Abstract
Objective
Clinically, electroacupuncture (EA) improves cerebral ischemic injury, but its mechanism remains unknown. The aim of this study was to confirm the protective effects of EA on focal cerebral ischemia (FCI)-induced injury and the possible mechanism.
Methods
Sprague-Dawley (SD) rats served as the FCI model and were divided into the sham, model, EA, AG490 and EA+AG490 groups. Rats in the EA and EA+AG490 groups were acupunctured at the Baihui (GV20) and Dazhui (GV14) acupoints, and those in the AG490 and EA+AG490 groups were administered an intracerebroventricular injection of AG490 (a Janus-tyrosine kinase-2 (JAK-2) phosphorylation inhibitor). Neurological deficits and morphological changes in the ischemic cortex were observed through neurological deficit scoring and HE staining, respectively, and neuronal apoptosis was examined using the TUNEL assay. Transmission electron microscopy was used to observe neuronal ultrastructure, and HIF-1α, erythropoietin (EPO), phosphorylated (p)-JAK2, p-STAT5, HSP70, Bax and Bcl-2 expression was measured by RT-PCR and immunohistochemistry.
Results
FCI model rats showed obvious neurological deficits and neuronal apoptosis compared with sham rats. EA alleviated FCI-induced neurological deficits, improved neuronal ultrastructure, reduced neuronal apoptosis, and induced HIF-1α, EPO, p-JAK2, p-STAT5, HSP70 and Bcl-2 expression in a time-dependent manner. In contrast, AG490 treatment impaired the effects of EA on neurological deficits, neuronal apoptosis and HIF-1α, EPO, p-JAK2, p-STAT5, HSP70, Bax and Bcl-2 expression.
Conclusion
EA at GV20 and GV14 could improve neurological deficits and reduce neuronal apoptosis, thereby improving FCI-induced injury, which may be related to enhancing the EPO-JAK2-STAT5 pathway.
Keywords: electroacupuncture, focal cerebral ischemia, apoptosis, EPO-JAK2-STAT5 pathway, AG490
Introduction
Ischemic stroke is a major disabling disease and the third leading cause of death in North America, Europe, and Asia.1 In patients who have an ischemic stroke, there is a substantial rate of recurrence.2 The middle cerebral artery (MCA) is the artery that is most often occluded, which leads to a sharply demarcated infarct and to scattered neuronal injury in the adjacent cortical tissue.3 Despite advances in the understanding of the pathophysiology of cerebral ischemia, therapeutic options remain limited.4 Only intravenous tissue plasminogen activator (rt-PA) and endovascular thrombectomy for large-vessel occlusion are currently used to treat ischemic stroke, but the therapeutic window is only 3 h.5,6 To address the current shortage of therapeutic approaches for treating stroke, it is critical to identify new potential therapeutic methods. Clinically, acupuncture is increasingly widely used in the treatment of ischemic stroke in Asia,7,8 but the therapeutic mechanisms are still not clearly understood.
Recently, studies showed that erythropoietin (EPO) and its receptor (EPOR) play critical roles in neuronal survival, and their expression level markedly change after ischemic injury.9 Several studies have reported that EPO promotes neuronal survival and reduces neurological dysfunction in rodent models of stroke.10,11 Under ischemic conditions, high levels of HIF-1α regulate the transcription of EPO, which induces several pathways associated with neuroprotection.9 The binding of EPO and EPOR can induce the autophosphorylation of EPOR-associated Janus-tyrosine kinase-2 (JAK-2), and JAK-2 activation leads to the phosphorylation of several downstream signaling pathways, including the transcription factor signal transducers and activators of transcription 5 (STAT5), Ras-mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3K).12 The JAK2-STAT pathway is one of the important pathways that regulates cellular development and survival.13 Han et al reported that acupuncture preconditioning enhanced the expression of EPO in neurons, glia and vascular endothelial cells in the ischemic peripheral zone, and EPO was involved in acupuncture preconditioning-induced neuroprotection following focal cerebral ischemia (FCI).14 Xu et al reported that EA stimulation at Baihui (GV20) and Zusanli (ST36) exerted neuroprotective effects possibly by regulating the EPO-mediated JAK2/STAT3 pathway and downstream apoptotic pathways in a cerebral ischemia rat model.15
In our previous study, we found that EA at GV20 and Dazhui (GV14) promoted neuronal repair in the cerebral cortex by reducing the expression of phosphorylated JAK2 and STAT3.16 However, the therapeutic mechanism of EA at GV20 and GV14 is not well understood. Are there other mechanisms associated with effect of EA at GV20 and GV14 on neuronal repair? In this study, an FCI model was established by middle cerebral artery occlusion (MCAO) via the heat-coagulation method, and EA was performed at GV20 and GV14 to reveal the other neural mechanisms associated with the therapeutic effects of EA on cerebral ischemia.
Materials and Methods
Animals and Reagents
Adult Sprague-Dawley (SD) rats (male: female=1:1, 200–250 g) were used in this study. Animals (Certificate No: SXCK: 2013–0034) were purchased from the Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine. Rats were housed under a 12/12-h light/dark cycle at a constant room temperature of 22 ± 2°C. AG490 (Sigma-Aldrich, #658401, Germany) was dissolved in saline with 3% DMSO.
Intracerebroventricular Injection of AG490
Intracerebroventricular injection of AG490 was performed as previously described.16 AG490 group rats were subjected to an intracerebroventricular infusion of AG490 20 min prior to MCAO. First, the rats were anesthetized by 2% isoflurane. Then, 25-μL osmotic minipumps were filled with 10 μL of AG490 and connected to an Alzet brain infusion stainless steel cannula via peristaltic tubing. The cannula was stereotaxically implanted into the lateral ventricle (bregma, 0.8 mm posterior, −4.8 mm dorsoventral, −1.5 mm lateral).17 Intracerebroventricular injection lasted for 10 min, and the needle was maintained for 2 min. After that, penicillin was used for disinfection following injection.
Modeling and EA Treatment
Heat coagulation-induced MCAO was used to establish the FCI model. After being anesthetized using 2% isoflurane, the rats were fixed on the surgical table, and then an incision was made at the median position between the ear and eye to isolate the temporal muscle and expose the middle cerebral artery. Finally, a heated stainless steel wire was used for occlusion. For sham group rats, the middle cerebral arteries were only exposed without occlusion. After suturing the skin, analgesics and antibiotics were injected intraperitoneally to prevent postoperative pain and infection. The neurological function score was used to evaluate the success of the model. Neurological deficits were scored according to Zea-Longa scoring standards as follows:18 0 points: no neurological deficit symptoms; 1: slight neurological deficits, unable to fully extend the contralateral front paw; 2 points: moderate neurological deficits, turning to the opposite side when walking; 3 points: severe neurological deficits, walking while dumping to the opposite side; and 4 points: cannot move spontaneously, loss of consciousness. A score of 1 to 4 indicates successful establishment of the cerebral ischemia model. Finally, 300 model rats were successfully established. Then, the model rats were randomly divided into four groups: the model group, EA group, AG490 group and EA+AG490 group. Each group was divided into five subgroups (5 time points): 2 h, 12 h, 24 h, 48 h and 72 h.
One hour post-FCI, the rats in the EA and EA+AG490 groups were treated with EA. The acupoints used were Baihui (GV20, in the median of the parietal bone) and Dazhui (GV14, in the median of the back, below the spinous process of the seventh cervical and first thoracic vertebrae). The 0.3 mm × 25 mm needles (Suzhou Medical Equipment Factory, Suzhou, China) were connected to a G-6805 electric acupuncture apparatus (Qingdao Xinsheng Medical Instrument Factory, Qingdao, China) and vertically inserted approximately 15 mm at Baihui and 7.5 mm at Dazhui. The electric acupuncture apparatus was set at 4/20 Hz and 1–2 mA for 30 min once daily at the same time.16
Neurological Deficit Scores and Sample Collection
At 12, 24, 48 and 72 h after EA treatment, the rats were subjected to neurological deficit scoring and then euthanized by overdose isoflurane anesthesia. The chest was opened, the heart was exposed for left ventricle aortic cannulation, and perfusion was performed with 200 mL of normal saline and 300 mL of 4% paraformaldehyde/phosphate-buffered saline (pH 7.4). The brain tissues were harvested for TUNEL, electron microscopy and immunohistochemical assays. The brain tissues of the the remaining nonperfused rats were collected for RT-PCR.
TUNEL Assay
A terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) kit (TBD science, #TBD2020POD, Tianjin, China) was used to evaluate neuronal apoptosis. Brain tissues were fixed with 4% paraformaldehyde for 2 h and then soaked in a 20% sucrose solution until the tissues sank. Coronal sections were made horizontally, 10 μm in diameter, using a cryostat freezing microtome (HM550, Microm, Walldorf, Germany). Images were taken with a fluorescence microscope at 400× magnification. Five fields were randomly chosen, and the percentage of apoptotic nuclei (TUNEL-positive) among the total nuclei (DAB-labeled) was calculated (TUNEL index= (TUNEL-positive cells/DAB-labeled cells) ×100).
RT-PCR
Total RNA was extracted from the FCI zone using TRIzol reagent (Invitrogen, USA). A cDNA synthesis kit (Takara, Dalian, China) was used to synthesize cDNA, and then RT-PCR was performed to measure the mRNA expression levels using SYBR Green and a LightCycler 480 detection system (Roche, USA). The primers are shown in Table 1. GAPDH mRNA levels were used for normalization. The mRNA levels were analyzed and are expressed as relative mRNA levels based on the CT value, which was then converted to fold change.
Table 1.
Primer Sequences Used for RT-PCR
| Gene Names | Primer Names | Sequences | Length |
|---|---|---|---|
| GAPDH | F | AGTGCCAGCCTCGTCTCATA | 96 |
| R | ATGAAGGGGTCGTTGATGGC | ||
| HSP70 | F | TGCCCTATCCAGATCCTGCT | 142 |
| R | CTCTACTAAGGCCGCACTGG | ||
| HIF-1α | F | GCGGCGAGAACGAGAAGAAA | 132 |
| R | GGGGAAGTGGCAACTGATGA | ||
| EPO | F | AGAAGATCTGGCCTGGCATC | 90 |
| R | GCAACAGCCATAGCTGGAAGT | ||
| JAK2 | F | GAGAAGGACCAGACTCCCCT | 70 |
| R | GCACGCACTTCGGTAAGAAC | ||
| Bax | F | AAGACAGGGGCCTTTTTGCTA | 83 |
| R | TCCAAGGTCAGCTCAGGTGT | ||
| Bcl-2 | F | ATGTGTGTGGAGAGCGTCAA | 143 |
| R | GGGCCGTACAGTTCCACAAA | ||
| STAT5 | F | CACGACGCGAGATTTCTCCA | 143 |
| R | GTGGATGCATTGACGAACCA |
Immunohistochemistry
Coronal sections were fixed in acetone for 15 min, permeabilized in 0.3% Triton X-100 for 5 min, and then blocked in 5% bovine serum albumin for 30 min. The sections were then incubated with anti-HIF-1α antibodies (1: 200, Abcam, ab216842, USA), anti-EPO antibodies (1: 200, Abcam, ab226956, USA), anti-p-JAK2 antibodies (1: 100; Abcam, ab32101, USA), anti-p-STAT5 antibodies (1: 200; Abnova, California, USA), anti-HSP70 antibodies (1: 100, Abcam, ab2787, USA), anti-Bax antibodies (1: 200, Abcam, ab32503, USA) and anti-Bcl-2 antibodies (1: 100, Abcam, ab194583, USA) at 37°C for 2 h. After being washed, the sections were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at 37°C. Nuclear staining was performed with Hoechst 33258 (1:8; BLKW, Beijing, China) for 5 min, followed by dehydration and blocking. Five fields were randomly chosen, and images were taken using an inverted microscope. Images were analyzed for mean optical density (MOD) using Image-Pro Plus 6.0 software (Media Cybernetics, USA).
Statistical Analysis
SPSS 17.0 software (IBM Corp, USA) was used for statistical analyses, and GraphPad Prism 5 (GraphPad Software, USA) was used to draw the graphs. The data are presented as the mean ± standard deviation (SD). Statistically significant differences between groups were determined by Student’s t-tests. Multiple comparisons were made among ≥3 groups using 1-way ANOVA followed by the Bonferroni post hoc test. When the data were not normally distributed, the nonparametric Dunnett’s T3 post hoc test was used. P<0.05 was considered statistically significant.
Results
EA Improves Cerebral Ischemia-Induced Neurological Deficits
At 12, 24, 48 and 72 h post-FCI, neurological deficits were scored according to Zea-Longa scoring standards, and the scores are shown in Table 2. For the sham group, the middle cerebral arteries were only exposed and were not occluded, and the rats exhibited no neurological deficits (the scores were all 0). Compared with those in the sham group, all rats in the model group showed obvious neurological deficits at 12, 24, 48 and 72 h post-FCI. After EA treatment, neurological deficits were significantly reduced, and the scores decreased significantly at 24, 48 and 72 h post-FCI (there was no obvious difference at 12 h). In contrast, AG490 treatment exacerbated neurological deficits, as indicated by significant increases in the scores. Additionally, after inhibiting the EPO-JAK2-STAT5 pathway, EA-mediated improvements in neurological deficits were significantly reduced. These results suggested that EA could improve cerebral ischemia-induced neurological deficits in a time-dependent manner.
Table 2.
Neurological Deficit Scores in Each Group (n=12)
| Groups | 12 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| Sham | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| Model | 2.05±0.34* | 2.22±0.36* | 2.48±0.37* | 2.60±0.21* |
| EA | 2.09±0.49 | 1.89±0.48 | 1.36±0.29# | 1.12±0.17# |
| AG490-NC | 2.11±0.31 | 2.27±0.32 | 2.53±0.32 | 2.70±0.25 |
| AG490 | 2.47±0.42# | 2.88±0.39# | 3.17±0.32# | 3.75±0.12# |
| EA+AG490 | 2.52±0.37& | 2.72±0.30& | 3.08±0.31& | 3.54±0.15&@ |
Notes: Compared to the sham group, *P<0.05; Compared to the model group, #P<0.05; Compared to the EA group, &P<0.05; Compared to the AG490 group, @P<0.05.
EA Reduces Neuronal Apoptosis in the Ischemic Cortex
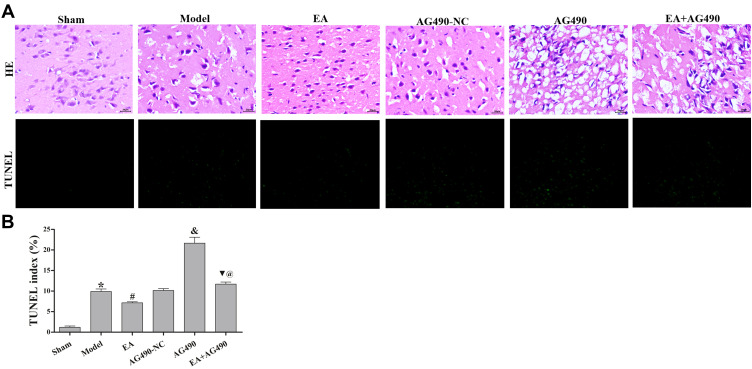
HE staining was performed to observe morphologic changes in the ischemic cortex (Figure 1A). After cerebral ischemia induction, nerve cells exhibited chromatin condensation, the number of nerve cells decreased, the cells were disordered, and the cells exhibited a small amount of mesh-like structures and cytoplasmic vacuole-like degeneration. After EA treatment, chromatin condensation was significantly improved, the number of neurons increased, and the cells were arranged in an orderly manner. However, chromatin condensation, the mesh-like structure and cytoplasmic vacuole-like degeneration were more obvious, and the number of neurons was significantly reduced after AG490 treatment. When the rats were treated with AG490, EA-mediated improvements in chromatin condensation, cytoplasmic vacuole-like degeneration and the increase in neuron counts were significantly reduced.
Figure 1.
Morphologic changes and neuronal apoptosis in the ischemic cortex. (A) Images show HE and TUNEL staining in each group (400×). (B). Statistical analysis of the TUNEL index in each group. The data are presented as the mean ± SD (n = 12). Compared to the sham group, *P<0.05. Compared to the model group, #P<0.05. Compared to the EA group, &P<0.05. Compared to the AG490 group, @P<0.05.
Furthermore, we quantified the effects of EA on neuronal apoptosis using a TUNEL assay. The TUNEL images and apoptosis rates at 72 h post-FCI are shown in Figure 1. The data showed that the TUNEL index increased from 1.20±0.42% in the sham group to 9.90 ± 0.85% in the model group (P<0.05). The TUNEL index was significantly decreased in the EA group (7.15±0.35%) and increased in the AG490 group (21.65±2.05%) compared to that in the model group. Additionally, when the rats were treated with AG490, EA did not decrease the TUNEL index (11.70±0.71%). Furthermore, EA had no significant effect on the TUNEL index at 2 h post-FCI, and EA significantly reduced the TUNEL index at 12 h post-FCI. These results indicated that EA reduced neuronal apoptosis in the ischemic cortex in a time-dependent manner.
EA Improves Neuronal Ultrastructure in the Ischemic Cortex
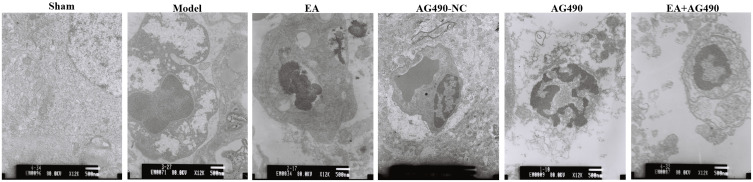
At 2 h, 12 h, 24 h, 48 h and 72 h post-FCI, transmission electron microscopy was used to observe neuronal ultrastructure. At 2 h post-FCI, there were no obvious changes in neuronal ultrastructure in any of the groups. At 12, 24, 48 and 72 h post-FCI, neuronal damage gradually increased in the model, AG490 and EA+AG490 groups, while neuronal damage was ameliorated in the EA group. At 72 h post-FCI (Figure 2), neurons were normal, the nuclear membranes were clear, nucleoli were regular, and there were many cytoplasmic organelles and intact mitochondria with cristae in the sham group. At 72 h post-FCI, the model group exhibited some neuronal chromatin condensation, cytoplasmic swelling, organelle loss, significantly distention of mitochondria with cristae loss, and widened perinuclear spaces. Compared with those of the model group, there were fewer swollen neurons, the loss of a small amount of mitochondrial cristae, and some slightly irregular nuclei in the EA group. However, there were more swollen neurons, more loss of mitochondrial cristae, and more irregular nuclei in the AG490 and EA+AG490 groups than in the other groups. The transmission electron microscopy images indicated that EA could improve neuronal ultrastructure in the ischemic cortex.
Figure 2.
Neuronal ultrastructure in the ischemic cortex at 72 h post-FCI was observed by transmission electron microscopy (uranyl acetate-lead citrate staining, 12, 000×).
EA Induces EPO-JAK2-STAT5 Pathway-Related Gene and Protein Expression in the Ischemic Cortex
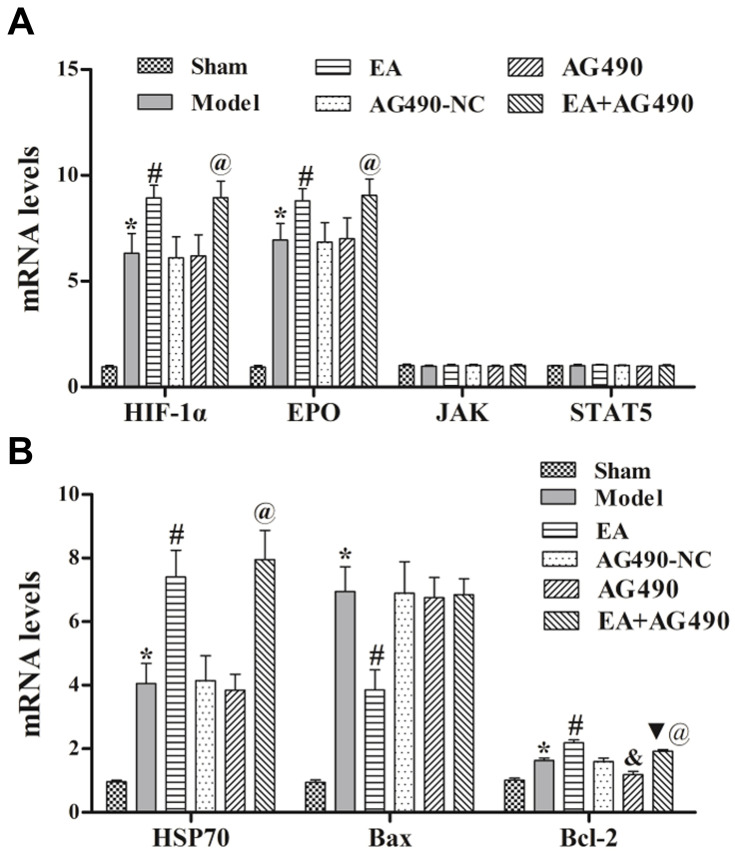
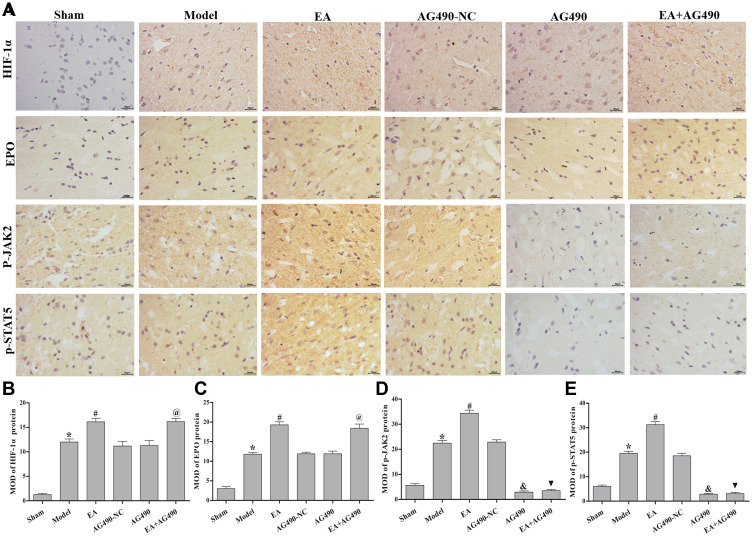
As shown in Figures 3A and 4, at 72 h post-FCI, EA treatment induced the activation of HIF-1α, EPO, p-JAK2 and p-STAT5 at the protein level compared with those in the model group. In comparison with those in the AG490-NC group, AG490 treatment decreased the protein levels of p-JAK2 and p-STAT5 but had no significant effects on HIF-1α, EPO, JAK2 or STAT5 mRNA expression. After treatment with AG490, the effects of EA on the levels of HIF-1α, EPO, p-JAK2 and p-STAT5 (EA+AG490 group) were significantly lower than those of EA alone (EA group). Additionally, at 2 and 12 h post-FCI, EA had no significant effects on HIF-1α, EPO, p-JAK2 and p-STAT5 expression. Twenty-four hours post-FCI, EA showed increasingly obvious effects on the expression of these factors. These results indicated that EA could induce EPO-JAK2-STAT5 pathway-related gene and protein expression in the ischemic cortex in a time-dependent manner, thereby activating the EPO-JAK2-STAT5 pathway.
Figure 3.
EPO-JAK2-STAT5 pathway-related (A) and apoptosis-related (B) gene expression in the ischemic cortex. qRT-PCR was performed to measure the relative mRNA levels of HIF-1α, EPO, JAK2, STAT5, HSP70, Bax and Bcl-2 at 72 h post-FCI. Compared to the sham group, *P<0.05. Compared to the model group, #P<0.05. Compared to the AG490-NC group, &P<0.05. Compared to the EA group, ▼P<0.05. Compared to the AG490 group, @P<0.05.
Figure 4.
EPO-JAK2-STAT5 pathway-related gene and protein expression in the ischemic cortex. (A) Immunohistochemistry was performed to measure the relative protein levels of HIF-1α, EPO, p-JAK2 and p-STAT5 at 72 h post-FCI (400×). (B) MOD of the HIF-1α protein level in each group. (C) MOD of the EPO protein level in each group. (D) MOD of the p-JAK2 protein level in each group. (E) MOD of the p-STAT5 protein level in each group. The data are presented as the mean ± SD (n = 12). Compared to the sham group, *P<0.05. Compared to the model group, #P<0.05. Compared to the AG490-NC group, &P<0.05. Compared to the EA group, ▼P<0.05. Compared to the AG490 group, @P<0.05.
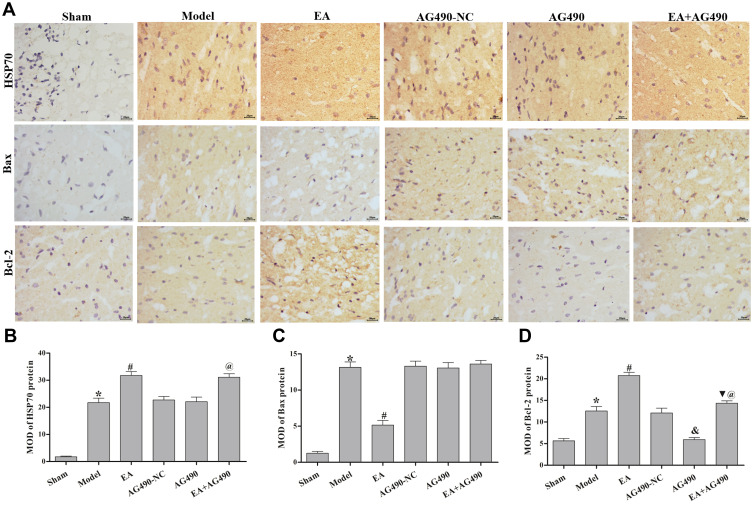
EA Inhibits Apoptosis-Related Gene and Protein Expression in the Ischemic Cortex
RT-PCR and immunohistochemistry were used to measure the mRNA and protein levels, respectively, of HSP70, Bax and Bcl-2 in the ischemic cortex. At 72 h post-FCI, compared with those in the model group, EA treatment induced mRNA and protein expression of HSP70 and Bcl-2, and inhibited the mRNA and protein expression of Bax (Figure 3B). Additionally, when the rats were treated with AG490, EA-mediated regulation of the mRNA and protein levels of HSP70, Bax and Bcl-2 was significantly reduced (Figure 5). Additionally, at 2 and 12 h post-FCI, EA had no significant effects on HSP70, Bax or Bcl-2 expression. Twenty-four hours post-FCI, EA showed increasingly obvious effects on the expression of these factors. These results indicated that EA could inhibit apoptosis-related gene and protein expression in cerebral ischemia model rats in a time-dependent manner, and this effect may be related to the EPO-JAK2-STAT5 pathway.
Figure 5.
Apoptosis-related protein expression in the ischemic cortex. (A) Immunohistochemistry was performed to measure the protein levels of HSP70, Bax and Bcl-2 at 72 h post-FCI (400×). (B) MOD of the HSP70 protein level in each group. (C) MOD of the Bax protein level in each group. (D) MOD of the Bcl-2 protein level in each group. The data are presented as the mean ± SD (n = 12). Compared to the sham group, *P<0.05. Compared to the model group, #P<0.05. Compared to the AG490-NC group, &P<0.05. Compared to the EA group, ▼P<0.05. Compared to the AG490 group, @P<0.05.
Discussion
Due to its verifiable effectiveness and lack of side effects, acupuncture is increasingly accepted as an alternative therapy worldwide. EA is widely used to treat disorders of the nervous system, such as ischemic stroke.19 The World Health Organization (WHO) recommends acupuncture as an alternative and complementary strategy for stroke treatment and improving stroke care. Clinical trials and meta-analyses have demonstrated the efficacy of acupuncture in improving neurological deficits and general well-being poststroke.19,20 The mechanisms underlying the beneficial effects of acupuncture on cerebral ischemia stroke rehabilitation remain unclear; fortunately, research on the therapeutic mechanisms of acupuncture is ongoing.21–26 Our previous study indicated that EA at GV20 and GV14 promoted neuronal repair in the cerebral cortex in FCI.16 In this study, we found that EA at GV20 and GV14 could improve neurological deficits (Table 2), reduce neuronal apoptosis (Figure 1), and improve neuronal ultrastructure (Figure 2) in a time-dependent manner, thereby ameliorating FCI-induced injury by enhancing the EPO-JAK2-STAT5 pathway. This study further examined the therapeutic mechanism of EA at GV20 and GV14 in FCI.
The JAK/STAT pathway is highly important in cell signal transduction.27 Four subtypes of JAK (JAK1, JAK2, JAK3, and Tyk2) proteins and seven subtypes of STAT (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6) proteins exist. Of these subtypes, the role of the JAK2-STAT5 pathway has attracted the attention of researchers. JAK2/STAT5/Bcl-xL signaling is essential for EPO-mediated protection against apoptosis, and activation of the JAK2/STAT5/Bcl-xL pathway can increase cell viability and reduce apoptosis in a PC12 neurological disease cell model.28 Breggia’s research indicated that the JAK2/Y343/STAT5 signaling axis is required for EPO-mediated protection against ischemic injury in renal tubular epithelial cells.29 EPO inhibits apoptosis by activating the PI3K-Akt axis or JAK2-STAT5 axis, and this antiapoptotic mechanism plays an important role in stroke, retinal diseases and myocardial infarction.30,31 Our study showed that FCI induced the expression of HIF-1α, EPO, p-JAK2 and p-STAT5, and EA further increased their expression levels. After AG490 treatment (to inhibit JAK2 phosphorylation), the EA-mediated increases in p-JAK2 and p-STAT5 were significantly reduced. Our results showed that EA could activate the EPO-JAK2-STAT5 pathway.
Poststroke, neuronal populations undergo apoptosis, and inhibiting apoptosis can reduce cell death and ischemic injury.27 Cerebral ischemia triggers a complex series of biochemical and molecular mechanisms that impair neurologic functions.32 Signaling cascades associated with apoptosis contribute to apoptosis and cell death after FCI. Studies have indicated that HSP70 directly regulates apoptosis and cell death by regulating the function of several proapoptotic proteins and indirectly by increasing the levels of the anti-death protein Bcl-2.33 In animal models of stroke and cell culture models, HSP70 overexpression has been shown to provide protection against cerebral ischemia.33,34 FCI can induce proto-oncogene expression, and the proto-oncogene Bcl-2 plays a key role in regulating apoptosis and cell death in neurons.35 After ischemia and trauma, the expression of several Bcl-2 family members (such as Bcl-2 and Bax) is altered in brain tissues, and alterations in the expression of Bcl-2 family genes (transgenic approaches, viral vectors, etc.) can modify neuronal cell death and neurological outcomes after injury.36 This study showed that EA could decrease neuronal apoptosis and Bax expression and increase HSP70/Bcl-2 expression, indicating that EA could inhibit proapoptotic proteins and promote antiapoptotic protein expression, thereby reducing neuronal apoptosis. After inhibiting JAK2 phosphorylation, the EA-mediated increase in Bcl-2 was significantly reduced, but there was no obvious inhibitory effect on HSP70 or Bax expression. These results show that EA may regulate neuronal apoptosis through multiple pathways, not just the EPO-JAK2-STAT5 pathway.
In conclusion, this study showed that EA at GV20 and GV14 could improve neurological deficits and reduce neuronal apoptosis, thereby improving FCI-induced injury, and the effect may be related to enhancing the EPO-JAK2-STAT5 pathway. This study provides two acupoints (GV20 and GV14) for the treatment of cerebral ischemic injury. Our team’s research has focused on the JAK2 protein and its related pathways, but there is a lack of research on other therapeutic mechanisms, such as the effects of EA on neuronal autophagy. Further research is needed to study other acupoints and signaling pathways to continuously improve our understanding of the mechanism by which EA can treat cerebral ischemia and provide additional therapeutic strategies for patients with cerebral ischemia.
Funding Statement
This work was supported by the Natural Science Foundation of Guangdong Province (No. 2015A030310375), and the Zhuang Lixing Guangdong Famous Traditional Chinese Medicine Inheritance Studio (2018, No. 5).
Ethical Approval
The protocol of animal care and experiments were approved by the Ethical Review Board of Guangzhou University of Chinese Medicine. The protocols of these animals were followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Disclosure
The authors declare no conflict of interest.
References
- 1.Higashida R, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109–e137. doi: 10.1161/01.STR.0000082720.85129.0A [DOI] [PubMed] [Google Scholar]
- 2.He Y, Cai Z, Zeng S, et al. Effect of fluoxetine on three-year recurrence in acute ischemic stroke: a randomized controlled clinical study. Clin Neurol Neurosurg. 2018;168:1–6. doi: 10.1016/j.clineuro.2018.02.029 [DOI] [PubMed] [Google Scholar]
- 3.Nedergaard M. Mechanisms of brain damage in focal cerebral ischemia. Acta Neurol Scand. 1988;77(2):81–101. doi: 10.1111/j.1600-0404.1988.tb05878.x [DOI] [PubMed] [Google Scholar]
- 4.Poustchi F, Amani H, Ahmadian Z, et al. Combination therapy of killing diseases by injectable hydrogels: from concept to medical applications. Adv Healthc Mater. 2020;10(3):2001571. doi: 10.1002/adhm.202001571 [DOI] [PubMed] [Google Scholar]
- 5.Yang JL, Mukda S, Chen SD. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018;16:263–275. doi: 10.1016/j.redox.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Shen J, Wang XM, et al. Scalp acupuncture for acute ischemic stroke: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:480950. doi: 10.1155/2012/480950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen PF, Kong L, Ni LW, et al. Acupuncture intervention in ischemic stroke: a randomized controlled prospective study. Am J Chin Med. 2012;40(4):685–693. doi: 10.1142/S0192415X12500516 [DOI] [PubMed] [Google Scholar]
- 9.Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev. 2009;62(1):99–108. doi: 10.1016/j.brainresrev.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 10.Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97(19):10526–10531. doi: 10.1073/pnas.97.19.10526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirén AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98(7):4044–4049. doi: 10.1073/pnas.051606598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma S, Chen J, Chen C, et al. Erythropoietin rescues memory impairment in a rat model of chronic cerebral hypoperfusion via the EPO-R/JAK2/STAT5/PI3K/Akt/GSK-3β pathway. Mol Neurobiol. 2018;55(4):3290–3299. doi: 10.1007/s12035-017-0568-5 [DOI] [PubMed] [Google Scholar]
- 13.O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109:S121–131. doi: 10.1016/S0092-8674(02)00701-8 [DOI] [PubMed] [Google Scholar]
- 14.Han XM, Wei HT, Liu SY. Involvement of erythropoietin expression in acupuncture preconditioning-induced ischemic tolerance. Adv Mater Res. 2012;554–556:1650–1655. doi: 10.4028/www.scientific.net/AMR.554-556.1650 [DOI] [Google Scholar]
- 15.Xu H, Zhang YM, Sun H, Chen SH, Si YK. Electroacupuncture at GV20 and ST36 exerts neuroprotective effects via the EPO-mediated JAK2/STAT3 pathway in cerebral ischemic rats. Evid Based Complement Alternat Med. 2017;2017:6027421. doi: 10.1155/2017/6027421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R, Xu N, Yi W, Huang K. Electroacupuncture effects on cortical neurons, as well as Janus kinase 2-signal transducer and activator of transcription 3 signal transduction pathway, in a rat model of cerebral ischemia. Neural Regen Res. 2012;7(6):457–462. doi: 10.3969/j.issn.1673-5374.2012.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paxinos G, Paxinos G. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Academic; 2007. [Google Scholar]
- 18.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.STR.20.1.84 [DOI] [PubMed] [Google Scholar]
- 19.Ouyang G, Jia SW, Wang F, Shi Y, Gao Z. [Effects of electroacupuncture of different frequencies on cerebral blood perfusion and cerebral function in the patient of stroke]. Zhongguo Zhen Jiu. 2005;25(11):776–778. [Chinese]. [PubMed] [Google Scholar]
- 20.Chavez LM, Huang SS, MacDonald I, Lin JG, Lee YC, Chen YH. Mechanisms of acupuncture therapy in ischemic stroke rehabilitation: a literature review of basic studies. Int J Mol Sci. 2017;18(11):2270. doi: 10.3390/ijms18112270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao J, Chen B, Gao Y, et al. Electroacupuncture enhances hippocampal NSCs proliferation in cerebral ischemia-reperfusion injured rats via activation of notch signaling pathway. Int J Neurosci. 2014;124(3):204–212. doi: 10.3109/00207454.2013.840781 [DOI] [PubMed] [Google Scholar]
- 22.Lan L, Tao J, Chen A, et al. Electroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathway. Int J Mol Med. 2013;31(1):75–80. doi: 10.3892/ijmm.2012.1184 [DOI] [PubMed] [Google Scholar]
- 23.Zhong S, Li Z, Huan L, Chen BY. Neurochemical mechanism of electroacupuncture: anti-injury effect on cerebral function after focal cerebral ischemia in rats. Evid Based Complement Alternat Med. 2009;6(1):51–56. doi: 10.1093/ecam/nem062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu NG, Yi W, Lai XS. [Effect of electro-acupuncture on calcium content in neurocytes of focal cerebral ischemia]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22(4):295–297. [Chinese]. [PubMed] [Google Scholar]
- 25.Wang F, Gao Z, Li X, et al. NDRG2 is involved in anti-apoptosis induced by electroacupuncture pretreatment after focal cerebral ischemia in rats. Neurol Res. 2013;35(4):406–414. doi: 10.1179/1743132813Y.0000000159 [DOI] [PubMed] [Google Scholar]
- 26.Yi W, Xu NG, Wang GB. [Experimental study on effects of electro-acupuncture in improving synaptic plasticity in focal cerebral ischemia rats]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26(8):710–714. [Chinese]. [PubMed] [Google Scholar]
- 27.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117(Pt 8):1281–1283. doi: 10.1242/jcs.00963 [DOI] [PubMed] [Google Scholar]
- 28.Ma R, Hu J, Huang C, Wang M, Xiang J, Li G. JAK2/STAT5/Bcl-xL signalling is essential for erythropoietin-mediated protection against apoptosis induced in PC12 cells by the amyloid β-peptide Aβ25-35. Br J Pharmacol. 2014;171(13):3234–3245. doi: 10.1111/bph.12672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breggia AC. The JAK2/Y343/STAT 5 signaling axis is required for erythropoietin-mediated protection against ischemic injury in renal tubular epithelial cells. The University of Maine; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karagiannis A, Tziomalos K. Neuroprotective properties of erythropoietin in cerebral ischemia. Cent Nerv Syst Agents Med Chem. 2006;6(3):153–161. doi: 10.2174/187152406778226734 [DOI] [Google Scholar]
- 31.Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009;111(6):483–495. [DOI] [PubMed] [Google Scholar]
- 32.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54(1):34–66. [DOI] [PubMed] [Google Scholar]
- 33.Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16(1):53–61. doi: 10.1097/00008506-200401000-00010 [DOI] [PubMed] [Google Scholar]
- 34.Sphn K, Uny J. Targeting expression of hsp70i to discrete neuronal populations using the Lmo-1 promoter: assessment of the neuroprotective effects of hsp70i in vivo and in vitro. Flow Metab. 2001;21:972–981. doi: 10.1097/00004647-200108000-00010 [DOI] [PubMed] [Google Scholar]
- 35.Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol. 2008;37(1):7–38. [DOI] [PubMed] [Google Scholar]
- 36.Graham SH, Chen J, Clark RS. Bcl-2 family gene products in cerebral ischemia and traumatic brain injury. J Neurotrauma. 2000;17(10):831–841. doi: 10.1089/neu.2000.17.831 [DOI] [PubMed] [Google Scholar]