Abstract
The consequences of climate change for biogeographic range dynamics depend on the spatial scales at which climate influences focal species directly and indirectly via biotic interactions. An overlooked question concerns the extent to which microclimates modify specialist biotic interactions, with emergent properties for communities and range dynamics. Here, we use an in-field experiment to assess egg-laying behaviour of a range-expanding herbivore across a range of natural microclimatic conditions. We show that variation in microclimate, resource condition and individual fecundity can generate differences in egg-laying rates of almost two orders of magnitude in an exemplar species, the brown argus butterfly (Aricia agestis). This within-site variation in fecundity dwarfs variation resulting from differences in average ambient temperatures among populations. Although higher temperatures did not reduce female selection for host plants in good condition, the thermal sensitivities of egg-laying behaviours have the potential to accelerate climate-driven range expansion by increasing egg-laying encounters with novel hosts in increasingly suitable microclimates. Understanding the sensitivity of specialist biotic interactions to microclimatic variation is, therefore, critical to predict the outcomes of climate change across species' geographical ranges, and the resilience of ecological communities.
Keywords: Aricia agestis, ectotherm, host shift, Lepidoptera, local adaptation, thermal biology
1. Background
Responses to climate change occur through a combination of geographical range shifts [1,2] and in situ plastic and genetic changes that modify the phenology, behaviour or resource use of phenotypes [1,3,4]. These changes determine the abundance, distribution and persistence of species and their biotic interactions [1,5–8]. Where biotic interactions are specialized (e.g. feeding by many phytophagous insects), they create locally suitable habitat patches with steep ‘suitability gradients’ at patch edges [9], embedded within a matrix of unsuitable habitats which limit dispersal and colonization. Specialist interspecific interactions can, therefore, constrain range expansion [10,11].
The effects of climate change on how individuals encounter, select and exploit resources, or on resource quality itself, could alter range dynamics by smoothing or steepening existing suitability gradients, for example by promoting or precluding certain biotic interactions [12–17]. Research on Lepidoptera host use suggests that range expansion itself promotes the incorporation of novel hosts in herbivore diets [18,19], while egg shortfall related to the availability of suitable (micro)habitats and climatic conditions is an important limiting factor at species' range margins [20,21]. Therefore, understanding how individuals’ behaviours are mediated by local conditions during species' interactions such as host selection represents a critical step in predicting ecological and evolutionary outcomes of climate change, but is often overlooked [22–25]. Assessments of responses to environmental change also rarely account for the sub-daily and sub-metre temporal and spatial resolutions over which interaction partners and climate vary [26,27]. Such fine-scale variation influences individual behaviour, resource acquisition and fitness, understanding of which may be critical to predict broader ecological responses to climate change [28–35].
In this paper, we consider how the steepness of habitat suitability gradients may be modified by individual responses to variation in microclimate and resource conditions. We use as a case study a specialist butterfly that has undergone a rapid range expansion associated with the evolution of its biotic interactions to exploit more widespread novel host plants [5,11]. Until the 1990s, the UK distribution of the brown argus butterfly (Aricia agestis, Lycaenidae) was largely restricted to calcareous grasslands, where it used the perennial common rockrose (Helianthemum nummularium, Cistaceae) as its main larval host [36]. Since then, populations have colonized formerly unsuitable regions by increasingly (and apparently exclusively) exploiting Geraniaceae, including the annuals Erodium cicutarium, Geranium dissectum and G. molle [5,11,14,37,38]. Studies suggest that warming has enabled increasing use of Geraniaceae and persistence of populations in areas that were previously too cool, coupled with evolutionary changes to increase the frequency of females using only Geraniaceae as hosts [5,11,14,37,39]. These analyses have focused on changes in coarse climate metrics (i.e. Central England Temperature). However, temperature variation at finer scales can dwarf that observed more broadly [26,40]. For example, ground-level temperatures of south-facing grasslands in England can be more than 15°C warmer than adjacent north-facing slopes [41]. Understanding how microclimate determines egg-laying behaviour in this species can, therefore, act as a model for the effects of warming on a biotic interaction that determines ecological and evolutionary range dynamics.
We test the extent to which within-site microclimatic temperature variation affects the egg-laying behaviour of individual butterflies on the novel host G. dissectum. We show that individual responses to variation in microclimate and the condition of host plants can generate 75-fold differences in egg-laying rates. These exogenous drivers of expressed fecundity could, therefore, have important impacts on broader-scale host use and range dynamics, by smoothing or steepening habitat suitability gradients at range margins.
2. Material and methods
(a) . Experimental approach
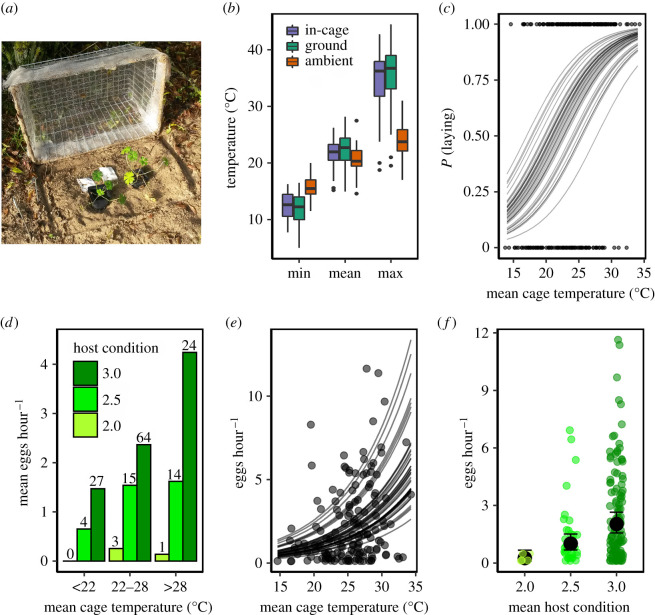
We carried out experiments on wild-caught female brown argus between 5 August and 7 September 2017 to test how natural microclimatic variation mediates in situ egg-laying behaviours on G. dissectum, a Geraniaceae species widely used as a larval host in recently established populations [14]. We established 25 experimental cages (figure 1a) in the dune system of Holkham National Nature Reserve (Norfolk, UK), in locations chosen to represent the local range of slopes and aspects (electronic supplementary material, appendix S1). At 30 min intervals, we measured in-cage ground-level temperatures (two dataloggers per cage), ambient temperatures (single datalogger with Stevenson screen 1.5 m above ground) and ground-level air temperatures (29 individual dataloggers at randomly selected locations across the site) (electronic supplementary material, appendix S1).
Figure 1.
(a) Experimental cage with two greenhouse-grown Geranium dissectum and iButton dataloggers. (b) Daily minimum, mean and maximum temperature across all cages (in-cage), compared with daily average across 29 iButtons distributed randomly at ground level around the site outside of cages (ground), and ambient temperature measured at 1.5 m above ground (ambient). (c) Probability of egg-laying increases with mean cage temperature (model LPfinal). Point clouds indicate exposures during which eggs were (1) and were not (0) laid, lines represent among-female variation. (d) Mean egg-laying rate grouped by host condition and mean cage temperature (range = 13.7–34.3°C; grouping for display only) during the relevant exposure; bar labels show sample size. (e) Marginal effects of mean cage temperature on egg-laying rate; lines show among-female variation (model LRfinal), points show raw data. (f) Egg-laying rate grouped by host condition, showing marginal effects (model LRfinal), 95% confidence intervals, and raw data (coloured points).
Cages contained greater than or equal to 95% bare ground, no natural host plants and two greenhouse-grown G. dissectum (experimental hosts) per cage (figure 1a; electronic supplementary material, appendix S1). Ground albedo and degree of thermal coupling between ground and air temperature will, therefore, have been similar between cages, and representative of microclimates in open dune areas [42], where A. agestis lay eggs on wild Geraniaceae at this site. Differences between cages in slope, aspect and topographical shading likely caused large variation in net radiation absorbed by the ground, thereby generating large variation in cage temperatures (microclimates) for a given ambient temperature. In-cage microclimates were representative of the range and averages of ambient and ground-level temperatures experienced at the site (figure 1b; electronic supplementary material, appendix S1).
All experimental hosts were watered daily and, though our experimental focus was on microclimatic temperature variation, we monitored host condition and phenophase every 2 days, to quantify temporal variation in plant traits that may influence acceptability for egg-laying. Host plant condition was visually assessed on a scale of 0–3 (poor–high quality for egg-laying, following [36]; see electronic supplementary material, appendix S1 for details and justification), and phenophase was recorded on a four-point scale describing whether the plant was in leaf, bud, flower or had set seed. Average plant condition within cages was maintained at ≥2.0 by replacing plants that deteriorated to category 2, and plants were typically replaced before flowers were visible (less than 5% of cage exposures included one flowering plant). This was achieved by growing 240 plants in four cohorts over a six-week period, so all plants used were similar in age, condition and phenophase. Adult female butterflies were captured and housed individually in mesh pots overnight prior to individual release into experimental cages (see electronic supplementary material, appendix S1 for husbandry).
Females were individually assigned to cage exposures each morning, in a pseudo-randomized manner to control for order effects (electronic supplementary material, appendix S1). A total of 109 females were exposed to host plant and thermal environments during 433 cage exposures. To avoid including data from unmated females, we use data from those 43 females which laid during at least one exposure. These females experienced 251 exposures (5.8 ± 2.8 (s.d.) exposures each) lasting on average 7 h 49 m (±47 m (s.d.)) per exposure. After each exposure, all experimental hosts were systematically searched for eggs; because there were only two plants per cage it was possible to find all eggs, which were removed to avoid double counting. Plant phenophase and the condition of the focal leaf and plant were recorded for each egg-laying location. Post-exposure, butterflies were housed overnight in mesh pots before release into a new cage on the following days. We consider data from all exposures occurring between the hours of 07:30 and 18:30 which included at least 6 h of favourable weather (electronic supplementary material, appendix S1).
(b) . Analysis
We modelled egg-laying probability per exposure using logistic regression with ‘lme4’ [43]. For exposures in which eggs were laid, hourly egg-laying rate was modelled using a gamma GLMM (log link) with ‘glmmTMB’ [44].
For both analyses, we considered female ID as a random intercept term (to account for individual variability due to factors such as age) and mean cage temperature (during the appropriate exposure for each cage) as a candidate random slope term representing among-individual variation in thermal sensitivity. As candidate fixed effects, we considered cage temperature (mean temperature of the relevant cage during the exposure) and its quadratic term, exposure number (whether it was the individual's first, second, etc., exposure), study day and cumulative eggs laid in prior exposures as scaled continuous predictors, and mean host plant condition and phenophase as ordered factors (with three- and five-factor levels respectively; electronic supplementary material, appendix S1). We also tested for a host condition–cage temperature interaction.
We constructed candidate model sets by considering all plausible parameter combinations, estimated parameters using maximum-likelihood, and used AIC-based model selection to determine model parsimony (see electronic supplementary material, appendix S1 for details and diagnostic checks). Random effects significance was tested with likelihood ratio tests (LRTs), and power to detect random slopes was tested with simulation-based power analyses (electronic supplementary material, appendix S1). We used R v. 3.5.1 [45–47].
3. Results
Egg-laying probability increased as a function of in-cage temperature (table 1 and figure 1c), such that the odds of laying increased by 27% per 1°C temperature increase. There was also a negative effect of study day (table 1 and electronic supplementary material, figure S4); candidate models showed limited support for positive effects of exposure number and host condition. There was no support for the effects of prior laying experience or host phenology in models of egg-laying probability or rate (table 1).
Table 1.
Summary of AIC analyses for GLMMs of egg-laying probability (LP) and rate (LR). Showing models with ΔAIC ≤ 6, including the best AIC model (MAIC), selected model (Mfinal) and null model (Mnull). Parameter estimates (with standard errors) are shown for the intercept (β0), study day (D), exposure number (E), mean cage temperature (T) and mean host condition (Q). Q is an ordered factor with orthogonal polynomial contrasts: estimates are presented for the linear (QL) and quadratic terms (QQ). Variance of the female ID random intercept term is denoted VRE. LL is the log-likelihood.
| model | model parameters |
LL | ΔAIC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β0 | D | E | T | QL | VRE | ||||
| LPAIC | 0.194 (0.351) | −0.877 (0.253) | 0.334 (0.212) | 1.090 (0.197) | 0.866 (0.647) | 0.035 (0.462) | 0.716 | −133.71 | 0.00 |
| LPa | 0.616 (0.215) | −0.855 (0.247) | 0.306 (0.209) | 1.023 (0.190) | — | — | 0.712 | −135.74 | 0.06 |
| LPfinal | 0.587 (0.213) | −0.648 (0.203) | — | 1.006 (0.188) | — | — | 0.736 | −136.83 | 0.25 |
| LPnull | 0.542 (0.195) | — | — | — | — | — | 0.664 | −162.12 | 46.83 |
| LRAIC | −0.231 (0.189) | −0.241 (0.118) | 0.205 (0.103) | 0.428 (0.084) | 1.593 (0.351) | −0.331 (0.243) | 0.223 | −248.84 | 0.00 |
| LRfinal | −0.207 (0.196) | — | — | 0.441 (0.085) | 1.458 (0.345) | −0.276 (0.241) | 0.279 | −251.12 | 0.57 |
| LRnull | 0.649 (0.126) | — | — | — | — | — | 0.266 | −269.38 | 31.08 |
Egg-laying rate increased as a function of in-cage temperature and host condition (figure 1d): by approximately 12% per 1°C (figure 1e), and by a factor of approximately 7.9 on good versus poor condition hosts (figure 1f). This equates to an egg-laying rate that is approximately 75 times higher on the best condition hosts in the warmest microclimate than on the poorest condition hosts in the coolest microclimate. Candidate models showed limited support for a positive effect of exposure number and a negative effect of study day (table 1).
The random effect variance (table 1) demonstrates between-individual variation in egg-laying probability (LRT = 9.016, p = 0.003) and rate (LRT = 10.903, p < 0.001). There was no support for inclusion of random slopes regarding temperature for laying probability (LRT = 0.065, p = 0.968) or rate (LRT = 0.974, p = 0.615): females differed in their fecundity overall but not in their sensitivity to temperature. Power analyses demonstrated low power to detect random slopes (5.6% in a model of laying probability; electronic supplementary material, appendix S1).
4. Discussion
We assessed egg-laying on a novel host across a temperature range that is representative of natural microclimates, but wider than the mean ambient temperature range typically experienced by the range-expanding brown argus butterfly across England [11]. Our data show that individual responses to variation in microclimates and host plant condition can combine to generate differences in egg-laying rates that are almost two orders of magnitude greater than population-average differences in egg-laying rates observed between host species [14].
Egg-laying females were remarkably sensitive to small variations in host condition, a factor we sought to minimize in our experiment. This is the first time such discrimination has been shown in the Geraniaceae hosts used in the brown argus' range expansion, and complements a previous [36] demonstration that females select lush green leaves (with thick mesophylls and high nitrogen content) when laying on the traditional perennial host, H. nummularium. Compared to H. nummularium, the condition of wild Geraniaceae hosts appears more temporally variable [48]. As annuals, Geraniaceae may be less reliable resources in terms of quality and availability at a fine spatial scale, even though they are more widely distributed at larger scales [37,49]. In this context, the bottom-up influences of plant phenotypic variation can provide a strong mechanistic basis for understanding population dynamic responses to global change in herbivores and plants [3,50,51].
A methodological concern is that cage experiments may eliminate the use of long-distance, pre-alighting cues for egg-laying site choice such as habitat structure or odour plumes of plant volatile compounds (e.g. [52]). Such cues could have altered the acceptability of the host plants with which the butterflies were confined (for example, relative to other host species). However, observations of eggs laid on natural hosts by free-flying brown argus suggest similar preferences regarding host condition [48]. These observations suggest either that the cage experiments do not introduce cue bias or that long-distance and short-distance cues are well-correlated, as observed in some other species [53]. Furthermore, G. dissectum is often preferred in direct choices between host species, though population-level host species preference varies between sites [14].
The odds and rate of egg-laying increased dramatically with microclimatic temperature (by 27% and 12% per 1°C, respectively). Warming may, therefore, increase population growth through increased fecundity, provided suitable hosts are available. At ecological margins, warming also increases the distribution and connectivity of microhabitats that are suitable for egg-laying [21]. Microclimatic variation could thereby drive range expansion at a faster rate than ambient temperatures would predict, and may account for recent range expansions by temperature-sensitive species across previously unsuitable landscapes [11,54,55]. Behavioural thermoregulation in ectotherms (e.g. basking), allows some thermal independence from the environment. However, many species (including the brown argus) are more dependent on microhabitat selection and their immediate thermal environment for thermoregulation [30]. Fine-scale temperature variation in the immediate proximity of resources may, therefore, have important effects on population responses to climate change [28–30,56,57].
In our experiment, the relationship between egg-laying rate and host condition did not vary with temperature. Though this experiment did not address inter-species host preferences, these results suggest that warming alone may not explain the concurrent host and range shifts observed in this species. Given the odds and rate of egg-laying increase dramatically with microclimatic temperature, warmer summers may increase the likelihood of females encountering and sampling alternative hosts in newly favourable microclimates, increasing the probability of host shifts during range expansion [18]. Larvae grow 10% larger and faster on Geraniaceae than on H. nummularium [5,58], provided temperatures are high and relatively stable. This may combine with increased fecundity to promote establishment and growth of populations using the novel host plants, once threshold temperatures are reached.
Beyond the potentially beneficial effects of warming on herbivore population growth, further warming may generate maladaptive behaviours. For example, if host condition is correlated with local temperature or moisture regimes then high egg-laying rates under warm, dry conditions may increase herbivore mortality through exposure to poor condition, desiccated hosts. Inflexible preferences for plants growing in drought-stressed habitats were maladaptive for Melitaea cinxia butterflies in an extremely dry year, reducing population persistence [59]. Given the capacity for behavioural responses to the environment to become maladaptive as climates change, there is a need for a better understanding of genetic variation among individuals and the potential for the evolution of novel behaviours [35]. With this in mind, although we found significant among-individual variation in fecundity (random intercepts), our experiment had insufficient power to detect significant among-individual variation in behaviour [60,61].
Here, we show how biotic interactions can be determined by individual responses to variation in microclimate and resource conditions. Spatial variation in microclimate may, therefore, be crucial in determining the steepness of habitat suitability gradients, which regulate rates of range expansion in fragmented landscapes [62]. Advances in modelling fine-scale spatial and temporal variation in microclimate can increasingly reveal when climatic conditions acting on individuals or biotic interactions regulate such range expansions [40,63,64]. Such approaches may permit a mechanistic understanding of range shifts, and higher resolution models of species distributions [48,65,66]. Incorporating robust evidence of the effects of microclimate and biotic interactions on range dynamics may thus improve understanding and prediction of ecological responses to climate change.
Acknowledgements
C. Milne, S. Speak, S. Henderson and Holkham estate staff assisted with fieldwork. Holkham Estate and Natural England kindly granted research permits. Thanks to Rosa Menéndez and Jon Bennie for helpful comments on the research, and to Susanne Foitzik, Ed Turner, and three anonymous reviewers for their contributions through the review process.
Ethics
This manuscript is based on original research on wild insects which adheres to the Royal Society's ethics standards, ASAB's Guidelines for the Use of Animals in Research, legal requirements in the United Kingdom and institutional guidelines at the University of Exeter. Permission to conduct fieldwork at Holkham NNR was granted by Holkham Estate and Natural England.
Data accessibility
The supporting data are available via the Dryad repository at https://doi.org/10.5061/dryad.3ffbg79j1 [46]. The supporting R code is available via Zenodo at https://doi.org/10.5281/zenodo.4898844 [47].
The data are provided in the electronic supplementary material [67].
Authors' contributions
J.E.S. conceived of and designed the study with input from I.M.D.M., J.B. and R.J.W. J.E.S. and A.J.E. carried out the fieldwork, and J.E.S. carried out the statistical analysis and drafted the manuscript. All authors helped to draft and/or critically revise the manuscript, gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
Research was funded by the Natural Environment Research Council (PhD studentship grant no. NE/L002434/1 to J.E.S.).
References
- 1.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637-669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 2.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024-1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 3.Visser ME, Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561-2569. ( 10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson SN, Jones TH. 2017. Global climate change and terrestrial invertebrates. Oxford, UK: John Wiley & Sons. [Google Scholar]
- 5.Thomas CD, Bodsworth EJ, Wilson RJ, Simmons AD, Davies ZG, Musche M, Conradt L. 2001. Ecological and evolutionary processes at expanding range margins. Nature 411, 577-581. ( 10.1038/35079066) [DOI] [PubMed] [Google Scholar]
- 6.Miller-Rushing AJ, Høye TT, Inouye DW, Post E. 2010. The effects of phenological mismatches on demography. Phil. Trans. R. Soc. B 365, 3177-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macgregor CJ, et al. 2019. Climate-induced phenology shifts linked to range expansions in species with multiple reproductive cycles per year. Nat. Commun. 10, 1-10. ( 10.1038/s41467-019-12479-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart JE, Illán JG, Richards SA, Gutiérrez D, Wilson RJ. 2019. Linking inter-annual variation in environment, phenology, and abundance for a montane butterfly community. Ecology 101, e02906. ( 10.1002/ecy.2906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bridle JR, Kawata M, Butlin RK. 2019. Local adaptation stops where ecological gradients steepen or are interrupted. Evol. Appl. 12, 1449-1462. ( 10.1111/eva.12789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merrill RM, Gutiérrez D, Lewis OT, Gutiérrez J, Díez SB, Wilson RJ. 2008. Combined effects of climate and biotic interactions on the elevational range of a phytophagous insect. J. Anim. Ecol. 77, 145-155. ( 10.1111/j.1365-2656.2007.01303.x) [DOI] [PubMed] [Google Scholar]
- 11.Pateman RM, Hill JK, Roy DB, Fox R, Thomas CD. 2012. Temperature-dependent alterations in host use drive rapid range expansion in a butterfly. Science 336, 1028-1030. ( 10.1126/science.1216980) [DOI] [PubMed] [Google Scholar]
- 12.Cudmore TJ, Björklund N, Carroll AL, Lindgren BS. 2010. Climate change and range expansion of an aggressive bark beetle: evidence of higher beetle reproduction in naïve host tree populations. J. Appl. Ecol. 47, 1036-1043. ( 10.1111/j.1365-2664.2010.01848.x) [DOI] [Google Scholar]
- 13.Rockwell RF, Gormezano LJ, Koons DN. 2011. Trophic matches and mismatches: can polar bears reduce the abundance of nesting snow geese in western Hudson Bay? Oikos 120, 696-709. ( 10.1111/j.1600-0706.2010.18837.x) [DOI] [Google Scholar]
- 14.Bridle JR, Buckley J, Bodsworth EJ, Thomas CD. 2014. Evolution on the move: specialization on widespread resources associated with rapid range expansion in response to climate change. Proc. R. Soc. B 281, 20131800. ( 10.1098/rspb.2013.1800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer MC, McBride CS. 2012. Geographic mosaics of species' association: a definition and an example driven by plant–insect phenological synchrony. Ecology 93, 2658-2673. ( 10.1890/11-2078.1) [DOI] [PubMed] [Google Scholar]
- 16.Moir ML, Hughes L, Vesk PA, Leng MC. 2014. Which host-dependent insects are most prone to coextinction under changed climates? Ecol. Evol. 4, 1295-1312. ( 10.1002/ece3.1021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forister ML, et al. 2020. Predicting patch occupancy reveals the complexity of host range expansion. Sci. Adv. 6, eabc6852. ( 10.1126/sciadv.abc6852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancaster LT. 2020. Host use diversification during range shifts shapes global variation in Lepidopteran dietary breadth. Nat. Ecol. Evol. 4, 963-969. ( 10.1038/s41559-020-1199-1) [DOI] [PubMed] [Google Scholar]
- 19.Singer MC, Parmesan C. 2020. Colonizations drive host shifts, diversification of preferences and expansion of herbivore diet breadth. bioRxiv, 2020.03.31.017830. ( 10.1101/2020.03.31.017830) [DOI]
- 20.Courtney SP, Duggan AE. 1983. The population biology of the orange tip butterfly Anthocharis cardamines in Britain. Ecol. Entomol. 8, 271-281. ( 10.1111/j.1365-2311.1983.tb00508.x) [DOI] [Google Scholar]
- 21.Davies ZG, Wilson RJ, Coles S, Thomas CD. 2006. Changing habitat associations of a thermally constrained species, the silver-spotted skipper butterfly, in response to climate warming. J. Anim. Ecol. 75, 247-256. ( 10.1111/j.1365-2656.2006.01044.x) [DOI] [PubMed] [Google Scholar]
- 22.Araújo MB, Rozenfeld A. 2014. The geographic scaling of biotic interactions. Ecography 37, 406-415. ( 10.1111/j.1600-0587.2013.00643.x) [DOI] [Google Scholar]
- 23.Jenkins DA, Lecomte N, Andrews G, Yannic G, Schaefer JA. 2020. Biotic interactions govern the distribution of coexisting ungulates in the Arctic Archipelago — a case for conservation planning. Glob. Ecol. Conserv. 24, e01239. ( 10.1016/j.gecco.2020.e01239) [DOI] [Google Scholar]
- 24.Staniczenko PPA, Sivasubramaniam P, Suttle KB, Pearson RG. 2017. Linking macroecology and community ecology: refining predictions of species distributions using biotic interaction networks. Ecol. Lett. 20, 693-707. ( 10.1111/ele.12770) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flores-Tolentino M, García-Valdés R, Saénz-Romero C, Ávila-Díaz I, Paz H, Lopez-Toledo L. 2020. Distribution and conservation of species is misestimated if biotic interactions are ignored: the case of the orchid Laelia speciosa. Sci. Rep. 10, 9542. ( 10.1038/s41598-020-63638-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suggitt AJ, Gillingham PK, Hill JK, Huntley B, Kunin WE, Roy DB, Thomas CD. 2011. Habitat microclimates drive fine-scale variation in extreme temperatures. Oikos 120, 1-8. ( 10.1111/j.1600-0706.2010.18270.x) [DOI] [Google Scholar]
- 27.Maclean IMD, Suggitt AJ, Wilson RJ, Duffy JP, Bennie JJ. 2017. Fine-scale climate change: modelling spatial variation in biologically meaningful rates of warming. Glob. Change Biol. 23, 256-268. ( 10.1111/gcb.13343) [DOI] [PubMed] [Google Scholar]
- 28.Suggitt AJ, et al. 2018. Extinction risk from climate change is reduced by microclimatic buffering. Nat. Clim. Change 8, 713-717. ( 10.1038/s41558-018-0231-9) [DOI] [Google Scholar]
- 29.Suggitt AJ, Wilson RJ, August TA, Fox R, Isaac NJB, Macgregor NA, Morecroft MD, Maclean IMD. 2015. Microclimate affects landscape level persistence in the British Lepidoptera. J. Insect Conserv. 19, 237-253. ( 10.1007/s10841-014-9749-y) [DOI] [Google Scholar]
- 30.Bladon AJ, et al. 2020. How butterflies keep their cool: physical and ecological traits influence thermoregulatory ability and population trends. J. Anim. Ecol. 89, 2440-2450. ( 10.1111/1365-2656.13319) [DOI] [PubMed] [Google Scholar]
- 31.Bladon AJ, Donald PF, Jones SEI, Collar NJ, Deng J, Dadacha G, Abebe YD, Green RE. 2019. Behavioural thermoregulation and climatic range restriction in the globally threatened Ethiopian bush-crow Zavattariornis stresemanni. Ibis 161, 546-558. ( 10.1111/ibi.12660) [DOI] [Google Scholar]
- 32.Weiss SB, Murphy DD, White RR. 1988. Sun, slope, and butterflies: topographic determinants of habitat quality for Euphydryas editha. Ecology 69, 1486-1496. ( 10.2307/1941646) [DOI] [Google Scholar]
- 33.Michielsen RJ, Ausems ANMA, Jakubas D, Pętlicki M, Plenzler J, Shamoun-Baranes J, Wojczulanis-Jakubas K. 2019. Nest characteristics determine nest microclimate and affect breeding output in an Antarctic seabird, the Wilson's storm-petrel. PLoS ONE 14, e0217708. ( 10.1371/journal.pone.0217708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadeau CP, Urban MC, Bridle JR. 2017. Climates past, present, and yet-to-come shape climate change vulnerabilities. Trends Ecol. Evol. 32, 786-800. ( 10.1016/j.tree.2017.07.012) [DOI] [PubMed] [Google Scholar]
- 35.Bridle J, van Rensburg A.. 2020. Discovering the limits of ecological resilience. Science 367, 626-627. ( 10.1126/science.aba6432) [DOI] [PubMed] [Google Scholar]
- 36.Bourn NAD, Thomas JA. 1993. The ecology and conservation of the brown argus butterfly Aricia agestis in Britain. Biol. Conserv. 63, 67-74. ( 10.1016/0006-3207(93)90075-C) [DOI] [Google Scholar]
- 37.Buckley J, Bridle JR. 2014. Loss of adaptive variation during evolutionary responses to climate change. Ecol. Lett. 17, 1316-1325. ( 10.1111/ele.12340) [DOI] [PubMed] [Google Scholar]
- 38.Buckley J, Butlin RK, Bridle JR. 2012. Evidence for evolutionary change associated with the recent range expansion of the British butterfly, Aricia agestis, in response to climate change. Mol. Ecol. 21, 267-280. ( 10.1111/j.1365-294X.2011.05388.x) [DOI] [PubMed] [Google Scholar]
- 39.Bodsworth EJ. 2002. Dispersal and behaviour of butterflies in response to their habitat. PhD thesis, University of Leeds, Leeds, UK. [Google Scholar]
- 40.Bramer I, et al. 2018. Advances in monitoring and modelling climate at ecologically relevant scales. Next Generation Biomonitoring, part 1 58, 101-161. ( 10.1016/bs.aecr.2017.12.005) [DOI] [Google Scholar]
- 41.Maclean IMD, Mosedale JR, Bennie JJ. 2019. Microclima: an R package for modelling meso- and microclimate. Methods Ecol. Evol. 10, 280-290. ( 10.1111/2041-210X.13093) [DOI] [Google Scholar]
- 42.Liddle MJ, Moore KG. 1974. The microclimate of sand dune tracks: the relative contribution of vegetation removal and soil compression. J. Appl. Ecol. 11, 1057-1068. ( 10.2307/2401765) [DOI] [Google Scholar]
- 43.Bates D, Maechler M, Bolker B, Walker S. 2018. lme4: Linear mixed-effects models using ‘Eigen’ and S4. R package version 1.1-19. See https://cran.r-project.org/web/packages/lme4/index.html.
- 44.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9, 378-400. ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 45.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. (https://www.R-project.org/). [Google Scholar]
- 46.Stewart JE, Maclean IMD, Edney AJ, Bridle J, Wilson RJ.. 2021. Data from: Microclimate and resource quality determine resource use in a range-expanding herbivore. Dryad Digital Repository. ( 10.5061/dryad.3ffbg79j1) [DOI] [PMC free article] [PubMed]
- 47.Stewart JE, Maclean IMD, Edney AJ, Bridle J, Wilson RJ.. 2021. R Code from: Microclimate and resource quality determine resource use in a range-expanding herbivore. Zenodo. ( 10.5281/zenodo.4898844) [DOI] [PMC free article] [PubMed]
- 48.Stewart JE. 2020. Linking phenology to population dynamics and distribution change in a changing climate. PhD Thesis, University of Exeter, Exeter, UK. [Google Scholar]
- 49.Kemp RJ, Hardy PB, Roy DB, Dennis RLH. 2008. The relative exploitation of annuals as larval host plants by European butterflies. J. Natural Hist. 42, 1079-1093. ( 10.1080/00222930801937608) [DOI] [Google Scholar]
- 50.Hunter MD, Varley GC, Gradwell GR. 1997. Estimating the relative roles of top-down and bottom-up forces on insect herbivore populations: a classic study revisited. Proc. Natl Acad. Sci. USA 94, 9176-9181. ( 10.1073/pnas.94.17.9176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price PW, Hunter MD. 2015. Population dynamics of an insect herbivore over 32 years are driven by precipitation and host-plant effects: testing model predictions. Environ. Entomol. 44, 463-473. ( 10.1093/ee/nvv039) [DOI] [PubMed] [Google Scholar]
- 52.Conchou L, Lucas P, Meslin C, Proffit M, Staudt M, Renou M. 2019. Insect odorscapes: from plant volatiles to natural olfactory scenes. Front. Physiol. 10, 972. ( 10.3389/fphys.2019.00972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schöps K, Hanski I. 2001. Population level correlation between pre-alighting and post-alighting host plant preference in the Glanville fritillary butterfly. Ecol. Entomol. 26, 517-524. ( 10.1046/j.1365-2311.2001.00357.x) [DOI] [Google Scholar]
- 54.Cannizzo ZJ, Griffen BD. 2019. An artificial habitat facilitates a climate-mediated range expansion into a suboptimal novel ecosystem. PLoS ONE 14, e0211638. ( 10.1371/journal.pone.0211638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bennie J, Hodgson JA, Lawson CR, Holloway CTR, Roy DB, Brereton T, Thomas CD, Wilson RJ. 2013. Range expansion through fragmented landscapes under a variable climate. Ecol. Lett. 16, 921-929. ( 10.1111/ele.12129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curtis RJ, Isaac NJB. 2015. The effect of temperature and habitat quality on abundance of the Glanville fritillary on the Isle of Wight: implications for conservation management in a warming climate. J. Insect Conserv. 19, 217-225. ( 10.1007/s10841-014-9738-1) [DOI] [Google Scholar]
- 57.Greenwood O, Mossman HL, Suggitt AJ, Curtis RJ, Maclean IMD. 2016. Using in situ management to conserve biodiversity under climate change. J. Appl. Ecol. 53, 885-894. ( 10.1111/1365-2664.12602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pateman RM. 2012. Climate change and habitat associations at species' range boundaries. PhD thesis, University of York, York, UK. http://etheses.whiterose.ac.uk/2910/1/Thesis_RM_Pateman_2012.pdf. [Google Scholar]
- 59.Salgado AL, DiLeo MF, Saastamoinen M. 2020. Narrow oviposition preference of an insect herbivore risks survival under conditions of severe drought. Funct. Ecol. 34, 1358-1369. ( 10.1111/1365-2435.13587) [DOI] [Google Scholar]
- 60.Schäpers A, Petrén H, Wheat CW, Wiklund C, Friberg M. 2017. Female fecundity variation affects reproducibility of experiments on host plant preference and acceptance in a phytophagous insect. Proc. R. Soc. B 284, 20162643. ( 10.1098/rspb.2016.2643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Villemereuil P, Gaggiotti OE, Mouterde M, Till-Bottraud I.. 2016. Common garden experiments in the genomic era: new perspectives and opportunities. Heredity 116, 249-254. ( 10.1038/hdy.2015.93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Platts PJ, Mason SC, Palmer G, Hill JK, Oliver TH, Powney GD, Fox R, Thomas C. 2019. Habitat availability explains variation in climate-driven range shifts across multiple taxonomic groups. Sci. Rep. 9, 15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bennie J, Wilson RJ, Maclean IMD, Suggitt AJ. 2014. Seeing the woods for the trees — when is microclimate important in species distribution models? Glob. Change Biol. 20, 2699-2700. ( 10.1111/gcb.12525) [DOI] [PubMed] [Google Scholar]
- 64.Nadeau CP, Urban MC, Bridle JR. 2017. Coarse climate change projections for species living in a fine-scaled world. Glob. Change Biol. 23, 12-24. ( 10.1111/gcb.13475) [DOI] [PubMed] [Google Scholar]
- 65.Rebaudo F, Faye E, Dangles O. 2016. Microclimate data improve predictions of insect abundance models based on calibrated spatiotemporal temperatures. Front. Physiol. 7, 139. ( 10.3389/fphys.2016.00139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schouten R, Vesk PA, Kearney MR. 2020. Integrating dynamic plant growth models and microclimates for species distribution modelling. Ecol. Modell 435, 109262. ( 10.1016/j.ecolmodel.2020.109262) [DOI] [Google Scholar]
- 67.Stewart JE, Maclean IMD, Edney AJ, Bridle J, Wilson RJ. 2021. Microclimate and resource quality determine resource use in a range-expanding herbivore. FigShare. 10.6084/m9.figshare.c.5527011. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Stewart JE, Maclean IMD, Edney AJ, Bridle J, Wilson RJ.. 2021. Data from: Microclimate and resource quality determine resource use in a range-expanding herbivore. Dryad Digital Repository. ( 10.5061/dryad.3ffbg79j1) [DOI] [PMC free article] [PubMed]
- Stewart JE, Maclean IMD, Edney AJ, Bridle J, Wilson RJ.. 2021. R Code from: Microclimate and resource quality determine resource use in a range-expanding herbivore. Zenodo. ( 10.5281/zenodo.4898844) [DOI] [PMC free article] [PubMed]
- Stewart JE, Maclean IMD, Edney AJ, Bridle J, Wilson RJ. 2021. Microclimate and resource quality determine resource use in a range-expanding herbivore. FigShare. 10.6084/m9.figshare.c.5527011. [DOI] [PMC free article] [PubMed]
Data Availability Statement
The supporting data are available via the Dryad repository at https://doi.org/10.5061/dryad.3ffbg79j1 [46]. The supporting R code is available via Zenodo at https://doi.org/10.5281/zenodo.4898844 [47].
The data are provided in the electronic supplementary material [67].