Abstract
Appropriate choice of vaccine vector is crucial for effective vaccine development. Rhabdoviral vectors, such as rabies virus and vesicular stomatitis virus, have been used in a variety of vaccine strategies. These viruses have small, easily manipulated genomes that can stably express foreign glycoproteins due to a well-established reverse genetics system for virus recovery. Both viruses have well-described safety profiles and have been demonstrated to be effective vaccine vectors. This review will describe how these Rhabdoviruses can be manipulated for use as vectors, their various applications as vaccines or therapeutics, and the advantages and disadvantages of their use.
Keywords: rabies virus, vesicular stomatitis virus, vaccine
Graphical Abstract
Introduction
The Rhabdoviridae family are bullet-shaped, negative-sense single-stranded RNA (ssRNA) viruses. They have a single segment genome that encodes five proteins: the nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and RNA dependent RNA polymerase (L) [1]. Two well-studied rhabdoviruses are rabies virus (RABV), from genus Lyssavirus, and vesicular stomatitis virus (VSV) from genus Vesiculovirus. While both viruses are neurotropic zoonoses, and have similar length genomes (~11–12kb) [1], they differ in their biology.
RABV infects a variety of mammals and is typically maintained in nature through mesocarnivores (e.g., dogs, coyotes, foxes, raccoons, skunks) and bats [2]. In addition to causing disease in animals, RABV also causes severe encephalitis in humans that, if untreated, is almost always fatal [3]. Prompt treatment with the rabies vaccine before the onset of symptoms avoids the disease in almost all individuals exposed to RABV [4]. The first record of rabies disease dates back to the 4th century BC [5], and while significant advances have been made to eradicate the virus, it is still prevalent in many parts of the world [2].
VSV is an arbovirus with a limited host range compared to RABV, only causing disease in cattle, horses, and swine [1,6]. Unlike RABV, which replicates slowly and does not kill the host cell [1], VSV replicates rapidly to high titers and is a lytic virus, an effect caused by the M protein, which blocks host messenger RNA (mRNA) export [7]. VSV has been shown to induce a strong interferon response [8], in contrast to RABV which evades the innate immune response [9].
Research on rhabdoviruses has benefited significantly from the development of reverse genetics systems to recover them from cDNA [10,11]. Briefly, a cDNA plasmid encoding the entire rhabdoviral anti-genome is transfected into cells along with individual plasmids encoding the N, P, and L proteins (Figure 1, 1). A T7 promoter controls the expression of the different viral genes from each plasmid. Initially, a recombinant vaccinia virus expressing a T7 RNA polymerase was utilized to express the viral genes via the T7-promotor. Currently, most investigators use plasmids expressing the T7 polymerase, under control of a cytomegalovirus (CMV) promoter, that is included in the transfection. In both cases, the T7 polymerase produces mRNAs of the individual viral proteins and a positive-sense full-length anti-genome (2). Once translated from their mRNAs, the N protein encapsulates the anti-genome and the polymerase complex composed of P and L proteins and transcribes a full-length viral genome (3). The viral genome then serves as a template for the transcription of the mRNAs as well as full-length anti-genomes (4). Viral particles can assemble after translation of these mRNAs and reverse transcription of the full-length anti-genomes (5). Figure 1 illustrates this process for RABV.
Figure 1. Rhabdoviral reverse genetics virus recovery system.
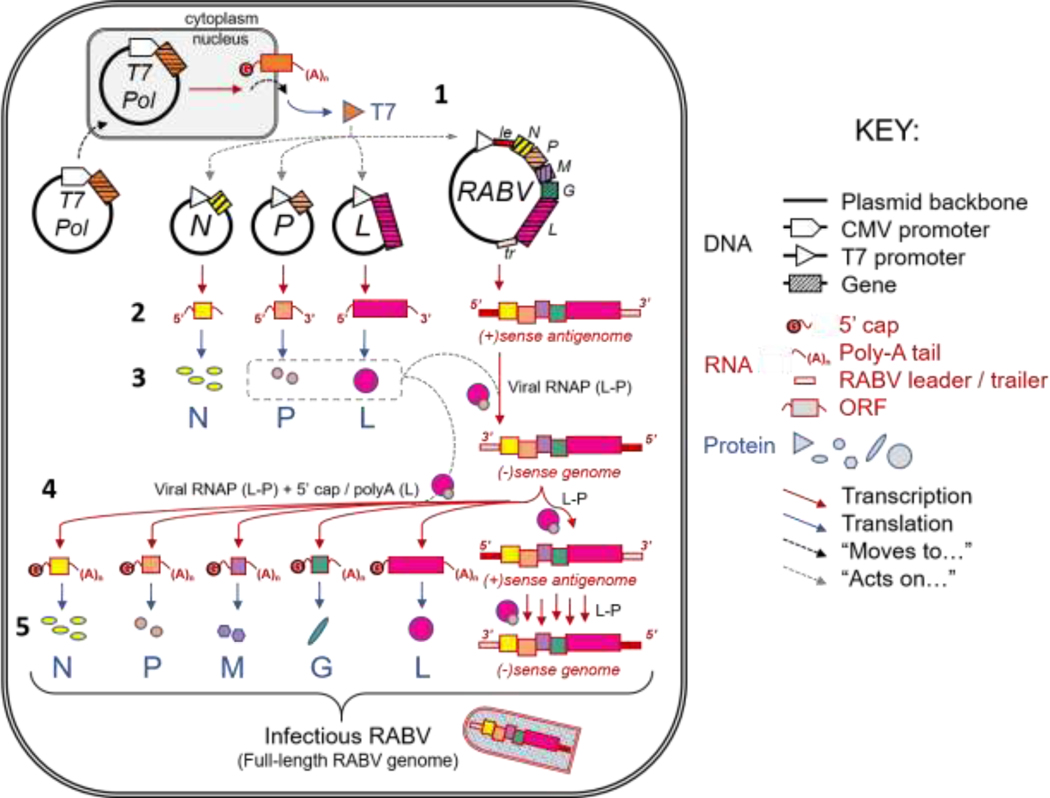
Diagram of virus recovery through cDNA reverse genetics system, exemplified by RABV. Abbreviations are defined as follows: Nucleoprotein (N), Phosphoprotein (P), Matrix Protein (M), Glycoprotein (G), RNA-dependent RNA polymerase (L), Cytomegalovirus (CMV), Open Reading Frame (ORF), T7 Polymerase (T7 Pol), RNA Polymerase (RNAP), Leader (le), Trailer (tr). Figure adapted from Davis, B.M. (unpublished).
The so-called reverse genetics system allows for manipulation of rhabdoviral genes and determination of their functions and host interactions [7,12]. It was first shown for RABV that foreign genes can be introduced into the genome and expressed utilizing short transcription start and stop signals [13] (Figure 2), and then similar findings were made for VSV [14]. A large number of foreign proteins have since been expressed from these recombinant viruses. For example, both viruses can express and incorporate the cellular receptors CD4 and CCR5 into their viral particles, allowing them to specifically target HIV-1 infected cells [15,16]. RABV and VSV can also carry foreign glycoproteins in their viral envelope that can act as a functional substitute for the rhabdoviral glycoproteins or be expressed simultaneously. Two examples are the currently U.S. Food and Drug Administration (FDA)-approved VSV-based Ebola virus vaccine [17] and a RABV expressing the Lassa Fever Virus glycoprotein complex [18].
Figure 2. Incorporation of foreign protein into Rhabdovirus.
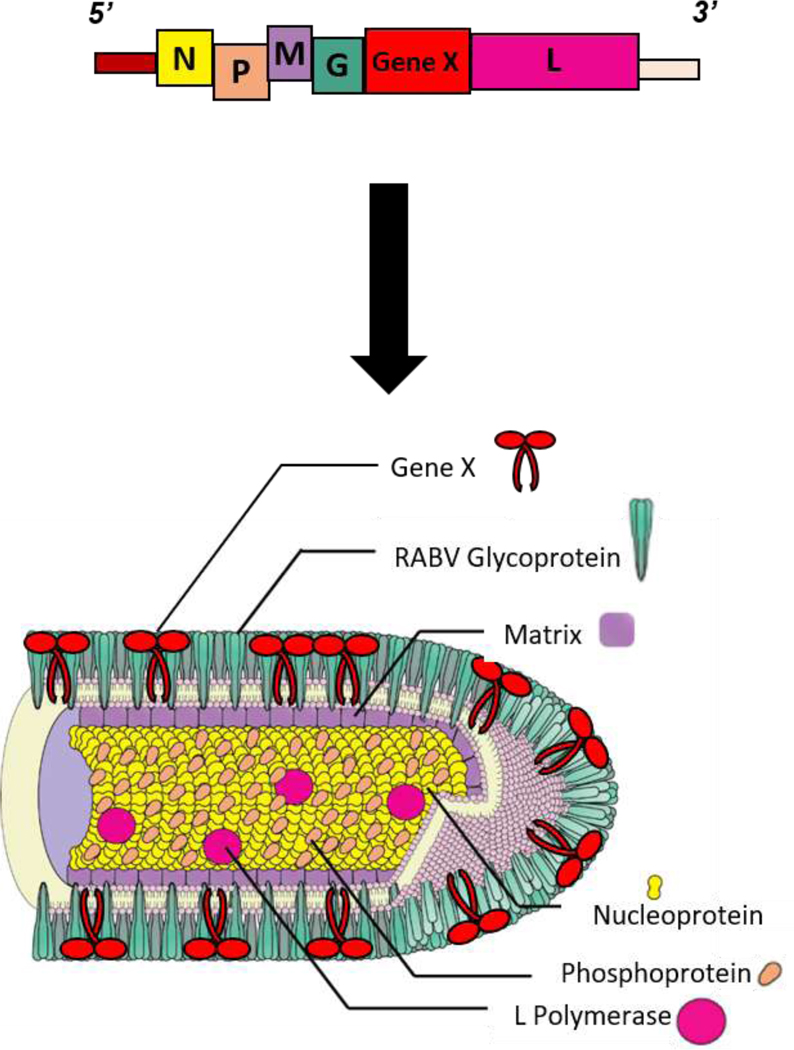
Diagram of how genetic engineering leads to foreign protein incorporation into rhabdovirus virions, exemplified by RABV. Abbreviations are defined as follows: Nucleoprotein (N), Phosphoprotein (P), Matrix Protein (M), Glycoprotein (G), RNA-dependent RNA polymerase (L). Figure adapted from Davis, B.M. et al. 2015 [184].
Because rhabdoviral genomes can be easily manipulated and can integrate foreign proteins in the viral envelope, they are excellent candidates for vaccine vectors. Examples of their use as vaccine vectors and further discussion of their advantages and disadvantages are discussed in the sections below.
Rabies Virus
The RABV vaccine has had a long history of successful use [19], however, no recombinant RABV-vectored vaccines have been developed for humans. This section will describe the history of the RABV vaccine, the qualities that support its use as a vaccine vector, and experimental vaccines that have been developed with this vector.
Human Rabies Vaccine
The first human rabies vaccine was discovered by Louis Pasteur in the 19th century and consisted of dried spinal cord from rabbits injected with RABV [20]. It protected some individuals from rabies, but it had many limitations ranging from incomplete viral inactivation to severe allergic reactions. Since then, a variety of other strategies have been tested, including other nerve tissue vaccines, such as the Semple and suckling mouse brain vaccines and avian embryo vaccines [19]. Once cell culture methods were developed, production was easier and more efficient, eventually leading to the development of four rabies vaccines that are currently World Health Organization (WHO) pre-qualified: Rabipur (purified chick embryo cell [PCEC] vaccine); Verorab (purified Vero cell rabies vaccine [PVRV]); RABIVAX-S (PVRV); and VaxiRab-N (PCEC) [21]. These vaccines are produced by infecting either chick embryo cells or Vero cells with the Flury LEP or Pitman-Moore strains of RABV, followed by virus purification and beta-propiolactone (BPL) inactivation [22–25]. All four vaccines efficiently induce rabies neutralizing antibody titers at a level considered protective, and are comparable to the previous gold standard rabies vaccine, the human diploid cell vaccine (HDCV) [4,19,26–28]. Additionally, vaccine safety has been demonstrated through use in essentially all patient populations with very few adverse effects (Reviewed for Rabipur in [29]).
Currently, human rabies vaccines are used world-wide in both pre- and post-exposure settings. Typically, rabies pre-exposure prophylaxis consists of 3 vaccine doses and is only recommended for those regularly exposed to the virus, such as laboratory workers and veterinarians [4]. Post-exposure prophylaxis (PEP) consists of 4–5 vaccine doses, and for the previously unvaccinated is typically accompanied with rabies immunoglobulin (RIG) treatment to promote immediate neutralization of the virus while the adaptive immune response to the vaccine develops [4,19,30]. When applied before symptom onset and following the proper dosing schedule, the rabies vaccine can prevent rabies disease in almost all cases [4,19,30]. Unfortunately, RABV is still prevalent in many parts of the world due to a lack of PEP accessibility [31].
Wildlife Rabies Vaccine
Rabies is maintained in nature through a variety of mammalian hosts, including bats, foxes, racoons and dogs [2]. Dogs are the major source of human rabies infections world-wide and are the major target for efforts attempting to end human disease caused by the virus [32–34]. In various studies, vaccination of at least 70% of the dog population in an endemic area effectively prevented rabies transmission [35–37]. The WHO has guidelines for carrying out campaigns to vaccinate dogs against rabies [38], which are based on similar efforts that were effective in North America, Europe, and some Latin American countries [39,40].
Dog and wildlife vaccination efforts may employ a combination of vaccine strategies, including live-attenuated, recombinant, and inactivated vaccines [4]. Typically, domestic animals are given injections of inactivated vaccines in a veterinary setting, as these vaccines pose little risk to the animal or humans in contact with it [4]. Given the difficulty and potential dangers in capturing and administering vaccine injections to wildlife, wildlife vaccination campaigns typically employ live-attenuated or recombinant oral vaccines in the form of bait [38,41,42]. The standard live-attenuated viruses used for wildlife vaccines are derived from either the Street Alabama Dufferin (SAD) strain or its derivative strain, Evelyn-Rokitnicki-Abelseth (ERA), and frequently contain an attenuating mutation at amino acid 333 in the glycoprotein [43,44]. The attenuated SAD strain is also used as a vaccine vector for other pathogens, as will be discussed in the next section.
RABV as a Vaccine Vector
Wide-spread use of modern rabies vaccines has highlighted their safety profile, ease of large-scale production, safe administration, and efficacy. Additionally, RABV shares endemic regions with several pathogens, thus increasing the potential impact of a bivalent vaccine in affected areas.
To use RABV as a vaccine vector the foreign gene of interest is typically inserted into the rabies genome and the native rabies glycoprotein (G) is either retained or removed. The rabies vectors used typically contain attenuating mutations, like the 333 amino acid mutation in RABV-G [12], or have a gene deleted to render them replication deficient (Figure 3). These attenuations ensure the viruses are safe to work with, produce, and administer [45,46]. Examples of these kinds of RABV-based vaccines include live recombinant vaccines against human immunodeficiency virus (HIV) [45] and Lagos Bat Virus [47], and a replication-deficient vaccine against lymphocytic choriomeningitis virus [48]. Live-attenuated vaccines are advantageous for the strong immune responses they induce, whereas replication-deficient viruses are safe for use in immunocompromised people because they cannot spread.
Figure 3. Schematic of replication competent vs. replication deficient rhabdoviruses.
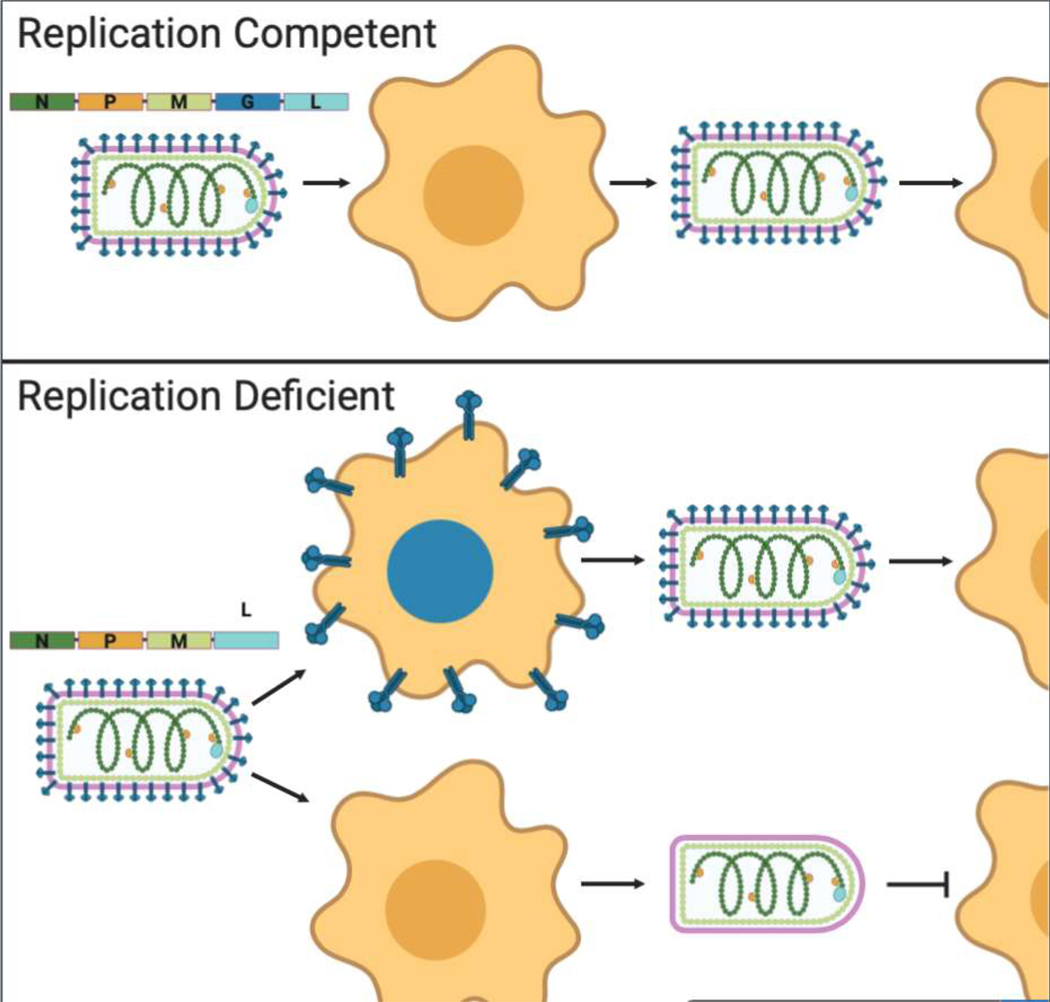
Top panel shows replication competent rhabodovirus that encodes for all five rhabdoviral proteins is able to undergo multiple rounds of infection. Bottom panel shows a replication deficient rhabdovirus whose genome does not encode for a glycoprotein. This virus can only undergo one round of infection unless the missing gene is supplemented, as illustrated by the cell expressing the blue glycoproteins. Abbreviations are defined as follows: Nucleoprotein (N), Phosphoprotein (P), Matrix Protein (M), Glycoprotein (G), RNA-dependent RNA polymerase (L). Created with BioRender.com
Inactivated RABV-based vaccines benefit from not requiring supplementation for production, unlike replication-deficient vaccines, and are safer to administer than live virus. For an inactivated vaccine to be effective, the foreign protein must incorporate into the membrane of the RABV virion. For many viral glycoproteins, no modifications are necessary to achieve this, as is the case for the Ebola virus (EBOV) [49] and Lassa fever virus glycoproteins [18]. Other proteins require modifications for successful virion incorporation. For example, Rift Valley Fever virus buds from the Golgi complex and its glycoproteins do not localize to the plasma membrane. Thus, this glycoprotein requires replacement of its transmembrane domain and cytoplasmic tail with that of RABV-G for incorporation into RABV virions [50]. Such modifications can also be applied to non-viral proteins, as was done for anthrax protective antigen (PA) [51]. Specifically, to allow the protein to integrate into RABV particles, a chimeric PA domain 4 protein was engineered to contain a signal sequence and the proximal 51 amino acids of the RABV-G ectodomain along with the RABV-G transmembrane domain and cytoplasmic tail. Table 1 lists experimental RABV-vectored vaccine strategies.
Table 1. RABV-based vaccine candidates.
Vaccines are listed chronologically, by the type of vaccine and the target antigen used.
| Vaccine Type | Vaccine Target | Refs. |
|---|---|---|
| Live attenuated and inactivated | Severe Acute Respiratory Syndrome Coronavirus 2 S1 Spike Protein | [52] |
| Inactivated | Rift Valley Fever Virus Glycoprotein | [50] |
| Inactivated | Marburg Virus Glycoprotein | [94] |
| Inactivated | Nipah Virus Glycoprotein | [95] |
| Inactivated | Lassa Fever Virus Glycoprotein | [18] |
| Replication-deficient | Lymphocytic Choriomeningitis Virus Glycoprotein | [48] |
| Live attenuated | Lagos Bat Virus Glycoprotein | [47] |
| Inactivated | Middle East Respiratory Syndrome Coronavirus Spike Protein | [96] |
| Inactivated | Canine Distemper Virus Glycoproteins | [97] |
| Live attenuated and inactivated | Canine Parvovirus Virion Protein 2 | [98] |
| Live attenuated and inactivated | Hendra Virus Glycoprotein | [92] |
| Live attenuated | Canine Distemper Virus Hemagglutinin Protein | [99] |
| Inactivated | Botulinum Neurotoxins, serotypes /A, /B and /E | [100,101] |
| Live attenuated and inactivated | Ebola Virus Glycoprotein | [49,102–105] |
| Live attenuated | Gonadotropin-Releasing Hormone (Immunocontraception) | [106] |
| Inactivated | Anthrax Protective Antigen | [51] |
| Live attenuated | Severe Acute Respiratory Syndrome Coronavirus Spike Protein | [107] |
| Replication-deficient | Simian Immunodeficiency Virus Env Protein | [108] |
| Live attenuated | Simian Immunodeficiency Virus GagPol Proteins | [109] |
| Live attenuated | Simian Immunodeficiency Virus Gag Protein | [110] |
| Live attenuated | Simian-Human Immunodeficiency Virus Env Protein | [110] |
| Live attenuated | HIV Env Protein | [111,112] |
| Live attenuated | HIV Chimeric Env Protein (gp120/gp41) | [113] |
| Live attenuated | HIV Gag-Pol or Gag-Pol and Env Proteins | [114] |
| Live attenuated and inactivated | Hepatitis C Envelope Proteins | [115] |
| Live attenuated | HIV Gag Protein | [45,112,116–118] |
| Inactivated | Rabies, Mokola and European bat lyssavirus 1 glycoproteins | [119] |
| Replication-deficient | Mokola Virus Glycoprotein | [120] |
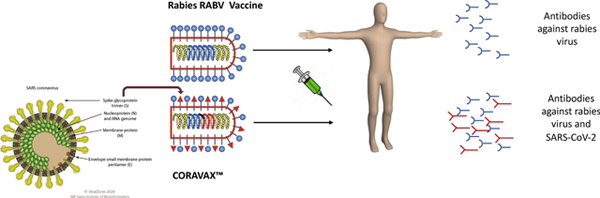
In response to the current Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) outbreak, one group has developed an inactivated recombinant rabies-based vaccine against SARS-CoV-2 [52]. The vaccine, CORAVAX™ uses the S1 domain of the SARS-CoV-2 spike protein that, as described above for the anthrax PA, was engineered to contain a RABV ectodomain, transmembrane domain and cytoplasmic tail for incorporation into RABV virions [52]. CORAVAX™ was shown to induce neutralizing antibodies in mice as both a live and inactivated vaccine [52]. This strategy is illustrated in the graphical abstract. Additional studies will be needed to determine whether this vaccine is protective against SARS-CoV-2 challenge and effective in humans.
Limitations
While there are many advantages to using RABV as a vaccine vector, there are some special considerations for their use. In particular, even though live RABV vaccine vectors are extremely safe, their use will be difficult because all risks must be eliminated before they can be applied in healthy populations. Additionally, RABV-G is highly immunogenic [53], and while this is ideal for rabies vaccines, the immune response to RABV-G could potentially interfere with the response to the foreign protein. In the case of foreign viral glycoproteins, this issue of RABV-G immunodominance can be avoided by removing RABV-G from the genome [18]. However, this would likely not be possible with other kinds of proteins, since glycoproteins are necessary for propagation of the virus.
Vesicular Stomatitis Virus
Since the establishment of the reverse genetics system, VSV has been used to develop biologic assays to study many different pathogens. Recently, the first recombinant VSV-based vaccine was approved for use in humans by the FDA [17], paving the way for future therapeutics using this platform. This section will discuss the numerous uses of VSV as a vector to study and treat various diseases.
VSV as a Tool
VSV vectors have a variety of applications. A common use is as a vector for the production of pseudoviruses, where foreign glycoproteins are incorporated into VSV virions either through genetic manipulation or infection of cells expressing a foreign glycoprotein with VSV lacking its native glycoprotein gene [54]. These pseudoviruses have been employed in virus neutralization assays [18,55], surrogate challenge viruses [18], and studies of foreign glycoprotein mediation of attachment and entry [56–58]. Since VSV is a biosafety level 2 (BSL-2) pathogen, VSV pseudoviruses are used in this way for studying BSL-4 restricted viruses.
The glycoprotein of VSV (VSV-G), has also been incorporated onto other viruses because of its stability and broad tissue and host tropism [59,60]. Specifically, VSV-G has been used to produce stable retro- and lentiviruses with better transduction efficacy for various applications, including gene therapies [61,62]. Another use of VSV-G is the preparation of virosomes (essentially VSV-G coated vesicles) for delivery of several therapeutic agents, such as antibodies and DNA, directly into cells [63,64]. These strategies employ VSV-G to deliver genome editing machinery and specific genes directly to cells therapeutically, most notably as anti-cancer treatments [65–67].
While the above-mentioned applications of VSV are widely used, the more well-known therapeutic uses of VSV are as a vaccine vector and anti-cancer oncolytic virus, both of which will be discussed in detail in the sections below.
VSV as a Vaccine Vector
In addition to the ability of VSV to stably express foreign genes [14], the virus is also advantageous as a vaccine vector because it does not typically cause disease in humans and can be produced efficiently due to its fast replication and high titers in tissue culture. Most vaccine strategies employ VSV as a replication competent vector and either insert a foreign gene into the VSV genome with the addition of vector attenuating mutations, or replace the native VSV-G with a foreign glycoprotein. This vector has been used to develop an assortment of experimental vaccines, including those against pulmonary tuberculosis [68], HIV [69], and most successfully, EBOV [70]. A more comprehensive list of VSV-vectored vaccine candidates can be found in Table 2.
Table 2. VSV-based vaccines candidates.
Vaccines are listed chronologically, stating the type of vaccine and the target antigen used.
| Vaccine Type | Vaccine Target | Refs. |
|---|---|---|
| Live attenuated | Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein | [85,86] |
| Live attenuated | Andes Virus and Sin Nombre Virus Glycoproteins | [121] |
| Live attenuated | Crimean-Congo Hemorrhagic Fever Virus Glycoprotein | [122] |
| Live attenuated | Porcine Epidemic Diarrhea Virus Spike Protein | [123] |
| Live attenuated | Ebola Virus Glycoprotein and Zika Virus Pre- Membrane and Envelope Proteins or Pre-Membrane and Soluble Envelope Proteins |
[124] |
| Live attenuated | Chikungunya Virus Envelope Polyprotein and Zika Virus Membrane-Envelope Glycoproteins | [125] |
| Live attenuated | Zika Virus Capsid Protein | [126] |
| Live attenuated | Middle East Respiratory Syndrome Coronavirus Spike Protein | [127] |
| Replication deficient | Ebola Virus Glycoprotein | [128] |
| Live attenuated | Venezuelan Equine Encephalitis Virus E2/E1 Glycoproteins | [129] |
| Live attenuated | Zika Virus Envelope Protein | [130] |
| Live attenuated | Enterovirus 71 VP1 Protein | [131] |
| Live attenuated | Dengue-2 Virus Pre-membrane and Envelope Proteins | [132] |
| Live attenuated | Porcine Reproductive and Respiratory Syndrome Virus Envelope Proteins GP5, M, GP4, GP3, GP2 and Nucleocapsid Protein |
[133] |
| Live attenuated | Lassa Virus Glycoprotein | [134] |
| Replication deficient | Mycobacterium ulcerans Proteins MUL2232 and MUL3720 | [135] |
| Live attenuated and inactivated | Hendra Virus Glycoprotein | [92] |
| Live attenuated | Nipah Virus Glycoprotein | [136] |
| Live attenuated | Bluetongue Virus Serotype 8 VP2 Protein | [137] |
| Live attenuated | Coxsackievirus B3 VP1 Protein | [138] |
| Live attenuated | Bundibugyo Ebolavirus Glycoprotein | [78] |
| Live attenuated | Andes Virus Glycoprotein | [139] |
| Live attenuated | Simian Retrovirus Type 2 Gag and Env Proteins | [140] |
| Live attenuated | Human Norovirus VP1 Protein | [141,142] |
| Live attenuated | Hepatitis B Virus Middle Envelope Surface Protein | [143,144] |
| Live attenuated | Vaccinia Virus B5R and L1R Proteins | [145] |
| Live attenuated | Human Immunodeficiency Virus gp160 Protein | [146] |
| Live attenuated | Influenza Virus Nucleoprotein and Hemagglutinin Protein | [147,148] |
| Replication deficient | Highly Pathogenic Avian Influenza Virus Hemagglutinin Protein | [149,150] |
| Live attenuated | West Nile Virus Envelope Glycoprotein | [151] |
| Replication deficient | Severe Acute Respiratory Syndrome Coronavirus Spike Protein | [152] |
| Live attenuated | Sudan Ebolavirus Glycoprotein | [153] |
| Live attenuated | Murine Cytomegalovirus Glycoprotein B | [154] |
| Live attenuated | Mycobacterium tuberculosis Ag85A Protein | [68] |
| Live attenuated | Human Paillomavirus Type 16 E7 Protein | [155] |
| Live attenuated | Human Immunodeficiency Virus Gag Protein | [156,157] |
| Live attenuated | Cottontail Rabbit Papillomavirus E1, E2, E6 and E7 Proteins | [158] |
| Live attenuated | Cottontail Rabbit Papillomavirus Early Protein E6 | [159,160] |
| Live attenuated | Yersinia pestis LcrV protein | [161,162] |
| Live attenuated | Human Immunodeficiency Virus gp120 Protein | [163] |
| Live attenuated and replication deficient | Human Immunodeficiency Virus Env Protein | [164] |
| Live attenuated | Marburg Virus Glycoprotein | [72,73,165] |
| Live attenuated | Ebola Virus Glycoprotein | [72,73] |
| Live attenuated | Severe Acute Respiratory Syndrome Coronavirus Spike Protein | [166] |
| Live attenuated | Human Immunodeficiency Virus gp41 and Porcine Endogenous Retrovirus p15E Proteins | [167] |
| Live attenuated | Simian Immunodeficiency Virus-Human Immunodeficiency Virus Env, Gag and Pol proteins |
[168,169] |
| Live attenuated | Cottontail Rabbit Papillomavirus L1 Protein | [170,171] |
| Live attenuated | Hepatitis C Virus Glycoproteins | [172] |
| Live attenuated and replication deficient | Respiratory Syncytial Virus G and F Proteins | [173,174] |
| Live attenuated | Bovine Viral Diarrhea Virus E2 Glycoprotein | [175] |
| Live attenuated | Measles Virus Hemagglutinin Protein | [176,177] |
| Live attenuated | Human Immunodeficiency Virus Env and Gag Proteins | [69,178,179] |
| Live attenuated | Influenza HA Protein | [93,180,181] |
| Live attenuated | Human Immunodeficiency Virus Env Protein | [111,182,183] |
In a recent example of a successful VSV-based vaccine, the replication-competent VSV-ZEBOV replaces the native VSV-G with the glycoprotein (GP) of EBOV (Zaire strain) [71–74]. VSV-ZEBOV mediates protection mainly through production of anti-EBOV-GP antibodies [75] and provides cross-protection against heterologous strains of Ebolavirus [76–78]. In various clinical trials evaluating the immunogenicity and safety of VSV-ZEBOV in humans [79–84], the vaccine was linked to some mild to moderate side-effects, but was otherwise shown to be safe and to induce an EBOV-GP specific immune response in humans, resulting in its approval by the U.S. FDA [17].
VSV has also been used to develop vaccines against SARS-CoV-2. Specifically, there have been two groups that have developed a recombinant VSV expressing a modified SARS-CoV-2 spike protein [85,86]. Both vaccine strategies elicited anti-SARS-CoV-2 antibodies and showed protection in either human angiotensin-converting enzyme-2 (ACE2) expressing mice [85], or hamsters challenged with SARS-CoV-2 [86]. Future studies will be needed to see whether these vaccines are safe and effective in humans.
VSV as an Oncolytic Virus
Another characteristic of VSV that has been exploited for therapeutic use is the virus’s ability to specifically kill cancer cells. VSV-G acts as a fusogenic membrane protein, which, when expressed in cells, causes syncytia formation and eventually cell death [87]. Many cancer cells down-regulate expression of various molecules in the interferon system, making them susceptible to VSV infection, as the virus is very sensitive to interferon and preferentially replicates in interferon deficient cells [88]. Additionally, due to VSV-G’s broad tissue tropism [1] and ability to express foreign genes [14] it can naturally infect a wide range of cells and be further targeted for specific cancer cell receptors or genes.
Numerous approaches have been taken to use VSV as an anti-cancer therapy (reviewed in [89]). Two recombinant VSV examples will be discussed here. In one, VSV-G was replaced with the Sindibis Virus glycoprotein modified to targeted the Her2/neu receptor, which is commonly overexpressed on breast cancer cells [90]. This virus, called rrVSV, protected mice in a Her2/neu receptor-dependent manner and, upon rechallenge with tumor cells lacking the Her2/neu receptor, some mice previously treated with rrVSV were protected from tumor regrowth, showing that they gained immunity against the tumor cells themselves. Another strategy used VSV-G in Moloney murine leukemia virus (MoMLV) either with or without the Gibbon ape leukemia virus (GALV) env gene [91]. These recombinant viruses both induced syncytia formation and cell death in various cell lines in vitro, highlighting their potential use as an anti-cancer therapy.
Limitations
As discussed above, VSV is typically used as a replication competent virus since it does not typically cause disease in humans. However, there is a concern for immunocompromised individuals and pregnant women who are more susceptible to infection from even attenuated live viruses. While some studies have tested the safety of these vectors in immunocompromised populations, such as VSV-ZEBOV trials in patients with HIV [83], there are many different immunocompromising conditions and more wide-spread testing should be conducted. Another concern when using replication competent VSV vectors is that much of the world is not endemic to VSV, and introducing live virus to these areas could have consequences for wildlife there, given that VSV causes disease in cattle, horses, and swine [1,6]. To address these concerns, testing needs to be done to ensure that these vectors will not undergo mutations resulting in restoration of virus pathogenesis. Using inactivated VSV-based vaccines, a strategy shown to induce immunogenicity in mice, could potentially overcome both of these issues [92,93].
Conclusions
As demonstrated throughout this review, RABV and VSV are attractive candidates for vaccine vectors. RABV and VSV are both well-studied and have well-established reverse genetics recovery systems [10,11] that can be used in combination with genome manipulation. This allows for foreign gene expression and incorporation into Rhabdovirus virions [13,14]. Both vectors have been successful vaccine vectors, as illustrated through the current use of the rabies vaccine world-wide [21] and FDA approval of VSV-ZEBOV [17]. Additionally, both vectors have excellent safety profiles for manufacturing and administration. Thus, in spite of the necessity of live virus handling during their production and other limitations, these viruses have a wide range of applications. Future studies are needed to see whether some of these limitations can be addressed. Overall, RABV and VSV are suitable vectors for the development of vaccines and other therapeutics.
Acknowledgements
The authors would like to thank Christine Fisher and Catherine Yankowski for their proofreading and feedback of the review and Benjamin Davis for allowing us to use his figures.
Footnotes
Conflict of interest
M.J.S. is an inventor on different Patents and Provisional Patent Application related to rhabdoviral vectors and vaccine. All remaining authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dietzschold B, Rupprecht CE, Fu ZF, Koprowski H: Rhabdoviruses. In Fields BN, and Knipe DM, and Howley PM, eds. Virology. . Lippincott-Raven Publishers; 1996:1137–1159. [Google Scholar]
- 2.Fooks AR, Banyard AC, Horton DL, Johnson N, McElhinney LM, Jackson AC: Current status of rabies and prospects for elimination. Lancet (London, England) 2014, 384:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson AC: Rabies pathogenesis. J Neurovirol 2002, 8:267–269. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization: WHO Expert Consultation on Rabies, Second Report. WHO; 2013. [PubMed] [Google Scholar]
- 5.Neville J: Rabies in the Ancient World. In Historical Perspective of Rabies in Europe and the Mediterranean Basin. Edited by King AA, Fooks AR, Aubert M, Wandeler AI. OIE; 2004:1–12. [Google Scholar]
- 6.HANSON RP: The natural history of vesicular stomatitis. Bacteriol Rev 1952, 16:179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blondel D, Harmison GG, Schubert M: Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J Virol 1990, 64:1716 LP – 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trottier MD, Lyles DS, Reiss CS: Peripheral, but not central nervous system, type I interferon expression in mice in response to intranasal vesicular stomatitis virus infection. J Neurovirol 2007, 13:433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang ZW, Sarmento L, Wang Y, Li X, Dhingra V, Tseggai T, Jiang B, Fu ZF: Attenuated Rabies Virus Activates, while Pathogenic Rabies Virus Evades, the Host Innate Immune Responses in the Central Nervous System. J Virol 2005, 79:12554–12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.*.Schnell MJ, Mebatsion T, Conzelmann KK: Infectious rabies viruses from cloned cDNA. EMBO J 1994, 13:4195–4203.First publication describing the generation of a negative-strand RNA virus from cDNA
- 11.Harty RN, Brown ME, Hayes FP, Wright NT, Schnell MJ: Vaccinia virus-free recovery of vesicular stomatitis virus. J Mol Microbiol Biotechnol 2001, 3:513–517. [PubMed] [Google Scholar]
- 12.Mebatsion T: Extensive Attenuation of Rabies Virus by Simultaneously Modifying the Dynein Light Chain Binding Site in the P Protein and Replacing Arg333 in the G Protein. J Virol 2001, 75:11496 LP – 11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.*.Mebatsion T, Schnell MJ, Cox JH, Finke S, Conzelmann KK: Highly stable expression of a foreign gene from rabies virus vectors. Proc Natl Acad Sci U S A 1996, 93:7310–7314.Rabies virus as an viral vector, publish at same time as the approach for VSV
- 14.*.Schnell MJ, Buonocore L, Kretzschmar E, Johnson E, Rose JK: Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci U S A 1996, 93:11359–11365.VSV as a viral vector, another key paper on rhabdoviral vectors
- 15.Mebatsion T, Finke S, Weiland F, Conzelmann K-K: A CXCR4/CD4 Pseudotype Rhabdovirus That Selectively Infects HIV-1 Envelope Protein-Expressing Cells. Cell 1997, 90:841–847. [DOI] [PubMed] [Google Scholar]
- 16.Okuma K, Fukagawa K, Kohma T, Takahama Y, Hamaguchi Y, Ito M, Tanaka Y, Buonocore L, Rose JK, Hamaguchi I: A recombinant vesicular stomatitis virus encoding CCR5-tropic HIV-1 receptors targets HIV-1-infected cells and controls HIV-1 infection. Microbes Infect 2017, 19:277–287. [DOI] [PubMed] [Google Scholar]
- 17.First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response. 2019.
- 18.*.Abreu-Mota T, Hagen KR, Cooper K, Jahrling PB, Tan G, Wirblich C, Johnson RF, Schnell MJ: Non-neutralizing antibodies elicited by recombinant Lassa–Rabies vaccine are critical for protection against Lassa fever. Nat Commun 2018, 9:4223.This is the first paper demonstrating that non-neutralizing antibodies are protective against Lassa fever.
- 19.Rupprecht CE, Nagarajan T, Ertl H: Current Status and Development of Vaccines and Other Biologics for Human Rabies Prevention. Expert Rev Vaccines 2016, 15:731–749. [DOI] [PubMed] [Google Scholar]
- 20.Geison GL: The Private Science of Louis Pasteur. Princeton Legacy Library; 2014. [Google Scholar]
- 21.World Health Organization: WHO Prequalified Vaccines. 2020, [Google Scholar]
- 22.Barth R, Gruschkau H, Jaeger O, Milcke L, Weinmann E: Purified chick embryo cell (PCEC) rabies vaccine for human use. Laboratory data. Behring Inst Mitt 1984, 76:142–54. [PubMed] [Google Scholar]
- 23.Fournier P, Montagnon B, Vincent-Falquet J-C, Ajjan N, Drucker J, Roumiantzeff M: A new vaccine produced from rabies virus cultivated on vero cells. In Improvements in rabies post-exposure treatment. Edited by Vodopija I, Nicholson KG, Smerdel S, Bijok U. Zagreb Institute of Public Health; 1985:115–121. [Google Scholar]
- 24.Patel PR, Patel PM: ADAPTATION OF PITMAN MOORE STRAIN OF RABIES VIRUS TO PRIMARY CHICK EMBRYO FIBROBLAST CELL CULTURES. 2010, [Google Scholar]
- 25.*.Kulkarni PS, Sahai A, Gunale B, Dhere RM: Development of a new purified vero cell rabies vaccine (Rabivax-S) at the serum institute of India Pvt Ltd. Expert Rev Vaccines 2017, 16:303–311.Detailed review of the development of Rabivax-S, a WHO prequalified human rabies vaccine.
- 26.Toovey S: Preventing rabies with the Verorab® vaccine: 1985–2005. Travel Med Infect Dis 2007, 5:327–348. [DOI] [PubMed] [Google Scholar]
- 27.Bose A, Munshi R, Tripathy RM, Madhusudana SN, Harish BR, Thaker S, Mahendra BJ, Gunale B, Gogtay NJ, Thatte UM, et al. : A randomized non-inferiority clinical study to assess post-exposure prophylaxis by a new purified vero cell rabies vaccine (Rabivax-S) administered by intramuscular and intradermal routes. Vaccine 2016, 34:4820–4826. [DOI] [PubMed] [Google Scholar]
- 28.Ashwath Narayana DH, Madhusudana SN, Sampath G, Tripathy RM, Sudarshan MK, Gangaboraiah, Ravish HS, Satapathy DM, Gowda G, Holla R, et al. : Safety and immunogenicity study of a new purified chick embryo cell rabies vaccine Vaxirab-N (Pitman-Moore strain) manufactured in India. Hum Vaccin Immunother 2014, 10:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giesen A, Gniel D, Malerczyk C: 30 years of rabies vaccination with Rabipur: a summary of clinical data and global experience. Expert Rev Vaccines 2015, 14:351–367. [DOI] [PubMed] [Google Scholar]
- 30.*.Fooks AR, Cliquet F, Finke S, Freuling C, Hemachudha T, Mani RS, Müller T, Nadin-Davis S, Picard-Meyer E, Wilde H, et al. : Rabies. Nat Rev Dis Prim 2017, 3.Comprehensive review about rabies disease, current preventative measures and potential erradication methods.
- 31.Wilde H, Lumlertdacha B, Meslin FX, Ghai S, Hemachudha T: Worldwide rabies deaths prevention—A focus on the current inadequacies in postexposure prophylaxis of animal bite victims. Vaccine 2016, 34:187–189. [DOI] [PubMed] [Google Scholar]
- 32.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, et al. : Estimating the Global Burden of Endemic Canine Rabies. PLoS Negl Trop Dis 2015, 9:e0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banyard AC, Horton DL, Freuling C, Müller T, Fooks AR: Control and prevention of canine rabies: The need for building laboratory-based surveillance capacity. Antiviral Res 2013, 98:357–364. [DOI] [PubMed] [Google Scholar]
- 34.*.Lavan RP, King AIM, Sutton DJ, Tunceli K: Rationale and support for a One Health program for canine vaccination as the most cost-effective means of controlling zoonotic rabies in endemic settings. Vaccine 2017, 35:1668–1674.A thorough article providing evidence for using canine vaccination as a method of controlling rabies virus.
- 35.Coleman PG, Dye C: Immunization coverage required to prevent outbreaks of dog rabies. Vaccine 1996, 14:185–186. [DOI] [PubMed] [Google Scholar]
- 36.Hampson K, Dushoff J, Cleaveland S, Haydon DT, Kaare M, Packer C, Dobson A: Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol 2009, 7:e53–e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leung T, Davis SA: Rabies Vaccination Targets for Stray Dog Populations. Front Vet Sci 2017, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization: Oral Vaccination of Dogs Against Rabies. Guidance for Research on Oral Rabies Vaccines and Field Application of Oral Vaccination of Dogs Against Rabies (ed. Meslin F). 2007. [Google Scholar]
- 39.Lankester F, Hampson K, Lembo T, Palmer G, Taylor L, Cleaveland S: Implementing Pasteur’s vision for rabies elimination. Science (80- ) 2014, 345:1562–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.*.FEHLNER-GARDINER C: Rabies control in North America – past, present and future. Rev Sci Tech l’OIE 2018, 37:421–437.Recent review of the efforts to control rabies virus in North America.
- 41.Commission E: The oral vaccination of foxes against rabies. Report of the Scientific Committee on Animal Health and Animal Welfare. 2002. [Google Scholar]
- 42.Rabies vaccines (live, oral) for foxes. In European Pharmacopoeia. . Council of Europe, European Directorate for the Quality of Medicines and Health Care; 2008:736–743. [Google Scholar]
- 43.Cliquet F, Gurbuxani JP, Pradhan HK, Pattnaik B, Patil SS, Regnault A, Begouen H, Guiot AL, Sood R, Mahl P, et al. : The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine 2007, 25:3409–3418. [DOI] [PubMed] [Google Scholar]
- 44.Cliquet F, Robardet E, Must K, Laine M, Peik K, Picard-Meyer E, Guiot A-L, Niin E: Eliminating Rabies in Estonia. PLoS Negl Trop Dis 2012, 6:e1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGettigan JP, Pomerantz RJ, Siler CA, McKenna PM, Foley HD, Dietzschold B, Schnell MJ: Second-Generation Rabies Virus-Based Vaccine Vectors Expressing Human Immunodeficiency Virus Type 1 Gag Have Greatly Reduced Pathogenicity but Are Highly Immunogenic. J Virol 2003, 77:237 LP – 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cenna J, Hunter M, Tan GS, Papaneri AB, Ribka EP, Schnell MJ, Marx PA, McGettigan JP: Replication-Deficient Rabies Virus–Based Vaccines Are Safe and Immunogenic in Mice and Nonhuman Primates. J Infect Dis 2009, 200:1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kgaladi J, Faber M, Dietzschold B, Nel LH, Markotter W: Pathogenicity and Immunogenicity of Recombinant Rabies Viruses Expressing the Lagos Bat Virus Matrix and Glycoprotein: Perspectives for a Pan-Lyssavirus Vaccine. Trop Med Infect Dis 2017, 2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.*.Takayama-Ito M, Lim C-K, Yamaguchi Y, Posadas-Herrera G, Kato H, Iizuka I, Islam MT, Morimoto K, Saijo M: Replication-incompetent rabies virus vector harboring glycoprotein gene of lymphocytic choriomeningitis virus (LCMV) protects mice from LCMV challenge. PLoS Negl Trop Dis 2018, 12:e0006398.This is the first paper describing a protective rabies-based vaccine against lymphocytic choriomeningitis virus.
- 49.Blaney JE, Wirblich C, Papaneri AB, Johnson RF, Myers CJ, Juelich TL, Holbrook MR, Freiberg AN, Bernbaum JG, Jahrling PB, et al. : Inactivated or Live-Attenuated Bivalent Vaccines That Confer Protection against Rabies and Ebola Viruses. J Virol 2011, 85:10605–10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.*.Zhang Hao, Feng Jin, Yan Chi, Wang Han, Wang Wong, et al. : Genetically Modified Rabies Virus Vector-Based Rift Valley Fever Virus Vaccine is Safe and Induces Efficacious Immune Responses in Mice. Viruses 2019, 11:919.This study showing that immunization of mice with a novel rabies-based rift valley fever virus vaccine induces antibodies and memory T cells.
- 51.Smith ME, Koser M, Xiao S, Siler C, McGettigan JP, Calkins C, Pomerantz RJ, Dietzschold B, Schnell MJ: Rabies virus glycoprotein as a carrier for anthrax protective antigen. Virology 2006, 353:344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.*.Kurup D, Wirblich C, Ramage H, Schnell MJ: Rabies virus-based COVID-19 vaccine CORAVAX induces high levels of neutralizing antibodies against SARS-CoV-2. npj Vaccines 2020, Accepted.The first rabies-based vaccine against Severe Acute Respiratory Syndrome Coronavirus 2.
- 53.Johnson N, Cunningham AF, Fooks AR: The immune response to rabies virus infection and vaccination. Vaccine 2010, 28:3896–3901. [DOI] [PubMed] [Google Scholar]
- 54.Whitt MA: Generation of VSV pseudotypes using recombinant ΔG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J Virol Methods 2010, 169:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Logan N, McMonagle E, Drew AA, Takahashi E, McDonald M, Baron MD, Gilbert M, Cleaveland S, Haydon DT, Hosie MJ, et al. : Efficient generation of vesicular stomatitis virus (VSV)-pseudotypes bearing morbilliviral glycoproteins and their use in quantifying virus neutralising antibodies. Vaccine 2016, 34:814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suda Y, Fukushi S, Tani H, Murakami S, Saijo M, Horimoto T, Shimojima M: Analysis of the entry mechanism of Crimean-Congo hemorrhagic fever virus, using a vesicular stomatitis virus pseudotyping system. Arch Virol 2016, 161:1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakata M, Tani H, Anraku M, Kataoka M, Nagata N, Seki F, Tahara M, Otsuki N, Okamoto K, Takeda M, et al. : Analysis of VSV pseudotype virus infection mediated by rubella virus envelope proteins. Sci Rep 2017, 7:11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogalin HB, Heldwein EE: Characterization of Vesicular Stomatitis Virus Pseudotypes Bearing Essential Entry Glycoproteins gB, gD, gH, and gL of Herpes Simplex Virus 1. J Virol 2016, 90:10321 LP – 10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seganti L, Superti F, Girmenia C, Melucci L, Orsi N: Study of receptors for vesicular stomatitis virus in vertebrate and invertebrate cells. Microbiologica 1986, 9:259–67. [PubMed] [Google Scholar]
- 60.Hastie E, Cataldi M, Marriott I, Grdzelishvili VZ: Understanding and altering cell tropism of vesicular stomatitis virus. Virus Res 2013, 176:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen ST, Iida A, Guo L, Friedmann T, Yee JK: Generation of packaging cell lines for pseudotyped retroviral vectors of the G protein of vesicular stomatitis virus by using a modified tetracycline inducible system. Proc Natl Acad Sci U S A 1996, 93:10057–10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lévy C, Verhoeyen E, Cosset F-L: Surface engineering of lentiviral vectors for gene transfer into gene therapy target cells. Curr Opin Pharmacol 2015, 24:79–85. [DOI] [PubMed] [Google Scholar]
- 63.Hug P, Sleight RG: Fusogenic virosomes prepared by partitioning of vesicular stomatitis virus G protein into preformed vesicles. J Biol Chem 1994, 269:4050–4056. [PubMed] [Google Scholar]
- 64.Mangeot P-E, Dollet S, Girard M, Ciancia C, Joly S, Peschanski M, Lotteau V: Protein transfer into human cells by VSV-G-induced nanovesicles. Mol Ther 2011, 19:1656–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.*.Mangeot PE, Risson V, Fusil F, Marnef A, Laurent E, Blin J, Mournetas V, Massouridès E, Sohier TJM, Corbin A, et al. : Genome editing in primary cells and in vivo using viral-derived Nanoblades loaded with Cas9-sgRNA ribonucleoproteins. Nat Commun 2019, 10:45.This study describes the development of a new vector for genome editing, Nanoblades, that can effectively edit cell lines and primary cells. These Nanoblades are murine leukemia virus-like particles expressing vesicular stomatitis virus and baboon endogenous retrovirus Rless glycoproteins that contain CRISPR-associated protein 9 single-guide RNAs.
- 66.*.Frank AM, Buchholz CJ: Surface-Engineered Lentiviral Vectors for Selective Gene Transfer into Subtypes of Lymphocytes. Mol Ther - Methods Clin Dev 2019, 12:19–31.A recent review discussing various strategies for lymphocyte gene therapy, specifically highlighting the use of lentiviral vectors.
- 67.*.Liu X, Li Y, Zhong Z, Tan H, Lin H, Chen S, Fu Y, Xu W, Wei C: Incorporation of Viral Glycoprotein VSV-G Improves the Delivery of DNA by Erythrocyte Ghost into Cells Refractory to Conventional Transfection. Appl Biochem Biotechnol 2017, 181:748–761.This study describes a novel erythrocyte ghost gene delivery system integrated with the vesicular stomatitis virus glycoprotein that successfully delivers DNA into a variety of cells through increased endocytosis.
- 68.Roediger EK, Kugathasan K, Zhang X, Lichty BD, Xing Z: Heterologous Boosting of Recombinant Adenoviral Prime Immunization With a Novel Vesicular Stomatitis Virus–vectored Tuberculosis Vaccine. Mol Ther 2008, 16:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, Montefiori D, Roberts A, Buonocore L, Rose JK: An Effective AIDS Vaccine Based on Live Attenuated Vesicular Stomatitis Virus Recombinants. Cell 2001, 106:539–549. [DOI] [PubMed] [Google Scholar]
- 70.Geisbert TW, Feldmann H: Recombinant Vesicular Stomatitis Virus–Based Vaccines Against Ebola and Marburg Virus Infections. J Infect Dis 2011, 204:S1075–S1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Möller P, Wagner R, Volchkov V, Klenk H-D, Feldmann H, Ströher U: Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol 2004, 78:5458–5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones SM, Feldmann H, Ströher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, et al. : Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 2005, 11:786–790. [DOI] [PubMed] [Google Scholar]
- 73.Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, Reed DS, Feldmann F, Grolla A, Ströher U, Fritz EA, Hensley LE, Jones SM, et al. : Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine 2008, 26:6894–6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu X, Fernando L, Alimonti JB, Melito PL, Feldmann F, Dick D, Ströher U, Feldmann H, Jones SM: Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS One 2009, 4:e5547–e5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marzi A, Engelmann F, Feldmann F, Haberthur K, Shupert WL, Brining D, Scott DP, Geisbert TW, Kawaoka Y, Katze MG, et al. : Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci 2013, 110:1893–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marzi A, Ebihara H, Callison J, Groseth A, Williams KJ, Geisbert TW, Feldmann H: Vesicular Stomatitis Virus–Based Ebola Vaccines With Improved Cross-Protective Efficacy. J Infect Dis 2011, 204:S1066–S1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Falzarano D, Feldmann F, Grolla A, Leung A, Ebihara H, Strong JE, Marzi A, Takada A, Jones S, Gren J, et al. : Single Immunization With a Monovalent Vesicular Stomatitis Virus–Based Vaccine Protects Nonhuman Primates Against Heterologous Challenge With Bundibugyo ebolavirus. J Infect Dis 2011, 204:S1082–S1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mire CE, Geisbert JB, Marzi A, Agans KN, Feldmann H, Geisbert TW: Vesicular Stomatitis Virus-Based Vaccines Protect Nonhuman Primates against Bundibugyo ebolavirus. PLoS Negl Trop Dis 2013, 7:e2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.ElSherif MS, Brown C, MacKinnon-Cameron D, Li L, Racine T, Alimonti J, Rudge TL, Sabourin C, Silvera P, Hooper JW, et al. : Assessing the safety and immunogenicity of recombinant vesicular stomatitis virus Ebola vaccine in healthy adults: a randomized clinical trial. Can Med Assoc J 2017, 189:E819–E827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Hu Z, Muñoz P, Moon JE, Ruck RC, Bennett JW, et al. : A Recombinant Vesicular Stomatitis Virus Ebola Vaccine. N Engl J Med 2017, 376:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khurana S, Fuentes S, Coyle EM, Ravichandran S, Davey RT, Beigel JH: Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat Med 2016, 22:1439–1447. [DOI] [PubMed] [Google Scholar]
- 82.Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, Yerly S, Dayer J-A, Kraehling V, Kasonta R, et al. : Phase 1 Trials of rVSV Ebola Vaccine in Africa and Europe. N Engl J Med 2016, 374:1647–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.*.Kennedy SB, Bolay F, Kieh M, Grandits G, Badio M, Ballou R, Eckes R, Feinberg M, Follmann D, Grund B, et al. : Phase 2 Placebo-Controlled Trial of Two Vaccines to Prevent Ebola in Liberia. N Engl J Med 2017, 377:1438–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.*.Halperin SA, Arribas JR, Rupp R, Andrews CP, Chu L, Das R, Simon JK, Onorato MT, Liu K, Martin J, et al. : Six-Month Safety Data of Recombinant Vesicular Stomatitis Virus–Zaire Ebola Virus Envelope Glycoprotein Vaccine in a Phase 3 Double-Blind, Placebo-Controlled Randomized Study in Healthy Adults. J Infect Dis 2017, 215:1789–1798.Two human clinical trials showing the safety and efficacy of the vesicular stomatitis virus-based Ebola vaccine, VSV-ZEBOV.
- 85.*.Case JB, Rothlauf PW, Chen RE, Kafai NM, Fox JM, Smith BK, Shrihari S, McCune BT, Harvey IB, Keeler SP, et al. : Replication-Competent Vesicular Stomatitis Virus Vaccine Vector Protects against SARS-CoV-2-Mediated Pathogenesis in Mice. Cell Host Microbe 2020, doi: 10.1016/j.chom.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.*.Yahalom-Ronen Y, Tamir H, Melamed S, Politi B, Shifman O, Achdout H, Vitner EB, Israeli O, Milrot E, Stein D, et al. : A single dose of recombinant VSV-ΔG-spike vaccine provides protection against SARS-CoV-2 challenge. bioRxiv 2020, doi: 10.1101/2020.06.18.160655.Two studies describing the development of vesicular stomatitis virus-based Severe Acute Respiratory Coronavirus 2 vaccines that were shown to be protective in animal models.
- 87.Bateman A, Bullough F, Murphy S, Emiliusen L, Lavillette D, Cosset F-L, Cattaneo R, Russell SJ, Vile RG: Fusogenic Membrane Glycoproteins As a Novel Class of Genes for the Local and Immune-mediated Control of Tumor Growth. Cancer Res 2000, 60:1492 LP – 1497. [PubMed] [Google Scholar]
- 88.Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, Bell JC: Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 2000, 6:821–825. [DOI] [PubMed] [Google Scholar]
- 89.*.Bishnoi S, Tiwari R, Gupta S, Byrareddy SN, Nayak D: Oncotargeting by Vesicular Stomatitis Virus (VSV): Advances in Cancer Therapy. Viruses 2018, 10:90.This is a recent review of how vesicular stomatitis virus can be applied as a cancer therapy.
- 90.Bergman I, Griffin JA, Gao Y, Whitaker-Dowling P: Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to Her2/neu. Int J Cancer 2007, 121:425–430. [DOI] [PubMed] [Google Scholar]
- 91.*.Jin SY, Jung Y-T: Construction of a replication-competent retroviral vector for expression of the VSV-G envelope glycoprotein for cancer gene therapy. Arch Virol 2020, doi: 10.1007/s00705-020-04585-8.This study demonstrated that a Moloney murine leukemia virus expressing vesicular stomatitis virus glycoprotein with or without gibbon ape leukemia virus env gene successfully infected and lysed of a variety of cell lines, including cancer cell lines, highlighting its potential use as a cancer therapy.
- 92.Kurup D, Wirblich C, Feldmann H, Marzi A, Schnell MJ: Rhabdovirus-Based Vaccine Platforms against Henipaviruses. J Virol 2015, 89:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, Kawaoka Y, Rose JK: Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol 1998, 72:4704–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.*.Keshwara R, Hagen KR, Abreu-Mota T, Papaneri AB, Liu D, Wirblich C, Johnson RF, Schnell MJ: A Recombinant Rabies Virus Expressing the Marburg Virus Glycoprotein Is Dependent upon Antibody-Mediated Cellular Cytotoxicity for Protection against Marburg Virus Disease in a Murine Model. J Virol 2019, 93:e01865–18.This article is the first to show that after immunization of mice with a rabies-based Marburg virus vaccine, protection against virus challenge is mediated through antibody-mediated cellular cytotoxicity.
- 95.*.Keshwara R, Shiels T, Postnikova E, Kurup D, Wirblich C, Johnson RF, Schnell MJ: Rabies-based vaccine induces potent immune responses against Nipah virus. npj Vaccines 2019, 4:15.This study describes the immune responses elicited by a novel rabies-based nipah virus vaccine.
- 96.*.Wirblich C, Coleman CM, Kurup D, Abraham TS, Bernbaum JG, Jahrling PB, Hensley LE, Johnson RF, Frieman MB, Schnell MJ: One-Health: a Safe, Efficient, Dual-Use Vaccine for Humans and Animals against Middle East Respiratory Syndrome Coronavirus and Rabies Virus. J Virol 2017, 91:e02040–16.This study showed that a rabies-based vaccine against Middle East Respiratory Syndrome Coronavirus was protective in a mouse challenge model.
- 97.da Fontoura Budaszewski R, Hudacek A, Sawatsky B, Krämer B, Yin X, Schnell MJ, von Messling V: Inactivated Recombinant Rabies Viruses Displaying Canine Distemper Virus Glycoproteins Induce Protective Immunity against Both Pathogens. J Virol 2017, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo J, Shi H, Tan Y, Niu X, Long T, Zhao J, Tian Q, Wang Y, Chen H, Guo X: Two potential recombinant rabies vaccines expressing canine parvovirus virion protein 2 induce immunogenicity to canine parvovirus and rabies virus. Vaccine 2016, 34:4392–4398. [DOI] [PubMed] [Google Scholar]
- 99.Wang F-X, Zhang S-Q, Zhu H-W, Yang Y, Sun N, Tan B, Li Z-G, Cheng S-P, Fu ZF, Wen Y-J: Recombinant rabies virus expressing the H protein of canine distemper virus protects dogs from the lethal distemper challenge. Vet Microbiol 2014, 174:362–371. [DOI] [PubMed] [Google Scholar]
- 100.Mustafa W, Al-Saleem FH, Nasser Z, Olson RM, Mattis JA, Simpson LL, Schnell MJ: Immunization of mice with the non-toxic HC50 domain of botulinum neurotoxin presented by rabies virus particles induces a strong immune response affording protection against high-dose botulinum neurotoxin challenge. Vaccine 2011, 29:4638–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hudacek AW, Al-Saleem FH, Willet M, Eisemann T, Mattis JA, Simpson LL, Schnell MJ: Recombinant rabies virus particles presenting botulinum neurotoxin antigens elicit a protective humoral response in vivo. Mol Ther - Methods Clin Dev 2014, 1:14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Papaneri AB, Wirblich C, Cann JA, Cooper K, Jahrling PB, Schnell MJ, Blaney JE: A replication-deficient rabies virus vaccine expressing Ebola virus glycoprotein is highly attenuated for neurovirulence. Virology 2012, 434:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blaney JE, Marzi A, Willet M, Papaneri AB, Wirblich C, Feldmann F, Holbrook M, Jahrling P, Feldmann H, Schnell MJ: Antibody Quality and Protection from Lethal Ebola Virus Challenge in Nonhuman Primates Immunized with Rabies Virus Based Bivalent Vaccine. PLOS Pathog 2013, 9:e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Willet M, Kurup D, Papaneri A, Wirblich C, Hooper JW, Kwilas SA, Keshwara R, Hudacek A, Beilfuss S, Rudolph G, et al. : Preclinical Development of Inactivated Rabies Virus–Based Polyvalent Vaccine Against Rabies and Filoviruses. J Infect Dis 2015, 212:S414–S424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnson RF, Kurup D, Hagen KR, Fisher C, Keshwara R, Papaneri A, Perry DL, Cooper K, Jahrling PB, Wang JT, et al. : An Inactivated Rabies Virus-Based Ebola Vaccine, FILORAB1, Adjuvanted With Glucopyranosyl Lipid A in Stable Emulsion Confers Complete Protection in Nonhuman Primate Challenge Models. J Infect Dis 2016, 214:S342–S354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu X, Franka R, Svoboda P, Pohl J, Rupprecht CE: Development of combined vaccines for rabies and immunocontraception. Vaccine 2009, 27:7202–7209. [DOI] [PubMed] [Google Scholar]
- 107.Faber M, Lamirande EW, Roberts A, Rice AB, Koprowski H, Dietzschold B, Schnell MJ: A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies. J Gen Virol 2005, 86:1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dunkel A, Shen S, LaBranche CC, Montefiori D, McGettigan JP: A Bivalent, Chimeric Rabies Virus Expressing Simian Immunodeficiency Virus Envelope Induces Multifunctional Antibody Responses. AIDS Res Hum Retroviruses 2015, 31:1126–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Faul EJ, Aye PP, Papaneri AB, Pahar B, McGettigan JP, Schiro F, Chervoneva I, Montefiori DC, Lackner AA, Schnell MJ: Rabies virus-based vaccines elicit neutralizing antibodies, poly-functional CD8+ T cell, and protect rhesus macaques from AIDS-like disease after SIVmac251 challenge. Vaccine 2009, 28:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McKenna PM, Koser ML, Carlson KR, Montefiori DC, Letvin NL, Papaneri AB, Pomerantz RJ, Dietzschold B, Silvera P, McGettigan JP, et al. : Highly Attenuated Rabies Virus–Based Vaccine Vectors Expressing Simian-Human Immunodeficiency Virus 89.6P Env and Simian Immunodeficiency Virus mac239 Gag Are Safe in Rhesus Macaques and Protect from an AIDS-Like Disease. J Infect Dis 2007, 195:980–988. [DOI] [PubMed] [Google Scholar]
- 111.Tan GS, McKenna PM, Koser ML, McLinden R, Kim JH, McGettigan JP, Schnell MJ: Strong cellular and humoral anti-HIV Env immune responses induced by a heterologous rhabdoviral prime–boost approach. Virology 2005, 331:82–93. [DOI] [PubMed] [Google Scholar]
- 112.McGettigan JP, Koser ML, McKenna PM, Smith ME, Marvin JM, Eisenlohr LC, Dietzschold B, Schnell MJ: Enhanced humoral HIV-1-specifc immune responses generated from recombinant rhabdoviral-based vaccine vectors co-expressing HIV-1 proteins and IL-2. Virology 2006, 344:363–377. [DOI] [PubMed] [Google Scholar]
- 113.McKenna PM, Pomerantz RJ, Dietzschold B, McGettigan JP, Schnell MJ: Covalently Linked Human Immunodeficiency Virus Type 1 gp120/gp41 Is Stably Anchored in Rhabdovirus Particles and Exposes Critical Neutralizing Epitopes. J Virol 2003, 77:12782–12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McGettigan JP, Naper K, Orenstein J, Koser M, McKenna PM, Schnell MJ: Functional Human Immunodeficiency Virus Type 1 (HIV-1) Gag-Pol or HIV-1 Gag-Pol and Env Expressed from a Single Rhabdovirus-Based Vaccine Vector Genome. J Virol 2003, 77:10889–10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Siler CA, McGettigan JP, Dietzschold B, Herrine SK, Dubuisson J, Pomerantz RJ, Schnell MJ: Live and Killed Rhabdovirus-Based Vectors as Potential Hepatitis C Vaccines. Virology 2002, 292:24–34. [DOI] [PubMed] [Google Scholar]
- 116.McGettigan JP, Sarma S, Orenstein JM, Pomerantz RJ, Schnell MJ: Expression and Immunogenicity of Human Immunodeficiency Virus Type 1 Gag Expressed by a Replication-Competent Rhabdovirus-Based Vaccine Vector. J Virol 2001, 75:8724–8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McGettigan JP, Foley HD, Belyakov IM, Berzofsky JA, Pomerantz RJ, Schnell MJ: Rabies Virus-Based Vectors Expressing Human Immunodeficiency Virus Type 1 (HIV-1) Envelope Protein Induce a Strong, Cross-Reactive Cytotoxic T-Lymphocyte Response against Envelope Proteins from Different HIV-1 Isolates. J Virol 2001, 75:4430–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lawrence TM, Wanjalla CN, Gomme EA, Wirblich C, Gatt A, Carnero E, García-Sastre A, Lyles DS, McGettigan JP, Schnell MJ: Comparison of Heterologous Prime-Boost Strategies against Human Immunodeficiency Virus Type 1 Gag Using Negative Stranded RNA Viruses. PLoS One 2013, 8:e67123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jallet C, Jacob Y, Bahloul C, Drings A, Desmezieres E, Tordo N, Perrin P: Chimeric Lyssavirus Glycoproteins with Increased Immunological Potential. J Virol 1999, 73:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mebatsion T, Schnell MJ, Conzelmann KK: Mokola virus glycoprotein and chimeric proteins can replace rabies virus glycoprotein in the rescue of infectious defective rabies virus particles. J Virol 1995, 69:1444 LP – 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.*.Warner BM, Stein DR, Jangra RK, Slough MM, Sroga P, Sloan A, Frost KL, Booth S, Chandran K, Safronetz D: Vesicular Stomatitis Virus-Based Vaccines Provide Cross-Protection against Andes and Sin Nombre Viruses. Viruses 2019, 11:645.This is the first study to show that vaccines against individual hantaviruses can provide cross-protection against heterologous hantaviruses.
- 122.*.Rodriguez SE, Cross RW, Fenton KA, Bente DA, Mire CE, Geisbert TW: Vesicular Stomatitis Virus-Based Vaccine Protects Mice against Crimean-Congo Hemorrhagic Fever. Sci Rep 2019, 9:7755.This study demonstrates that a vesicular stomatitis virus-based Crimean-Congo Hemorrhagic Fever virus vaccine is protective in a mouse challenge model.
- 123.*.Ke Y, Yu D, Zhang F, Gao J, Wang X, Fang X, Wang H, Sun T: Recombinant vesicular stomatitis virus expressing the spike protein of genotype 2b porcine epidemic diarrhea virus: A platform for vaccine development against emerging epidemic isolates. Virology 2019, 533:77–85.This article describes a novel recombinant vesicular stomatitis virus vaccine expressing a truncated porcine epidemic diarrhea viruses G2b spike protein and it’s efficacy in pigs.
- 124.*.Emanuel J, Callison J, Dowd KA, Pierson TC, Feldmann H, Marzi A: A VSV-based Zika virus vaccine protects mice from lethal challenge. Sci Rep 2018, 8:11043.This study demonstrates that a vesicular stomatitis virus expressing Ebola virus glycoprotein and Zika virus full-length pre-membrane and envelope proteins, or premembrane and soluble envelope proteins are protective in a lethal Zika virus mouse challenge model.
- 125.*.Chattopadhyay A, Aguilar PV, Bopp NE, Yarovinsky TO, Weaver SC, Rose JK: A recombinant virus vaccine that protects against both Chikungunya and Zika virus infections. Vaccine 2018, 36:3894–3900.This study showed that a bivalent vesicular stomatitis virus-based vaccine against Chikungunya virus and Zika virus was protective against both viruses in mouse challenge models.
- 126.Shi X, Hu J, Guo J, Wu C, Xiong S, Dong C: A Vesicular Stomatitis Virus-Based Vaccine Carrying Zika Virus Capsid Protein Protects Mice from Viral Infection. Virol Sin 2019, 34:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.*.Liu R, Wang J, Shao Y, Wang X, Zhang H, Shuai L, Ge J, Wen Z, Bu Z: A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization. Antiviral Res 2018, 150:30–38.This study describes the development of a vesicular stomatitis-based Middle East respiratory syndrome coronavirus vaccine that when administered to Rhesus monkeys, induced neutralizing antibodies and T cell responses.
- 128.*.Lennemann NJ, Herbert AS, Brouillette R, Rhein B, Bakken RA, Perschbacher KJ, Cooney AL, Miller-Hunt CL, Ten Eyck P, Biggins J, et al. : Vesicular Stomatitis Virus Pseudotyped with Ebola Virus Glycoprotein Serves as a Protective, Noninfectious Vaccine against Ebola Virus Challenge in Mice. J Virol 2017, 91.This study demonstrated that a noninfectious vesicular stomatitis virus pseudotyped with the Ebola virus glycoprotein is protective against Ebola virus challenge in a mouse model.
- 129.*.Nasar F, Matassov D, Seymour RL, Latham T, Gorchakov RV, Nowak RM, Leal G, Hamm S, Eldridge JH, Tesh RB, et al. : Recombinant Isfahan Virus and Vesicular Stomatitis Virus Vaccine Vectors Provide Durable, Multivalent, Single-Dose Protection against Lethal Alphavirus Challenge. J Virol 2017, 91.This study showed that both recombinant Isfahan virus and vesicular stomatitis virus expressing Venezuelan equine encephalitis virus and Eastern equine encephalitis virus E2/E1 surface proteins are protective in mouse challenge models.
- 130.*.Betancourt D, de Queiroz NMGP, Xia T, Ahn J, Barber GN: Cutting Edge: Innate Immune Augmenting Vesicular Stomatitis Virus Expressing Zika Virus Proteins Confers Protective Immunity. J Immunol 2017, 198:3023–3028.This study describes vesicular stomatitis virus-based vaccines expressing the Zika virus envelope protein with or without the Zika precursor to membrane protein that induced Zika virus neutralizing antibodies and protected pups of vaccinated mothers from lethal challenge.
- 131.Yan Q, Wu L, Chen L, Qin Y, Pan Z, Chen M: Vesicular stomatitis virus-based vaccines expressing EV71 virus-like particles elicit strong immune responses and protect newborn mice from lethal challenges. Vaccine 2016, 34:4196–4204. [DOI] [PubMed] [Google Scholar]
- 132.Lauretti F, Chattopadhyay A, de Oliveira França RF, Castro-Jorge L, Rose J, Fonseca BAL da: Recombinant vesicular stomatitis virus-based dengue-2 vaccine candidate induces humoral response and protects mice against lethal infection. Hum Vaccin Immunother 2016, 12:2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Eck M, Durán MG, Ricklin ME, Locher S, Sarraseca J, Rodríguez MJ, McCullough KC, Summerfield A, Zimmer G, Ruggli N: Virus replicon particles expressing porcine reproductive and respiratory syndrome virus proteins elicit immune priming but do not confer protection from viremia in pigs. Vet Res 2016, 47:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Safronetz D, Mire C, Rosenke K, Feldmann F, Haddock E, Geisbert T, Feldmann H: A Recombinant Vesicular Stomatitis Virus-Based Lassa Fever Vaccine Protects Guinea Pigs and Macaques against Challenge with Geographically and Genetically Distinct Lassa Viruses. PLoS Negl Trop Dis 2015, 9:e0003736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bolz M, Kerber S, Zimmer G, Pluschke G: Use of Recombinant Virus Replicon Particles for Vaccination against Mycobacterium ulcerans Disease. PLoS Negl Trop Dis 2015, 9:e0004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Prescott J, DeBuysscher BL, Feldmann F, Gardner DJ, Haddock E, Martellaro C, Scott D, Feldmann H: Single-dose live-attenuated vesicular stomatitis virus-based vaccine protects African green monkeys from Nipah virus disease. Vaccine 2015, 33:2823–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kochinger S, Renevey N, Hofmann MA, Zimmer G: Vesicular stomatitis virus replicon expressing the VP2 outer capsid protein of bluetongue virus serotype 8 induces complete protection of sheep against challenge infection. Vet Res 2014, 45:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu F, Fan X, Yue Y, Xiong S, Dong C: A vesicular stomatitis virus-based mucosal vaccine promotes dendritic cell maturation and elicits preferable immune response against coxsackievirus B3 induced viral myocarditis. Vaccine 2014, 32:3917–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brown KS, Safronetz D, Marzi A, Ebihara H, Feldmann H: Vesicular Stomatitis Virus-Based Vaccine Protects Hamsters against Lethal Challenge with Andes Virus. J Virol 2011, 85:12781–12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gautam R, Iyer A, Hunter M, Das A, Williams T, Dufour J, Apetrei C, Kousoulas KG, Marx PA: Vesicular Stomatitis Virus-Simian Retrovirus Type 2 Vaccine Protects Macaques from Detectable Infection and B-Cell Destruction. J Virol 2011, 85:5889–5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ma Y, Li J: Vesicular Stomatitis Virus as a Vector To Deliver Virus-Like Particles of Human Norovirus: a New Vaccine Candidate against an Important Noncultivable Virus. J Virol 2011, 85:2942–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ma Y, Duan Y, Wei Y, Liang X, Niewiesk S, Oglesbee M, Li J: Heat Shock Protein 70 Enhances Mucosal Immunity against Human Norovirus When Coexpressed from a Vesicular Stomatitis Virus Vector. J Virol 2014, 88:5122–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cobleigh MA, Buonocore L, Uprichard SL, Rose JK, Robek MD: A Vesicular Stomatitis Virus-Based Hepatitis B Virus Vaccine Vector Provides Protection against Challenge in a Single Dose. J Virol 2010, 84:7513–7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cobleigh MA, Wei X, Robek MD: A Vesicular Stomatitis Virus-Based Therapeutic Vaccine Generates a Functional CD8 T Cell Response to Hepatitis B Virus in Transgenic Mice. J Virol 2013, 87:2969–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Braxton CL, Puckett SH, Mizel SB, Lyles DS: Protection against Lethal Vaccinia Virus Challenge by Using an Attenuated Matrix Protein Mutant Vesicular Stomatitis Virus Vaccine Vector Expressing Poxvirus Antigens. J Virol 2010, 84:3552–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu K, Kim GN, Kang CY: Expression and processing of human immunodeficiency virus type 1 gp160 using the vesicular stomatitis virus New Jersey serotype vector system. J Gen Virol 2009, 90:1135–1140. [DOI] [PubMed] [Google Scholar]
- 147.Barefoot BE, Sample CJ, Ramsburg EA: Recombinant Vesicular Stomatitis Virus Expressing Influenza Nucleoprotein Induces CD8 T-Cell Responses That Enhance Antibody-Mediated Protection after Lethal Challenge with Influenza Virus. Clin Vaccine Immunol 2009, 16:488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ryder AB, Buonocore L, Vogel L, Nachbagauer R, Krammer F, Rose JK: A Viable Recombinant Rhabdovirus Lacking Its Glycoprotein Gene and Expressing Influenza Virus Hemagglutinin and Neuraminidase Is a Potent Influenza Vaccine. J Virol 2015, 89:2820–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kalhoro NH, Veits J, Rautenschlein S, Zimmer G: A recombinant vesicular stomatitis virus replicon vaccine protects chickens from highly pathogenic avian influenza virus (H7N1). Vaccine 2009, 27:1174–1183. [DOI] [PubMed] [Google Scholar]
- 150.Halbherr SJ, Brostoff T, Tippenhauer M, Locher S, Berger Rentsch M, Zimmer G: Vaccination with Recombinant RNA Replicon Particles Protects Chickens from H5N1 Highly Pathogenic Avian Influenza Virus. PLoS One 2013, 8:e66059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Iyer A V, Pahar B, Boudreaux MJ, Wakamatsu N, Roy AF, Chouljenko VN, Baghian A, Apetrei C, Marx PA, Kousoulas KG: Recombinant vesicular stomatitis virus-based west Nile vaccine elicits strong humoral and cellular immune responses and protects mice against lethal challenge with the virulent west Nile virus strain LSU-AR01. Vaccine 2009, 27:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kapadia SU, Simon ID, Rose JK: SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector. Virology 2008, 376:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Geisbert TW, Daddario-DiCaprio KM, Williams KJN, Geisbert JB, Leung A, Feldmann F, Hensley LE, Feldmann H, Jones SM: Recombinant Vesicular Stomatitis Virus Vector Mediates Postexposure Protection against Sudan Ebola Hemorrhagic Fever in Nonhuman Primates. J Virol 2008, 82:5664–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wilson SR, Wilson JH, Buonocore L, Palin A, Rose JK, Reuter JD: Intranasal Immunization With Recombinant Vesicular Stomatitis Virus Expressing Murine Cytomegalovirus Glycoprotein B Induces Humoral and Cellular Immunity. Comp Med 2008, 58:129–139. [PMC free article] [PubMed] [Google Scholar]
- 155.Liao JB, Publicover J, Rose JK, DiMaio D: Single-Dose, Therapeutic Vaccination of Mice with Vesicular Stomatitis Virus Expressing Human Papillomavirus Type 16 E7 Protein. Clin Vaccine Immunol 2008, 15:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cooper D, Wright KJ, Calderon PC, Guo M, Nasar F, Johnson JE, Coleman JW, Lee M, Kotash C, Yurgelonis I, et al. : Attenuation of Recombinant Vesicular Stomatitis Virus-Human Immunodeficiency Virus Type 1 Vaccine Vectors by Gene Translocations and G Gene Truncation Reduces Neurovirulence and Enhances Immunogenicity in Mice. J Virol 2008, 82:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fuchs JD, Frank I, Elizaga ML, Allen M, Frahm N, Kochar N, Li S, Edupuganti S, Kalams SA, Tomaras GD, et al. : First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Vesicular Stomatitis Virus Human Immunodeficiency Virus-1 gag Vaccine (HVTN 090). Open Forum Infect Dis 2015, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brandsma JL, Shylankevich M, Su Y, Roberts A, Rose JK, Zelterman D, Buonocore L: Vesicular Stomatitis Virus-Based Therapeutic Vaccination Targeted to the E1, E2, E6, and E7 Proteins of Cottontail Rabbit Papillomavirus. J Virol 2007, 81:5749–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brandsma JL, Shlyankevich M, Buonocore L, Roberts A, Becker SM, Rose JK: Therapeutic efficacy of vesicular stomatitis virus-based E6 vaccination in rabbits. Vaccine 2007, 25:751–762. [DOI] [PubMed] [Google Scholar]
- 160.Brandsma JL, Shlyankevich M, Su Y, Zelterman D, Rose JK, Buonocore L: Reversal of papilloma growth in rabbits therapeutically vaccinated against E6 with naked DNA and/or vesicular stomatitis virus vectors. Vaccine 2010, 28:8345–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Palin A, Chattopadhyay A, Park S, Delmas G, Suresh R, Senina S, Perlin DS, Rose JK: An optimized vaccine vector based on recombinant vesicular stomatitis virus gives high-level, long-term protection against Yersinia pestis challenge. Vaccine 2007, 25:741–750. [DOI] [PubMed] [Google Scholar]
- 162.Chattopadhyay A, Park S, Delmas G, Suresh R, Senina S, Perlin DS, Rose JK: Single-dose, virus-vectored vaccine protection against Yersinia pestis challenge: CD4+ cells are required at the time of challenge for optimal protection. Vaccine 2008, 26:6329–6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jiang P, Liu Y, Yin X, Yuan F, Nie Y, Luo M, Aihua Z, Liyin D, Ding M, Deng H: Elicitation of neutralizing antibodies by intranasal administration of recombinant vesicular stomatitis virus expressing human immunodeficiency virus type 1 gp120. Biochem Biophys Res Commun 2006, 339:526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Publicover J, Ramsburg E, Rose JK: A Single-Cycle Vaccine Vector Based on Vesicular Stomatitis Virus Can Induce Immune Responses Comparable to Those Generated by a Replication-Competent Vector. J Virol 2005, 79:13231–13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Daddario-DiCaprio KM, Geisbert TW, Geisbert JB, Ströher U, Hensley LE, Grolla A, Fritz EA, Feldmann F, Feldmann H, Jones SM: Cross-Protection against Marburg Virus Strains by Using a Live, Attenuated Recombinant Vaccine. J Virol 2006, 80:9659–9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kapadia SU, Rose JK, Lamirande E, Vogel L, Subbarao K, Roberts A: Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 2005, 340:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Luo M, Yuan F, Liu Y, Jiang S, Song X, Jiang P, Yin X, Ding M, Deng H: Induction of neutralizing antibody against human immunodeficiency virus type 1 (HIV-1) by immunization with gp41 membrane-proximal external region (MPER) fused with porcine endogenous retrovirus (PERV) p15E fragment. Vaccine 2006, 24:435–442. [DOI] [PubMed] [Google Scholar]
- 168.Ramsburg E, Rose NF, Marx PA, Mefford M, Nixon DF, Moretto WJ, Montefiori D, Earl P, Moss B, Rose JK: Highly Effective Control of an AIDS Virus Challenge in Macaques by Using Vesicular Stomatitis Virus and Modified Vaccinia Virus Ankara Vaccine Vectors in a Single-Boost Protocol. J Virol 2004, 78:3930–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Van Rompay KKA, Abel K, Earl P, Kozlowski PA, Easlick J, Moore J, Buonocore-Buzzelli L, Schmidt KA, Wilson RL, Simon I, et al. : Immunogenicity of viral vector, prime-boost SIV vaccine regimens in infant rhesus macaques: Attenuated vesicular stomatitis virus (VSV) and modified vaccinia Ankara (MVA) recombinant SIV vaccines compared to live-attenuated SIV. Vaccine 2010, 28:1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Reuter JD, Vivas-Gonzalez BE, Gomez D, Wilson JH, Brandsma JL, Greenstone HL, Rose JK, Roberts A: Intranasal Vaccination with a Recombinant Vesicular Stomatitis Virus Expressing Cottontail Rabbit Papillomavirus L1 Protein Provides Complete Protection against Papillomavirus-Induced Disease. J Virol 2002, 76:8900–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Roberts A, Reuter JD, Wilson JH, Baldwin S, Rose JK: Complete Protection from Papillomavirus Challenge after a Single Vaccination with a Vesicular Stomatitis Virus Vector Expressing High Levels of L1 Protein. J Virol 2004, 78:3196–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Buonocore L, Blight KJ, Rice CM, Rose JK: Characterization of Vesicular Stomatitis Virus Recombinants That Express and Incorporate High Levels of Hepatitis C Virus Glycoproteins. J Virol 2002, 76:6865–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kahn JS, Roberts A, Weibel C, Buonocore L, Rose JK: Replication-Competent or Attenuated, Nonpropagating Vesicular Stomatitis Viruses Expressing Respiratory Syncytial Virus (RSV) Antigens Protect Mice against RSV Challenge. J Virol 2001, 75:11079–11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Johnson JE, McNeil LK, Megati S, Witko SE, Roopchand VS, Obregon JH, Illenberger DM, Kotash CS, Nowak RM, Braunstein E, et al. : Non-propagating, recombinant vesicular stomatitis virus vectors encoding respiratory syncytial virus proteins generate potent humoral and cellular immunity against RSV and are protective in mice. Immunol Lett 2013, 150:134–144. [DOI] [PubMed] [Google Scholar]
- 175.Grigera P, Marzocca M, Capozzo AV, Buonocore L, Donis R, Rose J: Presence of bovine viral diarrhea virus (BVDV) E2 glycoprotein in VSV recombinant particles and induction of neutralizing BVDV antibodies in mice. Virus Res 2000, 69:3–15. [DOI] [PubMed] [Google Scholar]
- 176.Schlereth B, Rose JK, Buonocore L, ter Meulen V, Niewiesk S: Successful Vaccine-Induced Seroconversion by Single-Dose Immunization in the Presence of Measles Virus-Specific Maternal Antibodies. J Virol 2000, 74:4652–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schlereth B, Buonocore L, Tietz A, Meulen V ter, Rose JK, Niewiesk S: Successful mucosal immunization of cotton rats in the presence of measles virus-specific antibodies depends on degree of attenuation of vaccine vector and virus dose. J Gen Virol 2003, 84:2145–2151. [DOI] [PubMed] [Google Scholar]
- 178.Haglund K, Forman J, Kräusslich H-G, Rose JK: Expression of Human Immunodeficiency Virus Type 1 Gag Protein Precursor and Envelope Proteins from a Vesicular Stomatitis Virus Recombinant: High-Level Production of Virus-like Particles Containing HIV Envelope. Virology 2000, 268:112–121. [DOI] [PubMed] [Google Scholar]
- 179.Haglund K, Leiner I, Kerksiek K, Buonocore L, Pamer E, Rose JK: High-Level Primary CD8+ T-Cell Response to Human Immunodeficiency Virus Type 1 Gag and Env Generated by Vaccination with Recombinant Vesicular Stomatitis Viruses. J Virol 2002, 76:2730–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schwartz JA, Buonocore L, Roberts A, Suguitan A, Kobasa D, Kobinger G, Feldmann H, Subbarao K, Rose JK: Vesicular stomatitis virus vectors expressing avian influenza H5 HA induce cross-neutralizing antibodies and long-term protection. Virology 2007, 366:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.*.Furuyama W, Reynolds P, Haddock E, Meade-White K, Quynh Le M, Kawaoka Y, Feldmann H, Marzi A: A single dose of a vesicular stomatitis virus-based influenza vaccine confers rapid protection against H5 viruses from different clades. npj Vaccines 2020, 5:4.This study showed that a vesicular stomatitis virus-based H5N1 influenza virus vaccine was protective against challenge with a variety of H5 viruses in mice, implicating its potential as a pan-H5 incluenza vaccine.
- 182.Johnson JE, Schnell MJ, Buonocore L, Rose JK: Specific Targeting to CD4+ Cells of Recombinant Vesicular Stomatitis Viruses Encoding Human Immunodeficiency Virus Envelope Proteins. J Virol 1997, 71:5060–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rose NF, Roberts A, Buonocore L, Rose JK: Glycoprotein Exchange Vectors Based on Vesicular Stomatitis Virus Allow Effective Boosting and Generation of Neutralizing Antibodies to a Primary Isolate of Human Immunodeficiency Virus Type 1. J Virol 2000, 74:10903–10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Davis BM, Rall GF, Schnell MJ: Everything You Always Wanted to Know About Rabies Virus (But Were Afraid to Ask). Annu Rev Virol 2015, 2:451–471. [DOI] [PMC free article] [PubMed] [Google Scholar]


