Abstract
Aims:
To evaluate a deep oropharyngeal suction intervention (NO-ASPIRATE) in intubated patients on microaspiration, ventilator-associated events and clinical outcomes.
Design:
Prospective, two-group, single-blind, randomized clinical trial.
Methods:
The study was conducted between 2014 – 2017 in 513 participants enroled within 24 hr of intubation and randomized into NO-ASPIRATE or usual care groups. Standard oral care was provided to all participants every 4 hr and deep oropharyngeal suctioning was added to the NO-ASPIRATE group. Oral and tracheal specimens were obtained to quantify α-amylase as an aspiration biomarker.
Results:
Data were analysed for 410 study completers enrolled at least 36 hr: NO-ASPIRATE (N = 206) and usual care (N = 204). Percent of tracheal specimens positive for α-amylase, mean tracheal α-amylase levels over time and ventilator-associated events were not different between groups. The NO-ASPIRATE group had a shorter hospital length of stay and a subgroup with moderate aspiration at baseline had significantly lower α-amylase levels across time.
Conclusion:
Hospital length of stay was shorter in the NO-ASPIRATE group and a subgroup of intervention participants had lower α-amylase across time. Delivery of standardized oral care to all participants may have been an intervention itself and possibly associated with the lack of significant findings for most outcomes.
Impact:
This trial compared usual care to oral care with a deep suctioning intervention on microaspiration and ventilator-associated events, as this has not been systematically studied. Further research on the usefulness of α-amylase as an aspiration biomarker and the role of oral suctioning, especially for certain populations, is indicated.
Trial registration number:
Keywords: aspiration, aspiration biomarker, mechanical ventilation, nursing, pneumonia, ventilator associated events, α-amylase
1 |. INTRODUCTION
Although necessary treatments for many critically ill patients, endotracheal tube (ETT) intubation and mechanical ventilation (MV) are associated with complications such as ventilator-associated events (VAE) and ventilator-associated pneumonia (VAP). VAP is pneumonia that develops after MV for at least 48 hr (Hua et al., 2016). VAE are a broad range of complications associated with deterioration in oxygenation after 48 hr of MV (United States Department of Health & Human Services, Centers for Disease Control & Prevention, 2019b). Microaspiration, defined as leakage of secretions around the ETT cuff, is one risk factor for both VAP and VAE (Blot, Poelaert, & Kollef, 2014; Nseir, Zerimech, Jaillette, Artru, & Balduyck, 2011).
The ventilator bundle, a group of interventions to reduce complications in ventilated patients, has been adopted as a standard nursing practice and has improved outcomes worldwide (Rawat et al., 2017). Regular oral care is one component of the bundle. Oral hygiene includes cleansing, secretion removal and rinsing the mouth, but practices vary widely (Hua et al., 2016) and no large randomized trials have compared different methods of secretion removal. We hypothesized that a deep oropharyngeal suction intervention that reduced oral secretion volume, combined with usual oral care practices, would reduce microaspiration and VAE. The detailed protocol for the study has been published (Sole et al., 2019).
1.1 |. Background
1.1.1 |. Mechanical ventilation and complications
Approximately 13–20 million individuals worldwide require MV annually (Adhikari, Fowler, Bhagwanjee, & Rubenfeld, 2010). Complications associated with MV are common and include a longer duration of MV, longer stays in the hospital and intensive care unit (ICU) and higher mortality (Klompas et al., 2011).
Reported global VAP prevalence rate is 15.6% (Kollef et al., 2014). Determination of VAP is challenging and often based on subjective data. In 2013, the United States Centers for Disease Control and Prevention (CDC) refocused surveillance to assess for a broader range of complications associated with MV based on objective data, termed VAE (United States Department of Health & Human Services, Centers for Disease Control & Prevention, 2019b). VAE is determined by a deterioration in oxygenation after improvement or stability, laboratory indication of respiratory infection and clinical evidence suggesting infection or inflammation (United States Department of Health & Human Services, Centers for Disease Control & Prevention, 2019b). The VAE algorithm defines three sub-sets: ventilator-associated conditions (VAC), infection-related VAC and possible VAP (PVAP) (United States Department of Health & Human Services, Centers for Disease Control & Prevention, 2019b). Patients determined to have infection-related VAC must meet VAC criteria, have an abnormal fluctuation in temperature and/or white blood cell count and receive a new antimicrobial agent continued for more than four days (United States Department of Health & Human Services, Centers for Disease Control & Prevention, 2019a). VAE rates up to 28% have been reported (Hayashi et al., 2013; Klompas et al., 2012; Kobayashi, Uchino, Takinami, & Uezono, 2017; Kollef et al., 2014).
1.1.2 |. Study framework
This study is based on a physiological framework for development of aspiration (Sole et al., 2019). ETT intubation interferes with mucociliary clearance and cough and maintains the glottis in an open position (Craven & Steger, 1997). Inflation of the ETT cuff theoretically provides a seal to allow ventilation via the ETT and serves as a barrier to microaspiration. However, if the ETT cuff is not inflated sufficiently or cuff pressures vary, microaspiration of secretions into the lungs may occur. Oral secretions are colonized with potential pathogens and sometimes include gastric contents (Nseir, Zerimech, Fournier, et al., 2011). Microaspiration may trigger pulmonary aspiration syndromes that result in inflammation and infection (Marik, 2011). Our NO-ASPIRATE intervention was targeted to reduce secretion volume and microaspiration.
1.1.3 |. Alpha-amylase may be a biomarker for aspiration
Detection of microaspiration of oral secretions, indicated by the presence of α-amylase in tracheal and/or lung secretions, may serve as an important biomarker in ventilated patients at risk of developing VAE and VAP. Alpha-amylase is an enzyme that initiates the digestive process and is normally present in saliva and oral secretions but not in the lungs. Several researchers have identified α-amylase in tracheal secretions, indicating microaspiration of oral contents (Abu-Hasan, Elmallah, Neal, & Brookes, 2014; Dewavrin et al., 2014; Filloux et al., 2013; Qu et al., 2018; Samanta et al., 2018; Sole et al., 2012; Tripathi, Mirant-Borde, & Lee, 2011; Weiss, Moazed, DiBardino, Swaroop, & Wunderink, 2013).
Studies linking tracheal amylase to clinical outcomes are limited. Weiss et al. (2013) reported that secretions obtained via bronchoalveolar lavage of ventilated patients were positive for α-amylase within 72 hr after intubation. Levels of tracheal α-amylase were higher in patients diagnosed with VAP using American Thoracic Society guidelines (Samanta et al., 2018).
Additionally, the amount of α-amylase detected in tracheal secretions in relation to the value in oral secretions (tracheal/oral ratio) may indicate the potential load of α-amylase aspirated (Nandapalan, McIlwain, & Hamilton, 1995). Higher ratios indicate greater aspiration of oral secretions.
1.1.4 |. Effectiveness of oral suctioning and preliminary work
Oral suctioning is a potentially overlooked intervention to prevent microaspiration and has not been systematically studied. Oral suctioning practices and devices vary widely and frequency of oral suction ranges from every 4–12 hr (Sole & Bennett, 2011; Sole et al., 2003; Sole, Penoyer, Bennett, Bertrand, & Talbert, 2011). The volume of oral secretions can be high. In a 4-hr period, an average of 7.5 ml of oral secretions were retrieved and some patients had up to 25 ml (Sole, Su, et al., 2011).
VAP reduction and shorter MV duration were noted when oral suctioning was done immediately prior to turning patients (Chang, Tsai, & Lin, 2008; Chao, Chen, Wang, Lee, & Tsai, 2009). Similar findings were noted when continuous oral suctioning with a saliva ejector (similar to devices used in dental offices) was initiated (Chow, Kwok, Luk, Law, & Leung, 2012). Cutler and Sluman (2014) implemented a comprehensive oral care bundle that included oropharyngeal suction prior to turning and reported a 0.53 risk reduction in a historical control study. In quality improvement projects, oral suctioning was associated with a reduction in VAP when implemented every 4 hr with a disposable suction swab (Blamoun et al., 2009) or every 6 hr with an oropharyngeal suction catheter (Garcia et al., 2009).
Preliminary study findings showed that a catheter specifically designed to reach the oropharynx was the most effective device for secretion removal, especially in the posterior oropharynx (Sole, Penoyer, Bennett, & Bertrand, 2010). In a small sample, the number of patients with a tracheal specimen positive for α-amylase decreased when deep oropharyngeal suctioning was implemented in a 4-hr period (Sole et al., 2012).
Intubation with an ETT with a subglottic suction port (SS-ETT) is another strategy to reduce secretions that accumulate above the ETT cuff; however, outcomes of their use are mixed. Damas et al. (2015) found a reduction in VAP but not in VAC in a randomized trial evaluating subglottic suctioning. In a meta-analysis of 17 trials, Caroff, Li, Muscedere, and Klompas (2016) reported a 0.58 reduction in VAP (21%–13%) with the SS-ETT. No significant differences were noted for duration of MV, ICU length of stay (LOS), VAE and mortality. In a preliminary study, we found α-amylase in tracheal secretions in patients with both traditional and SS-ETT (Sole et al., 2014).
2 |. THE STUDY
2.1 |. Aims
The primary aim of this study was to evaluate the impact of the NO-ASPIRATE intervention versus usual care on microaspiration of oral contents in critically ill intubated patients (Aim 1). Secondary aims were to evaluate the impact of the NO-ASPIRATE intervention versus usual care on VAE rate, time to VAE occurrence and clinical outcomes (Aim 2), as well as explore changes in the tracheal/oral ratio of α-amylase between groups (Aim 3).
2.2 |. Hypotheses
Participants in the NO-ASPIRATE group will have significantly lower microaspiration (α-amylase levels), VAE rates and tracheal/oral α-amylase ratios compared with the control group. Time to development of VAE will also be longer and clinical outcomes will be improved in the NO-ASPIRATE group.
2.3 |. Design
The study design was a prospective, single-blind, randomized clinical trial. The NO-ASPIRATE study protocol has been published (Sole et al., 2019). The trial is registered at ClinicalTrials.gov and the identifier is NCT02284178.
2.4 |. Sample
Participants were patients in one of four critical care units (trauma, neuroscience, multi-system, or cardiac) at a tertiary care hospital in the southeast United States who were admitted between August 2014 - August 2017. Participants were recruited within 24 hr of intubation if they were 18 years of age or older and expected to be intubated for at least 36 hr. Potential participants were excluded if aspiration was documented during intubation or the patient was intubated to treat aspiration, non-traditional ventilation (e.g. oscillator) was required, reintubation occurred, patient unable to receive oral care (e.g. oral injury), history of lung or head/neck cancer, Sjögren’s syndrome, or prisoner status.
The target sample size was 600 participants randomized to either the NO-ASPIRATE or usual care group to yield 400 participants who were enrolled at least 36 hr (completers). Sample size estimates were based on preliminary data to detect a difference in mean α-amylase with an effect size of 0.25, power of 0.80 and alpha 0.05 and achieve a 15% reduction in the proportion of tracheal specimens that tested positive for α-amylase with a power of 0.87 at a significance level of 0.05. Attrition was lower than estimated and data collection stopped after 513 patients were enrolled, and 410 participants met the minimum 36-hr criterion.
Based on historical data, it was estimated that 65% of participants would be intubated with a SS-ETT. Therefore, stratified random sampling based on type of ETT was used. Participants were randomized using a computerized blocked randomization procedure, using different sized blocks, to ensure balanced assignment to groups stratified by ETT type (Craven, Chroneou, Zias, & Hjalmarson, 2009). The statistician generated the randomization order and assignment cards were placed in sealed numbered envelopes. Randomization occurred following informed consent. Group assignment was known only to study team members who delivered the intervention.
2.5 |. Study procedures
Trained, critical care registered nurses served as research coordinators and assistants (RAs) for the study. They were available approximately 18 hr per day/seven days a week to facilitate enrolment, data collection and delivery of the intervention. Following enrolment, the RA recorded baseline demographic and physiological data. Additional details were described in the published protocol (Sole et al., 2019).
2.6 |. Data collection
The RAs provided standard oral care to all participants using disposable components in a kit (Sage Products, Cary, IL). Standard care consisted of oral antisepsis and suctioning with a suction swab and toothbrushing every 12 hr with a suction toothbrush. Chlorhexidine gluconate was used every 12 hr during oral care if ordered.
In addition to the standard care, intervention participants (NO-ASPIRATE group) received deep oropharyngeal suctioning to the mouth and oropharynx every 4 hr using an oropharyngeal suction catheter (Sage, Cary, IL). A sham intervention was used for control participants; it was an imitation of the oropharyngeal suctioning for 45 s without occlusion of the suction port.
The 4-hr frequency of the intervention was based on preliminary work related to volume of oropharyngeal secretions. No difference in volume of oral secretions was noted between deep suctioning every 2 or 4 hr (Sole, Su, et al., 2011).
At enrolment and every 12 hr thereafter, the RAs collected oral and tracheal specimens into sterile traps (Medline, Mundelein, IL). Following hyperoxygenation, tracheal specimens were obtained via the closed ETT suction. Specimens were frozen at −20°C until assays were run. Data were collected until one of the following study end- points was met: extubation, tracheostomy, 14 days of enrolment or other exclusion criterion met.
Microaspiration was assessed using the value of α-amylase value from paired oral and tracheal specimens. Assays were performed following standard protocol by laboratory personnel who were blinded to study group (Sole et al., in press). A value of 392 U/L was considered positive for microaspiration (Dewavrin et al., 2014). Using the CDC criteria and the VAE Calculator (Version 3.0; 2015), VAE was determined as present or absent for up to two days be-yond the last intervention (United States Department of Health & Human Services, Centers for Disease Control & Prevention, 2019b). Ventilator data were recorded daily to observe for deteriorating oxygenation status and time to VAE in days was recorded. The tracheal to oral ratio was calculated and clinical outcomes were documented.
2.7 |. Ethical considerations
Institutional Review Board approval was obtained from the organization site and university in July of 2013 and was renewed annually. The study received funding from the National Institutes of Health in February of 2014. Either the legal proxy or patient (if able) provided consent to participate.
2.8 |. Data analysis
A significance level of 0.05 was determined a priori for all analyses. Baseline demographic data were compared between groups using independent sample t-test and chi-square test.
2.8.1 |. Aim 1
The α-amylase value for each tracheal and oral specimen was recorded in U/L. The value of each tracheal specimen was classified as either positive or negative and the percentage of positive specimens was calculated for each subject. Differences in percentages of positive specimens and values between groups over time were calculated using generalized estimating equation (GEE) methods.
2.8.2 |. Aim 2
Each subject was classified using the CDC algorithm as VAC positive or negative. If positive, assessment of infection-related VAC followed by PVAP was completed. The percentage of VAC-positive participants was compared between groups using chi-square test. The time to VAC was computed and compared between groups using the Kaplan–Meier with log-rank test. Clinical outcomes (length of stay and ventilator hours) were compared between groups using a t-test and discharge disposition was analysed with chi-square test.
2.8.3 |. Aim 3
The ratio of the tracheal value to the oral value of α-amylase for each paired sample was calculated. The values over time were compared between groups using GEE.
2.9 |. Validity and reliability/Rigour
CONSORT randomization guidelines and checklist were followed. An Operations Manual was developed to guide standardize study procedures. RAs were trained by the principal investigator or research coordinator and interrater reliability was established using a minimum kappa level of 0.90; retraining occurred quarterly. Fidelity of treatment and usual care was ensured by adhering to the existing protocols for daily interruption of sedation, head of bed elevation and ETT cuff pressure management. One co-investigator (AM) worked closely with the laboratory to monitor the protocol and review accuracy of results. Data validation features were used in REDCap™ to facilitate accurate data entry and data were audited regularly for accuracy.
3 |. RESULTS/FINDINGS
3.1 |. Participant flow
Figure 1 shows subject enrolment. During the 3-year data collection, 11,125 patients were assessed for eligibility; 2,283 met inclusion criteria; and 513 were enrolled and randomized to groups. Most of those eligible and not enrolled were secondary to inability to obtain consent during the short enrolment window (Sole et al., 2017). Demographic data for those eligible versus those consented were no different with one exception. Proxies of participants of Asian descent had a higher rate of decline (Sole et al., 2017).
FIGURE 1.
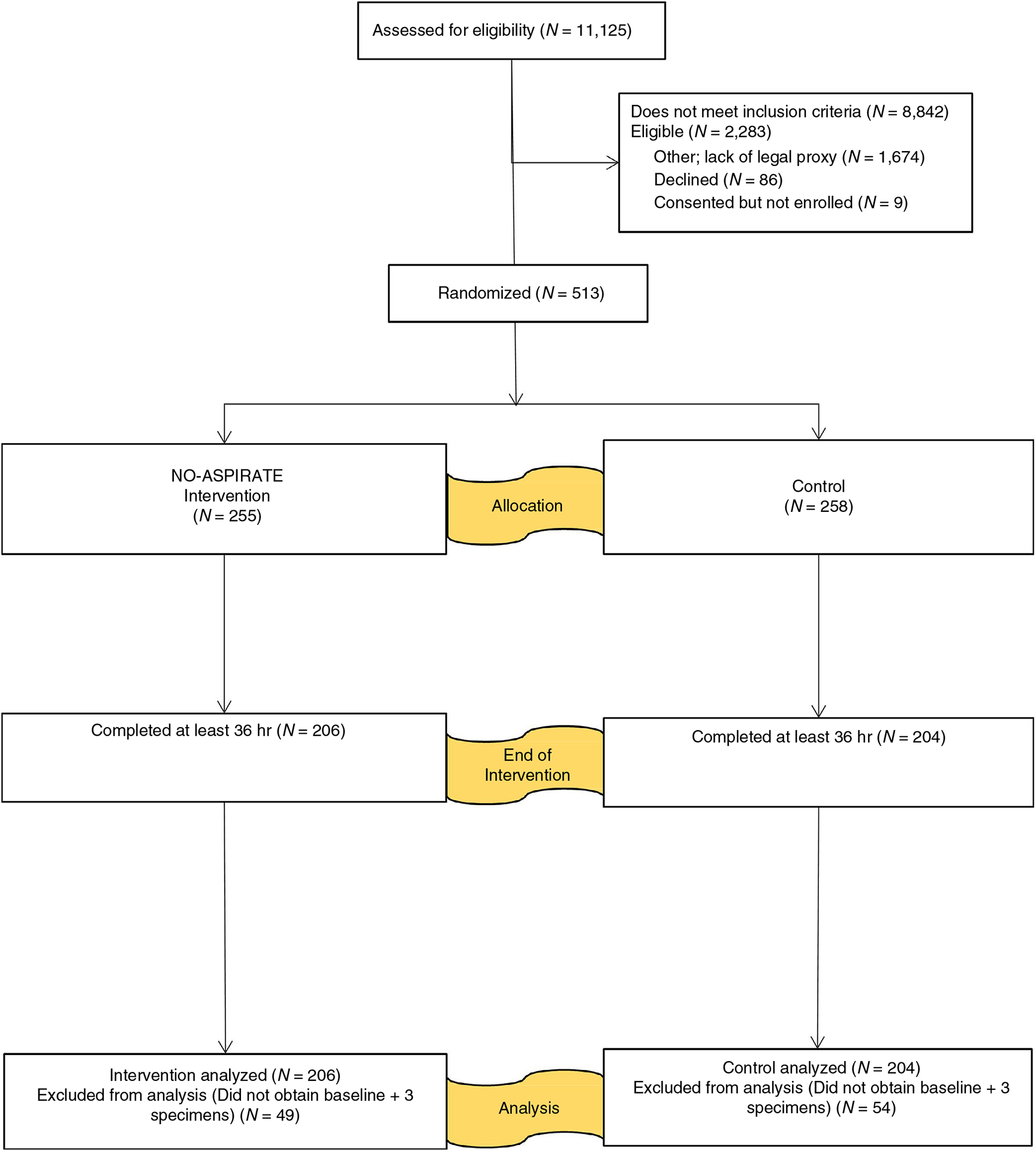
NO-ASPIRATE CONSORT flow diagram
Data were analysed for 410 subject completers: intervention (N = 206) and usual care (N = 204). No differences were found between completers versus non-completers for age, sex, race and ethnicity. Completers had a higher severity of illness (APACHE II 23.3 vs 20.9; p = .005) and fewer surgical and more trauma diagnoses (p = .011). These differences are expected.
No serious adverse events occurred. Minor protocol deviations (missed intervention or specimen) were recorded for 11 completers: NO-ASPIRATE N = 6 and control N = 5. All were included based on intent-to-treat analysis.
3.2 |. Demographic data
No significant differences in demographic variables (Table 1) were noted between groups (p > .050 for all variables). Most participants were white (74.1%; N = 304/410), male (59.0%; N = 242/410); and were intubated with a SS-ETT (85.1%; N = 349/410). Racial (25.9%; N = 106/410) and Hispanic ethnic minorities (18.5%; N = 76/410) were represented. Diagnostic classification was widely distributed with more patients having a medical diagnosis. Participants’ mean age was 58.6 years and they had a high severity of illness (mean Acute Physiologic Assessment and Chronic Health Evaluation II score of 23.3).
TABLE 1.
Demographics variable analysis
| Variable | Total (N = 410) | Control group (N = 204) | Intervention group (N = 206) | p value | |||
|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 58.6 | (18.9) | 57.4 | (19.2) | 59.8 | (18.6) | .188a |
| ETT type, n (%) | |||||||
| SS-ETT | 349 | (85.1) | 171 | (83.8) | 178 | (86.4) | .462a |
| Conventional ETT | 61 | (14.9) | 33 | (16.2) | 28 | (13.6) | |
| Gender, n (%) | |||||||
| Male | 242 | (59.0) | 118 | (57.8) | 124 | (60.2) | .628a |
| Female | 168 | (41.0) | 86 | (42.2) | 82 | (39.8) | |
| Race, n (%) | |||||||
| White | 304 | (74.1) | 149 | (73.0) | 155 | (75.2) | .541a |
| Black | 86 | (21.0) | 44 | (21.6) | 42 | (20.4) | |
| Asian | 18 | (4.4) | 9 | (4.4) | 9 | (4.4) | |
| Other | 2 | (0.5) | 2 | (1.0) | 0 | (0) | |
| Ethnicity, n (%) | |||||||
| Not Hispanic or Latino | 334 | (81.5) | 169 | (82.8) | 165 | (80.1) | .474a |
| Hispanic or Latino | 76 | (18.5) | 35 | (17.2) | 41 | (19.9) | |
| Diagnoses, n (%) | |||||||
| Medical and surgical | 151 | (36.8) | 66 | (32.4) | 85 | (41.3) | .173a |
| Trauma | 121 | (29.5) | 62 | (30.4) | 59 | (28.6) | |
| Neurological | 120 | (29.3) | 64 | (31.4) | 56 | (27.2) | |
| Comorbidities, n (%) | |||||||
| Diabetes | 103 | (25.1) | 56 | (27.5) | 47 | (22.8) | .279a |
| History of smoking | 95 | (23.2) | 43 | (21.1) | 52 | (25.2) | .318a |
| Chronic heart failure | 49 | (12.0) | 24 | (11.8) | 25 | (12.1) | .908a |
| Chronic renal failure | 43 | (10.5) | 21 | (10.3) | 22 | (10.7) | .899a |
| History of ETOH abuse | 41 | (10.0) | 18 | (8.8) | 23 | (11.2) | .429a |
| COPD | 33 | (8.0) | 17 | (8.3) | 16 | (7.8) | .833a |
| History of drug abuse | 27 | (6.6) | 13 | (6.4) | 14 | (6.8) | .863a |
| Immune-com-promise | 10 | (2.4) | 5 | (2.5) | 5 | (2.4) | .988a |
| Chronic liver failure | 8 | (2.0) | 4 | (2.0) | 4 | (1.9) | .989a |
| Acuity, mean (SD) | |||||||
| APACHE II | 23.3 | (7.5) | 22.7 | (0.5) | 23.0 | (0.5) | .691b |
| SAPS | 48.9 | (15.5) | 47.8 | (1.0) | 48.3 | (0.9) | .742b |
| ISS trauma only | 23.7 | (11.0) | 21.9 | (1.2) | 24.2 | (1.4) | .217b |
Note: Abbreviations: APACHE, acute physiology and chronic health evaluation; COPD, chronic obstructive pulmonary disease; ETOH, alcohol; ETT, endotracheal tube; ISS, Injury Severity Score; SAPS, Simplified Acute Physiology Score; SD, standard deviation.
Chi-Square.
t-test.
3.3 |. NO-ASPIRATE impact on microaspiration and VAE
The primary study aim was to compare the NO-ASPIRATE intervention versus usual care on microaspiration (Table 2). Among the NO-ASPIRATE participants, 75.2% of tracheal specimens were positive for α-amylase compared with 78.4% in the control group. GEE statistics found no difference in positive specimens (p = .684) or tracheal α- amylase value across time (p = .432). Mean α-amylase in the NO-ASPIRATE and control groups were 13,086 U/L and 15,298 U/L respectively.
TABLE 2.
Analysis of study aims
| Outcome | Control group (N = 204) | Intervention group (N = 206) | p value | ||
|---|---|---|---|---|---|
| Aim 1- Microaspiration | |||||
| Positive tracheal specimens for α-amylase, n (%) | 160 | (78.4) | 155 | (75.2) | .684a |
| Average tracheal α-amylase level, mean U/L (SD) | 15,298 | (36,643) | 13,086 | (25,288) | .432a |
| Aim 2- VAE | |||||
| VAC, n (%) | 32 | (15.7) | 31 | (15.0) | .858b |
| IVAC, n (%) | 17 | (8.3) | 16 | (7.8) | .977b |
| VAP, n (%) | 9 | (4.4) | 4 | (1.9) | .255b |
| Aim 3- Tracheal/Oral ratio of α-amylase | |||||
| Mean ratio with baseline, mean (SD) | 0.075 | (0.2) | 0.073 | (0.1) | .460a |
Note: Abbreviations: IVAC, infection-related ventilator-associated condition; SD, standard deviation; VAC, ventilator-associated condition; VAE, ventilator-associated event; VAP, ventilator-associated pneumonia.
GEE.
Chi-Square.
Additionally, the Local Weighted Regression (LOESS) method was used to explore trends of tracheal α-amylase values over time along with the 95% confidence bands. All available data for each subject were used for the LOESS curve fittings. The 95% confidence bands for mean tracheal α-amylase values overlap between groups at all points (Figure 2), reflecting no significant difference between groups.
FIGURE 2.
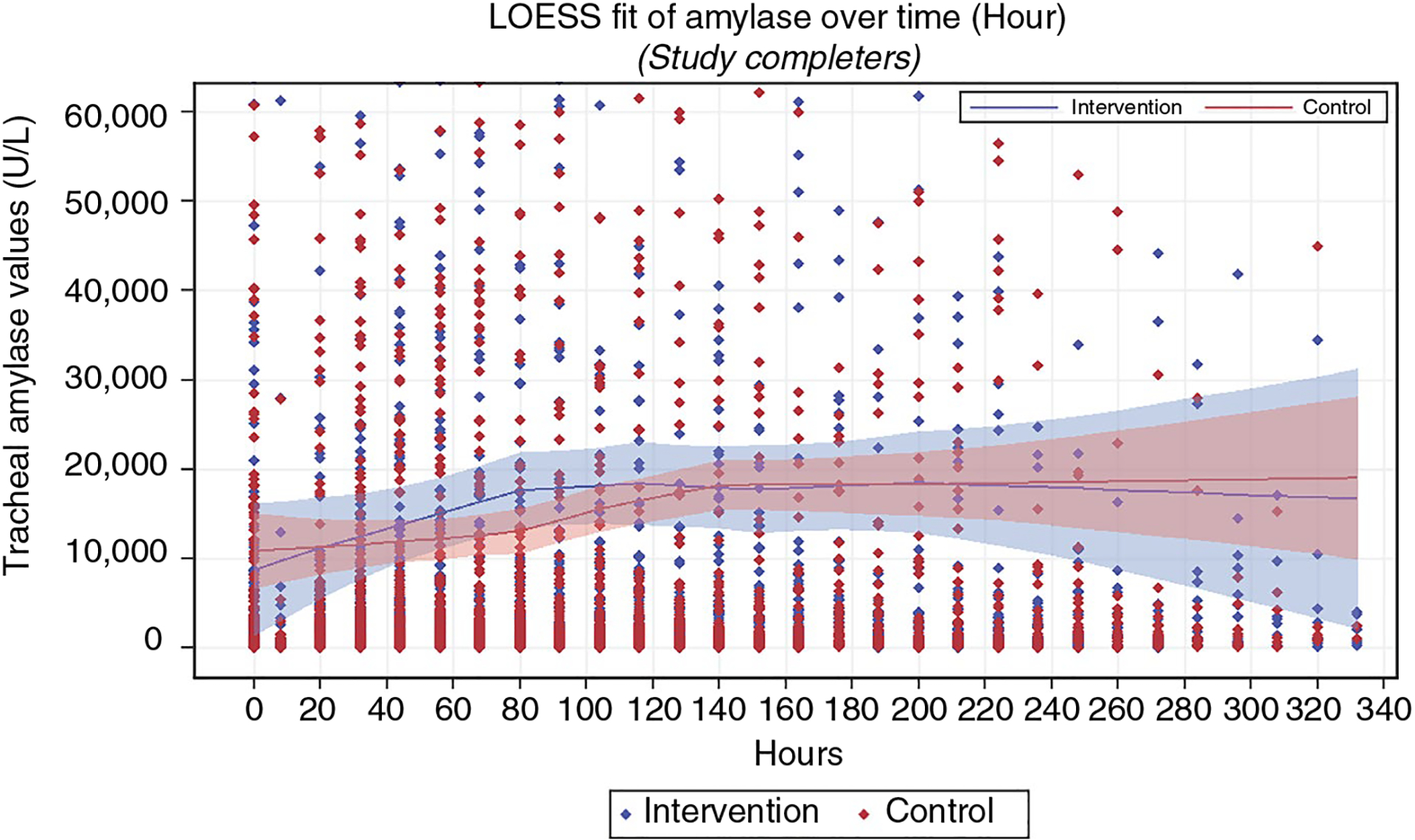
Local weighted regression analysis of α-amylase in all study completers
LOESS subgroup exploratory analysis was completed for four groups according to baseline α-amylase levels: Group 1–no aspiration (0 to 392 U/L), Group 2–low aspiration (392 to 1,499 U/L), Group 3–moderate (1,500 to 4,999 U/L) and Group 4–high (≥ 5,000 U/L). Although multiple cut-off points were evaluated, these categories were selected because they optimized equality of sample size between NO-ASPIRATE and control participants in each subgroup. Groups 1, 2 and 4 showed no difference in tracheal α-amylase values between groups at any time. NO-ASPIRATE participants in Group 3 (1,500 to 4,999 U/L) had a significantly lower tracheal α-amylase over time (Figure 3). The LOESS curves showed that 95% confidence bands for both groups overlapped with one another until the 90-hr mark. At this point, the 95% confidence bands differed between groups with values of the NO-ASPIRATE group decreasing and those of the control group increasing.
FIGURE 3.
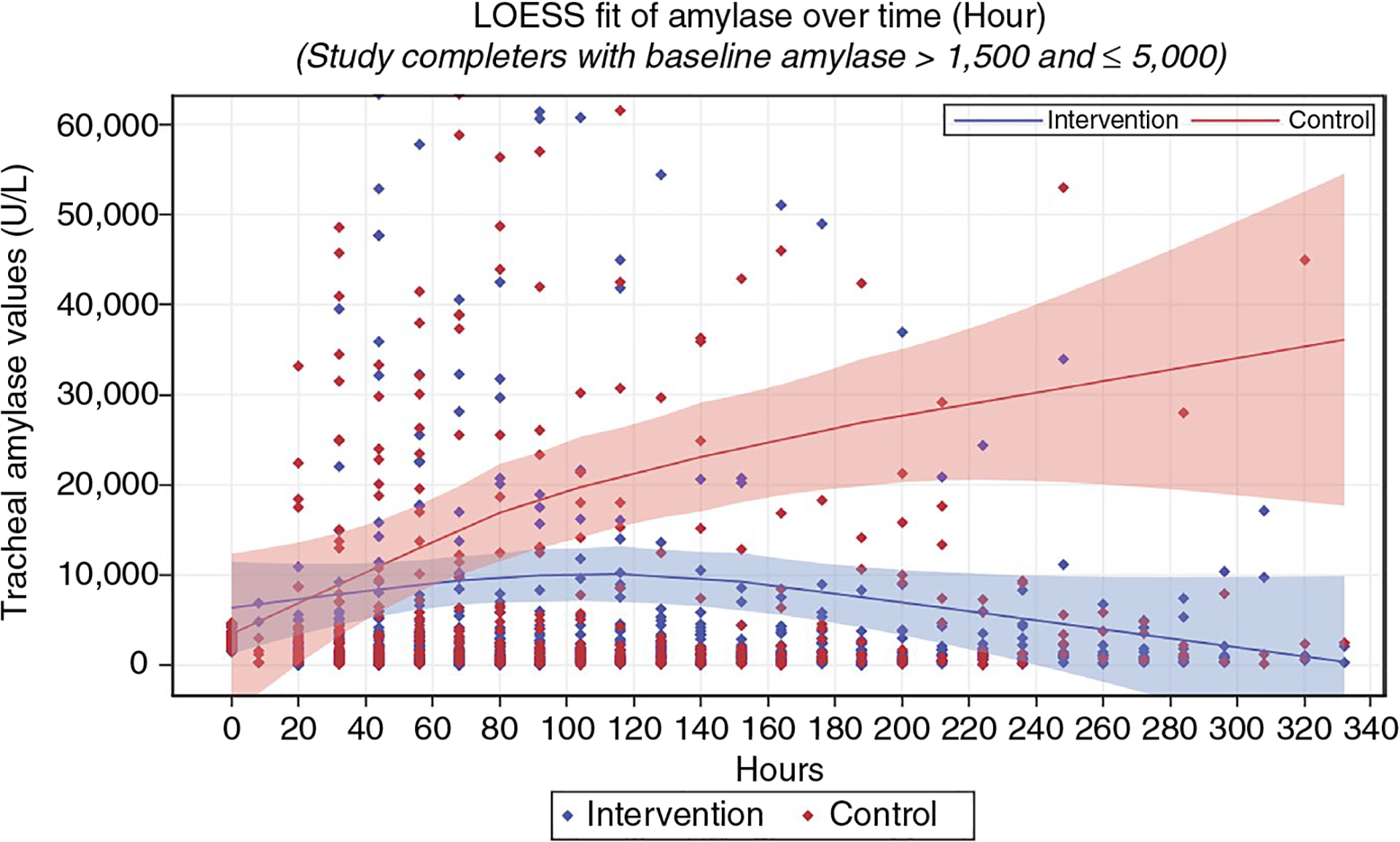
Local weighted regression subgroup analysis of α-amylase
3.4 |. NO-ASPIRATE impact on VAE and time to event
No significant differences were noted between groups on VAE rate and time to occurrence. The NO-ASPIRATE group had 15% (N = 31/206) of participants develop VAE in comparison to 15.7% (N = 32/204) from the usual care group (p = .858). The number of VAE patients progressed to PVAP was only 1.9% (N = 4/206) in the NO-ASPIRATE group and 4.4% (N = 9/204) in the control group (p = .255). The log-rank test of time to event found no differences between groups (p = .913); mean time to VAC event day was 4.9 days in the NO-ASPIRATE group compared with 5.7 days in the control group.
3.5 |. Clinical outcomes of NO-ASPIRATE intervention
The NO-ASPIRATE group had a significantly shorter mean hospital LOS compared with the usual care group (19.5 vs 23.4 days; p = .050) (Table 3). The ICU mean LOS was 9.8 days in the NO-ASPIRATE group compared with 10.8 days in the usual care group (p = .155). Duration of mechanical ventilation was 147.6 hr in the NO-ASPIRATE group and 159.3 hr in the control group (p = .214).
TABLE 3.
Clinical outcome variable analysis
| Variable | Control group (N = 204) | Intervention group (N = 206) | p value | ||
|---|---|---|---|---|---|
| Outcomes, mean (SD) | |||||
| Ventilator hours | 159.3 | (102.6) | 147.6 | (86.4) | .214a |
| Hospital LOS days | 23.4 | (23.1) | 19.5 | (16.5) | .050a |
| Total study days | 5.7 | (3.5) | 5.2 | (3.3) | .151a |
| ICU LOS in days | 10.8 | (7.7) | 9.8 | (6.6) | .155a |
| Discharge disposition, n (%) | .839b | ||||
| LTAC, rehab, or other | 57 | (27.9) | 65 | (31.6) | |
| Home | 47 | (23.0) | 49 | (23.8) | |
| Death | 49 | (24.0) | 41 | (19.9) | |
| Hospice | 28 | (13.7) | 30 | (14.6) | |
| Skilled nursing facility | 23 | (11.3) | 21 | (10.2) | |
Note: Abbreviations: ICU, intensive care unit; LOS, length of stay; LTAC, long-term acute care; SD, standard deviation.
t-test.
Chi-square.
No significant differences between groups were noted for discharge disposition (p = .839) (Table 3). The most frequent discharge disposition of patients was to a long-term acute care facility, rehabilitation centre or other similar setting (29.8%, N = 122/410). Although mortality was higher in the control group (24%, N = 49/204) compared with the NO-ASPIRATE group (19.9%, N = 41/206), the proportion was not significantly different (p = .314).
3.6 |. Exploration of tracheal-oral ratio
The final aim explored changes in the tracheal/oral ratio of α-amylase between groups over time. On average, 7% (ratio 0.07) of the oral α-amylase value was detected in the lungs and no significant differences were found in the value over time using GEE analysis (0.073), intervention vs 0.075, control; p = .460).
4 |. DISCUSSION
Outcomes of the NO-ASPIRATE intervention were compared with usual care. Over 75% of tracheal specimens were positive for tracheal α-amylase in both groups, which was higher than we expected. Only 38% of participants had a positive tracheal α-amylase in preliminary work; findings were based on a one-time assessment of a sample of 13 participants over 4 hr and not for the duration of mechanical ventilation (Sole et al., 2012). Findings are similar to other studies that used higher values to determine microaspiration. Using a tracheal α-amylase cut-point of 1,685 U/L, Millot et al. (2018) reported that 88% (SS-ETT) to 100% (traditional ETT) of specimens were positive in a sample of 100 participants during 24 hr after enrolment. Their study evaluated the SS-ETT; oral suctioning was not part of the protocol. Jaillette et al. (2017) reported microaspiration in 68.6% to 77.4% of participants in a study comparing aspiration with different types of ETT cuffs. They defined microaspiration as α-amylase levels greater than 1,685 IU/L in over 30% of tracheal aspirates.
Filloux et al. (2013) reported a tracheal/oral ratio of 5.5% while we found a ratio of 7.0%. Differences in sample size may be one reason for the difference as Filloux et al. (2013) reported data from only 26 intubated patients.
Tracheal α-amylase levels were lower in the NO-ASPIRATE group but not significant over time and no differences were noted in the tracheal-oral ratio. The inclusion of standard oral care to both groups every 4 hr may have been a contributing factor. The standard care included oral antisepsis with the suction swab, which may have adequately removed oral secretions. Findings may also have been different if the standardized kit had not been used. The kit included suction toothbrushes and swabs, both which help to reduce the secretion volume. Not all ICUs incorporate standardized kits, especially in international settings.
Nearly all participants (85.1%) were intubated with a SS-ETT, a potential confounding factor. However, these tubes may not prevent aspiration. Millot et al. (2018) reported that 85% of participants with a SS-ETT and 80% with a traditional ETT had microaspiration of oral secretions and median amylase levels (10,675 U/L vs 4,279 U/L) were higher in those with a SS-ETT.
Filloux et al. (2013) compared oral, subglottic and tracheal amylase levels in non-intubated patients and patients intubated with the SS-ETT. Tracheal amylase was detected in all intubated patients and 75% of non-intubated patients. Median values were significantly higher in intubated patients (6,691 U/L vs 191 U/L).
Much remains unknown regarding the prognostic ability α-amylase in detecting microaspiration (Dewavrin et al., 2014; Qu et al., 2018; Samanta et al., 2018; Weiss et al., 2013). Filloux et al. (2013) reported that a cut-off value of 1,832 U/L for tracheal amylase had a sensitivity of 88% and specificity of 100% for predicting microaspiration, yet the value that predicts clinical outcomes remains unknown.
A recent observational study found that median α-amylase levels obtained from bronchoalveolar specimens increased with pre-intubation risk factors, such as altered consciousness, cardiac arrest and prolonged transport. Participants with the highest risk for VAP (defined clinically) also had the highest median α-amylase level of 3,453 U/L (Samanta et al., 2018). In comparison, our participants had higher median values: NO-ASPIRATE–5,918 U/L and usual care–6,664 U/L. Technique (bronchoalveolar lavage vs ETT suctioning) may be reason for the difference in values and should be further explored. Filloux et al. (2013) reported a median value of 6,661 U/L for tracheal specimens, which are comparable to our findings.
Alpha-amylase was present in most tracheal specimens, suggesting that microaspiration is a continuous process in ventilated patients (Samanta et al., 2018). The duration of detecting α-amylase in the lungs following aspiration is also unknown, making interpretation of values challenging. Once secretions are aspirated, the tracheal α-amylase value should decrease over time. However, increases (spikes) in values over time in our sample indicated ongoing aspiration events. We will further explore the ability to detect large increases in tracheal α-amylase and higher tracheal oral ratios that indicate aspiration events.
ETT cuff pressure adjustment was not part of the study protocol. Respiratory therapists assigned to the patients measured and adjusted cuff pressure, usually every 12 hr. Although values within the recommended range of 20–30 cm H2O were recorded, it is not known what the values were between measurement periods. Cuff pressures often decrease over time and vary widely based on a variety of clinical factors (Nseir et al., 2007; Nseir, Zerimech, Fournier, et al., 2011; Sole, Su, et al., 2011). Fluctuations or reductions in ETT cuff pressure may diminish the protective seal provided by the cuff, allowing for aspiration.
The intervention was beneficial to a subgroup of patients who had tracheal α-amylase of 1,500 to 4,999 U/L at baseline (Figure 3). Values of patients whose tracheal α-amylase levels were lower at baseline remained low with or without the NO-ASPIRATE intervention. At a conceptual level, findings may be similar to disease staging in that interventions or treatments are only effective for certain subgroups. Factors such as ETT tube size, ETT cuff pressure, or other physiological variables may have influenced findings for these subgroups. Likewise, the intervention was not of benefit in reducing α-amylase levels in those with the highest α-amylase at baseline. These individuals likely had significant aspiration during intubation that went undetected and it is not known how long α-amylase remains active in the lungs once secretions are aspirated. If α-amylase remains high for an extended period, differences between groups will be challenging to detect. Findings warrant further research evaluating the impact of deep oropharyngeal suctioning in patients with baseline tracheal α-amylase levels of 1,500 to 4,999 U/L.
Both groups had comparable VAC rates and a low rate of PVAP (3.2%). Similar findings have been reported in the literature. Rates of VAC have ranged from 14% to 23% and PVAP from 1.6% to 9.3% (Klompas et al., 2011; Klompas, Li, Kleinman, Murphy, & Szumita, 2016). One quality improvement initiative evaluating the impact of spontaneous awakening trials and spontaneous breathing trials in a medical critical care population reported an 8.5% VAC rate and a 1.6% PVAP rate, which are lower (Balas et al., 2015). Our participants had high admission acuity levels and most were trauma and neurological patients, who often have the highest rates. Additionally, VAE is associated with many aetiologies, including atelectasis, fluid overload and acute respiratory distress syndrome (Klompas, 2015). Oral suctioning does not influence these aetiologies.
Our findings differ from others who found a reduction in VAP and shorter duration of mechanical ventilation with implementation of oral suctioning (Chang et al., 2008; Chao et al., 2009; Cutler & Sluman, 2014). Reasons for these findings may be differences in determining VAP and differences in oral suction practices and devices. Using a clinical diagnosis to confirm VAP, another study found VAP rates higher than ours of 12.8% and 8.5%, respectively, after performing intervention care with an oral care device every 8 hr (Ory et al., 2017). Our findings also differed from quality improvement findings reported in the United States (Blamoun et al., 2009; Garcia et al., 2009), which were conducted nearly 10 years ago. The consistent delivery of oral care every 4 hr, using a commercial kit, by the study team is a possible reasons that our findings did not affect VAC and PVAP. Since our VAC rates were similar between groups, the time to VAC did not differ either, which would be expected.
Participants in the intervention group had a shorter hospital LOS. Many factors have an impact on hospital LOS; therefore, it is challenging to interpret this finding. Clinically important (but not statistically significant) decreases were noted for ventilator hours and ICU LOS, which may have translated into a shorter hospital LOS. Systematic reviews of oral care found no significant impact on mortality outcomes, ICU LOS, or ventilator hours (Hua et al., 2016; Klompas, Speck, Howell, Greene, & Berenholtz, 2014), but the focus was not on oral suctioning.
Notably, the ICU LOS (mean 9.8 days) was shorter than stays reported in other intervention studies of ventilated patients. ICU LOS of 11–17 days have been reported in recent studies of similar patient populations (Balas et al., 2015; Jaillette et al., 2017; Samanta et al., 2018). The hospital LOS for our NO-ASPIRATE group (19.5 days) was also shorter compared with the mean hospital LOS of 21–22 days reported in other studies (Balas et al., 2015; Samanta et al., 2018). Decreased hospital LOS found in our study, potentially attributed to enhanced oropharyngeal suctioning and oral cleansing in MV patients, is of financial and clinical interest to hospitals and clinicians.
4.1 |. Limitations
Delivery of standard oral care every 4 hr to participants in both groups may have contributed to findings as this could be an intervention itself. Prior evidence shows that nursing staff do not uniformly comply with oral care recommendations in MV patients (Hsin-Lan, Li-Yu, & Chih-Cheng, 2014), despite oral care being a singular nursing intervention associated with a significantly reduced risk of VAE development (O’Horo et al., 2016). Our study shows the expected findings when standardized oral cleansing and oral suction with swabs are completed systematically every 4 hr in addition to the standard ventilator bundle.
Alpha-amylase as a biomarker is useful in detecting aspiration of oral secretions. However, its usefulness as a prognostic measure needs further study. Once aspiration occurs, the duration of α-am-ylase in the lungs requires further exploration to assist in interpretation of positive values over time. The cut-point value we used for microaspiration of oral secretions was lower than reported by other researchers, but our findings were similar.
The lack of control over ETT cuff pressures is another limitation. If the study were to be replicated, it is suggested that cuff pressure measurement and documentation prior to adjustment be included as part of the protocol.
Lastly, the effect sizes for key outcome variables were smaller than predicted in our initial power analysis. Therefore, the study may have been underpowered on select variables.
5 |. CONCLUSION
The NO-ASPIRATE intervention was beneficial to a subgroup of participants who had moderate (1,500 to 4,999 U/L) levels of α-amylase within 24 hr of intubation. Aside from this subgroup, no other significant differences in α-amylase were noted between groups. The importance of baseline α-amylase levels on relevant outcomes, such as VAE, ventilator hours and LOS, warrants additional study. Measurement of tracheal α-amylase levels is not part of routine care and obtaining a baseline specimen may be important to tailor interventions for the patient. The role of microaspiration of oral secretions and clinical outcomes is in its infancy.
No differences were found in the secondary study aim of VAE. VAE is attributed to many factors and microaspiration of oral secretions may not be as important as we hypothesized. Participants in the intervention group had a shorter hospital LOS. Since many factors also influence LOS, further exploration of this finding is warranted.
Although limited differences were noted between two different methods for removing oral secretions, our findings provide data related to expected findings when a standardized oral care protocol is provided by trained team members every 4 hr to ventilated patients. Outcomes support existing knowledge that oral care is an important nursing intervention that should be provided uniformly to MV patients.
ACKNOWLEDGMENTS
This study received financial support from the NIH in February of 2014. The NIH grant number is 1R01NR014508-01A1. No other acknowledgements are noted in this study.
Funding information
This study received financial support from the NIH. The NIH grant number for the NO-ASPIRATE study is 1R01NR014508-01A1.
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflicts of interest to declare.
REFERENCES
- Abu-Hasan M, Elmallah M, Neal D, & Brookes J (2014). Salivary amylase level in bronchoalveolar fluid as a marker of chronic pulmonary aspiration in children. Pediatric Allergy, Immunology & Pulmonology, 27, 115–119. 10.1089/ped.2014.0348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari NKJ, Fowler RA, Bhagwanjee S, & Rubenfeld GD (2010). Critical care and the global burden of critical illness in adults. Lancet, 376, 1339–1346. 10.1016/S0140-6736(10)60446-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas M, Klompas M, Li L, Kleinman K, Bruce C, Lankiewicz J, … Weinstein RA (2015). The preventability of ventilator-associated events the CDC prevention epicenters wake up and breathe collaborative. American Journal of Respiratory and Critical Care Medicine, 191, 292–301. 10.1164/rccm.201407-1394OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamoun J, Alfakir M, Rella ME, Wojcik JM, Solis RA, Anees Khan M, & DeBari VA (2009). Efficacy of an expanded ventilator bundle for the reduction of ventilator-associated pneumonia in the medical intensive care unit. American Journal of Infection Control, 37, 172–175. 10.1016/j.ajic.2008.05.010 [DOI] [PubMed] [Google Scholar]
- Blot SI, Poelaert J, & Kollef M (2014). How to avoid microaspiration? A key element for the prevention of ventilator-associated pneumonia in intubated ICU patients. BMC Infectious Diseases, 14(119), 1–6. 10.1186/1471-2334-14-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff DA, Li L, Muscedere J, & Klompas M (2016). Subglottic secretion drainage and objective outcomes: A systematic review and meta-analysis. Critical Care Medicine, 44, 830–840. 10.1097/CCM.0000000000001414 [DOI] [PubMed] [Google Scholar]
- Chang S-C, Tsai H-H, & Lin F-C (2008). Intermittent suction of oral secretions before each positional change may reduce ventilator-associated pneumonia: A pilot study. American Journal of the Medical Sciences, 336, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao YF, Chen YY, Wang KW, Lee RP, & Tsai H (2009). Removal of oral secretion prior to position change can reduce the incidence of ventilator-associated pneumonia for adult ICU patients: A clinical controlled trial study. Journal of Clinical Nursing, 18(1), 22–28. 10.1111/j.1365-2702.2007.02193.x [DOI] [PubMed] [Google Scholar]
- Chow MC, Kwok SM, Luk HW, Law JW, & Leung BP (2012). Effect of continuous oral suctioning on the development of ventilator-associated pneumonia: A pilot randomized controlled trial. International Journal of Nursing Studies, 49, 1333–1341. 10.1016/j.ijnurstu.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Craven DE, Chroneou A, Zias N, & Hjalmarson KI (2009). Ventilator-associated tracheobronchitis: The impact of targeted antibiotic therapy on patient outcomes. Chest, 135, 521–528. 10.1378/chest.08-1617 [DOI] [PubMed] [Google Scholar]
- Craven DE, & Steger KA (1997). Hospital-acquired pneumonia: Perspectives for the healthcare epidemiologist. Infection Control and Hospital Epidemiology, 18, 783–795. 10.2307/30141328 [DOI] [PubMed] [Google Scholar]
- Cutler LR, & Sluman P (2014). Reducing ventilator associated pneumonia in adult patients through high standards of oral care: A historical control study. Intensive & Critical Care Nursing, 30, 61–68. 10.1016/j.iccn.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Damas P, Frippiat F, Ancion A, Canivet J-L, Lambermont B, Layios N, … Ledoux D (2015). Prevention of ventilator-associated pneumonia and ventilator-associated conditions: A randomized controlled trial with subglottic secretion suctioning. Critical Care Medicine, 43(1), 22–30. 10.1097/CCM.0000000000000674 [DOI] [PubMed] [Google Scholar]
- Dewavrin F, Zerimech F, Boyer A, Maboudou P, Balduyck M, Duhamel A, & Nseir S (2014). Accuracy of alpha amylase in diagnosing microaspiration in intubated criticallyi-ll patients. PLoS ONE, 9, e90851. 10.1371/journal.pone.0090851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux B, Bedel A, Nseir S, Mathiaux J, Amadeo B, Clouzeau B, … Boyer A (2013). Tracheal amylase dosage as a marker for microaspiration: A pilot study. Minerva Anestesiologica, 79, 1003–1010. [PubMed] [Google Scholar]
- Garcia R, Jendresky L, Colbert L, Bailey A, Zaman M, & Majumder M (2009). Reducing ventilator-associated pneumonia through advanced oral-dental care: A 48-month study. American Journal of Critical Care, 18, 523–532. 10.4037/ajcc2009311 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Morisawa K, Klompas M, Jones M, Bandeshe H, Boots R, … Paterson DL (2013). Toward improved surveillance: The impact of ventilator-associated complications on length of stay and antibiotic use in patients in intensive care units. Clinical Infectious Diseases, 56, 471–477. 10.1093/cid/cis926 [DOI] [PubMed] [Google Scholar]
- Hsin-Lan L, Li-Yu Y, & Chih-Cheng L (2014). Factors related to compliance among critical care nurses with performing oral care protocols for mechanically ventilated patients in the intensive care unit. American Journal of Infection Control, 42, 533–535. 10.1016/j.ajic.2014.01.023 [DOI] [PubMed] [Google Scholar]
- Hua F, Xie H, Worthington HV, Furness S, Zhang Q, & Li C (2016). Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database of Systematic Reviews, 10(7), 1–137. 10.1002/14651858.CD008367.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillette E, Girault C, Brunin G, Zerimech F, Behal H, Chiche A, … Nseir S (2017). Impact of tapered-cuff tracheal tube on microaspiration of gastric contents in intubated critically ill patients: A multicenter cluster-randomized cross-over controlled trial. Intensive Care Medicine, 43, 1562–1571. 10.1007/s00134-017-4736-x [DOI] [PubMed] [Google Scholar]
- Klompas M (2015). Potential Strategies to Prevent Ventilator-associated Events. American Journal of Respiratory and Critical Care Medicine, 192(12), 1420–1430. 10.1164/rccm.201506-1161CI [DOI] [PubMed] [Google Scholar]
- Klompas M, Khan Y, Kleinman K, Evans RS, Lloyd JF, Stevenson K, … Platt R (2011). Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS ONE, 6(3), 1–7. 10.1371/journal.pone.0018062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M, Li L, Kleinman K, Murphy MV, & Szumita P (2016). Associations between different sedatives and ventilator-associated events, length of stay and mortality in patients who were mechanically ventilated. Chest, 149, 1373–1379. 10.1378/chest.15-1389 [DOI] [PubMed] [Google Scholar]
- Klompas M, Magill S, Robicsek A, Strymish JM, Kleinman K, Evans RS, … Platt R (2012). Objective surveillance definitions for ventilator-associated pneumonia. Critical Care Medicine, 40, 3154–3161. 10.1097/CCM.0b013e318260c6d9 [DOI] [PubMed] [Google Scholar]
- Klompas M, Speck K, Howell MD, Greene LR, & Berenholtz SM (2014). Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: Systematic review and meta-analysis. JAMA Internal Medicine, 174, 751–761. 10.1001/jamainternmed.2014.359 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Uchino S, Takinami M, & Uezono S (2017). The impact of ventilator-associated events in critically ill subjects with prolonged mechanical ventilation. Respiratory Care, 62, 1379–1386. 10.4187/respcare.05073 [DOI] [PubMed] [Google Scholar]
- Kollef MH, Chastre J, Fagon J-Y, François B, Niederman MS, Rello J, … Rehm C (2014). Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Critical Care Medicine, 42, 2178–2187. 10.1097/CCM.0000000000000510 [DOI] [PubMed] [Google Scholar]
- Marik PE (2011). Pulmonary aspiration syndromes. Current Opinion in Pulmonary Medicine, 17, 148–154. 10.1097/MCP.0b013e32834397d6 [DOI] [PubMed] [Google Scholar]
- Millot G, Boddaert P, Parmentier-Decrucq E, Palud A, Balduyck M, Maboudou P, … Nseir S (2018). Impact of subglottic secretion drainage on microaspiration in critically ill patients: A prospective observational study. Annals of Translational Medicine, 6, 1–8. 10.21037/atm.2018.10.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandapalan V, McIlwain JC, & Hamilton J (1995). A study of alpha-amylase activity in tracheobronchial secretions of seriously ill patients with tracheostomies. Journal of Laryngology and Ontology, 109, 640–643. 10.1017/S0022215100130907 [DOI] [PubMed] [Google Scholar]
- Nseir S, Duguet A, Copin M-C, De Jonckheere J, Zhang M, Similowski T, & Marquette C-H (2007). Continuous control of endotracheal cuff pressure and tracheal wall damage: A randomized controlled animal study. Critical Care, 11, R109. 10.1186/cc6142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nseir S, Zerimech F, Fournier C, Lubret R, Ramon P, Durocher A, & Balduyck M (2011). Continuous control of tracheal cuff pressure and microaspiration of gastric contents in critically ill patients. American Journal of Respiratory & Critical Care Medicine, 184, 1041–1047. 10.1164/rccm.201104-0630OC [DOI] [PubMed] [Google Scholar]
- Nseir S, Zerimech F, Jaillette E, Artru F, & Balduyck M (2011). Microaspiration in intubated critically ill patients: Diagnosis and prevention. Infectious Disorders Drug Targets, 11, 413–423. [DOI] [PubMed] [Google Scholar]
- O’Horo JC, Lan H, Thongprayoon C, Schenck L, Ahmed A, Dziadzko M, … Sampathkumar P (2016). “Bundle” practices and ventilator-associated events: Not enough. Infection Control & Hospital Epidemiology, 37, 1453–1457. 10.1017/ice.2016.207 [DOI] [PubMed] [Google Scholar]
- Ory J, Raybaud E, Chabanne R, Cosserant B, Faure JS, Guérin R, … Traore O (2017). Comparative study of 2 oral care protocols in intensive care units. American Journal of Infection, 45(3), 245–250. 10.1016/j.ajic.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Qu G-P, Fang X-Q, Xu Y-P, Shi M, Wang Y, Gong M-L, & Fang H-M (2018). Predictive value of α-amylase in tracheal aspirates for ventilator-associated pneumonia in elderly patients. The Clinical Respiratory Journal, 12, 1685–1692. 10.1111/crj.12729 [DOI] [PubMed] [Google Scholar]
- Rawat N, Yang T, Ali KJ, Catanzaro M, Cohen MD, Farley DO, … Berenholtz SM (2017). Two-state collaborative study of a multifaceted intervention to decrease ventilator-associated events. Critical Care Medicine, 45, 1208–1215. 10.1097/CCM.0000000000002463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta S, Poddar B, Azim A, Singh RK, Gurjar M, & Baronia AK (2018). Significance of mini bronchoalveolar lavage fluid amylase level in ventilator-associated pneumonia: A prospective observational study. Critical Care Medicine, 46(1), 71–78. 10.1097/CCM.00000000002774 [DOI] [PubMed] [Google Scholar]
- Sole ML, & Bennett M (2011). Suctioning and airway management practices (STAMP 2011) of registered nurses (RNs) and respiratory care practitioners (RCPs). Critical Care Medicine, 39(12), A341. 10.1097/01.ccm.0000408627.24229.88 [DOI] [PubMed] [Google Scholar]
- Sole ML, Byers JF, Ludy JE, Zhang Y, Banta CM, & Brummel K (2003). A multisite survey of suctioning techniques and airway management practices. American Journal of Critical Care, 12, 220–232. [PubMed] [Google Scholar]
- Sole ML, Conrad J, Bennett M, Middleton A, Hay K, Ashworth S, & Indulal Mehta D (2014). Pepsin and amylase in oral and tracheal secretions: A pilot study. American Journal of Critical Care, 23(4), 334–338. 10.4037/ajcc2014292 [DOI] [PubMed] [Google Scholar]
- Sole M, Middleton A, Allen K, Mehta D, Bennett M, Conrad J, & Ashworth S (2012). Pepsin and salivary amylase in oral and tracheal secretions: A pilot study. Critical Care Medicine, 40, U127. [DOI] [PubMed] [Google Scholar]
- Sole ML, Middleton A, Deaton L, Bennett M, Talbert S, & Penoyer D (2017). Enrollment challenges in critical care nursing research. American Journal of Critical Care, 26, 395–400. 10.4037/ajcc2017511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole ML, Penoyer DA, Bennett M, & Bertrand J (2010). Oropharyngeal secretion removal in intubated patients. Paper presented at Southern Nursing Research Society Annual Research Conference Austin, TX. [Google Scholar]
- Sole ML, Penoyer DA, Bennett M, Bertrand J, & Talbert S (2011). Oropharyngeal secretion volume in intubated patients: The importance of oral suctioning. American Journal of Critical Care, 20(6), E141–E145. 10.4037/ajcc2011178 [DOI] [PubMed] [Google Scholar]
- Sole ML, Su X, Talbert S, Penoyer DA, Kalita S, Jimenez E, … Bennett M (2011). Evaluation of an intervention to maintain endotracheal tube cuff pressure within therapeutic range. American Journal of Critical Care, 20, 109–118. 10.4037/ajcc2011661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole ML, Talbert S, Yan X, Penoyer D, Mehta D, Bennett M, … Emery KP (2019). Nursing oral suction intervention to reduce aspiration and ventilator events (NO-ASPIRATE): A randomized clinical trial. Journal of Advanced Nursing, 75(5), 1108–1118. 10.1111/jan.13920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Mirant-Borde MC, & Lee A (2011). Amylase in bronchoalveolar lavage as a potential marker of oropharyngeal-to-pulmonary aspiration. American Journal of Respiratory and Critical Care Medicine, 183, A4616. [Google Scholar]
- United States Department of Health and Human Services, Centers for Disease Control and Prevention (2019a). 2019 NHSN ventilator-associated event (VAE) checklist. Retrieved from https://www.cdc.gov/nhsn/pdfs/checklists/vae-checklist-508.pdf.
- United States Department of Health and Human Services, Centers for Disease Control and Prevention (2019b). Ventilator-associated event (VAE). Retrieved from https://www.cdc.gov/nhsn/PDFs/pscManual/10-VAE_FINAL.pdf.
- Weiss CH, Moazed F, DiBardino D, Swaroop M, & Wunderink RG (2013). Bronchoalveolar lavage amylase is associated with risk factors for aspiration and predicts bacterial pneumonia. Critical Care Medicine, 41, 765–773. 10.1097/CCM.0b013e31827417bc [DOI] [PubMed] [Google Scholar]


