ABSTRACT
In recent years, studies investigating the role of the gut microbiota in health and diseases have increased enormously – making it essential to deepen and question the research methodology employed. Fecal microbiota transplantation (FMT) in rodent studies (either from human or animal donors) allows us to better understand the causal role of the intestinal microbiota across multiple fields. However, this technique lacks standardization and requires careful experimental design in order to obtain optimal results. By comparing several studies in which rodents are the final recipients of FMT, we summarize the common practices employed. In this review, we document the limitations of this method and highlight different parameters to be considered while designing FMT Studies. Standardizing this method is challenging, as it differs according to the research topic, but avoiding common pitfalls is feasible. Several methodological questions remain unanswered to this day and we offer a discussion on issues to be explored in future studies.
KEYWORDS: Fecal microbiota transplantation, rodent, human, germ-free, antibiotics, gnotobiotic, microbiota depletion, methods, experimental design
Introduction
The human gastrointestinal (GI) tract houses a dynamic population of microorganisms, known as the gut microbiota, which have a major impact on the host during homeostasis and disease.1 By comparing conventional rodents with germ free ones (i.e. devoid of all microorganisms), an important role of the intestinal microbiota is attributed to the symptoms expressed and prominent features of numerous diseases.2 The information garnered from this approach has been supplemented with supporting evidence from a number of complementary experimental strategies. However, despite the invaluable information gathered from preclinical studies, in the majority of cases, causality remains unclear. Recently, we have begun to understand how host physiology and behavior – in rodents – can be affected solely by the transplantation of fecal microbiota from a donor (human, rodent, etc.). This method has become common for assessing causality arising from the complex host-microbiota interactions, as it is a powerful way to understand the strong involvement of intestinal microorganisms on our overall health. Indeed, fecal microbiota transplantation (FMT) studies have implicated the gut microbiota in gastrointestinal disorders like irritable bowel syndrome (IBS)3 and metabolic disorders such as Type 2 Diabetes and Obesity.4 Even more surprisingly, FMT studies highlighted the potential involvement of the intestinal microbiota in CNS diseases including: neurodegenerative disorders such as Parkinson’s disease,5 multiple sclerosis,6 Alzheimer’s disease;7 developmental disorders like autism spectrum disorder8 but also some psychiatric conditions including schizophrenia9 and depression.10 FMT involves many steps and requires careful experimental design. Indeed, laboratories using this method to study different topics adapt the experimental parameters to meet the requirements of their own research topic and experimental readouts. FMT studies are the result of a number of important experimental design choices: human or rodent donors, rodent strain and sex, recipient animal models (e.g.: germ-free (GF), antibiotics- or laxative-treated, conventional animal etc.), factors influencing the recipient animals (feeding, housing conditions), fecal slurry preparation (processing, storage, concentration, administration method) and the method used to ascertain the degree and stability of engraftment (see Figure 1). The lack of explicit explanations around the rationale for the choices made in the selection of these parameters is reflected in a great deal of variation in experimental protocols that often complicates the interpretation of results and makes inter-study comparison difficult. In addition, the constant emergence of new information regarding the best way to approach a specific parameter of the FMT process, makes it challenging to plan best practice during FMT. For example, new information has recently become available on the establishment of microbiota in gnotobiotic animals.11 Careful consideration of these factors is critical to obtain reliable and robust results. While FMT has undoubtedly led to improvements in our understanding of health and disease, this rapidly evolving scientific approach is undermined by the lack of standardization in its methods, application, and interpretation. As such, the intention of this review is to equip basic, clinical and translational scientists with a clear understanding of the principles of the FMT technique in preclinical research with the aim to increase the quality of research outcomes and their translational relevance. This review focuses only on rodent recipients as this is the most widely used model in pre-clinical research to assess causality of the intestinal microbiota. By identifying key elements in FMT methodology that are subject to variable implementation, we hope to provide recommendations based on the state of the art in this field and highlight the promise and pitfalls of this approach as it is increasingly used in attempts to define a potential causal role of the gut microbiota in various aspects of health and disease. Our goal is to provide scientists wishing to use this technique in rodent research models with an overview of what has been done so far, the problems encountered and the best way to approach this technique according to the questions under evaluation. We believe that although it may be appropriate for studies to adopt bespoke protocols according to the topic being studied, certain principles and parameters of the experimental design can be further considered and standardized to best optimize the reliability of this method in each particular context. Clinical guidelines for the use of FMT in the treatment of disorders (e.g. C. difficile infection) are outside the scope of this review. However, relevant clinical findings are included where appropriate as some factors are given more consideration in the clinical literature than preclinical studies.
Figure 1.
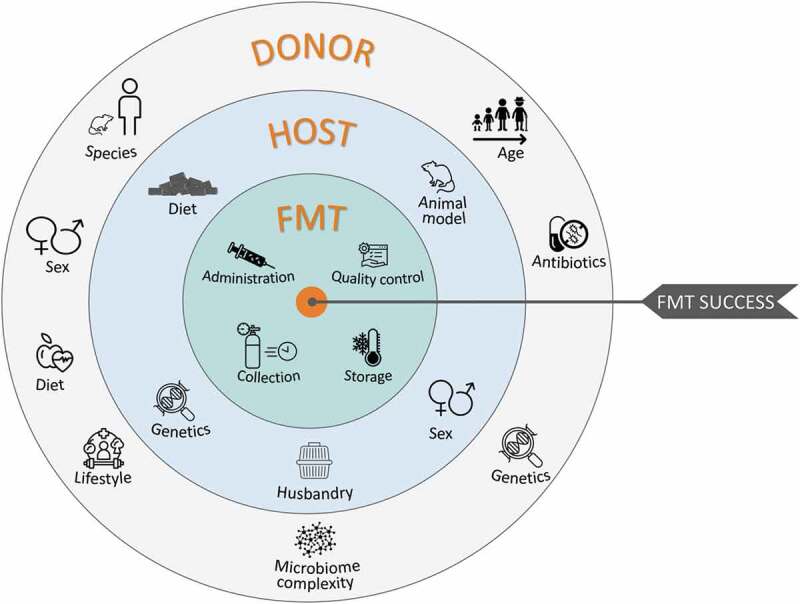
Parameters influencing the experimental design of fecal microbiota transplant studies
Animal models
Several animal models are used in FMT studies, each with advantages and limitations (see Figure 2). Researchers need to carefully consider the goals of their studies to determine the most suitable approach. (See Table 1 and 3)
Figure 2.
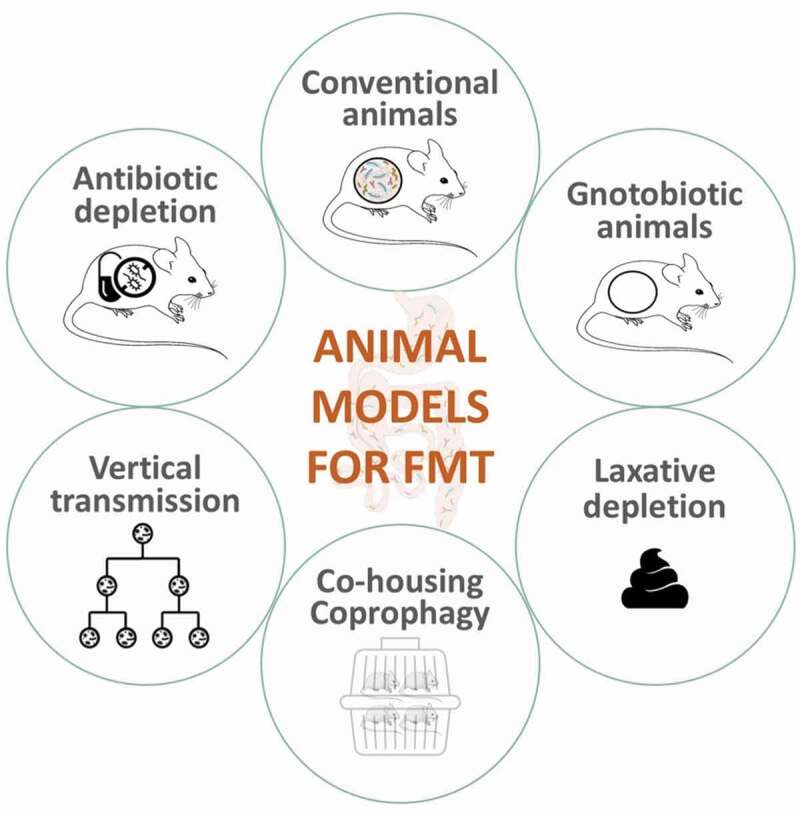
Most commonly used animal models as recipients for fecal microbiota transplants
Table 1.
Frequently used antibiotics for gut microbiota depletion and their characteristics
| Antibiotics | Spectrum of activity | Intestinal Absorption |
|---|---|---|
| Ampicillin (± sulbactam) |
Broad spectrum (mostly Gram +) |
Extensive 30–55% |
| Ciprofloxacin | Broad spectrum | Extensive |
| Streptomycin | Broad spectrum | Poor |
| Neomycin | Broad spectrum | Poor |
| Kanamycin | Broad spectrum | Unknown |
| Imipenem (Cilastatin) |
Broad spectrum: both aerobic and anaerobic bacteria |
Poor |
| Vancomycin | Gram-positive Bacteria | Poorly absorbed from GI, systemic absorption (up to 60%) may occur following intraperitoneal administration |
| Metronidazole | Narrow spectrum: anaerobic bacteria |
Complete: lipophilic , rapidly and widely distributed after absorption. |
| Gentamicin | Mostly Gram negative | Poor30 |
| Colistin | Gram negative Bacilli | Very poor absorption from gastrointestinal tract |
Table 2.
Advantages and limitations: administration of antibiotics
| Advantages | Limitations | |
|---|---|---|
| Drinking water (Table 4) |
|
|
| Oral gavage (Table 5) |
|
|
| Combination (Table 6) |
|
Table 3.
Comparisons of different animal models for FMT
| Advantages | Limitations | |
|---|---|---|
| Gnotobiotic animals GF |
|
|
| Gnotobiotic animals -Defined microbiota (SPF) |
|
|
| Gnotobiotic animals -Defined microbiota (ASF) |
|
|
| Antibiotic-treated animals |
|
|
| Conventional animals |
|
|
| Laxative-depletion |
|
|
| Vertical microbiota transmission model |
|
|
| Bedding material/coprophagy |
|
|
Gnotobiotic animals
Gnotobiotic animals are used as a resource to understand the role of the intestinal microbiota in relevant conditions. Starting a GF line – rodents devoid of all microorganisms – requires aseptic removal of a pup from the mother to avoid any exposure to its mother’s microorganisms. The animals are reared in sterile cages and reproduce under aseptic conditions.2 The term “gnotobiotic animals” includes GF animals but also GF animals recolonized with a defined microbiota to study specific germs (mono-associated or defined microbiota animals).12 Gnotobiotic rodents are fundamental tools to understand the role of the gut microbiota and have been employed in a number of studies to successfully demonstrate the adoptive transfer of various behavioral phenotypes.
GF rodents are raised in isolators and are devoid of all microorganisms including: bacteria, viruses, fungi, archaea, protozoa and other parasites – within the range of detection limits used (sequencing- or cultured-based techniques). The use of this animal model has contributed greatly to the microbiome field. It is an excellent model for understanding the extent to which the intestinal microbiota impacts on health and overall physiology. Therefore, it appears as a reliable recipient for FMT studies. The most beneficial aspect of using this model to receive an exogenous intestinal microbiota is the lack of competition with resident microbes to colonize the gut. However, it is also important to note that a GF state leads to abnormal development in many features of interest: immune system development,13 enteric nervous development,2 neurodevelopmental deficits2 and altered behavioral responses.2 This renders interpretation challenging as it has been shown that the introduction of a complex microbiota will sometimes normalize those aspects of the host physiology that are compromised by growing up GF. Based on this knowledge, it is advisable not to rely solely on comparisons between GF animals and ex-GF animals. For example, the use of both conventionally raised and ex-GF animals (i.e. born and delivered under GF conditions and subsequently removed from isolators for colonization) as control groups, when comparing to GF animals, can help parse a role for the gut microbiota in the abnormal development of germ-free mice vs an ongoing contribution during adulthood. Moreover, standardized behavioral tests can require some adjustments for germ-free rodents – as they exhibit abnormal behavior – to avoid floor and ceiling effects. For instance, GF mice exhibit an exaggerated HPA-axis response. Therefore, understanding the effect of a fecal transplant, where it is anticipated to activate the stress-response system on their response to a restraint stress paradigm, will be more challenging.14 Additionally, the cost of breeding and handling animals under sterile conditions is significant.15 Detailed protocols for maintaining and generating GF animals are available16 but the requirement for a dedicated facility and expertise means access is not routine in many institutions. To counteract the expenses of isolators, a cost-effective solution would be the use of individually ventilated cages to house GF mice according to Lange et al. protocol.12 They present a protocol for the use of a conventional IVC system operating under containment mode and provide details for cage changing and sampling without compromising the sterile status. Importantly, the use of IVC system might have an impact for behavioral studies.17
(ii) Defined microbiota animals are generated by colonizing GF animals with one or more specific microorganisms. Specific Pathogen Free (SPF) rodents,18 widely used in FMT studies, are defined as animals free from specific pathogens but otherwise having an undefined microbiota. Differences exist between breeding establishments with regard to the list of excluded pathogens in SPF mice used, therefore not allowing for proper standardization of SPF animals.18 With the hope of standardization between studies, Schaedler et al.19 proposed the “Altered Schaedler Flora (ASF)” rodent model, representing a simpler model of commensal microorganisms composed of only eight bacterial species. (Methodology papers regarding this model can be found in Dewhirst et al. and Biggs et al.20 review). ASF rodent use has evolved since 1965 toward a controlled and simplified model of a more complex microbiota to study microbiome-host interactions.21 However, in some studies, this low diversity of microorganism can result in intermediate phenotypes between GF and SPF.22 This model is limited in its ability to fully predict the impact of the intestinal microbiota on its host since it is only a simplified representation of the gut microbiota.
In conclusion, GF rodents are an insightful ‘knock-out’ model that have helped to highlight the importance of a rich and diverse microbiota for health and diseases. Rodents colonized with a simplified microbiota are useful for standardizing research between groups and provide a simplified model for studying microbiome-host interaction, thereby reducing the tremendous variability that exists when studying conventionally raised rodents. It also reduced developmental abnormalities seen in GF animals. One study raises important concerns on the use of SPF mice for translational studies in the immunology field, given that this could compromise the establishment of a fully mature adult-like immune system.23 Limitations of the use of gnotobiotic animals led to the proliferation of alternative microbiota-depletion approaches.
Antibiotic-treated animals
The antibiotic-depleted microbiota model is an alternative to gnotobiotic rodents widely used for a number of reasons: it is simpler in terms of experimental design and less expensive, it does not require access to specialized housing equipment, and it circumvents the limitations of a GF animal model. Antibiotics are a simple and accessible way to induce gut microbiota depletion – even though it never fully removes all microorganisms. It has been suggested that the use of juvenile mice subjected to initial microbiota depletion constitutes a valid alternative to GF mice in microbiota transfer studies.24 ‘Pseudo-depletion’ of gut microbes can be adjusted depending on: the type of antibiotics used (bactericidal or bacteriostatic) or targeted microbes (gram-negative or positive bacteria, narrow- or broad-spectrum antibiotics). When targeting specific types of bacteria, it is important to consider how it impacts the overall ecological network and might cause the multiplication of some strains at the expense of others. For an effective depletion of gut bacteria, an antibiotic cocktail containing several antibiotics that together give a broad spectrum of activity is necessary: after 2 weeks of broad-spectrum antibiotic administration, 72% to 86% reduction in bacterial load was reported.25,26 One study compared the depletion of gut microbes by administering a single broad-spectrum antibiotic (Ampicillin, Doxycycline or Ciprofloxacin) and concluded that each option individually elicited unique taxonomic changes27 which reinforces the view that an antibiotic cocktail would be more appropriate than a single broad-spectrum antibiotic. Another study compared 16S copies number in fecal pellets after 2 weeks of administration of either: an antibiotic cocktail (1 g/L ampicillin, 1 g/L metronidazole, 1 g/L neomycin, 0.5 g/L vancomycin), a single antibiotic (1 g/L ciprofloxacin) or a combination of antibiotics (1 g/L neomycin and 1 g/L metronidazole; 0.5 g/L vancomycin and 1 g/L ampicillin). Only the antibiotic cocktail and the combination vancomycin/ampicillin were significantly different than vehicle-treated animals.28 The normal commensal organisms of rodents are mainly gram-positive bacteria.29 Hence, the use of limited-spectrum antibiotics that target only gram-positive bacteria, for example, may lead to the proliferation of gram-negative bacteria.29 The antibiotic cocktail can be supplemented with antifungals (amphotericin B, pimaricin, natamycin) to prevent fungal proliferation during antibiotic treatment. Table 1. offers an overview of frequently used antibiotics for gut microbiota depletion.
Administration
The antibiotic cocktail – drugs and dosage – to be used for a significant standardized knockdown of gut bacteria still needs to be determined: this incomplete and variable depletion of the microbiome is currently a biological limitation of this approach. However, it may be advanageous that antibiotics used for depletion studies are non-absorbable by the gut (see Table 1). A short-term oral gavage treatment with non-absorbable antibiotics has been proposed (ampicillin, bacitracin, meropenem, neomycin, vancomycin) – except for ampicillin which was able to reach the systemic circulation but was undetectable in the brain.31 The dose, frequency and cocktail of antibiotics must be considered to induce a depletion of the intestinal microbiota that could approach a germ-free state, although there are a lack of studies specifically examining the impact of all the different antibiotic cocktails that have been used to date (see Table 1 for some estimates of depletion). Tables 4, 5 and 6 offers a summary of doses/frequency, concentration and antibiotic cocktails used in the literature so far. In general, it appears that 7 days of antibiotic administration is sufficient to ensure a severe microbiota depletion.37 This is likely a reasonable time to avoid antibiotic resistance and overgrowth of pathogenic bacteria. If the treatment is short enough (e.g. 5–7 days) and followed by FMT, the side effects of the antibiotics can be reversed. Unpublished data from our laboratory suggests that substantial knockdown can happen even after 24 hours of an antibiotic cocktail (Vancomycin, Imipenem, Gentamicin, Ampicillin) in mice.
Table 4.
Drinking water – protocols for antibiotic-induced gut depletion
| DURA-TION | ANTIBIOTIC COCKTAILS CONCENTRATIONS | DISEASES (ICD 10) |
PUBLICA-TIONS |
|---|---|---|---|
| 2 days | streptomycin (500 g/ L) | Diseases of the circulatory system (Stroke) | 32 |
| ciprofloxacin (0.2 g/L) + metronidazole (1 g/L) |
Endocrine, nutritional and metabolic diseases (Obesity) | 33 | |
| 5 days | ampicillin (0.5 g/L) | Infectious disease | 34 |
| 1 week | ampicillin (1 g/L) | GI disorder (due to antibiotic- and chemotherapy-induced gut dysbiosis) | 35 |
| 1 or 2 weeks | amoxicillin–clavulanic acid (1 g/L) |
Diseases of the circulatory system (Stroke) | 36 |
| 7 days | Systemic antibiotic cocktail: ampicillin (1 g/L) + cefoperazone sodium salt (1 g/L) + clindamycin (1 g/L) OR Non-absorbable antibiotic cocktail: ertapenem sodium (1 g/L) + neomycin sulfate (1 g/L) + vancomycin hydrochloride (1 g/L) |
FMT protocol | 37 |
| 7 days | ampicillin (0.01 g/1 L) + metronidazol (0.01 g/L) + neomycin (0.01 g/L) |
Diseases of the digestive system (Hepatic steatosis) | 38 |
| 10 days | vancomycin (1 g/L) + metronidazole (1 g/L) + polymyxin B (1 g/L) + cefotaxime (2 g/L) |
FMT protocol | 39 |
| cefoxitin (1 g/L) + gentamicin (1 g/L) + metronidazole (1 g/L) + vancomycin (1 g/L) |
Immune system | 40 | |
| 10–14 days | ampicillin (1 g/L) + metronidazole (1 g/L) + neomycin sulfate (1 g/L) + vancomycin (0.5 g/L) |
Infectious disease | 41 |
| 14 days | ampicillin (1 g/L) + metronidazole (1 g/L) + neomycin (1 g/L) + vancomycin (0.5 g/L) |
Immune system | 42 |
| 14 days | ampicillin (1 g/L) + metronidazole (1 g/L) + neomycin sulfate (1 g/L) |
Diseases of the nervous system (Alzheimer's disease) | 43 |
| 14 days | ampicillin (1 g/L) + metronidazole (1 g/L) + neomycin (1 g/L) + vancomycin (0.5 g/L) supplemented with 1% (wt/vol) glucose. |
Immune system | 44 |
| 14 days | ampicillin (1 g/L) + metronidazole (1 g/L) + neomycin (1 g/L) + vancomycin (0.5 g/L) |
Endocrine, nutritional and metabolic diseases (Obesity) | 45 |
| 14 days | ampicillin (1 g/L) + metronidazole (1 g/L) + neomycin sulfate (1 g/L) |
Mental and behavioral disorders (Depression) | 46 |
| 14 days | ampicillin (1 g/L) + vancomycin (0.5 g/L) + neomycin (1 g/L) + metronidazole (1 g/L) |
Antibiotic effects | 47 |
| 2–3 weeks | ampicillin (1 g/l) + streptomycin (5 g/l) + colistin (1 mg/ml) or vancomycin alone (0.25 g/L) or imipenem alone (0.25 g/L) or colistin alone (2.103 U/ml) |
Neoplasms (Cancer) | 48 |
| 2–3 weeks | ampicillin (1 g/L) + vancomycin (0.5 g/L) + neomycin (1 g/L) + metronidazole (1 g/L) |
Infectious diseases | 49 |
| 3 weeks | ampicillin (1 g/L) + neomycin (1 g/L) + streptomycin (1 g/L) + kanamycin (1 g/L) and/or anti-fungal cocktail drinking water amphotericin (0.2 g/L) + fluconazole (0.5 g/L) + 5-fluorocytosine (0.5 g/L) |
Infectious diseases | 50 |
| 3 weeks | ampicillin (1 g/L) + metronidazole (1 g/L) + neomycin sulfate (1 g/L) + vancomycin (0.5 g/L) |
Infectious diseases | 51 |
| 3 weeks | vancomycin (0.5 g/l) + ampicillin (1 g/l) + kanamycin (1 g/l) + metronidazole(1 g/l) |
Immune system | 52 |
| 3 weeks | ampicillin (1 g/L) + metronidazole (1 g/L) + neomycin (1 g/L) + vancomycin (0.5 g/L) |
Immune system | 53 |
| 3 weeks | vancomycin (0.5 g/L) + neomycin sulfate (1 g/L)+ ampicillin (1 g/L) + metronidazole (1 g/L) |
Immune system | 54 |
| 3 weeks | ampicillin (1 g/L) + metronidazole (1 g/L) + vancomycin (0.5 g/L) + neomycin trisulfate (1 g/L) |
Diseases of the digestive system | 55 |
| 4 weeks | ampicillin (1 g/L) + vancomycin (0.5 g/L) + polymyxin (0.1 g/L) |
Immune system | 56 |
| 4 weeks | ampicillin (1 g/L) + neomycin (1 g/L) + metronidazole (1 g/L) + vancomycin (0.5 g/L) |
Diseases of the musculoskeletal system and connective tissue | 57 |
| 4 weeks | vancomycin (0.5 g/L) + neomycin (1 g/L) + ampicillin (1 g/L) + metronidazole (1 g/L) |
Diseases of the digestive system | 58 |
| 4 weeks | ampicillin (1 g/L) + neomycin (1 g/L) + metronidazole (1 g/L) + vancomycin (0.5 g/L) or vancomycin (0.5 g/L) alone |
Bone formation | 59 |
| 4–5 weeks | ampicillin (1 g/L) + neomycin (0.5 g/L) + streptomycin (0.5 g/L) + vancomycin (0.5 g/L) |
Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 60 |
| 7 weeks | ampicillin (1 g/L) + vancomycin (0.5 g/L) + ciprofloxacin (0.2 g/L) + imipenem plus cilastatin (250 mg/L) + metronidazole (1 g/L) |
Mental and behavioral disorders (depression) | 61 |
Table 5.
Oral gavage – protocols for antibiotic-induced gut depletion
| DURATION | ANTIBIOTIC COCKTAILS CONCENTRATIONS |
DISEASES (ICD 10) |
PUBLICATIONS |
|---|---|---|---|
| 3 days | ampicillin (1 g/L) + neomycin (0.5 g/L) + vancomycin (0.5 g/L) + metronidazole (1 g/L) |
Aging | 62 |
| 3 days | ampicillin (500 mg) + vancomycin (250 mg) + neomycin (500 mg) + metronidazole (250 mg) |
Diseases of the digestive system | 63 |
| 3 days | ampicillin (1 g/l) + streptomycin (5 g/L) + colistin (1 g/L) + vancomycin (0.25 g/L) |
Neoplasms (Cancer) | 64 |
| 5 days | ciprofloxacin (0.1 g/L) + ampicillin (0.5 g/L) |
Diseases of the digestive system | 65 |
| 5 days | ampicillin (200 mg/kg) + neomycin sulfate (200 mg/kg) + metronidazole (200 mg/kg) + vancomycin (100 mg/kg) |
Infectious diseases | 66 |
| 7 days | ampicillin (1 g/ml) + metronidazole (1 g/ml) + neomycin sulfate (1 g/ml) + vancomycin (0.5 g/ml) |
Diseases of the nervous system (Encephalomyelitis) | 67 |
| 7 days | ampicillin (200 mg/kg) + neomycin (200 mg/kg) + metronidazole (200 mg/kg) + vancomycin (100 mg/kg) |
FMT protocol | 24 |
| 10 days | metronidazole (0.1 mg/g bodyweight) + ampicillin (0.26 mg/g bodyweight) + neomycin (0.26 mg/g bodyweight) + vancomycin (0.13 mg/g bodyweight) |
Disease of the circulatory system | 68 |
| 11 days | ampicillin (43.2 mg) + bacitracin (108.0 mg) + meropenem (21.6 mg) + neomycin (108.0 mg) + vancomycin (6.48 mg) in 4,5 mL of distilled water |
Antibiotic effects | 31 |
| 14 days | ampicillin (1 g/L) + neomycin sulfate (1 g/L) + metronidazole (1 g/L) |
Mental and behavioral disorders (depression) | 69 |
| 14 days | ampicillin (1 g/L) + neomycin sulfate (1 g/L) + metronidazole (1 g/L) |
Mental and behavioral disorders (depression) | 70 |
| 14 days | ampicillin (0.2 g/L) + neomycin (0.2 g/L) + metronidazole (0.2 g/L) + vancomycin (0.1 g/L) |
Neoplasms (Cancer) | 71 |
| 14 days | ampicillin (1 g/L) + vancomycin (0.5 g/L) + neomycin (1 g/L) + metronidazole (1 g/L) + ciprofloxacin (1 g/L) in some experiments |
Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 47 |
| 14 days | ampicillin (0.25 mg/day) + gentamicin (0.25 mg/day) + metronidazole (0.25 mg/day) + neomycin (0.25 mg/day) + vancomycin (0.125 mg/day) |
Mental and behavioral disorders (depression) | 72 |
| 14 days | ampicillin (2.5 g/L) + metronidazole (2.5 g/L) + neomycin (2.5 g/L) + vancomycin (1.0 g/L) |
Sleep | 73 |
| 3 weeks | ampicillin (1 g/L) + metronidazole (1 g/L) + neomycin (1 g/L) + vancomycin (0.5 g/L) |
Immune system | 74 |
Table 6.
Combined oral gavage and drinking water administration – protocols for antibiotic-induced gut depletion
| DURATION | ANTIBIOTIC COCKTAILS CONCENTRATIONS |
DISEASES (ICD 10) |
PUBLICATIONS |
|---|---|---|---|
| 5 consecutive days of gavage + 5 weeks in drinking water |
Oral gavage: ampicillin, neomycin, metronidazole and vancomycin for 5 days (0.2 mL: 10 mg of each antibiotic per mouse per day) Drinking water: ampicillin (1 g/L) + neomycin (1 g/L) + metronidazole: (1 g/L) + vancomycin: (0.5 g/L) for 5 weeks |
Endocrine, nutritional and metabolic diseases (Obesity) | 75 |
| 7 days |
Oral gavage: vancomycin (50 mg/kg) + neomycin (100 mg/kg) + metronidazole (100 mg/kg); Drinking water: Ampicillin (1 g/L) |
Disease of the circulatory system (Stoke, Seizure) | 76 |
| 14 days |
Oral gavage: vancomycin (5 g/L) + neomycin, (10 g/L) + metronidazole (10 g/L) + amphotericinB (0.1 g/L); Drinking water: ampicillin (1 g/l) + last 3 days: omeprazole via oral gavage (50 mg/kg, once a day) |
Disease of the nervous system (Epilepsy) | 77 |
| 14 days |
Oral gavage: 3 days of amphotericin-B (1 mg/kg BW) every 12 h + from day 3 Drinking water: ampicillin (1 g/L) + Oral gavage: every 12 h vancomycin (50 mg/kg BW) + neomycin (100 mg/kg BW) + metronidazole (100 mg/kg BW) + + amphotericin-B (1 mg/kg BW) |
Antibiotic-depletion protocol | 26 |
| 14 days |
Oral gavage: Vancomycin (250 mg) + neomycin-sulfate (500 mg) + ampicillin (500 mg) + metronidazole (500 mg) supplemented with 10 g grape Kool-Aid in 500 mL water + Driking water: Ampicillin (1 g/L) |
Disease of the respiratory system (Influenza) | 78 |
| 17 days |
Oral gavage: 3 days of amphotericin-B (1 mg/kg BW) every 12 h + from day 3 Drinking water: ampicillin ad libitum (1 g/L) + Oral gavage: every 12 h vancomycin (50 mg/kg BW) + neomycin (100 mg/kg BW) + metronidazol (100 mg/kg BW) + + amphotericin-B (1 mg/kg BW) |
FMT Protocol | 79 |
Antibiotics can be administered for gut bacteria depletion via several routes: drinking water, oral gavage or a combination of both and intraperitoneal injection. By comparing them, it was observed that oral treatment – by drinking water or oral gavage – but not intraperitoneal injection treatment substantially reduced gut commensal microbiota.67 Administration of antibiotics in drinking water is very common as it is easy and noninvasive for the animal. However, ad libitum administration prevents control over the rate of consumption and consideration should be given to the stability of the antibiotics in water. It is recommended to record daily water intake to get an estimate of their antibiotic consumption, even though a number of rodents are usually caged together.
Oral gavage is a more reliable method for controlling the frequency and dose of antibiotics administered but is not ideal for long periods of antibiotic administration. This technique requires practice to minimize adverse events associated with it80 such as habituation to handling prior to the study.80 If large volumes are required, a slow delivery rate is recommended.80 Without habituation, rodents can show signs of stress as long as 1 h after gavage.81 Oral gavage with sucrose-precoated gavage needles can measurably decreases signs of stress thereby improving animal welfare during this procedure;82 even though sucrose consumption might be a confounding factor in some studies. A comparison of mice receiving: a single-course systemic antibiotic cocktail, a non-absorbable antibiotic cocktail and a three-course antibiotic cocktail altering systemic and non-absorbable antibiotics has shown that multiple courses of alternating antibiotic cocktails allowed sustained engraftment of human gut microbiota – numerically comparable to the colonization of GF mice.37 However, for the same human donor, the gut microbiota composition of GF or antibiotic-treated mice recipients differed significantly. This implies that regardless of the model chosen, the engraftment dynamics will be different since they have different gastrointestinal environment. This information is also important when interpreting the results. It is possible to combine administration by drinking water and oral gavage, allowing administration according to the pharmacokinetics of the antibiotics. Table 2 summarizes advantages and limitations of each based on Turner et al.81 and Morton et al.83 work.
Use of antibiotics: important considerations
Some important aspects need to be considered when designing an antibiotic depletion experiment. Antibiotic consumption may produce temporary side effects or in some cases permanent ones – especially at critical developmental stages1. Side effects of antibiotic-induced depletion are not yet fully understood. For example, some antibiotics can alter metabolic homeostasis (ampicillin, vancomycin, metronidazole, neomycin),84 alter hippocampal neurogenesis (ampicillin + sublactam, vancomycin, ciprofloxacin, imipenem plus cilastatin, metronidazole),85 anxiety and cognitive behaviors (ampicillin, vancomycin, metronidazole, ciproflaxin, neomycin or ampicillin, bacitracin, meropenem, imipenem, neomycin and vancomycin).86 Important side effects also include immune system perturbations.1 Studies focusing on the gut-brain axis can be compromised by the use of compounds able to cross the blood-brain barrier or those that are readily absorbed from the gastrointestinal tract. We know, for example, that metronidazole absorbed in the intestine can accumulate in the brain, where it may exert undesirable effects.1 If your study focuses on bacteriophage, it is important to consider phage-bacterial network interactions following antibiotic treatment.87
Three major considerations have to be taken into account regarding antibiotic-induced alterations in the gut: 1) depletion of the gut microbiota, 2) direct effects of antibiotics on host tissues and 3) effects of remaining antibiotic-resistant microbes. These alterations are dependent on the duration of the treatment and dosage of antibiotics and may lead to emergence of bacterial resistance. Indeed, bacteria compete with each other via a wide range of mechanisms, including the secretion of antibiotics.88 A disrupted healthy microbiota can lead to an expanded abundance of pathobionts – virulent species in the resident microbiota – resulting in aberrant pathobiont-induced innate immune signaling.89 To minimize bacterial resistance following an antibiotic treatment, high doses of antibiotics over a fairly short duration is preferable.90 This is consistent with studies suggesting that a short course of antibiotic treatment is sufficient to induce complete depletion without significant side effects.37 Antibiotics affect the microbiota of various host species in different ways.37 Functional studies on the gut microbiota that use antibiotic treatment must mention the limitations of this model and strive to control for the potential confounding effects as much as possible.
Direct supplementation in conventionally colonized animals
Conventionally colonized animals are reared in an animal facility with their commensal microbiota. A few studies preferred not to use GF mice or a microbiota depletion-model and simply performed a fecal transfer in mice harboring their commensal microbiota.91–93 By reconstructing colonization patterns of human fecal microbes in mice with different genotypes (C57BL6/J vs. NSG) and with or without gut microbiota depletion using antibiotics, Zhou et al. found that mouse genotypes and native gut microbiota exert different selective pressures on exogenous colonizers, considered by the authors as colonization resistance.91 They concluded that depleting the mouse gut microbiota promotes colonization of human microbes.91 Conversely, another study looked at the results of transplantation in the same species (rat) when the recipient is treated with or without antibiotics. They concluded that antibiotic intake prior to transplantation did not increase the establishment of the donor phylotypes and that an indigenous gut microbiota can be reshaped without the use of antibiotics.94 To date, very few studies transferred microbiota into rodents simultaneously harboring their native commensal microbiota. In some cases, the use of conventional animals for FMT studies seems to be an adequate solution to limit the variables of the study. For instance, in the context of an infectious disease95 or after inducing a disease by injecting a chemical compound that mimics the symptoms.5 FMT in conventional animals can restore or help to improve the symptoms of a disease.
Laxative-depletion
More recently, the use of laxatives as an alternative approach to counteract limitations of both gnotobiotic and antibiotic-treated rodents have been considered in FMT experiments. For example, it has been shown in conventional mice that the gut microbiota can be depleted by laxative exposure: 4 bowel cleansings with PolyEthylene Glycol (PEG) were used to empty the intestines and transiently decrease bacterial load by 90%.96 However, as there is little research using this method yet, it makes it difficult to compare across studies. More detailed studies on the influence of the PEG (or other possible laxatives) are necessary to allow us to discern the limitations of this model. However, three pre-treatment conditions (antibiotics, laxatives or no pre-treatment) have been recently compared.97 Interestingly, and perhaps not unexpectedly, antibiotics induced a more effective depletion of the intestinal bacterial community in the colon than induced by laxative treatment.97 However, disparities exists between the concentration of laxative (PEG) used in this versus other studies.24 It appears that laxative-induced depletion can be a valid method to induce gut microbiota depletion – however, at least 170 mg of PEG is needed to obtain a significant bacterial load reduction in adult mice.96 Moreover, its systemic effect or direct impact on the intestine is not yet well characterized. In humans, laxative depletion is the most popular method of depleting the microbiota in patients requiring FMT following C. difficile infection; however, it may simply reflect the common practice of bowel preparation prior to colonoscopy. At present, this method provides an alternative in newborns rodents or studies where antibiotic depletion or GF state is not suitable. However, it is unclear if this method can be successfully applied to all pathological conditions.
Vertical microbiota transmission models
In 1995, Hirayama et al. demonstrated that GF mice, transplanted with microbiota from a human donor, could transmit the human gut microbiota to their offspring – thus circumventing the side effects of using a GF model by studying the next generation.98 Indeed, after conducting a long-term assessment of the transmission modes of bacterial genera – 11 generations – they showed that the majority of the murine gut microbiota was vertically inherited.99 More specifically, obligate anaerobes tended to be transmitted vertically whereas obligate aerobes tended to be transmitted horizontally. Since then, other studies demonstrated that bacteria were transferred to the offspring naturally from the mother and used it as a model of FMT in rodents.100,101 This model was later refined by spreading the corresponding FMT inocula on the abdominal and nipple regions several days after birth.102
Given that GF mice have many physiological, immune and neurodevelopmental alterations at baseline it makes it difficult to use them for certain causal inferences.2 Vertical transmission to the next generation can obviate these effects at least partially. In this regard, Sharon et al. revealed that colonizing GF mice with fecal microbiota from autistic human donors was sufficient to promote core behavioral symptoms in their offspring.103 The maternal gut environment during pregnancy is a contributor to metabolic programming of the offspring (i.e. can confer resistance to obesity).104 It is therefore plausible that administering short-chain fatty acid-producing bacteria in mothers might influence prenatal development of the metabolic and neural systems. Overall, this model allows a natural transmission of an “exogenous” microbiota without deficiency in development – as opposed to a GF development. In contrast, it has been shown in humans that viromes are unique to individuals regardless of their genetic relationship.1 However, the lack of information on the transmission of microbiota from the mother to the offspring prevents conclusions regarding the validity of this method for FMT studies.
Bedding material, coprophagy and co-housing
Due to the coprophagic nature of rodents, some studies transfer the microbiota of donor rodents by transferring used bedding from the donor mouse to the cage of the recipient. This technique can also be used as booster inoculation, after gavaging rodents with FMT once, cages of recipient mice can be replenished with dirty bedding and fresh fecal pellets from donors several times a week.105 The recipient mice can also be recolonized by swabbing their mouths with homogenized donor fecal pellets prior to placing bedding and feces from them into the cages of the recipients.54
However, co-housing donor and recipient animals might lead to an untargeted and uncontrolled colonization. For example, results from a study where mice were caged together after receiving either a microbiota from a healthy or an underweight human donor, showed that colonization was mainly from mice receiving a healthy microbiota to mice with an undernourished microbiota but not the opposite.106 This suggests that it is challenging to control the outcome of a microbial transfer using this method of colonization. Moreover, it is possible that this method allows the transfer of aerobic and facultative anaerobic microorganisms but might not transfer strict anaerobes due to their exposure to an oxygenated environment. Currently, there are not enough studies comparing different methods of microbial transfer which prevents us from fully understanding the effectiveness of this method compared to the others.
Comparisons of different models
The ideal donor
FMT treatments have become prominent tools in research due to their use in Clostridioides difficile infection, as antibiotic treatment induces a large disruption of the commensal gut microbiota community and functionality whereby the pathogen is then able to proliferate and cause disease.107 For this reason, a healthy and intact gut microbiota can outcompete and replace C. difficile in the intestinal habitat and this leads to effective treatment and resolution of the infection.108 The extent to which microbial engraftment is necessary compared to treatments that influence a shift in the microbiome of the recipient is likely dependent on the goal of the treatment. It appears that engraftment is less important if the purpose of the FMT is to treat infections such as C. difficile;109 whereas certain microbial taxa may need to be present from engraftment in order to introduce specific functional outputs (eg. Ruminococcaceae, Verrucomicrobiaceae, and Lachnospiraceae) in the case of cognitive improvement and reduction in inflammation following FMT in cirrhotic patients110- which is still to be fully established in FMT pre-clinical studies.
The goal of these studies is to better understand the influence of the gut microbiota in the desired model and to maximize the efficient use of animals, materials and labor. Herein we identify the route to optimally recapitulate the microbiota of the donor to recipient through FMT. These considerations are intended for both human and animal donors. It is necessary to characterize the donor in terms specific to the study as well as general health and lifestyle traits that may be pertinent. Reportable and easily obtained descriptors that have been identified to affect the gut microbiota should be collected when identifying donors: such as age, sex, diet, general health, medications, etc. Undoubtedly more information leads to more detailed selection criteria, however, donor information should be carefully considered and tailored to the goals of the study. Similarly, donor exclusion criteria include recent antibiotic use, illness, disorder, medication use, age and chronic disease. Additional factors should be considered dependent on the specific research goals of each study. It is best to analyze the gut microbiota of the donor before FMT to the recipient as this allows for the association of the donor with specific taxa and functional groups with the phenotype being studied. Then, individual donors with a combination of the highest phenotypic scores and selected gut microbiota profile traits can be selected for use. Furthermore, it is unknown to what extent the gut microbiota will be linked with different diseases and disorders, therefore, whether specific microbial groups are implicated in the causality or result as a downstream consequence of the phenotype will impact the results. It may be desirable to use phenotypic extremes of a population for the recapitulation of a donor profile in the recipient animal. However, in some cases, like in a highly heterogenous population, randomly chosen donors may function better. Patient donors commonly take medication for either the target phenotype of the study or other comorbidities; this should be accounted for in the study design. Ideally, fecal microbiota should be collected from donors without medication since some compounds are able to impact gut microbiota significantly,111 and therefore the medication remodeled gut microbiota can potentially be transferred to the recipient by FMT and exert downstream effects in the model. If donors without medication cannot be found – which is a difficult criterion to fulfill in some populations – then it is paramount to adequately control for the potentially confounding factors.
Microbiome composition, stability, and engraftment are determined by a number of factors112 such as genes, environment, diet and immune function that can impact how well the FMT is received. Donor microbiome composition and complexity (i.e. diversity) appear to be major determinants of transplant efficacy.113 Patients who have a successful clinical response to FMT (i.e. responders) usually exhibit a higher microbial diversity than those who do not (ie. non-responders).114 Conventional theory is that there are certain microbes fundamental to healthy functionality of the gut microbiota, which, through mutualistic mechanisms (like microbial cross feeding and pathogen exclusion), aid the colonization of other commensal microbes.115 However, proving this theory has proven to be an elusive and arduous task as there is tremendous inter- and intra-individual heterogeneity dependent on a multitude of different factors. One explanation is that the groups necessary to maintain a healthy microbiota are composed of guilds that provide specific functions116 within the gut while important clusters and specific members are commonly referred to as enterotypes and keystone species (e.g. Bacteroidaceae, Bifidobacteriaceae, Lactobacillaceae, Eubacteriaceae, Prevotellaceae).117 Furthermore, emerging evidence suggests that some FMT donors may result in higher efficacy to treat C. difficile, irritable bowel syndrome, and inflammatory bowel disease. These individuals are frequently described as super-donors.118 Common trends found in super donor FMTs are high microbial diversity, richness, and the presence of keystone species within the microbiota composition (e.g., butyrate producers within Clostridium clusters IV and XIVa).113,119 However, more research must be done to identify, evaluate, and utilize this concept to our advantage and to elucidate whether there are similar factors that influence cross-species FMT.
The ideal recipient
Rodent recipient models
This review focuses only on rodent recipients as it is the most widely used model in pre-clinical research to assess causality of the intestinal microbiota. Rodents are commonly used as recipients of microbiota from human donors even though they might not be the most appropriate model for a successful engraftment given their disparities – compared to monkeys or pigs for example.120 Indeed, porcine models were used successfully but are not within the repertoire of most research groups in the field.121 On the same note, colonizing zebrafish with either zebrafish,122 mice122or human123 gut microbiota has also been the focus of FMT research. However, the transfer of behavioral phenotypes from human or rodent donors to rodent recipients has been demonstrated most frequently.9,10
When FMT is performed within the same species, there are fewer physiological dissimilarities within the GI tract (pH, morphology, diet etc.)120 and a greater likelihood that the microbiota of the recipient will resemble that of the donor. However, it seems likely that donor-specific taxa reliably colonize recipients only when rich donor material is transferred to mice originally colonized with a simpler microbiota.124 Several studies have compared humanized rodent models and concluded that rats outperformed mouse models – in terms of colonization efficiency – because they are more similar to humans and capture more human microbial species.125 Spatial organization of bacterial communities across and along the GI tract complicates microbiome analysis. The sample type used to extract bacterial genetic information is of crucial importance. Along the gastrointestinal tract, a gradient of pH, bile acid concentrations, oxygen levels and antibacterial products exists. Therefore, the microbial composition of feces and intestinal tissue is intrinsically different, because they are dictated by the radial gradient of oxygen and substrates provided by the host.126 Consequently, when a rodent is colonized by the microbiota originating from different gut regions of a donor – in this case a pig – different colonization patterns are obtained depending on the site of origin of the donor inoculum: jejunal, ileal, cecal, colonic, fecal or whole-intestinal microbiota.127 It was demonstrated that the microbiota of a specific intestinal region selectively colonizes the corresponding intestinal region of the recipients; leaving the whole-intestinal microbiota inoculum as the most promising solution to reconstitute the entire microbiota of the whole gastrointestinal tract. For ethical and practical reasons, it may not always be feasible to collect tissue (biopsy) samples. In addition, fecal matter is usually collected to meet practical standards for the donors in terms of storage and shipment, and some investigators use samples that were collected anaerobically.
Sex differences
Despite sex being an important variable affecting the gut microbiota,128 the majority of scientific publications still present results from males only. It has been demonstrated that sex differences and hormonal effects on gut microbiota composition are important; by comparing 89 different inbred strains of mice – including C57BL/6 which exhibited high sex-specific differences and are widely used in FMT studies.129 Both sex and strains have a significant effect on the gut microbiota. To test the evolvement of sex hormones, they performed a gonadectomy in 3 different strains and were able to identify clear hormonal effects on gut microbiota composition. Markle and colleagues investigated how a male donor would influence a female recipient – with particular regard to age.130 Their data showed that in young female recipients, testosterone elevation was compatible with normal breeding behavior but not in adults; suggesting that if inter-sex transplantation needs to be done, puberty is probably a more appropriate period. Indeed, sex-specific differences in gut microbiota composition became evident at puberty and most apparent in adult mice. FMT to GF mice from same or opposite sex was done to investigate the stability of engraftment at 1 and 4 weeks following FMT administration; it appears that for both the microbiota first adapted to the sex of the recipient at week 1, then at week 4, gut microbiota composition was similar to the sex of the donor, regardless of the sex of the recipient.131 Therefore, sex-specific differences need to be considered while designing an FMT experiment to ensure valid conclusions for both genders: it is preferable that donors and recipients be of the same sex.129
Age of the recipient
The next important consideration is the age of the recipient rodents that allows the most efficient transfer of the donors’ gut microbiota. By transferring microbiota from a donor mice into juvenile or adult SPF mice, it was suggested that the engraftment was more efficient in juveniles rather than adults.24 This observation seems plausible given that the rodent microbiota is more stable at a later stage of life.132 With these collective results, they show that the use of juvenile mice subjected to initial microbiota depletion constitutes a valid alternative to GF mice in microbiota transfer studies.24 However, additional research should be conducted to understand the impact of age on microbiota transplant success. Indeed, age-related changes in the intestinal microbiota can have a significant impact on metabolism, immunity and behavior.133 If antibiotic-depletion is used during early life in a recipient animal, this can be associated with long-lasting metabolic consequences,134 even if the microbiota recovers following colonization. FMT experiments on rodents with a significant age difference influenced behavior,135 inflammation,105 neurogenesis,136 and intestinal morphology.136 When planning an experimental design with human donors, the ideal option would be to match the stage of life between human and rodent, or at least acknowledge the implications that the age of the model plays in the engraftment of the FMT and the measured outcomes.
Housing conditions
Important considerations such as housing conditions need to be discussed in an FMT experiment. First of all, rodents are coprophagic, therefore they cannot be caged together if they receive FMT from different donors unless this is an intentional experimental intervention. By comparing metagenome composition of mice depending on: strains from different suppliers, housing laboratory and low- or high- fat diets; it was shown that mouse provider and housing conditions had a pronounced effect on the composition of the gut microbiota.137 Although the mouse supplier is a factor that cannot be controlled when comparing between studies, housing condition is. On the other hand, when one becomes interested in human-to-mice FMT success with regard to housing conditions and mice coprophagy; it was found that in mice inoculated with the same donors and caged together in a very controlled environment – 2 mice per cage – the spread of microbes between cages occurs within each isolator.91 However, individually ventilated cages might be sufficient to prevent bacteria transfers between cages for at least 9 weeks when basic hygiene measures are applied when handling mice,24 but may compromise some behavioral readouts.17 Furthermore, cohabitation of mice with different phenotypes (e.g. lean vs. obese or aged vs young) can lead to important metabolic changes.105
These results imply that animals from the same experimental group must be in the same cage, as cross-contamination could occur and affect the results. To prevent coprophagy, collars can be used. However, coprophagy in rodents is beneficial for their metabolism; preventing it would affect a healthy energy balance, microbial diversity and downstream behaviors.138 When examining differences over broad range of animal facilities using standardized procedures while maintaining a constant host genetic background (C57BL/6 mice); increased variation was observed among mice held in open cages, in rooms where other strains are present, or in a less restrictive access policy.139 Furthermore, the gut microbiota of experimental animals is also influenced by contamination through shed skin or dust particles carried by other animals, caretakers, or scientists alike. The genus Propionibacterium is an indicator of human skin contamination of the mouse microbiota. Despite all efforts to maintain hygiene and standardize conditions and procedure, each facility, and even each room in a facility, harbors its own unique combination of a multitude of variable factors which will give rise to distinct microbiota configurations. The impact of these differences is an important but often unknown factor in FMT studies.
Diet
Diet has a great influence on intestinal microbes,112 and therefore demands particular attention for studies involving changes in gut microbial population. Many studies have demonstrated that FMT procedures from an animal or human with a specific diet to another animal can dramatically impact host physiology and metabolism; and thereby be an important confounding factor if not considered properly. Therefore, it is important to consider how the diet of the donor may affect the recipient140 but also the extent to which the diet of the recipient may affect the outcome of the microbial transfer.141 This was demonstrated elegantly in 2013, by transplanting the microbiota of human twin pairs discordant for kwashiorkor – a severe form of protein malnutrition – into GF mice. As expected, transplantation of the microbiota of the kwashiorkor co-twins in mice resulted in a transfer of phenotype compared to mice harboring the healthy microbiota of the sibling. Surprisingly, the weight loss phenotype was not the only the result of the microbiota transplant, but rather depended on the combination of the diet of the recipient (chow, low protein or high protein diet) and the transplanted microbiota. If the diet switched from low-protein to high-protein in recipient animals, all animals rapidly gain weight again.140 The fact that diet had such a significant impact in this study compared to fecal transplantation can also be explained by the fact that there was only one gavage for fecal transplantation followed by 63 days of different diet.
These results indicate that the microbiota of donors and the diet of both the donor and the recipient has a huge impact on the transfer of phenotype in gnotobiotic animals. Turnbaugh et al. also demonstrated that colonization of human or murine microbiota in GF mice is greatly influenced by diet: they demonstrated that a switch of diet – a low-fat, plant polysaccharide-rich or a high-fat, high sugar “western” diet – was able to shift the structure of the microbiota within a single day and altered microbiome gene expression of the mice.100 Moreover, if human donors are used, inter-species feeding differences should be considered as it shapes the gut microbiota differently.142 For example, when assessing the stability of human fecal transplantation in GF mice with a “humanized” diet – as opposed to mice on a regular chow diet: it was found that a humanized diet allowed a better retention of human gut microbiota in recipient mice101 and a decrease in enterotype changes.143 For gnotobiotic rodents, the diet can be treated: autoclaved or irradiated. It is important to know that these treatments influence the quality of the diet and no longer contain all the nutrients necessary for the development of a healthy microbiota.139 In such cases, a laboratory autoclavable rodent diet can be used, as it is supplemented with higher levels of nutrients to compensate losses during autoclaving. We might underestimate the impact of diet when conducting an FMT study, which is especially important for human-to-rodent FMT. It must be kept in mind that humans and rodents have very distinct diets, in addition to having many physiological differences, making it a challenge to obtain a humanized-microbiota model in rodents with a high percentage of microbial transmission.
Host and genetics
The current literature lacks information about which strain of rodents are best suitable for fecal microbiota transplant whilst genetics influence gut microbiota composition in widely used laboratory mouse strains.144 Table 7 summarizes which strains of rodents have been used over the past 30 years according to the topic of the study. If some information are needed to estimate the microbial composition of a specific strain of mice in healthy condition, a database has been compiled called “Murine Microbiome Database” with 9 common strains of laboratory mice.458 It was demonstrated that colonization patterns differed between mouse strains by comparing 23 different strains of gnotobiotic mice receiving the ASF gut microbiota.459 This illustrates how a transplant of only 8 bacterial strains can vary depending on host genetics. Korach-Rechtman et al.460 went even further by crossbreeding two strains of mice BALB/c and C57BL/6 J and studying the F1 offspring derived from their reciprocal crossbreeding (♀C57BL/6 J × ♂BALB/c; ♀BALB/c × ♂C57BL/6 J). They demonstrated that twelve taxa were shown to have genetically controlled gut persistence. This is in agreement with previous studies showing that some bacterial phylotypes appear to be discriminative and strain-specific to each mouse line used.461 While the two genetically distinct parental inbred lines presented important microbiota differences, their hybrids offspring presented a very similar microbiota; highlighting the importance of genetic effect on microbiota composition. Finally, they analyzed to what extent the inherited microbiota would be disrupted by co-habitation with one of the parental strains, allowing bacterial transfer by co-habitation and coprophagy. Interestingly, some taxa were modified by cohabitation but returned to their inherited microbiota composition after separation. This suggests that for a microbial phenotype of the donor to be sustainable over time, the microbiota of the donor must be inoculated repeatedly throughout the whole duration of the FMT experiment (e.g.: co-habitation, several inoculations across time).
Table 7.
Rodent strains used in preclinical FMT studies over the last 30 years in the most prevalent topics
| DISEASES | DONORS | RECIPITENTS | REFERENCES |
|---|---|---|---|
| Aging | Mice | Mice: C57BL/6 | 32,43,105,136,145,146 |
| Mice: C57BL/6 J | 147 | ||
| Mice: C57BL/6N | 62 | ||
| Mice: BALB/c | 105,148 | ||
| Mice: Swiss Webster | 149 | ||
| Mice: SAMP8 | 150 | ||
| Mice/C3H/HeN | 151 | ||
| Rats | Rats: Dahl | 152 | |
| Rats: Sprague-Dawley | 153 | ||
| Human | Mice: C57BL/6 | 154 | |
| Mice: C57BL/6 J | 155,156 | ||
| Alcohol-related disorders | Mice | Mice: C57BL/6 | 157,158 |
| Mice: C57Bl/6 J | 159,160 | ||
| Rats | Rats: Sprague Dawley | 161 | |
| Human donors | Mice: C57BL/6 J | 162,163 | |
| Mice: NSG | 164 | ||
| Alzheimer’s disease | Mice | Mice: C57BL/6 | 43 |
| Mice: ADLPAPT | 165 | ||
| Mice: SAMP8 mice | 150 | ||
| Mice: APPswe/PS1dE9 transgenic (Tg) mouse model | 7 | ||
| Rats | Rats: Sprague Dawley | 166 | |
| Human | Mice: C57BL/6N | 167 | |
| Autism-spectrum disorder | Mice | Mice: C57BL/6 J | 168,169 |
| Human | Mice: C57BL/6 J | 103 | |
| Mice: C57BL/6N | 170 | ||
| Cancer | Mice | Mice: C57BL/6 | 171–182 |
| Mice: C57BL/6 J | 35,183–186 | ||
| Mice: ICR | 187 | ||
| Mice: Swiss webster | 173,174,188 | ||
| Mice: BALB/c | 171,189–192 | ||
| Rats | Rats: Sprague-Dawley | 193,194 | |
| Human | Mice: C57BL/6 | 71,195–197 | |
| Mice: C57BL/6 J | 198 | ||
| Mice: C57BL/6, ALB/c or IQI | 199 | ||
| Mice: 5TGM1 | 200 | ||
| Mice/C3H/HeN | 201 | ||
| Cognition | Mice | Mice: C57BL/6 | 43,145,202 |
| Rats | Rats: Sprague-Dawley | 153 | |
| Colitis | Mice | Mice: C57BL/6 | 74,178,179,203–236 |
| Mice: C57BL/6 j | 95,237–247 | ||
| Mice: C57BL/6NTac | 248 | ||
| Mice: C57BL/6 NCr | 249 | ||
| Mice: Swiss Webster | 229,250–252 | ||
| Mice: BALB/c | 191,239,253–261 | ||
| Mice: CBA/CaJ | 230 | ||
| Mice: 129SvEv | 262 | ||
| Mice: CBA and Swiss Jim Lambert (SJL) | 263 | ||
| Rats | Rats: Sprague-Dawley | 264–267 | |
| Mice: BALB/c | 261 | ||
| Human | Mice: C57BL/6 | 268 | |
| Mice: C57BL/6 J | 268–270 | ||
| Mice and Rats: BALB/c and Sprague-Dawley | 271 | ||
| Clostridium Difficile infection | Mice | Mice: C57BL/6 | 272 |
| Mice: Swiss-Webster | 273 | ||
| Human | Mice: C57BL/6 | 274–276 | |
| Mice: C57BL/6 J | 277,278 | ||
| Diabetes | Mice | Mice: C57BL/6 | 279 |
| Mice: C57BL/6 J | 279 | ||
| Mice: C57BL/6 NTac | 280 | ||
| Mice: db/dd and C57BL/Ks | 281 | ||
| Mice: Kunming | 282 | ||
| Mice: NOD | 283–285 | ||
| Mice: NOR mice | 285 | ||
| Mice: BALB/c | 286 | ||
| Rats | Rats: Sprague Dawley | 287 | |
| Human | Mice: C57BL/6 J | 288 | |
| Mice: NOD | 289 | ||
| Mice: db/db and db/m unknown genetic background | 290 | ||
| Depression | Mice | Mice: C57BL/6 | 61,291,292 |
| Mice: C57BL/6 J | 293 | ||
| Mice: BALB/c | 294 | ||
| Rats | Rats: Sprague Dawley | 10,295,296 | |
| Rats: Long–Evans | 297 | ||
| Rats: Flinders sensitive line and Flinders resistant line | 298 | ||
| Rats Lewis | 299 | ||
| Rats: Wistar | 300 | ||
| Mice: C57BL/6 | 46 | ||
| Human | Mice: Kunming | 301,302 | |
| Exercise | Mice | Mice: C57BL/6 J | 303 |
| Mice: C57BL/6 JNarl | 33 | ||
| Irritable Bowel Syndrome/Irritable Bowel Disease | Mice | Mice: C57BL/6 | 174,251,304 |
| Mice: Swiss Webster | 174,251 | ||
| Rats | Rats: Sprague-Dawley | 305 | |
| Rats: Long-Evans | 306 | ||
| Rats: Wistar | 307 | ||
| Human donors | Mice: C57BL/6 | 308–311 | |
| Mice: ATG16L1T300A KO mice unknown genetic background | 312 | ||
| Rats: Sprague-Dawley | 313 | ||
| Rats: Fisher 344 albinos | 314 | ||
| Liver-associated conditions | Mice | Mice: C57BL/6 | 315–321 |
| Mice: C57BL/6 J | 322–327 | ||
| Mice: DBA/2 J | 327 | ||
| Mice: IRC | 328 | ||
| Mice: Swiss Webster | 329 | ||
| Rats | Mice: C57BL/6 | 330 | |
| Rats: Sprague-Dawley | 331 | ||
| Human | Mice: C57BL/6 | 38 | |
| Mice: C57BL/6 J | 96,163,332,333 | ||
| Mice: SAMP | 334 | ||
| Mice: Swiss/NIH | 335 | ||
| Rats: Sprague Dawley | 336 | ||
| Rats: F344 | 337 | ||
| Malnutrition | Mice | Mice: C57BL/6 | 338 |
| Mice: A/J and C57BL/6 J | 339 | ||
| Human | Mice: C57BL/6 J | 106,340,341 | |
| Metabolic syndrome | Mice | Mice: C57BL/6 | 342–345 |
| Mice: C57BL/6 J | 346,347 | ||
| Mice: C57BL/6 N | 348 | ||
| Mice: ICR | 347,349,350 | ||
| Mice: Swiss Webster | 251,351,352 | ||
| Mice: BALB/c | 353 | ||
| Human donors | Mice: Swiss-Webster | 352 | |
| Multiple sclerosis | Mice | Mice: C57BL/6 | 354,355 |
| Mice: C57BL/6 J | 356,357 | ||
| Rats | Rats: Dark Agouti | 358 | |
| Human | Mice: C57BL/6 | 359 | |
| Nonalcoholic Fatty Liver Disease (NAFLD) | Mice | Mice: C57BL/6 | 360,361 |
| Mice: C57BL/6 J | 362 | ||
| Nonalcoholic steatohepatitis (NASH) |
Mice | Mice: C57BL/6 | 363 |
| Obesity | Mice | Mice: C57BL/6 | 75,141,222,364–373 |
| Mice: C57BL/6 J | 346,374–391 | ||
| C57Bl/6 N | 392–395 | ||
| Mice: C57BL/6 NTac | 396 | ||
| Mice: C57BL/6 JNarl | 33 | ||
| Mice: SwissWebster | 149,397 | ||
| Mice: ICR | 398 | ||
| Mice: ob/ob | 399 | ||
| Mice: Atg7 | 400 | ||
| Rats | Rats: Sprague Dawley | 401–406 | |
| Rats: Wistar | 45 | ||
| Rats: LZ and ZDF | 407 | ||
| Pigs | Mice: C57BL/6 J | 408 | |
| Human | Mice: C57BL/6 | 409,410 | |
| Mice: C57BL/6 J | 411–415 | ||
| Mice: Swiss Webster | 416,417 | ||
| Mice: db/db and db/m unknown genetic background | 290 | ||
| Pancreatitis | Mice | Mice: C57BL/6 | 418–420 |
| Mice: C57BL/6 J | 421,422 | ||
| Mice: MRL/MpJ | 423 | ||
| Mice: NOD/MrkTac | 424 | ||
| Parkinson | Mice | Mice: C57BL/6 | 5 |
| Mice: C57BL/6 J | 425 | ||
| Polycystic ovary syndrome | Mice | Mice: C57BL/6 J | 426 |
| Rats | Rats: Sprague-Dawley | 427 | |
| Human | Mice: C57BL/6 | 428 | |
| Schizophrenia | Human donors | Mice: C57BL/6 J | 429,430 |
| Mice: Kunming | 431 | ||
| Sepsis | Mice | Mice: C57BL/6 | 432–435 |
| Mice: C57BL/6 J | 436 | ||
| Mice: Swiss Webster | 435 | ||
| Rats | Rats: Sprague-Dawley | 437,438 | |
| Rats: Wistar | 439,440 | ||
| Human | Mice: C57BL/6 | 66 | |
| Stress | Mice | Mice: C57BL/6 | 441 |
| Mice: C57BL/6 J | 72,293,442,443 | ||
| Mice: SKG and BALB/c | 444 | ||
| Mice: BALB/c | 14 | ||
| Rats | Rats: Wistar-Kyoto and Spontaneously Hypertensive Rats | 445 | |
| Rats: Sprague-Dawley | 446–448 | ||
| Human | Mice: C57BL6/J and NSG | 449 | |
| Stroke | Mice | Mice: C57BL/6 | 32,36,146,450 |
| Mice: C57BL/6 J | 451 | ||
| Mice: BALB/c | 452 | ||
| Rats | Rats: Sprague Dawley | 453,454 | |
| Rats: Dahl | 455 | ||
| Human | Mice: C57BL/6 | 456,457 |
To conclude, this study shows that some taxa might be present in the offspring independently of genetic transmission; but also, that relationships of genetic dominance and recessivity exists which influence the microbial composition of the offspring. This has been exemplified in a study showing that administration of Lactobacillus johnsonii level decreased rapidly after oral administration in BALB/c mice but not C57BL/6 J mice.462 Interestingly, inter-strain colonization of BALB/c and NIH Swiss mice into GF mice was able to alter behavioral phenotype to resemble the behavior corresponding to the microbiota of the donor.463Although FMT between different mouse strains may result in similar intestinal microorganisms between donor and recipient,339,464 transplantation is optimal if the mouse strains are similar since the host will not exert as much selective pressure on the microbiome of the donor.465
Lastly, the use of knock-out rodents has been coupled with FMT experiments. Interestingly, deletion of a single gene can cause substantial alterations in the microbiota466 and thereby be an important confounding factor. In some cases, FMT can reduce the severity of a phenotype in WT animals. For example, in experimental necrotizing enterocolitis, which was reduced in severity in WT but not Grx1-/- mice.203 This suggests that an important gene can modulate the benefits of FMT, opening up the possibility of specifically targeting a mechanism of interest. Therefore, such study design requires appropriate control groups or preliminary data comparing the composition of the gut microbiome of the wild-type and knock-out mouse models. Further information is required regarding how different strains of rodents differ in terms of microbial composition and how it can influence the outcomes of FMT protocols.
FMT induced microbial, metabolic and immune changes in the host
The genetic background and microbial profile of the host plays a fundamental role in associated microbial, metabolic and immune changes following FMT. Indeed, FMT experiments not only induce changes in the microbial profile of the host, but also causes important metabolic and immune changes. This was explored more extensively in clinical studies where it was shown, for example, that the metabolic benefits associated with a transfer of intestinal microbiota from a lean individual to an obese patient with metabolic syndrome is driven by baseline microbial composition of the host.467 However, it is unclear how the colonization of a GF animal following different FMT preparations will affect its immune system and intestinal morphology. Indeed, the intestine comprises multiple mechanisms to ensure a good balance between the preservation of the bacterial members of the microbiota and the elimination of pathogens. Distinct sites in the gastrointestinal tract are composed of different cell types (including Paneth and goblet cells) and mechanisms of action (mucus secretion, immune activation) that act together to preserve location-specific intestinal homeostasis.
To elucidate the immune mechanisms involved in colonization, some researchers have analyzed innate and adaptive immune responses following colonization in GF mice.468 To start with, they observed a time- and region-dependent enrichment of genes involved in innate and adaptive immune responses – mainly involving T cells – with the largest proportion of differentially expressed genes being involved in the development of the mucosal immune system. They concluded that a novel state of homeostasis was achieved 30 days post-colonization in a region-dependent manner: homeostasis appeared to be established after 8 to 16 days in the colon, whereas in the jejunum and ileum 16 to 30 days were required.468 Importantly, 4 days post-colonization represented an important turning point: strong induction of innate immune functions followed by the stimulation of adaptive immune response, secretion of antimicrobial peptides by Paneth cells and biochemical changes in the mucosal barrier. At the anatomical level, FMT reduced cecum weight considerably, enhanced crypt depths in the whole intestine and increased connective tissue cells in the lamina propria in both the jejunum and the ileum.468 However, one study investigated bacterial colonization of GF mice depending on whether the inoculum of the donor (pig) was provided from the jejunum, the ileum, the cecum, the colon, feces or whole intestine.127 They found that bacterial colonization across different gut segments resulted in anatomical differences in the gut following FMT of the recipient mouse. A similar study with a mouse-to-mouse FMT would be interesting to discern inter- and intra-species FMT differences in FMT grafting according to the origin of the inoculum.
Regarding the impact of colonization on metabolic changes, it appears that the introduction of an exogenous microbiota into a GF mouse greatly impacts host fat storage by affecting hepatic lipogenesis and adipocytes leading to an increase in body fat content despite a reduction in food consumption.469 These are critical points of information when phenotypic, metabolic or behavioral changes are observed in studies on FMT, as the involvement of the intestinal microbiota can be direct – through secreted or metabolized molecules – or indirect by modifying the immune and physiological environment of the intestine, which has repercussions on the extra-intestinal organs. Moreover, it seems like colonization is time- and region- specific468 which implies that depending on the aim of the study, one should wait from 1 to 4 weeks for the newly established gut microbiota to be stable but also for its environment to achieve a new state of homeostasis.
Fecal microbiota transplant preparation
To enhance comparability in the human microbiome field, it is necessary to coordinate and provide standard operating procedures. Some researchers in the field have greatly contributed by launching the International Human Microbiome Standards project where an entire section is dedicated to human sample collection and processing standards and provides procedures for different conditions (http://www.human-microbiome.org/). Regarding FMT from rodent donors, there is currently no equivalent level of standardization and guidance on minimum requirements. Establishing standardized methodologies is critical, with many studies indicating that techniques for processing stool samples vary depending on the experimental design, feasibility and facilities. Figure 3 above summarizes key steps for FMT from donor sample collection to the tractability of the microbial transplant.
Figure 3.
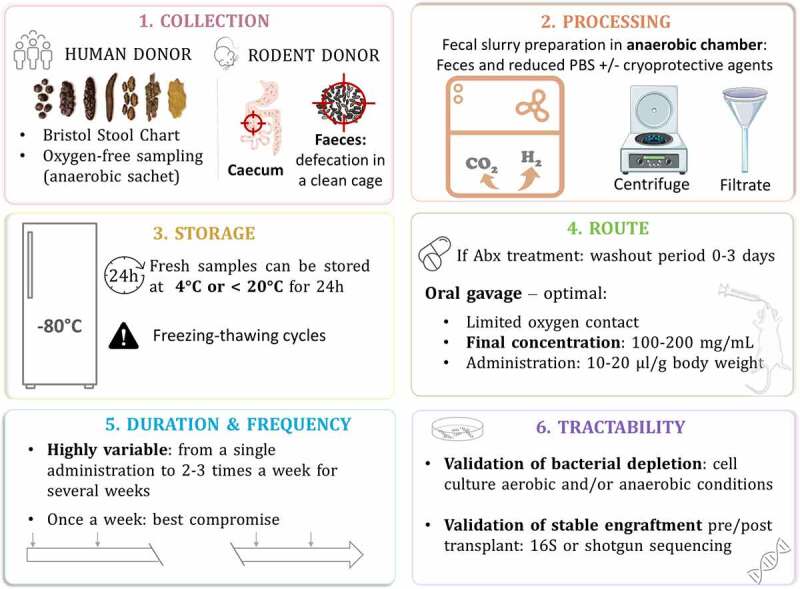
Fecal microbiota transfer: from donor sample collection to the tractability of the microbial transplant
Collection
Microorganisms in the colon are mostly strict anaerobes and oxygen is detrimental to their survival. Collection steps need to take this into consideration. If the donor is human, the feces can be contained in the sample box with an anaerobic sachet that will keep the sample in an oxygen-free environment. The Bristol Stool Chart can be used to record the appearance of collected material, with scaling ranging from 1 (constipation) to 7 (diarrhea).470 International Human Microbiome Standards project offers some guidelines for collection of human samples under different circumstances.471 Ideally, to maximize the preservation of microorganisms, all fecal samples should be kept at 4°C or on ice after collection and during transportation (but should not be frozen as freeze-thaw cycle are damaging for microorganisms); then extracted ideally within 1 hour of collection or a maximum of 24 hours.472 However, some logistical constraints prevent researchers from doing this. Successful transfer of an autistic phenotype by FMT was possible even when the sample was frozen directly at −80°C and processed just prior to transplantation.103
Regarding the collection of mouse feces, donor mice can be placed in a clean cage and allowed to defecate normally. The amount of feces needed for the inoculum preparation would depend on the experimental design of the study. Researchers can then use individual sterile tools to collect and place fecal pellets in a sterile cryo-vial prior to processing in an anaerobic chamber.
Processing
Several studies compared the quality of donor samples when used fresh or frozen for FMT studies. Here again, the methods used to determine the quality of the samples differ between studies and therefore leads to different conclusions. One study found no significant differences in terms of richness, diversity and community structure in mice receiving FMT prepared from frozen donor feces; however, frozen samples were stored for a short time at −80°C.124 Another study found no differences in aerobic and anaerobic populations usinfigug culture-based analysis between the fresh, snap or −80°C frozen samples. However, they treated all samples with a maximum recovery diluent used to support a maximal recovery of microorganisms. This suggests that frozen samples can constitute a good alternative to the use of fresh sample, but with addition of protective solution prior to freezing.
Unlike previous publications, Papanicolas et al.473 showed a significant effect of freeze-thaw cycles which reduced viability to 23% – despite the use of a cryoprotective agent (glycerol) without significantly affecting taxa richness in the sample. Interestingly, they observed an important inter-donor variation in the impact of sample processing. Indeed, microbiota composition can vary from one donor to another in terms of richness of microorganisms vulnerable to oxygen exposure and freezing. Therefore, if the goal of the study is to transfer viable bacteria, we recommend processing the fecal sample in an anaerobic chamber and with the use of a cryoprotective agent if a freezing step is required to enhance bacterial survival. Even when processed in anaerobic conditions, a considerable proportion of bacteria will be damaged or deceased.473 First, the stool sample needs to be homogenized manually to avoid analytical biases due to the heterogeneity of fecal samples. Then, the fecal slurry can be homogenized in autoclaved reduced PBS and glycerol supplemented with L-cysteine hydrochloride, filtered and/or centrifuged.474 The addition of cryoprotective agents such as glycerol, skim milk, maltodextrin, yeast extract and antioxidants – like sodium ascorbate or cysteine – can help reduce damaging effects caused by stresses due to freezing affecting both physical and biological bacteria properties.475 Regarding fecal slurry preparation with rodent donors, most studies processed samples by diluting it with autoclaved, filtered water and homogenized the whole preparation using a tissue lyzer. Homogenates should then be passed through a 30–70 µm pore-size nylon filter to remove large particulate and fibrous matter to generate fresh fecal slurries.
Storage
For human samples, if time before processing to transplantation exceeds 24 hours, fresh samples should not be exposed to temperatures above 20°C, and refrigeration at 4°C can be a safe option.476 Importantly, the freeze-thaw cycles are more deleterious to bacteria than duration of cryoconservation.473 For preservation of bacterial community structure, fecal samples should be frozen within 2 days of collection and up to 2 years at −80°C which leads to minimal changes in the microbial community.477 By analyzing DNA of fecal samples after storage at −80°C for 14 years, it was concluded that microbial profiles are preserved and robust to this extended storage period.478
Route
When rodents are treated with antibiotics, generally a washout period of up to 72 hours is allowed without antibiotic consumption prior to the first FMT administration. The majority of FMT studies in rodents introduce the fecal material by oral gavage: a dose-controlled and efficient option to administer fecal microbes without damaging anaerobes because the contact with oxygen is limited. The volume generally administered in rodents is 200 μL at a final concentration ranging from 100 to 200 mg/mL.
Duration and frequency
These are the most variable parameter across studies. The frequency and duration of FMT administration range from a single administration to twice or three times a week for several weeks. To date, there is a lack of studies comparing transplantation according to the frequency of FMT administration. It has been suggested that repeated gavage at very short intervals (i.e. daily) disturbs the newly established ecosystem and that FMT once a week might be a good compromise.96 Importantly, several studies highlighted that repeated gavage instead of a single gavage increases similarity to donor microbiota.11,96 A stable microbial graft can be achieved only when the whole intestine has reached a new state of homeostasis with its new bacterial population. A few studies demonstrated that it can be achieved in 28–30 days after the first inoculation.11,468 Interestingly, this seems region-dependent where it takes 8–16 days in the colon and 16–30 days in the small intestine.468
Tractability
Validation of bacterial load is advisable to establish the extent to which depletion is achieved and maintained. It can be done before and after antibiotic treatment, but also every week after the first transplant to see how the exogenous microbiota is being engrafted. Culture-based methods are useful to assess the colony-forming units (CFUs) from fecal samples plated in aerobic and/or anaerobic conditions on nonselective media. In most cases, quantitative PCR of the gene encoding 16S rRNA is used as it allows for culture-independent assessment of gastrointestinal bacterial load.
By using different sub-regions of the 16S rRNA gene, one study showed that sequencing full length of 16S rRNA provides a real and significant advantage over sequencing commonly targeted variable regions to provide taxonomic resolution at species and strain level.479 Shotgun metagenomics is an exhaustive method to quantify microbial populations and to assess functionality. It is important to monitor the establishment of the engrafted microbiota, in order to be able to assess the extent to which an exogenous microbiota can be transferred.
Emphasis on the experimental design
Each step is critical when designing an FMT study as they all significantly influence the final outcome and data generated. Figure 4 provides key questions to be answered at the early stage of the experimental design.
Figure 4.

Key questions when designing an FMT experiment
To begin with, consideration should be given on how to collect and process donor stool samples in order to minimize its impact on microbial survival in the gut. Secondly, deciding on which animal model to use as a recipient of FMT should depend on its limitations and whether it fits the goals of the study. The initial starting point of the microbiota was developed extensively in the first parts of the review. However, several possibilities for transmission exists: it can be autologous (microbial transfer from the same animal) or heterologous (microbial transfer from a donor distinct from the recipient). If heterologous, it can be done vertically – from a parent to an offspring – or horizontally.
To be sure that the changes one sees are specific to the transplanted-associated microbiota, each experimental group should differ by only one variable between them, which is not always the case in FMT studies. The common independent variable in FMT experiments is the intestinal microbiota: the goal is to compare two experimental groups that do not have the same intestinal microbiota in order to compare the dependent variable(s) of interest (e.g. behavioral tests, molecular measurements etc.). Therefore, it is important that all the experimental group are treated similarly (e.g.: antibiotic treatment, laxative depletion, diet, housing conditions etc.) but differs only by the FMT treatment (control or disease of interest).
The same is true for GF animals that one wishes to recolonize with a microbiota of interest. A control group comprising only conventional animals will be insufficient to determine whether the differences are due to the GF status of the animal or as a result of its newly established microbiota. (see section: Gnotobiotic Animals)
Combination of two or more animal models as recipients of FMT (GF, gut microbiota-depleted, transmission via F1 generation etc.) would allow the study to be more robust if we see the effect of interest in two different animal models. GF studies could also be supplemented with microbiota-depleted animal experiments to highlight a specific time-window where the effect is expected. To better understand a mechanism of interest, it is possible to recolonize GF animals with a specific consortium of microbes tosee whether they are responsible for the effect observedafter a full FMT.
In addition, FMT experiments can lead to a wide range of experimental designs: some studies are designed to have one recipient per donor, whereas other experimental designs pool fecal samples from several donors for FMT into one recipient animal. To account for experimental and individual variability, you can also have several recipients per donor or per pooled samples. With such variability in experimental designs, it is becoming more difficult to compare across studies and to determine which method is the most appropriate. Walter et al. highlighted an important bias of experimental designs in FMT studies, that has a huge impact on statistical analysis and thereby, the conclusions it led to.480 Indeed, in FMT studies, sample size calculation can be challenging, and the experimental unit may not necessarily necessarily equal the number of recipient animals. More commonly, the sample size of the study corresponds to the number of donors per animal or group of animals if the samples are not pooled together.480 Therefore, if one donor is used to gavage several mice, the sample size would be ‘N = 1' as the group of mice receiving FMT from the same donor will be technical rather than biological replicates. If the donor samples are pooled before inoculation in rodents, it would limit the potential understanding understanding of inter-individual variability of the donors. Indeed, pooled FMTs do not resemble individual donors, but rather a combination of multiple donors, which might not lead to an accurate animal model for representation.
Importantly, it emphasized that the transfer of different gut microbial populations via FMT does not necessarily produce the same output in recipient mice, since the gut microbial profile of the recipient plays an important role in FMT engraftment – suggesting that technical replicates for each donor are necessary.124 Where possible, researchers should monitor the successful engraftment of the transplanted donor microbiota to the recipient animal (preferably at species level), and not solely focus on whether phenotypes of interest were successfully transferred, as we do not know the extent to which the transplanted microbiome must be engrafted in order to achieve a significant phenotypic or molecular change. 16S or shotgun sequencing methods require DNA extraction from feces samples to analyze gut bacterial composition. If using a microbiota-depletion model (e.g. laxative or antibiotic treatment), depletion should be monitored at different stages either by cell culture or 16S sequencing.
Once again, FMT studies present a disparity in DNA extraction methods – with some methods performing better than others. By carrying out replicate DNA extractions (with different researchers, different reagents, different protocols), it was concluded that it contributes negligibly and still leads to consistent results.477 DNA extraction kits can be chosen accordingly to the purpose of study, but it is preferable to include a repeated bead-beating step and a heating step for sample lysis and controls (internal and external) must be included.481 Verification of DNA quality and quantity from fecal samples is highly recommended before sequencing as it will directly affect downstream results.
If the desired effects are transmitted, the next question would be to understand what elements of the FMT preparation is most likely to transmit the desired effects as the preparation will include: bacteria, viruses, fungi, protists, archaea, microbial components and metabolites. Indeed, there is still much to be discovered about the viable and active components within the transplant.473 The fecal inocula used for FMT are typically filtered or centrifuged prior to transplantation. Many active components are remaining within the fecal filtrate, including small molecules, metabolites, nucleic acids, and viruses that are capable of freely passing through the typical filter pore size (30–70 µm). It is possible to use size stratification filtration techniques combined with other methods to target specific molecules or viruses.482 The effects of more intensive filtering and transplantation of only fecal metabolites has not yet been tested. It is important to note that these biologically active small compounds and viruses are also components, at least initially, in FMTs. It remains unclear if they persist for a sufficient period of time or at the concentrations necessary to impact experimental readouts. The most predominant viral entity in the gastrointestinal tract and feces is the bacteriophage (or simply phage), which infect and multiply within bacteria.483 Early evidence indicates that fecal virome transplants are capable of changing the gut microbiota of the recipient and impacting host health.484 This calls into question whether the host is responding to the microbiota, directly, to the virome transplant or a combination of both. Unraveling this interaction is another avenue for future research and will likely offer more treatment options.
More rigorous and critical approach for inferring causality in the microbiome field is needed along with recommendations that might help researchers with planning a correct experimental design and appropriate statistical approach for future FMT study.480 Future efforts should also focus on implementing minimum reporting requirements for preclinical FMT methodology to improve reproducibility and consistency across the field.
Conclusion
FMT is a powerful tool to understand the involvement of gut microorganisms in health and diseases. Today, we can study the impact of the gut microbes without germ-free facilities, however we must rely on alternatives such as antibiotic or laxative treatments to deplete gut bacteria prior to FMT. These methods have been helpful to characterize the involvement of microorganisms in numerous diseases; however, it should be coupled with targeted mechanistic studies to fully understand the impact of these microbes and to what extent they are implicated in the phenotype of interest. To understand fully the impact of the intestinal microbiota in health and disease, we need to deepen our knowledge and find new methods to investigate the mechanisms of action of the relevant microorganisms.
In the meantime, experimental protocols require better design to counter the biases associated with this method and to establish rules of standardization, especially in the case of human-microbiota associated rodents. To ensure maximum bacterial survival, we recommend that sample collection and processing – when possible – should use anaerobic conditions at both stages of collection and processing,473 even though there is evidence of achieved phenotype transfers without. Furthermore, it is important to acknowledge the current gaps and limitations of metagenomic sequencing to be able to interpret published data with caution.485 Biases in microbiome studies can be introduced at every step: from sample collection to data analysis – all of which can impact interpretation and discovery.
Recently, a mathematical model was proposed to quantify bias, to partition bias into steps such as DNA extraction and PCR amplification, and to reason through the effects of bias on downstream statistical analyses.486 Future research is needed to improve this technique and to understand the best experimental approach one can use to answer a specific question and understand: how genetics and sex of the rodents influences the transplantation, gut microbiota depletion agents (side effects, concentrations to be used, frequency of administration, length of treatment) and their impacts on fecal transplantation, what components of the fecal or cecal microbiota are having the desired effect in an FMT and limitations of sequencing and culturing techniques.
In conclusion, it is imperative to maximize the standardization of FMT studies – from experimental design to statistical analysis – to be able to compare different studies and to discern whether changes observed are the cause, the consequence or indirectly related to the area of study in question. FMT is a good model to assess causality but we need a more complete understanding of specific mechanisms involved. This understanding will enable us to translate this research for the benefit of human conditions and diseases, by providing microbiota-based treatments with the help of defined consortia or with specific strains administered in probiotics or by diet.
Acknowledgments
The authors would like to thank Dr. Sarah Nicolas for critical reviewing of the manuscript and Ms Audrey Gheorghe for her advice on figures. Figures uses icons from thenounproject.com (creators: Adrien Coquet, Azam Ishaq, IQON, Mette Galaxy, Sewon Park, Vectorstall, Marie Van den Broeck, The Icon Z, Vectors Point, Sumit Saengthong, Alberto LM, Anagaja Design, Bernd Lakenbrik, SBTS, Prettycons, EliRatus) licensed under a Creative Commons Attribution 3.0 CCBY license.
APC Microbiome Ireland is a research center funded by Science Foundation Ireland (SFI), through the Irish Government’s National Development Plan (grant no. 12/RC/2273_P2). GC and JFC are supported by the Irish Health Research Board (Grant number ILP-POR-2017-013) and by Horizon 2020 (DISCOvERIE, Grant agreement ID: 848228). CEG is supported by European Foundation for the Advancement in Neurosciences, Geneva, Switzerland.
Funding Statement
This work was supported by the Health Research Board [ILP-POR-2017-013]; Horizon 2020 Framework Programme [DISCOvERIE 848228]; Horizon 2020 Framework Programme [DISCOvERIE 848228]; European Foundation for the Advancement in Neurosciences; Science Foundation Ireland [12/RC/2273_P2]; Science Foundation Ireland [12/RC/2273_P2].
Disclosure statement
Gerard Clarke has spoken at meetings sponsored by food (Probi) and pharmaceutical companies (Janssen Ireland) and received research funding from Pharmavite, and this support neither influenced nor constrained the contents of this manuscript and John F. Cryan received research support from Cremo, Pharmavite, Dupont and Nutricia and has spoken at meetings sponsored by food and pharmaceutical companies, and this support neither influenced nor constrained the contents of this manuscript. Cassandra E. Gheorghe, Nathaniel L. Ritz, Jason A. Martin and Hannah R. Wardill declare they have no competing interests.
Disclosure of interest
The authors report no conflict of interest.
References
- 1.Cryan JF, O'Riordan K, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–51. [DOI] [PubMed] [Google Scholar]
- 2.Luczynski P, McVey Neufeld K, Seira Oriach C, Clarke G, Dinan TG, Cryan JF. Assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol. 2016;19(8):1–17. doi: 10.1093/ijnp/pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weingarden AR, Vaughn BP.. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8(3):238–252. doi: 10.1080/19490976.2017.1290757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartstra AV, Bouter KEC, Bäckhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38(1):159–165. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- 5.Sun M-F, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, Cui C, Shen YQ. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun. 2018;70:48–60. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. U. S. A. 2017;114(40):10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J, Xu J, Ling Y, Wang F, Gong T, Yang C, Ye S, Ye K, Wei D, Song Z et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry. 2019 5;9(1):189. doi: 10.1038/s41398-019-0525-3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):1–16. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, Sun Q, Fan Y, Xie Y, Yang Z, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. 2019;25(11):2905–2918. doi: 10.1038/s41380-019-0475-4. [DOI] [PubMed] [Google Scholar]
- 10.Kelly JR, Borre Y, O' Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, et al.Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–118. [DOI] [PubMed] [Google Scholar]
- 11.Choo JM, Rogers GB. Establishment of murine gut microbiota in gnotobiotic mice. iScience. 2021;24(102049):102049. doi: 10.1016/j.isci.2021.102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange ME, Uwiera RRE, Inglis GD. Housing gnotobiotic mice in conventional animal facilities. Curr. Protoc. Mouse Biol. 2019;9(e59):e59. doi: 10.1002/cpmo.59. [DOI] [PubMed] [Google Scholar]
- 13.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004;558(1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallapaty S. Gnotobiotics: getting a grip on the microbiome boom. Lab Anim. (NY). 2017;46(10):373–377. doi: 10.1038/laban.1344. [DOI] [PubMed] [Google Scholar]
- 16.Arvidsson C, Hallén A, Bäckhed F. Generating and analyzing germ-free mice. Curr. Protoc. Mouse Biol. 2012;2(4):307–316. doi: 10.1002/9780470942390.mo120064. [DOI] [PubMed] [Google Scholar]
- 17.Burman O, Buccarello L, Redaelli V, Cervo L. The effect of two different Individually Ventilated Cage systems on anxiety-related behaviour and welfare in two strains of laboratory mouse. Physiol Behav. 2014;124:92–99. doi: 10.1016/j.physbeh.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Dobson GP, Letson HL, Biros E, Morris J. Specific pathogen-free (SPF) animal status as a variable in biomedical research: have we come full circle? EBioMedicine. 2019;41:42–43. doi: 10.1016/j.ebiom.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaedler RW, Dubs R, Costello R. Association of germ free mice with bacteria isolated from normal mice. J. Exp. Med. 1965;122(1):77–82. doi: 10.1084/jem.122.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewhirst FE, Chien CC, Paster BJ, Ericson RL, Orcutt RP, Schauer DB, Fox JG. Phylogeny of the defined murine microbiota: altered schaedler flora. Appl Environ Microbiol. 1999;65(8):3287–3292. doi: 10.1128/AEM.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wymore Brand M, Wannemuehler MJ, Phillips GJ, Proctor A, Overstreet AM, Jergens AE, Orcutt RP, Fox JG. The altered schaedler flora: continued applications of a defined murine microbial community. ILAR J. 2015;56(2):169–178. doi: 10.1093/ilar/ilv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stecher B, Chaffron S, Käppeli R, Hapfelmeier S, Freedrich S, Weber TC, Kirundi J, Suar M, McCoy KD, von Mering C, et al. Like will to like: abundances of closely related species can predict susceptibility to intestinal colonization by pathogenic and commensal bacteria. PLoS Pathog. 2010;6(e1000711):e1000711. doi: 10.1371/journal.ppat.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Roy T, Debédat J, Marquet F, Da-Cunha C, Ichou F, Guerre-Millo M, Kapel N, Aron-Wisnewsky J, Clément K. Comparative evaluation of microbiota engraftment following fecal microbiota transfer in mice models: age, kinetic and microbial status matter. Front. Microbiol. 2019;9. doi: 10.3389/fmicb.2018.03289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, Ribeiro EA, Russo SJ, Nestler EJ. Alterations of the host microbiome affect behavioral responses to cocaine. Sci Rep. 2016;6(35455). doi: 10.1038/srep35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA, Johansen FE. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One. 2011;6(3):e17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabral DJ, Penumutchu S, Reinhart EM, Zhang C, Korry BJ, Wurster JI, Nilson R, Guang A, Sano WH, Rowan-Nash AD, et al. Microbial metabolism modulates antibiotic susceptibility within the murine gut microbiome. Cell Metab. 2019;30(800–823.e7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science. 2015;347(6219):266–269. doi: 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adamcak A, Otten B. Rodent Therapeutics. Rodent Ther. Vet. Clin. North Am. Exot. Anim. Pract. 2000;3(1):221–237. doi: 10.1016/S1094-9194(17)30102-0. [DOI] [PubMed] [Google Scholar]
- 30.Hu Z, Tawa R, Konishi T, Shibata N, Takada K. A novel emulsifier, labrasol, enhances gastrointestinal absorption of gentamicin. Life Sci. 2001;69(24):2899–2910. doi: 10.1016/S0024-3205(01)01375-3. [DOI] [PubMed] [Google Scholar]
- 31.Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B, Zinser E, Bordag N, Magnes C, Fröhlich E, et al. Cognitive Impairment by Antibiotic-Induced Gut Dysbiosis: analysis of Gut Microbiota-Brain Communication. Brain Behav Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 2018;84(1):23–36. doi: 10.1002/ana.25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai ZL, Tseng CH, Ho HJ, Cheung CKY, Lin JY, Chen YJ, Cheng FC, Hsu YC, Lin JT, El-Omar EM, et al. Fecal microbiota transplantation confers beneficial metabolic effects of diet and exercise on diet-induced obese mice. Sci. Rep. 2018;8(1):15625. doi: 10.1038/s41598-018-33893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SG, Becattini S, Moody TU, Shliaha PV, Littmann ER, Seok R, Gjonbalaj M, Eaton V, Fontana E, Amoretti L, et al. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nature. 2019;572(7771):665–669. doi: 10.1038/s41586-019-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bastard Q, Ward T, Sidiropoulos D, Hillmann BM, Chun CL, Sadowsky MJ, Knights D, Montassier E. Fecal microbiota transplantation reverses antibiotic and chemotherapy-induced gut dysbiosis in mice. Scientific Reports. 2018;8(1). doi: 10.1038/s41598-018-24342-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016;22(5):516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staley C, Kaiser T, Beura LK, Hamilton MJ, Weingarden AR, Bobr A, Kang J, Masopust D, Sadowsky MJ, Khoruts A. Stable engraftment of human microbiota into mice with a single oral gavage following antibiotic conditioning. Microbiome. 2017;5(87). doi: 10.1186/s40168-017-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J, Heymes C, Luque JL, Anthony E, Barton RH, et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018;24(7):1070–1080. doi: 10.1038/s41591-018-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.. Assessing the viability of transplanted gut microbiota by sequential tagging with D-amino acid-based metabolic probes. Nat Commun. 2019;10(1317). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, et al. Priming of Natural Killer Cells by Nonmucosal Mononuclear Phagocytes Requires Instructive Signals from Commensal Microbiota. Immunity. 2012;37(1):171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 2017;8(1):1512. doi: 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burrello C, Garavaglia F, Cribiù FM, Ercoli G, Bosari S, Caprioli F, Facciotti F. Short-term oral antibiotics treatment promotes inflammatory activation of colonic invariant natural killer T and Conventional CD4(+) T Cells. Front Med. 2018;5:21. doi: 10.3389/fmed.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhan G, Yang N, Li S, Huang N, Fang X, Zhang J, Zhu B, Yang L, Yang C, Luo A. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging (Albany NY). 2018;10(6):1257–1267. doi: 10.18632/aging.101464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emal D, Rampanelli E, Stroo I, Butter LM, Teske GJ, Claessen N, Stokman G, Florquin S, Leemans JC, Dessing MC. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2017;28(5):1450–1461. doi: 10.1681/ASN.2016030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guirro M, Costa A, Gual-Grau A, Herrero P, Torrell H, Canela N, Arola L. Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: a multiomics approach. PLoS One. 2019;14(9):e0218143. doi: 10.1371/journal.pone.0218143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang C, Fang X, Zhan G, Huang N, Li S, Bi J, Jiang R, Yang L, Miao L, Zhu B, et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl Psychiatry. 2019;9(1):57. doi: 10.1038/s41398-019-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josefsdottir KS, Baldridge MT, Kadmon CS, King KY. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. 2017;129(6):729–739. doi: 10.1182/blood-2016-03-708594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CPet al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, Van Treuren W, Pruss K, Stabler SR, Lugo K, et al. A gut commensal-produced metabolite mediates colonization resistance to salmonella infection. Cell Host Microbe. 2018;24(2):296–307.e7. doi: 10.1016/j.chom.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Z, Zou J, Zhang Z, Zhao X, Noriega J, Zhang B, Zhao C, Ingle H, Bittinger K, Mattei LM, et al. Segmented Filamentous Bacteria Prevent and Cure Rotavirus Infection. Cell. 2019;179(3):644–658.e13. doi: 10.1016/j.cell.2019.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, Levy M, Salame TM, Weiner A, David E, et al. The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome. Cell. 2016;166(5):1231–1246.e13. doi: 10.1016/j.cell.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 53.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansson ME, Jakobsson HE, Holmén-Larsson J, Schütte A, Ermund A, Rodríguez-Piñeiro AM, Arike L, Wising C, Svensson F, Bäckhed F, et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. 2015;18(5):582–592. doi: 10.1016/j.chom.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim S-H, Cho B-H, Kiyono H, Jang Y-S. Microbiota-derived butyrate suppresses group 3 innate lymphoid cells in terminal ileal Peyer’s patches. Sci. Rep. 2017;7(1):3980. doi: 10.1038/s41598-017-02729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye Z, Zhang N, Wu C, Zhang X, Wang Q, Huang X, Du L, Cao Q, Tang J, Zhou C, et al. A metagenomic study of the gut microbiome in Behcet’s disease. Microbiome. 2018;6(1):135. doi: 10.1186/s40168-018-0520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park JH, Kotani T, Konno T, Setiawan J, Kitamura Y, Imada S, Usui Y, Hatano N, Shinohara M, Saito Y, et al. Promotion of intestinal epithelial cell turnover by commensal bacteria: role of short-chain fatty acids. PLoS One. 2016;11(5):e0156334. doi: 10.1371/journal.pone.0156334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci. 2016;113(47):E7554–E7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15(3):374–381. doi: 10.1016/j.chom.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Huang R, Cheng M, Wang L, Chao J, Li J, Zheng P, Xie P, Zhang Z, Yao H. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome. 2019;7(1):116. doi: 10.1186/s40168-019-0733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bárcena C, Valdés-Mas R, Mayoral P, Garabaya C, Durand S, Rodríguez F, Fernández-García MT, Salazar N, Nogacka AM, Garatachea N, et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019;25(8):1234–1242. doi: 10.1038/s41591-019-0504-5. [DOI] [PubMed] [Google Scholar]
- 63.Cao H, Liu X, An Y, Zhou G, Liu Y, Xu M, Dong W, Wang S, Yan F, Jiang K, et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci. Rep. 2017;7(1):10322. doi: 10.1038/s41598-017-10835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science (80-). 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 65.Hughes KR, Schofield Z, Dalby MJ, Caim S, Chalklen L, Bernuzzi F, Alcon-Giner C, Le Gall G, Watson AJM, Hall LJ. The early life microbiota protects neonatal mice from pathological small intestinal epithelial cell shedding. FASEB J. 2020;34(5):7075–7088. doi: 10.1096/fj.202000042R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Z, Li N, Fang H, Chen X, Guo Y, Gong S, Niu M, Zhou H, Jiang Y, Chang P, et al. Enteric dysbiosis is associated with sepsis in patients. FASEB J. 2019;33(11):12299–12310. doi: 10.1096/fj.201900398RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of Gut Commensal Microflora in the Development of Experimental Autoimmune Encephalomyelitis. J Immunol. 2009;183(10):6041–6050. doi: 10.4049/jimmunol.0900747. [DOI] [PubMed] [Google Scholar]
- 68.Brandsma E, Kloosterhuis NJ, Koster M, Dekker DC, Gijbels MJJ, van der Velden S, Ríos-Morales M, van Faassen MJR, Loreti MG, de Bruin A, et al. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ. Res. 2019;124(1):94–100. doi: 10.1161/CIRCRESAHA.118.313234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J, Bi JJ, Guo GJ, Yang L, Zhu B, Zhan GF, Li S, Huang NN, Hashimoto K, Yang C, et al. Abnormal composition of gut microbiota contributes to delirium-like behaviors after abdominal surgery in mice. CNS Neurosci. Ther. 2019;25(6):685–696. doi: 10.1111/cns.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du HX, Liu Y, Zhang LG, Zhan CS, Chen J, Zhang M, Chen XG, Zhang L, Liang CZ. Abnormal gut microbiota composition is associated with experimental autoimmune prostatitis-induced depressive-like behaviors in mice. Prostate. 2020;80(9):663–673. doi: 10.1002/pros.23978. [DOI] [PubMed] [Google Scholar]
- 71.Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 2017;153(6):1621–1633.e6. doi: 10.1053/j.gastro.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 72.Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, Li X. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress. 2019;22(5):592–602. doi: 10.1080/10253890.2019.1617267. [DOI] [PubMed] [Google Scholar]
- 73.Lendrum J, Seebach B, Klein B, Liu S. Sleep and the gut microbiome: antibiotic-induced depletion of the gut microbiota reduces nocturnal sleep in mice. bioRxiv. 2017:199075. doi: 10.1101/199075. [DOI] [Google Scholar]
- 74.Chen L, He Z, Iuga AC, Martins Filho SN, Faith JJ, Clemente JC, Deshpande M, Jayaprakash A, Colombel JF, Lafaille JJ, et al. Diet Modifies Colonic Microbiota and CD4+ T-Cell Repertoire to Induce Flares of Colitis in Mice With Myeloid-Cell Expression of Interleukin 23. Gastroenterology. 2018;155(4):1177–1191.e16. doi: 10.1053/j.gastro.2018.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang S, Huang M, You X, Zhao J, Chen L, Wang L, Luo Y, Chen Y. Gut microbiota mediates the anti-obesity effect of calorie restriction in mice. Sci. Rep. 2018;8(1):13037. doi: 10.1038/s41598-018-31353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell. 2018;173(7):1728–1741.e13. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Medel-Matus J-S, Shin D, Dorfman E, Sankar R, Mazarati A. Facilitation of kindling epileptogenesis by chronic stress may be mediated by intestinal microbiome. Epilepsia Open. 2018;3(2):290–294. doi: 10.1002/epi4.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, Esaulova E, Artyomov MN, Morales DJ, Holtzman MJ, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science (80-). 2017;357(6350):498–502. doi: 10.1126/science.aam5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hintze KJ, Cox JE, Rompato G, Benninghoff AD, Ward RE, Broadbent J, Lefevre M. Broad scope method for creating humanized animal models for animal health and disease research through antibiotic treatment and human fecal transfer. Gut Microbes. 2014;5(2):183–191. doi: 10.4161/gmic.28403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turner PV, Vaughn E, Sunohara-Neilson J, Ovari J, Leri F. Oral gavage in rats: animal welfare evaluation. J. Am. Assoc. Lab. Anim. Sci. 2012;51:25–30. [PMC free article] [PubMed] [Google Scholar]
- 81.Turner PV, Brabb T, Pekow C, Vasbinder MA. Administration of substances to laboratory animals: routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011;50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 82.Hoggatt AF, Hoggatt J, Honerlaw M, Pelus LM. A spoonful of sugar helps the medicine go down: a novel technique to improve oral gavage in mice. J. Am. Assoc. Lab. Anim. Sci. 2010;49:329–334. [PMC free article] [PubMed] [Google Scholar]
- 83.Morton DB, Jennings M, Buckwell A, Ewbank R, Godfrey C, Holgate B, Inglis I, James R, Page C, Sharman I, et al. Refining procedures for the administration of substances. Lab. Anim. 2001;35(1):1–41. doi: 10.1258/0023677011911345. [DOI] [PubMed] [Google Scholar]
- 84.Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, Knight R, Panda S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun. 2018;9(2872). doi: 10.1038/s41467-018-05336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, et al. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15(9):1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 86.Desbonnet L, Clarke G, Traplin A, O'Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 87.Modi SR, Lee HH, Spina CS, Collins JJ. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature. 2013;499(7457):219–222. doi: 10.1038/nature12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8(1):15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multi-drug resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med. 2012;18(5):799–806. doi: 10.1038/nm.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martinez MN, Papich MG, Drusano GL. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 2012;56(6):2795–2805. doi: 10.1128/AAC.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou W, Chow KH, Fleming E, Oh J. Selective colonization ability of human fecal microbes in different mouse gut environments. ISME J. 2019;13(3):805–823. doi: 10.1038/s41396-018-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pearson-Leary J, Zhao C, Bittinger K, Eacret D, Luz S, Vigderman AS, Dayanim G, Bhatnagar S. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry. 2019;1. doi: 10.1038/s41380-019-0380-x. [DOI] [PubMed] [Google Scholar]
- 93.Nicolas S, Blasco‐Baque V, Fournel A, Gilleron J, Klopp P, Waget A, Ceppo F, Marlin A, Padmanabhan R, Iacovoni JS, et al. Transfer of dysbiotic gut microbiota has beneficial effects on host liver metabolism. Molecular Systems Biology. 2017;13(3):921. doi: 10.15252/msb.20167356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Manichanh C, Reeder J, Gibert P, Varela E, Llopis M, Antolin M, Guigo R, Knight R, Guarner F. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 2013;1411–1419. doi: 10.1101/gr.107987.110.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heimesaat MM, Plickert R, Fischer A, Göbel UB, Bereswill S. Can microbiota transplantation abrogate murine colonization resistance against Campylobacter jejuni ? Eur. J. Microbiol. Immunol. (Bp). 2013;3(1):36–43. doi: 10.1556/EuJMI.3.2013.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wrzosek L, Ciocan D, Borentain P, Spatz M, Puchois V, Hugot C, Ferrere G, Mayeur C, Perlemuter G, Cassard AM. Transplantation of human microbiota into conventional mice durably reshapes the gut microbiota. Sci. Rep. 2018;8(1):6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ji SK, Yan H, Jiang T, Guo CY, Liu JJ, Dong SZ, Yang KL, Wang YJ, Cao ZJ, Li SL. Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting xenomicrobiota colonization. Front Microbiol. 2017;8:1–9. doi: 10.3389/fmicb.2017.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hirayama K, Miyaji K, Kawamura S, Itoh K, Takahashi E, Mitsuoka T. Development of intestinal flora of human-flora-associated mice in the intestine of their offspring. Exp. Anim. 1995;44(3):219–222. doi: 10.1538/expanim.44.219. [DOI] [PubMed] [Google Scholar]
- 99.Moeller AH, Suzuki TA, Phifer-Rixey M, Nachman MW. Transmission modes of the mammalian gut microbiota. Science. 2018;362(6413):453–457. doi: 10.1126/science.aat7164. [DOI] [PubMed] [Google Scholar]
- 100.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14–6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moreno-Indias I, Lundberg R, Krych L, Metzdorff SB, Kot W, Sørensen DB, Nielsen DS, Hansen CHF, Hansen AK. A humanized diet profile may facilitate colonization and immune stimulation in human microbiota-colonized mice. Front Microbiol. 2020;11(1336). doi: 10.3389/fmicb.2020.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moreno-Indias I, Lundberg R, Krych L, Metzdorff SB, Kot W, Sørensen DB, Nielsen DS, Hansen CHF, Hansen AK. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat Commun. 2020;11(2577). doi: 10.1038/s41467-020-16431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, Zink EM, Casey CP, Taylor BC, Lane CJ, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;30;177(6):1600-1618.e17. doi: 10.1016/j.cell.2019.05.004. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kimura I,Kimura I, Miyamoto J, Ohue-Kitano R, Watanabe K, Yamada T, Onuki M, Aoki R, Isobe Y, Kashihara D, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science (80-). 2020;367(6481):eaaw8429. doi: 10.1126/science.aaw8429. [DOI] [PubMed] [Google Scholar]
- 105.Stebegg M, Silva-Cayetano A, Innocentin S, Jenkins TP, Cantacessi C, Gilbert C, Linterman MA. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat. Commun. 2019;10(1):1–13. doi: 10.1038/s41467-019-10430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351(6275):aad3311–aad3311. doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weingarden A, González A, Vázquez-Baeza Y, Weiss S, Humphry G, Berg-Lyons D, Knights D, Unno T, Bobr A, Kang J, et al. Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome. 2015;3(10). doi: 10.1186/s40168-015-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smillie CS, Sauk J, Gevers D, Friedman J, Sung J, Youngster I, Hohmann EL, Staley C, Khoruts A, et al. Strain Tracking Reveals the Determinants of Bacterial Engraftment in the Human Gut Following Fecal Microbiota Transplantation. Cell Host Microbe. 2018;23(229–240.e5):229–240.e5. doi: 10.1016/j.chom.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Staley C, Kelly CR, Brandt LJ, Khoruts A, Sadowsky MJ. Complete microbiota engraftment is not essential for recovery from recurrent Clostridium difficile infection following fecal microbiota transplantation. MBio. 2016;7(6):e01965–16. doi: 10.1128/mBio.01965-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bajaj JS, Salzman N, Acharya C, Takei H, Kakiyama G, Fagan A, White MB, Gavis EA, Holtz ML, Hayward M, et al. Microbial functional change is linked with clinical outcomes after capsular fecal transplant in cirrhosis. JCI Insight. 2019;4(24). doi: 10.1172/jci.insight.133410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang W, Qu W, Wang H, Yan H. Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl Psychiatry. 2021;11(131). doi: 10.1038/s41398-021-01254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502–e7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kump P, Wurm P, Gröchenig HP, Wenzl H, Petritsch W, Halwachs B, Wagner M, Stadlbauer V, Eherer A, Hoffmann KM, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2018;47(1):67–77. doi: 10.1111/apt.14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vaughn BP, Vatanen T, Allegretti JR, Bai A, Xavier RJ, Korzenik J, Gevers D, Ting A, Robson SC, Moss AC. Increased Intestinal Microbial Diversity Following Fecal Microbiota Transplant for Active Crohn’s Disease. Inflamm. Bowel Dis. 2016;22(9):2182–2190. doi: 10.1097/MIB.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8(51). doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 117.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol. 2019;9(2). doi: 10.3389/fcimb.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vermeire S, Joossens M, Verbeke K, Wang J, Machiels K, Sabino J, Ferrante M, Van Assche G, Rutgeerts P, Raes J. Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J Crohn’s Colitis. 2016;10(4):387–394. doi: 10.1093/ecco-jcc/jjv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nguyen TLA, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015;8(1):1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Canibe N, O’Dea M, Abraham S. Potential relevance of pig gut content transplantation for production and research. J Anim Sci Biotechnol. 2019;10(55). doi: 10.1186/s40104-019-0363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal Gut Microbiota Transplants from Zebrafish and Mice to Germ-free Recipients Reveal Host Habitat Selection. Cell. 2006;127(2):423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Valenzuela MJ, Caruffo M, Herrera Y, Medina DA, Coronado M, Feijóo CG, Muñoz S, Garrido D, Troncoso M, Figueroa G, et al. Evaluating the Capacity of Human Gut Microorganisms to Colonize the Zebrafish Larvae (Danio rerio). Front Microbiol. 2018;9(1032). doi: 10.3389/fmicb.2018.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ericsson AC, Personett AR, Turner G, Dorfmeyer RA, Franklin CL. Variable Colonization after Reciprocal Fecal Microbiota Transfer between Mice with Low and High Richness Microbiota. Front Microbiol. 2017;8(196). doi: 10.3389/fmicb.2017.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wos-Oxley M, Bleich A, Oxley AP, Kahl S, Janus LM, Smoczek A, Nahrstedt H, Pils MC, Taudien S, Platzer M, et al. Comparative evaluation of establishing a human gut microbial community within rodent models. Gut Microbes. 2012;3(3):1–16. doi: 10.4161/gmic.19934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–63.e8. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li N, Zuo B, Huang S, Zeng B, Han D, Li T, Liu T, Wu Z, Wei H, Zhao J, Wang J. Spatial heterogeneity of bacterial colonization across different gut segments following inter-species microbiota transplantation. Microbiome. 2020;8(161). doi: 10.1186/s40168-020-00917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim YS, Unno T, Kim BY, Park MS. Sex differences in gut microbiota. World J. Men?s Heal. 2020;38(1):48–60. doi: 10.5534/wjmh.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, Lusis AJ. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7(4):313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 131.Fransen F, van Beek AA, Borghuis T, Meijer B, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HF, de Jonge MI, Faas MM, Boekschoten MV, et al. The Impact of Gut Microbiota on Gender-Specific Differences in Immunity. Front Immunol. 2017;8(754). doi: 10.3389/fimmu.2017.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schloss PD, Schubert AM, Zackular JP, Iverson KD, Young VB, Petrosino JF. Stabilization of the murine gut microbiome following weaning. Gut Microbes. 2012;3(4):383–393. doi: 10.4161/gmic.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol. Rev. 2016;40(1):117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.D'Amato A, Di Cesare Mannelli L, Lucarini E, Man AL, Le Gall G, Branca JJV, Ghelardini C, Amedei A, Bertelli E, Regoli M, et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. bioRxiv. 2020;866459. doi: 10.1101/866459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kundu P, Lee HU, Garcia-Perez I, Tay EXY, Kim H, Faylon LE, Martin KA, Purbojati R, Drautz-Moses DI, et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci. Transl. Med. 2019;11(eaau4760):eaau4760. doi: 10.1126/scitranslmed.aau4760. [DOI] [PubMed] [Google Scholar]
- 137.Xiao L, Feng Q, Liang S, Sonne SB, Xia Z, Qiu X, Li X, Long H, Zhang J, Zhang D, et al. A catalog of the mouse gut metagenome. Nat. Biotechnol. 2015;33(10):1103. doi: 10.1038/nbt.3353. [DOI] [PubMed] [Google Scholar]
- 138.Bo TB, Zhang XY, Kohl KD, Wen J, Tian SJ, Wang DH. Coprophagy prevention alters microbiome, metabolism, neurochemistry, and cognitive behavior in a small mammal. ISME J. 2020;14(10):2625–2645. doi: 10.1038/s41396-020-0711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rausch P, Basic M, Batra A, Bischoff SC, Blaut M, Clavel T, Gläsner J, Gopalakrishnan S, Grassl GA, Günther C, et al. Analysis of factors contributing to variation in the C57BL/6J fecal microbiota across German animal facilities. Int. J. Med. Microbiol. 2016;306(5):343–355. doi: 10.1016/j.ijmm.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 140.Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, et al. Gut Microbiomes of Malawian Twin Pairs Discordant for Kwashiorkor. Science (80-). 2013;339(6119):548–554. doi: 10.1126/science.1229000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schroeder BO, Birchenough GMH, Ståhlman M, Arike L, Johansson MEV, Hansson GC, Bäckhed F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe. 2018;23(1):27–40.e7. doi: 10.1016/j.chom.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yanze L, Wenming C, Gao NL, Xing-Ming Zhao W-HC. Consistent alterations of human fecal microbes after transplanted to germ-free mice. Genomics ProteomicsBioinformatics 2018;III:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Campbell JH, Foster CM, Vishnivetskaya T, Campbell AG, Yang ZK, Wymore A, Palumbo AV, Chesler EJ, Podar M. Host genetic and environmental effects on mouse intestinal microbiota. ISME J. 2012;6(11):2033–2044. doi: 10.1038/ismej.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee J, Venna VR, Durgan DJ, Shi H, Hudobenko J, Putluri N, Petrosino J, McCullough LD, Bryan RM. Young versus aged microbiota transplants to germ-free mice: increased short-chain fatty acids and improved cognitive performance. Gut Microbes. 2020;12(1):1–14. doi: 10.1080/19490976.2020.1814107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee J, d'Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, Hassan A, Graf J, Petrosino J, Putluri N, et al. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Poststroke Recovery in Aged Mice. Circ. Res. 2020;127(4):453–465. doi: 10.1161/CIRCRESAHA.119.316448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pan F, Zhang L, Li M, Hu Y, Zeng B, Yuan H, Zhao L, Zhang C. Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice. Microbiome. 2018;6(54). doi: 10.1186/s40168-018-0440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rogier R, Ederveen THA, Boekhorst J, Wopereis H, Scher JU, Manasson J, Frambach SJCM, Knol J, Garssen J, van der Kraan PM, et al. Aberrant intestinal microbiota due to IL-1 receptor antagonist deficiency promotes IL-17- and TLR4-dependent arthritis. Microbiome. 2017;5(1):63. doi: 10.1186/s40168-017-0278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Binyamin D, Werbner N, Nuriel-Ohayon M, Uzan A, Mor H, Abbas A, Ziv O, Teperino R, Gutman R, Koren O. The aging mouse microbiome has obesogenic characteristics. Genome Med. 2020;12(1):87. doi: 10.1186/s13073-020-00784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cui B, Su D, Li W, She X, Zhang M, Wang R, Zhai Q. Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: implications for Alzheimer’s disease. J Neuroinflammation. 2018;15(1):190. doi: 10.1186/s12974-018-1223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Albouery M, Buteau B, Grégoire S, Cherbuy C, Pais de Barros JP, Martine L, Chain F, Cabaret S, Berdeaux O, Bron AM, et al. Age-Related Changes in the Gut Microbiota Modify Brain Lipid Composition. Front Cell Infect Microbiol. 2019;9:444. doi: 10.3389/fcimb.2019.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47(6):187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li Y, Ning L, Yin Y, Wang R, Zhang Z, Hao L, Wang B, Zhao X, Yang X, Yin L, et al. Age-related shifts in gut microbiota contribute to cognitive decline in aged rats. Aging (Albany NY). 2020;12(9):7801–7817. doi: 10.18632/aging.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen Y, Zhang S, Zeng B, Zhao J, Yang M, Zhang M, Li Y, Ni Q, Wu D, Li Y. Transplant of microbiota from long-living people to mice reduces aging-related indices and transfers beneficial bacteria. Aging (Albany NY). 2020;12(6):4778–4793. doi: 10.18632/aging.102872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Planer JD, Peng Y, Kau AL, Blanton LV, Ndao IM, Tarr PI, Warner BB, Gordon JI. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature. 2016;534(7606):263–266. doi: 10.1038/nature17940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fielding RA, Reeves AR, Jasuja R, Liu C, Barrett BB, Lustgarten MS. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp Gerontol. 2019;127:110722. doi: 10.1016/j.exger.2019.110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xiao HW, Ge C, Feng GX, Li Y, Luo D, Dong JL, Li H, Wang H, Cui M, Fan SJ. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol Lett. 2018;287:23–30. doi: 10.1016/j.toxlet.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Samuelson DR, Shellito JE, Maffei VJ, Tague ED, Campagna SR, Blanchard EE, Luo M, Taylor CM, Ronis MJJ, Molina PE, et al. Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae. PLoS Pathog. 2017;13(6):e1006426. doi: 10.1371/journal.ppat.1006426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.JJiang Y , Liu Y , Gao M , Xue M , Wang Z , Liang H . Nicotinamide riboside alleviates alcohol-induced depression-like behaviours in C57BL/6J mice by altering the intestinal microbiota associated with microglial activation and BDNF expression. Food Funct. 2020;11(1):378–391. doi: 10.1039/C9FO01780A. [DOI] [PubMed] [Google Scholar]
- 160.Uebanso T, Kano S, Yoshimoto A, Naito C, Shimohata T, Mawatari K, Takahashi A. Effects of Consuming Xylitol on Gut Microbiota and Lipid Metabolism in Mice. Nutrients. 2017;14;9(7):756. doi: 10.3390/nu9070756.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yu L, Wang L, Yi H, Wu X. Beneficial effects of LRP6-CRISPR on prevention of alcohol-related liver injury surpassed fecal microbiota transplant in a rat model. Gut Microbes. 2020;11(4):1015–1029. doi: 10.1080/19490976.2020.1736457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhao W, Hu Y, Li C, Li N, Zhu S, Tan X, Li M, Zhang Y, Xu Z, Ding Z, et al. Transplantation of fecal microbiota from patients with alcoholism induces anxiety/depression behaviors and decreases brain mGluR1/PKC ε levels in mouse. Biofactors. 2020;46(1):38–54. doi: 10.1002/biof.1567. [DOI] [PubMed] [Google Scholar]
- 163.Wrzosek L, Ciocan D, Hugot C, Spatz M, Dupeux M, Houron C, Lievin-Le Moal V, Puchois V, Ferrere G, Trainel N, et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut. 2021;70(7):1299–1308. doi: 10.1136/gutjnl-2020-321565. [DOI] [PubMed] [Google Scholar]
- 164.Samuelson DR, Siggins RW, Ruan S, Amedee AM, Sun J, Zhu QK, Marasco WA, Taylor CM, Luo M, Welsh DA, Shellito JE. Alcohol consumption increases susceptibility to pneumococcal pneumonia in a humanized murine HIV model mediated by intestinal dysbiosis. Alcohol. 2019;80:33–43. doi: 10.1016/j.alcohol.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim MS, Kim Y, Choi H, Kim W, Park S, Lee D, Kim DK, Kim HJ, Choi H, Hyun DW, et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut. 2020;69(2):283–294. doi: 10.1136/gutjnl-2018-317431. [DOI] [PubMed] [Google Scholar]
- 166.Zhou H, Tai J, Xu H, Lu X, Meng D. Xanthoceraside Could Ameliorate Alzheimer’s Disease Symptoms of Rats by Affecting the Gut Microbiota Composition and Modulating the Endogenous Metabolite Levels. Front Pharmacol. 2019;10:1035. doi: 10.3389/fphar.2019.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fujii Y, Nguyen TTT, Fujimura Y, Kameya N, Nakamura S, Arakawa K, Morita H. Fecal metabolite of a gnotobiotic mouse transplanted with gut microbiota from a patient with Alzheimer’s disease. Biosci. Biotechnol. Biochem. 2019;83(11):2144–2152. doi: 10.1080/09168451.2019.1644149. [DOI] [PubMed] [Google Scholar]
- 168.Goo N, Bae HJ, Park K, Kim J, Jeong Y, Cai M, Cho K, Jung SY, Kim DH, Ryu JH. The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. 2020;262:118497. doi: 10.1016/j.lfs.2020.118497. [DOI] [PubMed] [Google Scholar]
- 169.Li Y, Luo ZY, Hu YY, Bi YW, Yang JM, Zou WJ, Song YL, Li S, Shen T, Li SJ, et al. The gut microbiota regulates autism-like behavior by mediating vitamin B(6) homeostasis in EphB6-deficient mice. Microbiome. 2020;8(1):120. doi: 10.1186/s40168-020-00884-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen K, Fu Y, Wang Y, Liao L, Xu H, Zhang A, Zhang J, Fan L, Ren J, Fang B. Therapeutic Effects of the In Vitro Cultured Human Gut Microbiota as Transplants on Altering Gut Microbiota and Improving Symptoms Associated with Autism Spectrum Disorder. Microb. Ecol. 2020;80(2):475–486. doi: 10.1007/s00248-020-01494-w. [DOI] [PubMed] [Google Scholar]
- 171.Lee B, Lee J, Woo MY, Lee MJ, Shin HJ, Kim K, Park S. Modulation of the Gut Microbiota Alters the Tumour-Suppressive Efficacy of Tim-3 Pathway Blockade in a Bacterial Species- and Host Factor-Dependent Manner. Microorganisms. 2020;8(9):1395. doi: 10.3390/microorganisms8091395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, Chan AWH, Wei H, Yang X, Sung JJY, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2020. doi: 10.1136/gutjnl-2019-319664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Almeida RR, Vieira RS, Castoldi A, Terra FF, Melo ACL, Canesso MCC, Lemos L, Cipelli M, Rana N, Hiyane MI, et al. Host dysbiosis negatively impacts IL-9-producing T-cell differentiation and antitumour immunity. Br J Cancer. 2020;123(4):534–541. doi: 10.1038/s41416-020-0915-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Paik J, Meeker S, Hsu CC, Seamons A, Pershutkina O, Snyder JM, Brabb T, Maggio-Price L. Validation studies for germ-free Smad3-/- mice as a bio-assay to test the causative role of fecal microbiomes in IBD. Gut Microbes. 2020;11(1):21–31. doi: 10.1080/19490976.2019.1611151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhu Z, Huang J, Li X, Xing J, Chen Q, Liu R, Hua F, Qiu Z, Song Y, Bai C, et al. Gut microbiota regulate tumor metastasis via circRNA/miRNA networks. Gut Microbes. 2020;12(1):1788891. doi: 10.1080/19490976.2020.1788891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nie X, Li L, Yi M, Qin W, Zhao W, Li F, Wu B, Yuan X. et al. The Intestinal Microbiota Plays as a Protective Regulator Against Radiation Pneumonitis. Radiat. Res. 2020;194(1):52–60. doi: 10.1667/RR15579.1. [DOI] [PubMed] [Google Scholar]
- 177.Alrafas HR, Busbee PB, Chitrala KN, Nagarkatti M, Nagarkatti P. Alterations in the Gut Microbiome and Suppression of Histone Deacetylases by Resveratrol Are Associated with Attenuation of Colonic Inflammation and Protection Against Colorectal Cancer. J. Clin. Med. 2020;9(6):1796. doi: 10.3390/jcm9061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Toubai T, Fujiwara H, Rossi C, Riwes M, Tamaki H, Zajac C, Liu C, Mathew AV, Byun J, Oravecz-Wilson K, et al. Host NLRP6 exacerbates graft-versus-host disease independent of gut microbial composition. Nat. Microbiol. 2019;4(5):800–812. doi: 10.1038/s41564-019-0373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sougiannis AT, VanderVeen BN, Enos RT, Velazquez KT, Bader JE, Carson M, Chatzistamou I, Walla M, Pena MM, Kubinak JL, et al. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain Behav Immun. 2019;80:44–55. doi: 10.1016/j.bbi.2019.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Uribe-Herranz M, Bittinger K, Rafail S, Guedan S, Pierini S, Tanes C, Ganetsky A, Morgan MA, Gill S, Tanyi JL, et al. Gut microbiota modulates adoptive cell therapy via CD8α dendritic cells and IL-12. JCI Insight. 2018;3. doi: 10.1172/jci.insight.94952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jala VR, Maturu P, Bodduluri SR, Krishnan E, Mathis S, Subbarao K, Wang M, Jenson AB, Proctor ML, Rouchka EC, et al. Leukotriene B4-receptor-1 mediated host response shapes gut microbiota and controls colon tumor progression. Oncoimmunology. 2017;6(e1361593):e1361593. doi: 10.1080/2162402X.2017.1361593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gong Y, Dong R, Gao X, Li J, Jiang L, Zheng J, Cui S, Ying M, Yang B, Cao J, He Q. Neohesperidin prevents colorectal tumorigenesis by altering the gut microbiota. Pharmacol. Res. 2019;148(104460):104460. doi: 10.1016/j.phrs.2019.104460. [DOI] [PubMed] [Google Scholar]
- 184.Cui M, Xiao H, Li Y, Zhou L, Zhao S, Luo D, Zheng Q, Dong J, Zhao Y, Zhang X, et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 2017;9(4):448–461. doi: 10.15252/emmm.201606932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vicente-Dueñas C, Janssen S, Oldenburg M, Auer F, González-Herrero I, Casado-García A, Isidro-Hernández M, Raboso-Gallego J, Westhoff P, Pandyra AA, et al. An intact gut microbiome protects genetically predisposed mice against leukemia. Blood. 2020;136(18):2003–2017. doi: 10.1182/blood.2019004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ubeda C, Bucci V, Caballero S, Djukovic A, Toussaint NC, Equinda M, Lipuma L, Ling L, Gobourne A, No D, et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 2013;81(3):965–973. doi: 10.1128/IAI.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang P, Liu J, Xiong B, Zhang C, Kang B, Gao Y, Li Z, Ge W, Cheng S, Hao Y, et al. Microbiota from alginate oligosaccharide-dosed mice successfully mitigated small intestinal mucositis. Microbiome. 2020;8(1):112. doi: 10.1186/s40168-020-00886-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Poutahidis T, Varian BJ, Levkovich T, Lakritz JR, Mirabal S, Kwok C, Ibrahim YM, Kearney SM, Chatzigiagkos A, Alm EJ, et al. Dietary microbes modulate transgenerational cancer risk. Cancer Res. 2015;75(7):1197–1204. doi: 10.1158/0008-5472.CAN-14-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shi L, Sheng J, Wang M, Luo H, Zhu J, Zhang B, Liu Z, Yang X. Combination Therapy of TGF-β Blockade and Commensal-derived Probiotics Provides Enhanced Antitumor Immune Response and Tumor Suppression. Theranostics. 2019;9(14):4115–4129. doi: 10.7150/thno.35131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chang CW, Lee HC, Li LH, Chiang Chiau JS, Wang TE, Chuang WH, Chen MJ, Wang HY, Shih SC, Liu CY, et al. Fecal Microbiota Transplantation Prevents Intestinal Injury, Upregulation of Toll-Like Receptors, and 5-Fluorouracil/Oxaliplatin-Induced Toxicity in Colorectal Cancer. Int J Mol Sci. 2020 Jan 8;21(2):386. doi: 10.3390/ijms21020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang Z, Hua W, Li C, Chang H, Liu R, Ni Y, Sun H, Li Y, Wang X, Hou M, et al. Protective Role of Fecal Microbiota Transplantation on Colitis and Colitis-Associated Colon Cancer in Mice Is Associated With Treg Cells. Front Microbiol. 2019;10:2498. doi: 10.3389/fmicb.2019.02498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang X, Zhao S, Song X, Jia J, Zhang Z, Zhou H, Fu H, Cui H, Hu S, Fang M, et al. Inhibition effect of glycyrrhiza polysaccharide (GCP) on tumor growth through regulation of the gut microbiota composition. . Journal of Pharmacological Sciences. 2018;137(4):324–332. doi: 10.1016/j.jphs.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 193.Chen H, Zhang F, Li R, Liu Y, Wang X, Zhang X, Xu C, Li Y, Guo Y, Yao Q. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed Pharmacother. 2020;124:109829. doi: 10.1016/j.biopha.2020.109829. [DOI] [PubMed] [Google Scholar]
- 194.Wu R, Mei X, Ye Y, Xue T, Wang J, Sun W, Lin C, Xue R, Zhang J, Xu D. Zn(II)-curcumin solid dispersion impairs hepatocellular carcinoma growth and enhances chemotherapy by modulating gut microbiota-mediated zinc homeostasis. Pharmacol Res. 2018;3:104454. doi: 10.1016/j.phrs.2019.104454. [DOI] [PubMed] [Google Scholar]
- 195.Sui H, Zhang L, Gu K, Chai N, Ji Q, Zhou L, Wang Y, Ren J, Yang L, Zhang B, et al. YYFZBJS ameliorates colorectal cancer progression in Apc(Min/+) mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell Commun. Signal. 2020;18(1):113. doi: 10.1186/s12964-020-00596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Baxter NT, Zackular JP, Chen GY, Schloss PD. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome. 2014;2(1):20. doi: 10.1186/2049-2618-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019;178(4):795–806.e12. doi: 10.1016/j.cell.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li L, Li X, Zhong W, Yang M, Xu M, Sun Y, Ma J, Liu T, Song X, Dong W, et al. Gut microbiota from colorectal cancer patients enhances the progression of intestinal adenoma in Apc(min/+) mice. EBioMedicine. 2019;48:301–315. doi: 10.1016/j.ebiom.2019.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 200.Jian X, Zhu Y, Ouyang J, Wang Y, Lei Q, Xia J, Guan Y, Zhang J, Guo J, He Y, et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome. 2020;8(1):74. doi: 10.1186/s40168-020-00854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sobhani I, Bergsten E, Couffin S, Amiot A, Nebbad B, Barau C, de'Angelis N, Rabot S, Canoui-Poitrine F, Mestivier D, et al. Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures. Proc. Natl. Acad. Sci. U. S. A. 2019;116(48):24285–24295. doi: 10.1073/pnas.1912129116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Su A, Yang W, Zhao L, Pei F, Yuan B, Zhong L, Ma G, Hu Q. Flammulina velutipes polysaccharides improve scopolamine-induced learning and memory impairment in mice by modulating gut microbiota composition. Food Funct. 2018;9:1424–1432. [DOI] [PubMed] [Google Scholar]
- 203.Li X, Li X, Shang Q, Gao Z, Hao F, Guo H, Guo C. Fecal microbiota transplantation (FMT) could reverse the severity of experimental necrotizing enterocolitis (NEC) via oxidative stress modulation. Free Radic Biol Med. 2017;108:32–43. doi: 10.1016/j.freeradbiomed.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 204.Brinkman BM, Becker A, Ayiseh RB, Hildebrand F, Raes J, Huys G, Vandenabeele P. Gut microbiota affects sensitivity to acute DSS-induced colitis independently of host genotype. Inflamm. Bowel Dis. 2013;19(12):2560–2567. doi: 10.1097/MIB.0b013e3182a8759a. [DOI] [PubMed] [Google Scholar]
- 205.Yin A, Luo Y, Chen W, He M, Deng JH, Zhao N, Cao L, Wang L. FAM96A Protects Mice From Dextran Sulfate Sodium (DSS)-Induced Colitis by Preventing Microbial Dysbiosis. Front Cell Infect Microbiol. 2019;9:381. doi: 10.3389/fcimb.2019.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Spalinger MR, Schwarzfischer M, Hering L, Shawki A, Sayoc A, Santos A, Gottier C, Lang S, Bäbler K, Geirnaert A, et al. Loss of PTPN22 abrogates the beneficial effect of cohousing-mediated fecal microbiota transfer in murine colitis. Mucosal Immunol. 2019;12(6):1336–1347. doi: 10.1038/s41385-019-0201-1. [DOI] [PubMed] [Google Scholar]
- 207.Lee C, Hong SN, Paik NY, Kim TJ, Kim ER, Chang DK, Kim YH. CD1d Modulates Colonic Inflammation in NOD2-/- Mice by Altering the Intestinal Microbial Composition Comprising Acetatifactor muris. J Crohns Colitis. 2019;13(8):1081–1091. doi: 10.1093/ecco-jcc/jjz025. [DOI] [PubMed] [Google Scholar]
- 208.Mishima Y, Oka A, Liu B, Herzog JW, Eun CS, Fan TJ, Bulik-Sullivan E, Carroll IM, Hansen JJ, Chen L, et al. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J. Clin. Invest. 2019;129(9):3702–3716. doi: 10.1172/JCI93820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Burrello C, Giuffrè MR, Macandog AD, Diaz-Basabe A, Cribiù FM, Lopez G, Borgo F, Nezi L, Caprioli F, Vecchi M, et al. Fecal Microbiota Transplantation Controls Murine Chronic Intestinal Inflammation by Modulating Immune Cell Functions and Gut Microbiota Composition. Cells. 2019;8(6):517. doi: 10.3390/cells8060517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.He Y, Yu H, Ge Y, Li X, Jiang M, Liu Y, Li X, Wang Y, Guo M, Qin X, et al. Bacterial β-glucuronidase alleviates dextran sulfate sodium-induced colitis in mice: a possible crucial new diagnostic and therapeutic target for inflammatory bowel disease. Biochem. Biophys. Res. Commun. 2019;513(2):426–433. doi: 10.1016/j.bbrc.2019.03.196. [DOI] [PubMed] [Google Scholar]
- 211.Goethel A, Turpin W, Rouquier S, Zanello G, Robertson SJ, Streutker CJ, Philpott DJ, Croitoru K. Nod2 influences microbial resilience and susceptibility to colitis following antibiotic exposure. Mucosal Immunol. 2019;12(3):720–732. doi: 10.1038/s41385-018-0128-y. [DOI] [PubMed] [Google Scholar]
- 212.Zhao M, Xiong X, Ren K, Xu B, Cheng M, Sahu C, Wu K, Nie Y, Huang Z, Blumberg RS, et al. Deficiency in intestinal epithelial O-GlcNAcylation predisposes to gut inflammation. EMBO Mol. Med. 2018;10(8). doi: 10.15252/emmm.201708736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Selvanantham T, Lin Q, Guo CX, Surendra A, Fieve S, Escalante NK, Guttman DS, Streutker CJ, Robertson SJ, Philpott DJ, et al. NKT Cell-Deficient Mice Harbor an Altered Microbiota That Fuels Intestinal Inflammation during Chemically Induced Colitis. J. Immunol. 2016;197(11):4464–4472. doi: 10.4049/jimmunol.1601410. [DOI] [PubMed] [Google Scholar]
- 214.Ward NL, Phillips CD, Nguyen DD, Shanmugam NK, Song Y, Hodin R, Shi HN, Cherayil BJ, Goldstein AM. Antibiotic Treatment Induces Long-lasting Changes in the Fecal Microbiota that Protect Against Colitis. Inflamm. Bowel Dis. 2016;22(10):2328–2340. doi: 10.1097/MIB.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Meisel M, Mayassi T, Fehlner-Peach H, Koval JC, O'Brien SL, Hinterleitner R, Lesko K, Kim S, Bouziat R, Chen L, et al. Interleukin-15 promotes intestinal dysbiosis with butyrate deficiency associated with increased susceptibility to colitis. ISME J. 2017;11(1):15–30. doi: 10.1038/ismej.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sun X, Winglee K, Gharaibeh RZ, Gauthier J, He Z, Tripathi P, Avram D, Bruner S, Fodor A, Jobin C. Microbiota-Derived Metabolic Factors Reduce Campylobacteriosis in Mice. Gastroenterology. 2018;154(6):1751–1763.e2. doi: 10.1053/j.gastro.2018.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schulfer AF, Battaglia T, Alvarez Y, Bijnens L, Ruiz VE, Ho M, Robinson S, Ward T, Cox LM, Rogers AB, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat. Microbiol. 2018;3(2):234–242. doi: 10.1038/s41564-017-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Darnaud M, Dos Santos A, Gonzalez P, Augui S, Lacoste C, Desterke C, De Hertogh G, Valentino E, Braun E, Zheng J, et al. Enteric Delivery of Regenerating Family Member 3 alpha Alters the Intestinal Microbiota and Controls Inflammation in Mice With Colitis. Gastroenterology. 2018;154(4):1009–1023.e14. doi: 10.1053/j.gastro.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 219.Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y, et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell. 2015;163(6):1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Runtsch MC, Hu R, Alexander M, Wallace J, Kagele D, Petersen C, Valentine JF, Welker NC, Bronner MP, Chen X, et al. MicroRNA-146a constrains multiple parameters of intestinal immunity and increases susceptibility to DSS colitis. Oncotarget. 2015;6(30):28556–28572. doi: 10.18632/oncotarget.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.He Y, Li X, Yu H, Ge Y, Liu Y, Qin X, Jiang M, Wang X. The Functional Role of Fecal Microbiota Transplantation on Dextran Sulfate Sodium-Induced Colitis in Mice. Front Cell Infect Microbiol. 2019;9:393. doi: 10.3389/fcimb.2019.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nagy-Szakal D, Mir SA, Harris RA, Dowd SE, Yamada T, Lacorazza HD, Tatevian N, Smith CW, de Zoeten EF, Klein J, et al. Loss of n-6 fatty acid induced pediatric obesity protects against acute murine colitis. FASEB J Off Publ Fed Am Soc Exp Biol. 2015;29:3151–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jacob N, Jacobs JP, Kumagai K, Ha CWY, Kanazawa Y, Lagishetty V, Altmayer K, Hamill AM, Von Arx A, Sartor RB, et al. Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal Immunol. 2018;11(5):1466–1476. doi: 10.1038/s41385-018-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ihekweazu FD, Fofanova TY, Queliza K, Nagy-Szakal D, Stewart CJ, Engevik MA, Hulten KG, Tatevian N, Graham DY, Versalovic J, et al. Bacteroides ovatus ATCC 8483 monotherapy is superior to traditional fecal transplant and multi-strain bacteriotherapy in a murine colitis model. Gut Microbes. 2019;10(4):504–520. doi: 10.1080/19490976.2018.1560753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shanmugam NKN, Trebicka E, Fu -L-L, Shi HN, Cherayil BJ. Intestinal inflammation modulates expression of the iron-regulating hormone hepcidin depending on erythropoietic activity and the commensal microbiota. J. Immunol. 2014;193(3):1398–1407. doi: 10.4049/jimmunol.1400278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ihara S, Hirata Y, Serizawa T, Suzuki N, Sakitani K, Kinoshita H, Hayakawa Y, Nakagawa H, Ijichi H, Tateishi K, et al. TGF-β Signaling in Dendritic Cells Governs Colonic Homeostasis by Controlling Epithelial Differentiation and the Luminal Microbiota. J. Immunol. 2016;196(11):4603–4613. doi: 10.4049/jimmunol.1502548. [DOI] [PubMed] [Google Scholar]
- 227.Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee JC, Lee HY, Kim TK, Kim MS, Park YM, Kim J, Park K, Kweon MN, Kim SH, Bae JW, et al. Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis. PLoS One. 2017;12(11):e0187515. doi: 10.1371/journal.pone.0187515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gogokhia L, Buhrke K, Bell R, Hoffman B, Brown DG, Hanke-Gogokhia C, Ajami NJ, Wong MC, Ghazaryan A, Valentine JF, et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe. 2019;25(2):285–299.e8. doi: 10.1016/j.chom.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gupta S, Basu S, Bal V, Rath S, George A. Gut IgA abundance in adult life is a major determinant of resistance to dextran sodium sulfate-colitis and can compensate for the effects of inadequate maternal IgA received by neonates. Immunology. 2019;158(1):19–34. doi: 10.1111/imm.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang L, An J, Song S, Mei M, Li W, Ding F, Liu S. Electroacupuncture preserves intestinal barrier integrity through modulating the gut microbiota in DSS-induced chronic colitis. Life Sci. 2020;261:118473. doi: 10.1016/j.lfs.2020.118473. [DOI] [PubMed] [Google Scholar]
- 232.Yan Y, Zhou X, Guo K, Zhou F, Yang H. Chlorogenic Acid Protects Against Indomethacin-Induced Inflammation and Mucosa Damage by Decreasing Bacteroides-Derived LPS. Front Immunol. 2020;11:1125. doi: 10.3389/fimmu.2020.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu Y, Luo L, Luo Y, Zhang J, Wang X, Sun K, Zeng L. Prebiotic Properties of Green and Dark Tea Contribute to Protective Effects in Chemical-Induced Colitis in Mice: a Fecal Microbiota Transplantation Study. J. Agric. Food Chem. 2020;68(23):6368–6380. doi: 10.1021/acs.jafc.0c02336. [DOI] [PubMed] [Google Scholar]
- 234.Pickert G, Wirtz S, Matzner J, Ashfaq-Khan M, Heck R, Rosigkeit S, Thies D, Surabattula R, Ehmann D, Wehkamp J, et al. Wheat Consumption Aggravates Colitis in Mice via Amylase Trypsin Inhibitor-mediated Dysbiosis. Gastroenterology. 2020;159(1):257–272.e17. doi: 10.1053/j.gastro.2020.03.064. [DOI] [PubMed] [Google Scholar]
- 235.Wu H, Rao Q, Ma GC, Yu XH, Zhang CE, Ma ZJ. Effect of Triptolide on Dextran Sodium Sulfate-Induced Ulcerative Colitis and Gut Microbiota in Mice. Front Pharmacol. 2019;10:1652. doi: 10.3389/fphar.2019.01652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chen Y, Zhang L, Hong G, Huang C, Qian W, Bai T, Song J, Song Y, Hou X. Probiotic mixtures with aerobic constituent promoted the recovery of multi-barriers in DSS-induced chronic colitis. Life Sci. 2020;240:117089. [DOI] [PubMed] [Google Scholar]
- 237.Zou J, Zhao X, Shi Z, Zhang Z, Vijay-Kumar M, Chassaing B, Gewirtz AT. Critical role of innate immunity to flagellin in absence of adaptive immunity. J Infect Dis. 2020. doi: 10.1093/infdis/jiaa521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hu S, Peng L, Kwak YT, Tekippe EM, Pasare C, Malter JS, Hooper LV, Zaki MH. The DNA Sensor AIM2 Maintains Intestinal Homeostasis via Regulation of Epithelial Antimicrobial Host Defense. Cell Rep. 2015;13(9):1922–1936. doi: 10.1016/j.celrep.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tian Z, et al. Beneficial Effects of Fecal Microbiota Transplantation on Ulcerative Colitis in Mice. Dig. Dis. Sci. 2016;61(8):2262–2271. doi: 10.1007/s10620-016-4060-2. [DOI] [PubMed] [Google Scholar]
- 240.Lee KW, Kim M, Lee CH. Treatment of Dextran Sulfate Sodium-Induced Colitis with Mucosa-Associated Lymphoid Tissue Lymphoma Translocation 1 Inhibitor MI-2 Is Associated with Restoration of Gut Immune Function and the Microbiota. Infect Immun. 2018;86(12). doi: 10.1128/IAI.00091-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kiyohara H, Sujino T, Teratani T, Miyamoto K, Arai MM, Nomura E, Harada Y, Aoki R, Koda Y, Mikami Y, et al. Toll-Like Receptor 7 Agonist-Induced Dermatitis Causes Severe Dextran Sulfate Sodium Colitis by Altering the Gut Microbiome and Immune Cells. Cell. Mol. Gastroenterol. Hepatol. 2019;7(1):135–156. doi: 10.1016/j.jcmgh.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Reinoso Webb C, den Bakker H, Koboziev I, Jones-Hall Y, Rao Kottapalli K, Ostanin D, Furr KL, Mu Q, Luo XM, Grisham MB. Differential Susceptibility to T Cell-Induced Colitis in Mice: role of the Intestinal Microbiota. Inflamm. Bowel Dis. 2018;24(2):361–379. doi: 10.1093/ibd/izx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chen L, Wilson JE, Koenigsknecht MJ, Chou WC, Montgomery SA, Truax AD, Brickey WJ, Packey CD, Maharshak N, Matsushima GK, et al. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat. Immunol. 2017;18(5):541–551. doi: 10.1038/ni.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016;22(6):598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Guo X-K, Ou J, Liang S, Zhou X, Hu X. Epithelial Hes1 maintains gut homeostasis by preventing microbial dysbiosis. Mucosal Immunol. 2018;11(3):716–726. doi: 10.1038/mi.2017.111. [DOI] [PubMed] [Google Scholar]
- 246.Wu J, Wei Z, Cheng P, Qian C, Xu F, Yang Y, Wang A, Chen W, Sun Z, Lu Y. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics. 2020;10(23):10665–10679. doi: 10.7150/thno.43528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Shin JH, Lee YK, Shon WJ, Kim B, Jeon CO, Cho JY, Morse HC 3rd, Choi EY, Shin DM. Gut microorganisms and their metabolites modulate the severity of acute colitis in a tryptophan metabolism-dependent manner. Eur. J. Nutr. 2020;59(8):3591–3601. doi: 10.1007/s00394-020-02194-4. [DOI] [PubMed] [Google Scholar]
- 248.Zachariassen LF, Hansen AK, Krych L, Nielsen DS, Holm TL, Tougaard P, Hansen CHF. Cesarean section increases sensitivity to oxazolone-induced colitis in C57BL/6 mice. Mucosal Immunol. 2019;12(6):1348–1357. doi: 10.1038/s41385-019-0207-8. [DOI] [PubMed] [Google Scholar]
- 249.Yoshimura T, McLean MH, Dzutsev AK, Yao X, Chen K, Huang J, Gong W, Zhou J, Xiang Y, H Badger J, et al. The Antimicrobial Peptide CRAMP is essential for colon homeostasis by maintaining microbiota Balance. J. Immunol. 2018;200(6):2174–2185. doi: 10.4049/jimmunol.1602073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bernardazzi C, Xu H, Tong H, Laubitz D, Figliuolo da Paz V, Curiel L, Ghishan FK. An indisputable role of NHE8 in mucosal protection. Am. J. Physiol. Gastrointest. Liver Physiol. 2020;319(4):G421–G431. doi: 10.1152/ajpgi.00246.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mir SA, Nagy-Szakal D, Dowd SE, Szigeti RG, Smith CW, Kellermayer R. Prenatal methyl-donor supplementation augments colitis in young adult mice. PLoS One. 2013;8(8):e73162. doi: 10.1371/journal.pone.0073162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Dziarski R, Park SY, Kashyap DR, Dowd SE, Gupta D. Pglyrp-regulated gut microflora prevotella falsenii, parabacteroides distasonis and bacteroides eggerthii enhance and alistipes finegoldii attenuates colitis in mice. PLoS One. 2016;11(1):e0146162. doi: 10.1371/journal.pone.0146162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhao D, Dai W, Tao H, Zhuang W, Qu M, Chang YN. Polysaccharide isolated from Auricularia auricular-judae (Bull.) prevents dextran sulfate sodium-induced colitis in mice through modulating the composition of the gut microbiota. J. Food Sci. 2020;85(9):2943–2951. doi: 10.1111/1750-3841.15319. [DOI] [PubMed] [Google Scholar]
- 255.Wu M, Li P, An Y, Ren J, Yan D, Cui J, Li D, Li M, Wang M, Zhong G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol Res. 2019;150:104489. doi: 10.1016/j.phrs.2019.104489. [DOI] [PubMed] [Google Scholar]
- 256.Cui H, Cai Y, Wang L, Jia B, Li J, Zhao S, Chu X, Lin J, Zhang X, Bian Y, et al. Berberine regulates Treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon. Front Pharmacol. 2018;9:571. doi: 10.3389/fphar.2018.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Su C, Su L, Li Y, Long SR, Chang J, Zhang W, Walker WA, Xavier RJ, Cherayil BJ, Shi HN. Helminth-induced alterations of the gut microbiota exacerbate bacterial colitis. Mucosal Immunol. 2018;11(1):144–157. doi: 10.1038/mi.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Alrafas HR, Busbee PB, Nagarkatti M, Nagarkatti PS. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 2019;106(2):467–480. doi: 10.1002/JLB.3A1218-476RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fan Q, Guan X, Hou Y, Liu Y, Wei W, Cai X, Zhang Y, Wang G, Zheng X, Hao H. Paeoniflorin modulates gut microbial production of indole-3-lactate and epithelial autophagy to alleviate colitis in mice. Phytomedicine. 2020;79:153345. doi: 10.1016/j.phymed.2020.153345. [DOI] [PubMed] [Google Scholar]
- 260.Ji J, Ge X, Chen Y, Zhu B, Wu Q, Zhang J, Shan J, Cheng H, Shi L. Daphnetin ameliorates experimental colitis by modulating microbiota composition and T(reg)/T(h)17 balance. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33:9308–9322. [DOI] [PubMed] [Google Scholar]
- 261.Zhou J, Zhou Z, Ji P, Ma M, Guo J, Jiang S. Effect of fecal microbiota transplantation on experimental colitis in mice. Exp Ther Med. 2019;17:2581–2586. doi: 10.3892/etm.2019.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Harrison CA, Laubitz D, Ohland CL, Midura-Kiela MT, Patil K, Besselsen DG, Jamwal DR, Jobin C, Ghishan FK, Kiela PR. Microbial dysbiosis associated with impaired intestinal Na(+)/H(+) exchange accelerates and exacerbates colitis in ex-germ free mice. Mucosal Immunol. 2018;11(5):1329–1341. doi: 10.1038/s41385-018-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Faivre B, Bellenger J, Rieu A, Guivier E, Galan M, Ollivier A, Poloni L, Sorci G. Disentangling the effect of host genetics and gut microbiota on resistance to an intestinal parasite. Int. J. Parasitol. 2019;49(11):873–883. doi: 10.1016/j.ijpara.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 264.Sun Z, Li J, Dai Y, Wang W, Shi R, Wang Z, Ding P, Lu Q, Jiang H, Pei W, et al. Indigo naturalis alleviates dextran sulfate sodium-induced colitis in rats via altering gut microbiota. Front Microbiol. 2020;11(731). doi: 10.3389/fmicb.2020.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang MX, Lin L, Chen YD, Zhong YP, Lin YX, Li P, Tian X, Han B, Xie ZY, Liao QF. Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol Res. 2020;159:104978. doi: 10.1016/j.phrs.2020.104978. [DOI] [PubMed] [Google Scholar]
- 266.Yan ZX, Gao XJ, Li T, Wei B, Wang PP, Yang Y, Yan R. Fecal Microbiota Transplantation in Experimental Ulcerative Colitis Reveals Associated Gut Microbial and Host Metabolic Reprogramming. Appl. Environ. Microbiol. 2018;84(14). doi: 10.1128/AEM.00434-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gao XJ, Li T, Wei B, Yan ZX, Hu N, Huang YJ, Han BL, Wai TS, Yang W, Yan R. Bacterial outer membrane vesicles from dextran sulfate sodium-induced colitis differentially regulate intestinal UDP-glucuronosyltransferase 1A1 partially through toll-like receptor 4/mitogen-activated protein kinase/phosphatidylinositol 3-kinase pathway. Drug Metab. Dispos. 2018;46(3):292–302. doi: 10.1124/dmd.117.079046. [DOI] [PubMed] [Google Scholar]
- 268.Natividad JM, Pinto-Sanchez MI, Galipeau HJ, Jury J, Jordana M, Reinisch W, Collins SM, Bercik P, Surette MG, Allen-Vercoe E, et al. Ecobiotherapy rich in firmicutes decreases susceptibility to colitis in a humanized gnotobiotic mouse model. Inflamm. Bowel Dis. 2015;21(8):1883–1893. doi: 10.1097/MIB.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 269.Seishima J, Iida N, Kitamura K, Yutani M, Wang Z, Seki A, Yamashita T, Sakai Y, Honda M, Yamashita T, et al. Gut-derived Enterococcus faecium from ulcerative colitis patients promotes colitis in a genetically susceptible mouse host. Genome Biol. 2019;20(1):252. doi: 10.1186/s13059-019-1879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.von Klitzing E, Ekmekciu I, Bereswill S, Heimesaat MM. Acute ileitis facilitates infection with multidrug resistant Pseudomonas aeruginosa in human microbiota-associated mice. Gut Pathog. 2017;9(1):4. doi: 10.1186/s13099-017-0154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lleal M, Sarrabayrouse G, Willamil J, Santiago A, Pozuelo M, Manichanh C. A single faecal microbiota transplantation modulates the microbiome and improves clinical manifestations in a rat model of colitis. EBioMedicine. 2019;48:630–641. doi: 10.1016/j.ebiom.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Frisbee AL, Saleh MM, Young MK, Leslie JL, Simpson ME, Abhyankar MM, Cowardin CA, Ma JZ, Pramoonjago P, Turner SD, et al. IL-33 drives group 2 innate lymphoid cell-mediated protection during Clostridium difficile infection. Nat. Commun. 2019;10(1):2712. doi: 10.1038/s41467-019-10733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lichtman JS, Ferreyra JA, Ng KM, Smits SA, Sonnenburg JL, Elias JE. Host-Microbiota Interactions in the Pathogenesis of Antibiotic-Associated Diseases. Cell Rep. 2016;14(5):1049–1061. doi: 10.1016/j.celrep.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mullish BH, McDonald JAK, Pechlivanis A, Allegretti JR, Kao D, Barker GF, Kapila D, Petrof EO, Joyce SA, Gahan CGM, et al. Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut. 2019;68(10):1791–1800. doi: 10.1136/gutjnl-2018-317842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.McDonald JAK, Mullish BH, Pechlivanis A, Liu Z, Brignardello J, Kao D, Holmes E, Li JV, Clarke TB, Thursz MR, et al. Inhibiting growth of clostridioides difficile by restoring valerate, produced by the intestinal microbiota. Gastroenterology. 2018;155(5):1495–1507.e15. doi: 10.1053/j.gastro.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Collins J, Auchtung JM, Schaefer L, Eaton KA, Britton RA. Humanized microbiota mice as a model of recurrent Clostridium difficile disease. Microbiome. 2015;3(1):35. doi: 10.1186/s40168-015-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jiang ZD, Alexander A, Ke S, Valilis EM, Hu S, Li B, DuPont HL. Stability and efficacy of frozen and lyophilized fecal microbiota transplant (FMT) product in a mouse model of Clostridium difficile infection (CDI). Anaerobe. 2017;48:110–114. doi: 10.1016/j.anaerobe.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 278.Liu H, Tian R, Wang H, Feng S, Li H, Xiao Y, Luan X, Zhang Z, Shi N, Niu H, et al. Gut microbiota from coronary artery disease patients contributes to vascular dysfunction in mice by regulating bile acid metabolism and immune activation. J. Transl. Med. 2020;18(1):382. doi: 10.1186/s12967-020-02539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Yu F, Han W, Zhan G, Li S, Xiang S, Zhu B, Jiang X, Yang L, Luo A, Hua F, et al. Abnormal gut microbiota composition contributes to cognitive dysfunction in streptozotocin-induced diabetic mice. Aging (Albany NY). 2019;11(10):3262–3279. doi: 10.18632/aging.101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Rasmussen TS, Mentzel CMJ, Kot W, Castro-Mejía JL, Zuffa S, Swann JR, Hansen LH, Vogensen FK, Hansen AK, Nielsen DS. Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut. 2020;69(12):2122–2130. gutjnl-2019-320005. doi: 10.1136/gutjnl-2019-320005. [DOI] [PubMed] [Google Scholar]
- 281.Wei Z, Shen P, Cheng P, Lu Y, Wang A, Sun Z. Gut Bacteria Selectively Altered by Sennoside A Alleviate Type 2 Diabetes and Obesity Traits. Oxid Med Cell Longev. 2020;2020:2375676. doi: 10.1155/2020/2375676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wang H, Lu Y, Yan Y, Tian S, Zheng D, Leng D, Wang C, Jiao J, Wang Z, Bai Y. Promising treatment for type 2 diabetes: fecal microbiota transplantation reverses insulin resistance and impaired islets. Front Cell Infect Microbiol. 2020;9:1–10. doi: 10.3389/fcimb.2019.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Pearson JA, Tai N, Ekanayake-Alper DK, Peng J, Hu Y, Hager K, Compton S, Wong FS, Smith PC, Wen L. Norovirus changes susceptibility to Type 1 diabetes by altering intestinal microbiota and immune cell functions. Front Immunol. 2019;10:2654. doi: 10.3389/fimmu.2019.02654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mullaney JA, Stephens JE, Geeling BE, Hamilton-Williams EE. Early-life exposure to gut microbiota from disease-protected mice does not impact disease outcome in type 1 diabetes susceptible NOD mice. Immunol. Cell Biol. 2019;97(1):97–103. doi: 10.1111/imcb.12201. [DOI] [PubMed] [Google Scholar]
- 285.Brown K, Godovannyi A, Ma C, Zhang Y, Ahmadi-Vand Z, Dai C, Gorzelak MA, Chan Y, Chan JM, Lochner A, et al. Prolonged antibiotic treatment induces a diabetogenic intestinal microbiome that accelerates diabetes in NOD mice. ISME J. 2016;10(2):321–332. doi: 10.1038/ismej.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Velmurugan G, Ramprasath T, Swaminathan K, Mithieux G, Rajendhran J, Dhivakar M, Parthasarathy A, Babu DD, Thumburaj LJ, Freddy AJ, et al. Gut microbial degradation of organophosphate insecticides-induces glucose intolerance via gluconeogenesis. Genome Biol. 2017;18(8). doi: 10.1186/s13059-016-1134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Bauer PV, Duca FA, Waise TMZ, Dranse HJ, Rasmussen BA, Puri A, Rasti M, O'Brien CA, Lam TKT. Lactobacillus gasseri in the Upper Small Intestine Impacts an ACSL3-Dependent Fatty Acid-Sensing Pathway Regulating Whole-Body Glucose Homeostasis. Cell Metab. 2018;27(3):572–587.e6. doi: 10.1016/j.cmet.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 288.Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, Turnbaugh JA, Verdin E, Hall KD, Leibel RL, et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell. 2020;181(6):1263–1275.e16. doi: 10.1016/j.cell.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Neuman V, Cinek O, Funda DP, Hudcovic T, Golias J, Kramna L, Petruzelkova L, Pruhova S, Sumnik Z. Human gut microbiota transferred to germ-free NOD mice modulate the progression towards type 1 diabetes regardless of the pace of beta cell function loss in the donor. Diabetologia. 2019;62(7):1291–1296. doi: 10.1007/s00125-019-4869-2. [DOI] [PubMed] [Google Scholar]
- 290.Zhang PP, Li LL, Han X, Li QW, Zhang XH, Liu JJ, Wang Y. Fecal microbiota transplantation improves metabolism and gut microbiome composition in db/db mice. Acta Pharmacol. Sin. 2020;41(5):678–685. doi: 10.1038/s41401-019-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yan T, Nian T, Liao Z, Xiao F, Wu B, Bi K, He B, Jia Y. Antidepressant effects of a polysaccharide from okra (Abelmoschus esculentus (L) Moench) by anti-inflammation and rebalancing the gut microbiota. Int J Biol Macromol. 2020;144:427–440. doi: 10.1016/j.ijbiomac.2019.12.138. [DOI] [PubMed] [Google Scholar]
- 292.Wang S, Ishima T, Zhang J, Qu Y, Chang L, Pu Y, Fujita Y, Tan Y, Wang X, Hashimoto K. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J Neuroinflammation. 2020;17(241). doi: 10.1186/s12974-020-01916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Siopi E, Chevalier G, Katsimpardi L, Saha S, Bigot M, Moigneu C, Eberl G, Lledo PM. Changes in gut microbiota by chronic stress impair the efficacy of fluoxetine. Cell Rep. 2020;30(11):3682–3690.e6. doi: 10.1016/j.celrep.2020.02.099. [DOI] [PubMed] [Google Scholar]
- 294.Chen P, Hei M, Kong L, Liu Y, Yang Y, Mu H, Zhang X, Zhao S, Duan J. One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food Funct. 2019;10(12):8161–8171. doi: 10.1039/C9FO01178A. [DOI] [PubMed] [Google Scholar]
- 295.Lv WJ, Wu XL, Chen WQ, Li YF, Zhang GF, Chao LM, Zhou JH, Guo A, Liu C, Guo SN. The gut microbiome modulates the changes in liver metabolism and in inflammatory processes in the brain of chronic unpredictable mild stress rats. Oxid Med Cell Longev. 2019;2019:7902874. doi: 10.1155/2019/7902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zhang F, Chen H, Zhang R, Liu Y, Kong N, Guo Y, Xu M.5-Fluorouracil induced dysregulation of the microbiome-gut-brain axis manifesting as depressive like behaviors in rats. Biochim. Biophys. Acta. Mol. Basis Dis. 2020;1866(10):165884. doi: 10.1016/j.bbadis.2020.165884. [DOI] [PubMed] [Google Scholar]
- 297.Pearson-Leary J, Zhao C, Bittinger K, Eacret D, Luz S, Vigderman AS, Dayanim G, Bhatnagar S. The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats. Mol Psychiatry. 2020;25(5):1068–1079. doi: 10.1038/s41380-019-0380-x. [DOI] [PubMed] [Google Scholar]
- 298.Tillmann S, Abildgaard A, Winther G, Wegener G. Altered fecal microbiota composition in the Flinders sensitive line rat model of depression. Psychopharmacology (Berl). 2019;236(5):1445–1457. doi: 10.1007/s00213-018-5094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Schmidt EKA, Torres-Espin A, Raposo PJF, Madsen KL, Kigerl KA, Popovich PG, Fenrich KK, Fouad K. Fecal transplant prevents gut dysbiosis and anxiety-like behaviour after spinal cord injury in rats. PLoS One. 2020;15(1):e0226128. doi: 10.1371/journal.pone.0226128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Marcondes Ávila PR, Fiorot M, Michels M, Dominguini D, Abatti M, Vieira A, de Moura AB, Behenck JP, Borba LA, Botelho MEM, et al. Effects of microbiota transplantation and the role of the vagus nerve in gut-brain axis in animals subjected to chronic mild stress. J Affect Disord. 2020;277:410–416. doi: 10.1016/j.jad.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 301.Wang H, Liu L, Rao X, Zeng B, Yu Y, Zhou C, Zeng L, Zheng P, Pu J, Xu S, et al. Integrated phosphoproteomic and metabolomic profiling reveals perturbed pathways in the hippocampus of gut microbiota dysbiosis mice. Transl Psychiatry. 2020;10(1):346. doi: 10.1038/s41398-020-01024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Qi X, Zhong X, Xu S, Zeng B, Chen J, Zang G, Zeng L, Bai S, Zhou C, Wei H, et al. Extracellular matrix and oxidative phosphorylation: important role in the regulation of hypothalamic function by gut microbiota. Front Genet. 2020;11:520. doi: 10.3389/fgene.2020.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Okamoto T, Morino K, Ugi S, Nakagawa F, Lemecha M, Ida S, Ohashi N, Sato D, Fujita Y, Maegawa H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019;316(5):E956–E966. doi: 10.1152/ajpendo.00510.2018. [DOI] [PubMed] [Google Scholar]
- 304.Liu YJ, Tang B, Wang FC, Tang L, Lei YY, Luo Y, Huang SJ, Yang M, Wu LY, Wang W, et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. 2020;10(12):5225–5241. doi: 10.7150/thno.43716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ma J, Li J, Qian M, He N, Cao Y, Liu Y, Wu K, He S. The comprehensive pathophysiological changes in a novel rat model of postinflammatory visceral hypersensitivity. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33:13560–13571. [DOI] [PubMed] [Google Scholar]
- 306.Botschuijver S, Roeselers G, Levin E, Jonkers DM, Welting O, Heinsbroek SEM, de Weerd HH, Boekhout T, Fornai M, Masclee AA, et al. Intestinal Fungal Dysbiosis Is Associated With Visceral Hypersensitivity in Patients With Irritable Bowel Syndrome and Rats. Gastroenterology. 2017;153(4):1026–1039. doi: 10.1053/j.gastro.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 307.Li J, Cui H, Cai Y, Lin J, Song X, Zhou Z, Xiong W, Zhou H, Bian Y, Wang L. Tong-Xie-Yao-Fang Regulates 5-HT Level in Diarrhea Predominant Irritable Bowel Syndrome Through Gut Microbiota Modulation. Front Pharmacol. 2018;9:1110. doi: 10.3389/fphar.2018.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Touw K, Ringus DL, Hubert N, Wang Y, Leone VA, Nadimpalli A, Theriault BR, Huang YE, Tune JD, Herring PB, et al. Mutual reinforcement of pathophysiological host-microbe interactions in intestinal stasis models. Physiol. Rep. 2017;5(6):e13182. doi: 10.14814/phy2.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Britton GJ, Contijoch EJ, Spindler MP, Aggarwala V, Dogan B, Bongers G, San Mateo L, Baltus A, Das A, Gevers D, et al. Defined microbiota transplant restores Th17/RORγt + regulatory T cell balance in mice colonized with inflammatory bowel disease microbiotas. Proc. Natl. Acad. Sci. U. S. A. 2020;117(35):21536–21545. doi: 10.1073/pnas.1922189117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Torres J, Hu J, Seki A, Eisele C, Nair N, Huang R, Tarassishin L, Jharap B, Cote-Daigneault J, Mao Q, et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut. 2020;69(1):42–51. doi: 10.1136/gutjnl-2018-317855. [DOI] [PubMed] [Google Scholar]
- 311.Chen YJ, Wu H, Wu SD, Lu N, Wang YT, Liu HN, Dong L, Liu TT, Shen XZ. Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroenterol. Hepatol. 2018;33(11):1844–1852. doi: 10.1111/jgh.14281. [DOI] [PubMed] [Google Scholar]
- 312.Lavoie S, Conway KL, Lassen KG, Jijon HB, Pan H, Chun E, Michaud M, Lang JK, Gallini Comeau CA, et al. The Crohn’s disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. Elife. 2019;8. doi: 10.7554/eLife.39982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Jia Q, Zhang L, Zhang J, Pei F, Zhu S, Sun Q, Duan L. Fecal Microbiota of Diarrhea-Predominant Irritable Bowel Syndrome Patients Causes Hepatic Inflammation of Germ-Free Rats and Berberine Reverses It Partially. Biomed Res Int. 2019;2019:4530203. doi: 10.1155/2019/4530203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Crouzet L, Gaultier E, Del'Homme C, Cartier C, Delmas E, Dapoigny M, Fioramonti J, Bernalier-Donadille A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2013;25(4):e272–82. doi: 10.1111/nmo.12103. [DOI] [PubMed] [Google Scholar]
- 315.Liu R, Kang JD, Sartor RB, Sikaroodi M, Fagan A, Gavis EA, Zhou H, Hylemon PB, Herzog JW, Li X, et al. Neuroinflammation in Murine Cirrhosis Is Dependent on the Gut Microbiome and Is Attenuated by Fecal Transplant. Hepatology. 2020;71(2):611–626. doi: 10.1002/hep.30827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wang Y, Zhao W, Shi J, Wang J, Hao J, Pang X, Huang X, Chen X, Li Y, Jin R, et al. Intestinal microbiota contributes to altered glucose metabolism in simulated microgravity mouse model. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33:10140–10151. [DOI] [PubMed] [Google Scholar]
- 317.Xie Y, Matsumoto H, Kennedy S, Newberry EP, Moritz W, DeBosch BJ, Moley KH, Rubin DC, Warner BW, Kau AL, et al. Impaired Chylomicron Assembly Modifies Hepatic Metabolism Through Bile Acid-Dependent and Transmissible Microbial Adaptations. Hepatology. 2019;70(4):1168–1184. doi: 10.1002/hep.30669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sheng L, Jena PK, Hu Y, Liu HX, Nagar N, Kalanetra KM, French SW, French SW, Mills DA, Wan YY. Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation. J. Pathol. 2017;243(4):431–441. doi: 10.1002/path.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 2017;7(1):1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Khan N, Vidyarthi A, Nadeem S, Negi S, Nair G, Agrewala JN. Alteration in the Gut Microbiota Provokes Susceptibility to Tuberculosis. Front Immunol. 2016;7:529. doi: 10.3389/fimmu.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Cao X, Han Y, Gu M, Du H, Song M, Zhu X, Ma G, Pan C, Wang W, Zhao E, Foodborne Titanium Dioxide Nanoparticles Induce Stronger Adverse Effects in Obese Mice than Non-Obese Mice: gut Microbiota Dysbiosis, Colonic Inflammation, and Proteome Alterations. Small. 2020;16(36):e2001858. doi: 10.1002/smll.202001858. [DOI] [PubMed] [Google Scholar]
- 322.Wang P, Wang J, Li D, Ke W, Chen F, Hu X. Targeting the gut microbiota with resveratrol: a demonstration of novel evidence for the management of hepatic steatosis. J Nutr Biochem. 2020;81:108363. doi: 10.1016/j.jnutbio.2020.108363. [DOI] [PubMed] [Google Scholar]
- 323.Bereswill S, Escher U, Grunau A, Kühl AA, Dunay IR, Tamas A, Reglodi D, Heimesaat MM. Pituitary Adenylate Cyclase-Activating Polypeptide-A Neuropeptide as Novel Treatment Option for Subacute Ileitis in Mice Harboring a Human Gut Microbiota. Front Immunol. 2019;10:554. doi: 10.3389/fimmu.2019.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ferrere G, Wrzosek L, Cailleux F, Turpin W, Puchois V, Spatz M, Ciocan D, Rainteau D, Humbert L, Hugot C, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J. Hepatol. 2017;66(4):806–815. doi: 10.1016/j.jhep.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 325.Murakami M, Tognini P, Liu Y, Eckel-Mahan KL, Baldi P, Sassone-Corsi P. Gut microbiota directs PPAR γ-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep. 2016;17(9):1292–1303. doi: 10.15252/embr.201642463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Shen TC, Chehoud C, Ni J, Hsu E, Chen YY, Bailey A, Laughlin A, Bittinger K, Bushman FD, Wu GD. Dietary Regulation of the Gut Microbiota Engineered by a Minimal Defined Bacterial Consortium. PLoS One. 2016;11(5):e0155620. doi: 10.1371/journal.pone.0155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Ahn IS, Lang JM, Olson CA, Diamante G, Zhang G, Ying Z, Byun HR, Cely I, Ding J, Cohn P, et al. Host Genetic Background and Gut Microbiota Contribute to Differential Metabolic Responses to Fructose Consumption in Mice. J. Nutr. 2020;150(10):2716–2728. doi: 10.1093/jn/nxaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Yang M, Fukui H, Eda H, Xu X, Kitayama Y, Hara K, Kodani M, Tomita T, Oshima T, Watari J, et al. Involvement of gut microbiota in association between GLP-1/GLP-1 receptor expression and gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312(4):G367–G373. doi: 10.1152/ajpgi.00232.2016. [DOI] [PubMed] [Google Scholar]
- 329.Celaj S, Gleeson MW, Deng J, O'Toole GA, Hampton TH, Toft MF, Morrison HG, Sogin ML, Putra J, Suriawinata AA, et al. The microbiota regulates susceptibility to Fas-mediated acute hepatic injury. Lab. Invest. 2014;94(9):938–949. doi: 10.1038/labinvest.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Qin C, Zhang H, Zhao L, Zeng M, Huang W, Fu G, Zhou W, Wang H, Yan H. Microbiota transplantation reveals beneficial impact of berberine on hepatotoxicity by improving gut homeostasis. Sci. China. Life Sci. 2018;61(12):1537–1544. doi: 10.1007/s11427-017-9202-0. [DOI] [PubMed] [Google Scholar]
- 331.Zhao Y, Tang Y, Chen L, Lv S, Liu S, Nie P, Aguilar ZP, Xu H. Restraining the TiO(2) nanoparticles-induced intestinal inflammation mediated by gut microbiota in juvenile rats via ingestion of Lactobacillus rhamnosus GG. Ecotoxicol Environ Saf. 2020;206:111393. doi: 10.1016/j.ecoenv.2020.111393. [DOI] [PubMed] [Google Scholar]
- 332.Escher U, Giladi E, Dunay IR, Bereswill S, Gozes I, Heimesaat MM. Anti-inflammatory Effects of the Octapeptide NAP in Human Microbiota-Associated Mice Suffering from Subacute Ileitis. Eur. J. Microbiol. Immunol. (Bp). 2018;8(2):34–40. doi: 10.1556/1886.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, Puchois V, Martin JC, Lepage P, Le Roy T, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65(5):830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 334.Basson AR, Gomez-Nguyen A, Menghini P, Buttó LF, Di Martino L, Aladyshkina N, Osme A, LaSalla A, Fischer D, Ezeji JC, et al. Human Gut Microbiome Transplantation in Ileitis Prone Mice: a Tool for the Functional Characterization of the Microbiota in Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2020;26(3):347–359. doi: 10.1093/ibd/izz242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Galvão MF, Bastos RW, Acurcio LB, Nascimento BB, Sandes SHC, Arantes RME, Souza MR, Martins FS, Vieira LQ, Nicoli JR. Evaluation of colonisation resistance in stool of human donors using ex vivo, in vitro and in vivo assays. Benef Microbes. 2017;8(2):217–230. doi: 10.3920/BM2016.0027. [DOI] [PubMed] [Google Scholar]
- 336.Wang WW, Zhang Y, Huang XB, You N, Zheng L, Li J. Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride-induced acute hepatic dysfunction. World J. Gastroenterol. 2017;23(38):6983–6994. doi: 10.3748/wjg.v23.i38.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Rungapamestry V, Rabot S, Fuller Z, Ratcliffe B, Duncan AJ. Influence of cooking duration of cabbage and presence of colonic microbiota on the excretion of N-acetylcysteine conjugates of allyl isothiocyanate and bioactivity of phase 2 enzymes in F344 rats. Br. J. Nutr. 2008;99(4):773–781. doi: 10.1017/S0007114507841134. [DOI] [PubMed] [Google Scholar]
- 338.Lin DM, Koskella B, Ritz NL, Lin D, Carroll-Portillo A, Lin HC. Transplanting Fecal Virus-Like Particles Reduces High-Fat Diet-Induced Small Intestinal Bacterial Overgrowth in Mice. Front Cell Infect Microbiol. 2019;9:348. doi: 10.3389/fcimb.2019.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.SSafari Z, Bruneau A, Monnoye M, Mariadassou M, Philippe C, Zatloukal K, Gérard P. Murine Genetic Background Overcomes Gut Microbiota Changes to Explain Metabolic Response to High-Fat Diet. Nutrients. 2020;12(287):287. doi: 10.3390/nu12020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kau AL, Planer JD, Liu J, Rao S, Yatsunenko T, Trehan I, Manary MJ, Liu TC, Stappenbeck TS, Maleta KM, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci. Transl. Med. 2015;7(276):276ra24. doi: 10.1126/scitranslmed.aaa4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Cowardin CA, Ahern PP, Kung VL, Hibberd MC, Cheng J, Guruge JL, Sundaresan V, Head RD, Barile D, Mills DA, et al. Mechanisms by which sialylated milk oligosaccharides impact bone biology in a gnotobiotic mouse model of infant undernutrition. Proc. Natl. Acad. Sci. U. S. A. 2019;116(24):11988–11996. doi: 10.1073/pnas.1821770116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Zeng SL, Li SZ, Xiao PT, Cai YY, Chu C, Chen BZ, Li P, Li J, Liu EH. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020;6(1):eaax6208. doi: 10.1126/sciadv.aax6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Su CW, Chen CY, Jiao L, Long SR, Mao T, Ji Q, O'Donnell S, Stanton C, Zheng S, Walker WA, et al. Helminth-Induced and Th2-Dependent Alterations of the Gut Microbiota Attenuate Obesity Caused by High-Fat Diet. Cell. Mol. Gastroenterol. Hepatol. 2020;10(4):763–778. doi: 10.1016/j.jcmgh.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Kaliannan K, Robertson RC, Murphy K, Stanton C, Kang C, Wang B, Hao L, Bhan AK, Kang JX. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome. 2018;6(1):205. doi: 10.1186/s40168-018-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Kapur R, Kim M, Rebetz J, Hallström B, Björkman JT, Takabe-French A, Kim N, Liu J, Shanmugabhavananthan S, Milosevic S, et al. Gastrointestinal microbiota contributes to the development of murine transfusion-related acute lung injury. Blood Adv. 2018;2(13):1651–1663. doi: 10.1182/bloodadvances.2018018903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Chen S, Li X, Liu L, Liu C, Han X. Ophiopogonin D alleviates high-fat diet-induced metabolic syndrome and changes the structure of gut microbiota in mice. FASEB J Off Publ Fed Am Soc Exp Biol. 2018;32:1139–1153. [DOI] [PubMed] [Google Scholar]
- 347.Xie Z, Jiang H, Liu W, Zhang X, Chen D, Sun S, Zhou C, Liu J, Bao S, Wang X, et al. The triterpenoid sapogenin (2α-OH-Protopanoxadiol) ameliorates metabolic syndrome via the intestinal FXR/GLP-1 axis through gut microbiota remodelling. Cell Death Dis. 2020;11(9):770. doi: 10.1038/s41419-020-02974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Kim TT, Parajuli N, Sung MM, Bairwa SC, Levasseur J, Soltys CM, Wishart DS, Madsen K, Schertzer JD, Dyck JRB. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am. J. Physiol. Endocrinol. Metab. 2018;315(4):E511–E519. doi: 10.1152/ajpendo.00471.2017. [DOI] [PubMed] [Google Scholar]
- 349.Han J, Wang X, Tang S, Lu C, Wan H, Zhou J, Li Y, Ming T, Wang ZJ, Su X. Protective effects of tuna meat oligopeptides (TMOP) supplementation on hyperuricemia and associated renal inflammation mediated by gut microbiota. FASEB J Off Publ Fed Am Soc Exp Biol. 2020;34:5061–5076. [DOI] [PubMed] [Google Scholar]
- 350.Zhan J, Ma X, Liu D, Liang Y, Li P, Cui J, Zhou Z, Wang P. Gut microbiome alterations induced by tributyltin exposure are associated with increased body weight, impaired glucose and insulin homeostasis and endocrine disruption in mice. Environ Pollut. 2020;266:115276. doi: 10.1016/j.envpol.2020.115276. [DOI] [PubMed] [Google Scholar]
- 351.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, Zmora N, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159(3):514–529. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 352.Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 353.Xu Y, Ai C, Jiang P, Sun X, Liu Y, Jiang G, Song S. Oligosaccharides from Gracilaria lemaneiformis better attenuated high fat diet-induced metabolic syndrome by promoting the Bacteroidales proliferation. Food Funct. 2020;11(1):1049–1062. doi: 10.1039/C9FO01996K. [DOI] [PubMed] [Google Scholar]
- 354.Chitrala KN, Guan H, Singh NP, Busbee B, Gandy A, Mehrpouya-Bahrami P, Ganewatta MS, Tang C, Chatterjee S, Nagarkatti P, et al. CD44 deletion leading to attenuation of experimental autoimmune encephalomyelitis results from alterations in gut microbiome in mice. Eur. J. Immunol. 2017;47(7):1188–1199. doi: 10.1002/eji.201646792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Takata K, Tomita T, Okuno T, Kinoshita M, Koda T, Honorat JA, Takei M, Hagihara K, Sugimoto T, Mochizuki H,et al. Dietary Yeasts Reduce Inflammation in Central Nerve System via Microflora. Ann. Clin. Transl. Neurol. 2015;2(1):56–66. doi: 10.1002/acn3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Chen H, Chen Z, Shen L, Wu X, Ma X, Lin D, Zhang M, Ma X, Liu Y, Wang Z, et al. Fecal microbiota transplantation from patients with autoimmune encephalitis modulates Th17 response and relevant behaviors in mice. Cell Death Discov. 2020;6(1):75. doi: 10.1038/s41420-020-00309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Liu S, Rezende RM, Moreira TG, Tankou SK, Cox LM, Wu M, Song A, Dhang FH, Wei Z, Costamagna G, et al. Oral Administration of miR-30d from Feces of MS Patients Suppresses MS-like Symptoms in Mice by Expanding Akkermansia muciniphila. Cell Host Microbe. 2019;26(6):779–794.e8. doi: 10.1016/j.chom.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Stanisavljević S, Dinić M, Jevtić B, Đedović N, Momčilović M, Đokić J, Golić N, Mostarica Stojković M, Miljković Đ. Gut Microbiota Confers Resistance of Albino Oxford Rats to the Induction of Experimental Autoimmune Encephalomyelitis. Front Immunol. 2018;9:942. doi: 10.3389/fimmu.2018.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Li K, Wei S, Hu L, Yin X, Mai Y, Jiang C, Peng X, Cao X, Huang Z, Zhou H, et al. Protection of Fecal Microbiota Transplantation in a Mouse Model of Multiple Sclerosis. Mediators Inflamm. 2020;2020:2058272. doi: 10.1155/2020/2058272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G, et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019;30(4):675–688.e7. doi: 10.1016/j.cmet.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 361.Petrov PD, García-Mediavilla MV, Guzmán C, Porras D, Nistal E, Martínez-Flórez S, Castell JV, González-Gallego J, Sánchez-Campos S, Jover R. A Network Involving Gut Microbiota, Circulating Bile Acids, and Hepatic Metabolism Genes That Protects Against Non-Alcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019;63(20):e1900487. doi: 10.1002/mnfr.201900487. [DOI] [PubMed] [Google Scholar]
- 362.Porras D, Nistal E, Martínez-Flórez S, Olcoz JL, Jover R, Jorquera F, González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. Functional Interactions between Gut Microbiota Transplantation, Quercetin, and High-Fat Diet Determine Non-Alcoholic Fatty Liver Disease Development in Germ-Free Mice. Mol. Nutr. Food Res. 2019;63(8):e1800930. doi: 10.1002/mnfr.201800930. [DOI] [PubMed] [Google Scholar]
- 363.Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L, et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019;71(6):1216–1228. doi: 10.1016/j.jhep.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Morissette A, Kropp C, Songpadith JP, Junges Moreira R, Costa J, Mariné-Casadó R, Pilon G, Varin TV, Dudonné S, Boutekrabt L, et al. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2020;318(6):E965–E980. doi: 10.1152/ajpendo.00560.2019. [DOI] [PubMed] [Google Scholar]
- 365.Chagwedera DN, Ang QY, Bisanz JE, Leong YA, Ganeshan K, Cai J, Patterson AD, Turnbaugh PJ, Chawla A. Nutrient Sensing in CD11c Cells Alters the Gut Microbiota to Regulate Food Intake and Body Mass. Cell Metab. 2019;30(2):364–373.e7. doi: 10.1016/j.cmet.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lu JF, Zhu MQ, Zhang H, Liu H, Xia B, Wang YL, Shi X, Peng L, Wu JW. Neohesperidin attenuates obesity by altering the composition of the gut microbiota in high-fat diet-fed mice. FASEB J Off Publ Fed Am Soc Exp Biol. 2020. doi: 10.1096/fj.201903102RR. [DOI] [PubMed] [Google Scholar]
- 367.Luo Y, Chen GL, Hannemann N, Ipseiz N, Krönke G, Bäuerle T, Munos L, Wirtz S, Schett G, Bozec A. Microbiota from Obese Mice Regulate Hematopoietic Stem Cell Differentiation by Altering the Bone Niche. Cell Metab. 2015;22(5):886–894. doi: 10.1016/j.cmet.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 368.Tashiro H, Cho Y, Kasahara DI, Brand JD, Bry L, Yeliseyev V, Abu-Ali G, Huttenhower C, Shore SA. Microbiota Contribute to Obesity-related Increases in the Pulmonary Response to Ozone. Am. J. Respir. Cell Mol. Biol. 2019;61(6):702–712. doi: 10.1165/rcmb.2019-0144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Ji Y, Sun S, Goodrich JK, Kim H, Poole AC, Duhamel GE, Ley RE, Qi L. Diet-induced alterations in gut microflora contribute to lethal pulmonary damage in TLR2/TLR4-deficient mice. Cell Rep. 2014;8(1):137–149. doi: 10.1016/j.celrep.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Liu MT, Huang YJ, Zhang TY, Tan LB, Lu XF, Qin J. Lingguizhugan decoction attenuates diet-induced obesity and hepatosteatosis via gut microbiota. World J. Gastroenterol. 2019;25(27):3590–3606. doi: 10.3748/wjg.v25.i27.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Andriessen EM, Wilson AM, Mawambo G, Dejda A, Miloudi K, Sennlaub F, Sapieha P. Gut microbiota influences pathological angiogenesis in obesity-driven choroidal neovascularization. EMBO Mol. Med. 2016;8(12):1366–1379. doi: 10.15252/emmm.201606531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Parséus A, Sommer N, Sommer F, Caesar R, Molinaro A, Ståhlman M, Greiner TU, Perkins R, Bäckhed F. Microbiota-induced obesity requires farnesoid X receptor. Gut. 2017;66(3):429–437. doi: 10.1136/gutjnl-2015-310283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, Ojcius DM, Lu CC, Young JD, Lai HC. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68(2):248–262. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- 374.Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013;5(178):178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Kang C, Wang B, Kaliannan K, Wang X, Lang H, Hui S, Huang L, Zhang Y, Zhou M, Chen M, et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Wang J, Wang P, Li D, Hu X, Chen F. Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur. J. Nutr. 2020;59(2):699–718. doi: 10.1007/s00394-019-01938-1. [DOI] [PubMed] [Google Scholar]
- 377.Arita S, Inagaki-Ohara K. High-fat-diet-induced modulations of leptin signaling and gastric microbiota drive precancerous lesions in the stomach. Nutrition. 2019;67–68:110556. doi: 10.1016/j.nut.2019.110556. [DOI] [PubMed] [Google Scholar]
- 378.Li X, Chen P, Zhang P, Chang Y, Cui M, Duan J. Protein-Bound β-glucan from Coriolus Versicolor has Potential for Use Against Obesity. Mol. Nutr. Food Res. 2019;63(7):e1801231. doi: 10.1002/mnfr.201801231. [DOI] [PubMed] [Google Scholar]
- 379.Ba Q, Li M, Chen P, Huang C, Duan X, Lu L, Li J, Chu R, Xie D, Song H, et al. Sex-Dependent Effects of Cadmium Exposure in Early Life on Gut Microbiota and Fat Accumulation in Mice. Environ. Health Perspect. 2017;125(3):437–446. doi: 10.1289/EHP360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Rabot S, Membrez M, Blancher F, Berger B, Moine D, Krause L, Bibiloni R, Bruneau A, Gérard P, Siddharth J, et al. High fat diet drives obesity regardless the composition of gut microbiota in mice. Sci. Rep. 2016;6(1):32484. doi: 10.1038/srep32484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Soto M, Herzog C, Pacheco JA, Fujisaka S, Bullock K, Clish CB, Kahn CR. Gut microbiota modulate neurobehavior through changes in brain insulin sensitivity and metabolism. Mol Psychiatry. 2018;23(12):2287–2301. doi: 10.1038/s41380-018-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Chaplin A, Parra P, Laraichi S, Serra F, Palou A. Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol. Nutr. Food Res. 2016;60(2):468–480. doi: 10.1002/mnfr.201500480. [DOI] [PubMed] [Google Scholar]
- 383.Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, Deng L, Bry L, Gordon JI, Kahn CR. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22(3):516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Liu Z, Wang N, Ma Y, Wen D. Hydroxytyrosol improves obesity and insulin resistance by modulating gut microbiota in high-fat diet-induced obese mice. Front Microbiol. 2019;10:390. doi: 10.3389/fmicb.2019.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Xu Y, Wang N, Tan HY, Li S, Zhang C, Zhang Z, Feng Y. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics. 2020;10(24):11302–11323. doi: 10.7150/thno.47746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Foley KP, Zlitni S, Duggan BM, Barra NG, Anhê FF, Cavallari JF, Henriksbo BD, Chen CY, Huang M, Lau TC, et al. Gut microbiota impairs insulin clearance in obese mice. Mol. Metab. 2020;42(101067):101067. doi: 10.1016/j.molmet.2020.101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Jing N, Liu X, Jin M, Yang X, Hu X, Li C, Zhao K. Fubrick tea attenuates high-fat diet induced fat deposition and metabolic disorder by regulating gut microbiota and caffeine metabolism. Food Funct. 2020;11(8):6971–6986. doi: 10.1039/D0FO01282C. [DOI] [PubMed] [Google Scholar]
- 388.Pérez-Matute P, Íñiguez M, de Toro M, Recio-Fernández E, Oteo JA. Autologous fecal transplantation from a lean state potentiates caloric restriction effects on body weight and adiposity in obese mice. Sci. Rep. 2020;10(1):9388. doi: 10.1038/s41598-020-64961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Zoll J, Read MN, Heywood SE, Estevez E, Marshall JPS, Kammoun HL, Allen TL, Holmes AJ, Febbraio MA, Henstridge DC. Fecal microbiota transplantation from high caloric-fed donors alters glucose metabolism in recipient mice, independently of adiposity or exercise status. Am. J. Physiol. Endocrinol. Metab. 2020;319(1):E203–E216. doi: 10.1152/ajpendo.00037.2020. [DOI] [PubMed] [Google Scholar]
- 390.Pereira FV, Melo ACL, Silva MB, de Melo FM, Terra FF, Castro IA, Perandini LA, Miyagi MT, Sato FT, Origassa CST, et al. Interleukin-6 and the gut microbiota influence melanoma progression in obese mice. Nutr Cancer. 2020;1–10. doi: 10.1080/01635581.2020.1764982. [DOI] [PubMed] [Google Scholar]
- 391.Wang P, Gao J, Ke W, Wang J, Li D, Liu R, Jia Y, Wang X, Chen X, Chen F, et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic Biol Med. 2020;156:83–98. doi: 10.1016/j.freeradbiomed.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 392.Anhê FF, Nachbar RT, Varin TV, Trottier J, Dudonné S, Le Barz M, Feutry P, Pilon G, Barbier O, Desjardins Y, et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut. 2019;68(3):453–464. doi: 10.1136/gutjnl-2017-315565. [DOI] [PubMed] [Google Scholar]
- 393.Lee H, Lee Y, Kim J, An J, Lee S, Kong H, Song Y, Lee CK, Kim K. Modulation of the gut microbiota by metformin improves metabolic profiles in aged obese mice. Gut Microbes. 2018;9(2):155–165. doi: 10.1080/19490976.2017.1405209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Kim J, Lee H, An J, Song Y, Lee CK, Kim K, Kong H. Alterations in Gut Microbiota by Statin Therapy and Possible Intermediate Effects on Hyperglycemia and Hyperlipidemia. Front Microbiol. 2019;10:1947. doi: 10.3389/fmicb.2019.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Sung MM, Kim TT, Denou E, Soltys CM, Hamza SM, Byrne NJ, Masson G, Park H, Wishart DS, Madsen KL, et al. Improved glucose homeostasis in obese mice treated with resveratrol is associated with alterations in the gut microbiome. Diabetes. 2017;66(2):418–425. doi: 10.2337/db16-0680. [DOI] [PubMed] [Google Scholar]
- 396.Lützhøft DO, Sánchez-Alcoholado L, Tougaard P, Junker Mentzel CM, Kot W, Nielsen DS, Hansen AK. Short communication: gut microbial colonization of the mouse colon using faecal transfer was equally effective when comparing rectal inoculation and oral inoculation based on 16S rRNA sequencing. Res Vet Sci. 2019;126:227–232. doi: 10.1016/j.rvsc.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 397.Rinott E, Youngster I, Yaskolka Meir A, Tsaban G, Zelicha H, Kaplan A, Knights D, Tuohy K, Fava F, Scholz MU, et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology. 2020;160(1):158–173.e10. doi: 10.1053/j.gastro.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Duan Y, Zhong Y, Xiao H, Zheng C, Song B, Wang W, Guo Q, Li Y, Han H, Gao J, et al. Gut microbiota mediates the protective effects of dietary β-hydroxy-β-methylbutyrate (HMB) against obesity induced by high-fat diets. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33:10019–10033. [DOI] [PubMed] [Google Scholar]
- 399.Sun -S-S, Wang K, Ma K, Bao L, Liu H-W. An insoluble polysaccharide from the sclerotium of Poria cocos improves hyperglycemia, hyperlipidemia and hepatic steatosis in ob/ob mice via modulation of gut microbiota. Chin. J. Nat. Med. 2019;17(1):3–14. doi: 10.1016/S1875-5364(19)30003-2. [DOI] [PubMed] [Google Scholar]
- 400.Yang JY, Lee YS, Kim Y, Lee SH, Ryu S, Fukuda S, Hase K, Yang CS, Lim HS, Kim MS, et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017;10(1):104–116. doi: 10.1038/mi.2016.42. [DOI] [PubMed] [Google Scholar]
- 401.Nettleton JE, Cho NA, Klancic T, Nicolucci AC, Shearer J, Borgland SL, Johnston LA, Ramay HR, Noye Tuplin E, Chleilat F, et al. Maternal low-dose aspartame and stevia consumption with an obesogenic diet alters metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring. Gut. 2020;69(10):1807–1817. doi: 10.1136/gutjnl-2018-317505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Klancic T, Laforest-Lapointe I, Choo A, Nettleton JE, Chleilat F, Noye Tuplin EW, Alukic E, Cho NA, Nicolucci AC, Arrieta MC, et al. Prebiotic oligofructose prevents antibiotic-induced obesity risk and improves metabolic and gut microbiota profiles in rat dams and offspring. Mol. Nutr. Food Res. 2020;64(16):e2000288. doi: 10.1002/mnfr.202000288. [DOI] [PubMed] [Google Scholar]
- 403.Sun W, Guo Y, Zhang S, Chen Z, Wu K, Liu Q, Liu K, Wen L, Wei Y, Wang B, et al. Fecal Microbiota Transplantation Can Alleviate Gastrointestinal Transit in Rats with High-Fat Diet-Induced Obesity via Regulation of Serotonin Biosynthesis. Biomed Res Int. 2018;2018:8308671. doi: 10.1155/2018/8308671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wang J-H, Kim B-S, Han K, Kim H. Ephedra-treated donor-derived gut microbiota transplantation ameliorates high fat diet-induced obesity in rats. Int J Environ Res Public Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Di Luccia B, Crescenzo R, Mazzoli A, Cigliano L, Venditti P, Walser JC, Widmer A, Baccigalupi L, Ricca E, Iossa S. Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS One. 2015;10(8):e0134893. doi: 10.1371/journal.pone.0134893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Zhu Y, Zhang JY, Wei YL, Hao JY, Lei YQ, Zhao WB, Xiao YH, Sun AD. The polyphenol-rich extract from chokeberry (Aronia melanocarpa L.) modulates gut microbiota and improves lipid metabolism in diet-induced obese rats. Nutr. Metab. (Lond). 2020;17(1):54. doi: 10.1186/s12986-020-00473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Zhang L, Zhou W, Zhan L, Hou S, Zhao C, Bi T, Lu X. Fecal microbiota transplantation alters the susceptibility of obese rats to type 2 diabetes mellitus. Aging (Albany NY). 2020;12(17):17480–17502. doi: 10.18632/aging.103756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Yang H, Xiang Y, Robinson K, Wang J, Zhang G, Zhao J, Xiao Y. Gut Microbiota Is a Major Contributor to Adiposity in Pigs. Front Microbiol. 2018;9:3045. doi: 10.3389/fmicb.2018.03045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Rodriguez DM, Benninghoff AD, Aardema NDJ, Phatak S, Hintze KJ. Basal diet determined long-term composition of the gut microbiome and mouse phenotype to a greater extent than fecal microbiome transfer from lean or obese human donors. Nutrients. 2019;11(7):1630. doi: 10.3390/nu11071630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Fouladi F, Brooks AE, Fodor AA, Carroll IM, Bulik-Sullivan EC, Tsilimigras MCB, Sioda M, Steffen KJ. The Role of the Gut Microbiota in Sustained Weight Loss Following Roux-en-Y Gastric Bypass Surgery. Obes. Surg. 2019;29(4):1259–1267. doi: 10.1007/s11695-018-03653-y. [DOI] [PubMed] [Google Scholar]
- 411.Dugas LR, Bernabé BP, Priyadarshini M, Fei N, Park SJ, Brown L, Plange-Rhule J, Nelson D, Toh EC, Gao X, et al. Decreased microbial co-occurrence network stability and SCFA receptor level correlates with obesity in African-origin women. Sci. Rep. 2018;8(1):17135. doi: 10.1038/s41598-018-35230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Arnoriaga-Rodríguez M, Mayneris-Perxachs J, Burokas A, Pérez-Brocal V, Moya A, Portero-Otin M, Ricart W, Maldonado R, Fernández-Real JM. Gut bacterial ClpB-like gene function is associated with decreased body weight and a characteristic microbiota profile. Microbiome. 2020;8(1):59. doi: 10.1186/s40168-020-00837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Rodriguez J, Hiel S, Neyrinck AM, Le Roy T, Pötgens SA, Leyrolle Q, Pachikian BD, Gianfrancesco MA, Cani PD, Paquot N, et al. Discovery of the gut microbial signature driving the efficacy of prebiotic intervention in obese patients. Gut. 2020;69(11):1975–1987. doi: 10.1136/gutjnl-2019-319726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Liu Y, Wang Y, Ni Y, Cheung CKY, Lam KSL, Wang Y, Xia Z, Ye D, Guo J, Tse MA, et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab. 2020;31(1):77–91.e5. doi: 10.1016/j.cmet.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 415.Arnoriaga-Rodríguez M, Mayneris-Perxachs J, Burokas A, Contreras-Rodríguez O, Blasco G, Coll C, Biarnés C, Miranda-Olivos R, Latorre J, Moreno-Navarrete JM, et al. Obesity impairs short-term and working memory through gut microbial metabolism of aromatic amino acids. Cell Metab. 2020;32(4):548–560.e7. doi: 10.1016/j.cmet.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 416.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Olsson LM, Poitou C, Tremaroli V, Coupaye M, Aron-Wisnewsky J, Bäckhed F, Clément K, Caesar R. Gut microbiota of obese subjects with Prader-Willi syndrome is linked to metabolic health. Gut. 2020;69(7):1229–1238. doi: 10.1136/gutjnl-2019-319322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.van den Berg FF, van Dalen D, Hyoju SK, van Santvoort HC, Besselink MG, Wiersinga WJ, Zaborina O, Boermeester MA, Alverdy J. Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate. Gut. 2020. doi: 10.1136/gutjnl-2019-320430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Li X, He C, Li N, Ding L, Chen H, Wan J, Yang X, Xia L, He W, Xiong H, et al. The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut Microbes. 2020;11(6):1774–1789. doi: 10.1080/19490976.2020.1770042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Zhu Y, He C, Li X, Cai Y, Hu J, Liao Y, Zhao J, Xia L, He W, Liu L, et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J. Gastroenterol. 2019;54(4):347–358. doi: 10.1007/s00535-018-1529-0. [DOI] [PubMed] [Google Scholar]
- 421.Wang J, Wang P, Tian H, Tian F, Zhang Y, Zhang L, Gao X, Wang X. Aryl hydrocarbon receptor/IL-22/Stat3 signaling pathway is involved in the modulation of intestinal mucosa antimicrobial molecules by commensal microbiota in mice. Innate Immunity. 2018;24(5):297–306. doi: 10.1177/1753425918785016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Couturier-Maillard A, Froux N, Piotet-Morin J, Michaudel C, Brault L, Le Bérichel J, Sénéchal A, Robinet P, Chenuet P, Jejou S, et al. Interleukin-22-deficiency and microbiota contribute to the exacerbation of Toxoplasma gondii-induced intestinal inflammation. Mucosal Immunol. 2018;11(4):1181–1190. doi: 10.1038/s41385-018-0005-8. [DOI] [PubMed] [Google Scholar]
- 423.Kamata K, Watanabe T, Minaga K, Hara A, Yoshikawa T, Okamoto A, Yamao K, Takenaka M, Park A-M, Kudo M, et al. Intestinal dysbiosis mediates experimental autoimmune pancreatitis via activation of plasmacytoid dendritic cells. International Immunology. 2019;31(12):795–809. doi: 10.1093/intimm/dxz050. [DOI] [PubMed] [Google Scholar]
- 424.Hansen CHF, Larsen CS, Petersson HO, Zachariassen LF, Vegge A, Lauridsen C, Kot W, Krych Ł, Nielsen DS, Hansen AK, et al. Targeting gut microbiota and barrier function with prebiotics to alleviate autoimmune manifestations in NOD mice. Diabetologia. 2019;62(9):1689–1700. doi: 10.1007/s00125-019-4910-5. [DOI] [PubMed] [Google Scholar]
- 425.Zhou ZL, Jia XB, Sun MF, Zhu YL, Qiao CM, Zhang BP, Zhao LP, Yang Q, Cui C, Chen X, et al. Neuroprotection of Fasting Mimicking Diet on MPTP-Induced Parkinson’s Disease Mice via Gut Microbiota and Metabolites. Neurother J Am Soc Exp Neurother. 2019;16:741–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Lindheim L, Manti M, Fornes R, Bashir M, Czarnewski P, Diaz OE, Seifert M, Engstrand L, Villablanca EJ, Obermayer-Pietsch B, et al. Reproductive and behavior dysfunction induced by maternal androgen exposure and obesity is likely not gut microbiome-mediated. . Journal of the Endocrine Society. 2018;2(12):1363–1380. doi: 10.1210/js.2018-00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y, Tang L. Association between polycystic ovary syndrome and gut microbiota. PLoS One. 2016;11(4):e0153196. doi: 10.1371/journal.pone.0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y, Liang X, Wang L, et al. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nature Medicine. 2019;25(8):1225–1233. doi: 10.1038/s41591-019-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, Sun Q, Fan Y, Xie Y, Yang Z, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. . Molecular Psychiatry. 2020;25(11):2905–2918. doi: 10.1038/s41380-019-0475-4. [DOI] [PubMed] [Google Scholar]
- 430.Zhu F, Ju Y, Wang W, Wang Q, Guo R, Ma Q, Sun Q, Fan Y, Xie Y, Yang Z, et al. Metagenome-wide association of gut microbiome features for schizophrenia. . Nature Communications. 2020;11(1):1612. doi: 10.1038/s41467-020-15457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, Liu Y, Cheng K, Zhou C, Wang H, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. . Science Advances. 2019;5(2):eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Kim SM, DeFazio JR, Hyoju SK, Sangani K, Keskey R, Krezalek MA, Khodarev NN, Sangwan N, Christley S, Harris KG, et al. Fecal microbiota transplant rescues mice from human pathogen mediated sepsis by restoring systemic immunity. Nature Communications. 2020;11(1):2354. doi: 10.1038/s41467-020-15545-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Fay KT, Klingensmith NJ, Chen CW, Zhang W, Sun Y, Morrow KN, Liang Z, Burd EM, Ford ML, Coopersmith CM. The gut microbiome alters immunophenotype and survival from sepsis. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33:11258–11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Gong S, Yan Z, Liu Z, Niu M, Fang H, Li N, Huang C, Li L, Chen G, Luo H, et al. Intestinal microbiota mediates the susceptibility to polymicrobial sepsis-induced liver injury by granisetron generation in mice. Hepatology. 2019;69(4):1751–1767. doi: 10.1002/hep.30361. [DOI] [PubMed] [Google Scholar]
- 435.Morffy Smith CD, Gong M, Andrew AK, Russ BN, Ge Y, Zadeh M, Cooper CA, Mohamadzadeh M, Moore JM. Composition of the gut microbiota transcends genetic determinants of malaria infection severity and influences pregnancy outcome. EBioMedicine. 2019;44:639–655. doi: 10.1016/j.ebiom.2019.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Bradley KC, Finsterbusch K, Schnepf D, Crotta S, Llorian M, Davidson S, Fuchs SY, Staeheli P, Wack A. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28(1):245–256.e4. doi: 10.1016/j.celrep.2019.05.105. [DOI] [PubMed] [Google Scholar]
- 437.Li S, Xu N, Hua R, Niu X, Lyu C, Li M, Li J. [Fecal microbiota transplantation regulates the cholinergic anti-inflammatory pathway in cerebral cortex of septic rats through intestinal microbiota]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31(9):1102–1107. doi: 10.3760/cma.j..2095-4352.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 438.Li S, Lv J, Li J, Zhao Z, Guo H, Zhang Y, Cheng S, Sun J, Pan H, Fan S, et al. Intestinal microbiota impact sepsis associated encephalopathy via the vagus nerve. Neurosci Lett. 2018;662:98–104. doi: 10.1016/j.neulet.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 439.Ávila PRM, Michels M, Vuolo F, Bilésimo R, Burger H, Milioli MVM, Sonai B, Borges H, Carneiro C, Abatti M, et al. Protective effects of fecal microbiota transplantation in sepsis are independent of the modulation of the intestinal flora. Nutrition. 2020;73:110727. doi: 10.1016/j.nut.2020.110727. [DOI] [PubMed] [Google Scholar]
- 440.Assimakopoulos SF, Papadopoulou I, Bantouna D, de Lastic AL, Rodi M, Mouzaki A, Gogos CA, Zolota V, Maroulis I. Fecal Microbiota Transplantation and Hydrocortisone Ameliorate Intestinal Barrier Dysfunction and Improve Survival in a Rat Model of Cecal Ligation and Puncture-Induced Sepsis. Shock. 2020. doi: 10.1097/SHK.0000000000001566. [DOI] [PubMed] [Google Scholar]
- 441.Zaborin A, Krezalek M, Hyoju S, Defazio JR, Setia N, Belogortseva N, Bindokas VP, Guo Q, Zaborina O, Alverdy JC. Critical role of microbiota within cecal crypts on the regenerative capacity of the intestinal epithelium following surgical stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312(2):G112–G122. doi: 10.1152/ajpgi.00294.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Jang H-M, Lee K-E, Lee H-J, Kim D-H. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci. Rep. 2018;8(1):13897. doi: 10.1038/s41598-018-31764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Yoshikawa K, Kurihara C, Furuhashi H, Takajo T, Maruta K, Yasutake Y, Sato H, Narimatsu K, Okada Y, Higashiyama M, et al. Psychological stress exacerbates NSAID-induced small bowel injury by inducing changes in intestinal microbiota and permeability via glucocorticoid receptor signaling. J. Gastroenterol. 2017;52(1):61–71. doi: 10.1007/s00535-016-1205-1. [DOI] [PubMed] [Google Scholar]
- 444.Rehaume LM, Mondot S, Aguirre de Cárcer D, Velasco J, Benham H, Hasnain SZ, Bowman J, Ruutu M, Hansbro PM, McGuckin MA, et al. ZAP-70 genotype disrupts the relationship between microbiota and host, leading to spondyloarthritis and ileitis in SKG mice. Arthritis Rheumatol (Hoboken, N J). 2014;66(10):2780–2792. doi: 10.1002/art.38773. [DOI] [PubMed] [Google Scholar]
- 445.Toral M, Robles-Vera I, de la Visitación N, Romero M, Yang T, Sánchez M, Gómez-Guzmán M, Jiménez R, Raizada MK, Duarte J. Critical Role of the Interaction Gut Microbiota - Sympathetic Nervous System in the Regulation of Blood Pressure. Front Physiol. 2019;10:231. doi: 10.3389/fphys.2019.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Nishimura N, Tanabe H, Komori E, Sasaki Y, Inoue R, Yamamoto T. Transplantation of high hydrogen-producing microbiota leads to generation of large amounts of colonic hydrogen in recipient rats fed high amylose maize starch. Nutrients. 2018;10(2):144. doi: 10.3390/nu10020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Yang CQ, Guo XS, Ji-Li, Wei ZB, Zhao L, Zhao GT, Sheng ST. Rifaximin improves visceral hyperalgesia via trpv1 by modulating intestinal flora in the water avoidance stressed rat. Gastroenterol. Res. Pract. 2020;2020(4078681):1–9. doi: 10.1155/2020/4078681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Shan B, Ai Z, Zeng S, Song Y, Song J, Zeng Q, Liao Z, Wang T, Huang C, Su D. Gut microbiome-derived lactate promotes to anxiety-like behaviors through GPR81 receptor-mediated lipid metabolism pathway. Psychoneuroendocrinology. 2020;117:104699. doi: 10.1016/j.psyneuen.2020.104699. [DOI] [PubMed] [Google Scholar]
- 449.Zhou W, Chow K-H, Fleming E, Oh J. Selective colonization ability of human fecal microbes in different mouse gut environments. ISME J. 2019;13:805–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Buchta Rosean C, Bostic RR, Ferey JCM, Feng TY, Azar FN, Tung KS, Dozmorov MG, Smirnova E, Bos PD, Rutkowski MR. Preexisting commensal dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor-positive breast cancer. Cancer Res. 2019;79(14):3662–3675. doi: 10.1158/0008-5472.CAN-18-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36:7428–7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Cheung MK, Yue GGL, Tsui KY, Gomes AJ, Kwan HS, Chiu PWY, Lau CBS. Discovery of an interplay between the gut microbiota and esophageal squamous cell carcinoma in mice. Am. J. Cancer Res. 2020;10(8):2409–2427. [PMC free article] [PubMed] [Google Scholar]
- 453.Zhang F, Zhai M, Wu Q, Jia X, Wang Y, Wang N. Protective effect of tong-qiao-huo-xue decoction on inflammatory injury caused by intestinal microbial disorders in stroke rats. Biol. Pharm. Bull. 2020;43(5):788–800. doi: 10.1248/bpb.b19-00847. [DOI] [PubMed] [Google Scholar]
- 454.Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, Tang L, Ye L, Li X, Cai Z, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. 2019;148:104403. doi: 10.1016/j.phrs.2019.104403. [DOI] [PubMed] [Google Scholar]
- 455.Zhou GF, Jiang YH, Ma DF, Wang YC, Yang JL, Chen JY, Chi CY, Han XW, Li ZY, Li X. Xiao-Qing-Long Tang Prevents Cardiomyocyte Hypertrophy, Fibrosis, and the Development of Heart Failure with Preserved Ejection Faction in Rats by Modulating the Composition of the Gut Microbiota. Biomed Res Int. 2019;2019:9637479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Xia GH, You C, Gao XX, Zeng XL, Zhu JJ, Xu KY, Tan CH, Xu RT, Wu QH, Zhou HW, et al. Stroke Dysbiosis Index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front Neurol. 2019;10(397). doi: 10.3389/fneur.2019.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, Putignani L, Fischer M, Keller JJ, Costello SP, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Yang J, Park S, Park S, Baek I, Chun J. Introducing Murine Microbiome Database (MMDB): a curated database with taxonomic profiling of the healthy mouse gastrointestinal microbiome. Microorganisms. 2019;7(11):480. doi: 10.3390/microorganisms7110480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Deloris Alexander A, Orcutt RP, Henry JC, Baker J Jr, Bissahoyo AC, Threadgill DW. Quantitative PCR assays for mouse enteric flora reveal strain-dependent differences in composition that are influenced by the microenvironment. Mamm Genome. 2006;17(11):1093–1104. doi: 10.1007/s00335-006-0063-1. [DOI] [PubMed] [Google Scholar]
- 460.Korach-Rechtman H, Freilich S, Gerassy-Vainberg S, Buhnik-Rosenblau K, Danin-Poleg Y, Bar H, Kashi Y. Murine genetic background has a stronger impact on the composition of the gut microbiota than maternal inoculation or exposure to unlike exogenous microbiota. Appl. Environ. Microbiol. 2019;85(18). doi: 10.1128/AEM.00826-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, Zhang M, Oh PL, Nehrenberg D, Hua K, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl. Acad. Sci. U. S. A. 2010;107(44):18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Buhnik-Rosenblau K, Danin-Poleg Y, Kashi Y. Predominant effect of host genetics on levels of Lactobacillus johnsonii bacteria in the mouse gut. Appl. Environ. Microbiol. 2011;77(18):6531–6538. doi: 10.1128/AEM.00324-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(599–609.e3):599–609.e3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 464.Lundberg R, Toft MF, Metzdorff SB, Hansen CHF, Licht TR, Bahl MI, Hansen AK. Human microbiota-transplanted C57BL/6 mice and offspring display reduced establishment of key bacteria and reduced immune stimulation compared to mouse microbiota-transplantation. Sci Rep. 2020;10(7805). doi: 10.1038/s41598-020-64703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Montgomery TL, Künstner A, Kennedy JJ, Fang Q, Asarian L, Culp-Hill R, D'Alessandro A, Teuscher C, Busch H, Krementsov DN. Interactions between host genetics and gut microbiota determine susceptibility to CNS autoimmunity. Proc Natl Acad Sci. 2020;117(44):27516–27527. doi: 10.1073/pnas.2002817117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Pu Y, Tan Y, Qu Y, Chang L, Wang S, Wei Y, Wang X, Hashimoto K. A role of the subdiaphragmatic vagus nerve in depression-like phenotypes in mice after fecal microbiota transplantation from Chrna7 knock-out mice with depression-like phenotypes. Brain. Behav. Immun. 2021;94:318–326. doi: 10.1016/j.bbi.2020.12.032. [DOI] [PubMed] [Google Scholar]
- 467.Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26(611–619.e6):611–619.e6. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 468.El Aidy S, van Baarlen P, Derrien M, Lindenbergh-Kortleve DJ, Hooiveld G, Levenez F, Doré J, Dekker J, Samsom JN, Nieuwenhuis EE, et al. Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 2012;5(5):567–579. doi: 10.1038/mi.2012.32. [DOI] [PubMed] [Google Scholar]
- 469.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U. S. A. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997;32(9):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 471.Dore J, Ehrlich SD, Levenez F, Pelletier E, Alberti A, Bertrand L, Bork P, Costea PI, Sunagawa S, Guarner F, et al. IHMS Sop 02 V1 : standard operating procedure for fecal samples self ‐ collection. Int Hum Microbiome Stand. 2015;2(12). [Google Scholar]
- 472.Gratton J, Phetcharaburanin J, Mullish BH, Williams HR, Thursz M, Nicholson JK, Holmes E, Marchesi JR, Li JV. Optimized sample handling strategy for metabolic profiling of human feces. Anal. Chem. 2016;88(9):4661–4668. doi: 10.1021/acs.analchem.5b04159. [DOI] [PubMed] [Google Scholar]
- 473.Papanicolas LE, Choo JM, Wang Y, Leong LEX, Costello SP, Gordon DL, Wesselingh SL, Rogers GB. Bacterial viability in faecal transplants: which bacteria survive? EBioMedicine. 2019;41:509–516. doi: 10.1016/j.ebiom.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.O'Donnell MM, Rea MC, O'Sullivan Ó, Flynn C, Jones B, McQuaid A, Shanahan F, Ross RP. Preparation of a standardised faecal slurry for ex-vivo microbiota studies which reduces inter-individual donor bias. J Microbiol Methods. 2016;129:109–116. doi: 10.1016/j.mimet.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 475.Béal C, Fonseca F. Freezing of Probiotic Bacteria. Adv Probiotic Technol. 2015;978-1–4987:179–212. [Google Scholar]
- 476.Burz SD, Abraham AL, Fonseca F, David O, Chapron A, Béguet-Crespel F, Cénard S, Le Roux K, Patrascu O, Levenez F, et al. A guide for ex vivo handling and storage of stool samples intended for fecal microbiota transplantation. Sci. Rep. 2019;9(1):1–16. doi: 10.1038/s41598-019-45173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Shaw AG, Sim K, Powell E, Cornwell E, Cramer T, McClure ZE, Li MS, Kroll JS. Latitude in sample handling and storage for infant faecal microbiota studies: the elephant in the room? Microbiome. 2016;4(1):1–14. doi: 10.1186/s40168-016-0186-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Kia E, Wagner Mackenzie B, Middleton D, Lau A, Waite DW, Lewis G, Chan YK, Silvestre M, Cooper GJ, Poppitt SD, et al. Integrity of the human faecal microbiota following long-term sample storage. PLoS One. 2016;11(e0163666):e0163666. doi: 10.1371/journal.pone.0163666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Johnson JS, Spakowicz DJ, Hong BY, Petersen LM, Demkowicz P, Chen L, Leopold SR, Hanson BM, Agresta HO, Gerstein M, et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019;10(1):1–11. doi: 10.1038/s41467-019-13036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Walter J, Armet AM, Finlay BB, Shanahan F. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell. 2020;180(2):221–232. doi: 10.1016/j.cell.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 481.Thomas V, Clark J, Doré J. Fecal microbiota analysis: an overview of sample collection methods and sequencing strategies. Future Microbiol. 2015;10(9):1485–1504. doi: 10.2217/fmb.15.87. [DOI] [PubMed] [Google Scholar]
- 482.Deng L, Silins R, Castro-Mejía JL, Kot W, Jessen L, Thorsen J, Shah S, Stokholm J, Bisgaard H, Moineau S, et al. A protocol for extraction of infective viromes suitable for metagenomics sequencing from low volume fecal samples. Viruses. 2019;11(667):667. doi: 10.3390/v11070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Sausset R, Petit MA, Gaboriau-Routhiau V, De Paepe M. New insights into intestinal phages. Mucosal Immunol. 2020;13(2):205–215. doi: 10.1038/s41385-019-0250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Hsu BB, Gibson TE, Yeliseyev V, Liu Q, Lyon L, Bry L, Silver PA, Gerber GK. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe. 2019;25(803–814.e5):803–814.e5. doi: 10.1016/j.chom.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Gloor GB, Macklaim JM, Pawlowsky-Glahn V, Egozcue JJ. Microbiome datasets are compositional: and this is not optional. Front Microbiol. 2017;8:1–6. doi: 10.3389/fmicb.2017.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.McLaren MR, Willis AD, Callahan BJ. Consistent and correctable bias in metagenomic sequencing experiments. Elife. 2019;8:1–31. doi: 10.7554/eLife.46923. [DOI] [PMC free article] [PubMed] [Google Scholar]


