Abstract
Big data approaches have profoundly influenced state-of-the-art in many fields of research, with toxicology being no exception. Here, we use Parkinson’s disease as a window through which to explore the challenges of a dual explosion of metabolomic data addressing the myriad environmental exposures individuals experience and genetic analyses implicating many different loci as risk factors for disease. We argue that new experimental approaches are needed to convert the growing body of omics data into molecular mechanisms of disease that can be therapeutically targeted in specific patients. We outline one attractive strategy, which capitalizes on the rapid generation time and advanced molecular tools available in the fruit fly, Drosophila, to provide a platform for mechanistic dissection and drug discovery.
Keywords: gene-environment interactions, Parkinson’s disease, precision medicine, GWAS, exposome, Drosophila genetics
Parkinson’s disease is the second most common neurodegenerative disorder, after Alzheimer’s disease, and affects 1% of individuals by age 65, rising to nearly 5% of people by age 85 (de Lau and Breteler, 2006; Marras et al., 2018; Yang et al., 2019). Although dopamine agonists can ameliorate the motor symptoms of Parkinson’s disease, there are currently no effective disease-modifying therapies, leading to a significant burden of suffering for patients and their caregivers. The financial cost to patients, families, the health care system and society are also substantial. The economic burden of Parkinson’s disease, including costs associated with reduced or lost employment and medical expenses, has been estimated at $52 billion per year in the United States (Yang et al., 2019). These figures will only increase as our population ages. By 2030, the number of people with Parkinson’s disease in the United States is expected to reach 1.2 million (Marras et al., 2018) with a substantial accompanying increase in societal costs unless successful treatments are introduced into clinical practice.
Patients with Parkinson’s disease classically manifest clinically with the triad of tremor (often termed “pill rolling”), bradykinesia (slowness of movement) and rigidity. These motor manifestations are linked to pathology in the dopaminergic nigrostriatal system, with significant loss of dopaminergic neurons in the substantia nigra pars compacta. In patients with typical Parkinson’s disease nigral dopaminergic neurons also display eosinophilic cytoplasmic inclusion bodies known as Lewy bodies and Lewy neurites, which are comprised of insoluble aggregates containing the synaptic protein α-synuclein (Shulman et al., 2011). Current treatments for Parkinson’s disease primarily involve pharmacological replacement of lost nigrostriatal dopamine to manage symptom severity. However, therapeutic options to slow or prevent dopaminergic neuronal death are lacking, due at least in part to an incomplete mechanistic understanding of disease etiology. Thus, there is an urgent need to define mechanistic pathways in detail so that they can be exploited to develop treatment strategies that slow or halt the progression of the disease.
ETIOLOGY OF PARKINSON’S DISEASE: COMPLEX INTERPLAY OF GENES, CELLS, TISSUES, AND ENVIRONMENT
Epidemiology, genetics, and neuropathology have all provided important clues to the pathogenesis of Parkinson’s disease. Spurred by the observation in the early 1980s that individuals exposed to the meperidine analog byproduct 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in synthetic heroin, developed a clinical and neuropathological syndrome strikingly similar to Parkinson’s disease (Langston et al., 1983) environmental influences on the disorder have been investigated at length. Multiple epidemiological studies have suggested a protective role for caffeine and nicotine (Ascherio and Schwarzschild, 2016). Particularly, strong evidence supports a role for pesticide exposure in promoting Parkinson's disease (Ascherio and Schwarzschild, 2016; Chen and Ritz, 2018). Although causality is challenging to address with epidemiological data alone, additional support has been provided for a role of pesticides, in particular rotenone and paraquat, by the ability of these toxic compounds to mimic the dopaminergic neurodegeneration characteristic of Parkinson's disease when administered to rodents (Zeng et al., 2018).
In addition to the challenges of inferring cause and effect from epidemiological data, determining the compound or compounds contributing to pathology is a significant challenge. Individuals are not exposed to a single neurotoxic compound in isolation. Rather, there are a variety of exposures throughout the lifespan that may influence disease development. Integration of traditional toxicological exposures with lifestyle factors and endogenous responses to the stimuli has been encompassed in the broad concept of the exposome (Niedzwiecki et al., 2019). Careful history and environmental toxicological data can provide important information on individual and group exposomes (Ritz et al., 2016, 2017).
More recently, unbiased metabolomic profiling approaches have begun to provide a unprecedented wealth of data on current and past environmental exposures, as well as endogenous responses to those exposures. Mass spectrometry-based analysis can now identify more than 20 000 compounds from complex fluid and tissue samples, including molecules derived from endogenous metabolism, diet, the microbiome and drugs (Niedzwiecki et al., 2019). Importantly, the unbiased mass spectrometry approach is not limited by preexisting hypotheses regarding specific environmental exposures and thus has the ability to detect novel compounds associated with environmental toxicity. However, identifying specific compounds remains a challenge. Analytical standards are not available for most detected compounds, which are reported based on mass, charge and column retention time. Approaches to address the key challenge of identifying metabolites in complex mixtures include improved computation methods for assigning annotation confidence, analysis of fragmentation patterns and development of expanded chemical databases that include both chemicals from the environment and endogenous metabolites (Walker et al., 2019).
In addition, integrating and interpreting the large datasets produced by unbiased high-resolution mass spectrometry analyses presents significant challenges. Significant computational power is required to for data analysis and storage. Conceptually, the tools of system biology, including network-based representations to connect uncharacterized signals to known biological pathways and assess responses of individuals and groups to environmental influences, will be needed to distill actionable insights from large-scale untargeted metabolomics (Vermeulen et al., 2020; Walker et al., 2019). Despite these challenges, the stage has now been set for a comprehensive characterization of environmental exposures across the lifespan of the organism (Niedzwiecki et al., 2019). As information on exposures of many individuals, together with network-based prediction regarding interactions and cellular pathways affected, accumulates the need for complex in vivo experimental model systems with adequate throughput to test emerging hypotheses will grow more and more acute.
The increasing availability of environmental exposure data has been met with an equivalent expansion of information on genetic influences on disease. In the past, Parkinson’s disease was traditionally considered to be a prime example of a “non-genetic” disease, based largely on discordance in twin studies. However, over the past 20 years or so unequivocal evidence has gradually accumulated for a genetic component in the disorder. Traditional linkage approaches slowly identified a relatively small number of highly penetrant recessive and dominant gene mutations that could cause Parkinson’s disease (Shulman et al., 2011), although the numbers of patients with these clearly genetic forms of the disease remained quite small.
With the more recent advent of genome-wide association studies (GWASs) evidence started to emerge that a patient’s genetic makeup could influence the much more common, apparently sporadic form of Parkinson’s disease as well. Power is critical for gene identification through GWAS. Initial studies were relatively small and identified only a handful of genes that met the p < 10−8 statistical threshold require for genome-wide significance. Recognition that lack of statistical power has played a key role in the failure to define genetic influences in many common, apparently sporadic human diseases has led the field to increase sample size through increasingly large meta-analyses. In Parkinson’s disease, the most recently reported GWAS incorporated 17 datasets containing 7.8 million single nucleotide polymorphisms in 37 688 cases and 1.4 million controls. Ninety independent genome-wide significant signals were identified, which explained 16–34% of disease risk, depending on the prevalence of the disorder (Nalls et al., 2019). Like the large-scale environmental exposure data, the new genetic data also pose significant challenges for understanding disease mechanisms at a precise molecular level. Mechanistic analysis of the 90 separate genes in the pathogenesis of Parkinson’s disease is a daunting prospect using conventional in vivo models. In addition, GWAS peaks are typically broad, and assigning a specific gene underlying disease risk can be difficult. Further, defining the cell type in which the locus acts can be difficult. Importantly, the molecular mechanism through which the variant influences disease requires definition. Thus, the multiple challenges provided by recent large-scale omics approaches defining a multitude of both environmental and genetic risk factors for Parkinson’s disease demand innovative new approaches to functional analysis.
Successful development of appropriate models to examine gene-environment interactions also requires consideration of the complex gene-environment interactions that influence the degree and nature of specific genetic influences on Parkinson’s disease pathogenesis. In a recent study of participants in the UK Biobank, mathematical modeling incorporating both genetic and environmental risk factors suggests that the excess risk of Parkinson’s disease associated with diabetes is more important in individuals with lower genetic risk. In fact, diabetes, or its treatment, may be protective in the context of higher genetic risk (Jacobs et al., 2020).
Gene-environment interactions are likely at play in non-cell autonomous as well as cell-specific contexts. Increasing evidence implicates the function of the immune system as particularly important in Parkinson’s disease. Many genes linked to the disorder are expressed in one or more cells types of immune cells. LRRK2, in particular, has been shown to regulate innate immunity and control cytokine production (Kline et al., 2021). Neurotoxicants implicated in Parkinson’s disease, including rotenone and pyrethroids, can also influence cytokine production and the function of a range of cytokine producing cells (Kline et al., 2021; Rabaneda-Lombarte et al., 2020; Sarkar et al., 2017). The contribution of both genetic and environmental risk factors thus most likely represents the integration of traditionally studied and better understood cell autonomous effects in neurons coupled with more recently recognized noncell autonomous effects of cells resident in or circulating through the brain. Indeed, an additional layer of complexity is added by recent data suggesting that Parkinson’s disease can be influenced by the composition of the gut microbiota (Romano et al., 2021; Sampson et al., 2016), perhaps by modulating the spread of the α-synuclein protein from the myenteric plexus to the brain (Uemura et al., 2018). Thus, gene-environment interactions may also influence signaling between organ systems.
Gene-environment interactions do not simply reflect environmental influences working on a backdrop of pre-defined gene expression profiles determined by an individual’s germline DNA sequences. Rather, environment can sculpt the transcriptome in Parkinson’s disease models via transcription factor regulatory cascades (Song et al., 2020), changes in expression or activity of DNA methylation and histone modifying enzymes (Urbizu and Beyer, 2020) and through direct damage to DNA (Sanders et al., 2017). Indeed, the genomes of postmitotic cells in the central nervous system, are likely both more heterogenous at baseline and less static than previously thought. Somatic mutations (Verheijen et al., 2018) and epigenetically regulated transposon activation (Sun et al., 2018) have recently been implicated in the pathogenesis of brain diseases. Although investigation of these is at an early stage in Parkinson’s disease (Leija-Salazar et al., 2020; Pfaff et al., 2020), such somatic brain mosaicism likely represents an additional substrate for complexity in gene-environment interactions in Parkinson’s disease.
DROSOPHILA MODEL FOR DISSECTION OF GENE-ENVIRONMENT INTERACTIONS IN PARKINSON’S DISEASE
Given the substantial challenges the large amount of new environmental and genetic data present for defining underlying molecular mechanisms and developing rational therapies, we suggest here a new approach to investigate gene-environment interactions (Figure 1). We propose taking advantage of the rapid generation time and well-developed experimental tools available in the fruit fly Drosophila to identify and then molecularly dissect the mechanisms underlying the interaction of Parkinson’s disease-linked loci with specific environmental factors. We focus here on pesticides as well-documented environmental exposure linked to Parkinson’s disease, but the general approach is applicable to the wide range of exogenous and endogenous factors comprising the exposome, including metabolic (Girard et al., 2020; Sarkar et al., 2020) microbiological (Westfall et al., 2019), and behavioral (Seugnet et al., 2009) factors.
Figure 1.
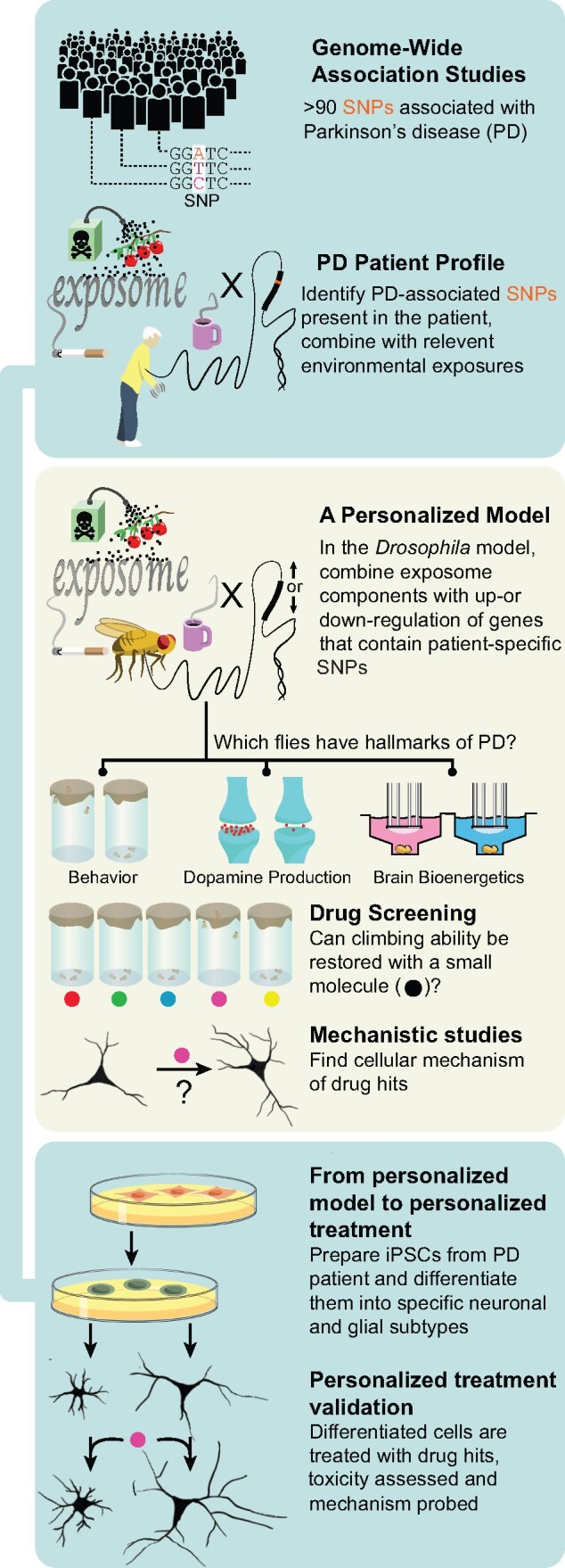
Proposed model for studying gene-environment interactions.
Our method uses Parkinson’s disease genetics and epidemiology as a starting point. From the genetic perspective, we propose altering expression of Drosophila orthologs of genes identified by GWAS in patients. Our primary goal is to integrate GWAS with exposome-related factors, but we note that the rapid genetics and cell-type specific expression systems (Olsen and Feany, 2019) available in flies allow investigation of the causal genes underlying GWAS peaks and their cell type specificity (Sarkar et al., 2020). An important advantage of the Drosophila system is the ability to perform unbiased screening at a relatively high-throughput level. Genetic screens focused on human disease models are logically carried out at the level of conserved genome scale such that all Drosophila genes with human orthologs are tested. Ortholog searches using the current reference genome assembly (http://flybase.org/; FB2021_01) identify 8,869 Drosophila genes with human orthologs. Several conserved genome-scale transgenicRNA interference (RNAi)-based screen have been reported in Drosophila models of human neurological disease (Lohr et al., 2020; Wang et al., 2018), supporting the feasibility of unbiased screening of all genome-wide significant Parkinson’s GWAS genes, even in the context of modulating environmental exposure. Of course, a more targeted approach focusing on a conserved set of mechanisms such as mitochondrial dysfunction, or more common genetic risk factors is also possible.
Although we emphasize interrogation of GWAS candidates given their more recent discovery, important influence on common forms of Parkinson’s disease and poorly characterized nature, integration with better characterized Mendelian factors can also be incorporated in the approach. Well-characterized Drosophila models of α-synucleinopathy (Feany and Bender, 2000; Ordonez et al., 2018), LRRK2-associated disease (Liu et al., 2008; Venderova et al., 2009), parkin (Greene et al., 2003), and PINK1 (Clark et al., 2006; Park et al., 2006) deficiency have been described and used extensively to probe pathogenic mechanisms associated with both common forms of Parkinson’s disease as well as rarer Mendelian variants.
Flies with conventional mutant alleles or transgenic manipulation of Parkinson’s disease-associated genetic variants are then exposed to environmental factors altering the risk of Parkinson’s disease. Substantial evidence suggests that Drosophila are a useful model for studying environmental risk factors associated with Parkinson’s disease. Flies fed with rotenone (Coulom and Birman, 2004) or paraquat (Chaudhuri et al., 2007) mimic key features of Parkinson’s disease in patients and have been used to identify signaling pathways involved in neurotoxicant-mediated neurodegeneration (Hajji et al., 2019), including inflammation (Maitra et al., 2019), and propose new treatment strategies (Ortega-Arellano et al., 2017). Interestingly, Drosophila models of Parkinson’s disease have previously been used to suggest that the neuroprotective effects of coffee and cigarette smoking are independent of caffeine or nicotine, although the protective compound(s) were not identified (Trinh et al., 2010). Here, we specifically suggest that the higher throughput of the Drosophila system compared with other in vivo models will allow the experimental identification of causative compounds in complex environmental exposures (Figure 1).
Testing of thousands of genetic variants is feasible in Drosophila models of neurological disease (Lohr et al., 2020; Wang et al., 2018), as discussed earlier. However, as methods for identification of compounds measured in unbiased high-resolution mass spectrometry-based metabolomics improves, the number of compounds identified, and combination of compounds predicted by increasingly sophisticated network analyses to impact key biological pathways, will certainly exceed the capacity for testing in Drosophila. Prioritization of compounds and combinations of compounds may logically be dictated by a number of factors. The degree of predicted impact of the environmental compound or factor will undoubtedly be important. Specific mechanistic connections may be predicted based on known biological connections of the gene, environmental exposure, or both. Nonetheless, although entirely comprehensive and unbiased combinational examination of all known and suspected genetic and environmental factors will not be possible, the experimental design most likely to reveal truly novel, unexpected biological insights will incorporate unbiased elements. For example, all currently identified genome-wide significant genetic risk factors (90 to date with p < 10−8) could be tested for interaction with a specified environmental toxin. Using the propose system for exclusively hypothesis-based experiments will fail to capture the full power of unbiased metabolomics and high-throughput genetics.
Once a genetic manipulation is selected, and environmental exposure is applied, a wide range of Parkinson’s disease-relevant phenotypes can then be used to interrogate the functional effects of the combined factors. Simple locomotor tests such as climbing have been a mainstay of analysis of Drosophila models of Parkinson’s disease, correlating with the prominence of motor phenotypes in patients reflecting the loss of substantia nigra motor neurons (Feany and Bender, 2000; Ordonez et al., 2018). However, patients with Parkinson’s disease display a wide range of nonmotor symptoms. In point of fact, nonmotor symptoms include the earliest and most clinically challenging manifestations of Parkinson’s disease. Many of these nonmotor symptoms can also be modeled and assessed at scale in Drosophila. Olfactory and sleep abnormalities some of the earliest symptoms of Parkinson’s disease in patients and are seen in multiple Drosophila models of the disorder (Chambers et al., 2013; Poddighe et al., 2013). Cognitive dysfunction has been previously underappreciated but is particularly devastating for patients and families. Parkinson’s disease model Drosophila display impairments in a variety of cognitive task and these deficits can be ameliorated by pharmacological therapies (Julienne et al., 2017; Naz et al., 2020).
Importantly, behavioral analyses can be complemented by a variety of biochemical and neuropathological assessments. The throughput available in the Drosophila system allows simple behavioral tests to be coupled with selected biochemical measurements in the screening setting (Figure 1). In particular, dopamine levels can be measured by high performance liquid chromatography. A new innovation allows bioenergetic analysis of single brains using the AgilentXFe96 metabolic analyzer (Neville et al., 2018; Sarkar et al., 2020). These higher throughput techniques can be coupled with assessment of degeneration of dopaminergic and nondopaminergic neurons, as well as formation of α-synuclein inclusion bodies, for a more selected subgroup of experimental conditions (Ordonez et al., 2018). Together, these approaches allow for relatively rapid screening coupled with mechanistic analysis of gene-environment interactions.
Our model is particularly well-suited to a personalized medicine approach (Figure 1). Genome sequencing is used to identify patient-specific gene variants predisposing to Parkinson’s disease onset or progression. Environmental exposures are assessed using metabolomic analyses. These personalized risk factors are then combined in a specific Drosophila model, as we have discussed. The screening potential of the fly system is then again realized to identify compounds with therapeutic benefit to the precise combination of risk factors experienced by an individual patient. Although Drosophila is best recognized as an outstanding genetic model system, drug screening in flies is also an established and useful approach (Roy et al., 2020; Wang et al., 2016).
In terms of personalized medicine approaches, flies and other genetic model organisms are best established in assessing putative causal variants identified by genome sequencing, and exploring the mechanisms of action of these variants at the basic cell biological level (Link and Bellen, 2020). However, successes in engineering models with complex disease-like phenotypes and using these models to target therapies at an individual patient level have been reported. The Cagan laboratory has pioneered efforts to develop personalized cancer models in flies (Kasai and Cagan, 2010). Building on work demonstrating that expression of oncogenic RAS in Drosophila epithelia promotes transformation-associated phenotypes including excess proliferation, multilayering, altered apoptosis and senescence, and dissemination of transformed cells to distant sites (Bangi et al., 2016), Bangi et al. (2016) introduced alterations mimicking a total of nine genetic aberrations identified in one patient’s metastatic, KRAS-mutant colorectal carcinoma. Robotic high-throughput chemical screens were then performed to identify the novel combination of trametinib plus zoledronate, which improved survival of the Drosophila “avatars.” Notably, a partial, though unfortunately not durable, response was observed when treating the patient with trametinib plus zoledronate. In a similar approach, a patient’s metastatic, poorly drug responsive adenoid cystic carcinoma was modeled by incorporating 5 major disease-associated genetic variants in a personalized Drosophila avatar, which was then screened chemically using high-throughput robotic technology. A 3 drug combination effective in the fly model was forwarded to the patient as part of a clinical trial, resulting in sustained stabilization and partial metabolic normalization of the tumor over 12 months of follow-up (Bangi et al., 2021). Thus, compounds identified through drug screening in flies modeling the combined genetic and environmental risk factors predisposing to Parkinson’s disease (Figure 1) may represent attractive therapeutic candidates for use in patients following additional vertebrate validation studies and may also serve as chemical tools for mechanistic pathway analysis in both Drosophila and vertebrate model systems.
Compounds and pathways identified in flies will be forwarded for validation in vertebrate experimental systems (Figure 1). Fortunately, recent advances in stem cell biology make preparation of induced pluripotent stem cell models of specific genetic variants, including in the context of neurons derived from individual patients, feasible (Marotta et al., 2020). Patient-derived pluripotent stem cells (iPSCs) can be differentiated into a wide variety of cell types, including dopaminergic neurons. These powerful human experimental cell tools have shown significant promise in unraveling underlying mechanisms of Parkinson’s disease pathogenesis (Marotta et al., 2020) including the contribution of environmental toxins (Simmnacher et al., 2020). These precisely tailored stem cell models are thus attractive models to investigate molecular mechanisms of gene-environment interactions and test the relevance of candidate compounds identified through screening in Drosophila.
Though undoubtedly powerful, cell culture models lack important cellular interactions, anatomic complexity and aging available in the in vivo system. Although organoid models show promise in addressing many of these experimental limitations (Marotta et al., 2020), vertebrate animal models of Parkinson’s disease suitable for interrogation of gene-environment interactions will play an important role in verifying and extending results from the Drosophila and cell culture systems. Rats treated with rotenone develop key physiological and pathological hallmarks of Parkinson’s disease, including motor deficits, mitochondrial dysfunction, and α-synuclein aggregation (Betarbet et al., 2000). Experimental manipulation of the levels of α-synuclein in rotenone treated rats can influence neurotoxicity (Naughton et al., 2017; Zharikov et al., 2015) suggesting that the model can be used to probe additional gene-environment interactions discovered in the Drosophila system. Similarly, the herbicide paraquat has been used to model Parkinson’s disease and investigation potential therapies in mice (Ishola et al., 2019). Finally, a variety of nonhuman primate models of Parkinson’s disease exist, including animals exposed to MPTP, manganese, and elevated levels of α-synuclein through viral transduction (Chia et al., 2020). Although the primate models best replicate important anatomic, physiological, and genomic features relevant to disease pathogenesis, the time and other resources needed will substantially limit investigations of gene-environment interactions (Table 1).
Table 1.
Advantages and Drawbacks of Different Model Systems
| Model | Advantages | Drawbacks |
|---|---|---|
| Drosophila |
|
|
| Transformed cell lines |
|
|
| Patient-derived iPSCs |
|
|
| Rodent models |
|
|
| Nonhuman Primate |
|
|
Although we have organized our discussion following a hierarchical system (Figure 1, Table 1) for heuristic value, cross species, cross disease (Sarkar et al., 2020) and multiomic data integration are powerful tools to outline conserved, therapeutically targetable disease mechanisms. For example, TransposeNet is a recently developed computational tool for interpreting genetic screening data from model organisms to understand human disease. TransposeNet maps genetic hits from the model organism to their human homologs and then builds networks linking the genetic hits and other human genes. Using TransposeNet to analyze results from yeast screens for modifiers of α-synuclein identified known genetic modifiers of α-synuclein neurotoxicity (Khurana et al., 2017) and two druggable nodes that can influence α-synuclein toxicity in mammalian models of Parkinson’s disease: Calcineurin subunit B, (Caraveo et al., 2014) targeted by FK506, and Nedd4-dependent N-arylbenzimidazole (Tardiff et al., 2013).
Although conservation of disease mechanisms even from yeast to mammals supports the utility of model organism screening as a starting point for disease mechanism and therapeutic target discovery, not all findings will be consistent across model organisms (Table 1), or pertinent in the authentic human disease. Fortunately, the large and growing collections of diverse patient-derived data can help interpret model organism data and prioritize pathways for therapeutic development. In the near future techniques like spatial transcriptomics (Vickovic et al., 2019) will be able to provide fine grain detail on gene expression profiles in patient tissue. These high-resolution spatial data will allow, for example, transcriptional activation of a target pathway to be compared between selective vulnerable substantia nigra pars compact dopaminergic neurons with relatively resistant ventral tegmental dopamine cells. Large clinical databases can also be harnessed. In one example, the comprehensive prescribing information contained in the Norwegian Prescription Database was used to support the clinical relevance of cell culture data demonstrating control of α-synuclein gene expression by ß2-adrenoreceptor activation (Mittal et al., 2017). Such integrative and diverse data integration offers an attractive path for moving the best therapeutic candidate targets and compounds toward the clinic.
Our proposed model proposed (Figure 1) has many important strengths, but there are also limitations. Drosophila orthologs will not exist for all human genes implicated in Parkinson’s disease through traditional linkage studies. Although basic neuronal cell biology is well conserved from flies to humans, as are many aspects of dopaminergic function, not all cell biology relevant to Parkinson’s disease may be conserved in flies. For example, specific subtypes of human dopaminergic neurons are selectively vulnerable in Parkinson’s disease and it is not yet clear if similar selective vulnerability exists in Drosophila. Fly glia subserve many of the same functions as vertebrate glia but do not form dense myelin. Thus, a critical role for any gene or environmental fact on myelination or any other glial function not conserved in flies will not be accessible to experimental investigation.
SUMMARY AND FUTURE DIRECTIONS
Here, we propose Drosophila as a starting point in an integrated system with the throughput required to study the mechanistic basis of gene-environment interactions in Parkinson’s disease at scale and to move these insights toward personalized therapies. Our proposal comes in response to emerging challenges in big data: the more than 90 loci identified as genetic risk factors for Parkinson’s disease (Nalls et al., 2019) and the emerging ability to identify vast numbers of chemicals through high-resolution mass spectrometry (Niedzwiecki et al., 2019) allowing the definition of an individual patient’s exposome in unprecedented detail. We envision tailoring Drosophila “avatars” to individual patient genetic makeup and environmental risk factors. These personalized in vivo models can then be used to screen for therapeutic compounds specifically effective in the context of an individual’s genetic composition and exposure history. The recent development of similarly personalized induced pluripotent stem cell models allows rapid translation from the invertebrate screening model to patient cells. Although we have focused here on a discussion of the most validated genetic and environmental risk factors associated with Parkinson’s disease, we note that our flexible model system can incorporate new concepts in the pathogenesis of Parkinson’s disease and the exposome, such as host-microbiome interactions, as they emerge. We envision our approach as an innovative, powerful and flexible adjunct to more conventional approaches to understanding gene-environment interactions in Parkinson’s disease as we move toward the important goal of disease modifying, patient-specific therapies for a common and debilitating neurodegenerative disease.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
Brigham Research Institute Microgrant and the Colgate-Palmolive Postdoctoral Fellowship Award in In Vitro Toxicology (S.S.) and NIH-NINDS (R01-NS098821 to M.B.F.).
REFERENCES
- Ascherio A., Schwarzschild M. A. (2016). The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 15, 1257–1272. [DOI] [PubMed] [Google Scholar]
- Bangi E., Murgia C., Teague A. G. S., Sansom O. J., Cagan R. L. (2016). Functional exploration of colorectal cancer genomes using Drosophila. Nat. Commun. 7, 13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangi E., Smibert P., Uzilov A. V., Teague A. G., Gopinath S., Antipin Y., Chen R., Hecht C., Gruszczynski N., Yon W. J., et al. (2021). A Drosophila platform identifies a novel, personalized therapy for a patient with adenoid cystic carcinoma. iScience 24, 102212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R., Sherer T. B., MacKenzie G., Garcia-Osuna M., Panov A. V., Greenamyre J. T. (2000). Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 3, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Caraveo G., Auluck P. K., Whitesell L., Chung C. Y., Baru V., Mosharov E. V., Yan X., Ben-Johny M., Soste M., Picotti P., et al. (2014). Calcineurin determines toxic versus beneficial responses to α-synuclein. Proc. Natl. Acad. Sci. U.S.A. 111, E3544–E3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. P., Call G. B., Meyer D., Smith J., Techau J. A., Pearman K., Buhlman L. M. (2013). Nicotine increases lifespan and rescues olfactory and motor deficits in a Drosophila model of Parkinson’s disease. Behav. Brain Res. 253, 95–102. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Bowling K., Funderburk C., Lawal H., Inamdar A., Wang Z., O’Donnell J. M. (2007). Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J. Neurosci. 27, 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Ritz B. (2018). The search for environmental causes of Parkinson’s disease: Moving forward. J. Parkinsons Dis. 8, S9–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia S. J., Tan E.-K., Chao Y.-X. (2020). Historical perspective: Models of Parkinson’s disease. Int. J. Mol. Sci. 21, 2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. E., Dodson M. W., Jiang C., Cao J. H., Huh J. R., Seol J. H., Yoo S. J., Hay B. A., Guo M. (2006). Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441, 1162–1166. [DOI] [PubMed] [Google Scholar]
- Coulom H., Birman S. (2004). Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 24, 10993–10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau L. M. L., Breteler M. M. B. (2006). Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535. [DOI] [PubMed] [Google Scholar]
- Feany M. B., Bender W. W. (2000). A Drosophila model of Parkinson’s disease. Nature 404, 394–398. [DOI] [PubMed] [Google Scholar]
- Girard V., Goubard V., Querenet M., Seugnet L., Pays L., Nataf S., Dufourd E., Cluet D., Mollereau B., Davoust N. (2020). Spen modulates lipid droplet content in adult Drosophila glial cells and protects against paraquat toxicity. Sci. Rep. 10, 20023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. C., Whitworth A. J., Kuo I., Andrews L. A., Feany M. B., Pallanck L. J. (2003). Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. U.S.A. 100, 4078–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajji K., Mteyrek A., Sun J., Cassar M., Mezghani S., Leprince J., Vaudry D., Masmoudi-Kouki O., Birman S. (2019). Neuroprotective effects of PACAP against paraquat-induced oxidative stress in the Drosophila central nervous system. Hum. Mol. Genet. 28, 1905–1918. [DOI] [PubMed] [Google Scholar]
- Ishola I. O., Akataobi O. E., Alade A. A., Adeyemi O. O. (2019). Glimepiride prevents paraquat-induced Parkinsonism in mice: Involvement of oxidative stress and neuroinflammation. Fundam. Clin. Pharmacol. 33, 277–285. [DOI] [PubMed] [Google Scholar]
- Jacobs B. M., Belete D., Bestwick J., Blauwendraat C., Bandres-Ciga S., Heilbron K., Dobson R., Nalls M. A., Singleton A., Hardy J., et al. (2020). Parkinson’s disease determinants, prediction and gene-environment interactions in the UK Biobank. J. Neurol. Neurosurg. Psychiatry 91, 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julienne H., Buhl E., Leslie D. S., Hodge J. J. L. (2017). Drosophila PINK1 and parkin loss-of-function mutants display a range of non-motor Parkinson’s disease phenotypes. Neurobiol. Dis. 104, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai Y., Cagan R. (2010). Drosophila as a tool for personalized medicine: A primer. Per. Med. 7, 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana V., Peng J., Chung C. Y., Auluck P. K., Fanning S., Tardiff D. F., Bartels T., Koeva M., Eichhorn S. W., Benyamini H., et al. (2017). Genome-scale networks link neurodegenerative disease genes to α-synuclein through specific molecular pathways. Cell Syst. 4, 157–170.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline E. M., Houser M. C., Herrick M. K., Seibler P., Klein C., West A., Tansey M. G. (2021). Genetic and environmental factors in Parkinson’s disease converge on immune function and inflammation. Mov. Disord. 36, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. (1983). Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980. [DOI] [PubMed] [Google Scholar]
- Leija-Salazar M., Pittman A., Mokretar K., Morris H., Schapira A. H., Proukakis C. (2020). Investigation of somatic mutations in human brains targeting genes associated with Parkinson’s dis.. Front. Neurol. 11, 570424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link N., Bellen H. J. (2020). Using Drosophila to drive the diagnosis and understand the mechanisms of rare human diseases. Development 147, dev191411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang X., Yu Y., Li X., Wang T., Jiang H., Ren Q., Jiao Y., Sawa A., Moran T., et al. (2008). A Drosophila model for LRRK2-linked parkinsonism. Proc. Natl. Acad. Sci. U.S.A. 105, 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr K. M., Frost B., Scherzer C., Feany M. B. (2020). Biotin rescues mitochondrial dysfunction and neurotoxicity in a tauopathy model. Proc. Natl. Acad. Sci. U.S.A. 117, 33608–33618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U., Scaglione M. N., Chtarbanova S., O’Donnell J. M. (2019). Innate immune responses to paraquat exposure in a Drosophila model of Parkinson’s disease. Sci. Rep. 9, 12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta N., Kim S., Krainc D. (2020). Organoid and pluripotent stem cells in Parkinson’s disease modeling: An expert view on their value to drug discovery. Expert Opin. Drug Discov. 15, 427–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras C., Beck J. C., Bower J. H., Roberts E., Ritz B., Ross G. W., Abbott R. D., Savica R., Van Den Eeden S. K., Willis A. W., et al. ; Parkinson’s Foundation P4 Group. (2018). Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal S., Bjørnevik K., Im D. S., Flierl A., Dong X., Locascio J. J., Abo K. M., Long E., Jin M., Xu B., et al. (2017). β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science 357, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls M. A., Blauwendraat C., Vallerga C. L., Heilbron K., Bandres-Ciga S., Chang D., Tan M., Kia D. A., Noyce A. J., Xue A., et al. ; International Parkinson’s Disease Genomics Consortium. (2019). Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton C., O’Toole D., Kirik D., Dowd E. (2017). Interaction between subclinical doses of the Parkinson’s disease associated gene, α-synuclein, and the pesticide, rotenone, precipitates motor dysfunction and nigrostriatal neurodegeneration in rats. Behav. Brain Res. 316, 160–168. [DOI] [PubMed] [Google Scholar]
- Naz F., null R., Fatima M., Naseem S., Khan W., Mondal A. C., Siddique Y. H. (2020). Ropinirole silver nanocomposite attenuates neurodegeneration in the transgenic Drosophila melanogaster model of Parkinson’s disease. Neuropharmacology 177, 108216. [DOI] [PubMed] [Google Scholar]
- Neville K. E., Bosse T. L., Klekos M., Mills J. F., Weicksel S. E., Waters J. S., Tipping M. (2018). A novel ex vivo method for measuring whole brain metabolism in model systems. J. Neurosci. Methods 296, 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiecki M. M., Walker D. I., Vermeulen R., Chadeau-Hyam M., Jones D. P., Miller G. W. (2019). The exposome: Molecules to populations. Annu. Rev. Pharmacol. Toxicol. 59, 107–127. [DOI] [PubMed] [Google Scholar]
- Olsen A. L., Feany M. B. (2019). Glial α-synuclein promotes neurodegeneration characterized by a distinct transcriptional program in vivo. Glia 67, 1933–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordonez D. G., Lee M. K., Feany M. B. (2018). α-Synuclein induces mitochondrial dysfunction through spectrin and the actin cytoskeleton. Neuron 97, 108–124.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Arellano H. F., Jimenez-Del-Rio M., Velez-Pardo C. (2017). Minocycline protects, rescues and prevents knockdown transgenic parkin Drosophila against paraquat/iron toxicity: Implications for autosomic recessive juvenile parkinsonism. Neurotoxicology 60, 42–53. [DOI] [PubMed] [Google Scholar]
- Park J., Lee S. B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M., Kim J.-M., et al. (2006). Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161. [DOI] [PubMed] [Google Scholar]
- Pfaff A. L., Bubb V. J., Quinn J. P., Koks S. (2020). An increased burden of highly active retrotransposition competent L1s is associated with Parkinson’s disease risk and progression in the PPMI cohort. Int. J. Mol. Sci. 21, 6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddighe S., Bhat K. M., Setzu M. D., Solla P., Angioy A. M., Marotta R., Ruffilli R., Marrosu F., Liscia A. (2013). Impaired sense of smell in a Drosophila Parkinson’s model. PLoS One 8, e73156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaneda-Lombarte N., Blasco-Agell L., Serratosa J., Ferigle L., Saura J., Solà C. (2020). Parkinsonian neurotoxicants impair the anti-inflammatory response induced by IL4 in glial cells: Involvement of the CD200-CD200R1 ligand-receptor pair. Sci. Rep. 10, 10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B. R., Chatterjee N., Garcia-Closas M., Gauderman W. J., Pierce B. L., Kraft P., Tanner C. M., Mechanic L. E., McAllister K. (2017). Lessons learned from past gene-environment interaction successes. Am. J. Epidemiol. 186, 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B. R., Paul K. C., Bronstein J. M. (2016). Of pesticides and men: A California story of genes and environment in Parkinson’s Disease. Curr Environ Health Rep. 3, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano S., Savva G. M., Bedarf J. R., Charles I. G., Hildebrand F., Narbad A. (2021). Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B., Han J., Hope K. A., Peters T. L., Palmer G., Reiter L. T. (2020). An unbiased drug screen for seizure suppressors in duplication 15q syndrome reveals 5-HT1A and dopamine pathway activation as potential therapies. Biol. Psychiatry 88, 698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson T. R., Debelius J. W., Thron T., Janssen S., Shastri G. G., Ilhan Z. E., Challis C., Schretter C. E., Rocha S., Gradinaru V., et al. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167, 1469–1480.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L. H., Paul K. C., Howlett E. H., Lawal H., Boppana S., Bronstein J. M., Ritz B., Greenamyre J. T. (2017). Editor’s highlight: Base excision repair variants and Pesticide exposure increase Parkinson’s disease risk. Toxicol. Sci. 158, 188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Malovic E., Harishchandra D. S., Ghaisas S., Panicker N., Charli A., Palanisamy B. N., Rokad D., Jin H., Anantharam V., et al. (2017). Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson’s disease. NPJ Parkinsons Dis. 3, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Murphy M. A., Dammer E. B., Olsen A. L., Rangaraju S., Fraenkel E., Feany M. B. (2020). Comparative proteomic analysis highlights metabolic dysfunction in α-synucleinopathy. NPJ Parkinsons Dis. 6, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L., Galvin J. E., Suzuki Y., Gottschalk L., Shaw P. J. (2009). Persistent short-term memory defects following sleep deprivation in a Drosophila model of Parkinson disease. Sleep 32, 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman J. M., De Jager P. L., Feany M. B. (2011). Parkinson’s disease: Genetics and pathogenesis. Annu. Rev. Pathol. 6, 193–222. [DOI] [PubMed] [Google Scholar]
- Simmnacher K., Krach F., Schneider Y., Alecu J. E., Mautner L., Klein P., Roybon L., Prots I., Xiang W., Winner B. (2020). Unique signatures of stress-induced senescent human astrocytes. Exp. Neurol. 334, 113466. [DOI] [PubMed] [Google Scholar]
- Song I. Y., Snyder A. M., Kim Y., Neely E. B., Wade Q. W., Connor J. R. (2020). The Nrf2-mediated defense mechanism associated with HFE genotype limits vulnerability to oxidative stress-induced toxicity. Toxicology 441, 152525. [DOI] [PubMed] [Google Scholar]
- Sun W., Samimi H., Gamez M., Zare H., Frost B. (2018). Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat. Neurosci. 21, 1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff D. F., Jui N. T., Khurana V., Tambe M. A., Thompson M. L., Chung C. Y., Kamadurai H. B., Kim H. T., Lancaster A. K., Caldwell K. A., et al. (2013). Yeast reveal a “druggable” Rsp5/Nedd4 network that ameliorates α-synuclein toxicity in neurons. Science 342, 979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K., Andrews L., Krause J., Hanak T., Lee D., Gelb M., Pallanck L. (2010). Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson’s disease through an NRF2-dependent mechanism. J. Neurosci. 30, 5525–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura N., Yagi H., Uemura M. T., Hatanaka Y., Yamakado H., Takahashi R. (2018). Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol. Neurodegener. 13, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbizu A., Beyer K. (2020). Epigenetics in lewy body diseases: Impact on gene expression, utility as a biomarker, and possibilities for therapy. Int. J. Mol. Sci. 21, 4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venderova K., Kabbach G., Abdel-Messih E., Zhang Y., Parks R. J., Imai Y., Gehrke S., Ngsee J., Lavoie M. J., Slack R. S., et al. (2009). Leucine-rich repeat kinase 2 interacts with parkin, DJ-1 and PINK-1 in a Drosophila melanogaster model of Parkinson’s disease. Hum. Mol. Genet. 18, 4390–4404. [DOI] [PubMed] [Google Scholar]
- Verheijen B. M., Vermulst M., van Leeuwen F. W. (2018). Somatic mutations in neurons during aging and neurodegeneration. Acta Neuropathol. (Berl.) 135, 811–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen R., Schymanski E. L., Barabási A.-L., Miller G. W. (2020). The exposome and health: Where chemistry meets biology. Science 367, 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickovic S., Eraslan G., Salmén F., Klughammer J., Stenbeck L., Schapiro D., Äijö T., Bonneau R., Bergenstråhle L., Navarro J. F., et al. (2019). High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 16, 987–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. I., Valvi D., Rothman N., Lan Q., Miller G. W., Jones D. P. (2019). The metabolome: A key measure for exposome research in epidemiology. Curr. Epidemiol. Rep. 6, 93–103. [PMC free article] [PubMed] [Google Scholar]
- Wang L., Hagemann T. L., Messing A., Feany M. B. (2016). An in vivo pharmacological screen identifies cholinergic signaling as a therapeutic target in glial-based nervous system disease. J. Neurosci. 36, 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xia J., Li J., Hagemann T. L., Jones J. R., Fraenkel E., Weitz D. A., Zhang S.-C., Messing A., Feany M. B. (2018). Tissue and cellular rigidity and mechanosensitive signaling activation in Alexander disease. Nat. Commun. 9, 1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall S., Lomis N., Prakash S. (2019). A novel synbiotic delays Alzheimer’s disease onset via combinatorial gut-brain-axis signaling in Drosophila melanogaster. PLoS One 14, e0214985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Schmiel L., Zhou M., Cintina I., Spencer D., Hogan P.. 2019. The Economic Burden of Parkinson’s Disease. Available at: https://www.parkinson.org/sites/default/files/2019%20Parkinson%27s%20Economic%20Burden%20Study%20-%20FINAL.pdf.
- Zeng X.-S., Geng W.-S., Jia J.-J. (2018). Neurotoxin-induced animal models of Parkinson disease: Pathogenic mechanism and assessment. ASN Neuro. 10, 1759091418777438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharikov A. D., Cannon J. R., Tapias V., Bai Q., Horowitz M. P., Shah V., El Ayadi A., Hastings T. G., Greenamyre J. T., Burton E. A. (2015). shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson’s disease model. J. Clin. Invest. 125, 2721–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]


