Abstract
PURPOSE
Patients with Diffuse Large B-cell Lymphoma (DLBCL) in need of immediate therapy are largely under-represented in clinical trials. The diagnosis-to-treatment interval (DTI) has recently been described as a metric to quantify such patient selection bias, with short DTI being associated with adverse risk factors and inferior outcomes. Here, we characterized the relationships between DTI, circulating tumor DNA (ctDNA), conventional risk factors, and clinical outcomes, with the goal of defining objective disease metrics contributing to selection bias.
PATIENTS AND METHODS
We evaluated pretreatment ctDNA levels in 267 patients with DLBCL treated across multiple centers in Europe and the United States using Cancer Personalized Profiling by Deep Sequencing. Pretreatment ctDNA levels were correlated with DTI, total metabolic tumor volumes (TMTVs), the International Prognostic Index (IPI), and outcome.
RESULTS
Short DTI was associated with advanced-stage disease (P < .001) and higher IPI (P < .001). We also found an inverse correlation between DTI and TMTV (RS = −0.37; P < .001). Similarly, pretreatment ctDNA levels were significantly associated with stage, IPI, and TMTV (all P < .001), demonstrating that both DTI and ctDNA reflect disease burden. Notably, patients with shorter DTI had higher pretreatment ctDNA levels (P < .001). Pretreatment ctDNA levels predicted short DTI independent of the IPI (P < .001). Although each risk factor was significantly associated with event-free survival in univariable analysis, ctDNA level was prognostic of event-free survival independent of DTI and IPI in multivariable Cox regression (ctDNA: hazard ratio, 1.5; 95% CI [1.2 to 2.0]; IPI: 1.1 [0.9 to 1.3]; −DTI: 1.1 [1.0 to 1.2]).
CONCLUSION
Short DTI largely reflects baseline tumor burden, which can be objectively measured using pretreatment ctDNA levels. Pretreatment ctDNA levels therefore have utility for quantifying and guarding against selection biases in prospective DLBCL clinical trials.
INTRODUCTION
The diagnosis-to-treatment interval (DTI), measured as the time between pathologic diagnosis and initiation of immunochemotherapy, has recently been shown to be associated with prognostic clinical factors and outcomes in patients with diffuse large B-cell lymphoma (DLBCL).1 Short DTI was demonstrated to be associated with adverse clinical risk factors and inferior event-free survival (EFS). The underlying phenomenon is that patients with short DTI are enriched for patients with an acute clinical presentation in need of immediate treatment initiation and aggressive disease biology.
CONTEXT
Key Objective
In patients diagnosed with diffuse large B-cell lymphoma (DLBCL), is pretreatment circulating tumor DNA (ctDNA) level a more objective measure of clinical urgency and treatment outcome than the diagnosis-to-treatment interval (DTI)?
Knowledge Generated
Both DTI and ctDNA levels were found to be associated with conventional measures of tumor burden such as stage, the International Prognostic Index (IPI), and total metabolic tumor volume.
ctDNA levels predicted short DTI better than the IPI and were independently prognostic of event-free and overall survival in multivariable models also including the IPI and DTI.
Relevance
Collectively, our data suggest that ctDNA more objectively measures disease burden than DTI and could therefore have immediate utility for quantifying and mitigating selection biases in prospective diffuse large B-cell lymphoma clinical trials.
Intriguingly, the prognostic impact of DTI was independent of the International Prognostic Index (IPI), suggesting that widely applied prognostic scores do not adequately reflect disease aggressiveness and other factors considered for clinical decision making. There is evidence that DTI can confound patient selection for clinical trials, because the inability to delay therapy competes with the extensive screening and consent processes required for trial enrollment, with this conflict potentially leading to the exclusion of patients with short DTI. DTI may therefore be a valuable metric to measure the extent of selection bias by which a clinical trial can be affected. However, DTI may be affected not only by tumor-related biological factors but also by unrelated factors including health insurance type, access to care, holidays, and treatment delivery logistics.2-5 Thus, despite the simplicity of DTI and its ease of measurement, this lack of objectivity limits its clinical utility for risk stratification.
Circulating tumor DNA (ctDNA) is an emerging biomarker in B-cell lymphoma. Pretreatment ctDNA levels have been shown to reflect tumor burden and predict treatment outcomes.6-8 In this study, we sought to analyze whether pretreatment ctDNA levels in patients diagnosed with DLBCL may be a more objective measure of clinical urgency than DTI.
PATIENTS AND METHODS
Patients and Sample Collection
We included 267 patients with an initial diagnosis of a DLBCL according to the 2008 WHO classification9 including T cell/histiocyte rich large B-cell lymphoma. Patients with an antecedent low-grade lymphoma with histologic transformation were considered eligible. All patients were treated with combination immunochemotherapy with curative intent (rituximab plus cyclophosphamide, adriamycin, vincristine, and prednisone [R-CHOP] [75%]; dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, adriamycin, and rituximab [19%]; and others [6%]). We separately performed subgroup analyses for patients with de novo DLBCL (excluding transformed lymphomas and T cell/histiocyte rich large B-cell lymphoma) treated with standard R-CHOP (n = 185). Patients were treated at cancer centers across North America and Europe (Stanford Cancer Center, CA; MD Anderson Cancer Center, TX; Dijon, France; Novara, Italy; National Cancer Institute, MD, and within the phase III multicenter PETAL trial10; Supplemental Fig S1 [Data Supplement, online only]). One hundred sixty-three patients (61%) were evaluable for total metabolic tumor volume (TMTV) assessed by 18F-labeled fluorodeoxyglucose positron emission tomography and/or computed tomography (Supplemental Fig S2 [Data Supplement]). Baseline and early molecular response data from a subset of the studied patients (n = 156; 58%) described here were reported in a previous publication from our group.7 Peripheral blood samples were collected in K2EDTA tubes and processed according to local standards to isolate plasma before freezing. In 17 previously reported patients, serum samples were used for analyses after confirming consistency of ctDNA concentration measurements.7 The study was approved by the local institutional review board of each institution, and all patients provided written informed consent.
Sequencing and ctDNA Quantification
Somatic alterations for ctDNA quantitation were called from cell-free DNA (cfDNA) or tumor specimens, if available.7,11,12 Hybrid capture was done using targeted panels specifically optimized for B-cell lymphomas. Quantitative levels of ctDNA were measured in haploid genome equivalents per milliliter (hGE/mL), determined as the product of total cfDNA concentration and the mean allele fraction of somatic mutations, expressed in log scale (log hGE/mL).7 When considering ctDNA a binary variable, we used the previously defined threshold introduced by Kurtz et al,7 which dichotomized ctDNA levels > 2.5 log hGE/mL as high and the others as low. Cell-of-origin (COO) subtypes were inferred from sequencing data as previously described.6,13
DTI
The DTI was calculated as the time between biopsy and the initiation of immunochemotherapy. Steroid and/or other prephase treatment before immunochemotherapy was variably administered according to local standards. Per the convention introduced by Maurer et al,1 we considered patients with DTI ≤ 14 days as having short DTI in analyses requiring binarization.
Statistical Analysis
Continuous variables were compared using Wilcoxon rank-sum tests or one-way analysis of variance on ranks. Pearson and nonparametric Spearman correlations were used to correlate continuous variables as noted. Time-to-event variables were visualized using the Kaplan-Meier method; log-rank tests were applied to compare survival between cohorts. We used logistic regression to model the probability of binary variables. Cox proportional hazards regression was used to assess the impact of risk factors on outcome variables. For regression analyses, units are defined as follows: ctDNA: log10 hGE/mL, TMTV: log10 mL, IPI: five-point scale, and DTI: –weeks. EFS and overall survival (OS) were calculated from time of treatment initiation. OS events were death from any cause; EFS events were progression or relapse, unplanned treatment of lymphoma, and death resulting from any cause.
RESULTS
Distribution of DTI and ctDNA Levels
We measured DTI and ctDNA levels in 267 patients with a pathologic diagnosis of DLBCL (Fig 1A, Table 1, and Supplemental Tables S1 and S2 [Data Supplement]). The median DTI in our cohort was 21 days (range, 0-154 days), which is comparable with two previously reported cohorts described by Maurer et al1 (Fig 1B). The distribution of DTI by treatment center varied significantly (P = .002; Fig 1C). In contrast, no significant difference in ctDNA levels was observed across treatment centers (median: 2.3 log hGE/mL; Fig 1D), despite significant variations of the timepoint that blood samples were taken relative to the initiation of therapy (P = .001; Supplemental Fig S3 [Data Supplement]). Importantly, 80% of pretreatment blood samples evaluated for ctDNA were collected within 7 days of therapy, and we did not observe a strong correlation between DTI and the sample-to-treatment interval (RP = 0.12).
FIG 1.
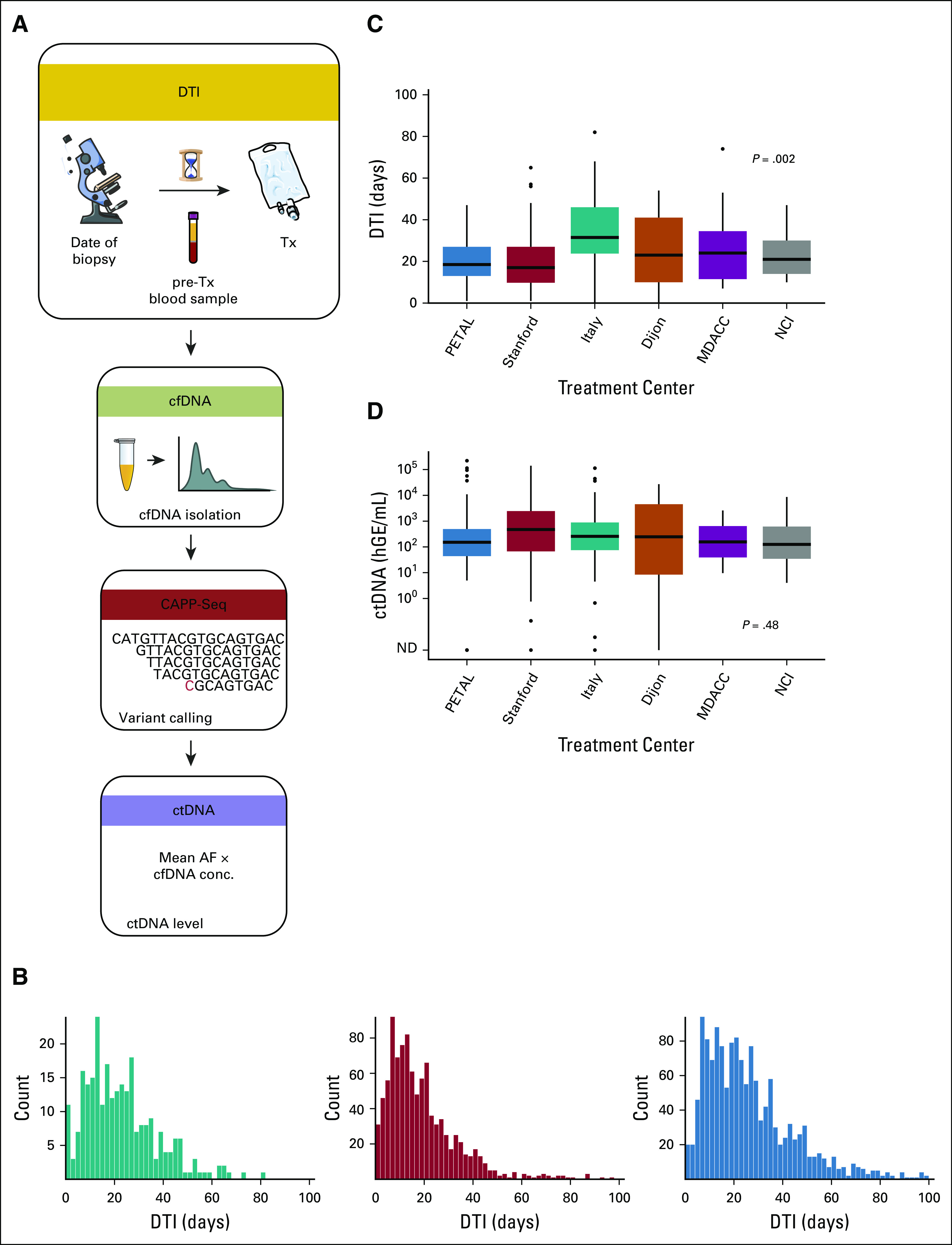
Study and cohort description. (A) Study description: pretreatment levels of ctDNA from 267 patients with pathologic diagnosis of a DLBCL were measured using CAPP-Seq and correlated with the DTI. ctDNA levels were calculated as product of total cfDNA and mean AF of tumor-derived mutations detected in the blood. (B) Distribution of DTI in this study (green) and two previously reported studies (MER [red] and LYSA [blue]).1 (C) DTI and (D) pretreatment ctDNA levels across treatment centers. P values are derived from one-way ANOVA on ranks. AF, allelic fraction; ANOVA, analysis of variance; CAPP-Seq, cancer personalized profiling by deep sequencing; cfDNA, cell-free DNA; ctDNA, circulating tumor-derived DNA; DLBCL, diffuse large B-cell lymphoma; DTI, diagnosis-to-treatment interval; hGE, haploid genome equivalents; LYSA, Lymphoma Study Association; MER, Molecular Epidemiology Resource; ND, not detected; Tx, treatment.
TABLE 1.
Patient Characteristics
DTI Is Associated With Tumor Burden
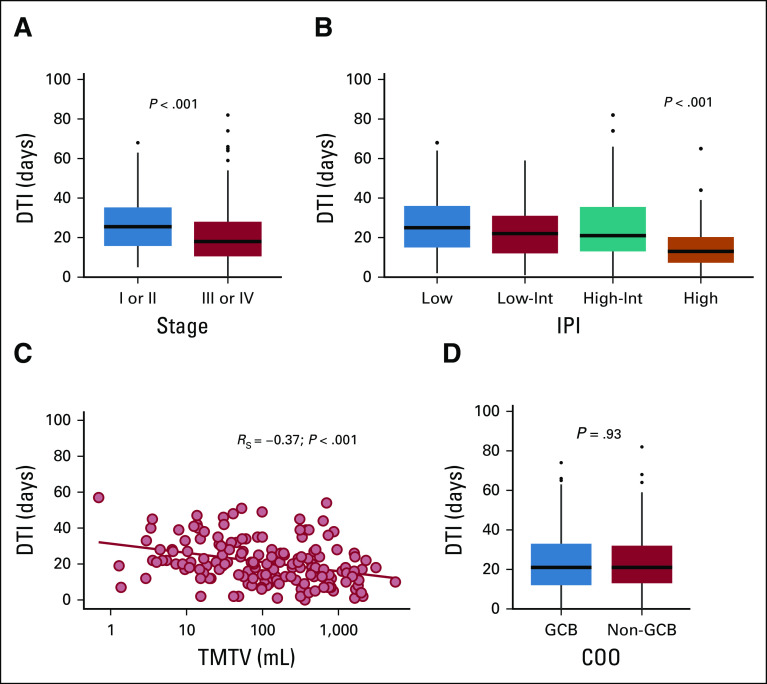
To validate and extend the findings of Maurer et al,1 we correlated DTI with clinical risk factors. Patients with advanced-stage disease (Ann Arbor stage III or IV) had shorter DTI than patients with limited stage (median 18 days v 26 days; P < .001; Fig 2A). Similarly, higher IPI was associated with shorter DTI (median: low, 25 days; low-intermediate [low-int], 22 days; high-int, 21 days; high, 13 days; P < .001; Fig 2B). Higher TMTV was significantly correlated with shorter DTI (RS = −0.37; P < .001, Fig 2C). No significant difference in DTI was found between GCB and non-GCB COO subtypes (21 days v 21 days; P = .93; Fig 2D). Similarly, we did not observe a significant association between mutations in individual genes and DTI (data not shown).
FIG 2.
Correlation of the DTI with clinical variables. DTI by (A) stage, (B) IPI, (C) TMTV, and (D) COO subtype. COO subtype was inferred from DNA genotypes from CAPP-Seq.6,13 COO subtypes were visualized from 218 patients after exclusion of featureless specimens. P values of categorical comparisons are derived from Mann-Whitney U test or one-way ANOVA on ranks. Spearman correlation was used to assess association between DTI and TMTV. ANOVA, analysis of variance; CAPP-Seq, cancer personalized profiling by deep sequencing; COO, cell-of-origin; DTI, diagnosis-to-treatment interval; GCB, germinal center B-cell subtype; IPI, International Prognostic Index; TMTV, total metabolic tumor volume.
Pretreatment ctDNA Levels Strongly Correlate With Tumor Burden
We next correlated pretreatment ctDNA levels with clinical risk factors to validate previous findings from our group7 in this larger patient cohort. Patients with advanced-stage disease had significantly higher ctDNA levels (median 2.5 v 1.8 log hGE/mL; P < .001; Fig 3A). Also, IPI was strongly associated with ctDNA levels (median log hGE/mL: low, 1.8; low-int, 2.2; high-int, 2.5; high, 3.4; P < .001; Fig 3B). Moreover, ctDNA levels and TMTV were highly correlated (RS = 0.6, P < .001; Fig 3C). In contrast, when analyzing total cfDNA levels, wherein we did not account for which molecules were tumor-derived as in ctDNA measurements, we observed a weaker correlation with tumor burden (RS = 0.25; P = .002; Supplemental Fig S4 [Data Supplement]). No significant difference in ctDNA levels was observed between COO subgroups (P = .61; Fig 3D) or individual gene mutations (data not shown). Supplemental Figure S5 (Data Supplement) summarizes the results separating the newly profiled patients from those previously reported.7
FIG 3.
Correlation of pretreatment ctDNA levels with clinical variables. Pretreatment ctDNA level by (A) stage, (B) IPI, (C) TMTV, and (D) COO subtype. COO subtype was inferred from DNA genotypes from CAPP-Seq.6,13 COO subtypes were visualized from 218 patients after exclusion of featureless specimens. P values of categorical comparisons are derived from Mann-Whitney U test or one-way ANOVA on ranks, respectively. Spearman correlation was used to assess association between ctDNA and TMTV. ANOVA, analysis of variance; CAPP-Seq, cancer personalized profiling by deep sequencing; COO, cell-of-origin; ctDNA, circulating tumor-derived DNA; GCB, germinal center B-cell subtype; hGE, haploid genome equivalents; IPI, International Prognostic Index; ND, not detected; TMTV, total metabolic tumor volume.
Pretreatment ctDNA Levels Independently Predict Short DTI
Since our data demonstrate that both DTI and pretreatment ctDNA levels reflect disease burden, we investigated the relationship between these two risk factors. Interestingly, we found that short DTI was associated with higher levels of ctDNA (median log hGE/mL: DTI ≤ 14 days 2.9 v DTI > 14 days 2.1, P < .001; RS = −0.33, P < .001; Fig 4A). In univariable logistic regression, ctDNA and IPI individually predicted short DTI (odds ratios, OR [95% CI] ctDNA: 1.9 [1.5 to 2.5], P < .001; IPI: 1.4 [1.2 to 1.7], P < .001). Of note, ctDNA retained its statistical significance in multivariable analysis (OR, 1.7 [1.3 to 2.3], P < .001; Fig 4B and Supplemental Table S3 [Data Supplement]).
FIG 4.
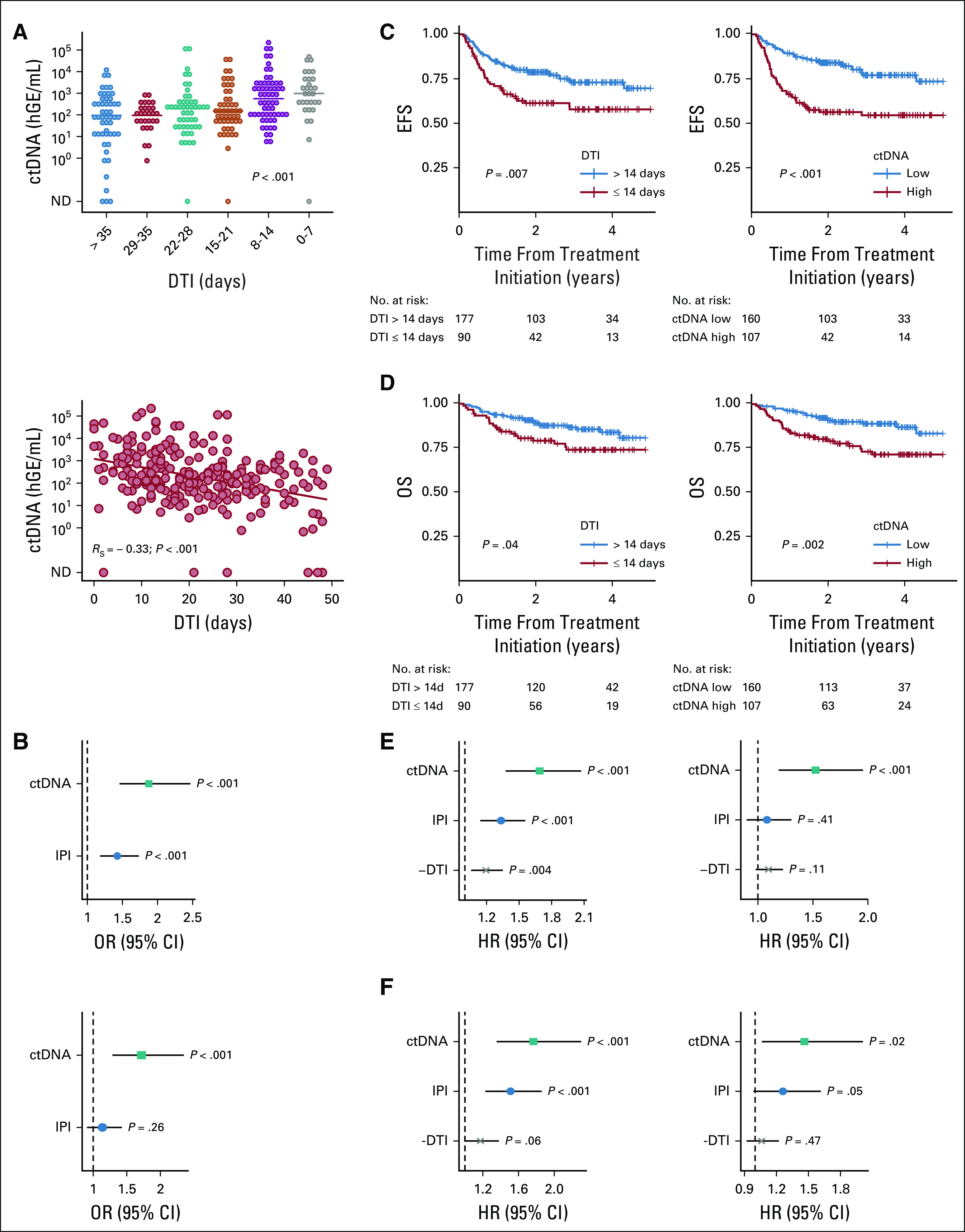
Association between DTI and ctDNA, and EFS and OS. (A) Pretreatment ctDNA levels by DTI in (top) weekly bins and as (bottom) continuous variable. P values are derived from one-way ANOVA on ranks and Spearman correlation, respectively. (B) (Top) Univariable and (bottom) multivariable logistic regression to predict short DTI (≤ 14 days). (C) Kaplan-Meier curve and log-rank P value for EFS in years by (left) DTI binarized at 14 days and (right) pretreatment ctDNA level binarized at 2.5 log hGE/mL.7 (D) Kaplan-Meier curve and log-rank P value for OS in years by (left) DTI binarized at 14 days and (right) pretreatment ctDNA level binarized at 2.5 log hGE/mL.7 (E) (Left) Univariable and (right) multivariable Cox regression to predict EFS. (F) (Left) Univariable and (right) multivariable Cox regression to predict OS. For regression analyses, ctDNA was used in log space, IPI as score ranging from 0 to 5, and DTI per week increment. ANOVA, analysis of variance; ctDNA, circulating tumor-derived DNA; DTI, diagnosis-to-treatment interval; EFS, event-free survival; hGE, haploid genome equivalents; HR, hazard ratio; IPI, International Prognostic Index; ND, not detected; OR, odds ratio; OS, overall survival.
Pretreatment ctDNA Levels Independently Predict Survival
We confirmed that short DTI is associated with inferior EFS (P = .007; Fig 4C) and OS (P = .04; Fig 4D). Similarly, when binarizing pretreatment ctDNA levels at a previously defined threshold,7 patients with high levels of ctDNA had significantly shorter EFS (P < .001; Fig 4C) and OS (P = .002; Fig 4D). Importantly, the threshold for ctDNA binarization validated when restricting the analysis to patients who were not part of the training set (Supplemental Fig S6 [Data Supplement]).
We then investigated the impact of DTI and ctDNA as continuous variables on treatment outcomes. In univariable Cox regression, ctDNA levels, IPI, and DTI were individually prognostic of EFS (hazard ratio, HR ctDNA: 1.7 [1.4 to 2.1], P < .001; IPI: 1.3 [1.1 to 1.6], P < .001; −DTI: 1.2 [1.1 to 1.4], P = .004), whereas in multivariable analysis, only ctDNA levels remained significantly associated with EFS (HR ctDNA: 1.5 [1.2 to 2.0], P < .001; IPI: 1.1 [0.9 to 1.3], P = .41; −DTI: 1.1 [1.0 to 1.2], P = .11; Fig 4E and Supplemental Table S4 [Data Supplement]). In corresponding analyses for OS, ctDNA, IPI, and DTI were prognostic in the univariable setting (HR ctDNA: 1.7 [1.3 to 2.3], P < .001; IPI: 1.5 [1.2 to 1.9], P < .001; −DTI: 1.2 [1.0 to 1.4], P = .06) and again only ctDNA level remained significant in multivariable Cox regression (HR ctDNA: 1.4 [1.0 to 2.0], P = .02; IPI: 1.3 [1.0 to 1.6], P = .05; −DTI: 1.1 [0.9 to 1.2], P = .47; Fig 4F and Supplemental Table S4 [Data Supplement]). The independent prognostic impact of ctDNA levels on EFS held up when adding TMTV to the multivariable model in the subset of patients evaluable for all risk factors (Supplemental Fig S7 and Tables S3-S4 [Data Supplement]).
Prognostic Impact of ctDNA and DTI in De Novo DLBCL Treated With R-CHOP
Finally, we validated key findings reported above in the subset of patients with de novo DLBCL treated with R-CHOP. DTI and ctDNA levels by cohort are depicted in Figures 5A and 5B. Similar to the full cohort, both DTI and ctDNA were associated with conventional measures of tumor burden, as shown for TMTV and DTI (RS = −0.37; P < .001; Fig 5C) as well as the IPI and ctDNA (P < .001; Fig 5D). DTI was correlated with ctDNA (RS = −0.28; P < .001; Fig 5E), and pretreatment ctDNA levels predicted short DTI independent of the IPI (OR, 1.8 [1.3 to 2.5], P < .001 [univariable] and OR, 1.6 [1.1 to 2.3], P = .01 [multivariable]; Fig 5F and Supplemental Table S5 [Data Supplement]). Univariable and multivariable Cox regressions confirmed the independent prognostic associations between pretreatment ctDNA levels and EFS (univariable: HR, 1.8 [1.3 to 2.3], P < .001 and multivariable: HR, 1.5 [1.1 to 2.1], P = .01; Fig 5G and Supplemental Table S6 [Data Supplement]), approaching significance in a multivariable model for OS (HR, 1.4 [1.0 to 2.0], P = .08; Fig 5H and Supplemental Table S6 [Data Supplement]). Consistent results from Cox regressions were obtained when adjusting for treatment center (Supplemental Fig S8 and Table S7 [Data Supplement]). In ROC analyses for EFS, ctDNA had a higher area under the curve than DTI, both in the full cohort and in the de novo DLBCL subset (0.67 v 0.59; P = .049 [full cohort] and 0.70 v 0.58; P = .027 [DLBCL/R-CHOP], respectively; Supplemental Fig S9 [Data Supplement]).
FIG 5.
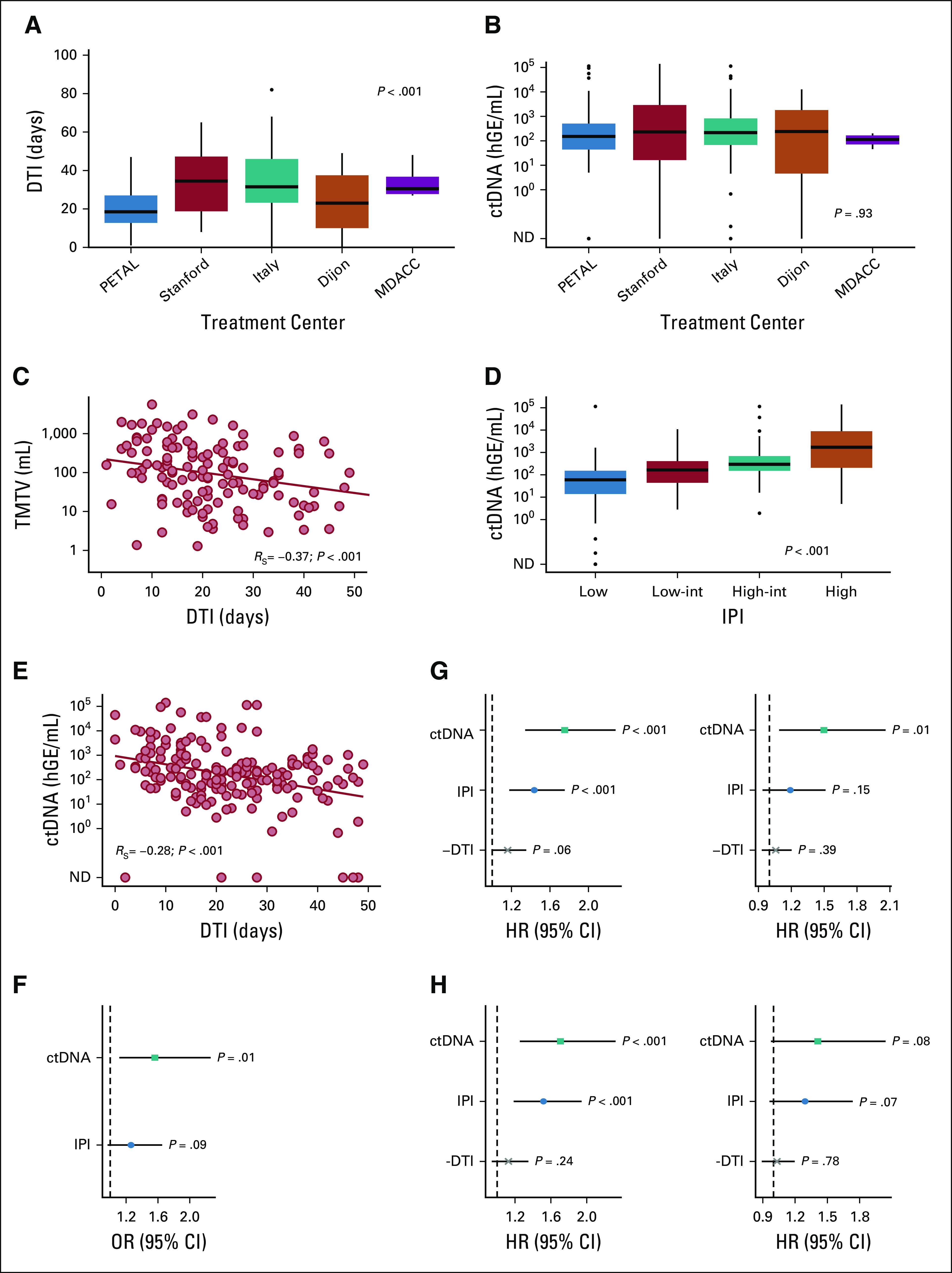
DTI, ctDNA, and their impact on EFS and OS in patients with de novo DLBCL treated with R-CHOP. (A) DTI and (B) pretreatment ctDNA levels across treatment centers. (C) DTI by TMTV. (D) Pretreatment ctDNA levels by IPI. (E) Pretreatment ctDNA levels by DTI as continuous variable. (F) Multivariable logistic regression to predict short DTI (≤ 14 days). (G) (Left) Univariable and (right) multivariable Cox regression to predict EFS. (H) (Left) Univariable and (right) multivariable Cox regression to predict OS. P values of categorical comparisons are derived from one-way ANOVA on ranks. Spearman correlation was used to assess the association between continuous variables. For regression analyses, ctDNA was used in log space, IPI as score ranging from 0 to 5, and DTI per week increment. One hundred eighty-five patients with de novo DLBCL treated with R-CHOP were included in all analyses not including TMTV, whereas TMTV was evaluable in a subset of 132 patients. ANOVA, analysis of variance; ctDNA, circulating tumor-derived DNA; DLBCL, diffuse large B-cell lymphoma; DTI, diagnosis-to-treatment interval; EFS, event-free survival; hGE, haploid genome equivalents; HR, hazard ratio; IPI, International Prognostic Index; ND, not detected; OR, odds ratio; OS, overall survival; R-CHOP, rituximab plus cyclophosphamide, adriamycin, vincristine, and prednisone; TMTV, total metabolic tumor volume.
DISCUSSION
The subset of patients with DLBCL with aggressive disease in need of immediate therapy is likely under-represented in clinical trials, especially because the urgency of acute clinical presentations may not be compatible with the screening and consent processes required by study protocols. The resulting selection bias can in part be captured by the DTI metric, defined as time between diagnosis and treatment initiation.1,14,15 Although the simplicity of DTI makes it an attractive marker, it can be confounded by a number of clinical, biological, and logistical factors, which limits its clinical utility for patient selection. Specifically, although both aggressive disease burden and disease-unrelated pattern-of-care factors can be associated with adverse outcomes, they can have opposite relationships with DTI, resulting in delayed versus accelerated treatment initiation, respectively. Here, we demonstrate that both DTI and pretreatment ctDNA levels reflect disease burden, with short DTI being associated with higher ctDNA levels. When correlated with treatment outcomes, ctDNA levels better predict EFS than DTI and other clinical risk factors. In requiring only a single assessment, pretreatment ctDNA levels may therefore be a more objective measure of clinical urgency and disease aggressiveness than DTI.
Improving on R-CHOP for unselected patients with newly diagnosed DLBCL has proven to be difficult. Promising results of phase II trials frequently do not translate into significant improvements of outcome in larger phase III trials.16 One potential explanation for this observation is that treatment outcomes observed in the control arms of clinical trials are often better than those expected based on historic controls, possibly because of selection bias artificially inflating survival. In DLBCL, recent randomized studies reported 2-year EFS rates between 70% and 80%17-20 which compares rather favorably with historic controls.21-23 This is particularly remarkable since two of the aforementioned trials selectively enrolled patients with activated B-cell subtype,18,19 which is generally associated with adverse prognosis.24 In fact, studies requiring extensive molecular profiling before enrollment may be particularly affected by selection bias since constraints during the initial work up which delay therapy can inherently result in the preferential inclusion of patients with favorable disease biology.1 Excluding patients with the highest unmet medical needs from clinical trials impairs the power to detect treatment benefits of novel therapeutic approaches. Hence, to assess the degree to which trials adequately capture representative populations of patients with DLBCL, objective metrics of disease are required.
The association between short DTI and survival has been demonstrated in both clinical trial cohorts and real-world populations in a large number of patients.1,14,15 Patients with short DTI have been shown to disproportionately have adverse clinical (eg, IPI, lactate dehydrogenase; Eastern Cooperative Oncology Group) and molecular features (eg, double-hit status).1,15 Importantly, Maurer et al1 found the prognostic impact of DTI to be independent of the IPI. In our study, we found a significant correlation between DTI and both stage and IPI, confirming previous findings. However, to our knowledge, our study is the first to describe an association between DTI and TMTV. We therefore conclude that DTI reflects tumor burden beyond factors contributing to the IPI.
A major challenge in using DTI for between-trial comparisons is that DTI is not only determined by disease-related factors but also influenced by disease-unrelated factors. For example, in our study population, we found significant differences in DTI between treatment centers in patients with similar ctDNA levels, indicating that DTI is heavily influenced by regional practice differences despite similar levels of tumor burden. This lack of consistency potentially hampers the clinical utility of DTI as risk factor and could impair its use as a tool to make comparisons between clinical trials. Moreover, since DTI is inherently affected by subjective judgments of treating providers, it cannot be objectively used to prospectively stratify patients. Indeed, active decisions to delay therapy in patients with nonacute clinical presentation can pose medical and ethical challenges for patient selection using DTI.
ctDNA is an emerging biomarker in B-cell lymphoma. Pretreatment ctDNA levels have been shown to reflect tumor burden and predict treatment outcomes.6-8,25 In this study, we validated previous findings that ctDNA levels are significantly correlated with stage, IPI, and TMTV, suggesting that ctDNA is an objective measure of tumor burden. Interestingly, we also demonstrated that short DTI is associated with higher levels of ctDNA and that ctDNA predicts short DTI independent of the IPI. Although this generally held true when adding TMTV to the multivariable model, these data need to be interpreted with caution since TMTV was only evaluable in a subset of cases.
In this study, we independently validated a previously defined threshold to discriminate high versus low pretreatment ctDNA levels7 with prognostic impact for both EFS and OS. In univariate Cox regressions, ctDNA, DTI, and IPI were each separately prognostic of EFS. Of note, in analogy to findings from Maurer et al,1 DTI retained its prognostic impact on EFS when only adjusting for the IPI. However, in multivariable analysis including all three indices, only ctDNA retained its prognostic significance, demonstrating its value as an independent risk factor. Importantly, we confirmed the prognostic impact of ctDNA on EFS in patients with de novo DLBCL treated with standard R-CHOP.
The fact that our cohort consists of patients treated across different centers, instead of a homogenous cohort treated within a single prospective clinical trial, may serve as a limitation of this study. However, this strategy also mimics the real-world issues in the management of DLBCL, wherein different centers have different standards for clinical workup that can affect DTI as well as blood collection and therapy. Our results therefore demonstrate the robustness of pretreatment ctDNA levels as biomarker in DLBCL in this context.
Based on our findings, pretreatment ctDNA levels may be used to both detect and avoid selection biases within clinical trials. ctDNA is a robust and objective metric using a single assessment blinded to time-based appraisals and can therefore be used to compare average disease burden between study arms and between populations of subjects in different clinical trials. Moreover, ctDNA may guide patient stratification within clinical trials, where allocation to study arms based on high versus low ctDNA tumor burden could be imagined. Such an approach could prevent the preferential inclusion of patients with favorable treatment outcomes and help to make clinical trial cohorts more representative of real-world patients. As an alternative or complementary approach, tumor burden assessments by positron emission tomography (TMTV) could also potentially be used to achieve similar goals. Future clinical trials should therefore compare, and possibly combine, both methods.13
Although multiple independent studies have demonstrated the promise of ctDNA detection for evaluating risk and measuring responses in lymphoma,6-8,26-28 these studies have generally involved retrospective analyses of prospectively collected specimens. Although this is useful for assay development, the field now faces significant logistic hurdles toward implementation of such ctDNA assays for prospective use, including real-time sample processing and reporting, to enable the use of standardized ctDNA levels as integral biomarkers to address prespecified hypotheses.29 Furthermore, although various groups have reported on the utility of ctDNA quantitation in B-cell lymphomas,6-8,26-28 existing assays have targeted different portions of the genome using distinct methodologies and comparisons between groups and methods remain lacking. An important next step will involve efforts to harmonize and standardize ctDNA profiling,30 similar to work done in leukemias.31,32
Collectively, our data suggest that ctDNA more objectively measures disease burden than DTI and could therefore have immediate utility to quantify potential selection biases in prospective DLBCL clinical trials.
David M. Kurtz
Stock and Other Ownership Interests: Foresight Diagnostics
Consulting or Advisory Role: Roche Molecular Diagnostics, Genentech
Patents, Royalties, Other Intellectual Property: Patent filings on ctDNA detection and methods for treatment selection based on statistical frameworks, assigned to Stanford University
Ulrich Dührsen
Honoraria: Roche Pharma AG, Janssen-Cilag, Novartis, Amgen, CPT Cellex Patient Treatment GmbH
Consulting or Advisory Role: Takeda, Gilead Sciences, Abbvie
Research Funding: Celgene
Travel, Accommodations, Expenses: Abbvie, Janssen-Cilag
Andreas Hüttmann
Honoraria: Takeda
Consulting or Advisory Role: Takeda
Travel, Accommodations, Expenses: Takeda, Roche Pharma AG
Christine Schmitz
Research Funding: Novartis
Travel, Accommodations, Expenses: Abbvie
Brian J. Sworder
Stock and Other Ownership Interests: Allogene Therapeutics
Patents, Royalties, Other Intellectual Property: Dr Sworder has patents related to methods of resistance to immunotherapy for lymphoma
Barzin Y. Nabet
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Patents, Royalties, Other Intellectual Property: Dr Nabet has a patent pending related to immunomodulatory nucleic acids, Provisional patent related to noninvasive means to predict immune checkpoint blockade outcomes
Olivier Casasnovas
Honoraria: Roche/Genentech, Takeda, Gilead Sciences, Bristol-Myers Squibb, Merck, Abbvie, Celgene, Janssen, Amgen
Consulting or Advisory Role: Roche/Genentech, Takeda, Gilead Sciences, Bristol-Myers Squibb, Merck, Abbvie, Celgene, Janssen, Incyte
Research Funding: Roche/Genentech, Gilead Sciences, Takeda
Travel, Accommodations, Expenses: Roche/Genentech, Takeda, Gilead Sciences, Janssen
Jason R. Westin
Consulting or Advisory Role: Novartis, Celgene, Genentech/Abbvie, Kite/Gilead, Janssen Scientific Affairs, Amgen, MorphoSys, Juno Therapeutics, Curis
Research Funding: Celgene, Janssen, Genentech, Novartis, Kite/Gilead, Bristol-Myers Squibb, Curis
Gianluca Gaidano
Honoraria: Janssen, Abbvie, Sunesis Pharmaceuticals, AstraZeneca
Consulting or Advisory Role: Janssen, Abbvie, AstraZeneca, Sunesis Pharmaceuticals
Speakers' Bureau: Janssen, Abbvie
Travel, Accommodations, Expenses: Janssen, Amgen
Davide Rossi
Honoraria: Abbvie, Janssen, AstraZeneca
Research Funding: Abbvie, Janssen, AstraZeneca
Michel Meignan
Honoraria: Roche
Travel, Accommodations, Expenses: Roche
Maximilian Diehn
Stock and Other Ownership Interests: CiberMed, Foresight Diagnostics
Consulting or Advisory Role: Roche, AstraZeneca, Illumina, Reflexion Medical, Gritstone Oncology, BioNTech AG, Novartis, Genentech
Research Funding: Varian Medical Systems, Illumina
Patents, Royalties, Other Intellectual Property: Patent filings on ctDNA detection assigned to Stanford University, Patent filings on tumor treatment resistance mechanisms assigned to Stanford University
Travel, Accommodations, Expenses: Roche, Varian Medical Systems, AstraZeneca
Ash A. Alizadeh
Leadership: Lymphoma Research Foundation
Stock and Other Ownership Interests: CiberMed, CAPP Medical, Forty Seven, Foresight Diagnostics
Honoraria: Roche, Janssen Oncology
Consulting or Advisory Role: Celgene, Roche/Genentech, Gilead Sciences, Cibermed, Foresight Diagnostics
Research Funding: Celgene
Patents, Royalties, Other Intellectual Property: Patent filings on ctDNA detection, assigned to Stanford University
Travel, Accommodations, Expenses: Roche, Gilead Sciences
No other potential conflicts of interest were reported.
Listen to the podcast by Dr Maurer at jcopodcast.libsynpro.com
PRIOR PRESENTATION
Presented at the 2019 Annual Meeting of the American Society of Hematology, Orlando, FL, December 7-10, 2019 (abstract 627).
SUPPORT
Supported by the National Cancer Institute (R01CA188298 and R01CA233975 to M.D. and A.A.A., 1-K08-CA241076-01 to D.M.K.), the US National Institutes of Health Director's New Innovator Award Program (1-DP2-CA186569 to M.D.), the US National Institutes of Health, the Virginia and D.K. Ludwig Fund for Cancer Research (M.D. and A.A.A.), the CRK Faculty Scholar Fund (M.D.), the Bakewell Foundation (M.D. and A.A.A.), the Damon Runyon Cancer Research Foundation (PST#09-16 to D.M.K.), the SDW/DT and Shanahan Family Foundations (A.A.A.), and Stand Up To Cancer (M.D. and A.A.A.). The PETAL trial was supported by grants from the Deutsche Krebshilfe (107592, 110515). S.A. was funded by a Dr Mildred Scheel postdoctoral fellowship of the Deutsche Krebshilfe (57406718). A.A.A. is a Scholar of The Leukemia & Lymphoma Society.
S.A., C.W.M. and D.M.K. contributed equally to this work as first authors. M.M., M.D., and A.A.A. contributed equally as senior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Stefan Alig, Charles W. Macaulay, David M. Kurtz, Michel Meignan, Maximilian Diehn, Ash A. Alizadeh
Financial support: Ash A. Alizadeh
Administrative support: Ash A. Alizadeh
Provision of study materials or patients: David M. Kurtz, Ulrich Dührsen, Andreas Hüttmann, Gianluca Gaidano, Davide Rossi, Mark Roschewski, Wyndham H. Wilson, Ash A. Alizadeh
Collection and assembly of data: Stefan Alig, Charles W. Macaulay, David M. Kurtz, Ulrich Dührsen, Andreas Hüttmann, Michael C. Jin, Brian J. Sworder, Joanne Soo, Alexander F. M. Craig, Olivier Casasnovas, Gianluca Gaidano, Davide Rossi, Wyndham H. Wilson, Michel Meignan, Ash A. Alizadeh
Data analysis and interpretation: Stefan Alig, Charles W. Macaulay, David M. Kurtz, Christine Schmitz, Brian J. Sworder, Andrea Garofalo, Mohammad Shahrokh Esfahani, Barzin Y. Nabet, Florian Scherer, Olivier Casasnovas, Jason R. Westin, Mark Roschewski, Wyndham H. Wilson, Michel Meignan, Maximilian Diehn, Ash A. Alizadeh
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Short Diagnosis-to-Treatment Interval Is Associated With Higher Circulating Tumor DNA Levels in Diffuse Large B-Cell Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David M. Kurtz
Stock and Other Ownership Interests: Foresight Diagnostics
Consulting or Advisory Role: Roche Molecular Diagnostics, Genentech
Patents, Royalties, Other Intellectual Property: Patent filings on ctDNA detection and methods for treatment selection based on statistical frameworks, assigned to Stanford University
Ulrich Dührsen
Honoraria: Roche Pharma AG, Janssen-Cilag, Novartis, Amgen, CPT Cellex Patient Treatment GmbH
Consulting or Advisory Role: Takeda, Gilead Sciences, Abbvie
Research Funding: Celgene
Travel, Accommodations, Expenses: Abbvie, Janssen-Cilag
Andreas Hüttmann
Honoraria: Takeda
Consulting or Advisory Role: Takeda
Travel, Accommodations, Expenses: Takeda, Roche Pharma AG
Christine Schmitz
Research Funding: Novartis
Travel, Accommodations, Expenses: Abbvie
Brian J. Sworder
Stock and Other Ownership Interests: Allogene Therapeutics
Patents, Royalties, Other Intellectual Property: Dr Sworder has patents related to methods of resistance to immunotherapy for lymphoma
Barzin Y. Nabet
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Patents, Royalties, Other Intellectual Property: Dr Nabet has a patent pending related to immunomodulatory nucleic acids, Provisional patent related to noninvasive means to predict immune checkpoint blockade outcomes
Olivier Casasnovas
Honoraria: Roche/Genentech, Takeda, Gilead Sciences, Bristol-Myers Squibb, Merck, Abbvie, Celgene, Janssen, Amgen
Consulting or Advisory Role: Roche/Genentech, Takeda, Gilead Sciences, Bristol-Myers Squibb, Merck, Abbvie, Celgene, Janssen, Incyte
Research Funding: Roche/Genentech, Gilead Sciences, Takeda
Travel, Accommodations, Expenses: Roche/Genentech, Takeda, Gilead Sciences, Janssen
Jason R. Westin
Consulting or Advisory Role: Novartis, Celgene, Genentech/Abbvie, Kite/Gilead, Janssen Scientific Affairs, Amgen, MorphoSys, Juno Therapeutics, Curis
Research Funding: Celgene, Janssen, Genentech, Novartis, Kite/Gilead, Bristol-Myers Squibb, Curis
Gianluca Gaidano
Honoraria: Janssen, Abbvie, Sunesis Pharmaceuticals, AstraZeneca
Consulting or Advisory Role: Janssen, Abbvie, AstraZeneca, Sunesis Pharmaceuticals
Speakers' Bureau: Janssen, Abbvie
Travel, Accommodations, Expenses: Janssen, Amgen
Davide Rossi
Honoraria: Abbvie, Janssen, AstraZeneca
Research Funding: Abbvie, Janssen, AstraZeneca
Michel Meignan
Honoraria: Roche
Travel, Accommodations, Expenses: Roche
Maximilian Diehn
Stock and Other Ownership Interests: CiberMed, Foresight Diagnostics
Consulting or Advisory Role: Roche, AstraZeneca, Illumina, Reflexion Medical, Gritstone Oncology, BioNTech AG, Novartis, Genentech
Research Funding: Varian Medical Systems, Illumina
Patents, Royalties, Other Intellectual Property: Patent filings on ctDNA detection assigned to Stanford University, Patent filings on tumor treatment resistance mechanisms assigned to Stanford University
Travel, Accommodations, Expenses: Roche, Varian Medical Systems, AstraZeneca
Ash A. Alizadeh
Leadership: Lymphoma Research Foundation
Stock and Other Ownership Interests: CiberMed, CAPP Medical, Forty Seven, Foresight Diagnostics
Honoraria: Roche, Janssen Oncology
Consulting or Advisory Role: Celgene, Roche/Genentech, Gilead Sciences, Cibermed, Foresight Diagnostics
Research Funding: Celgene
Patents, Royalties, Other Intellectual Property: Patent filings on ctDNA detection, assigned to Stanford University
Travel, Accommodations, Expenses: Roche, Gilead Sciences
No other potential conflicts of interest were reported.
REFERENCES
- 1.Maurer MJ, Ghesquieres H, Link BK, et al. Diagnosis-to-Treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials J Clin Oncol 361603–16102018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lapointe-Shaw L, Abushomar H, Chen XK, et al. Care and outcomes of patients with cancer admitted to the hospital on weekends and holidays: A retrospective cohort study J Natl Compr Canc Netw 14867–8742016 [DOI] [PubMed] [Google Scholar]
- 3.Han X, Jemal A, Flowers CR, et al. Insurance status is related to diffuse large B-cell lymphoma survival Cancer 1201220–12272014 [DOI] [PubMed] [Google Scholar]
- 4.Pulte D, Jansen L, Brenner H.Survival disparities by insurance type for patients aged 15-64 years with non-Hodgkin lymphoma Oncologist 20554–5612015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flowers CR, Nastoupil LJ.Socioeconomic disparities in lymphoma Blood 1233530–35312014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scherer F, Kurtz DM, Newman AM, et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci Transl Med. 2016;8:364ra155. doi: 10.1126/scitranslmed.aai8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtz DM, Scherer F, Jin MC, et al. Circulating tumor DNA measurements as early outcome predictors in diffuse large B-cell lymphoma J Clin Oncol 362845–28532018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spina V, Bruscaggin A, Cuccaro A, et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma Blood 1312413–24252018 [DOI] [PubMed] [Google Scholar]
- 9.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. ed 4. Lyon, France: IARC; 2008. [Google Scholar]
- 10.Duhrsen U, Muller S, Hertenstein B, et al. Positron emission tomography-guided therapy of aggressive non-Hodgkin lymphomas (PETAL): A multicenter, randomized phase III trial J Clin Oncol 362024–20342018 [DOI] [PubMed] [Google Scholar]
- 11.Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage Nat Med 20548–5542014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman AM, Lovejoy AF, Klass DM, et al. Integrated digital error suppression for improved detection of circulating tumor DNA Nat Biotechnol 34547–5552016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz DM, Esfahani MS, Scherer F, et al. Dynamic risk profiling using serial tumor biomarkers for personalized outcome prediction Cell 178699–713.e192019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camus V, Dubois S, Jardin F, et al. Prognostic impact of diagnosis to treatment interval (DTI) in diffuse large B-cell lymphoma patients: A real-life monocentric study Leuk Lymphoma 60839–8412019 [DOI] [PubMed] [Google Scholar]
- 15.Szafer-Glusman E, Liu J, Peale FV, Jr, et al. A simulation analysis to evaluate the effect of prospective biomarker testing on progression-free survival (PFS) in DLBCL. Blood. 2017;130:419. [Google Scholar]
- 16.Iacoboni G, Zucca E, Ghielmini M, et al. Methodology of clinical trials evaluating the incorporation of new drugs in the first-line treatment of patients with diffuse large B-cell lymphoma (DLBCL): A critical review Ann Oncol 291120–11292018 [DOI] [PubMed] [Google Scholar]
- 17.Vitolo U, Trněný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma J Clin Oncol 353529–35372017 [DOI] [PubMed] [Google Scholar]
- 18.Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma J Clin Oncol 371285–12952019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard JP, Kolibaba KS, Reeves JA, et al. Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-Cell-Like diffuse large B-cell lymphoma J Clin Oncol 353538–35462017 [DOI] [PubMed] [Google Scholar]
- 20.Bartlett NL, Wilson WH, Jung SH, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: Clinical outcomes of the phase III intergroup trial Alliance/CALGB 50303 J Clin Oncol 371790–17992019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma N Engl J Med 346235–2422002 [DOI] [PubMed] [Google Scholar]
- 22.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: A randomised controlled trial (RICOVER-60) Lancet Oncol 9105–1162008 [DOI] [PubMed] [Google Scholar]
- 23.Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group Lancet Oncol 121013–10222011 [DOI] [PubMed] [Google Scholar]
- 24.Scott DW, Mottok A, Ennishi D, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies J Clin Oncol 332848–28562015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohers E, Viailly PJ, Becker S, et al. Non-invasive monitoring of diffuse large B-cell lymphoma by cell-free DNA high-throughput targeted sequencing: Analysis of a prospective cohort. Blood Cancer J. 2018;8:74. doi: 10.1038/s41408-018-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roschewski M, Dunleavy K, Pittaluga S, et al. Circulating tumour DNA and CT monitoring in patients with untreated diffuse large B-cell lymphoma: A correlative biomarker study Lancet Oncol 16541–5492015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkozy C, Huet S, Carlton VE, et al. The prognostic value of clonal heterogeneity and quantitative assessment of plasma circulating clonal IG-VDJ sequences at diagnosis in patients with follicular lymphoma Oncotarget 88765–87742017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtz DM, Green MR, Bratman SV, et al. Noninvasive monitoring of diffuse large B-cell lymphoma by immunoglobulin high-throughput sequencing Blood 1253679–36872015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtz DM.Prognostication with circulating tumor DNA: Is it ready for prime time? Hematology 201947–522019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi D, Spina V, Bruscaggin A, et al. Liquid biopsy in lymphoma Haematologica 104648–6522019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawstron AC, Böttcher S, Letestu R, et al. Improving efficiency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL Leukemia 27142–1492013 [DOI] [PubMed] [Google Scholar]
- 32.Zalcberg I, D'Andrea MG, Monteiro L, et al. Multidisciplinary diagnostics of chronic lymphocytic leukemia: European Research Initiative on CLL—ERIC recommendations Hematol Transfus Cell Ther 42269–2742020 [DOI] [PMC free article] [PubMed] [Google Scholar]