Abstract
Background
Stroke and thromboembolic events occurring among patients taking direct oral anticoagulants (DOACs) have been associated with low concentrations of DOACs. Enzyme-inducing antiseizure medications (EI-ASMs) are associated with enhanced cytochrome-P450-mediated metabolism and enhanced P-glycoprotein-mediated transport.
Objective
The aim of this study was to evaluate the effect of concomitant EI-ASM use on DOAC peak concentrations in patients treated in clinical care.
Methods
We performed a retrospective cohort study of patients treated with DOACs for atrial fibrillation and venous thromboembolic disease in an academic general hospital. In total, 307 patients treated with DOACs between August 2015 and January 2020 were reviewed. Clinical characteristics and peak DOAC plasma concentrations of patients co-treated with an EI-ASM were compared with those of patients not treated with an EI-ASM. An apixaban dose score (ADS) was defined to account for apixaban dosage and the number of apixaban dose-reduction criteria.
Results
In total, 177 peak DOAC plasma concentrations (including apixaban, rivaroxaban, and dabigatran) from 131 patients were measured, including 24 patients co-treated with an EI-ASM and 107 controls not treated with an EI-ASM. The proportion of patients with DOAC concentrations below the expected range was significantly higher among EI-ASM users than among patients not taking an EI-ASM (37.5 vs. 9.3%, respectively; p = 0.0004; odds ratio 5.82; 95% confidence interval [CI] 2.03–16.66). Most of these patients were treated with apixaban (85%); however, sensitivity analysis results were also significant (p = 0.031) for patients with non-apixaban DOACs. In patients co-treated with apixaban and an EI-ASM, median apixaban peak concentration was 106 ng/mL (interquartile range [IQR] 71–181) compared with 150 ng/mL (IQR 94–222) in controls (p = 0.019). In multivariable analysis, EI-ASM use was associated with 6.26-fold increased odds for apixaban concentration below the expected range (95% CI 2.19–17.90; p = 0.001). Apixaban concentrations were significantly associated with EI-ASM use, moderate enzyme inhibitor use, and ADS.
Conclusions
Concurrent EI-ASM and DOAC use presents a possible risk for DOAC concentrations below the expected range. The clinical significance of the interaction is currently unclear.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40263-021-00795-z.
Key Points
| Low direct oral anticoagulant (DOAC) plasma concentrations can lead to treatment failure, but factors causing low DOAC concentrations are not yet fully established. |
| In a cohort of 131 patients treated with DOACs, including apixaban (85%), rivaroxaban, and dabigatran, we analyzed the association between DOAC concentration and the use of enzyme-inducing antiseizure medications (EI-ASMs). |
| The proportion of patients with lower than expected DOAC peak concentrations was significantly higher in patients co-treated with EI-ASMs. These results were consistent among all DOACs. |
| Concomitant treatment with EI-ASMs was associated with statistically significantly decreased apixaban levels. |
| In multivariable analysis, EI-ASM use was associated with more than 6-fold increased odds for apixaban concentrations below the expected range. |
Introduction
Direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists (VKAs) in the prevention of thromboembolic events associated with atrial fibrillation (AF) and venous thromboembolism (VTE), mainly because they are simpler to use and have a reduced risk of intracranial hemorrhage [1–5]. Failure of DOAC treatment results in severe morbidity and mortality. This may include cerebrovascular accident (CVA) or systemic embolism in patients with AF and include recurrent pulmonary embolism (PE) or deep vein thrombosis (DVT) in patients with prior VTE.
DOAC treatment failure may be associated with low DOAC concentrations. In a recent study in patients treated with DOACs who were admitted with ischemic CVA, the affected size of CVA, defined according to the National Institutes of Health Stroke Scale, inversely correlated with DOAC concentrations [6]. In another observational study, ten (1.8%) patients taking DOACs experienced thromboembolic events in a 1-year follow-up. All ten patients had DOAC serum concentrations in the lowest quartile of trough drug concentrations [7]. In the post hoc analysis of the AVERROES trial, patients within the lowest decile of anti-factor Xa (anti-Xa) activity calibrated for apixaban had a statistically significantly greater risk of stroke than those with higher anti-Xa activity (p = 0.013) [8]. Yet, the factors associated with risk of low DOAC concentrations are not well-defined.
Anecdotal reports describe co-prescription of DOACs and enzyme-inducing antiseizure medications (EI-ASMs) as a plausible culprit of DOAC treatment failure [9–15]. Few of these reports have also reported low concentrations of DOACs [9, 13, 15]. Clinical failure of anticoagulant treatment with an EI-ASM has been reported with apixaban [9, 13], rivaroxaban [11, 12, 14, 15], and dabigatran [10]. Studies on the effect of EI-ASMs on anticoagulation outcomes in patients treated with DOACs are scarce. Using the US FDA Adverse Event Reporting system, we previously described an increased odds of reporting DOAC treatment failure with concomitant use of EI-ASMs with apixaban, rivaroxaban, or edoxaban [16].
EI-ASMs are associated with increased metabolism of cytochrome-P450 (CYP) substrates and enhanced transport of the adenosine triphosphate-binding cassette sub-family B member 1, commonly known as P-glycoprotein (P-gp), substrates. Some frequently used antiseizure medications (ASMs) are strong CYP3A4 inducers, including carbamazepine, phenytoin, phenobarbital, and primidone. These ASMs and other CYP3A4 inducers, such as rifampicin and hypericum perforatum, increase the metabolism of a wide range of CYP3A4 substrates such as apixaban and rivaroxaban and the transport of P-gp substrates, including dabigatran, apixaban, rivaroxaban, and edoxaban.
As suggested by cases reporting low DOAC concentrations in patients treated with concurrent EI-ASMs [9, 13, 15, 17–20] and a small series [21], the mechanism for this interaction is likely to involve reduced DOAC concentrations and area under the curve (AUC). Such a mechanism is thus far supported only by studies in healthy subjects performed during the early phases of DOAC development. Rifampicin, a model for enzyme-inducing and P-gp-inducing drugs, has been shown to significantly decrease concentrations and AUCs of apixaban [22], rivaroxaban [23], edoxaban [24], and dabigatran [25] in healthy subjects.
We hypothesize that co-treatment with a DOAC and an EI-ASM is associated with a higher proportion of patients with DOAC concentrations below the reported 5th percentile compared with patients not treated with an EI-ASM. In addition, we hypothesize that concurrent treatment with DOACs and EI-ASMs is associated with lower DOAC concentrations, independent of DOAC dose and dose-adjustment criteria [26, 27]. The aim of the current study was to evaluate the effect of EI-ASM use on DOAC concentrations in patients treated with DOACs.
Methods
Study Design
We performed a retrospective cohort study including patients hospitalized in the Hadassah Medical Center between August 2015 and January 2020 with a prescription for a DOAC during hospitalization and whose records were reviewed by clinical pharmacists as part of the institutional DOAC monitoring program. Employing this program, clinical pharmacists review all DOAC orders in the hospital for potentially inappropriate prescribing during hospitalization. When potentially inappropriate prescribing or a drug-related problem was identified, the clinical pharmacist provided consultation on management options. A detailed description of this program has been previously published [21, 28]. The Hadassah Medical Center is a large general hospital affiliated with the Hebrew University that serves the population of Jerusalem and the surrounding area. Patients from whom blood samples were obtained for measurement of DOAC plasma concentration were included. The study was approved by the institutional review board of the Hadassah University Hospital. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Participants
Patients with an active prescription for apixaban, dabigatran, or rivaroxaban (the DOACs available in Israel) were included if measurement of DOAC plasma concentration was reported in the electronic medical record (EMR). Data collected included sociodemographic details, medical history and full medication history, as well as laboratory data, including DOAC concentration (see Sect. 2.3).
Results were included in the analysis if the following criteria were fulfilled:
Patients were treated with the same DOAC dose for more than 3 days.
A measurement of DOAC concentration was documented in the EMR.
Administration of the DOAC was documented in the EMR on the morning of concentration testing, at a time corresponding to peak drug concentration (tmax) [23]. DOAC concentration measurement was considered peak concentration if there was documentation of DOAC administration in the EMR (3–4 h for dabigatran or 4–5 h for apixaban or rivaroxaban) prior to the documented laboratory test time. This time window was chosen based on a pilot investigation where we observed a 1.5- to 2-h processing period between obtaining the blood sample and recording the result in the EMR, in addition to the previously reported 1- to 2-h tmax for dabigatran and 2- to 4-h tmax for apixaban and rivaroxaban [23].
Blood test was not performed because of acute bleeding or as part of pre-procedure testing.
Determination of Direct Oral Anticoagulant (DOAC) Plasma Concentrations
A calibrated anti-Xa-based assay was used to determine rivaroxaban and apixaban concentrations, and a thrombin time (TT)-based assay was used to establish dabigatran concentrations, in accordance with the previously published methods [29].
Data Collection
We collected sociodemographic and medical data. Sociodemographic data included age, sex, and body weight. Medical data included background medical history including International Classification of Diseases, Ninth Edition hospital discharge diagnoses, and diagnoses and indication(s) for DOAC administration. Laboratory data included serum creatinine and DOAC concentration(s). Medication history included all medications used regularly.
Data Analysis
The estimated glomerular filtration rate was calculated from serum creatinine using the Chronic Kidney Disease—Epidemiology Collaboration equation [30].
DOAC concentrations were classified as being below, within, or above the expected range. The expected ranges for apixaban and rivaroxaban was defined as a measurement between the 5th and 95th percentile, and for dabigatran between the 10th and 90th percentile as reported by analyses of phase III studies [31, 32] and are provided in Table 1 in the electronic supplementary material (ESM).
A list of CYP3A4 EI-ASMs (including phenytoin, phenobarbital, and carbamazepine) was compiled based on the DrugBank database [33]. Moderate or strong CYP3A4 inhibitors included dronedarone, verapamil, diltiazem, ciprofloxacin, and clindamycin based on the DrugBank database [34], as well as amiodarone [35].
Patients were considered to be consuming enzyme-inducing or enzyme-inhibiting drugs if their administration was documented in the EMR. The lists were then reviewed by two clinical pharmacists (AP, RG) and a clinical pharmacologist (MM) [16]. Among patients taking DOACs, those also treated with EI-ASMs were compared with those not receiving any EI-ASMs.
Apixaban Dose Score
In this study, the apixaban dose score (ADS) was defined to model apixaban plasma concentrations while accounting for a patient’s dose of apixaban and for the criteria requiring dose adjustments (Table 1), effectively translating the current dose-adjustment guidelines into a numerical scale [26, 27]. We used the ADS to evaluate the effect of drug interactions associated with low apixaban concentrations, independent of the clinically used apixaban dose-adjustment recommendations.
Table 1.
Apixaban dose score
| ADS | Apixaban dose | No. of dose adjustment criteriaa |
|---|---|---|
| − 2 | 2.5 mg BID | 0 |
| − 1 | 2.5 mg BID | 1 |
| 0 | 2.5 mg BID | 2 |
| + 1 | 2.5 mg BID | 3 |
| 0 | 5 mg BID | 0 |
| + 1 | 5 mg BID | 1 |
| + 2 | 5 mg BID | 2 |
| + 3 | 5 mg BID | 3 |
ADS apixaban dose score, BID twice daily
a Dosing as per guidelines
This score ranged from − 2 to +3 and was constructed to model the expected relative dose an individual received compared with the standard recommended apixaban dose. The standard recommended apixaban dose was the zero-reference point of the score. Thus, the zero-reference point of the score represented a dose of 5 mg twice daily (BID) for a patient with no dose-reduction criteria and 2.5 mg BID for a patient with two of three dose-reduction criteria (as per dosing guidelines). The presence of each additional dose-reduction criterion therefore added a point to the score.
A dose of 2.5 mg BID for a patient with no dose-reduction criteria would result in an ADS of − 2, and if a single criterion were present, the score would be − 1, both representing under-dosing of apixaban, relative to a patient’s characteristics.
Statistical Analysis
Univariate statistics were used to describe cohort characteristics, numbers and percent were used for categorical variables, and means or medians with measures of spread were used for continuous variables. Bivariate comparisons were made for the characteristics of patients treated with EI-ASMs compared with those who did not receive EI-ASMs and for the characteristics of patients with respect to DOAC concentrations (below, within, and above expected range). Since most of the patients were treated with apixaban (85%), a sensitivity analysis of the observed bivariate association between EI-ASMs and DOAC concentration was performed, whereby this association was tested separately among patients treated with apixaban and those treated with non-apixaban DOACs. The statistical significance of differences between groups was determined by the appropriate tests: Chi-squared test and Fisher’s exact test for categorical variables and the t-test and analysis of variance or Mann–Whitney U test and Kruskal–Wallis test for continuous variables.
Multivariable Analyses of Apixaban Plasma Concentration
Since most of the patients studied (85%) were treated with apixaban, the multivariable association of EI-ASM with apixaban plasma concentration was evaluated using a series of mixed-effects models with random intercepts to account for multiple measurements within individuals.
Two groups of models were used, one evaluating predictors of apixaban concentrations as a continuous measure and the other evaluating predictors of the risk for apixaban concentrations below the expected range, as a binary measure, with stepwise addition of variables of interest, as follows:
Group I—Apixaban concentrations (dependent variable, continuous)
Model A evaluated the relationship between apixaban concentration and ADS using a mixed-effects linear regression model. Apixaban concentrations were skewed and therefore log-transformed to facilitate accurate modelling.
Model B included terms for ADS and EI-ASM.
Model C included terms for ADS, EI-ASM, and moderate or strong CYP3A4 inhibitors.
Group II—Risk of apixaban concentrations below expected range (vs. patients with concentrations within and above the range), (dependent variable, categorical)
Model A evaluated the association of drug concentrations below the range with ADS using a mixed-effects logistic regression model.
Model B included terms for ADS and EI-ASM.
Model C included terms for ADS, EI-ASM, and moderate or strong CYP3A4 inhibitors.
The small number of patients using rivaroxaban and dabigatran meant it was not feasible to model the relationship between EI-ASMs and rivaroxaban and dabigatran concentrations.
p values < 0.05 were defined as significant for all tests. Model assumptions were evaluated analytically and graphically and judged to be adequately met. Analyses were performed using R, version 3.6.3 and the “lme4” and “lmerTest” packages.
Results
Patients' Characteristics
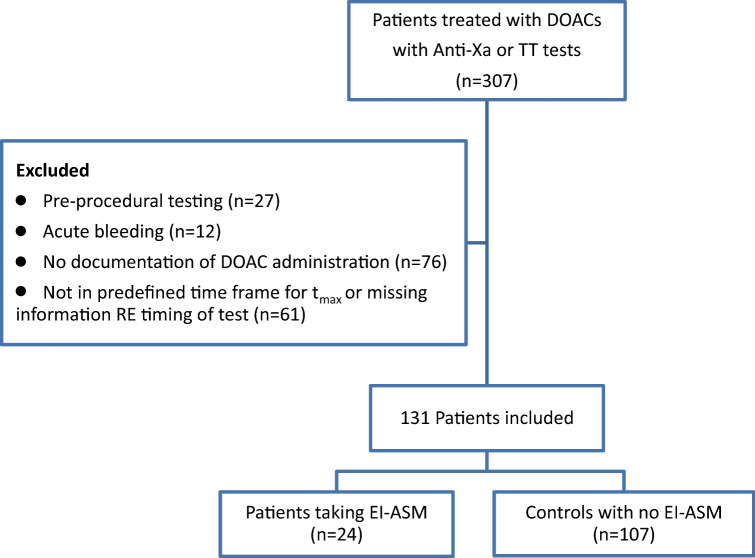
In total, 307 patients treated with DOACs with anti-Xa and TT tests were identified and reviewed (Fig. 1). In 76 patients, there was no documentation of DOAC administration, and, in 61 patients, the concentration test was not performed in the pre-defined timeframe or documentation was not available. In 27 patients, the test was performed pre-procedure, and in 12 patients the test was performed in the context of acute bleeding.
Fig. 1.
Consort diagram shows identification of patients who fulfilled inclusion criteria according to the study protocol. anti-Xa anti-factor Xa, DOAC direct oral anticoagulant, EI-ASM enzyme-inducing antiseizure medication, tmax time to peak drug concentration, TT thrombin time
In total, 131 patients with 177 DOAC plasma concentration tests fulfilling inclusion criteria were included in the analysis, including 152 apixaban tests (111 patients), 16 rivaroxaban tests (14 patients), and nine dabigatran tests (six patients). Eight patients had laboratory measurements of more than one DOAC.
The 131 patients included 66 females and 65 males. The indication for treatment with DOACs was AF in 116 patients (88.5%) and VTE in 13 patients (9.9%). In two patients, the indication was unknown. In total, 24 patients were taking EI-ASMs and 107 patients were not. Of the 24 patients on EI-ASMs, ten were treated with carbamazepine, four with phenobarbital (including one patient co-treated with topiramate), three with phenytoin, two with oxcarbazepine, and four with primidone. One patient was treated with a phenobarbital–oxcarbazepine combination. Indications for EI-ASM use included convulsive disorder (n = 14), trigeminal neuralgia (n = 3), post-herpetic neuralgia (n = 1), and tremor (otherwise unspecified) (n = 5). In one patient, the indication was unknown.
The proportion of patients with DOAC concentrations below the expected range was significantly higher among EI-ASM users than among patients not taking an EI-ASM (37.5 vs. 9.3%, respectively; p = 0.0004; odds ratio [OR] 5.82; 95% confidence interval [CI] 2.03–16.66).
In total, 35 patients were treated with moderate CYP3A4 inhibitors (dronedarone, verapamil, or amiodarone) and none with strong CYP3A4 inhibitors. There were no statistically significant differences in general clinical features between patients treated with an EI-ASM and those not treated with an EI-ASM. In particular, median age, body mass index, and creatinine level, as well as proportion of reduced dosing per guidelines, did not differ significantly.
There was no difference between patients treated with or without an EI-ASM in the use of moderate CYP3A4 or P-gp inhibitors. Among 24 patients in the group receiving EI-ASMs, nine patients were also taking enzyme inhibitors. Among 107 patients not treated with EI-ASMs, 26 were also treated with enzyme inhibitors (p = 0.21) (Table 2).
Table 2.
Patient characteristicsa
| Characteristics | All patients (n = 131) | Patients with an EI-ASM (n = 24) | Patients without an EI-ASM (n = 107) | p valueb |
|---|---|---|---|---|
| Female | 66 (50.4) | 9 (37.5) | 57 (53.3) | 0.242 |
| Age (years) | 80 (70–85) | 77 (64–83) | 80 (73–86) | 0.326 |
| Weight (kg) | 69 (58–80) | 70.7 (60–80) | 68 (58–80) | 0.783 |
| BMI | 25.2 (22–30) | 24 (20.75–31.25) | 25.4 (22.2–30) | 0.704 |
| Creatinine (mmol/L) | 90 (70–126) | 90 (73–114) | 89 (70–132) | 0.752 |
| DOAC | 1.000 | |||
| Apixaban | 111 (84.7) | 21 (87.5) | 90 (84.1) | |
| Rivaroxaban | 14 (10.7) | 2 (8.3) | 12 (11.2) | |
| Dabigatran | 6 (4.6) | 1 (4.2) | 5 (4.7) | |
| Indication | 0.327 | |||
| AF | 116 (88.5) | 20 (83.3) | 96 (89.7) | |
| VTE | 13 (9.9) | 3 (12.5) | 10 (9.3) | |
| Unknown | 2 (1.5) | 1 (4.2) | 1 (0.9) | |
| DOAC dosec | 0.354 | |||
| Standard dose | 48 (36.6) | 11 (45.8) | 37 (34.6) | |
| Reduced dose | 83 (63.4) | 13 (54.2) | 70 (65.4) | |
| Moderate CYP3A4 or P-gp inhibitors | 0.21 | |||
| Yes | 35 | 9 (37.5) | 26 (24.3) | |
| No | 96 | 15 (62.5) | 81 (75.7) |
Data are presented as n (%) or median (interquartile range)
AF atrial fibrillation, BMI body mass index, CYP cytochrome P450, DOAC direct oral anticoagulant, EI-ASM enzyme-inducing antiseizure medication, P-gp P-glycoprotein, VTE venous thromboembolism
aFor patients with more than one test, characteristics are presented for time of first DOAC measurement
bCalculated using Chi-squared and Fisher’s exact test for categorical measures and the Mann–Whitney U test for continuous measures
cDosing as per guidelines
Clinical Events
Of the 131 patients receiving DOACs, eight were admitted with stroke, one with PE, and none with DVT, all among the patients not treated with EI-ASMs (p = 0.21). The patient who presented with PE had a peak DOAC concentration below the range, whereas patients presenting with stroke had peak DOAC concentrations within the range. Following admission, five additional stroke events were recorded (one in a patient treated with an EI-ASM), and no additional PE or DVT events were observed.
Factors Associated with DOAC Peak Plasma Concentrations
The first measurement of DOAC concentration was used to determine the odds of the DOAC level being below the expected range.
Table 3 describes the bivariate associations between the first measurement of DOAC concentration and patients’ characteristics. Among the first measurement in the 131 patients, 91 tests (69.5%) were within the range, 19 tests (14.5%) were below the range, and 21 tests (16.0%) were above the range.
Table 3.
Bivariate relationship between patients’ characteristics (n = 131) and direct oral anticoagulant levels at first measurement
| Variable | Levels below expected range (n = 19 [14.5%]) | Levels within range (n = 91 [69.5%]) | Levels above expected range (n = 21 [16.0%]) | p valuea |
|---|---|---|---|---|
| Female | 5 (26.3) | 48 (52.7) | 13 (61.9) | 0.06 |
| Age (years) | 80 (72–87) | 80 (70–84) | 79 (74–86) | 0.90 |
| Weight (kg) | 72.8 (62.3–90.3) | 68.9 (59.5–81.3) | 65 (55.5–76.4) | 0.39 |
| Creatinine (mmol/L) | 90 (66–103.5) | 86.5 (70–123.8) | 110 (86.8–169.8) | 0.1 |
| DOAC doseb | 0.96 | |||
| Standard dose | 6 (31.6) | 34 (37.4) | 8 (38.1) | |
| Reduced dose | 13 (68.4) | 57 (62.6) | 13 (61.9) | |
| Patients treated with EI-ASM, n = 24 | 9 (37.5) | 15 (62.5) | 0 (0.0) | 0.0004 |
| Patients without EI-ASM, n = 107 | 10 (9.3) | 76 (71.0) | 21 (19.6) |
Data are presented as n (%) or median (interquartile range)
DOAC direct oral anticoagulant, EI-ASM enzyme-inducing antiseizure medication
aCalculated using Chi-squared and Fisher’s exact test for categorical measures, and Kruskal–Wallis test for continuous measures
bDosing as per guidelines
The proportion of patients with DOAC concentrations below the expected range was significantly higher among EI-ASM users than among non-users of EI-ASM (37.5 vs. 9.3%, respectively; OR 5.82; 95% CI 2.03–16.66; p = 0.0004).
In sensitivity analysis testing of this association, separately among patients treated with apixaban and among those with non-apixaban DOACs, EI-ASM use was associated with below-range DOAC concentrations in both subgroups (p = 0.004 and p = 0.031, respectively).
Factors Associated with Apixaban Peak Plasma Concentration
Among 111 patients treated with apixaban, 21 were treated with an EI-ASM, of whom six (28.6%) had concentrations below the range, whereas among the 90 not treated with an EI-ASM, only six (6.7%) had concentrations below the range (p = 0.004). Median apixaban peak concentration was 106 ng/mL (interquartile range [IQR] 71–181) in patients with an EI-ASM and 150 ng/mL (IQR 94–222) in patients without an EI-ASM (p = 0.019).
Apixaban concentrations among patients co-treated with EI-ASMs did not significantly differ between the various EI-ASMs (Kruskal–Wallis p = 0.305).
Of the 111 patients treated with apixaban, 33 were co-treated with moderate CYP3A/P-gp inhibitors. Apixaban peak plasma concentrations were significantly higher among patients treated with moderate CYP3A/P-gp inhibitors than among patients not treated with such inhibitors (median 172.5 [IQR 120.9–241.3] vs. 124.4 [79.2–206.4], respectively; p = 0.017, Mann–Whitney U test).
Multivariate Analyses
Factors Associated with Variability in Apixaban Concentrations
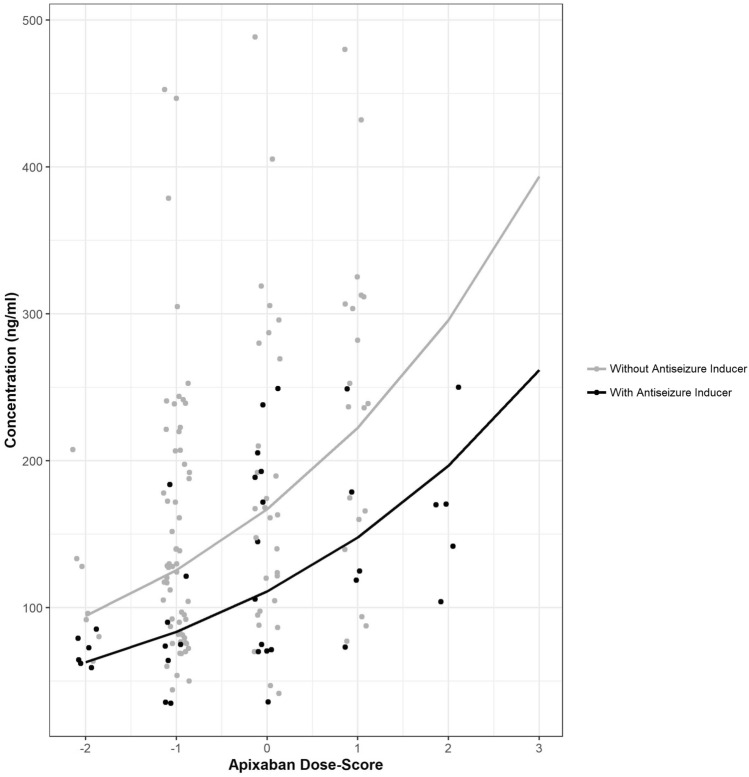
We used linear regression models to analyze factors associated with apixaban concentration (Table 4). The presence of EI-ASMs and moderate CYP3A4 inhibitors, as well as the ADS, were significantly associated with apixaban concentrations. EI-ASM use was significantly associated with reduced apixaban concentration (p<0.001) across the ADS range (Fig. 2).
Table 4.
The impact of enzyme-inducing antiseizure medications on apixaban levels (n = 152)
| Model | ADS | EI-ASMs | Moderate inhibitor |
|---|---|---|---|
| A | 0.28 (0.17–0.37)** | – | – |
| B | 0.29 (0.19–0.38)** | – 0.41 (– 0.63 to – 0.18)** | – |
| C | 0.28 (0.18–0.37)** | – 0.42 (– 0.64 to – 0.21)** | 0.26 (0.06–0.45)* |
Data are presented as coefficient (95% confidence interval). Model A evaluated the relation between log apixaban levels and apixaban dose score using a mixed-effects linear regression model. Model B included terms for ADS and EI-ASM. Model C included terms for ADS, EI-ASM, and moderate CYP3A4 inhibitors
ADS apixaban dose score, CYP cytochrome P450, EI-ASM enzyme-inducing antiseizure medications
*p < 0.05, **p < 0.001
Fig. 2.
Plot of concentration of apixaban by apixaban dose score, colored by presence of enzyme-inducing antiseizure medications (EI-ASM). Lines are population-level mixed-effect linear regression lines for apixaban log-transformed concentration by dose score stratified by the presence of inducing EI-ASM, and y-axis is apixaban concentration (n = 152)
Factors Associated with Odds of Apixaban Concentrations Below the Expected Range
In logistic regression models (Table 5) including ADS, the presence of EI-ASM, and the presence of moderate CYP3A4 inhibitors, only the presence of EI-ASM was associated with the odds for apixaban concentrations below the expected range, with more than a sixfold increase in odds (OR 6.26 [95% CI 2.19–17.90]; p < 0.001).
Table 5.
The impact of enzyme-inducing antiseizure medications on the odds of direct oral anticoagulant levels below the expected range (n = 152)
| Model | ADS | EI-ASM | Moderate inhibitor |
|---|---|---|---|
| A | 0.16 (0.02–1.06) | – | – |
| B | 0.80 (0.49–1.31) | 6.11 (2.19–17.0)* | – |
| C | 0.79 (0.48–1.28) | 6.26 (2.19–17.9)* | 0.23(0.05–1.09) |
Data are presented as odds ratio (95% confidence interval). Model A evaluated the association of levels below the range with apixaban dose score using a mixed-effects logistic regression model. Model B included terms for apixaban dose score and the presence of EI-AMSs. Model C included terms for apixaban dose score, EI-ASMs, and moderate CYP3A4 inhibitors
ADS apixaban dose score, CYP cytochrome P450, EI-ASM enzyme-inducing antiseizure medications
*p < 0.001
Discussion
Our study investigated factors associated with low DOAC peak plasma concentrations in patients treated with DOACs, including apixaban (85% of the patients), rivaroxaban, and dabigatran. The data indicated that use of EI-ASMs was significantly associated with DOAC concentrations below the expected range (OR 5.82 [95% CI 2.03–16.66]).
Among patients treated with apixaban, treatment with an EI-ASM remained associated with apixaban concentration independently from apixaban dose and criteria for dosage adjustment (as represented by ADS) and CYP3A4 moderate inhibitor use. The presence of EI-ASMs was associated with reduced apixaban concentrations, and the effect was consistent throughout the ADS range, as reflected by the effect of EI-ASMs on the intercept of the ADS–concentration curve (Fig. 2). As expected, the impact of ADS (reflecting dose reduction criteria for apixaban) on apixaban concentrations was statistically significant; however, in addition to the dose reduction criteria for apixaban, drug interactions with EI-ASMs were significantly correlated with reduced apixaban concentrations. Thus, the consumption of EI-ASMs may reflect variability in apixaban concentrations that is not currently addressed by dosage recommendations.
We have previously shown that concurrent use of EI-ASMs and apixaban or rivaroxaban is associated with increased odds of reporting anticoagulation treatment failure, including recurrent VTE and CVA, compared with concurrent use of ASMs that are not enzyme inducers and apixaban or rivaroxaban [16]. Other reports have also suggested that DOAC treatment failure is associated with low DOAC concentrations [6–9, 13, 15, 17]. However, there is poor concordance among drug compendia regarding the clinical significance of DOAC–EI-ASM interactions [36], indicating the need for real-world evidence and quality data [37].
The current study sheds light on the mechanism by which EI-ASMs may be associated with clinical thrombotic outcomes in patients treated with DOACs. Incorporating our results, this interaction is likely pharmacokinetic, resulting in low DOAC concentrations.
A major attraction of DOACs is their more predictable dose–response relationship and fewer drug–drug interactions compared with VKAs such as warfarin. Thus, DOAC treatment does not require routine monitoring of drug concentration or effect [26]. However, the lack of routine monitoring can be a disadvantage when significant drug interactions are suspected. In patients treated with VKAs, the interaction with EI-ASMs is well documented, and international normalized ratio monitoring in these patients can direct VKA dosing. However, similar strategies in DOACs have not been studied. Identifying factors associated with low DOAC concentrations can help tag patients at risk for treatment failure. Hence, these factors can direct clinicians to find alternatives to treatment with EI-ASMs or DOACs or select patients for DOAC concentration measurement.
Although DOACs have fewer drug–drug interactions than VKAs, they still have significant drug interactions that are related to the specific metabolic pathways of each agent. CYP enzymes, mainly CYP3A4, are responsible for apixaban and rivaroxaban metabolism. Thus, induction of these enzymes can result in reduced rivaroxaban and apixaban concentrations and effect. Rivaroxaban, apixaban, and dabigatran are substrates of P-gp. Induction of P-gp can also affect drug concentrations, as exhibited by concurrent use of rifampicin and dabigatran in healthy volunteers [25]. Our findings may be explained by induction of P-gp, CYP3A4, or both.
Our current findings support our previous study, which reported that the use of moderate CYP3A inhibitors was correlated with higher DOAC concentrations [38]. The concordance between the previous [38] and current data regarding the effect of enzyme inhibitors on DOAC concentrations may also validate the data used in the current analyses and our findings on the effect of EI-ASMs on DOAC concentrations.
In the current study, we did not observe a difference in clinical events (including stroke, DVT, and PE), either on admission or following hospitalization, between patients treated with EI-ASMs and patients not treated with EI-ASMs. This may be related both to the small number of events observed and to the effect of modifications in DOAC dosage performed following DOAC plasma concentration measurements during hospitalization. Previous studies by our group [16] and by others have suggested that DOAC treatment failure is associated with low DOAC concentrations [6–9, 13, 15, 17]. The current study was designed to examine the effect of EI-ASM treatment on DOAC plasma concentrations, and the results support the relationship between low DOAC peak concentrations and EI-ASM use. A larger study is required to determine the effect of DOAC concentration on the risk of thrombotic and embolic events in patients treated with EI-ASMs.
Concomitant use of EI-ASMs in patients treated with DOACs is not surprising, as significant comorbidity and polypharmacy is common in patients treated with DOACs. This combination can occur in the context of a stroke-induced seizure. Co-prescription of a DOAC and an EI-ASM has been reported to occur in 1.4% of patients in the hospital setting [21], warranting further research to evaluate the clinical implications of these potential drug interactions.
Limitations and Strengths
Our study has several limitations. First, it was retrospective and observational, so, although we observed an increased odds of DOAC plasma concentrations below the expected range with EI-ASM use, we cannot establish a causal relationship. Our study identified the correlation between EI-ASM use and apixaban concentration in a modest sample size, but, to our knowledge, this is the largest study addressing this question. Although similar associations were observed among patients with non-apixaban DOACs, the modest sample size precluded assessment of the association for each specific DOAC agent.
Our study was performed in a clinical setting, and indications for sampling were diverse. Thus, we excluded patients in whom blood samples were obtained in the context of acute bleeding, suspected overdose, or suspected non-compliance, and only included samples obtained after documented drug administration at a time corresponding to DOAC tmax.
DOAC plasma concentrations were calculated using calibrated anti-Xa and TT assays [26]. There are no validated target ranges for DOAC concentrations. Even so, therapeutic efficacy is doubtlessly compromised below some threshold. Indeed, clinical guidelines have suggested using the lower range of DOAC concentrations to guide the decisions regarding planned surgical intervention in patients at high bleeding risk receiving DOACs [26], and DOAC concentrations have been suggested to guide the use of recombinant tissue plasminogen activator in selected DOAC users presenting with acute ischemic stroke [39].
Our study also has several strengths. It includes the largest number of DOAC concentration tests in patients co-treated with an EI-ASM. We also used an ADS that included a combination of apixaban dose and apixaban dose-reduction criteria, resulting in a numerical scale that controlled for known factors affecting apixaban concentration. Thus, any additional factor, such as consumption of an EI-ASM or enzyme inhibitor, reflected variability not addressed by current dosage recommendations.
Regulatory Implications
Current recommendations for apixaban, rivaroxaban, and dabigatran warn against the concomitant use of these medications with EI-ASMs [26]. According to European Medicines Agency guidelines, the use of apixaban with strong CYP3A4-inducing drugs should be avoided in VTE treatment but can be used “with caution” in AF and VTE prophylaxis [40]. According to the European Cardiology Society, drug concentration measurement can be used to guide therapy in patients with significant drug interactions that cannot be avoided [26]. However, it is not specified whether “sub-therapeutic” DOAC concentrations can be an off-label indication for administration of higher DOAC doses. Our study, demonstrating reduced DOAC concentrations with EI-ASMs, provides the basis to support such a future strategy.
Conclusions
All currently approved DOACs are substrates of P-gp and/or CYP3A4; however, little is known regarding the management of DOACs in patients with commonly used antiseizure medications that induce the expression of P-gp and CYP3A4. Our study shows that this interaction results in potentially sub-therapeutic DOAC concentrations and supports recommendations that this drug combination should be avoided when possible. VKAs can be a useful alternative for patients taking EI-ASMs who need oral anticoagulation. Further study may determine the role of drug concentration measurement in patients in whom treatment with both a DOAC and an EI-ASM is considered necessary.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
None.
Conflict of interest
A Perlman is an employee at K-health Inc; the work presented in this manuscript is not related to his work at K-health. DE Singer is an employee of Massachusetts General Hospital. Dr. Singer has received research funding from Bristol Myers Squibb and has served as a paid consultant and/or member of advisory boards for Boehringer Ingelheim, Bristol Myers Squibb, Fitbit, Johnson and Johnson, Merck, and Pfizer. M Muszkat has received honoraria from Roche and a research grant from Pfizer Independent Global Medical Grant. R Goldstein, L Cohen, B Hirsh-Raccah, D Hakimian, I Matok, and Y Kalish have no conflicts of interest that are directly relevant to the content of this article.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The study was approved by the Hadassah Medical Organization Institutional Review Board and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent
This observational study was exempt from obtaining consent from the Hadassah Medical Organization institutional review board.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AP, RG, BH-R, LCC, DH, IM, YK, DS, and MM. The first draft of the manuscript was written by AP and RG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Footnotes
The original online version of this article was revised due to a retrospective Open Access order.
Amichai Perlman, Rachel Goldstein have contributed equally to this work.
Change history
8/3/2021
A Correction to this paper has been published: 10.1007/s40263-021-00844-7
References
- 1.Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc [Internet] 2016;15:4. doi: 10.1161/JAHA.116.003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 4.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 5.Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol. 2015;262:516–522. doi: 10.1007/s00415-014-7462-0. [DOI] [PubMed] [Google Scholar]
- 6.Macha K, Marsch A, Siedler G, Breuer L, Strasser EF, Engelhorn T, et al. Cerebral ischemia in patients on direct oral anticoagulants: plasma levels are associated with stroke severity. Stroke. 2019;50:873–879. doi: 10.1161/STROKEAHA.118.023877. [DOI] [PubMed] [Google Scholar]
- 7.Testa S, Paoletti O, Legnani C, Dellanoce C, Antonucci E, Cosmi B, et al. Low drug levels and thrombotic complications in high-risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16:842–848. doi: 10.1111/jth.14001. [DOI] [PubMed] [Google Scholar]
- 8.Bhagirath V, Eikelboom J, Hirsh J, Coppens M, Ginsberg J, Vanassche T, et al. Apixaban-calibrated anti-FXa activity in relation to outcome events and clinical characteristics in patients with atrial fibrillation: results from the AVERROES Trial. TH Open. 2017;01:e139–e145. doi: 10.1055/s-0037-1613679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Gennaro L, Lancellotti S, De Cristofaro R, De Candia E. Carbamazepine interaction with direct oral anticoagulants: help from the laboratory for the personalized management of oral anticoagulant therapy. J Thromb Thrombolysis. 2019;48:528–531. doi: 10.1007/s11239-019-01866-1. [DOI] [PubMed] [Google Scholar]
- 10.Hager N, Bolt J, Albers L, Wojcik W, Duffy P, Semchuk W. Development of Left Atrial Thrombus After Coadministration of Dabigatran Etexilate and Phenytoin. Can J Cardiol. 2017;33:554.e13–554.e14. doi: 10.1016/j.cjca.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Serra W, Li Calzi M, Coruzzi P. Left atrial appendage thrombosis during therapy with rivaroxaban in elective cardioversion for permanent atrial fibrillation. Clin Pract [Internet]. 2015 [cited 2020 Jun 7];5. http://www.clinicsandpractice.org/index.php/cp/article/view/788. Accessed 1 Oct 2020. [DOI] [PMC free article] [PubMed]
- 12.Burden T, Thompson C, Bonanos E, Medford AR. Lesson of the month 2: pulmonary embolism in a patient on rivaroxaban and concurrent carbamazepine. Clin Med. 2018;18:103–105. doi: 10.7861/clinmedicine.18-1-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King PK, Stump TA, Walkama AM, Ash BM, Bowling SM. Management of phenobarbital and apixaban interaction in recurrent cardioembolic stroke. Ann Pharmacother. 2018;52:605–606. doi: 10.1177/1060028018759938. [DOI] [PubMed] [Google Scholar]
- 14.Risselada AJ, Visser MJ, van Roon EN. Pulmonary embolism due to interaction between rivaroxaban and carbamazepine. Ned Tijdschr Geneeskd. 2013;157:A6568. [PubMed] [Google Scholar]
- 15.Stöllberger C, Finsterer J. Recurrent venous thrombosis under rivaroxaban and carbamazepine for symptomatic epilepsy. Neurol Neurochir Pol. 2017;51:194–196. doi: 10.1016/j.pjnns.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Perlman A, Wanounou M, Goldstein R, Choshen Cohen L, Singer DE, Muszkat M. Ischemic and thrombotic events associated with concomitant Xa-inhibiting direct oral anticoagulants and antiepileptic drugs: analysis of the FDA Adverse Event Reporting System (FAERS) CNS Drugs. 2019;33:1223–1228. doi: 10.1007/s40263-019-00677-5. [DOI] [PubMed] [Google Scholar]
- 17.Dagan G, Perlman A, Hochberg-Klein S, Kalish Y, Muszkat M. Managing direct oral anticoagulants in patients with antiepileptic medication. Can J Cardiol. 2018;34:1534.e1–1534.e3. doi: 10.1016/j.cjca.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Chin PKL, Wright DFB, Zhang M, Wallace MC, Roberts RL, Patterson DM, et al. Correlation between trough plasma dabigatran concentrations and estimates of glomerular filtration rate based on creatinine and cystatin C. Drugs RD. 2014;14:113–123. doi: 10.1007/s40268-014-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiggins BS, Northup A, Johnson D, Senfield J. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacother J Hum Pharmacol Drug Ther. 2016;36:e5–7. doi: 10.1002/phar.1698. [DOI] [PubMed] [Google Scholar]
- 20.Becerra AF, Amuchastegui T, Tabares AH. Decreased rivaroxaban levels in a patient with cerebral vein thrombosis receiving phenytoin. Case Rep Hematol. 2017;2017:1–3. doi: 10.1155/2017/4760612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perlman A, Hochberg-Klein S, Choshen Cohen L, Dagan G, Hirsh-Raccah B, Horwitz E, et al. Management strategies of the interaction between direct oral anticoagulant and drug-metabolizing enzyme inducers. J Thromb Thrombolysis. 2019;47:590–595. doi: 10.1007/s11239-018-01804-7. [DOI] [PubMed] [Google Scholar]
- 22.Vakkalagadda B, Frost C, Byon W, Boyd RA, Wang J, Zhang D, et al. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of factor Xa. Am J Cardiovasc Drugs. 2016;16:119–127. doi: 10.1007/s40256-015-0157-9. [DOI] [PubMed] [Google Scholar]
- 23.Mueck W, Schwers S, Stampfuss J. Rivaroxaban and other novel oral anticoagulants: pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring. Thromb J. 2013;11:10. doi: 10.1186/1477-9560-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendell J, Chen S, He L, Desai M, Parasramupria DA. The effect of rifampin on the pharmacokinetics of edoxaban in healthy adults. Clin Drug Investig. 2015;35:447–453. doi: 10.1007/s40261-015-0298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Härtter S, Koenen-Bergmann M, Sharma A, Nehmiz G, Lemke U, Timmer W, et al. Decrease in the oral bioavailability of dabigatran etexilate after co-medication with rifampicin. Br J Clin Pharmacol. 2012;74:490–500. doi: 10.1111/j.1365-2125.2012.04218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 27.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Perlman A, Horwitz E, Hirsh-Raccah B, Aldouby-Bier G, Fisher Negev T, Hochberg-Klein S, et al. Clinical pharmacist led hospital-wide direct oral anticoagulant stewardship program. Isr J Health Policy Res. 2019;8:19. doi: 10.1186/s13584-019-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rottenstreich A, Zacks N, Kleinstern G, Raccah BH, Roth B, Da’as N, et al. Direct-acting oral anticoagulant drug level monitoring in clinical patient management. J Thromb Thrombolysis. 2018;45:543–549. doi: 10.1007/s11239-018-1643-0. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eikelboom JW, Quinlan DJ, Hirsh J, Connolly SJ, Weitz JI. Laboratory monitoring of non-vitamin k antagonist oral anticoagulant use in patients with atrial fibrillation: a review. JAMA Cardiol. 2017;2:566. doi: 10.1001/jamacardio.2017.0364. [DOI] [PubMed] [Google Scholar]
- 32.Gosselin R, Adcock D, Bates S, Douxfils J, Favaloro E, Gouin-Thibault I, et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118:437–450. doi: 10.1055/s-0038-1627480. [DOI] [PubMed] [Google Scholar]
- 33.Cytochrome P-450 CYP3A4 Inducers. DrugBank [Internet]. [cited 2020 Jun 9]. https://www.drugbank.ca/categories/DBCAT003896. Accessed 9 June 2020.
- 34.Cytochrome P-450 CYP3A4 Inhibitors (moderate). DrugBank [Internet]. [cited 2020 Jul 1]. https://www.drugbank.ca/categories/DBCAT002648. Accessed 1 July 2020.
- 35.Raval AN, Cigarroa JE, Chung MK, Diaz-Sandoval LJ, Diercks D, Piccini JP, et al. Management of patients on non-vitamin K Antagonist oral anticoagulants in the acute care and periprocedural setting: a scientific statement from the American Heart Association. Circulation [Internet]. 2017;13:5. doi: 10.1161/CIR.0000000000000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acton EK, Willis AW, Gelfand MA, Kasner SE. Poor concordance among drug compendia for proposed interactions between enzyme-inducing antiepileptic drugs and direct oral anticoagulants. Pharmacoepidemiol Drug Saf. 2019;28:1534–1538. doi: 10.1002/pds.4896. [DOI] [PubMed] [Google Scholar]
- 37.Galgani A, Palleria C, Iannone LF, De Sarro G, Giorgi FS, Maschio M, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. doi: 10.3389/fneur.2018.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsh Raccah B, Rottenstreich A, Zacks N, Muszkat M, Matok I, Perlman A, et al. Drug interaction as a predictor of direct oral anticoagulant drug levels in atrial fibrillation patients. J Thromb Thrombolysis. 2018;46:521–527. doi: 10.1007/s11239-018-1738-7. [DOI] [PubMed] [Google Scholar]
- 39.Drouet L, Bal dit Sollier C, Steiner T, Purrucker J. Measuring non-vitamin K antagonist oral anticoagulant levels: When is it appropriate and which methods should be used? Int J Stroke. 2016;11:748–758. doi: 10.1177/1747493016659671. [DOI] [PubMed] [Google Scholar]
- 40.Eliquis-EPAR Product information [Internet]. European Medicines Agency; [cited 2020 Jun 9].: https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf. Accessed 9 June 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.