Abstract
Peripubertal exposure of male rodents to the phthalate metabolite mono-(2-ethylhexyl) phthalate (MEHP) causes testicular inflammation, spermatocyte apoptosis, and disruption of the blood-testis barrier. The MEHP-induced inflammatory response in the testis includes an infiltration of macrophages and neutrophils, although the cause and purpose of this response is unknown. Recently, a population of testicular macrophages known as peritubular macrophages that are phenotypically distinct from those resident in interstitium was described in mice. Peritubular macrophages aggregate near the spermatogonial stem cell niche and are believed to stimulate their differentiation. We hypothesized that if testicular peritubular macrophages do indeed stimulate spermatogonial differentiation, MEHP exposure would result in an increase of peritubular macrophages to stimulate the replacement of lost spermatocytes. Male rats were exposed to 700 mg/kg MEHP or corn oil (vehicle control) via oral gavage at postnatal day 28 and euthanized at 48 h, 1 or 2 weeks later. Seminiferous tubules were stained with immunofluorescent markers for macrophages (major histocompatibility complex class II [MHC-II+]) and undifferentiated spermatogonia (PLZF). Peritubular macrophages were observed in rat testis: MHC-II+ cells on the surface of seminiferous tubules with heterogeneous morphology. Quantification of MHC-II+ cells revealed that, unlike in the mouse, their numbers did not increase through puberty (2-week period). MEHP increased macrophage presence by 6-fold 48 h after exposure and remained elevated by 2-fold 2 weeks after exposure. An increase of differentiating spermatogonia occurred 2 weeks after MEHP exposure. Taken together, our results suggest that peritubular macrophages play a crucial role in the testis response to acute injury and the subsequent recovery of spermatogenesis.
Keywords: peritubular macrophage, testis, spermatogenesis, phthalate, toxicant
Di-(2-ethylhexyl) phthalate (DEHP) is a ubiquitous plasticizer that is not chemically bound to its substrates, which results in its release and eventual ingestion by humans and animals (Hanawa et al., 2000; Takehisa et al., 2005). Mono-(2-ethylhexyl) phthalate (MEHP) is the primary metabolite of DEHP and has been observed at measurable concentrations in a majority of newborns (Latini et al., 2003) and in prepubertal children from those observed in the United States (Silva et al., 2004) and Germany (Becker et al., 2004). MEHP is considered an endocrine disrupting chemical that is correlated with reduced free testosterone (Pan et al., 2006) and increased prolactin and estradiol (Li et al., 2011) in adult male humans. MEHP persists in measurable quantities in semen (Han et al., 2009) and urine and is correlated with a decrease in sperm quality (Wang et al., 2015). Hence, one of the primary concerns regarding MEHP exposure in humans is the deleterious effect it may have on fertility and reproductive function.
Experimental animal models provide a majority of the mechanistic evidence that links phthalate exposure to adverse fertility outcomes. In the testis, MEHP exposure primarily affects Sertoli cells (Boekelheide et al., 1989) which sequester meiotic, and therefore antigenic, germ cells behind a selective blood-testis barrier (BTB). The BTB protects germ cells from the immune system, transfers necessary nutrients and information from the endocrine system, and cleans up apoptotic germ cells and waste products created during spermatogenesis (reviewed in França et al., 2016). Acute high dose MEHP exposure in rodents results in the disruption of the Sertoli cell cytoskeleton (Richburg and Boekelheide, 1996). A secondary consequence of this disruption is the dysregulation of the apoptosis inducing CD95/CD95L signaling system (Lee et al., 1999) which results in the widespread death of maturing spermatocytes (Richburg et al., 1999). In addition, MEHP exposure causes disruption in the gap junctions between Sertoli cells that form the BTB (Yao et al., 2010) which is, in part, regulated by sTNF-α (Yao et al., 2007), a compound that is capable of initiating an inflammatory immune response.
A brief, but large, monocyte influx appears coincident with widespread spermatocyte apoptosis following MEHP exposure (Murphy et al., 2014). This influx is at least in part elicited by canonical pro-inflammatory cytokines (eg, IL-1α, IL-6 and monocyte chemoattractant protein-1 [MCP-1]) released by Sertoli cells and not by spermatocyte apoptosis itself (Stermer et al., 2017). Although the infiltrating monocytes observed due to acute MEHP exposure are inflammatory in phenotype, they do not contribute to or exacerbate spermatocyte apoptosis (Voss et al., 2018).
The purpose or function of the monocyte infiltration into the testes due to MEHP- induced injury remains unknown. Macrophages, which mature from naïve monocytes, make up a large portion of all cells in the testicular interstitium and are the most abundant immune cell type in the testis (Niemi et al., 1986). Testicular macrophages can be broadly categorized as those that are resident to the testes and those that infiltrate into the testes as a part of an inflammatory response. Resident testicular macrophages perform a number of essential functions during development and adulthood (reviewed in Bhushan and Meinhardt, 2017; Hedger, 2002). These functions include morphogenesis via vascularization during organogenesis (DeFalco et al., 2014), assisting in the steroidogenesis of testosterone (Hutson, 1992), and aiding spermatogenesis and maintaining an immunosuppressive environment (Hayes et al., 1996; Kern et al., 1995) presumably to protect the integrity of the meiotic spermatogonial niche. Even under inflammatory conditions, resident testicular macrophages have a unique response and tend to polarize towards an antiinflammatory phenotype; in response to LPS (Kern et al., 1995), bacteria (Bhushan et al., 2011), or IL-4 (Winnall et al., 2011). Recently, a novel role for testicular macrophages was proposed in mice. A subset of macrophages were observed on the surface of seminiferous tubules, concentrated in areas of undifferentiated spermatogonia, and were found to aid in the differentiation of spermatogonia (DeFalco et al., 2015). This specialized population of macrophages, called peritubular macrophages, was found to be derived exclusively from infiltrating populations of monocytes (Mossadegh-Keller et al., 2017). Therefore, we hypothesize that peritubular macrophages aid in the recovery of the spermatogonial niche, rather than cause additional damage as previously predicted (Voss et al., 2018). To test this hypothesis, first the presence of peritubular macrophages were evaluated in the rat testis, then their morphological and expression phenotype were characterized, and finally, it was determined if the presence of these cells correlated with an expansion of the population of undifferentiated spermatogonia as an indication of their influence on the spermatogonial niche.
MATERIALS AND METHODS
Animals and treatment
All animal work was conducted using humane procedures that were preapproved by the Institutional Animal Care and Use Committee at The University of Texas at Austin and followed NIH guidelines (AUP-201900115). Males Fischer CDF344 rats were purchased from Harlan (Indianapolis, Indiana) and shipped to The University of Texas at Austin at postnatal day (PND) 25. The inbred Fischer CDF344 rats were chosen for these studies based on the many years of data and experience we have using this strain of rat in interrogating the cellular and molecular mechanisms perturbed by phthalate exposure. Using this inbred rat strain allows for reduced variability in experiments and, as a result, allows for the favorable reduction in the numbers of animals needed for these studies. Peripubertal age rats were chosen for this study as MEHP is well known to incite a robust macrophage infiltration in these young animals (Murphy et al., 2014). Upon arrival, animals were maintained in a temperature-controlled housing facility on a 12:12 L:D cycle with ad libitum access to food and standard rat chow. After 3 days of habituation, on PND 28, males were randomly assigned to 1 of 2 treatment exposure groups; vehicle control (VEH) or MEHP.
Each animal was exposed via oral gavage to 700 mg/kg MEHP dissolved in corn oil or an equivalent volume of corn oil (VEH). Based on previous research, we estimate our exposure model results in an estimated approximately 100 µM MEHP within the testis (Thomas and Thomas, 1984). The acute high dose phthalate exposure model utilized in this article elicits a well-described, rapid and specific disruption in Sertoli cell function. The utility of the acute high dose exposure model is that it allows for a quick identification of end points due to its robust altered phenotype. A total of 7 animals were used for each treatment group across 2 cohorts; first cohort = 3 animals/treatment, second cohort = 4 animals/treatment. Following gavage, animals were checked for general health but were otherwise left undisturbed in their home cage until sacrifice 48 h after treatment, 1 week after treatment, or 2 weeks after treatment. Animals were euthanized by CO2 asphyxiation followed by cervical dislocation. The testes were rapidly removed and weighed. One testis was randomly selected for whole tubule immunofluorescence and was immediately placed in cold PBS (Gibco, Cat no. 10010-023) on ice. The other testis was submerged in Bouin’s fixative on a gentle rocker overnight. Bouin’s was replaced with lithium-saturated ethanol the following day and every subsequent day until the solution and the testis were clear and saved for future experiments.
Seminiferous tubules were isolated within 1-h of sacrifice from testes that had been placed in cold PBS on ice. The testis was decapsulated by puncturing and tearing the tunica and the tubules evacuated into cold PBS. The tubules were gently teased apart and the interstitial tissue removed with blunt forceps. The tubules were then washed 4 times in ice cold PBS to further remove interstitial tissue and cells. The tubules were then fixed in 4% paraformaldehyde (Electron Microscopy Sciences, 15710-S) overnight at 4°C, washed an additional 4 times in cold PBS, and stored in cold PBS at 4°C until use. This methodology (imaging isolated tubules) was chosen to observe peritubular macrophages because it removes interstitial tissue and the many macrophages that occupy the interstitial compartment. Using testes cross sections make it nearly impossible to differentiate between macrophages that are in the interstitium and those that are actually on the surface of seminiferous tubules (DeFalco et al., 2015).
Immunofluorescence staining
Immunofluorescence staining was performed in 96-well plates on seminiferous tubules with primary antibodies major histocompatibility complex class II [MHC class II RT1D (BioLegend, Clone OX-17, 205401, 1:200)], cluster of differentiation 68 (CD68 [Bio Rad, MCA341R, 1:200]) is a marker for circulating monocytes, zinc finger and BTB domain-containing protein 16 (ZBTB16/PLZF [Santa Cruz Biotechnology, sc-22839, 1:200]) is marker for differentiating spermatogonia and secondary antibodies Alexa 488 (Invitrogen, A11001, 1:500) and Alexa 568 (Invitrogen, A11036, 1:500). Hoechst 33342 (Life Technologies, H3570, 1:10 000) was used as a DNA/nuclei counter stain. Stained tubules were mounted whole on super frost slides with Flouromount-G (Southern Biotech, 0100-01) and sealed with standard clear nail polish. Slides were stored at −20°C until imaging.
Confocal microscopy and cell count
Slides were imaged on a Zeiss scanning confocal microscope (Zeiss, LSM 710). Tubule selection for imaging was performed exclusively under the Hoechst fluorescence filter to prevent any bias in macrophage or spermatogonia density. The only selection criteria for image capture were that the tubule not be damaged or distorted by the tubule isolation and staining processes. Images that were used to count macrophages and spermatogonia were taken at 20× (425.10 × 425.10 μm) and with a depth of 15 μm in 3 μm increments to ensure the surface of the seminiferous epithelium and spermatogonia were captured. Z-stacks were flattened in ImageJ (version 1.49T) using the “Max Intensity” method. Positively stained cells in flattened images were then counted with EBImage (version 4.24.0) and R (version 3.5.3). Cell count was normalized to tubule area and is represented as cell counts per 105 pixel area. An aggregate cell count was calculated across 15 individual tubule observations within each animal by summing the cell count and tubule area of each image and calculating the density of positive cells (no. cells/105 area). This methodology considers each animal as the individual statistical unit rather than each tubule observation as the individual statistical unit and avoids violations of nonindependence assumptions within statistical tests. Counts were intermittently verified by manual counting in ImageJ to ensure accuracy.
Statistics
Cell counts were compared between treatments and within age using a Wilcoxon Ranked-Sum test because the data were not normally distributed and were considered significant if p < .01. Graphs were made in R with ggplot2 (version 3.1.1) and edited only for style in Adobe Illustrator (version CS5). Images for morphological and co-staining analyses were taken at 63× (134.95 × 134.95 μm) at z-depths that varied depending on the depth of the cell being imaged.
RESULTS
Localization and Morphology of Peritubular Macrophage
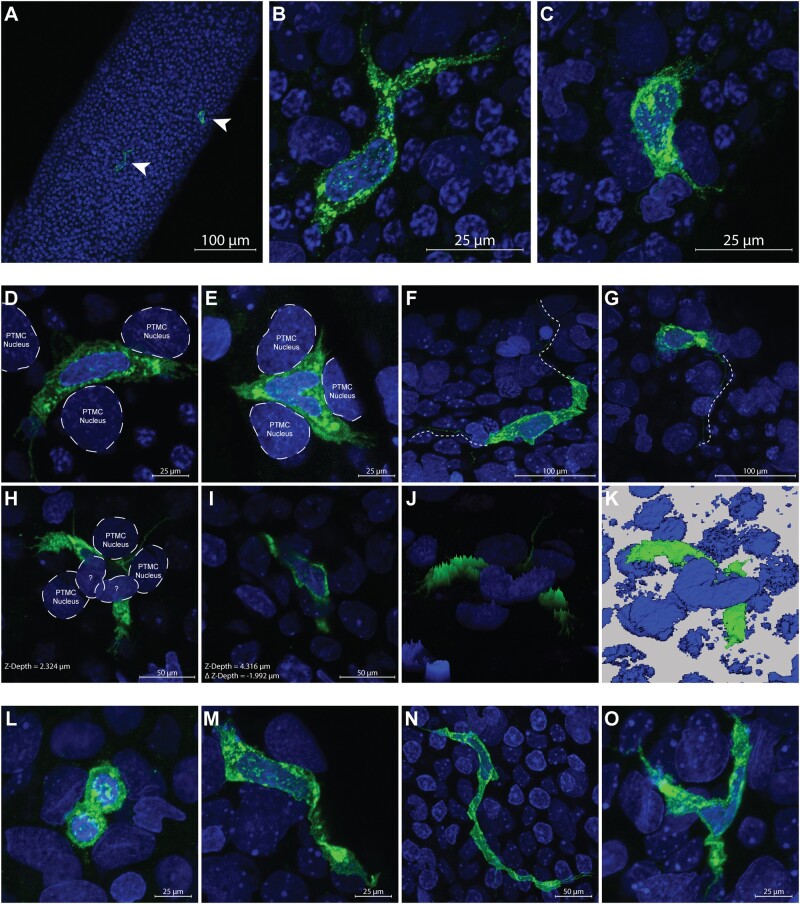
Seminiferous tubules from PND30 Fischer CD344 male rats were separated from interstitial tissue, subjected to immunofluorescence for MHCII, and mounted whole on slides for analysis on a confocal microscope. A population of MHCII+ cells was observed on the surface of seminiferous tubules (20×—Figure 1A, from VEH group). Closer observation of these cells revealed irregular morphology of the cell membrane and nucleus (63×—Figs. 1B and 1C, from VEH group). MHCII+ cells were sparse and found on average once every 2 randomly captured (425.1 × 425.1 μm, average tubule area 102 084 μm2) windows. These cells were located on the surface of tubules often overlying or nestled in-between, in the same plane, or in close proximity to the nuclei of what are presumed to be peritubular myoid cells (Figure 1D, from VEH group) and (Figure 1E) by their location and unique nuclei morphology. Occasionally, long (approximately 50–100 μm) protrusions originating from MHCII+ cells appeared to extend around or between the nuclei of apparent peritubular myoid cells (Figs. 1F and 1G, from VEH group). In rare instances the nuclei of an MHCII+ cell was observed under the nuclei of an unknown cell type (Figs. 1H and 1I, from MEHP group).
Figure 1.
A, major histocompatibility complex class II (MHCII+) peritubular macrophages vehicle control (VEH—green—arrow heads) are shown on the surface of a whole seminiferous tubule at 20×; scale bar = 100 μm. B and C, Up close of (A; 63×; scale bar = 25 μm) confocal images show 2 MHCII+ peritubular macrophages from VEH animals with heterogeneous morphology on the surface of a seminiferous tubule. D and E, Two separate macrophages D (from VEH) and E (from mono-(2-ethylhexyl) phthalate [MEHP]) are shown at high magnification (63×; scale bar = 25 μm) nestled between the nuclei of what appear to be peritubular myoid cells (PTMCs), identified by their location and unique nuclei morphology. The macrophage cell surface marker MHCII can be seen to occupy space in the same plane and between the presumed PTMC nuclei. F and G, Long (>100 μm) extensions can be seen protruding bidirectionally or unidirectionally (respectively) from MHCII+ cells from VEH animals (offset dashed/dotted line), sometimes meandering under the nuclei of other cells (63×; scale bar = 25 μm). H and I, An MHCII+ cell from MEHP group is shown in 2 different scanning planes (H = Z-Depth 2.324 μm and I = Z-Depth 4.316 μm) that is nestled between 3 presumed PTMC nuclei and under the nuclei of 2 cells unknown type (63×; scale bar = 50 μm). J, A 3D brightness histogram and K, a reconstructed 3D image shows an alternative view of an MHCII+ cell under the nuclei of 2 cells of unknown type from MEHP-treated animals. The various morphologies of MHCII+ peritubular macrophages observed on the surface of seminiferous tubules is shown; (L) circular from VEH group (63×; scale bar = 25 μm); (M) spindeloid from MEHP group (63×; scale bar = 25 μm); (N) elongated from MEHP group (63×; scale bar = 50 μm); (O) stellate from MEHP group (63×; scale bar = 25 μm).
The morphology of MHCII+ cells was heterogeneous. The majority of MHCII+ cells observed on the surface of the seminiferous tubule were either circular (Figure 1L, from MEHP group) or spindeloid (Figure 1M, from VEH group, Heinrich et al., 2017) in morphology. Observation of MHCII+ cells with elongated or stellate morphology was rare but was occasionally observed (Figure 1N, from VEH group and Figure 1O, from VEH group, respectively).
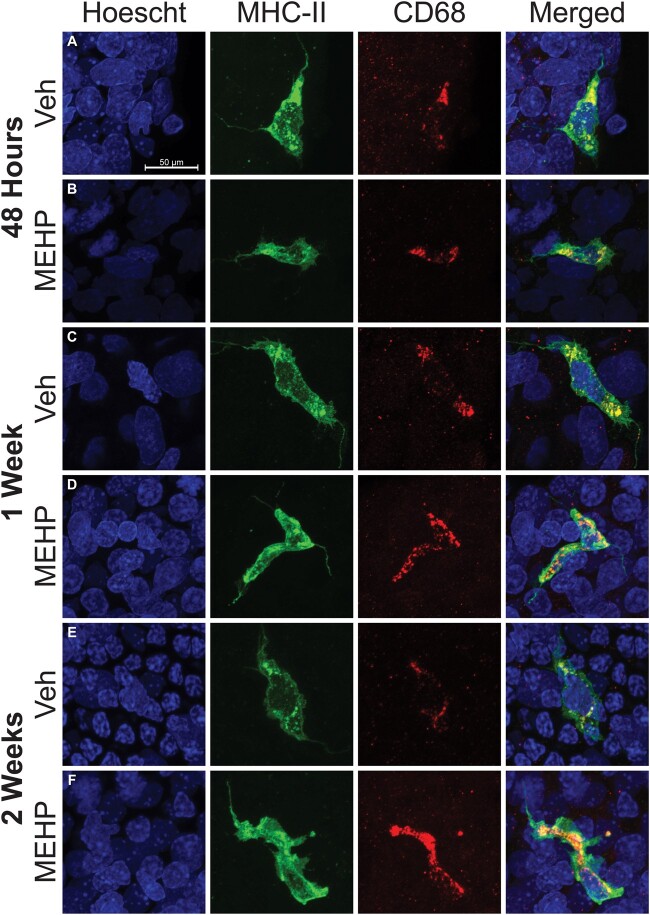
Peritubular Macrophages Are MHCII+ and CD68+
In order to determine if the MHCII+ cells observed were in fact derived from the monocyte lineage, tubules were double stained with MHCII+ and the lysosomal protein CD68. All observed MHCII+ cells on the surface of seminiferous tubules were CD68+ regardless of the morphology of MHCII+ cells (Figure 2). All MHCII+ cells remained CD68+ regardless of the age or treatment of the animal analyzed (PND30—Figs. 2A and 2B, PND35—Figs. 2C and 2D, and PND42—Figs. 2E and 2F).
Figure 2.
Double staining of peritubular macrophages with major histocompatibility complex class II (MHCII; green) and CD68 (red) are shown at all treatment and age endpoints; (A, B 48 h after mono-(2-ethylhexyl) phthalate (MEHP) exposure, (C, D) 1 week after MEHP exposure, and (E, F) 2 weeks after MEHP exposure. The Hoescht (blue) nuclei counter stain is used in all images and the scale bar shown in (A) is the same for all images. All MHCII+ macrophages are also CD68+ regardless of treatment, age, or time after exposure. All imaged were taken at 63×. The scale bar (50 μm) represented in (A) applies to all images in this figure.
MEHP Treatment Increases Number of Peritubular Macrophages
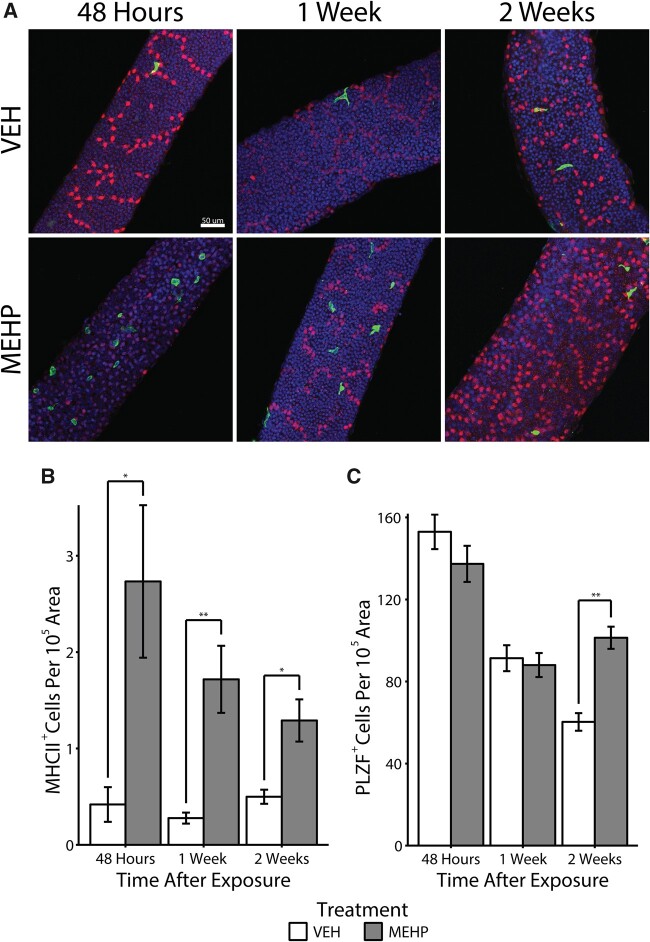
We hypothesized that if the function of peritubular macrophages is indeed to stimulate the differentiation of spermatogonial cells, then depletion of maturing germ cells via MEHP treatment would induce the mobilization of peritubular macrophages to the seminiferous tubules. PND28 male rats were exposed to either MEHP or corn oil (VEH) and sacrificed after 48 h, 1, or 2 weeks with no further manipulation and the number of MHCII+ cells, controlled for by tubule area, was counted (n = 7 per treatment per age, Figure 3A). After 48 h, the population of MHCII+ was significantly elevated approximately 6.5-fold compared with VEH males of the same age (U = 3, p = .002, Figure 3B). The number of MHCII+ cells began to decline but remained significantly elevated in MEHP animals approximately 6-fold after 1 week (U = 0, p = .0003) and approximately 2.5-fold after 2 weeks (U = 6, p = .005). Age did not affect the number of MHCII+ cells in VEH animals as determined by a Kruskal-Wallis test (H[2] = 4.1039, p = .1285). Subsequent pairwise Kruskal-Wallis rank sum tests, adjusted with the Benjamini-Hochberg method, also failed to identify any differences in MHCII+ cell counts across age and within VEH animals.
Figure 3.
A, Representative micrographs of major histocompatibility complex class II (MHCII+; green) peritubular macrophages are shown on the surface of seminiferous tubules in close proximity to PLZF+ (red) spermatogonia in both vehicle control (VEH) and mono-(2-ethylhexyl) phthalate (MEHP)-treated rats after 48 h after exposure to MEHP. All images in this figure were taken at 20×. The scale bar (50 μm) represented in the top left panel applies to all images in this figure. B, The number of MHCII+ cells, normalized to tubule area, is shown. *p < .01 and **p < .001. C, The number of PLZF+ spermatogonia in VEH and MEHP-treated male rats is shown normalized to tubule area (**p < .001).
Finally, we stained whole tubules with PLZF to determine if spermatogonial number was affected by MEHP treatment. We found no difference in the number of PLZF+ cells at either the 48-h or 1-week time point. However, the number of PLZF+ cells was increased due to MEHP exposure at the 2-week time point (U = 3, p < .001, Figure 3C).
DISCUSSION
Testicular macrophages perform an eclectic set of essential functions in the testes that span the life cycle (reviewed in Bhushan and Meinhardt, 2017). During development, testicular macrophages aid in the organogenesis of the testis by directing vascularization (DeFalco et al., 2014). During adolescence, testicular macrophages stimulate the differentiation and proliferation of Leydig cells, the prime steroid-hormone producing cell in the testis (Gaytan et al., 1994). In adulthood, testicular macrophages aid in the ultimate role of the testes, spermatogenesis, by aiding in the steroidogenesis of testosterone (Hutson, 1992). Throughout the lifespan, testicular macrophages help maintain an immune-privileged environment in the testis and therefore they protect sanctity of spermatogenesis (Kern and Maddocks, 1995). Recently, a newly discovered function was ascribed to testicular macrophages. A subset of testicular macrophages (peritubular macrophages) was identified in mice that localize to the surface of seminiferous tubules near the spermatogonial niche that when depleted disrupts spermatogonial differentiation (DeFalco et al., 2015).
The aim of this work is to describe the peritubular subset of macrophages in the rat testis for the first time, characterize their morphology and distribution, and observe their newly ascribed function in an injury model. Although previous models have shown that depleting macrophages from the testis reduces the number of differentiating spermatogonia (DeFalco et al., 2015), here we demonstrate that depletion of spermatocytes by exposure to MEHP mobilizes peritubular macrophages to the surface of seminiferous tubules in large numbers for at least 2 weeks. This increase is concurrent with an increase in the number of PLZF positive cells that marks undifferentiated and early stage differentiating spermatogonia. This work is significant in that it is one of the first demonstrations that peritubular macrophages are specifically mobilized by testicular injury and the large-scale loss of spermatocytes.
MEHP is a well-described Sertoli cell toxicant (Richburg and Boekelheide, 1996), which causes widespread loss of spermatocytes (Richburg et al., 1999). The loss of spermatocytes due to MEHP exposure is age- (younger > older), species- (rat > mouse), and dose-dependent (Murphy et al., 2014). The loss of spermatocytes is concurrent with a massive but transient (approximately 48 h) monocyte influx that is congruent with the degree of spermatocyte loss; the monocyte influx is more robust in younger animals, larger in rats compared with mice and larger at higher doses of MEHP (Murphy et al., 2014). It was initially hypothesized that because the monocyte influx induced by MEHP was proportional to the loss of spermatocytes, this large-scale inflammatory immune response might exacerbate, or be the cause of, the loss of spermatocytes and thus account for the specificity of the observed effects. This was found not to be the case. The depletion of circulating monocytes, which neutralizes the MEHP-induced monocyte influx, has no effect on spermatocyte apoptosis (Voss et al., 2018). The precipitating cause and the functional purpose of this influx is not yet fully understood, but our current results suggest that at least one consequence is an increase in the long-term (2 weeks) presence of peritubular macrophages.
Given that the only known function of peritubular macrophages is to stimulate the differentiation of spermatogonia, and that a loss of spermatocytes would necessitate their replacement by the induction of spermatogonial differentiation, it is reasonable to suggest that the increase in peritubular macrophages we observe is to stimulate spermatogonial differentiation to replace the lost spermatocytes. Our data indirectly support this hypothesis; 2 weeks after MEHP exposure, a time point where peritubular macrophages remain elevated, the number of PLZF+ cells (differentiating spermatogonia) is increased. Furthermore, the distribution of peritubular macrophages is uneven and sporadic with higher densities in a few locations, similar to what would be expected of the spermatogonial stem cell niche distribution (de Rooij, 2017). This hypothesis requires additional experiments and evidence, including colocalization of peritubular macrophages and the spermatogonial stem cell niche, which are currently underway in our laboratory.
Peritubular macrophages may, of course, have functions other than, or in addition to, the stimulation of spermatogonial differentiation. There are 2 primary immunoreactivity profiles of macrophages that populate the testes in rats; tissue resident testicular-macrophages (CD68−/CD163+) and newly arrived monocyte-like testicular macrophages (CD68+/CD163−; reviewed in: Hedger, 2002). These 2 populations have distinct expression phenotypes that represent antiinflammatory tissue resident macrophages (CD68−/CD163+) and “alternatively activated macrophages” with suppressed inflammatory activity (CD68+/CD163−; Winnall et al., 2011).
Here, we reveal that all peritubular macrophages in rats, regardless of treatment, are CD68+. This observation suggests that peritubular macrophages conform to the “alternatively activated” phenotype described above and are seeded in the testis from circulating monocytes. That we observe a marked increase in peritubular macrophages that are exclusively CD68+ within 48 h of treatment further supports this hypothesis, as tissue resident macrophages would be expected to be CD68−. These results are consistent with previous observations in mice that found macrophages are exclusively seeded from bone-marrow derived circulating monocytes using lineage-tracing techniques (Mossadegh-Keller et al., 2017). Determining a definitive immunoreactive profile for peritubular macrophages in future experiments is essential so that further characterization can be performed (eg, fluorescent activated cell sorting to allow for macrophage separation and transcriptome profiling). We attempted multiple CD163 antibodies and titrations but staining was not identified in any of the MHCII+ cells. It’s possible that either peritubular macrophages are not CD163+ or that our protocol needs further optimization. Efforts are currently underway to describe the CD163 phenotype of peritubular macrophages in rats, which will further resolve the phenotype of these macrophages.
The location and morphology of peritubular macrophages also provide some evidence of their possible function. Sertoli cells can perform macrophage-like functions and therefore serve an immune cell role in the seminiferous epithelium; phagocytosis and ingestion of apoptotic spermatocytes (Nakanishi and Shiratsuchi, 2004) and microbe detection via the Toll-like receptor (Riccioli et al., 2006). Sertoli cells actively participate in the clearance of apoptotic spermatocytes (Tay et al., 2007) and secrete the powerful MCP-1 (Stermer et al., 2017) specifically due to MEHP-induced injury. Under normal physiological conditions, Sertoli cells can induce germ cell apoptosis and ingest said cells such that the meiotic and immunogenic remnants remain sequestered behind the BTB (reviewed in Print and Loveland, 2000). It is, however, possible that in extreme cases of spermatocyte loss, such as those induced by acute MEHP exposure, Sertoli cells are not capable of keeping up with the processing of apoptotic bodies. A possible consequence of this scenario is that if the BTB becomes compromised, an effect observed due to MEHP exposure (Yao et al., 2010), an autoantigenic immune response could be mounted against the “nonself” antigens and cause further damage. Sertoli cells can act as antigen presenting cells which diminishes inflammatory immune responses (Dal Secco et al., 2008). Peritubular macrophages reside in such close proximity to Sertoli cells, that they are themselves antigen presenting cells, and that they respond specifically to an event with widespread apoptosis, suggests that they may play a role in the tolerization of inflammatory immune responses in certain catastrophic scenarios. Direct macrophage cell-cell contact and communication in the testis is not unheard of. Interstitial macrophages are known to directly communicate with Leydig cells and aid in steroidogenesis (Hutson, 1990). That peritubular macrophages could contact and interact with Sertoli cells would not be implausible and our observations suggest that, at minimum, peritubular macrophages are positioned to do so. Extensive additional work is needed to confirm this hypothesis including the localization of peritubular macrophages and the extent to which they might penetrate the PTMC layer.
Taken together, the results presented demonstrate that a specialized subcategory of testicular macrophage is recruited to the seminiferous tubules in response to acute testicular injury in rats. This work is fundamental to understanding the mechanisms by which infertility and azoospermia occur in adult males. Correlational observations in humans consistently note an increased presence of CD68+ macrophages in infertile men (Frungieri et al., 2002; Goluža et al., 2014). These studies suggest the presence of CD68+ macrophages may contribute to or be the cause of infertility. These studies, however, are retrospective in nature and may misinterpret the significance of the increased presence of macrophages. Although more experimentation is needed, our data, and that of our predecessors, suggest instead that CD68+ peritubular macrophages initially infiltrate the testis in response to the loss of maturing spermatocytes and act to stimulate spermatogonial differentiation.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
AUTHORS’ CONTRIBUTIONS
Conceptualization: J.H.R., R.T., and J.J.L.P.V.; Methodology: R.G., R.T., and J.J.L.P.V.; Investigation: R.G., R.T., J.J.L.P.V., and S.N.H.; Formal Analysis: R.G.; Writing—original draft: R.G.; Writing—review and editing: R.G., R.T., and J.H.R.; Funding Acquisition: J.H.R
ACKNOWLEDGMENTS
The authors recognize and appreciate Xin Fang for her critical review and suggested edits.
FUNDING
The National Institutes of Health/NIEHS (R01ES016591) and The Center for Molecular Carcinogenesis and Toxicology.
REFERENCES
- Becker K., Seiwert M., Angerer J., Heger W., Koch H. M., Nagorka R., Roßkamp E., Schlüter C., Seifert B., Ullrich D. (2004). DEHP metabolites in urine of children and DEHP in house dust. Int. J. Hyg. Environ. Health 207, 409–417. [DOI] [PubMed] [Google Scholar]
- Bhushan S., Hossain H., Lu Y., Geisler A., Tchatalbachev S., Mikulski Z., Schuler G., Klug J., Pilatz A., Wagenlehner F., et al. (2011). Uropathogenic E. coli induce different immune response in testicular and peritoneal macrophages: Implications for testicular immune privilege. PLoS One 6, e28452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan S., Meinhardt A. (2017). The macrophages in testis function. J. Reprod. Immunol. 119, 107–112. [DOI] [PubMed] [Google Scholar]
- Boekelheide K., Neely M. D., Sioussat T. M. (1989). The Sertoli cell cytoskeleton: A target for toxicant-induced germ cell loss. Toxicol. Appl. Pharmacol. 101, 373–389. [DOI] [PubMed] [Google Scholar]
- Dal Secco V., Riccioli A., Padula F., Ziparo E., Filippini A. (2008). Mouse Sertoli cells display phenotypical and functional traits of antigen-presenting cells in response to interferon gamma. Biol. Reprod. 78, 234–242. [DOI] [PubMed] [Google Scholar]
- de Rooij D. G. (2017). The nature and dynamics of spermatogonial stem cells. Development 144, 3022–3030. [DOI] [PubMed] [Google Scholar]
- DeFalco T., Bhattacharya I., Williams A. V., Sams D. M., Capel B. (2014). Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 111, E2384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco T., Potter S. J., Williams A. V., Waller B., Kan M. J., Capel B. (2015). Macrophages contribute to the spermatogonial niche in the adult testis. Cell Rep. 12, 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França L. R., Hess R. A., Dufour J. M., Hofmann M. C., Griswold M. D. (2016). The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 4, 189–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frungieri M. B., Calandra R. S., Lustig L., Meineke V., Köhn F. M., Vogt H. J., Mayerhofer A. (2002). Number, distribution pattern, and identification of macrophages in the testes of infertile men. Fertil. Steril. 78, 298–306. [DOI] [PubMed] [Google Scholar]
- Gaytan F., Bellido C., Aguilar E., van Rooijen N. (1994). Requirement for testicular macrophages in Leydig cell proliferation and differentiation during prepubertal development in rats. J. Reprod. Fertil. 102, 393–399. [DOI] [PubMed] [Google Scholar]
- Goluža T., Boscanin A., Cvetko J., Kozina V., Kosović M., Bernat M. M., Kasum M., Kaštelan Ž., Ježek D. (2014). Macrophages and Leydig cells in testicular biopsies of azoospermic men. Biomed. Res. Int. 2014, 828697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. W., Lee H., Han S. Y., Lim D. S., Jung K. K., Kwack S. J., Kim K. B., Lee B. M. (2009). An exposure assessment of di-(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DBP) in human semen. J. Toxicol. Environ. Health A 72, 1463–1469. [DOI] [PubMed] [Google Scholar]
- Hanawa T., Muramatsu E., Asakawa K., Suzuki M., Tanaka M., Kawano K., Seki T., Juni K., Nakajima S. (2000). Investigation of the release behavior of diethylhexyl phthalate from the polyvinyl-chloride tubing for intravenous administration. Int. J. Pharm. 210, 109–115. [DOI] [PubMed] [Google Scholar]
- Hayes R., Chalmers S. A., Nikolic-Paterson D. J., Atkins R. C., Hedger M. P. (1996). Secretion of bioactive interleukin 1 by rat testicular macrophages in vitro. J. Androl. 17, 41–49. [PubMed] [Google Scholar]
- Hedger M. P. (2002). Macrophages and the immune responsiveness of the testis. J. Reprod. Immunol. 57, 19–34. [DOI] [PubMed] [Google Scholar]
- Heinrich F., Lehmbecker A., Raddatz B. B., Kegler K., Tipold A., Stein V. M., Kalkuhl A., Deschl U., Baumgärtner W., Ulrich R., et al. (2017). Morphologic, phenotypic, and transcriptomic characterization of classically and alternatively activated canine blood-derived macrophages in vitro. PLoS One 12, e0183572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson J. C. (1990). Changes in the concentration and size of testicular macrophages during development. Biol. Reprod. 43, 885–890. [DOI] [PubMed] [Google Scholar]
- Hutson J. C. (1992). Development of cytoplasmic digitations between Leydig cells and testicular macrophages of the rat. Cell Tissue Res. 267, 385–389. [DOI] [PubMed] [Google Scholar]
- Kern S., Maddocks S. (1995). Indomethacin blocks the immunosuppressive activity of rat testicular macrophages cultured in vitro. J. Reprod. Immunol. 28, 189–201. [DOI] [PubMed] [Google Scholar]
- Kern S., Robertson S. A., Mau V. J., Maddocks S. (1995). Cytokine secretion by macrophages in the rat testis. Biol. Reprod. 53, 1407–1416. [DOI] [PubMed] [Google Scholar]
- Latini G., De Felice C., Presta G., Del Vecchio A., Paris I., Ruggieri F., Mazzeo P. (2003). In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ. Health Perspect. 111, 1783–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Richburg J. H., Shipp E. B., Meistrich M. L., Boekelheide K. (1999). The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology 140, 852–858. [DOI] [PubMed] [Google Scholar]
- Li S., Dai J., Zhang L., Zhang J., Zhang Z., Chen B. (2011). An association of elevated serum prolactin with phthalate exposure in adult men. Biomed. Environ. Sci. 24, 31–39. [DOI] [PubMed] [Google Scholar]
- Mossadegh-Keller N., Gentek R., Gimenez G., Bigot S., Mailfert S., Sieweke M. H. (2017). Developmental origin and maintenance of distinct testicular macrophage populations. J. Exp. Med. 214, 2829–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. J., Stermer A. R., Richburg J. H. (2014). Age- and species-dependent infiltration of macrophages into the testis of rats and mice exposed to mono-(2-ethylhexyl) phthalate (MEHP)1. Biol. Reprod. 91, 247–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y., Shiratsuchi A. (2004). Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: Mechanisms and consequences. Biol. Pharm. Bull. 27, 13–16. [DOI] [PubMed] [Google Scholar]
- Niemi M., Sharpe R. M., Brown W. R. (1986). Macrophages in the interstitial tissue of the rat testis. Cell Tissue Res. 243, 337–344. [DOI] [PubMed] [Google Scholar]
- Pan G., Hanaoka T., Yoshimura M., Zhang S., Wang P., Tsukino H., Inoue K., Nakazawa H., Tsugane S., Takahashi K. (2006). Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): A cross-sectional study in China. Environ. Health Perspect. 114, 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Print C. G., Loveland K. L. (2000). Germ cell suicide: New insights into apoptosis during spermatogenesis. Bioessays 22, 423–430. [DOI] [PubMed] [Google Scholar]
- Riccioli A., Starace D., Galli R., Fuso A., Scarpa S., Palombi F., De Cesaris P., Ziparo E., Filippini A. (2006). Sertoli cells initiate testicular innate immune responses through TLR activation. J. Immunol. 177, 7122–7130. [DOI] [PubMed] [Google Scholar]
- Richburg J. H., Boekelheide K. (1996). Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol. Appl. Pharmacol. 137, 42–50. [DOI] [PubMed] [Google Scholar]
- Richburg J. H., Nañez A., Gao H. (1999). Participation of the Fas-signaling system in the initiation of germ cell apoptosis in young rat testes after exposure to mono-(2-ethylhexyl) phthalate. Toxicol. Appl. Pharmacol. 160, 271–278. [DOI] [PubMed] [Google Scholar]
- Silva M. J., Barr D. B., Reidy J. A., Malek N. A., Hodge C. C., Caudill S. P., Brock J. W., Needham L. L., Calafat A. M. (2004). Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ. Health Perspect. 112, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stermer A. R., Murphy C. J., Ghaffari R., Di Bona K. R., Voss J. J., Richburg J. H. (2017). Mono-(2-ethylhexyl) phthalate-induced Sertoli cell injury stimulates the production of pro-inflammatory cytokines in Fischer 344 rats. Reprod. Toxicol. 69, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehisa H., Naoko E., Masahiko S., Katsuhide T., Moriyuki O., Keizoh S., Mutsuko T., Kenji K., Shin’ichiro N., Toshio O. (2005). Release behavior of diethylhexyl phthalate from the polyvinyl-chloride tubing used for intravenous administration and the plasticized PVC membrane. Int. J. Pharm. 297, 30–37. [DOI] [PubMed] [Google Scholar]
- Tay T. W., Andriana B. B., Ishii M., Choi E. K., Zhu X. B., Alam M. S., Tsunekawa N., Kanai Y., Kurohmaru M. (2007). Phagocytosis plays an important role in clearing dead cells caused by mono(2-ethylhexyl) phthalate administration. Tissue Cell 39, 241–246. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Thomas M. J. (1984). Biological effects of di-(2-ethylhexyl) phthalate and other phthalic acid esters. Crit. Rev. Toxicol. 13, 283–317. [DOI] [PubMed] [Google Scholar]
- Voss J. J. L. P., Stermer A. R., Ghaffari R., Tiwary R., Richburg J. H. (2018). MEHP-induced rat testicular inflammation does not exacerbate germ cell apoptosis. Reproduction 156, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-X., You L., Zeng Q., Sun Y., Huang Y.-H., Wang C., Wang P., Cao W.-C., Yang P., Li Y.-F., et al. (2015). Phthalate exposure and human semen quality: Results from an infertility clinic in China. Environ. Res. 142, 1–9. [DOI] [PubMed] [Google Scholar]
- Winnall W. R., Muir J. A., Hedger M. P. (2011). Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. J. Leukoc. Biol. 90, 133–143. [DOI] [PubMed] [Google Scholar]
- Yao P.-L., Lin Y.-C., Richburg J. H. (2010). Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol. Reprod. 82, 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P.-L., Lin Y.-C., Sawhney P., Richburg J. H. (2007). Transcriptional regulation of FasL expression and participation of sTNF-alpha in response to sertoli cell injury. J. Biol. Chem. 282, 5420–5431. [DOI] [PubMed] [Google Scholar]