Abstract
Background
Previous studies have reached mixed conclusions regarding the association between metabolic syndrome (MS) and osteoporosis. We aimed to perform a meta-analysis based on published studies that explored the association between osteoporosis and MS.
Methods
To identify related literature, a systematic search of PubMed, Cochrane Library, and EMBASE databases from inception to June 2020 was performed. Original studies that reported the risk estimates of osteoporosis morbidity for two or three categories of bone mineral density (BMD) in patients with MS were selected. Two independent investigators screened and selected the articles. Summary odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using random-effects models.
Results
Of 2632 identified studies, nine cross-sectional studies with 14 datasets were eligible for our meta-analysis. In seven studies (10 datasets), the summarized ORs of osteoporosis for MS were 0.72 (95% CI: 0.52–0.99). Subgroup analyses by gender showed that significant inverse associations were observed only in men (OR = 0.72, 95% CI: 0.55–0.96) but not in women (OR = 0.70, 95% CI: 0.41–1.22). The definition of MS, the source of the study population, and the adjustment of covariates affected the estimates. In two studies (4 datasets), there was no evidence for an association between MS and decreased BMD.
Conclusions
Our findings demonstrated that MS was significantly associated with a lower osteoporosis risk. There might be gender differences in the association between MS and osteoporosis. In addition, the association was likely to relate to the definition of MS, the source of the study population, and the adjustment of covariates.
1. Background
Metabolic syndrome (MS) is a cluster of conditions characterized by abdominal obesity, hypertension, insulin resistance, and dyslipidemia (elevated triglyceride (TG) and low high-density lipoprotein cholesterol (HDL-C) levels) [1]. From the perspectives of both individual clinicians and the health of the general public, MS is an attributable risk to the epidemic of diabetes, stroke, and coronary heart disease [2–4]. With increasing longevity, the number of people who are at risk for MS is progressively increasing in most countries. In the US National Health and Nutrition Examination Survey, more than one in three adults have MS [5].
Osteoporosis, regarded as reduced bone mineral density (BMD) and quality and an increased risk of fractures, is one of the most common chronic diseases, affecting nearly 200 million people worldwide [6]. In order to define osteoporosis, the World Health Organization (WHO) has categorized potential patients into 3 classifications according to changes in BMD obtained by dual-energy X-ray absorptiometry (DEXA) T score: normal BMD (+1 to −1), osteopenia or low bone mass (−1 to −2.5), and osteoporosis (−2.5 or below) [7].
As the world population is progressively aging, MS and osteoporosis are more likely to coexist in the same patient [8], which may have an impact both on the quality of life and on healthcare resources. Studies have shown that insulin resistance and bone metabolism are linked. Insulin signaling regulates osteoclast differentiation and osteoblast activity [9, 10], which may damage the bone quality and cause the development of osteoporosis. Two relevant meta-analyses on the association between MS and BMD were published previously [11, 12]. However, according to the WHO diagnostic criteria, we consider it is more clinically significant that BMD (continuity variable) is divided into osteoporosis and osteopenia (categorical variable). In addition, three studies on the association of MS and osteoporosis were published recently [13–15].
Therefore, we performed a systematic review and meta-analysis of available studies that assess the association between MS and osteoporosis in the general population. Our objective was to explore whether there is an association between MS and osteoporosis and to find out whether there are any factors that may affect these associations.
2. Methods
2.1. Search Strategy
PubMed, Cochrane Library, and EMBASE databases were searched from inception to June 2020 using the following terms: “metabolic syndrome” or “insulin resistance syndrome” or “plurimetabolic syndrome” or “syndrome X” or “MS” and “osteoporosis” or “osteopenia” or “bone mineral density” or “BMD” or “bone density” or “bone mineral contents” or “metabolic bone diseases.” Reference lists of retrieved articles and relevant reviews were manually searched. In September 2020, the databases were searched again using the same search criteria for additional studies. No language restrictions and study design restrictions were applied.
2.2. Eligibility and Study Selection
Inclusion criteria for our study were as follows: studies that were published as original reports, studies that reported the diagnostic criteria of MS, studies that reported the diagnostic criteria of osteoporosis, studies that reported the outcome of interest as osteoporosis or osteopenia (excluded studies that reported only BMD), and studies that reported risk estimates of osteoporosis or osteopenia and their corresponding 95% confidence interval (CI) for MS (or data to calculate them).
Title and abstract screening were performed for each article to remove obviously irrelevant and duplicated reports. Articles deemed potentially eligible by title and abstract screening were reexamined by full-text review according to the above inclusion criteria. The eligibility of articles was finally determined by 2 independent authors. Any discrepancies were resolved through discussion. For studies appearing in more than one publication, the most recent publication was included to avoid duplicate observation, unless more inclusive or detailed data were found in other publications.
2.3. Data Extraction
Data were extracted using a standardized data collection form. The following items were extracted from each study: first author's surname, publication year, country or region of the study origin, study design, number of participants, gender, mean age, the detection method of BMD, site of BMD, diagnostic criteria of MS, diagnostic criteria of osteoporosis or osteopenia, risk estimates and their corresponding 95% CI, and adjustment for potentially confounding factors. The models with the most covariate adjustment from each study were selected and used for the meta-analysis. If a study did not clearly mention any above key points, we contacted the authors of the primary reports to request any unpublished data (we contacted six authors for clarification and to obtain further data and one replied). Discrepancies were resolved by discussion.
2.4. Assessment of Bias Risk
A subjective assessment of methodological quality for observational studies was evaluated by two authors using the Agency for Health Care Research and Quality (AHRQ) method list, which is a quality assessment tool for the cross-sectional study [16]. Eleven questions were answered. If the answer is “No” or “Unclear,” the score of the item is “0;” if the answer is “Yes,” the score of the item is “1.” The quality of the study is evaluated as follows: low quality = 0 − 3; moderate quality = 4 − 7; high quality = 8 − 11.
2.5. Statistical Analysis
Study-specific findings were combined using the random-effects model by DerSimonian and Laird. We estimated unadjusted risk estimates using the reported numbers of participants for the studies in which only this information and no model results were published. Heterogeneity across studies was examined by using the Q and I2 statistic (significance level at p < 0.10) [17]. Because clinical characteristics were not consistent between men and women, all our analyses are compared by gender. A sensitivity analysis was performed to assess the influence of the individual studies on the overall results by omitting one study at a time. Subgroup analysis was performed to find factors that may explain heterogeneity or difference in outcome between each study. Potential publication bias was assessed by Egger's test and Begg's test. All analyses were performed using STATA version 12.0.
3. Results
3.1. Literature Search and Study Characteristics
A total of 2,632 references were identified following an electronic search and seven were identified by manual searching, of which 437 were duplicates, 2,042 were not relevant, and 61 were conference abstracts and so were excluded at the initial screening of the title and abstract. By full-text review, 90 more studies were excluded: 44 studies only reported the component of MS, 27 studies did not report the outcome of interest, 18 studies were reviews, and one study did not report the diagnostic criteria of MS. The complete study selection process is described in Figure 1.
Figure 1.
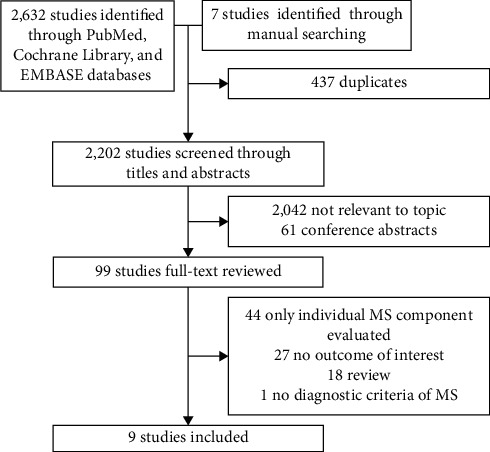
Results of information search.
Ultimately, nine cross-sectional studies with 12,987 individuals were eligible for our meta-analysis [13–15, 18–23]. Seven studies compared the association between MS and osteoporosis, and two studies compared the association between MS and decreased BMD (included both osteopenia and osteoporosis). A summary of the included studies is given in Table 1. Included studies were published between 2013 and 2018 and were performed in China, Iran, Germany, Korea, and Morocco. The mean age ranged from 52 to 71 years. Five studies reported multiple datasets by gender, one study only reported male participants, and the remaining three only reported female participants. MS was diagnosed according to the National Cholesterol Education Program's Adult Treatment Panel III (NCEPATP III) [24] in six studies, the Chinese Diabetes Society (CDS) [25] in one study, and harmonization criteria [26] in two studies. All studies measured BMD by DEXA and diagnosed osteoporosis according to the WHO definition.
Table 1.
Characteristics of included studies.
| Authors, year | Country (study period) | Study design | Sample size (male %) | Age (mean ± SD) | Metabolic syndrome diagnostic criteria | Outcome (diagnostic criteria) | BMD | |
|---|---|---|---|---|---|---|---|---|
| Measurement | Site | |||||||
| Kim et al. 2013 [18] | Korean (2008–2010) | Cross-sectional study | 3,207 (46.7) | 52.0 ± 0.4 | Harmonized criteria | Normal BMD vs. decreased BMDa (WHO criteria) | DEXA | Femoral neck, lumbar spine, and total hip |
|
| ||||||||
| Maghraoui et al. 2014 [19] | Morocco (2012–2013) | Cross-sectional study | 270 (0.0) | 61.0 ± 7.8 | NCEPATP III | Nonosteoporosisb vs. osteoporosis (WHO criteria) | DEXA | Femoral neck, lumbar spine, and total hip |
|
| ||||||||
| Lee et al. 2015 [20] | Korea (2010–2011) | Cross-sectional study | 3305 (46.5) | 63.0 ± 8.4 | NCEPATP III | Nonosteoporosis vs. osteoporosis (WHO criteria) | DEXA | Femoral neck and lumbar spine |
|
| ||||||||
| Eckstein et al. 2016 [21] | Germany (2011–2014) | Cross-sectional study | 1,402 (48.9) | 68.1 ± 3.5 | Harmonized criteria | Nonosteoporosis vs. osteoporosis (WHO criteria) | DEXA | Femoral neck, lumbar spine, and total hip |
|
| ||||||||
| Abbasi et al. 2017 [22] | Iran (NA) | Cross-sectional study | 143 (0.0) | 56.8 ± 7.8 | NCEPATP III | Nonosteoporosis vs. osteoporosis (WHO criteria) | DEXA | Femoral neck and lumbar spine |
|
| ||||||||
| Heidari et al. 2017 [23] | Iran (2011–2012) | Cross-sectional study | 553 (100.0) | 70.7 ± 7.7 | NCEPATP III | Nonosteoporosis vs. osteoporosis (WHO criteria) | DEXA | Femoral neck and lumbar spine |
|
| ||||||||
| Chen et al. 2018 [15] | China (2011–2016) | Cross-sectional study | 938 (0.0) | 61.2 ± 13.8 | CDS | Nonosteoporosis vs. osteoporosis (WHO criteria) | DEXA | Femoral neck, lumbar spine, and total hip |
|
| ||||||||
| Loke et al. 2018 [13] | Taiwan (2014–2015) | Cross-sectional study | 1162 (59.5) | 59.9 ± 7.3 | NCEPATP III | Normal BMD vs. decreased BMD (WHO criteria) | DEXA | Radius head, femoral neck, and total hip |
|
| ||||||||
| Lin et al. 2018 [14] | Taiwan (NA) | Cross-sectional study | 2007 (52.1) | 58.9 ± NA | NCEPATP III | Nonosteoporosis vs. osteoporosis (WHO criteria) | DEXA | Femoral neck, lumbar spine, and total hip |
Note. SD, standard deviance; BMD, bone mineral density; DEXA, dual-energy X-ray absorptiometry; NA, not available; CDS, Chinese Diabetes Society; NCEPATP III, the National Cholesterol Education Program Adult Treatment Panel III criteria. aDecreased BMD includes both osteopenia and osteoporosis. bNonosteoporosis includes both normal BMD and osteopenia.
As given in Table 2, the adjusted confounders varied across the included studies. Six studies adjusted for a wide range of risk factors for osteoporosis, including age, body mass index (BMI), calcium intake, physical activity, educational level, alkaline phosphatase, nonalcoholic fatty liver disease, smoking status, alcohol consumption, and so on.
Table 2.
Covariates adjusted for models of the associations between metabolic syndrome and osteoporosis.
| Authors, year | Covariates |
|---|---|
| Kim et al. 2013 [18] | Men and premenopausal women adjusted for age, BMI, WBC count, alkaline phosphatase, smoking, alcohol intake, PHA, self-reported health status, daily calcium intake, chronic disease, rheumatoid arthritis, cancer, and parental osteoporosis; postmenopausal women further adjusted for years since menopause and postmenopausal hormone therapy |
|
| |
| Maghraoui et al. 2014 [19] | Age, BMI, years since menopause, and number of pregnancies |
|
| |
| Lee et al. 2015 [20] | Age, calcium intake, serum 25-OH vitamin D level, serum parathyroid hormone level, smoking status, alcohol consumption, PHA, hormone replacement therapy (in women), and muscle mass |
|
| |
| Eckstein et al. 2016 [21] | None |
|
| |
| Abbasi et al. 2017 [22] | None |
|
| |
| Heidari et al. 2017 [23] | Age, BMI, muscle strength, PHA, educational level, history of fractures, abdominal obesity, smoking, and other biochemical parameters |
|
| |
| Chen et al. 2018 [15] | Age, serum total cholesterol, alkaline phosphatase, and nonalcoholic fatty liver disease |
|
| |
| Loke et al. 2018 [13] | None |
|
| |
| Lin et al. 2018 [14] | Age, aspartate aminotransferase, creatinine, hemoglobin, and exercise status |
Note. BMI, body mass index; WBC, white blood cell; PHA, physical activities.
3.2. Quality Assessment of Included Studies
The quality of the included studies was evaluated by the AHRQ list (Table 3). The total score ranged from 1 to 11. All studies had high or moderate quality (mean score = 8.1).
Table 3.
The methodological quality of the included studies.
| Authors, year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Score | Quality level |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional studiesa | |||||||||||||
| Kim et al. 2013 [18] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 | High |
| Maghraoui et al. 2014 [19] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 | High |
| Lee et al. 2015 [20] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 | High |
| Eckstein et al. 2016 [21] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 8 | High |
| Abbasi et al. 2017 [22] | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 6 | Moderate |
| Heidari et al. 2017 [23] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 8 | High |
| Chen et al. 2018 [15] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 | High |
| Loke et al. 2018 [13] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 8 | High |
| Lin et al. 2018 [14] | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 7 | Moderate |
aThe Agency for Healthcare Research and Quality (AHRQ) was used to assess the study quality for cross-sectional studies.
3.3. The Association between MS and Osteoporosis
As shown in Figure 2, ten datasets compared nonosteoporosis to osteoporosis with or without MS; there was a borderline significant association between MS and osteoporosis (OR = 0.72, 95% CI: 0.52–0.99, p=0.046) when combining all ORs with the random-effects model, and a high degree of heterogeneity (I2 = 73%, p < 0.001) between these studies. The result of Egger's test (p=0.198) and Begg's test (p=0.869) does not indicate publication bias among studies of MS and osteoporosis. Sensitivity analysis (one study was omitted per round) did not significantly alter the results, ranging from 0.63 (95% CI: 0.50–0.80) to 0.78 (95% CI: 0.57–1.08).
Figure 2.
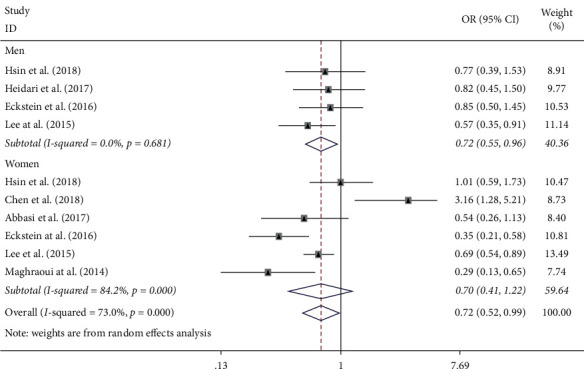
Forest plot showing the association between MS and the risk of osteoporosis (osteoporosis vs. nonosteoporosis).
Notably, subgroup analyses by gender revealed that a significant inverse relationship was observed merely in men (OR = 0.72, 95% CI: 0.55–0.96, p=0.023) but not in women (OR = 0.70, 95% CI: 0.41–1.22, p=0.210). There was no evidence of statistical heterogeneity in the male subgroup (I2 = 0%, p=0.681) but substantial heterogeneity in the female subgroup (I2 = 84.2%, p < 0.001).
3.4. The Association between MS and Decreased BMD
As shown in Figure 3, four datasets compared normal BMD to decreased BMD (includes both osteopenia and osteoporosis) with or without MS; there was no evidence for an association between MS and decreased BMD (OR = 1.01, 95% CI: 0.54–1.92, p=0.969) when combining all ORs with the random-effects model. High heterogeneity (I2 = 83.9%, p < 0.001) existed among the studies.
Figure 3.
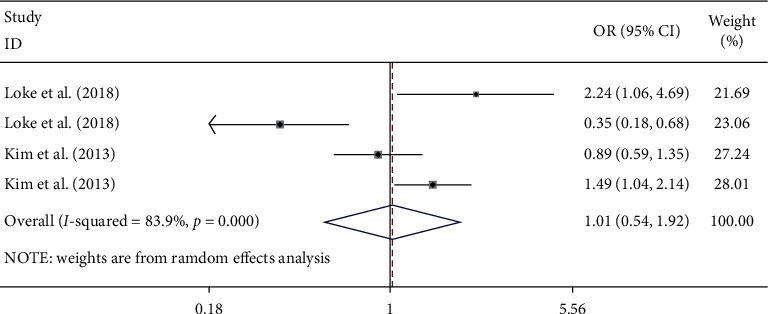
Forest plot showing the association between MS and the risk of decreased BMD.
3.5. Subgroup Analyses
As given in Table 4, heterogeneity was further explored by subgroup analyses based on the definition of MS (NCEPATP III vs. CDS vs. harmonization criteria), country (Asian vs. non-Asian countries), studies' quality (high vs. moderate), and confounding factors (adjusted vs. unadjusted).
Table 4.
Subgroup analyses for comparing nonosteoporosis to osteoporosis with or without MS.
| Group | No. of datasets | OR (95% CI) | P | P for heterogeneity | I 2 (%) |
|---|---|---|---|---|---|
| Definitions of MS | |||||
| NCEPATP III | 7 | 0.67 (0.54, 0.84) | <0.001 | 0.253 | 23.1 |
| CDS | 1 | 3.16 (1.28, 5.21) | 0.001 | — | — |
| Harmonization criteria | 2 | 0.54 (0.23, 1.30) | 0.169 | <0.001 | 82.1 |
| Country | |||||
| Asian | 7 | 0.86 (0.60, 1.23) | 0.404 | 0.003 | 69.6 |
| Non-Asian | 3 | 0.46 (0.23, 0.89) | 0.021 | 0.025 | 73.0 |
| Quality of studies | |||||
| Moderate | 3 | 0.80 (0.55, 1.15) | 0.231 | 0.400 | 0 |
| High | 7 | 0.70 (0.45, 1.08) | 0.108 | <0.001 | 80.6 |
| Confounding factors | |||||
| Adjusted | 7 | 0.81 (0.54, 1.21) | 0.303 | <0.001 | 75.5 |
| Unadjusted | 3 | 0.54 (0.31, 0.95) | 0.033 | <0.061 | 64.2 |
Note. MS, metabolic syndrome; OR, odds ratio; CI, confidence interval; NCEPATP III, the National Cholesterol Education Program Adult Treatment Panel III criteria; CDS, Chinese Diabetes Society.
There was a significant association between MS and osteoporosis in MS defined by NCEPATP III (OR = 0.67, 95% CI: 0.54–0.84, p < 0.001) in a non-Asian country (OR = 0.46, 95% CI: 0.23–0.89, p=0.021) and in an unadjusted model (OR = 0.54, 95% CI: 0.31–0.95, p < 0.033).
4. Discussion
This systematic review and meta-analysis found that MS was associated with reduced osteoporosis risk in men but not in women. In addition, the association between osteoporosis and MS was likely to relate to the definition of MS, the source of the study population, and the adjustment of covariates.
Two relevant meta-analyses on the association between MS and BMD were published previously [11, 12]. The meta-analysis from Xue et al. showed that MS may have a beneficial influence on BMD, and the meta-analysis by Zhou et al. showed a negative effect of MS on BMD in men but not in women. However, according to the WHO diagnostic criteria, we consider it is more clinically significant that BMD (continuity variable) is divided into osteoporosis and osteopenia (categorical variable). Osteopenia is a transition from normal BMD to osteoporosis, which is generally considered a reversible process [27]. Our meta-analysis compared normal BMD to decreased BMD (includes both osteopenia and osteoporosis), and there was no evidence for an association between MS and decreased BMD; however, the result was not robust because of the small number of studies and high heterogeneity.
Our analysis compared nonosteoporosis to osteoporosis and suggested a positive effect of MS on osteoporosis. Two relevant meta-analyses from Esposito et al. [28] and Yang et al. [29] also reported that MS was significantly associated with a lower fracture risk. MS is a cluster of diseases consisting of several disorders; the mechanism following the impacts of MS on osteoporosis is intricate and has not yet been studied thoroughly. The relationship between the various components of MS and osteoporosis has been widely investigated, but the results are inconclusive. As the component of MS, the results of studies on the association between obesity and osteoporosis are mostly inconsistent. Some studies reported obesity was a protective effect for osteoporosis in several studies generally [30, 31]. Because it is associated with a higher 17β-estradiol level and higher mechanical load, which may improve bone density. Other studies [32–36] have pointed out that inflammation-related factors released by visceral adipose tissue increase the risk of osteoporosis by inhibiting bone formation. Abnormality of calcium metabolism is a key factor linking hypertension and osteoporosis. Zhang et al. [37] found hypertension was independently and significantly associated with osteoporosis, while Mussolino et al. [38] found no significant association between blood pressure and BMD at any bone site. Additionally, there are also controversial reports on the effects of dyslipidemia [39–41] and insulin resistance [32, 42] on bone health. We believe that both positive and negative influences of the MS components on the bone exist in parallel. Therefore, the combined effect of the MS components on osteoporosis could be positive or insignificant.
The gender-specific differences regarding the association between MS and osteoporosis were observed in our analysis. Menopause may be the reason why there was no significant association between MS and osteoporosis for women. The majority of women included in our meta-analysis were older and postmenopausal. Menopause is one of the major risk factors for osteoporosis partly due to reduced estrogen production [43], which may dilute the benefit of MS on the bone. In addition, several study-level variables leading to heterogeneity were further found by subgroup analyses, such as the MS definition, the source of the study population, the quality of the study, and the adjustment of covariates. First, we obtained a significant association between MS and osteoporosis in the subjects diagnosed by NCEPATP III criteria rather than other criteria (includes CDS criteria and harmonization criteria). According to NCEPATP III or other criteria, participants were diagnosed with MS when they had three or more of the following: obesity, hypertension, low HDL-C levels, high fasting blood glucose, or high triglycerides. Although the other two criteria were similar to the NCEPATP III criteria, there were still differences. For example, the NCEPATP III criteria defined obesity by waist circumference, while the CDS defined obesity by BMI. Second, we obtained a significant association between MS and osteoporosis only in non-Asian subjects. In another meta-analysis, Xue et al. [13] also reported that MS may have a beneficial influence on BMD only in Caucasian populations. Genetic differences may explain the association between osteoporosis and MS be related to the source of the study population. The risk of osteoporosis has heritable factors, such as differences in bone geometry, size, and height [44, 45]. Third, we obtained a significant association between MS and osteoporosis only in the unadjusted model (univariate analysis). Although, in general, the adjusted models (multivariate analysis) were more accurate and deeper considered, we still accepted the unadjusted results to a certain extent. In the studies of the adjustment model, four datasets were adjusted for BMI, and one dataset was adjusted for serum total cholesterol. These adjustments distorted the clinical characteristics of MS, defined by obesity and dyslipidemia. In adjusting for these, the clinical sense of MS just disappeared or was at least essentially modified. However, this means that important confounding factors [46, 47] (such as BMI, physical activity, and dietary factors) are not controlled; this also is the reason that the finding should be interpreted with caution because the inverse associations of MS with osteoporosis risk could be attributed to the adoption of healthy lifestyles by subjects after being diagnosed MS.
Some limitations existed in our study. First, nonrandomized comparisons in observational studies may suffer from biases, which could impair the findings and thus weaken the strength of evidence. Second, most primary studies lacked data on important covariates, such as physical activities, vitamin D deficiency, and hormone therapy [48–50], which should be considered as a confounding factor when analyzing the association between MS and osteoporosis. Therefore, the risk of unmeasured confounding cannot be entirely ruled out. Third, although it would have been clinically meaningful to evaluate the effects of bone loss on different sites (total hip, femoral neck, and lumbar spine), we were unable to do so because of insufficient data. Fourth, in view of the heterogeneity, the random-effects model was used for meta-analyses, but in the comparison of normal BMD with decreased BMD, we included only four datasets in the analyses, so statistical power may be affected.
5. Conclusion
The findings of our meta-analyses suggested that individuals with MS demonstrate a lower risk of osteoporosis. Notably, subgroup analyses by gender showed that significant inverse associations were observed only in men but not in women. In addition, the association between osteoporosis and MS was likely to relate to the definition of MS, the source of the study population, and the adjustment of covariates. However, given the small number of studies mentioned above and the limitations of the study design, these findings must be interpreted carefully. The clinical significance of these findings remains uncertain and should be addressed in future well-designed prospective studies.
Acknowledgments
The authors thank Dr. Jiang for responding to inquiry about their publication.
Abbreviations
- AHRQ:
Agency for Health Care Research and Quality
- BMD:
Bone mineral density
- BMI:
Body mass index
- CDS:
Chinese Diabetes Society
- CI:
Confidence interval
- DEXA:
Dual-energy X-ray absorptiometry
- HDL-C:
High-density lipoprotein cholesterol
- MS:
Metabolic syndrome
- NCEPATP III:
National Cholesterol Education Program's Adult Treatment Panel III
- OR:
Odds ratio
- TG:
Triglyceride
- WHO:
World Health Organization.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
WL and YW were in charge of idea and designed the study. WL, LY, and JH searched the databases to find related articles. WL and CW analyzed those articles and wrote the article. All authors read and approved the final manuscript.
References
- 1.Yamagishi K., Iso H. The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiology and Health. 2017;39:e2017003–2017004. doi: 10.4178/epih.e2017003.e2017003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurka M. J., Guo Y., Filipp S. L., DeBoer M. D. Metabolic syndrome severity is significantly associated with future coronary heart disease in Type 2 diabetes. Cardiovascular Diabetology. 2018;17(1):p. 17. doi: 10.1186/s12933-017-0647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBoer M. D., Gurka M. J., Golden S. H., et al. Independent associations between metabolic syndrome severity and future coronary heart disease by sex and race. Journal of the American College of Cardiology. 2017;69(9):1204–1205. doi: 10.1016/j.jacc.2016.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decker J. J., Norby F. L., Rooney M. R., et al. Metabolic syndrome and risk of ischemic stroke in atrial fibrillation. Stroke. 2019;50(11):3045–3050. doi: 10.1161/strokeaha.119.025376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguilar M., Bhuket T., Torres S., Liu B., Wong R. J. Prevalence of the metabolic syndrome in the United States, 2003-2012. Jama. 2015;313(19):1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 6.Vidal M., Thibodaux R. J., Neira L. F. V., Messina O. D. Osteoporosis: a clinical and pharmacological update. Clinical Rheumatology. 2019;38(2):385–395. doi: 10.1007/s10067-018-4370-1. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Topics & Sites. Geneva, Switzerland: World Health Organization; 2016. http://www.who.int/chp/topics/en/http://www.who.int/chp/topics/Osteoporosis.pdf. [Google Scholar]
- 8.Wani K., Yakout S. M., Ansari M. G. A., et al. Metabolic syndrome in arab adults with low bone mineral density. Nutrients. 2019;11(6) doi: 10.3390/nu11061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulzele K., Riddle R. C., DiGirolamo D. J., et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferron M., Wei J., Yoshizawa T., et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J., Zhang Q., Yuan X., et al. Association between metabolic syndrome and osteoporosis: a meta-analysis. Bone. 2013;57(1):30–35. doi: 10.1016/j.bone.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Xue P., Gao P., Li Y. The association between metabolic syndrome and bone mineral density: a meta-analysis. Endocrine. 2012;42(3):546–554. doi: 10.1007/s12020-012-9684-1. [DOI] [PubMed] [Google Scholar]
- 13.Loke S.-S., Chang H.-W., Li W.-C. Association between metabolic syndrome and bone mineral density in a Taiwanese elderly population. Journal of Bone and Mineral Metabolism. 2018;36(2):200–208. doi: 10.1007/s00774-017-0826-7. [DOI] [PubMed] [Google Scholar]
- 14.Lin H.-H., Huang C.-Y., Hwang L.-C. Association between metabolic syndrome and osteoporosis in Taiwanese middle-aged and elderly participants. Archives of Osteoporosis. 2018;13(1):p. 48. doi: 10.1007/s11657-018-0467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D. Z., Xu Q. M., Wu X. X, et al. The combined effect of nonalcoholic fatty liver disease and metabolic syndrome on osteoporosis in postmenopausal females in eastern China. International Journal of Endocrinology. 2018;2018 doi: 10.1155/2018/2314769.2314769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng X., Zhang Y., Kwong J. S. W., et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. Journal of Evidence-Based Medicine. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H., Oh H. J., Choi H., Choi W. H., Lim S.-K., Kim J. G. The association between bone mineral density and metabolic syndrome: a Korean population-based study. Journal of Bone and Mineral Metabolism. 2013;31(5):571–578. doi: 10.1007/s00774-013-0446-9. [DOI] [PubMed] [Google Scholar]
- 19.Maghraoui A. E., Rezqi A., Mrahi S. E., Sadni S., Ghozlani I., Mounach A. Osteoporosis, vertebral fractures and metabolic syndrome in postmenopausal women. BMC Endocrine Disorders. 2014;14(1) doi: 10.1186/1472-6823-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K. Metabolic syndrome and osteoporosis in relation to muscle mass. Calcified Tissue International. 2015;97(5):487–494. doi: 10.1007/s00223-015-0033-2. [DOI] [PubMed] [Google Scholar]
- 21.Eckstein N., Buchmann N., Demuth I., et al. Association between metabolic syndrome and bone mineral density-data from the berlin aging study II (BASE-II) Gerontology. 2016;62(3):337–344. doi: 10.1159/000434678. [DOI] [PubMed] [Google Scholar]
- 22.Abbasi M., Farzam S. A., Mamaghani Z., Yazdi Z. Relationship between metabolic syndrome and its components with bone densitometry in postmenopausal women. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2017;11:S73–S76. doi: 10.1016/j.dsx.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Heidari B., Muhammadi A., Javadian Y., Bijani A., Hosseini R., Babaei M. Associated factors of bone mineral density and osteoporosis in elderly males. International Journal of Endocrinology and Metabolism. 2017;15(1) doi: 10.5812/ijem.39662.e39662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundy S. M., Cleeman J. I., Daniels S. R., et al. Diagnosis and management of the metabolic syndrome. Circulation. 2005;112(17):2735–2752. doi: 10.1161/circulationaha.105.169404. [DOI] [PubMed] [Google Scholar]
- 25.Weng J., Ji L., Jia W., et al. Standards of care for type 2 diabetes in China. Diabetes/metabolism Research and Reviews. 2016;32(5):442–458. doi: 10.1002/dmrr.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberti K. G. M. M., Eckel R. H., Grundy S. M., et al. Harmonizing the metabolic syndrome. Circulation. 2009;120(16):1640–1645. doi: 10.1161/circulationaha.109.192644. [DOI] [PubMed] [Google Scholar]
- 27.Yedavally-Yellayi S., Ho A. M., Patalinghug E. M. Update on osteoporosis. Primary Care: Clinics in Office Practice. 2019;46(1):175–190. doi: 10.1016/j.pop.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Esposito K., Chiodini P., Capuano A., Colao A., Giugliano D. Fracture risk and bone mineral density in metabolic syndrome: a meta-analysis. The Journal of Clinical Endocrinology & Metabolism. 2013;98(8):3306–3314. doi: 10.1210/jc.2013-1775. [DOI] [PubMed] [Google Scholar]
- 29.Yang L., Lv X., Wei D., Yue F., Guo J., Zhang T. Metabolic syndrome and the risk of bone fractures: a meta-analysis of prospective cohort studies. Bone. 2016;84:52–56. doi: 10.1016/j.bone.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Yang S., Shen X. Association and relative importance of multiple obesity measures with bone mineral density: the national health and nutrition examination survey 2005-2006. Archives of Osteoporosis. 2015;10(1):p. 14. doi: 10.1007/s11657-015-0219-2. [DOI] [PubMed] [Google Scholar]
- 31.Agbaht K., Gurlek A., Karakaya J., Bayraktar M. Circulating adiponectin represents a biomarker of the association between adiposity and bone mineral density. Endocrine. 2009;35(3):371–379. doi: 10.1007/s12020-009-9158-2. [DOI] [PubMed] [Google Scholar]
- 32.Neglia C., Argentiero A., Chitano G., et al. Diabetes and obesity as independent risk factors for osteoporosis: updated results from the ROIS/EMEROS registry in a population of five thousand post-menopausal women living in a region characterized by heavy environmental pressure. International Journal of Environmental Research and Public Health. 2016;13(11) doi: 10.3390/ijerph13111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adami G., Gatti D., Rossini M., et al. Risk of fragility fractures in obesity and diabetes: a retrospective analysis on a nation-wide cohort. Osteoporosis International. 2020;31(11):2113–2122. doi: 10.1007/s00198-020-05519-5. [DOI] [PubMed] [Google Scholar]
- 34.Roy B., Curtis M. E., Fears L. S., Nahashon S. N., Fentress H. M. Molecular mechanisms of obesity-induced osteoporosis and muscle atrophy. Frontiers in Physiology. 2016;7:p. 439. doi: 10.3389/fphys.2016.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma S., Tandon V., Mahajan S., Mahajan V., Mahajan A. Obesity: friend or foe for osteoporosis. Journal of Mid-life Health. 2014;5(1):6–9. doi: 10.4103/0976-7800.127782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka S., Kuroda T., Saito M., Shiraki M. Overweight/obesity and underweight are both risk factors for osteoporotic fractures at different sites in Japanese postmenopausal women. Osteoporosis International. 2013;24(1):69–76. doi: 10.1007/s00198-012-2209-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J., Zhang K., Shi H., Tang Z. A cross-sectional study to evaluate the associations between hypertension and osteoporosis in Chinese postmenopausal women. International Journal of Clinical and Experimental Medicine. 2015;8(11):21194–21200. [PMC free article] [PubMed] [Google Scholar]
- 38.Mussolino M., Gillum R. Bone mineral density and hypertension prevalence in postmenopausal women: results from the third national health and nutrition examination survey. Annals of Epidemiology. 2006;16(5):395–399. doi: 10.1016/j.annepidem.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 39.Dennison E. M., Syddall H. E., Aihie Sayer A., Martin H. J., Cooper C. Lipid profile, obesity and bone mineral density: the Hertfordshire cohort study. Qjm. 2007;100(5):297–303. doi: 10.1093/qjmed/hcm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adami S., Braga V., Zamboni M., et al. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcified Tissue International. 2004;74(2):136–142. doi: 10.1007/s00223-003-0050-4. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi T., Sugimoto T., Yano S., et al. Plasma lipids and osteoporosis in postmenopausal women. Endocrine Journal. 2002;49(2):211–217. doi: 10.1507/endocrj.49.211. [DOI] [PubMed] [Google Scholar]
- 42.Linda Kao W. H., Kammerer C. M., Schneider J. L., Bauer R. L., Mitchell B. D. Type 2 diabetes is associated with increased bone mineral density in Mexican-American women. Archives of Medical Research. 2003;34(5):399–406. doi: 10.1016/j.arcmed.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Yoldemir T., Erenus M. The impact of metabolic syndrome on bone mineral density in postmenopausal women. Gynecological Endocrinology. 2012;28(5):391–395. doi: 10.3109/09513590.2011.633656. [DOI] [PubMed] [Google Scholar]
- 44.Cauley J. A., Chalhoub D., Kassem A. M., Fuleihan G. E.-H. Geographic and ethnic disparities in osteoporotic fractures. Nature Reviews Endocrinology. 2014;10(6):338–351. doi: 10.1038/nrendo.2014.51. [DOI] [PubMed] [Google Scholar]
- 45.Lei S. F., Chen Y., Xiong D. H., Li L. M., Deng H. W. Ethnic difference in osteoporosis-related phenotypes and its potential underlying genetic determination. Journal of Musculoskeletal & Neuronal Interactions. 2006;6(1):36–46. [PubMed] [Google Scholar]
- 46.Yu C. Y., Chen F. P., Chen L. W., Kuo S. F., Chien R. N. Association between metabolic syndrome and bone fracture risk: a community-based study using a fracture risk assessment tool. Medicine. 2017;96(50):p. e9180. doi: 10.1097/MD.0000000000009180.e9180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominic E., Brozek W., Peter R. S, et al. Metabolic factors and hip fracture risk in a large Austrian cohort study. Bone Reports. 2020;12 doi: 10.1016/j.bonr.2020.100244.100244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reid I. R. A broader strategy for osteoporosis interventions. Nature Reviews Endocrinology. 2020;6 doi: 10.1038/s41574-020-0339-7. [DOI] [PubMed] [Google Scholar]
- 49.Chen L. R., Hou P. H., Chen K. H. Nutritional support and physical modalities for people with osteoporosis: current opinion. Nutrients. 2019;11(12) doi: 10.3390/nu11122848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu B., Cumming R. G., Seibel M. J., et al. Reproductive hormones and longitudinal change in bone mineral density and incident fracture risk in older men: the concord health and aging in men project. Journal of Bone and Mineral Research. 2015;30(9):1701–1708. doi: 10.1002/jbmr.2493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


