COVID-19 has ravished the world, with secondary consequences that are not yet possible to estimate. WHO and the European Extracorporeal Life Support Organization (ELSO) recommended extracorporeal membrane oxygenation (ECMO) early in the pandemic, according to the standard criteria. In March, 2020, the EuroELSO survey was established to report the use of ECMO and outcomes in patients with COVID-19 once per week.1
Several months into the pandemic, we learned empirically that steroids and thromboprophylaxis improved outcome, which was confirmed by subsequent studies.2, 3, 4 Data from the EuroECMO survey collected between March 12, 2020, and Sept 14, 2020 (ie, the first wave), and other multicentre aggregates showed favourable outcomes with survival of 55–60%.1, 5 Less encouraging outcomes were also reported, with survival rate of less than 30%.6
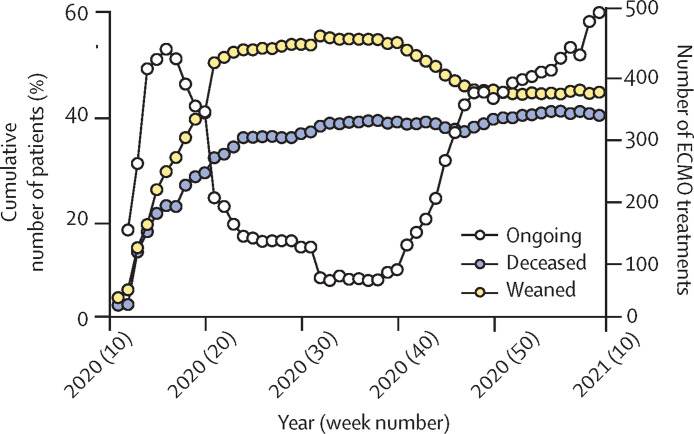
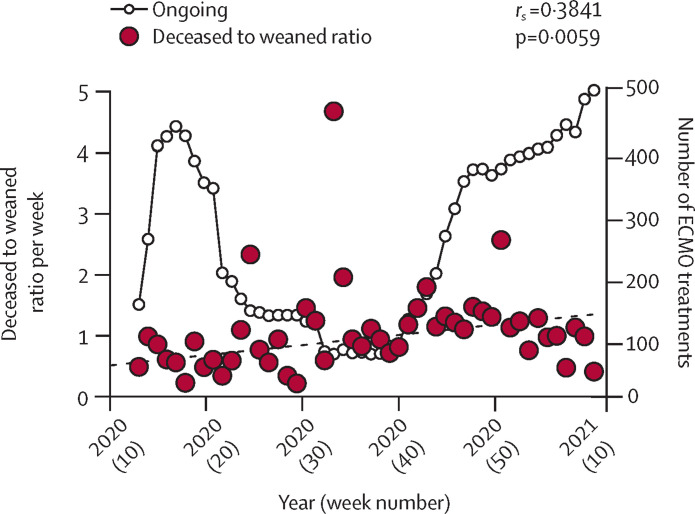
We analysed the continuous provision of ECMO for patients with COVID-19 during the first and second waves from the EuroECMO survey. Our results indicate that the clinical picture has changed during the second wave (between Sept 15, 2020, and March 8, 2021). Fatality and successful weaning curves approach each other, indicating an increase in mortality compared with weaning and survival (figure 1 ). An analysis of the deceased to weaned ratio during 2020 shows a significantly increasing trend over time (figure 2 ). During the spring and early summer of 2020, this ratio was less than 1—ie, the number of weaned (survivors from ECMO) was higher than the number of deceased. Currently, this ratio is more than 1, indicating worse outcome (p<0·006; median–median linear regression). The same pattern emerges concerning survival between first and second waves on the basis of data released on March 8, 2021. In the first wave, successful weaning was accomplished in 58% (841 of 1442) of patients, compared with 47% (718 of 1723; p<0·0001) in the second wave. Including deaths reported after successful weaning, survival was 53% (770) in the first wave and 44% (677; p<0.0001) in the second wave.
Figure 1.
Cumulative outcome of ECMO for COVID-19
ECMO support from March 12, 2020, to March 8, 2021. First wave, defined from disease onset to Sept 14, 2020. Second wave defined from Sept 15, 2020, to March 8, 2021. ECMO=extracorporeal membrane oxygenation.
Figure 2.
Relationship between survivors and patients deceased from COVID-19 supported with ECMO
Median–median linear regression (dashed line). First wave defined from disease onset to Sept 14, 2020. Second wave defined from Sept 15, 2020, to March 8, 2021. ECMO=extracorporeal membrane oxygenation.
Europe is entering the third wave of the COVID-19 pandemic. Most patients are given steroids during admission to hospital and other adjuvants might be used. Thus, when a patient is recognised as a candidate for ECMO, they are already receiving steroids. Data from the Intensive Care National Audit and Research Centre and a French study showed increased mortality in patients on mechanical ventilation during the second wave.4 Drawing any conclusions from these data might be premature, but the slope of clinical deterioration might be reduced. Patients could have been on mechanical ventilation for longer in the second wave than in the first wave with a selection bias towards patients who would not survive on mechanical ventilation or ECMO. The time on ECMO is longer in patients with COVID-19 than in patients with any other diagnoses, and the number of patients on long-term ECMO (>28 days) and the number of futile cases have also increased. Would awake ECMO and early extubation affect the disease course and improve outcomes? When the means to overcome this disease are not successful, should ECMO treatment be withdrawn or continued as a bridge to lung transplantation for suitable candidates? These questions evoke new clinical and ethical discussions.
One limitation of this primary analysis was that ECMO support was still ongoing in 25% of patients in the second wave compared with approximately 5% in the first wave, and the database did not allow for adjustment of time from mechanical ventilation to ECMO.
The ECMO community needs to be informed about these preliminary results. However, further prediction models are needed to enable identification of patients that might benefit most from ECMO.
Members of the collaborator groups are listed in the appendix (pp 1–12). LMB is an advisor for Eurosets and Xenios. RL is a consultant for Medtronic, Getinge, LivaNova, and Abiomed; and an advisor for Eurosets. All other authors declare no competing interests. We thank Peter Radell (Karolinska University Hospital, Stockholm, Sweden) for editing the English language.
Supplementary Material
References
- 1.Lorusso R, Combes A, Coco VL, De Piero ME, Belohlavek J. ECMO for COVID-19 patients in Europe and Israel. Intensive Care Med. 2021;47:344–348. doi: 10.1007/s00134-020-06272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Contou D, Fraissé M, Pajot O, Tirolien JA, Mentec H, Plantefève G. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? Crit Care. 2021;25:3. doi: 10.1186/s13054-020-03449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the extracorporeal life support organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.