Abstract
Eosinophils rapidly release extracellular filamentous chromatin fibers (extracellular traps, ETs) when they are stimulated. Reticulated ETs have been recently shown to affect secretion viscosity in eosinophilic inflammatory diseases. Here we report a 43-year-old woman with infiltrative shadows in both upper lungs that did not respond well to antibiotics. She admitted to occasional coughing and sputum, but had poor viscous regulation. Bronchoalveolar lavage fluid (BALF) collected from the upper left lobe showed many eosinophils (65%). She was diagnosed with chronic eosinophilic pneumonia, per previously reported criteria, and began treatment with prednisolone. The infiltration shadow gradually improved, and she was discharged 28 days after admission. Later, we immune-stained her BALF cell components with antibodies against major basic protein, an eosinophil granule protein, which showed a large number of agglomerating eosinophils; and antibodies against citrullinated histone H3 (CitH3-a marker for ETs), which showed CitH3-positive ETs, spread in a network. These findings confirmed that some BALF eosinophils released eosinophil ETs. This case shows the existence of ETs from BALF in patients with chronic eosinophilic pneumonia. Concentration of eosinophil ETs in eosinophilic inflammatory diseases may affect secretion viscosity in sputum, and so on.
Keywords: ETosis, Extracellular traps, Eosinophils, Pulmonary eosinophilia, Bronchoalveolar lavage fluid
INTRODUCTION
Chronic eosinophilic pneumonia (CEP) is characterized by accumulation of eosinophils and macrophages in the interstitium and alveoli. Although the role of eosinophils in CEP is unclear, production of inflammatory cytokines such as interleukin (IL)-5 and IL-25 activates eosinophils. Eosinophil infiltration and granule protein release in tissues are therefore thought to affect CEP pathology [1,2,3].
Extracellular-trap cell death (ETosis) is an apoptotic process originally found in neutrophils. Upon activation by various stimuli including cytokines and pathogens, cells release net-like filamentous chromatin fibers (extracellular traps, ETs) [4]. Reticulated ETs retain cytotoxic histones and granule proteins that enforce innate immunity by capturing and killing microorganisms [5]. Excessive ETs caused by neutrophilic inflammatory reactions can increase viscosity of secretions such as sputum and pus [6]. We previously reported that stimulated eosinophils may lead to ETosis (EETosis), causing eosinophils to release ETs [7]. As aggregated eosinophil ETs (EETs) are more stable than neutrophils, they may form highly viscous eosinophilic secretions, as found in allergic bronchopulmonary aspergillosis (ABPA), eosinophilic chronic rhinosinusitis (ECRS), and eosinophilic otitis media [8,9,10]. However, the presence of EETs in bronchoalveolar lavage fluids (BALFs) from CEP has not been previously recognized. Here, we assessed EETs in BALF from a patient with CEP.
CASE REPORT
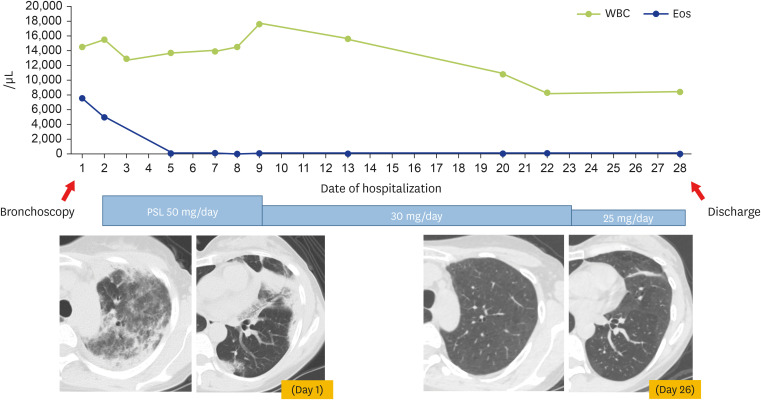
A 43-year-old woman presented to her primary-care physician with cough and sputum of 2-week duration, and a fever of 1-day duration. Chest x-ray showed an infiltration shadow in her left upper lung. She was diagnosed with pneumonia and treated with oral garenoxacin. As her symptoms and infiltration shadow did not improve, she consulted a nearby hospital. Blood tests showed elevated white blood cells with marked eosinophilia (white blood cell, 13,800/μL; eosinophils, 50.3%), and high levels of C-reactive protein (2.84 mg/dL) and immunoglobulin E (72 IU/mL). Test results for myeloperoxidase-specific antineutrophil cytoplasmic antibody (ANCA) and proteinase 3-ANCA were negative. Chest computed tomography showed infiltration shadows in both peripheral upper lungs. She was transferred to our department that day, for suspicion of eosinophilic pneumonia. She had no remarkable medical history, except for sinusitis 5 years previously. She also had no symptoms of asthma attacks or history of asthma or smoking. Her oxygen saturation was 96% in room air. Lung and bronchial sounds were normal. Examination of her heart, abdomen, and extremities showed no abnormalities. She did not have viscous sputum, as shown by few mucus plug formation. On her first day after admission (day 1), she received a bronchoscopy, during which we collected BALF from the upper left lobe. The BALF showed marked eosinophil elevation (total cell count, 1.79 × 106; neutrophils, 1%; eosinophils, 65%; lymphocytes, 15%; macrophages, 19%). No pathogenic or acid-fast bacteria were detected. She was diagnosed with CEP per previously reported criteria, and treated with prednisolone (PSL) (50 mg/day initially; reduced over time) [11]. Five days after starting PSL, the infiltration shadow started to improve. She was discharged on day 28 (Fig. 1).
Fig. 1. Post-hospital course in this case.
On first day after admission (day 1), this 43-year-old woman patient received a bronchoscopy. Bronchoalveolar lavage fluid (BALF) was collected from the upper left lobe. She was diagnosis with chronic eosinophilic pneumonia, and treated with prednisolone—initially at 50 mg/day, but reduced over time. On day 26, the shadow on her upper left lung had improved (per chest computed tomography [CT]), and she was discharged on day 28. Vertical axis: numbers of white blood cells and eosinophils; horizontal axis: days of hospitalization. Chest CT shows findings on her day of admission and 26 days after admission. PSL, prednisolone.
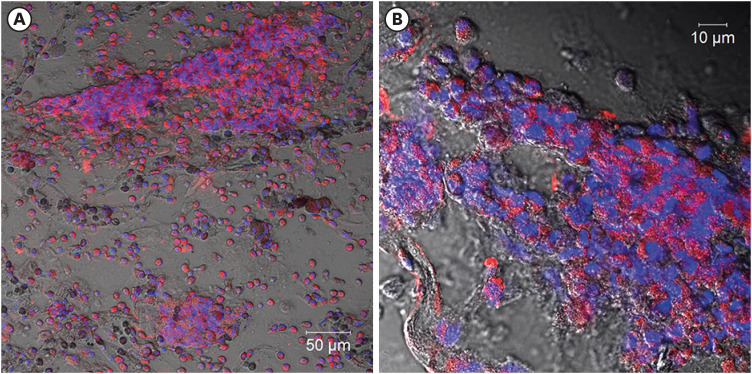
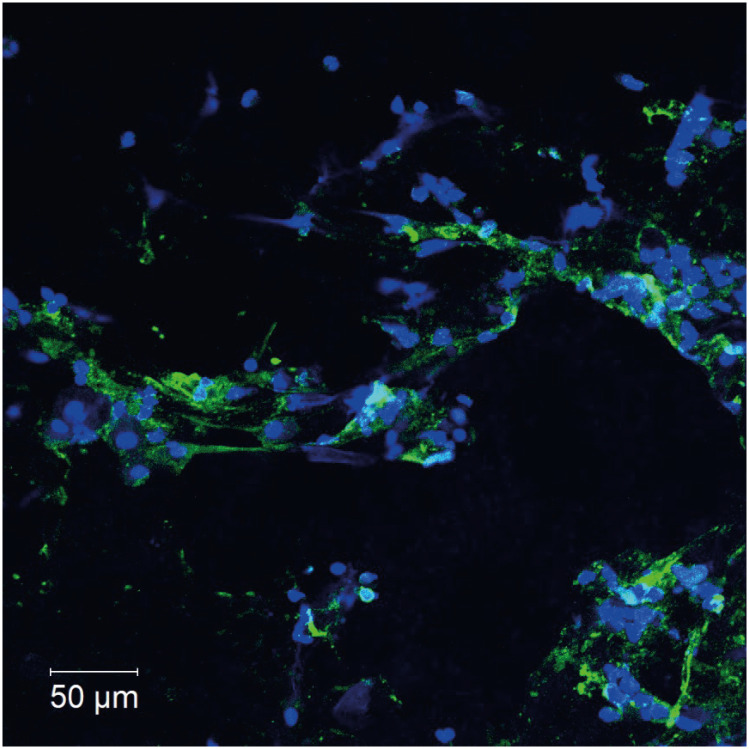
After her discharge, we smeared the BALF cells on slides, fixed them with paraformaldehyde, and immune-stained them, with antibodies against major basic protein, an eosinophil granule protein, which showed a large number of agglutinated eosinophils (Fig. 2A, B); and antibodies against citrullinated histone H3 (CitH3), a marker for ETs [10], which showed CitH3-positive ETs spread in a network. These finding confirmed that some eosinophils detected in BALF cause ETosis and released EETs (Fig. 3). The BALF specimen did not show Charcot-Leyden crystals.
Fig. 2. Major basic protein (MBP) immunostaining for localization of eosinophils in bronchoalveolar lavage fluid (BALF).
BALF taken from this patient before she received steroid treatment was applied to slides, which were fixed with paraformaldehyde and immunostained with MBP, an eosinophil granule protein. Red: MBP; blue: DNA. Images were obtained with a LSM780 confocal microscope (Carl Zeiss, Oberkochen, Germany). Scale bars are 50 µm (A) and 10 µm (B).
Fig. 3. Citrullinated histone H3 (CitH3) immunostaining to detect eosinophil extracellular traps in bronchoalveolar lavage fluid (BALF).
Immunofluorescent staining of citrullinated histone H3 (green) and DNA (blue) in BALF from this patient, which was collected before this patient received steroid treatment. The BALF was applied to slides, fixed with paraformaldehyde, incubated for 15 minutes in Tris-EDTA buffer in a microwave oven, blocked with 10% bovine serum albumin and 1% saponin coating phosphate-buffered saline for 30 minutes, and finally incubated with primary rabbit anti-CitH3 mAb (10 μg/mL, Abcam, Cambridge, UK), 90 minutes at room temperature [RT]) and with Alexa-488-conjugated antibodies (Life Technologies, Waltham, MA, USA; 30 minutes at RT) for the secondary incubation. Isotype-matched control antibodies and Hoechst 33342 (Life Technologies) were used for each experiment. Images were obtained using a LSM780 confocal microscope (Carl Zeiss, Oberkochen, Germany). CitH3-positive extracellular traps spread in a network, confirming that some cells have ETosis. Scale bars: 50 µm.
DISCUSSION
Patients with ABPA and ECRS often have viscous eosinophilic mucin [12,13,14]. We recently reported eosinophil ETs’ possible role in a mechanism to produce highly viscous eosinophilic mucin in ECRS [8]. EETs have been found to have thicker and stronger chromatin fibers than ETs from neutrophils [15]. EETs have been confirmed in secretion and tissue specimens from various eosinophilic diseases [8,9,10,16].
This case is the first to demonstrate the existence of EETs from BALF in patients with CEP. We tested the BALF using immunofluorescent staining with CitH3 (a marker for ETs [10]), and found CitH3-positive EETs spread in a network, confirming that some cells have EETosis. Generally, secretions from patients with eosinophilic pneumonia are less viscous than those of ABPA patients. Actually, our findings indicate that patients with eosinophilic pneumonia had fewer EETs than did previously reported cases of ABPA and ECRS [8,10]. This is consistent with this patient’s low mucus plug formation. In fact, she had mild sputum excretion, but it was not highly viscous. Differences in ET concentration in eosinophilic inflammation (EI) may affect secretion viscosity. Although EI in the lung interstitium is the main pathological condition in CEP, its pathophysiology may differ from that of diseases such as ABPA in which EI occurs in the lumen and forms mucus plugs [17]. The roles of EETs warrant further study, which may lead to novel therapies for severe EI.
ACKNOWLEDGEMENTS
The authors are grateful to Noriko Tan for her outstanding technical assistance. This study was funded in part by a Research Grant on Allergic Disease and Immunology from the Japan Agency for Medical Research and Development (JP18ek0410026 (SU)), Charitable Trust Laboratory Medicine Research Foundation of Japan (SU), and JSPS KAKENHI 15KK0329 (SU), 16K08926 (SU), and 17K09993(MT). We also thank Marla Brunker, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Footnotes
Conflict of Interest: SU received honoraria for lectures from AstraZeneca; and has received grant support from AstraZeneca and Maruho Co. Ltd. The other authors declare no competing financial interests.
- Conceptualization: Masahide Takeda, Shigeharu Ueki.
- Formal analysis: Masahide Takeda, Sho Sakamoto, Shigeharu Ueki, Miho Nara, Mineyo Fukuchi.
- Investigation: Masahide Takeda, Sho Sakamoto, Shigeharu Ueki, Yuji Okuda, Mariko Asano, Miho Nara, Mineyo Fukuchi.
- Methodology: Shigeharu Ueki, Yui Miyabe, Mineyo Fukuchi.
- Project administration: Masahide Takeda, Shigeharu Ueki.
- Writing - original draft: Masahide Takeda.
- Writing - review & editing: Shigeharu Ueki, Kazuhiro Sato, Katsutoshi Nakayama, Naoto Takahashi, Makoto Hirokawa.
References
- 1.Marchand E, Reynaud-Gaubert M, Lauque D, Durieu J, Tonnel AB, Cordier JF. Idiopathic chronic eosinophilic pneumonia. A clinical and follow-up study of 62 cases. The Groupe d'Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P) Medicine (Baltimore) 1998;77:299–312. doi: 10.1097/00005792-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki Y, Suda T. Eosinophilic pneumonia: a review of the previous literature, causes, diagnosis, and management. Allergol Int. 2019;68:413–419. doi: 10.1016/j.alit.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Crowe M, Robinson D, Sagar M, Chen L, Ghamande S. Chronic eosinophilic pneumonia: clinical perspectives. Ther Clin Risk Manag. 2019;15:397–403. doi: 10.2147/TCRM.S157882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brickmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V. Neutrophil extracellular traps in the second decade. J Innate Immun. 2018;10:414–421. doi: 10.1159/000489829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary disease: too much of a good things? Front Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121:2074–2083. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, Honda K, Ohta N, Yamamoto S, Takagi Y, Wada A, Weller PF. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016;137:258–267. doi: 10.1016/j.jaci.2015.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamura Y, Ikeda R, Hori T, Sasaki T, Miyabe Y, Fukuchi M, Sakamoto K, Ohta N, Kawase T, Katori Y, Ueki S. Sialodochitis fibrinosa: salivary duct obstruction by eosinophil extracellular traps? Oral Dis. 2020;26:1459–1463. doi: 10.1111/odi.13434. [DOI] [PubMed] [Google Scholar]
- 10.Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, Hebisawa A, Asano K, Figueiredo RT, Neves JS. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol. 2018;141:571–585. doi: 10.1016/j.jaci.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Matsuse H, Shimoda T, Fukushima C, Matsuo N, Sakai H, Takao A, Asai S, Kohno S. Diagnostic problems in chronic eosinophilic pneumonia. J Int Med Res. 1997;25:196–201. doi: 10.1177/030006059702500404. [DOI] [PubMed] [Google Scholar]
- 12.Asano K, Kamei K, Hebisawa A. Allergic bronchopulmonary mycosis - pathophysiology, histology, diagnosis, and treatment. Asia Pac Allergy. 2018;8:e24. doi: 10.5415/apallergy.2018.8.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson BJ. Eosinophilic mucin rhinosinusitis: a distinct clinico-pathological entity. Laryngoscope. 2000;110:799–813. doi: 10.1097/00005537-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Iino Y, Tomioka-Matsutani S, Matsubara A, Nakagawa T, Nonaka M. Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx. 2011;38:456–461. doi: 10.1016/j.anl.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Ueki S, Tokunaga T, Fujieda S, Honda K, Hirokawa M, Spencer LA, Weller PF. Eosinophil ETosis and DNA Traps: a New Look at Eosinophilic Inflammation. Curr Allergy Asthma Rep. 2016;16:54. doi: 10.1007/s11882-016-0634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda M, Ueki S, Yamamoto Y, Nara M, Fukuchi M, Nakayama K, Omori Y, Takahashi N, Hirokawa M. Hypereosinophilic syndrome with abundant Charcot-Leyden crystals in spleen and lymph nodes. Asia Pac Allergy. 2020;10:e24. doi: 10.5415/apallergy.2020.10.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueki S, Hebisawa A, Kitani M, Asano K, Neves JS. Allergic bronchopulmonary aspergillosis–a luminal hypereosinophilic disease with extracellular trap cell death. Front Immunol. 2018;9:2346. doi: 10.3389/fimmu.2018.02346. [DOI] [PMC free article] [PubMed] [Google Scholar]