determine obesity among people with lymphedema.1 Clinical recommendations include maintaining an ideal body weight among people with breast-cancer-related lymphedema and suggestions for appropriate compression garments to manage lymphedema (e.g., custom-made, flat-knit garments and separate overlapping garments for the lower limbs to facilitate application).1,47
It may be useful to explore evidence-based guidelines on managing obesity in the general population and to determine whether they apply to the population with lymphedema.48,49 Potentially effective interventions for obesity highlighted in the 2006 Canadian Clinical Practice Guidelines on the Management and Prevention of Obesity in Adults and Children include comprehensive lifestyle interventions, energy-reduced diets, and individualized physical activity programmes.48 However, the lack of any long-term benefits with current weight loss programmes and the risks associated with weight cycling suggest that a personalized, stigma-free approach focusing on healthy diet and active lifestyle instead of actual weight loss may be more appropriate.50 Further research is urgently needed to examine the effectiveness of tailored interventions to address obesity specifically among patients affected by lymphedema.
The strengths of our review include it being the first published study exploring obesity specifically among Canadian patients with lymphedema. Our method of using an electronic database in clinical practice is recognized as part of an international strategy to better delineate the characteristics of the population with lymphedema.51
There were two principal limitations of our study. First, patients with non-cancer-related lymphedema were likely under-represented in our sample. Second, the retrospective, cross-sectional study design limited our ability to make inferences about the relationships among the studied factors.
Conclusion
Physiotherapists have an important role to play in the early detection and comprehensive management of lymphedema. Our research on a Canadian population with lymphedema highlights the high prevalence of obesity, particularly in non-cancer cases of lymphedema. This finding warrants further attention to help prevent delayed referrals and the potentially life-threatening complications of cellulitis bacterial infections. Specialized multidisciplinary strategies addressing obesity in lymphedema may be valuable to successfully prevent and manage this combined condition.
Key Messages
What is already known on this topic
Lymphedema is a debilitating, lifelong condition of persistent swelling and inflammation, requiring tailored, multi-modal management involving specialized physiotherapists. An important interplay between lymphedema and obesity has been recognized.
What this study adds
To our knowledge, this is the first study to explore the link among obesity, lymphedema, and related factors, specifically in a Canadian population with lymphedema. Obesity is prevalent among Canadians with lymphedema and is associated with delayed referral and increased cellulitis rates.
References
- 1.International Lymphoedema Framework. Best practice for the management of lymphoedema. London: Medical Education Partnership; 2006. [Google Scholar]
- 2.Keast DH, Despatis M, Allen JO. et al. Chronic oedema/lymphoedema: under‐recognised and under‐treated. Int Wound J. 2015;12(3):328–33. 10.1111/iwj.12224. Medline:24618210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keast DH, Towers A. The rising prevalence of lymphedema in Canada: a continuing dialogue. Pathways, Canada’s Lymphedema Magazine. 2017:6–8.
- 4.Williams AF, Franks PJ, Moffatt CJ. Lymphoedema: estimating the size of the problem. J Palliat Med. 2005;19(4):300–13. 10.1191/0269216305pm1020oa. Medline:15984502 [DOI] [PubMed] [Google Scholar]
- 5.Trayes KP, Studdiford JS, Pickle S. et al. Edema: diagnosis and management. Am Fam Physician. 2013;88(2):102–10. [PubMed] [Google Scholar]
- 6.Cormier J, Feldman J, Askew R. et al. ALFP to update the best practice document. J Lymphoedema. 2010;5:68–71. [Google Scholar]
- 7.Stanton AW, Modi S, Bennett Britton TM. et al. Lymphatic drainage in the muscle and subcutis of the arm after breast cancer treatment. Breast Cancer Res Treat. 2009;117(3):549–57. 10.1007/s10549-008-0259-z. Medline:19052859 [DOI] [PubMed] [Google Scholar]
- 8.Jensen MR, Simonsen L, Karlsmark T. et al. Microvascular filtration is increased in the forearms of patients with breast cancer-related lymphedema. J Appl Physiol. 2013;114(1):19–27. 10.1152/japplphysiol.01116.2012. Medline:23123353 [DOI] [PubMed] [Google Scholar]
- 9.Modi S, Stanton AW, Svensson WE. et al. Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J Physiol. 2007;583(Pt 1):271–85. 10.1113/jphysiol.2007.130401. Medline:17569739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortimer P. Arm lymphoedema after breast cancer. Lancet Oncol. 2013;14(6):442–3. 10.1016/s1470-2045(13)70097-4. [DOI] [PubMed] [Google Scholar]
- 11.Rockson SG. Secondary lymphedema: is it a primary disease? Lymphat Res Biol. 2008;6(2):63–4. 10.1089/lrb.2008.6201. Medline:18564919 [DOI] [PubMed] [Google Scholar]
- 12.Finegold DN, Schacht V, Kimak MA. et al. HGF and MET mutations in primary and secondary lymphedema. Lymphat Res Biol. 2008;6(2):65–8. 10.1089/lrb.2008.1524. Medline:18564920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrell RE, Kimak MA, Lawrence EC. et al. Candidate gene analysis in primary lymphedema. Lymphat Res Biol. 2008;6(2):69–76. 10.1089/lrb.2007.1022. Medline:18564921 [DOI] [PubMed] [Google Scholar]
- 14.Beesley VL, Rowlands IJ, Hayes SC. et al. Incidence, risk factors and estimates of a woman’s risk of developing secondary lower limb lymphedema and lymphedema-specific supportive care needs in women treated for endometrial cancer. Gynecol Oncol. 2015;136(1):87–93. 10.1016/j.ygyno.2014.11.006. Medline:25448454 [DOI] [PubMed] [Google Scholar]
- 15.Passik S, Newman M, Brennan M. et al. Psychiatric consultation for women undergoing rehabilitation for upper-extremity lymphedema following breast cancer treatment. J Pain Symptom Manage. 1993;8(4):226–33. 10.1016/0885-3924(93)90132-F. Medline:7963764 [DOI] [PubMed] [Google Scholar]
- 16.Fu MR, Ridner SH, Hu SH. et al. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psychooncology. 2013;22(7):1466–84. 10.1002/pon.3201. Medline:23044512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas R, Hack TF, Quinlan E. et al. Loss, adaptation and new directions: the impact of arm morbidity on leisure activities following breast cancer. Can Oncol Nurs J. 2015;25(1):49–59. 10.5737/236880762514953. Medline:26642494 [DOI] [PubMed] [Google Scholar]
- 18.Schmitz KH, DiSipio T, Gordon LG. et al. Adverse breast cancer treatment effects: the economic case for making rehabilitative programs standard of care. Support Care Cancer. 2014;23(6):1807–17. 10.1007/s00520-014-2539-y. Medline:25471182 [DOI] [PubMed] [Google Scholar]
- 19.Shih YC, Xu Y, Cormier JN. et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27(12):2007–14. 10.1200/jco.2008.18.3517. Medline:19289624 [DOI] [PubMed] [Google Scholar]
- 20.Stout NL, Pfalzer LA, Springer B. et al. Breast cancer-related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2012;92(1):152–63. 10.2522/ptj.20100167. Medline:21921254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehrara BJ, Greene AK. Lymphedema and obesity: is there a link? Plast Reconstr Surg. 2014;134(1):154e–60e. 10.1097/prs.0000000000000268. Medline:25028830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest. 2014;124(3):915–21. 10.1172/jci71608. Medline:24590276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridner SH, Dietrich MS, Stewart BR. et al. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer. 2011;19(6):853–7. 10.1007/s00520-011-1089-9. Medline:21240649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz KH, Neuhouser ML, Agurs-Collins T. et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst. 2013;105(18):1344–54. 10.1093/jnci/djt223. Medline:23990667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Statistics Canada. Adjusting the scales: obesity in the Canadian population after correcting for respondent bias. Health at a Glance. 2014:1–10.
- 26.Helyer LK, Varnic M, Le LW. et al. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010;16(1):48–54. 10.1111/j.1524-4741.2009.00855.x. Medline:19889169 [DOI] [PubMed] [Google Scholar]
- 27.Greene AK, Grant FD, Slavin SA. et al. Obesity-induced lymphedema: clinical and lymphoscintigraphic features. Plast Reconstr Surg. 2015;135(6):1715–19. 10.1097/prs.0000000000001271. Medline:25724063 [DOI] [PubMed] [Google Scholar]
- 28.Vasileiou AM, Bull R, Kitou D. et al. Oedema in obesity; role of structural lymphatic abnormalities. Int J Obes (Lond). 2011;35(9):1247–50. 10.1038/ijo.2010.273. Medline:21266949 [DOI] [PubMed] [Google Scholar]
- 29.O’Malley E, Ahern T, Dunlevy C. et al. Obesity-related chronic lymphoedema-like swelling and physical function. QJM. 2015;108(3):183–7. 10.1093/qjmed/hcu155. Medline:25086107 [DOI] [PubMed] [Google Scholar]
- 30.Cucchi F, Rossmeislova L, Simonsen L. et al. A vicious circle in chronic lymphoedema pathophysiology? An adipocentric view. Obes Rev. 2017;18(10):1159–69. 10.1111/obr.12565. Medline:28660651 [DOI] [PubMed] [Google Scholar]
- 31.Shallwani SM, Hodgson P, Towers A. Comparisons between cancer-related and noncancer-related lymphedema: an overview of new patients referred to a specialized hospital-based center in Canada. Lymphat Res Biol. 2017;15(1):64–9. 10.1089/lrb.2016.0023. Medline:28135124 [DOI] [PubMed] [Google Scholar]
- 32.Health Canada. Canadian guidelines for body weight classification in adults. Ottawa: Health Canada; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vignes S, Vidal F, Arrault M. Specialized consultations in a hospital-based referral center for patients suspected of having limb lymphedema: impact on diagnosis. Vasc Med. 2017;22(4):331–6. 10.1177/1358863x17714884. Medline:28633618 [DOI] [PubMed] [Google Scholar]
- 34.Moffatt CJ, Franks PJ, Doherty DC. et al. Lymphoedema: an underestimated health problem. QJM. 2003;96(10):731–8. 10.1093/qjmed/hcg126. Medline:14500859 [DOI] [PubMed] [Google Scholar]
- 35.Sitzia J, Woods M, Hine P. et al. Characteristics of new referrals to twenty-seven lymphoedema treatment units. Eur J Cancer Care. 1998;7(4):255–62. 10.1046/j.1365-2354.1998.00112.x. Medline:9919113 [DOI] [PubMed] [Google Scholar]
- 36.Bogan LK, Powell JM, Dudgeon BJ. Experiences of living with non-cancer-related lymphedema: implications for clinical practice. Qual Health Res. 2007;17(2):213–24. 10.1177/1049732306297660. Medline:17220392 [DOI] [PubMed] [Google Scholar]
- 37.Morgan PA, Murray S, Moffatt CJ. et al. The challenges of managing complex lymphoedema/chronic oedema in the UK and Canada. Int Wound J. 2012;9(1):54–69. 10.1111/j.1742-481x.2011.00845.x. Medline:21848727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng J, Radina E, Fu MR. et al. Self-care status, symptom burden, and reported infections in individuals with lower-extremity primary lymphedema. J Nurs Scholarsh. 2015;47(2):126–34. 10.1111/jnu.12117. Medline:25475008 [DOI] [PubMed] [Google Scholar]
- 39.Cox NH. Oedema as a risk factor for multiple episodes of cellulitis/erysipelas of the lower leg: a series with community follow-up. Br J Dermatol. 2006;155(5):947–50. 10.1111/j.1365-2133.2006.07419.x. Medline:17034523 [DOI] [PubMed] [Google Scholar]
- 40.Al-Niaimi F, Cox N. Cellulitis and lymphoedema: a vicious cycle. J Lymphoedema. 2009;4(2):38–42. [Google Scholar]
- 41.Theofiles M, Maxson J, Herges L. et al. Cellulitis in obesity: adverse outcomes affected by increases in body mass index. J Prim Care Community Health. 2015;6(4):233–8. 10.1177/2150131915583659. Medline:25925834 [DOI] [PubMed] [Google Scholar]
- 42.Dalal A, Eskin-Schwartz M, Mimouni D. et al. Interventions for the prevention of recurrent erysipelas and cellulitis. Cochrane Database Syst Rev. 2017;6:CD009758. 10.1002/14651858.cd009758.pub2. Medline:28631307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Statistics Canada. Table 105-2023: Measured adult body mass index (BMI) (World Health Organization classification), by age group and sex, Canada and provinces, Canadian Community Health Survey – nutrition, occasional. [Internet] Ottawa: Statistics Canada; 2017 [cited 2018. February 1]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=1052023&&pattern=&stByVal=1&p1=1&p2=31&tabMode=dataTable&csid. [Google Scholar]
- 44.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the international society of lymphology. Lymphology. 2013;46(1):1–11. Medline:23930436 [PubMed] [Google Scholar]
- 45.Levenhagen K, Davies C, Perdomo M. et al. Diagnosis of upper quadrant lymphedema secondary to cancer: clinical practice guideline from the oncology section of the American Physical Therapy Association. Phys Ther. 2017;97(7):729–45. 10.1093/ptj/pzx050. Medline:28838217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armer JM, Hulett JM, Bernas M. et al. Best practice guidelines in assessment, risk reduction, management, and surveillance for post-breast cancer lymphedema. Curr Breast Cancer Rep. 2013;5(2):134–44. 10.1007/s12609-013-0105-0. Medline:26246870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris SR, Hugi MR, Olivotto IA. et al. Clinical practice guidelines for the care and treatment of breast cancer: 11. lymphedema. Can Med Assoc J. 2001;164(2):191–9. Medline:11332311 [PMC free article] [PubMed] [Google Scholar]
- 48.Lau DC, Douketis JD, Morrison KM. et al. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. CMAJ. 2007;176(8):S1–S13. 10.1503/cmaj.061409. Medline:17420481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garvey WT, Mechanick JI, Brett EM. et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(7):842–84. 10.4158/ep161356.esgl. Medline:27472012 [DOI] [PubMed] [Google Scholar]
- 50.Hale I. Long-term benefits of weight loss? Can Fam Physician. 2017;63(6):429–30. Medline:28615390 [PMC free article] [PubMed] [Google Scholar]
- 51.Moffatt C, Franks P, Keeley V. et al. The development and validation of the LIMPRINT methodology. Lymphat Res Biol. 2019;17(2):127–34. 10.1089/lrb.2018.0081. Medline:30995185 [DOI] [PMC free article] [PubMed] [Google Scholar]


