Abstract
Tuft cells—rare, secretory epithelial cells—generated scant immunologic interest until contemporaneous reports in 2016 linking tuft cells with type 2 immunity in the small intestine. These findings generated increasing scrutiny of these cells by the immunology community. Tuft cells have the capacity to produce an unusual spectrum of biological effector molecules, including interleukin 25 (IL-25); eicosanoids implicated in allergy, including cysteinyl leukotrienes and prostaglandin D2 (PGD2); and the neurotransmitter, acetylcholine (ACh). In most cases the extracellular signals which control tuft cell effector function are unknown, but signal transduction is thought to proceed via canonical, G-protein-coupled receptor (GPCR)-coupled pathways involving components of the signaling pathway by which Type II taste bud cells sense sweet, bitter and umami compounds. Tuft cells are ideally positioned as chemosensory sentinels, which can potentially detect and relay information from diverse luminal substances through what appear to be stereotyped outputs to populations of immune and neuronal cells to initiate both positive and aversive responses. Despite recent insights, numerous questions remain involving tuft cell lineage, diversity, effector mechanisms, and how tuft cells interface with the immunological niche across the tissues where they reside.
Introduction
Tuft cells were unfamiliar to most immunologists until three contemporaneous reports in 2016 identified these rare, chemosensory epithelial cells as a major constituent of the small intestinal response to parasitic helminths and protists1–3. Tuft cells were identified almost 100 years ago by the ‘tuft-like’ brush of apical microvilli that extended from solitary, bottle-shaped epithelial cells into the hollow lumen of mucosal organs of mammals. Lacking any understanding of their function, tuft cells were identified using morphologic criteria, whereby they are readily identifiable in many organs, including the airways (‘brush’ cells), nasopharyngeal cavities (‘microvillous’ cells), and the intestine (‘multivesicular’ cells). Recent reviews highlight older literature for interested readers4–6.
A major advance occurred in 2008, when mouse small intestine epithelial chemosensory cells, identified using a fluorescent reporter for expression of transient receptor potential cation channel subfamily M member 5 (TRPM5), a calcium-activated ion channel critical for signaling in Type II taste cells, were characterized using mRNA microarray analysis7. TRPM5+ tuft cells expressed canonical taste receptor signaling genes, as predicted, but also an array of neuronal and inflammatory gene pathways not previously associated with epithelial cells, including the enzymes involved in biosynthesis of acetylcholine (ACh) and eicosanoids, as well as the transcript for interleukin (IL)-25. This manuscript attracted little attention from the immunology community at the time, but set the stage for ready identification and characterization of these enigmatic cells. Prompted by increased interest in active roles of epithelial cells in initiating and propagating immune responses, and armed with more precise reagents, the 2016 reports identified a key role for tuft cells as the source of IL-25 implicated in intestinal immunity and showed these rare cells were dynamically regulated and central to type 2 immune circuits involving ILC2s and crypt epithelial cell progenitors (Fig. 1)1–3.
Figure 1. The small intestinal tuft cell ILC2 circuit.
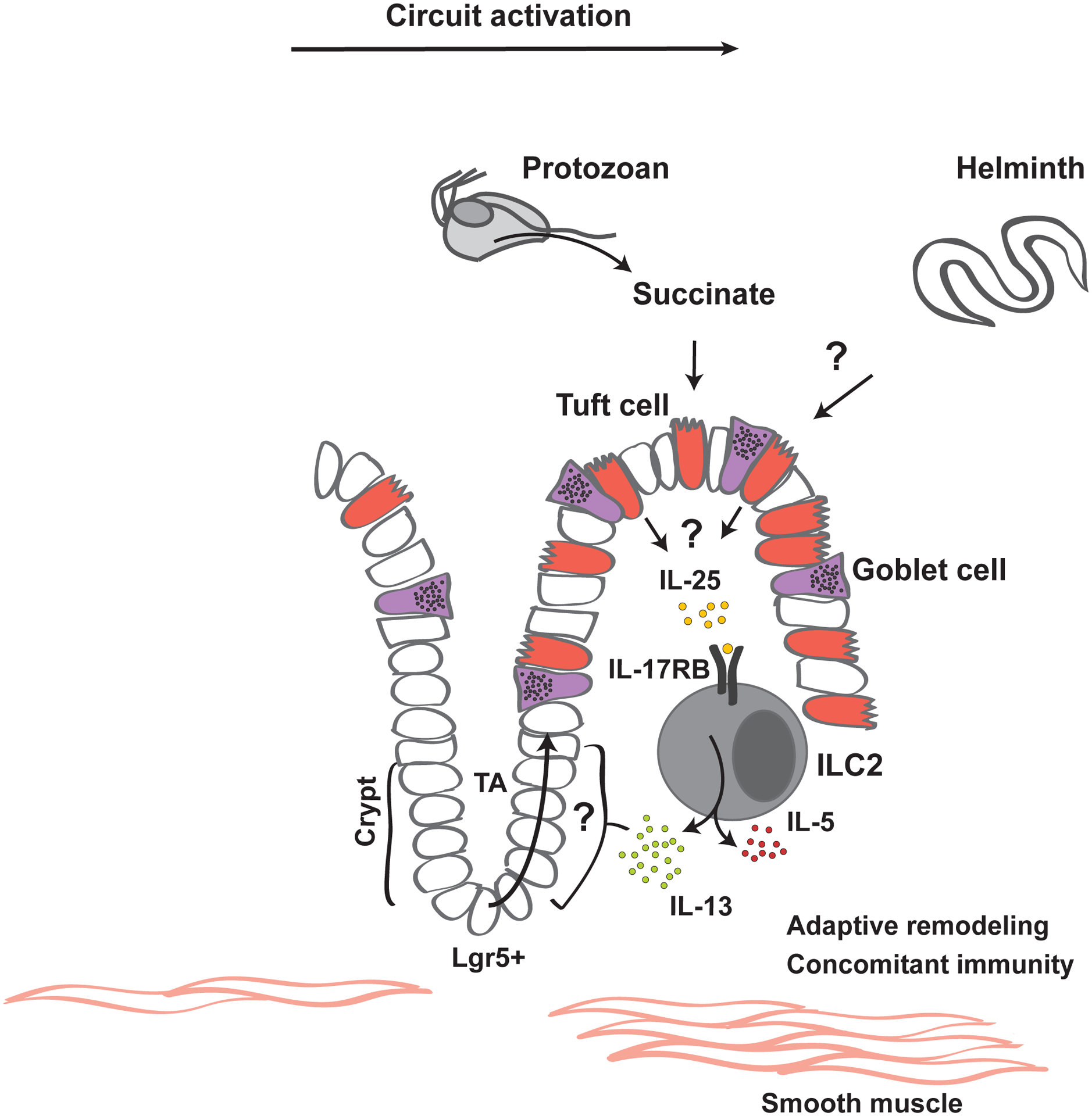
Rare gustatory epithelial tuft cells detect the presence of luminal pathosymbionts, including the protist Tritrichomonas and intestinal helminths. Tuft cells activate IL-17RB-expressing (IL-25R competent) ILC2s in the lamina propria in an IL-25-dependent manner, which involves taste sensation components; this, along with potential uncharacterized mechanisms, results in release of IL-25 from tuft cells. Activated ILC2s increase IL-13 expression, which acts on epithelial progenitor(s) to promote lineage specification towards tuft and goblet cells, thereby creating a feed-forward circuit through expansion of IL-25-expressing tuft cells.The metabolite succinate is sufficient for circuit activation; elevated succinate in vivo occurs downstream of colonization with Tritrichomonas, which are anaerobic protists that excrete succinate as a metabolic end-product. The identity of other tuft cell-activating ligands remains unknown, as succinate sensing is not required for circuit activation in response to helminths. Rapid epithelial replenishment, tuft cell gustatory signal transduction and rapid IL-13 expression by poised tissue-resident ILC2s contribute to the high sensitivity and dynamic nature of this circuit. Beyond the acute phases of increased tuft cell abundance and IL-13 production, more durable effects of circuit activation include smooth muscle hyperplasia, adaptive remodeling of epithelial and lamina propria compartments, and concomitant immunity, by which subsequent infections become attenuated. Additional ILC2-activating signals may contribute to modulating this circuit and might include tuft cell effectors (discussed in the text), IL-33 and neuropeptides, such a neuromedin U.
Here, we review the recent advances in phenotypic and functional aspects of tuft cells, emphasizing work that informs a more inclusive definition of the tuft cell lineage across tissues and elucidates upstream inputs and downstream outputs that interact with the immune system.
Definition and tissue distribution
Tuft cells have been defined with increasingly precise genetic markers and transcriptional analyses, and are readily identified based on a conserved core set of characteristics, summarized elsewhere8. Here, we define tuft cells as an epithelial lineage with (1) typical morphologic characteristics – bottle-shaped intraepithelial cells with apical microvilli – that (2) are dependent for their development on the transcription factor POU2F3, (3) utilize key constituents of the taste receptor signaling cascade, including TRPM5, and (4) express IL-25 and components of eicosanoid biosynthetic pathways, including cyclooxygenase (COX)-1 and -2, and arachidonate 5-lipoxygenase (ALOX5). Most tuft cells express choline acetyltransferase (ChAT) necessary for ACh synthesis and double cortin-like kinase 1 (DCKL1), a serine-threonine kinase involved in microtubule polymerization. Studies to date have fate-mapped tuft cells from basal epithelial progenitors in several tissues; no evidence for trans-differentiation from other differentiated epithelial cells has been reported.
In mouse, where these cells are best characterized, tuft cells are rare, primarily solitary cells in mucosal epithelia of the respiratory and gastrointestinal tracts. In the respiratory tract, tuft cells are present in both respiratory and olfactory epithelia of the nose, the trachea, and proximal airways. In the gastrointestinal tract, tuft cells are found in the stomach, throughout the small and large intestine, and within the pancreato-biliary system. Tuft cells are prevalent in mice at the gastric ridge delineating the transition from squamous to columnar epithelium—the gastric ridge is not present in humans, although solitary tuft cells are present in human gastric mucosa. Tuft cells are highly prevalent in gallbladder in a number of mammals, and found throughout the pancreato-biliary epithelia, comprising the extrahepatic bile ducts and the major pancreatic ducts. Tuft cells are also present in columnar epithelia of the urethra, auditory canals and nasal aspects of the conjunctiva.
The development of reporter mice (TRPM5, IL-25 and others), recognition of POU2F3 as a lineage-defining transcription factor, and the unique tuft cell transcriptional program have enabled recognition of additional specialized epithelial cell types as members of the tuft cell family. Type II taste cells share morphology, POU2F3-dependency and defining markers, including IL-25, that justifies considering these cells as tuft cells8, and supporting further investigations to understand the use of core taste signaling pathways by tuft cells in all tissues. A specialized population of thymic medullary epithelial cells was also recently described9,10 that shares POU2F3-dependency and TRPM5- dependent function.
Diversity and expression profiles
Despite the unique properties and functions of the tissues in which tuft cells are found, including diverse tissue-specific progenitor compartments, tuft cells express a remarkably uniform core gene expression profile when analyzed comparatively across distinct tissues11. This transcriptional similarity suggests conserved epithelial cell differentiation programs are enacted in diverse epithelia, driving convergence upon a highly similar tuft cell phenotype, which appears to be regulated, in part, by POU2F3 (BOX1: POU2F3 and identity)9,12. Among the core gene signature, IL-25 and biosynthetic pathways for soluble effector molecules—ACh, leukotrienes, and PGD2—feature prominently. As discussed below, functional studies of tuft cells have focused on production of IL-25 and, to a lesser extent, ACh. However, production of eicosanoids is an emerging area of interest with the potential to uncover additional tuft cell – immune cell circuits that may have relevance in inflammation or tissue homeostasis. An intriguing aspect of the tuft cell transcriptional program that remains largely unexplored is the unexpected expression of genes often considered to be specific for cells of hematopoietic origin, such as Vav1 and Ptprc (encoding CD45), which are important signaling components in immune cells.
BOX 1: POU2F3 and identity.
POU2F3 (SKN-1a, OCT11) is a subclass II Oct protein with affinity for a characteristic 8 bp consensus sequence [ATGC(A/T)AAT] (‘octamer motif’) and variants thereof81,82, and has emerged as a lineage-defining transcription factor for tuft cell specification. Although initially described to regulate keratinocyte differentiation in the skin, POU2F3 was later found to be expressed at high levels in TRPM5+ cells in taste buds and TRPM5+ epithelial tuft cells; these cells are absent in Pou2f3−/− mice3,83,84. Consistent with a function as a master regulator for tuft cell differentiation, accessible enhancer regions—examined in thymic tuft cells—are enriched in the POU2F3 binding motif suggesting that many tuft cell signature genes are likely directly regulated by POU2F39. Further, in a small cell lung cancer variant cell line with low neuroendocrine signature but high Pou2f3 expression, ChIP-seq analysis revealed that POU2F3 controls a set of well-known tuft cell-specific genes12, raising the intriguing possibility that malignant POU2F3-dependent cells in certain small cell lung cancer variants might arise from tuft cells. It will be important to perform similar ChIP-seq analysis in bona fide tuft cells, possibly in combination with SOX4, which is required for IL-13–induced tuft cell expansion in the small intestine85, and GFI1B, another tuft cell-specific transcription factor with yet unknown function86. How Pou2f3 expression is regulated, which likely occurs as one of the earliest steps in tuft cell specification from epithelial progenitor cells, and whether expression of POU2F3 is sufficient to confer tuft cell identity requires further study.
Although transcriptionally and morphologically similar across tissues, transcriptional profiling as determined by population-based and single cell RNA sequencing (scRNA-seq) has uncovered tuft cell heterogeneity both across8,11 and within tissues9,10,13–15. Among different tissues, heterogeneity has yet to be fully explored. Tissue-specific genes may give clues to tissue-specific functions. Such is the case for the G-protein-coupled receptor (GPCR) succinate receptor 1 (SUCNR1, GPR91), which is abundantly expressed in small intestinal tuft cells, enabling succinate-triggered immune circuit activation as described in more detail below11,16,17 (Fig 1, Fig 2a). The successful identification of a dedicated ligand-receptor pair with functional consequences for intestinal tuft cells suggests that other tissue-restricted receptors should be a focus of future studies.
Figure 2. GPCR signaling in tuft and tuft-like cells.
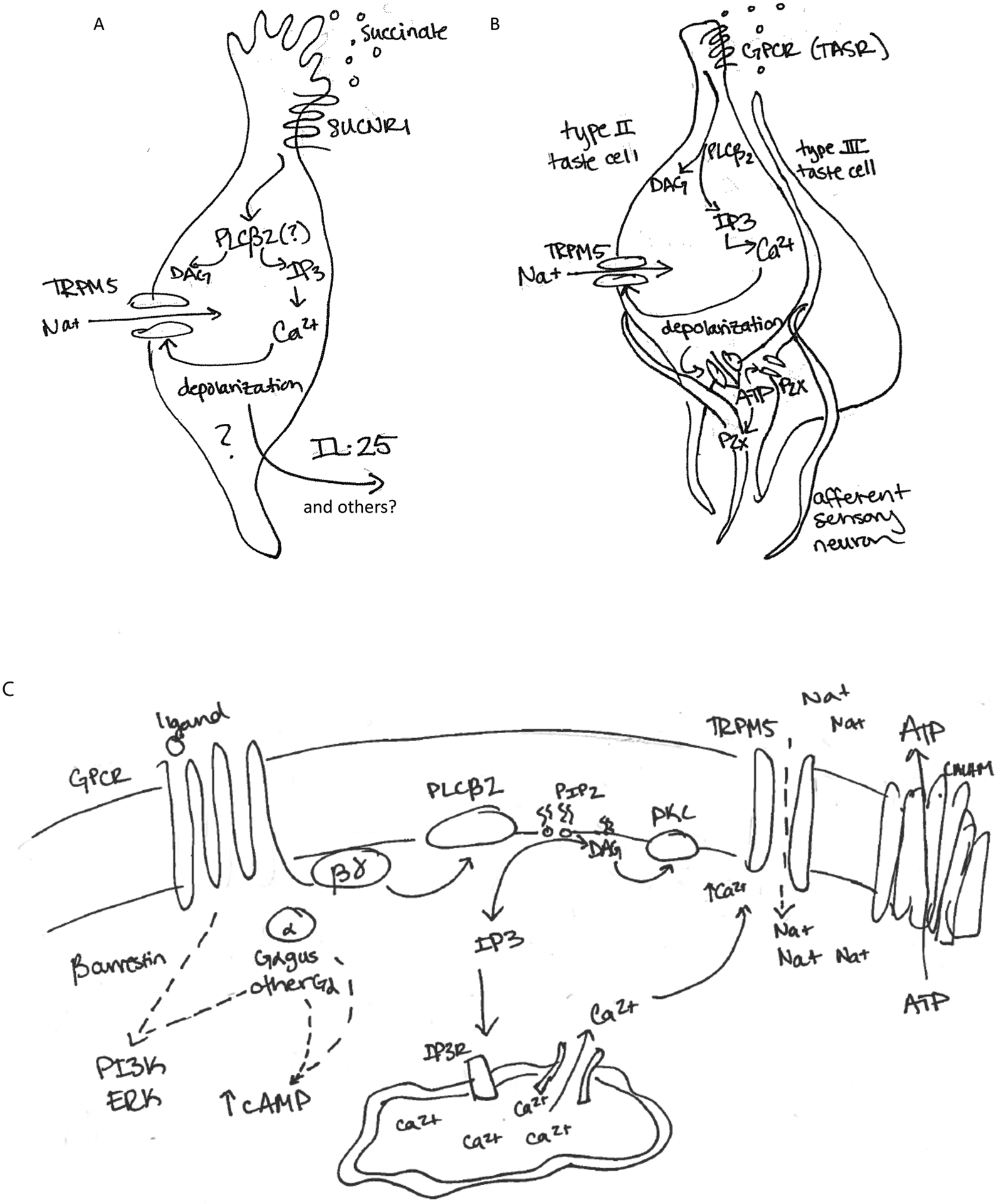
A) Through the use of knockout mice, it has been shown that effector functions (IL-25) of tuft cells downstream of succinate receptor ligand engagement are dependent on TRPM5 and the Gα protein α-gustducin (Gαgust). This suggests that typical taste receptor signaling pathways are involved in succinate responses, possibly including a depolarization-dependent mechanism for secretion of tuft cell effector molecules like IL-25 and/or other effector molecules. B) Taste receptor engagement on Type II taste cells activates a stereotypical taste transduction cascade to facilitate release of ATP which acts on afferent sensory neurons32,49. C) Taste receptor/GPCR ligand binding induces release of the Gα protein from the trimeric G protein complex. Gβγ activates PLCβ2 cleavage of PIP2 into IP3 and DAG. IP3 binds the IP3R on the endoplasmic reticulum, releasing intracellular calcium stores into the cytosol. Increased cytosolic calcium activates the inwardly rectifying sodium channel, TRPM5. Sodium influx depolarizes the cell body, and ATP is released via a CALHM1/3 channel. Additional signaling pathways engaged include DAG/calcium activation of PKC, Gα signaling, and additional calcium-dependent processes including activation of PLA.
Heterogeneity among tuft cells within the same tissue has been demonstrated in the thymus, the trachea, and the small intestine9,10,13,15. In the small intestine, Haber and colleagues propose the existence of two tuft cell subtypes (denoted tuft-1 and tuft-2). Though generally very similar, tuft-1 cells appear more poised to act as neuromodulating cells, while tuft-2 cells express higher levels of genes suggestive of immunological function13. A similar paradigm has emerged in analysis of tracheal tuft cells, which clustered into cells enriched in certain taste-transduction components and cells with higher expression of leukotriene-biosynthesis and immune-related genes; this diversity may be controlled by shifts in the expression of particular transcription factors15. Future studies will be necessary to functionally validate the consequences of these relatively modest differences in tuft cell gene expression, and to determine whether they arise stochastically, in association with differences in ontogeny8, or represent spatial signatures18. Notably, thymic tuft cells show heterogeneous expression of bitter taste receptors10, and it will be important to assess whether chemosensory receptor repertoire is a feature driving tuft cell heterogeneity in all tissues.
Tuft cell inputs.
Gene expression analysis and functional evidence suggest that GPCR signaling is a dominant mechanism whereby tuft cells perceive signals from the environment, although other inputs, including direct interaction with neighboring cells – possibly through cytospinules19 – cannot be excluded. Aspects of GPCR biology have been reviewed extensively20–23. We focus here on two types of GPCR inputs relevant for tuft cells.
Succinate.
The dicarboxylic acid succinate is an intermediate or end product in the metabolism of eukaryotes and prokaryotes. Intracellular succinate provides a link between the Krebs cycle and the mitochondrial respiratory chain, and is an important metabolic indicator of the mitochondrial status24. Extracellular succinate can be detected by a dedicated transmembrane GPCR, SUCNR1, also known as GPR9125. In 2018, three groups independently identified succinate as a potent tuft cell – ILC2 circuit agonist, which activates tuft cells in a SUCNR1-, IL-25-, and POU2F3-dependent manner to promote small intestinal ILC2 proliferation and IL-13 expression and subsequent tuft cell expansion11,16,17 (Fig. 1). Relative to tuft cells in other tissues, small intestinal tuft cells differentially express Sucnr1, also evident in prior work7,13, enabling activation of the circuit by succinate-producing luminal organisms, including the intestinal protist Tritrichomonas and certain bacteria; circuit activation required the canonical taste signaling transduction components TRPM5 and α-gustducin, suggestive of a depolarization-dependent mechanism for IL-25 release although this has not been shown11,16,17 (Fig. 2a). Although succinate is also released by intestinal helminths26, circuit activation as assessed by tuft cell expansion and worm clearance was unimpaired in Sucnr1−/− mice, implicating alternative or redundant pathways for tuft cell activation11. Notably, extracellular succinate levels are increased under certain inflammatory and hypoxic conditions, including in ischemia and reperfusion injury27, and in synovial fluid from rheumatoid arthritis patients28. Succinate can accumulate locally and in the circulation in the context of reduced oxidative phosphorylation, either due to lack of O2 or metabolic reprogramming, where it activates pro-inflammatory cytokine expression in macrophages by activation of HIF1α, in part through reverse electron transport and production of reactive oxygen species29,30. Further study is needed to evaluate whether tuft cell SUCNR1 is engaged in these conditions.
Taste ligands.
Among the three types (I, II, and III) of taste cells, which are localized together in pseudostratified columnar epithelial taste bud structures, Type II taste cells are strikingly similar to tuft cells, both morphologically and in their gene expression31. Type II taste cells express combinatorial arrays of GPCRs involved in taste reception for sweet (heterodimers of taste receptor type 1 member 2 (T1R2) and T1R3), umami (T1R1/T1R3 heterodimers) and bitter (T2Rs) tastants32. The genes for T1R1–3 are expressed in syntenic regions in both mouse and human. The genes for T2Rs represent a different class of GPCRs, expressed from ~30–40 functional genes with many additional pseudogenes. Simplistically, sweet and umami perceptions are oriented towards nutrient preferences for starch and protein, and are broadly conserved, whereas aversive responses to potential bitter toxins have expanded evolutionarily. Most functional T2Rs have been deorphanized using bitter compounds in calcium-activation screens, revealing that most receptors are broadly reactive to bitter compounds while others are selectively tuned to a single or highly limited repertoire of bitter tastants33. Activation of Type II taste cells with tastants leads to stimulation of the canonical taste transduction cascade—present in all tuft cells—which involves α-gustducin (GNAT3), phospholipase C β2 (PLCβ2), intracellular calcium mobilization, and activation of TRPM5, a calcium-activated cation channel that promotes Na+ entry to depolarize the cell32 (Fig. 2 b,c). In canonical taste transduction, TRPM5-dependent ATP release (discussed further below) from Type II taste cells is thought to be critical in taste sensation (Fig 2b). The role of additional common GPCR cascades, including cyclic AMP and β-arrestin pathways, has not been interrogated in tuft cells. Analysis by scRNA-seq revealed that multiple taste receptors are expressed in each Type II taste cell; expression of taste receptors in tuft cells outside the taste bud, though variable, has been noted in all tissues examined10,11,31. As discussed below, bitter T2R ligands may be activators of tuft cells; in this context, research has focused on tuft cell production of ACh.
Tuft cell outputs.
Acetylcholine.
Most tuft cells exhibit constitutive expression of choline acetyltransferase (ChAT). Highly expressed in cholinergic neurons, ChAT synthesizes the neurotransmitter ACh from mitochondrially-derived acetyl-CoA and extracellular choline (Fig. 3A). Concentration of ACh in vesicles is mediated by the vesicular ACh transporter, or VAChT (Slc18a3). Alhough not associated with the tuft cell core gene signature, Slc18a3 is encoded within the first intron of the highly conserved ChAT gene; mechanisms by which the genes might be regulated separately have been proposed34,35. Extracellular ACh activity is limited by acetyl- and butyrylcholinesterases36,37. Maintenance of ACh output requires choline import via the high-affinity choline uptake transporter, CHT1, considered to be rate-limiting in ACh synthesis38. Investigations of bitter T2R ligands, such as acyl-homoserine lactones used for quorum sensing in Gram-negative bacteria, demonstrated TRPM5-mediated ACh release from nasal tuft cells that elicited mast cell-mediated inflammation and a neuronal apneic response in mice39,40. Similar ligands generated tuft cell activation in human nasal epithelial cultures, prompting release of antimicrobial peptides from adjacent epithelial cells41. Activation of the T1R2/T1R3 sweet receptors using bacterial D-amino acids interfered with this pathway, suggesting that bacterial colonization of the upper respiratory tract could be modulated by epithelial cell taste receptor signaling42. A bitter Tas2R108 agonist, cycloheximide, elicited a drop in respiratory rate when applied to the trachea, where tuft cells were demonstrated to express ChAT and VAChT, suggesting a role for local ACh release on adjacent cholinergic neurons43. Elsewhere, bitter and umami ligands (denatonium and monosodium glutamate, respectively) elicited ACh release from urethral epithelia and led to bladder smooth muscle contraction, consistent with a pathway linking taste receptors with ACh activity in peripheral tuft cell populations44. Ligand-activated Type II taste cells also secrete ACh to drive muscarinic autocrine feedback amplification by enhancing ATP release45.
Figure 3. Biosynthetic pathways for putative tuft cell effector molecules.
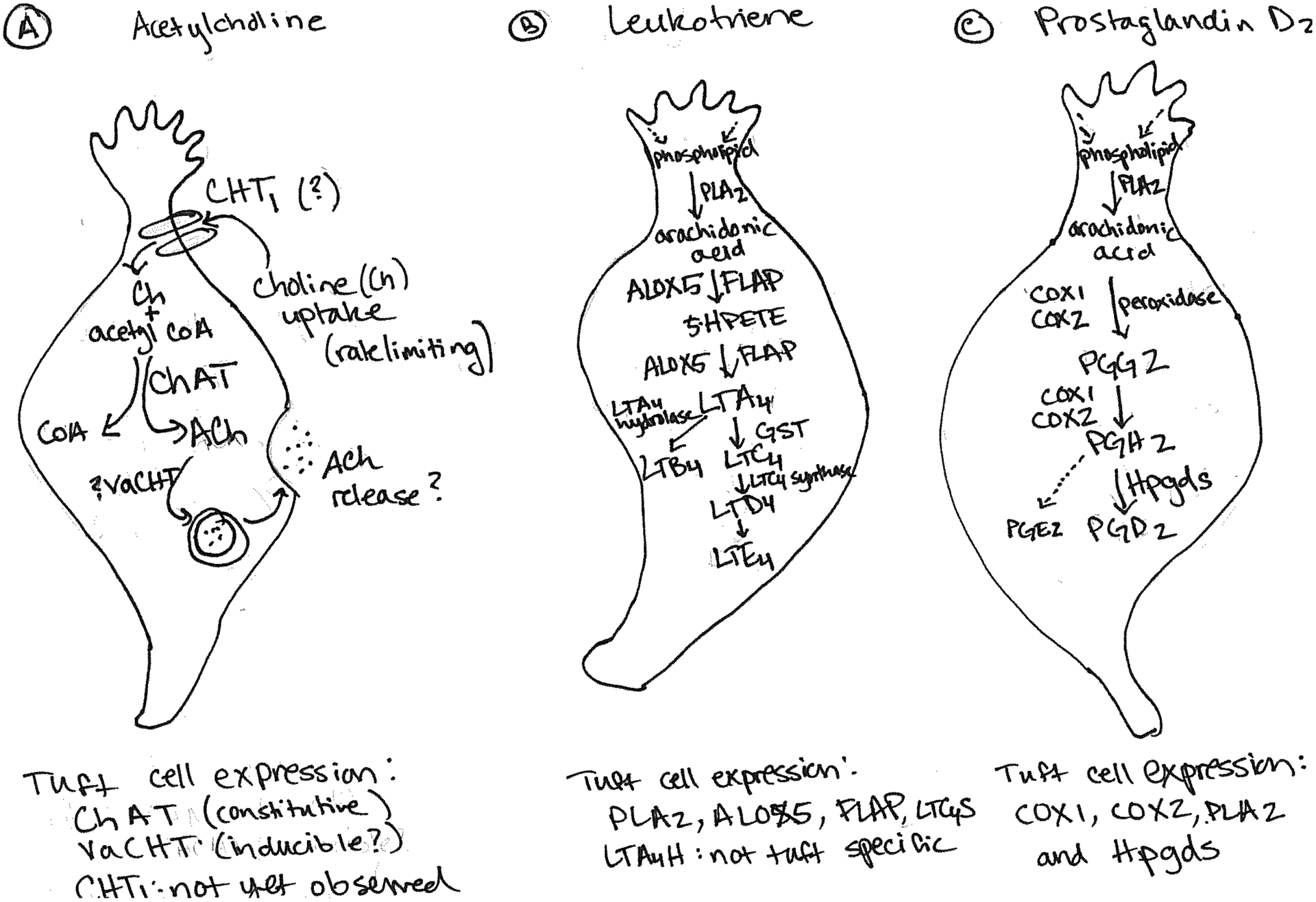
A) Biosynthesis of acetylcholine (ACh) requires extracellular choline, imported via the high affinity choline transporter, CHT1, and mitochondrial acetyl-CoA. Highly expressed in tuft cells, choline acetyltransferase (ChAT) produces ACh, which is then packaged into vesicles via the vesicular transporter VAChT. Sequencing data suggests that tuft cells in all tissues assayed to date lack expression of CHT1; VAChT expression may be inducible as noted in the text. B-C) Tuft cells express the critical enzymes for conversion of arachidonic acid into leukotrienes and PGD2, but do not express the synthase required for PGE2 production (B). While COX-1 is broadly expressed, among the small intestinal epithelium specific tuft cell staining has been observed78. COX-2 and HPGDS have also been validated as tuft cell markers by immunostaining, which may support constitutive activity of this pathway in tuft cells91. C) Similarly, tuft cells appear transcriptionally competent to produce leukotriene B4 (LTB4) and the cysteinyl leukotrienes leukotriene C4, D4 and E4 (LTC4, LTD4 and LTE4), arachidonic acid derivatives with multiple roles in immune function and inflammation69,92,93. Although likely, secretion of ACh, PGD2, and leukotriene has yet to be conclusively demonstrated from tuft cells.
Most tuft cells lack canonical constituents of the vesicular ACh transport sytem, however, as well as synaptic proteins or visible synapses in microscopic examination, raising questions regarding how tuft cell ACh might be stored, secreted and evade enzymatic degradation46. Cholinergic neurons densely innervate gastrointestinal and respiratory mucosa, with major effects on smooth muscle contractility and mucus production, while ACh itself is a potent goblet cell secretagogue, further confounding interpretation of the role of tuft cell-derived ACh. Many non-neuronal cells, including immune cells involved in type 2 immunity47, also express cholinergic pathway components. Studies of cell-intrinsic control of ChAT expression using floxed alleles in tuft cells (and conditional deletion of ACh receptors from possible target cells) will be necessary to establish firmly the role of ACh in tuft cell effector function.
ATP.
ATP functions as an important gustatory second-messenger in taste cells (Fig 2b,c)48. Taste-evoked action potentials open a voltage-gated nonselective channel to efflux ATP, which constitutes the requisite neurotransmitter for activation of P2X receptors on afferent gustatory neurons32. The ATP effluxing pore is a hexameric channel of calcium homeostasis modulator 1 (CALHM1) and CALHM3; the latter is essential to confer the fast gating kinetics for ATP release by the channel49. In mice, loss of CALHM1 or CALHM3 abolished GPCR-mediated taste reception, and was downstream of TRPM5 activation, similar to other tuft cell effector outputs49,50. Loss of CALMH1 attenuated ATP release and modulated ciliary beat frequency from mouse nasal epithelial monolayers in response to mechanical stimulation, but contributions from tuft cells were not assessed51. Purinoreceptors are engaged in numerous physiological processes and widely expressed, with clear roles in inflammation and immune cell function52, but the role for tuft cells (other than Type II taste cells) in ATP signaling has not been studied.
IL-25.
IL-25 (previously IL-17E) is a tuft cell lineage-defining cytokine as recently demonstrated by the constitutive expression of an IL-25 transcriptional reporter in tuft cells; in all tissues examined by reporter to-date, tuft cells appear to be the sole producers of IL-251–3. IL-25 belongs to the IL-17 family cytokines, which play key roles in epithelial biology and are important contributors to the crosstalk between luminal constituents, immune cells and epithelia53. Most of our understanding has been illuminated by studies of IL-17A, a key effector cytokine produced by ILC3s, γδ T cells and Th17 cells, and IL-17C, an epithelial-derived cytokine that intrinsically auto-amplifies IL-17A effects54. IL-17 cytokines are expressed in epithelia and immune cells of evolutionarily ancient organisms, such as lampreys55 and sea urchins56, and, in the latter, are early mediators of the response to inflammatory gut bacteria. Although the least related, human and mouse IL-25 were discovered based on sequence homology to IL-17 family members57,58. First implicated in allergic disorders due to its capacity to promote eosinophilia, goblet cell hyperplasia and IgE57, IL-25 is now known to be a major activating signal for ILC2s, promoting IL-5 and IL-13-mediated type 2 inflammation59–61, and recently described as a critical player in a tuft cell – ILC2 circuit that mediates small intestinal type 2 immune responses to luminal helminths and protists (Tritrichomonas muris)1–3. Whether IL-25 plays roles in response to other tissue commensal organisms or in other infectious contexts remains unexplored, in the small intestine and elsewhere (BOX2: Tuft cells in infectious disease).
BOX 2: Tuft cells and infectious diseases.
The role of tuft cells in type 2 host immune responses to pathogens is clear in the case of luminal parasitic infections. The role of tuft cells in other immune challenges, such as during bacterial or viral infection, has yet to be extensively studied, though the interaction of tuft cells with innate immune cells in parasitic responses suggests that other infections may engage similar or additional tuft cell-dependent immune circuits at barrier surfaces. Tuft cell chemosensory potential implicates them as key sensors of luminal signals, which are often associated with the presence of infectious organisms or the host response to pathogen invasion. Thus, tuft cells are positioned to orchestrate responses in the tissue that may balance aversive, defensive and adaptive processes. Examples of relevant luminal sensing include detection of bacterial quorum sensing and other molecules in upper airways and the urethra39,44, or the metabolite succinate in the small intestine, a major metabolic end product produced by protists and certain bacteria11,16,87. Tissue-specific detection capacities, encoded by tissue-specific receptor expression on tuft cells, could direct responses to relevant luminal stimuli, driving spatially restricted tuft cell-immune cell circuit activation. Such receptors, however, could also be co-opted by pathogens. In the case of murine norovirus infection, tuft cell-restricted expression of CD300lf in the intestinal epithelium serves as a critical co-receptor for murine norovirus—norovirus utilizes CD300lf to specifically infect intestinal tuft cells, promoting fecal shedding88,89. Tuft cells could also be indirectly involved in immune responses and infections. When elevated, as occurs in response to protist colonization, the increased tuft cell-dependent type 2 immune tone of the small intestine can serve to limit infections either locally, as occurs in co-infection of Tritrichomonas colonized mice with luminal helminths, or distally, as is the case when Tritrichomonas colonized mice are challenged with pathogenic bacteria16,90.
IL-25 signals through a heterodimeric receptor composed of IL-17RA and IL-17RB53; expression of the latter is enriched in ILC2s, in particular in the small intestine16,62,63. ILC2 depletion largely abrogates type 2 pathologies and anti-helminthic responses mediated by IL-251,60, suggesting that ILC2s are a major physiological target in the small intestine, although other cell types such as NKTs may also contribute64. Expression of Il17rb mRNA is present in tuft cells in multiple tissues and appears to be part of their core signature, but its role remains unknown7,9–11,13,14. Mice with conditional deletion of Il17rb in epithelial cells displayed normal worm clearance when infected with N. brasiliensis, suggesting that IL-17RB in tuft cells is not required for IL-25-mediated small intestinal type 2 responses (our own unpublished data).
Although a role for tuft cell-derived IL-25 in the small intestine is established, its function at other sites remains enigmatic. In the thymus, IL-25-expressing tuft cells comprise a subset of post-AIRE medullary thymic epithelial cells associated with Hassall’s corpuscles10. Thymic tuft cells were recently suggested to be important for establishing tolerance to tuft cell proteins and conditioning the type 2 cytokine thymic microenvironments necessary for development of certain invariant-like T cells populations, including NKT2 cells, EOMES+CD8+ single-positive thymocytes, and ILC2s; these populations were altered in mice lacking tuft cells (Pou2f3−/−) or taste signaling (Trpm5−/−), although the direct involvement of IL-25 was not assessed9,10. Thus epithelial circuits like the one in the small intestine thus far remain unidentified in other tissues. Whether tuft cell IL-25 secretion occurs constitutively or is regulated in the small intestine or elsewhere is unknown. Spatial segregation of tuft cells, which transit up the villi with epithelial progression, and their target cells in the small intestine lamina propria adds further complexity.
TSLP.
Thymic stromal lymphopoietin (TSLP) signals through a heterodimer of IL-7Rα and the TSLPR, CRLF2, (similar to the common γ chain cytokine receptor), and is considered an “epithelial cytokine”along with IL-33 and IL-25. Recent scRNA-seq approaches showed TSLP expression enriched in tuft cells among small intestinal and airway epithelial cells, suggesting that they might be a source of not one, but two important type 2-associated cytokines, namely IL-25 and TSLP13,15. Although tuft cell heterogeneity is incompletely understood8, the significantly higher expression of TSLP by tuft-2 compared to tuft-1 cells in the small intestine suggests that TSLP may be differentially regulated in subpopulations of tuft cells, in contrast to IL-25, which is expressed by all tuft cells. Whether tuft cells in other organs produce TSLP remains unknown. This may be of particular interest in the thymus where tuft cells are found in Hassall’s corpuscles9,10—TSLP expression in this anatomic location has been described as important for the instruction of DCs to induce regulatory T cell differentiation65, but in this previous work the source for TSLP was not identified. Further study is needed to evaluate the tissue-specific and relative contribution of different cellular sources of TSLP, which may also include keratinocytes, fibroblasts, and DCs. TSLP acts on a range of cells and has pleiotropic functions in cell development and activation, type 2-associated pathologies including allergies and asthma, and cancer; alternative promoter usage generates short and long forms of human TSLP, and proteolytic cleavage add further complexity66. Although the relevant sources in particular settings have yet to be determined using more specific tools, TSLP expression is associated with tissue stress and injury, in particular at barrier sites such as the skin and mucosal surfaces where it can amplify type 2 responses, in part by limiting the induction of type 1 responses67.
Eicosanoids.
Tuft cells express key enzymes in the biosynthetic pathways for lipid metabolites of arachidonic acid, which serve as critical modulators of immune function. Where assessed, tuft cells in all tissues express ALOX5 and its activating protein FLAP (Aloxap), COX-1 and -2, leukotriene C4 Synthase (Ltc4s), and prostaglandin D synthase (Hpgds)7,10,11,15. Expression of these enzymes suggests that tuft cells can produce cysteinyl leukotrienes and prostaglandin D2 (PGD2) (Fig. 3 bc). Multiple other cell types can be a source of these eicosanoids, which have pleiotropic effects on immune cells and non-immune cells68,69. In addition to mast cells, which are major producers of PGD2, platelets, dendritic cells, alveolar macrophages, TH2 cells, and osteoblasts express the PGD2 biosynthetic pathway68. PGD2 can be further metabolized into bio-active metabolites with anti-inflammatory effects, including the ligand for peroxisome proliferator-activated receptor-γ (PPARγ), 15-Deoxy-Δ(12,14)-PGJ2 (15d-PGJ2)70. The PGD2 receptor, DP2 or CRTH2 (chemoattractant receptor-homologous molecule), is expressed on both TH2 cells and ILC2s71–73. Thus, together with leukotrienes, which provide an NFAT-dependent activating signal for ILC2s74, this represents an attractive alternative “circuit” whereby tuft cells could engage tissue resident ILC2s either in the absence of IL-25 or to potentiate IL-25 signaling through the IL-17RB IL-25 receptor. Because CRTH2 may be more broadly expressed among tissues and cell types as compared to IL-17RB, this may allow tuft cells to more broadly impact tissue resident cells, including TH2 cells.
The relationship between eicosanoid production and tuft cell function remains incompletely studied. A recent report describing reduced colonic tuft and goblet cells in the context of impaired myeloid bacterial phagocytosis suggested that increased PGE2 resulted in decreased tuft cell frequency75. The mechanism whereby PGE2 affected tuft cell lineage specification or viability was not further investigated. Recent data suggests that tracheal tuft cells may also respond directly to LTE476. Tuft cells increased modestly when mice were treated with recombinant LTE4, or when challenged with fungal or house dust mite antigens. This effect was dependent on IL-25 and CysLT3R, but was STAT6-independent, suggesting a mechanism distinct from IL-4Ra-mediated tuft cell expansion in the small intestine (Fig. 1). Whether the “clusters” of induced tracheal tuft (or brush) cells represent de novo differentiation or proliferation of existing tuft cells was not addressed under these inflammatory conditions.
Miscellaneous.
In addition, tuft cells might be sources of angiotensinogen7 and endogenous opioids such as β-endorphin and Met-enkephalin (encoded by Pomc)77–79, which was inferred based on expression or immunostaining in tuft cells and, together with neural interactions, suggest roles beyond those in immune circuits. Further study is needed.
Conclusions and future directions
Despite growing interest in tuft cell biology over the past few years, many questions remain. While their function in promoting type 2 responses in the small intestine downstream of colonization with helminths or protists is firmly established, little is known about tuft cell function in other tissues or how potential tuft cell effector molecules beyond IL-25 (such as those covered here) might impact tissue homeostasis and inflammation. The existence of a dispersed, solitary, gustatory epithelial lineage that is conserved in mammals, and possibly in fish80, and their presence in most mucosal epithelia suggest distinct roles in tissue regulation and pathology, perhaps dependent on luminal signals unique to specific tissue environments. Tuft cells may also engage distinct cellular partners in other tissues, participating in tissue-specific tuft cell circuits which may or may not be dependent on type 2 cytokine signals. Tuft cells have the capacity to produce multiple effector molecules with broad immunomodulatory potential, as has been highlighted by recent transcriptomic approaches, and more functional assessment is warranted. Even as tuft cells have been established as a critical component of small intestinal type 2 immunity, new roles for tuft cells in immunological and non-immunological contexts in distinct tissues will undoubtedly emerge in the coming years.
Acknowledgements.
The authors are grateful for comments from lab members. This work was supported by funds from the NIH, HHMI, the SABRE Center at UCSF, and the Australian NHMCRC. C.S. is supported by fellowships from the Swiss National Science Foundation. C.E.O. is supported by the Gastroenterology T32 Training Grant at UCSF.
Disclosures.
The authors are unaware of any affiliation, memberships, funding or financial holdings that might be perceived as affecting the objectivity of this review.
References
- 1.von Moltke J, Ji M, Liang HE & Locksley RM Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225, doi: 10.1038/nature16161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howitt MR et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333, doi: 10.1126/science.aaf1648 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerbe F et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230, doi: 10.1038/nature16527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sbarbati A & Osculati F A new fate for old cells: brush cells and related elements. Journal of anatomy 206, 349–358, doi: 10.1111/j.1469-7580.2005.00403.x (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato A Tuft cells. Anatomical science international 82, 187–199, doi: 10.1111/j.1447-073X.2007.00188.x (2007). [DOI] [PubMed] [Google Scholar]
- 6.von Moltke J in Physiology of the Gastrointestinal Tract (Sixth Edition) 721–733 (Academic Press, 2018). [Google Scholar]
- 7.Bezençon C et al. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. The Journal of Comparative Neurology 509, 514–525, doi: 10.1002/cne.21768 (2008). [DOI] [PubMed] [Google Scholar]
- 8.O’Leary CE, Schneider C & Locksley RM Tuft Cells-Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry. Annu Rev Immunol, doi: 10.1146/annurev-immunol-042718-041505 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornstein C et al. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature 559, 622–626, doi: 10.1038/s41586-018-0346-1 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Miller CN et al. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 559, 627–631, doi: 10.1038/s41586-018-0345-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadjsombati MS et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 49, 33–41.e37, doi: 10.1016/j.immuni.2018.06.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YH et al. POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes & development 32, 915–928, doi: 10.1101/gad.314815.118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haber AL et al. A single-cell survey of the small intestinal epithelium. Nature 551, 333–339, doi: 10.1038/nature24489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plasschaert LW et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560, 377–381, doi: 10.1038/s41586-018-0394-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montoro DT et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560, 319–324, doi: 10.1038/s41586-018-0393-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider C et al. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 174, 271–284.e214, doi: 10.1016/j.cell.2018.05.014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei W et al. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc Natl Acad Sci U S A 115, 5552–5557, doi: 10.1073/pnas.1720758115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moor AE et al. Spatial Reconstruction of Single Enterocytes Uncovers Broad Zonation along the Intestinal Villus Axis. Cell 175, 1156–1167 e1115, doi: 10.1016/j.cell.2018.08.063 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Hoover B et al. The intestinal tuft cell nanostructure in 3D. Sci Rep 7, 1652, doi: 10.1038/s41598-017-01520-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum DM, Rasmussen SG & Kobilka BK The structure and function of G-protein-coupled receptors. Nature 459, 356–363, doi: 10.1038/nature08144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen JA & Roth BL Strategies to discover unexpected targets for drugs active at G protein-coupled receptors. Annu Rev Pharmacol Toxicol 51, 117–144, doi: 10.1146/annurev-pharmtox-010510-100553 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Tan JK, McKenzie C, Marino E, Macia L & Mackay CR Metabolite-Sensing G Protein-Coupled Receptors-Facilitators of Diet-Related Immune Regulation. Annu Rev Immunol 35, 371–402, doi: 10.1146/annurev-immunol-051116-052235 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Smith JS, Lefkowitz RJ & Rajagopal S Biased signalling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov 17, 243–260, doi: 10.1038/nrd.2017.229 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy MP & O’Neill LAJ Krebs Cycle Reimagined: The Emerging Roles of Succinate and Itaconate as Signal Transducers. Cell 174, 780–784, doi: 10.1016/j.cell.2018.07.030 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Gilissen J, Jouret F, Pirotte B & Hanson J Insight into SUCNR1 (GPR91) structure and function. Pharmacology & therapeutics 159, 56–65, doi: 10.1016/j.pharmthera.2016.01.008 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Muller M et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiol Mol Biol Rev 76, 444–495, doi: 10.1128/MMBR.05024-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chouchani ET et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435, doi: 10.1038/nature13909 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S et al. Global metabolite profiling of synovial fluid for the specific diagnosis of rheumatoid arthritis from other inflammatory arthritis. PLoS One 9, e97501, doi: 10.1371/journal.pone.0097501 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tannahill GM et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242, doi: 10.1038/nature11986 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills EL et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106, doi: 10.1038/s41586-018-0353-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sukumaran SK et al. Whole transcriptome profiling of taste bud cells. Sci Rep 7, 7595, doi: 10.1038/s41598-017-07746-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roper SD & Chaudhari N Taste buds: cells, signals and synapses. Nature reviews. Neuroscience 18, 485–497, doi: 10.1038/nrn.2017.68 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lossow K et al. Comprehensive Analysis of Mouse Bitter Taste Receptors Reveals Different Molecular Receptive Ranges for Orthologous Receptors in Mice and Humans. J Biol Chem 291, 15358–15377, doi: 10.1074/jbc.M116.718544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schutz B, Damadzic R, Weihe E & Eiden LE Identification of a region from the human cholinergic gene locus that targets expression of the vesicular acetylcholine transporter to a subset of neurons in the medial habenular nucleus in transgenic mice. Journal of neurochemistry 87, 1174–1183 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Nadorp B & Soreq H Predicted overlapping microRNA regulators of acetylcholine packaging and degradation in neuroinflammation-related disorders. Frontiers in molecular neuroscience 7, 9, doi: 10.3389/fnmol.2014.00009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girard E et al. Butyrylcholinesterase and the control of synaptic responses in acetylcholinesterase knockout mice. Life Sci 80, 2380–2385, doi: 10.1016/j.lfs.2007.03.011 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Lockridge O, Norgren RB Jr., Johnson RC & Blake TA Naturally Occurring Genetic Variants of Human Acetylcholinesterase and Butyrylcholinesterase and Their Potential Impact on the Risk of Toxicity from Cholinesterase Inhibitors. Chemical research in toxicology 29, 1381–1392, doi: 10.1021/acs.chemrestox.6b00228 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro FM et al. The “ins” and “outs” of the high-affinity choline transporter CHT1. Journal of neurochemistry 97, 1–12, doi: 10.1111/j.1471-4159.2006.03695.x (2006). [DOI] [PubMed] [Google Scholar]
- 39.Tizzano M et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A 107, 3210–3215, doi: 10.1073/pnas.0911934107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders CJ, Christensen M, Finger TE & Tizzano M Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci U S A 111, 6075–6080, doi: 10.1073/pnas.1402251111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee RJ et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest 124, 1393–1405, doi: 10.1172/jci72094 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee RJ et al. Bacterial d-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Science signaling 10, doi: 10.1126/scisignal.aam7703 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krasteva G et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci U S A 108, 9478–9483, doi: 10.1073/pnas.1019418108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deckmann K et al. Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc Natl Acad Sci U S A 111, 8287–8292, doi: 10.1073/pnas.1402436111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dando R & Roper SD Acetylcholine is released from taste cells, enhancing taste signalling. J Physiol 590, 3009–3017, doi: 10.1113/jphysiol.2012.232009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schutz B et al. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Frontiers in physiology 6, 87, doi: 10.3389/fphys.2015.00087 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosmans G et al. Cholinergic Modulation of Type 2 Immune Responses. Front Immunol 8, 1873, doi: 10.3389/fimmu.2017.01873 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finger TE et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310, 1495–1499, doi: 10.1126/science.1118435 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Ma Z et al. CALHM3 Is Essential for Rapid Ion Channel-Mediated Purinergic Neurotransmission of GPCR-Mediated Tastes. Neuron 98, 547–561 e510, doi: 10.1016/j.neuron.2018.03.043 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taruno A et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495, 223–226, doi: 10.1038/nature11906 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Workman AD et al. CALHM1-Mediated ATP Release and Ciliary Beat Frequency Modulation in Nasal Epithelial Cells. Sci Rep 7, 6687, doi: 10.1038/s41598-017-07221-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Virgilio F, Sarti AC & Grassi F Modulation of innate and adaptive immunity by P2X ion channels. Current opinion in immunology 52, 51–59, doi: 10.1016/j.coi.2018.03.026 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Song X, He X, Li X & Qian Y The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol Immunol 13, 418–431, doi: 10.1038/cmi.2015.105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swamy M & Hayday A Provocative exhibits at the Seventeen Gallery. Nature immunology 12, 1131–1133, doi: 10.1038/ni.2164 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Han Q et al. Characterization of Lamprey IL-17 Family Members and Their Receptors. Journal of immunology (Baltimore, Md. : 1950) 195, 5440–5451, doi: 10.4049/jimmunol.1500892 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buckley KM et al. IL17 factors are early regulators in the gut epithelium during inflammatory response to Vibrio in the sea urchin larva. Elife 6, 860, doi: 10.7554/eLife.23481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fort MM et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Lee J et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem 276, 1660–1664, doi: 10.1074/jbc.M008289200 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Moro K et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544, doi: 10.1038/nature08636 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Price AE et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A 107, 11489–11494, doi: 10.1073/pnas.1003988107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neill DR et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370, doi: 10.1038/nature08900 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ricardo-Gonzalez RR et al. Tissue signals imprint ILC2 identity with anticipatory function. Nature immunology 19, 1093–1099, doi: 10.1038/s41590-018-0201-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang Y et al. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359, 114–119, doi: 10.1126/science.aam5809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Terashima A et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. The Journal of experimental medicine 205, 2727–2733, doi: 10.1084/jem.20080698 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watanabe N et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436, 1181–1185, doi: 10.1038/nature03886 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Varricchi G et al. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front Immunol 9, 1595, doi: 10.3389/fimmu.2018.01595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harris NL & Loke P Recent Advances in Type-2-Cell-Mediated Immunity: Insights from Helminth Infection. Immunity 47, 1024–1036, doi: 10.1016/j.immuni.2017.11.015 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Harizi H The immunobiology of prostanoid receptor signaling in connecting innate and adaptive immunity. BioMed research international 2013, 683405, doi: 10.1155/2013/683405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh RK, Gupta S, Dastidar S & Ray A Cysteinyl leukotrienes and their receptors: molecular and functional characteristics. Pharmacology 85, 336–349, doi: 10.1159/000312669 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Shimura C et al. Dendritic cells express hematopoietic prostaglandin D synthase and function as a source of prostaglandin D2 in the skin. The American journal of pathology 176, 227–237, doi: 10.2353/ajpath.2010.090111 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wojno ED et al. The prostaglandin D(2) receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal immunology 8, 1313–1323, doi: 10.1038/mi.2015.21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xue L et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. The Journal of allergy and clinical immunology 133, 1184–1194, doi: 10.1016/j.jaci.2013.10.056 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang JE, Doherty TA, Baum R & Broide D Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. The Journal of allergy and clinical immunology 133, 899–901 e893, doi: 10.1016/j.jaci.2013.09.020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Moltke J et al. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. The Journal of experimental medicine 214, 27–37, doi: 10.1084/jem.20161274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyata N et al. Microbial Sensing by Intestinal Myeloid Cells Controls Carcinogenesis and Epithelial Differentiation. Cell Rep 24, 2342–2355, doi: 10.1016/j.celrep.2018.07.066 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bankova LG et al. The cysteinyl leukotriene 3 receptor regulates expansion of IL-25-producing airway brush cells leading to type 2 inflammation. Science immunology 3, doi: 10.1126/sciimmunol.aat9453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kokrashvili Z et al. Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology 137, 598–606, 606 e591–592, doi: 10.1053/j.gastro.2009.02.070 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gerbe F et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. The Journal of cell biology 192, 767–780, doi: 10.1083/jcb.201010127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delgiorno KE et al. Identification and manipulation of biliary metaplasia in pancreatic tumors. Gastroenterology 146, 233–244.e235, doi: 10.1053/j.gastro.2013.08.053 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morais S The Physiology of Taste in Fish: Potential Implications for Feeding Stimulation and Gut Chemical Sensing. Reviews in Fisheries Science & Aquaculture 25, 133–149, doi: 10.1080/23308249.2016.1249279 (2017). [DOI] [Google Scholar]
- 81.Tantin D Oct transcription factors in development and stem cells: insights and mechanisms. Development 140, 2857–2866, doi: 10.1242/dev.095927 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malik V, Zimmer D & Jauch R Diversity among POU transcription factors in chromatin recognition and cell fate reprogramming. Cellular and molecular life sciences : CMLS 75, 1587–1612, doi: 10.1007/s00018-018-2748-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y & Abe K Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci 14, 685–687, doi: 10.1038/nn.2820 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamashita J, Ohmoto M, Yamaguchi T, Matsumoto I & Hirota J Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice. PLoS One 12, e0189340, doi: 10.1371/journal.pone.0189340 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gracz AD et al. Sox4 Promotes Atoh1-Independent Intestinal Secretory Differentiation Toward Tuft and Enteroendocrine Fates. Gastroenterology 155, 1508–1523 e1510, doi: 10.1053/j.gastro.2018.07.023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bjerknes M et al. Origin of the brush cell lineage in the mouse intestinal epithelium. Developmental biology 362, 194–218, doi: 10.1016/j.ydbio.2011.12.009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lei W et al. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc Natl Acad Sci U S A, doi: 10.1073/pnas.1720758115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Orchard RC et al. Discovery of a proteinaceous cellular receptor for a norovirus. Science 353, 933–936, doi: 10.1126/science.aaf1220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilen CB et al. Tropism for tuft cells determines immune promotion of norovirus pathogenesis. Science 360, 204–208, doi: 10.1126/science.aar3799 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chudnovskiy A et al. Host-Protozoan Interactions Protect from Mucosal Infections through Activation of the Inflammasome. Cell 167, 444–456 e414, doi: 10.1016/j.cell.2016.08.076 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuga D et al. Tyrosine Phosphorylation of an Actin-Binding Protein Girdin Specifically Marks Tuft Cells in Human and Mouse Gut. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 65, 347–366, doi: 10.1369/0022155417702586 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kabata H, Moro K & Koyasu S The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunological reviews 286, 37–52, doi: 10.1111/imr.12706 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Lund SJ et al. Leukotriene C4 Potentiates IL-33-Induced Group 2 Innate Lymphoid Cell Activation and Lung Inflammation. Journal of immunology (Baltimore, Md. : 1950) 199, 1096–1104, doi: 10.4049/jimmunol.1601569 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


