ABSTRACT
Many conditions result in chronic interstitial lung disease (ILD), being classified as fibrosing ILDs, including idiopathic pulmonary fibrosis, connective tissue diseases, sarcoidosis, and fibrotic hypersensitivity pneumonitis. HRCT plays an important role in the clinical evaluation of fibrosing ILDs. Current treatment perspectives are encouraging and reinforce the need for HRCT scans of adequate technical quality for early detection of fibrosing ILD. Despite efforts in this regard, the significance and management of imaging findings of early interstitial lung abnormalities have yet to be clarified. After identification of CT findings consistent with fibrosing ILD, radiologists must be able to identify characteristic morphological patterns and, in some cases, features of specific clinical entities. In cases in which HRCT features are not sufficiently specific for a definitive diagnosis, HRCT can aid in selecting the best site for surgical lung biopsy. CT follow-up is useful for identifying progressive fibrosing ILDs and detecting complications unrelated to the underlying disease, including infections, acute exacerbations, and neoplasms. Automated quantification tools have clinical applicability and are likely to be available for use in imaging analysis in the near future. In addition, incorporation of CT evaluation into scoring systems based on clinical and functional parameters for staging fibrosing disease is likely to become valuable in determining prognosis. Knowledge of the clinical applications of CT evaluation is essential for specialists managing patients with fibrosing ILD and can have a positive impact on the clinical course of the disease.
Keywords: Tomography, X-ray computed; Diagnostic imaging; Pulmonary fibrosis
RESUMO
Inúmeras doenças determinam dano intersticial crônico no parênquima pulmonar e são agrupadas com a denominação de pneumopatias intersticiais fibrosantes, incluindo fibrose pulmonar idiopática, doenças do colágeno, sarcoidose, pneumonite por hipersensibilidade fibrótica etc. Entre os métodos complementares à avaliação clínica, a TCAR tem um papel relevante. Perspectivas atuais de tratamento são encorajadoras e reforçam a necessidade de realização de estudos com técnica adequada, visando a detecção confiável de acometimento intersticial fibrosante o mais precocemente possível. Embora esforços tenham sido direcionados nesse sentido, o significado e manejo de anormalidades pulmonares intersticiais incipientes, detectadas nos estudos de imagem, ainda não são claros. Uma vez detectado o acometimento fibrosante, é importante que o radiologista conheça aspectos característicos de determinados padrões morfológicos e reconheça elementos que possam apontar para entidades clínicas específicas. Em casos nos quais a especificidade dos achados não é suficiente para a suspeição diagnóstica, as imagens de TC servem de guia para a escolha de sítios para biópsia cirúrgica. O seguimento evolutivo é útil para a determinação de pneumopatias fibrosantes progressivas e para a detecção de complicações não relacionadas à doença de base, como infecções, exacerbação aguda e neoplasias. Ferramentas automatizadas de quantificação têm aplicabilidade clínica e devem estar acessíveis para a análise imagética no futuro próximo. Além disso, a incorporação da avaliação tomográfica a escores com parâmetros clínicos e funcionais de estadiamento do acometimento fibrosante poderá se tornar valiosa na determinação prognóstica. O conhecimento das diversas aplicabilidades clínicas do método tomográfico é fundamental aos especialistas que acompanham esses pacientes, podendo impactar positivamente sua trajetória clínica.
Descritores: Tomografia computadorizada por raios X, Diagnóstico por imagem, Fibrose pulmonar
INTRODUCTION
Fibrosis is the final consequence of cell injury, matrix injury, or both by a variety of mechanisms, including trauma, thermal injury, chemical injury, hypoxia, and immune-mediated injury. 1 In the lung parenchyma, repeated alveolar injury leads to fibrosing interstitial lung diseases (ILDs), including idiopathic pulmonary fibrosis (IPF), connective tissue disease (CTD)-associated fibrosing ILD (CTD-fILD), and fibrotic hypersensitivity pneumonitis (HP), as well as less common diseases such as idiopathic nonspecific interstitial pneumonia (NSIP), Langerhans cell histiocytosis, tobacco-related diseases, sarcoidosis, Erdheim-Chester disease, Hermansky-Pudlak syndrome, asbestosis, silicosis, drug reactions, and IgG4-related sclerosing disease. 1 , 2 Some patients develop a progressive phenotype characterized by self-sustaining fibrosis, decline in lung function, worsening quality of life, and, ultimately, early mortality, being designated progressive fibrosing ILD. 2
The differential diagnosis of fibrosing ILDs is difficult because clinical, radiological, and pathological characteristics often overlap, a multidisciplinary approach therefore being required. 3 - 5 In this context, it is essential to establish a definitive diagnosis because nonpharmacological and pharmacological treatment approaches (including corticosteroids, immunosuppressants, and, more recently, antifibrotic agents) are disease-specific. 6 - 8
The role of imaging in the diagnosis of fibrosing ILDs has evolved in recent decades, and HRCT currently plays an essential role in the diagnostic evaluation of fibrosing ILDs, being useful in the initial evaluation of fibrosing ILDs, in making a decision regarding the use of invasive diagnostic procedures, and in patient follow-up. The objective of the present study was to review the role of HRCT in the diagnostic evaluation of fibrosing ILDs.
DIAGNOSIS OF FIBROSING DISEASE
Technical parameters of CT examinations
CT scans of adequate technical quality are essential for accurate interpretation of ILD findings, low-quality CT images leading to misses and misinterpretations. 9 For volumetric image acquisition, the following parameters should be used: a) submillimetric collimation; b) thin-slice reconstructions (≤ 1.5 mm) with the use of a high-resolution filter; c) shortest rotation time and highest pitch, in order to reduce image acquisition time and movement artifacts; and d) use of tools for optimizing/reducing radiation dose. 10 , 11
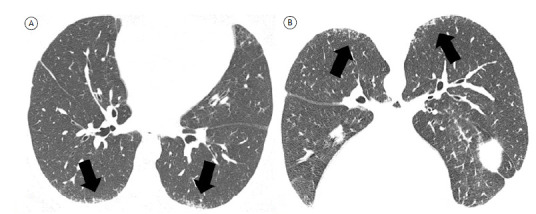
The first acquisition is obtained at maximal inspiration, volumetric CT acquisition being preferred because it allows multiplanar image reconstruction in the post-processing stage, clarifying disease distribution, facilitating differentiation between honeycombing and traction bronchiolectasis, and optimizing comparison with follow-up images. 10 - 12 The technical staff should be trained in providing patients with simple, clear respiratory instructions for chest CT examinations, the voice of the technician being preferred to the automatic instructions from the machine because submaximal inspiratory maneuvers can lead to misinterpretation of ground-glass attenuation and reticulation. 9 , 10 The second acquisition is obtained at end-expiration and is useful for identifying mosaic attenuation, which is an important finding in the diagnosis of fibrotic HP. 10 A third acquisition can be obtained with the patient in the prone position; it can be sequential or volumetric and can be limited to the lower lobes. 11 Prone CT imaging is useful in patients with clinical suspicion of fibrosing disease and only minor lung involvement, with normal or minimally abnormal chest X-rays, and particularly for distinguishing between position-induced changes and initial interstitial changes (Figure 1). 10
Figure 1. Axial HRCT scans with lung window settings. Note diffuse peripheral lower lobe reticular opacities (arrows in A) that are also seen on prone CT images (arrows in B), characterizing incipient interstitial lung disease.
CT findings
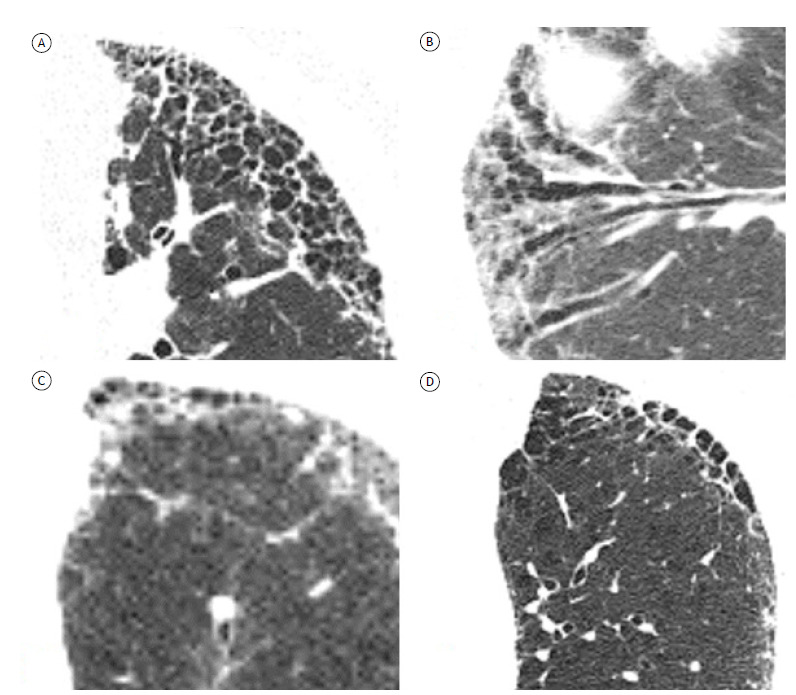
Common HRCT findings of fibrosing disease include reticular opacities, ground-glass opacities, traction bronchiolectasis, and honeycombing, the proportion and distribution of which vary. 10 , 11 Traction bronchiolectasis is an important finding for the diagnosis of fibrosing ILD and often overlaps with reticular or ground-glass opacities in patients with fibrosing lung disease. It is difficult to distinguish between traction bronchiolectasis and honeycombing in some cases, with significant interobserver variation (Figure 2). 13 The term honeycombing refers to clustered cystic airspaces of typically 3-10 mm in diameter with thick, well-defined walls. 14 , 15 Although honeycombing typically presents as multiple layers of cysts, a single layer of two or three cysts is enough for a diagnosis of honeycombing. 10 Ground-glass attenuation superimposed on reticular opacities or traction bronchiectasis can be seen in patients with fibrosing ILD. 10 However, the presence of “pure” ground-glass attenuation (i.e., without reticular opacities) might be related to inflammatory activity, and the presence of new bilateral ground-glass opacities in a patient with fibrosing ILD should raise the possibility of an acute exacerbation. 16 A crazy-paving pattern, micronodules, consolidations, and cysts are encountered in specific entities.
Figure 2. Axial HRCT scans with lung window settings, showing fibrosing interstitial lung disease in different patients. In A, typical honeycombing, presenting as multiple layers of cysts. In B, traction bronchiectasis in an oblique coronal plane. Note the usefulness of multiplanar reconstruction in differentiating between traction bronchiectasis and honeycombing. In C, early honeycombing, presenting as a single layer of cysts. In D, note the difficulty in differentiating between honeycombing and paraseptal emphysema.
Interstitial lung abnormalities and difficulties in early diagnosis
The widespread use of HRCT as a diagnostic tool has increased the detection of interstitial lung abnormalities (ILAs), which are incidental findings potentially consistent with ILD in individuals who are not clinically suspected of having ILD and who may or may not have clinical symptoms and functional limitations. 17 , 18 ILAs represent early stages of fibrosing ILD in some cases and are of particular interest because antifibrotic therapy has been shown to slow the progression of IPF even in individuals with less extensive disease. 19
ILAs are nondependent HRCT abnormalities affecting more than 5% of any lung zone (upper, middle, and lower lung zones being demarcated by the levels of the inferior aortic arch and right inferior pulmonary vein). CT findings in patients with ILAs include ground-glass opacities, reticular opacities, architectural distortion, traction bronchiectasis, honeycombing, and nonemphysematous cysts. 18 Morphological findings unrelated to ILAs include dependent lung atelectasis, focal paraspinal fibrosis in close contact with thoracic spine osteophytes, smoking-related centrilobular opacities in the absence of other findings, mild focal or unilateral opacities, interstitial edema (associated with congestive heart failure), and findings of aspiration (patchy ground-glass opacities and a tree-in-bud pattern). 18 ILAs are classified as follows: a) nonsubpleural; b) subpleural nonfibrotic (without architectural distortion, traction bronchiectasis, or honeycombing); and c) subpleural fibrotic (with architectural distortion and traction bronchiectasis, honeycombing, or both). 18
In patients with ILAs, risk factors for progression to ILD include clinical risk factors (smoking, other inhalational exposures, medications, radiation therapy, previous thoracic surgery, and changes in pulmonary function tests) and specific radiological features. In a study by Putman et al., 20 the presence of subpleural reticular opacities in the lower lobes and traction bronchiectasis significantly increased the likelihood of ILA progression, and all of the cases in which honeycombing was present progressed to ILD over the course of 5 years. Specific recommendations regarding management and follow-up have been published elsewhere. 18
DIFFERENTIAL DIAGNOSIS BASED ON CT FINDINGS: MORPHOLOGICAL PATTERNS AND SPECIFIC CLINICAL ENTITIES
After identification of CT findings consistent with fibrosing ILD, HRCT has an important role in narrowing diagnostic possibilities by identifying characteristic morphological patterns or, in some cases, indicating specific clinical entities (such as HP, sarcoidosis, and asbestosis). HRCT findings should be interpreted in the context of an integrated multidisciplinary approach, allowing a definitive diagnosis based on clinical and radiological findings in some cases and reinforcing the need for invasive diagnostic procedures in less typical cases. 3 , 4 After morphological evaluation by HRCT or surgical biopsy, potential causative factors such as environmental exposures, CTDs, and use of medications should be evaluated clinically. 4 In some cases, a final diagnosis will not be achieved for a number of reasons, including inadequate clinical, radiological, or pathological data and major discordance between clinical, radiological, and pathological findings; such cases should be classified under the category of “unclassifiable” idiopathic interstitial pneumonia. 4
Major morphological patterns of fibrosing ILD
Usual interstitial pneumonia
One of the key roles that HRCT plays in the diagnostic evaluation of fibrosing ILDs is determining the likelihood of a usual interstitial pneumonia (UIP) pattern. 3 , 4 , 10 , 11 A histopathological pattern of UIP is associated with multiple diagnoses, including drug-induced lung diseases, occupational diseases, CTDs, HP, and IPF. 3 , 21
An official American Thoracic Society (ATS), European Respiratory Society (ERS), Japanese Respiratory Society (JRS), and Asociación Latinoamericana de Tórax (ALAT, Latin American Thoracic Association) clinical practice guideline recommends that, in patients with clinical suspicion of IPF, HRCT features of the UIP pattern be categorized as follows (Figure 3):
Figure 3. Axial HRCT scans with lung window settings, showing fibrosing interstitial lung disease in patients suspected of having idiopathic pulmonary fibrosis and presenting with different HRCT features of the usual interstitial pneumonia (UIP) pattern. In A, UIP pattern, with extensive honeycombing (arrows), peripheral distribution, and basal predominance. In B, probable UIP pattern, with peripheral reticular opacities and traction bronchiolectasis (open arrows), without honeycombing. In C, indeterminate-for-UIP pattern, with diffuse axial distribution and central involvement (arrowheads), as well as areas of ground-glass opacity, together with reticular opacities. In D, a pattern suggestive of an alternative diagnosis, with extensive areas of ground-glass opacity and a mosaic pattern of lung attenuation (open arrowheads).
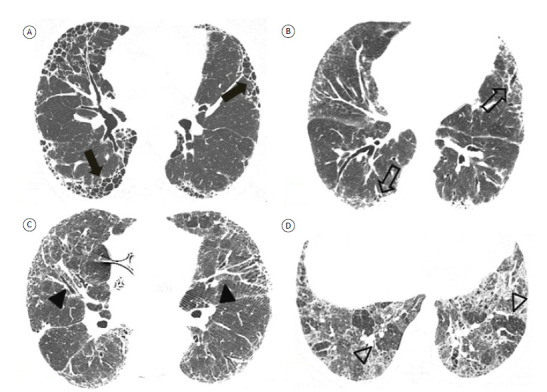
a) UIP pattern-the typical UIP HRCT pattern, with reticular opacities, traction bronchiolectasis, and honeycombing with peripheral distribution and basal predominance. There is high concordance between an HRCT pattern of UIP and a histopathological pattern of UIP. 10 , 11
b) probable UIP pattern-an HRCT pattern with reticular opacities and traction bronchiolectasis with peripheral distribution and an apicobasal gradient, without honeycombing. Studies have shown a high correlation between an HRCT pattern of probable UIP and a histopathological pattern of UIP. 10 , 11
c) indeterminate-for-UIP pattern-HRCT patterns that do not meet the criteria for a UIP or probable UIP pattern and that are not sufficiently characteristic to suggest a specific diagnosis. An HRCT pattern indeterminate for UIP includes mild ground-glass opacities that are not clearly more extensive than and are dissociated from reticular opacities, as well as ill-defined areas of mosaic attenuation or diffuse axial/zonal distribution. 22 , 23 Patients with evident but mild fibrosing disease, presumably related to early fibrosing changes, should also be included in this category, prone CT imaging being recommended to differentiate between dependent opacities and initial interstitial changes.
d) HRCT pattern suggestive of an alternative diagnosis-HRCT findings suggestive of diagnoses other than UIP/IPF, including atypical findings (including consolidation, extensive ground-glass attenuation in the absence of an exacerbation, well-defined mosaic attenuation, nodules, and cysts), atypical distribution (including upper-/mid-lung predominance, peribronchovascular involvement, and subpleural sparing), and other findings (including pleural plaques, pleural effusion, esophageal dilation, and extensive lymph node enlargement).
In specific contexts, a diagnosis of IPF can be made on the basis of clinical-radiological correlations; in other contexts, it can be made by correlating HRCT and biopsy findings. 10 , 11
NSIP
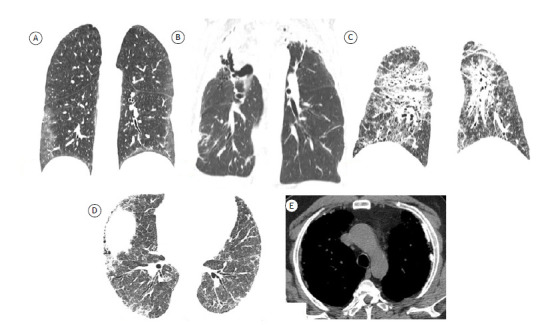
The NSIP pattern is associated with several diseases, including CTD-fILD, HP, drug-induced lung disease, infections, immunodeficiencies, and idiopathic ILD. 3 HRCT features suggestive of NSIP include ground-glass attenuation, reticular opacities, and traction bronchiolectasis in a predominantly basal and peripheral or diffuse distribution (Figure 4). Honeycombing is an uncommon finding, seen only in advanced cases. 3 , 24 , 25 Findings of NSIP and UIP overlap in some patients, subpleural sparing being suggestive of an NSIP pattern on HRCT. 24 , 25
Figure 4. Axial HRCT scans of the chest showing features related to different fibrosing diseases. In A, features suggestive of nonspecific interstitial pneumonia, with a predominance of ground-glass opacities. In B, features of pleuroparenchymal fibroelastosis confirmed by histopathology showing predominantly apical fibrosing disease with upper lobe volume loss and upward hilar retraction. In C, scan of a patient with sarcoidosis, showing fibrosing disease with an upper-lobe predominance. In D and E, scans of a patient with fibrosing lung disease caused by exposure to asbestos. Note the presence of pleural plaques (arrows in E).
Other morphological patterns of fibrosing ILD
There are several other morphological patterns of fibrosing ILD. Pleuroparenchymal fibroelastosis is a recently described rare fibrosing disease that can be idiopathic or associated with a variety of conditions, including complications of bone marrow or lung transplantation, autoimmune diseases, CTDs, infections (with Aspergillus spp. or nontuberculous mycobacteria), use of cancer treatment drugs, and occupational exposures. Typical HRCT findings include pleural thickening and subpleural consolidations with traction bronchiectasis, architectural distortion, and volume loss, typically in the upper lobes (Figure 4). Pneumothorax and recurrent infections are common in patients with pleuroparenchymal fibroelastosis, with disease progression occurring in most cases (60%). 4 , 26
Despite being classified as an acute/subacute interstitial pneumonia and the fact that the majority of patients recover completely with oral corticosteroids, organizing pneumonia can progress to residual or progressive interstitial fibrosis. 4 It is likely that some patients with fibrotic NSIP fall into this subgroup of patients. A mixed pattern of NSIP and organizing pneumonia can be seen in patients with antisynthetase syndrome-associated myositis. 4 , 27 , 28 Fibrotic patterns can also be observed in patients surviving episodes of (idiopathic or secondary) late-stage diffuse alveolar damage; architectural distortion, traction bronchiectasis, and cysts can occur in such patients, predominantly in nondependent lung regions. 3 , 4
Morphological patterns such as lymphocytic interstitial pneumonia, desquamative interstitial pneumonia, and smoking-related changes (airspace enlargement with fibrosis) can also be related to fibrotic HRCT features. 3 , 4
Specific clinical entities
Fibrotic HP
Fibrotic HP is a fibrosing ILD resulting from chronic exposure to specific antigens, the definitive diagnosis of which depends on a multidisciplinary approach. HRCT plays a central role in various algorithms for the diagnosis of fibrotic HP, 29 - 32 as well as in an official ATS/JRS/ALAT clinical practice guideline 33 for the diagnosis of fibrotic HP in adults. An HRCT pattern of fibrosing ILD and evidence of small airway disease are suggestive of a diagnosis of fibrotic HP. 33 Fibrosis is most severe in the mid or mid and lower lung zones or equally distributed in the three lung zones with relative basal sparing; on axial images, there is often no central or peripheral predominance of lung fibrosis. 33 Honeycombing can be present in a subpleural or peribronchovascular distribution and, less frequently, with a basal predominance. 24 HRCT features of small airway disease include centrilobular opacities, mosaic attenuation, air trapping, and the “three-density pattern” (formerly known as the “headcheese sign,” a combination of three attenuations on inspiratory CT images, i.e., areas of normal lung parenchyma, areas of ground-glass attenuation, and areas of hyperlucency, indicating an association between infiltrative lung disease and airway obstruction). 33 , 34 Mosaic attenuation has a high diagnostic value, particularly in pulmonary parenchymal segments without overt fibrosis.(10,24,29- 32) One recent study showed that the three-density pattern is specific for fibrotic HP, highlighting that areas of decreased attenuation can be seen in cases of IPF as well. 35 Other morphological patterns can be seen, including typical UIP and NSIP. 36 According to current guidelines, HRCT features in patients suspected of having fibrotic HP should be categorized as follows (Figure 5):
Figure 5. Axial HRCT scans with lung window settings (in A, D, and G), coronal reconstructions (in B, E, and H), and coronal minimum intensity projection reformatted images (in C, F, and I) for three patients with fibrotic hypersensitivity pneumonitis (HP). In A, B, and C, a typical HP pattern, with diffuse interstitial fibrosis axially and relatively spared lower lung zones, as well as marked mosaic attenuation in a predominantly caudal distribution, indicating small airway disease (in C). In D, E, and F, a compatible-with-HP pattern, with predominantly peripheral, basal interstitial fibrosis and extensive honeycombing (characteristic of usual interstitial pneumonia), as well as scattered areas of mosaic attenuation, constituting evidence of small airway disease (in F). In G, H, and I, an indeterminate-for-HP pattern, with areas of ground-glass opacity, reticular opacities, and traction bronchiectasis in a diffuse axial/craniocaudal distribution, as well as no evidence of small airway disease.
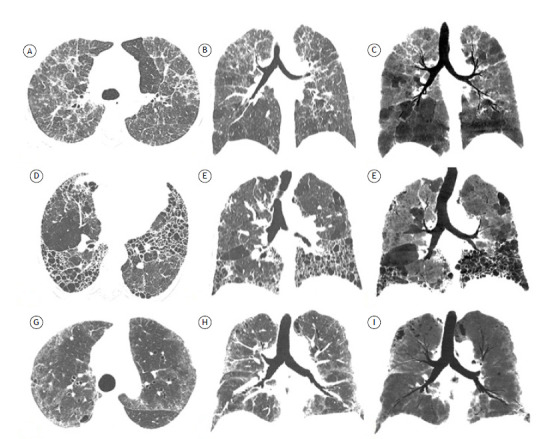
a) typical HP pattern-diffuse interstitial fibrosis with middle lung predominance or basal sparing, as well as abnormalities indicative of small airway disease.
b) compatible-with-HP pattern-variant patterns of interstitial fibrosis (UIP pattern and extensive ground-glass opacities with incipient fibrosis), peribronchovascular distribution with subpleural areas axially and upper lung predominance craniocaudally, as well as abnormalities indicative of small airway disease.
c) indeterminate-for-HP pattern-lone patterns (i.e., not accompanied by other findings suggestive of HP), including UIP pattern, probable UIP pattern, indeterminate pattern for UIP, fibrotic NSIP pattern, organizing pneumonia-like pattern, and truly indeterminate HRCT patterns.
Ancillary diagnostic tests such as bronchoalveolar lavage and surgical biopsy can be performed when a reliable diagnosis cannot be made on the basis of clinical and radiological analysis. 30 , 33
CTD-fILD
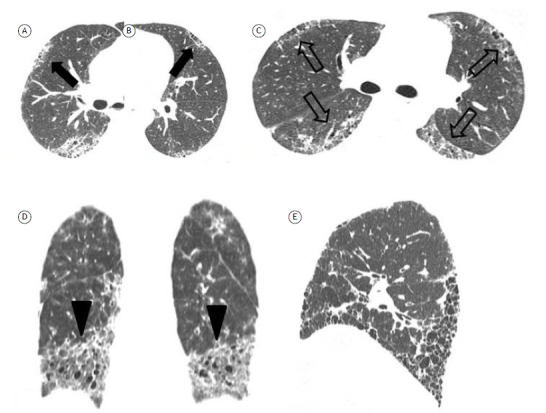
ILDs are associated with multiple CTDs, with varying prevalence across diseases. The estimated prevalence of HRCT findings consistent with ILD is 70-90% in systemic sclerosis, 15-70% in inflammatory myopathies (being more common in patients with antisynthetase antibodies), 4-68% in rheumatoid arthritis, 20-85% in mixed CTD, 10-30% in Sjögren’s syndrome, and up to 30% in systemic lupus erythematosus. 37 Although the patterns of fibrosis in CTD-fILD and other fibrosing ILDs are similar, Chung et al. 38 identified specific CT signs that are more common in the former than in the latter: the “anterior upper lobe” sign-concentration of fibrosis within the anterior aspect of the upper lobes (with relative sparing of the other aspects of the upper lobes) and concomitant lower lobe involvement; the “exuberant honeycombing” sign-extensive honeycomb-like cyst formation within the lungs constituting greater than 70% of the fibrotic portions of lung; and the “straight-edge” sign-isolation of fibrosis to the lung bases with sharp demarcation in the craniocaudal plane without substantial extension along the lateral margins of the lungs on coronal images (Figure 6). The authors 38 compared patients with UIP associated with IPF and patients with UIP associated with CTD-fILD (rheumatoid arthritis or systemic sclerosis in most cases). The positive predictive values of the anterior upper lobe, exuberant honeycombing, and straight-edge signs were 1.99 (p = 0.028), 3.69 (p < 0.001), and 4.22 (p < 0.001), respectively, and the signs were most common in patients with systemic sclerosis. Similarly, Walkoff et al. 39 evaluated the specificity of the “four corners” sign-pulmonary inflammation and/or fibrosis focally or disproportionately involving the bilateral anterolateral upper lobes and posterosuperior lower lobes-in a sample of patients with IPF and systemic sclerosis, and found a significant association between a confidently present four corners sign and a diagnosis of systemic sclerosis.
Figure 6. Specific CT signs of fibrosis in connective tissue disease-associated fibrosing interstitial lung disease. In A and B, the “anterior upper lobe” sign (arrows) in a patient with rheumatoid arthritis. In C, the “four corners” sign (open arrows)-fibrosis focally involving the bilateral anterolateral upper lobes and posterosuperior lower lobes-in a patient with systemic sclerosis. In D, the “straight-edge” sign (arrowheads)-isolation of fibrosis to the lung bases with sharp demarcation in the craniocaudal plane. In E, the “exuberant honeycombing” sign-extensive honeycombing constituting most of the fibrotic portions of lung.
Sarcoidosis
Approximately 20% of patients with sarcoidosis will develop fibrosing ILD over the course of the disease, with fibrocystic changes, including honeycombing. Typical fibrotic HRCT features include upper lobe and peribronchovascular fibrosis, as well as posterior retraction of the hila, together with mediastinal lymph node enlargement (Figure 4). 11 , 40
Asbestosis
Asbestos-related fibrosing ILD usually occurs 20 years or more after exposure. The changes of asbestosis are more pronounced in the lower lobes and subpleurally, and honeycombing can occur, albeit only in advance cases; imaging findings of asbestosis can be indistinguishable from those of various clinical entities, including IPF. 41 , 42 Akira et al. 42 studied HRCT scans of patients with asbestosis or IPF and concluded that subpleural dot-like or branching opacities, subpleural curvilinear lines, mosaic attenuation, and parenchymal bands are significantly more common in the former than in the latter. Although the parenchymal abnormalities found in patients with asbestosis can also be found in patients with other diseases, pleural plaques are characteristic of exposure to asbestos, being found in up to 80% of patients with asbestosis on radiographic studies (Figure 4). 41 The diagnosis of asbestosis is based on a thorough clinical and occupational history.
ROLE OF HRCT IN SELECTING THE BEST SITE FOR SURGICAL LUNG BIOPSY
The decision to perform a surgical lung biopsy must be made by a multidisciplinary team with experience in ILDs. In the diagnosis of IPF in particular, current guidelines recommend that surgical lung biopsy be performed in newly diagnosed ILD patients who have an HRCT pattern indeterminate for UIP or suggestive of an alternative diagnosis, conditional recommendations being made for performing surgical lung biopsy in those with a pattern of probable UIP. 10 , 11 , 43
When surgical lung biopsy is indicated, multiple biopsies should be obtained from two to three lobes, because the histological patterns on specimens obtained from different segments can be discordant. 10 , 11 HRCT is useful for prebiopsy evaluation and selection of the best sampling sites. In order to avoid the possibility that a biopsy specimen was taken from an area not representative of the predominant disease process, biopsy specimens should be obtained from areas that reflect the full spectrum of disease patterns as guided by HRCT evaluation or intraoperative inspection of the lung surface. Areas of honeycombing should be avoided because they show end-stage disease and are likely to be of little use when establishing a diagnosis. Areas that show intermediate or relatively preserved lung parenchyma, or areas of ground-glass opacity should ideally be selected for biopsy because the lung specimen must have fibrotic lung adjacent to normal lung for the pathologist to identify the UIP pattern. 10 , 44 , 45 Flaherty et al. 45 specifically addressed the role of histopathological sampling in differentiating between UIP and NSIP, demonstrating histological variability in surgical lung biopsy specimens from multiple lobes and reinforcing the need for having multiple lobes biopsied. Given that the prognosis is worse for UIP than for NSIP, sampling should be optimized so as not to miss areas most likely to demonstrate the histological pattern of UIP. 45
DISEASE SEVERITY ASSESSMENT, QUANTIFICATION, AND FOLLOW-UP
Disease severity assessment
Given that several fibrosing ILDs have an unpredictable natural history, staging systems can be useful in estimating prognosis and guiding management decisions (e.g., timing of pharmacological treatment and lung transplantation). The most widely used model for assessing chronic fibrosing diseases is a scoring system based on clinical and functional parameters (gender, age, FVC, and DLCO); the GAP model (Gender, Age, and lung Physiology) is most commonly used in patients with IPF and has been shown to be accurate in predicting survival. 46 , 47 Adaptations of this model have recently been tested to include HRCT. Ley et al. 48 replaced DLCO with a visual semiquantitative HRCT score and reported that the resulting model had a performance that was comparable to that of the original GAP model. Chahal et al. 49 evaluated the impact of adding a visual semiquantitative fibrotic score above or below 25% to the GAP model and found that the resulting model shows improved correlation with survival, especially in patients with mild disease. Other multidimensional indices including functional variables and CT evaluation have been proposed to stage systemic sclerosis-related fibrosing ILD, sarcoidosis, and other ILDs. 50 , 51
Quantification
Qualitative (visual) and semiquantitative imaging techniques can be used in order to assess ILDs in general and correlate with outcomes in a number of ILDs; however, they are highly dependent on visual analysis and are of limited use in detecting subtle changes in disease progression. Quantitative imaging, including histogram analysis and textural-based analysis coupled to machine learning, is a new and promising approach, allowing quantification of patterns such as ground-glass opacification, reticulation, and honeycombing. 52 In addition to quantification, automated analysis is useful for interpreting CT patterns, as demonstrated in a study by Walsh et al., 52 in which a deep learning algorithm had a performance comparable to that of thoracic radiologists in determining the likelihood of UIP in accordance with criteria specified in the 2011 ATS/ERS/JRS/ALAT guidelines. 53 A combination of automated and visual analysis is likely to be the optimal approach to disease staging and outcome prediction in fibrosing ILDs.
Follow-up
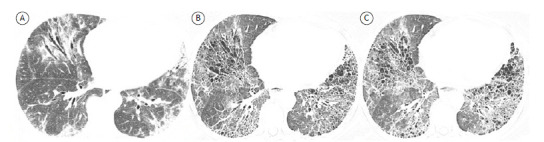
Serial CT can reveal changes in the extent of reticulation, traction bronchiectasis, and honeycombing, allowing identification of progressive fibrosing ILDs, which are associated with a worse prognosis. 52 However, the optimum time interval for CT follow-up has yet to be established, and currently there are no formal recommendations to sequencial HRCT follow-up in clinically stable patients. 51 Given that patients with various fibrosing ILDs have a slow disease progression, the longitudinal behavior of fibrosing ILDs can be more easily detected by comparing follow-up CT scans with initial CT scans rather than by comparing follow-up CT scans only (Figure 7). HRCT evaluation of disease progression is one of the recently proposed criteria for the definition of progressive fibrosing ILD. 54 Patients are required to meet at least one of the following criteria for progression of fibrosing ILD within 24 months: a reduction in FVC of at least 10% of the predicted value; a reduction in FVC of 5-10% of the predicted value and worsening of respiratory symptoms or an increased extent of fibrosis on HRCT; or worsening of respiratory symptoms and an increased extent of fibrosis on HRCT. It is important to define progressive fibrosing ILD because recent evidence shows a reduction in the rate of decline in FVC in non-IPF progressive fibrosing ILD patients undergoing treatment with nintedanib. 54
Figure 7. Axial HRCT scans. Initial CT scan (in A) and follow-up CT scans at six years (in B) and six and a half years (in C) in a patient with systemic sclerosis-related fibrosing interstitial lung disease. Although the follow-up scans apparently show no changes in disease progression, a comparison between the initial and follow-up scans reveals a progressive disease phenotype, underscoring the importance of comparing follow-up CT findings with initial CT findings.
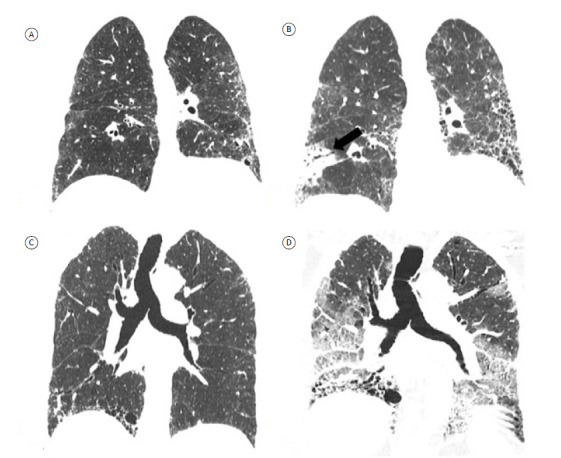
In addition to determining disease behavior, CT follow-up plays an important role in detecting complications such as pulmonary hypertension, pulmonary embolism, neoplasms, and coronary artery disease, all of which can have an impact on survival (Figure 8). 51
Figure 8. Coronal reconstructions of HRCT scans showing complications of fibrosing interstitial lung disease in two patients. In A and B, scans of a patient with progressive idiopathic pulmonary fibrosis. Initial CT scan (in A) and follow-up CT scan at seven years and 10 months (in B), showing disease progression and an irregular subpleural expansile neoplastic lesion in the right lower lobe, diagnosed as adenocarcinoma (arrow in B). In C and D, scans of a patient with progressive idiopathic pulmonary fibrosis. Initial CT scan (in C) and follow-up CT scan at 15 months (in D), showing disease progression and new bilateral ground-glass opacities, characterizing acute exacerbation of idiopathic pulmonary fibrosis.
ACUTE EXACERBATION OF FIBROSING ILD
Acute exacerbation of fibrosing ILD is defined in accordance with current criteria for IPF: an acute, clinically significant respiratory deterioration, typically less than 1 month in duration, categorized as pulmonary or extrapulmonary (pulmonary embolism, pneumothorax, pleural effusion, or any combination of the three). HRCT plays a major role in the diagnosis of acute exacerbations. In patients with episodes of inflammatory exacerbation, noninvasive criteria include HRCT findings of bilateral ground-glass opacities, consolidations, or a combination of the two superimposed on a background pattern consistent with fibrosing ILD but that are not explained by hydrostatic edema, regardless of whether the condition is idiopathic or caused by any other factor, including infection (Figure 8). CT angiography plays an important role in detecting episodes of acute exacerbation caused by pulmonary thromboembolism. 16 , 55
FINAL CONSIDERATIONS
The management of patients with fibrosing ILD is complex. In a multidisciplinary approach, CT evaluation is important at various stages of patient management, including early diagnosis, narrowing of the diagnostic possibilities (definition of morphological patterns and, eventually, pointing toward specific clinical entities), disease severity assessment, prognosis, and follow-up, as well as in identifying complications such as infections, neoplasms, and acute exacerbations. Many issues have yet to be resolved, including interobserver variation in the interpretation of scans, the optimum time interval for CT follow-up, and the significance of subclinical ILAs. New approaches involving automated techniques and machine learning can be useful at various stages, and their significance should be investigated in future studies.
Footnotes
Financial support: None.
REFERENCES
- 1.Larsen BT, Smith ML, Elicker BM, Fernandez JM, de Morvil GAA, Pereira CAC. Diagnostic Approach to Advanced Fibrotic Interstitial Lung Disease Bringing Together Clinical, Radiologic, and Histologic Clues. Arch Pathol Lab Med. 2017;141(7):901–915. doi: 10.5858/arpa.2016-0299-SA. [DOI] [PubMed] [Google Scholar]
- 2.Cottin V, Wollin L, Fischer A, Quaresma M, Stowasser S, Harari S. Fibrosing interstitial lung diseases knowns and unknowns. Eur Respir Rev. 2019;28(151):180100–180100. doi: 10.1183/16000617.0100-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic SocietyEuropean Respiratory Society American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001 [published correction appears in Am J Respir Crit Care Med2002 Aug 1;166(3):426]. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 4.Travis TD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG. An official American Thoracic Society/European Respiratory Society statement Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cottin V, Hirani NA, Hotchkin DL, Nambiar AM, Ogura T, Otaola M. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150):180076–180076. doi: 10.1183/16000617.0076-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis [published correction appears in N Engl. J Med. 2014;371(12):1172–1172. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 7.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis [published correction appears in N Engl. J Med. 2015;373(8):782–782. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 8.Richeldi L, Varone F, Bergna M, de Andrade J, Falk J, Hallowell R. Pharmacological management of progressive-fibrosing interstitial lung diseases a review of the current evidence. Eur Respir Rev. 2018;27(150):180074–180074. doi: 10.1183/16000617.0074-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bankier AA, O'Donnell CR, Boiselle PM. Quality initiatives Respiratory instructions for CT examinations of the lungs: a hands-on guide. Radiographics. 2008;28(4):919–931. doi: 10.1148/rg.284085035. [DOI] [PubMed] [Google Scholar]
- 10.Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR. Diagnostic criteria for idiopathic pulmonary fibrosis a Fleischner Society White Paper. Lancet Respir Med. 2018;6(2):138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 11.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ. Diagnosis of Idiopathic Pulmonary Fibrosis An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 12.Sverzellati N. Highlights of HRCT imaging in IPF. Respir Res. 2013;14(Suppl 1):S3–S3. doi: 10.1186/1465-9921-14-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266(3):936–944. doi: 10.1148/radiol.12112516. [DOI] [PubMed] [Google Scholar]
- 14.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 15.Silva CI, Marchiori E, Souza AS, Júnior, Müller NL. Comissão de Imagem da Sociedade Brasileira de Pneumologia e Tisiologia Illustrated Brazilian consensus of terms and fundamental patterns in chest CT scans. J Bras Pneumol. 2010;36(1):99–123. doi: 10.1590/s1806-37132010000100016. [DOI] [PubMed] [Google Scholar]
- 16.Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ. Exacerbation of Idiopathic Pulmonary Fibrosis An International Working Group Report. Am J Respir Crit Care Med. 2016;194(3):265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 17.Doyle TJ, Hunninghake GM, Rosas IO. Subclinical interstitial lung disease why you should care. Am J Respir Crit Care Med. 2012;185(11):1147–1153. doi: 10.1164/rccm.201108-1420PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M. Interstitial lung abnormalities detected incidentally on CT a Position Paper from the Fleischner Society. Lancet Respir Med. 2020;8(7):726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunninghake GM. Interstitial lung abnormalities erecting fences in the path towards advanced pulmonary fibrosis. Thorax. 2019;74(5):506–511. doi: 10.1136/thoraxjnl-2018-212446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Putman RK, Gudmundsson G, Axelsson GT, Hida T, Honda O, Araki T. Imaging Patterns Are Associated with Interstitial Lung Abnormality Progression and Mortality. Am J Respir Crit Care Med. 2019;200(2):175–183. doi: 10.1164/rccm.201809-1652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodnett PA, Naidich DP. Fibrosing interstitial lung disease A practical high-resolution computed tomography-based approach to diagnosis and management and a review of the literature. Am J Respir Crit Care Med. 2013;188(2):141–149. doi: 10.1164/rccm.201208-1544CI. [DOI] [PubMed] [Google Scholar]
- 22.Chung JH, Chawla A, Peljto AL, Cool CD, Groshong SD, Talbert JL. CT scan findings of probable usual interstitial pneumonitis have a high predictive value for histologic usual interstitial pneumonitis. Chest. 2015;147(2):450–459. doi: 10.1378/chest.14-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung JH, Oldham JM, Montner SM, Vij R, Adegunsoye A, Husain AN. CT-Pathologic Correlation of Major Types of Pulmonary Fibrosis Insights for Revisions to Current Guidelines. AJR Am J Roentgenol. 2018;210(5):1034–1041. doi: 10.2214/AJR.17.18947. [DOI] [PubMed] [Google Scholar]
- 24.Silva CIS, Müller NL, Lynch DA, Curran-Everett D, Brown KK, Lee KS. Chronic hypersensitivity pneumonitis differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology. 2008;246(1):288–297. doi: 10.1148/radiol.2453061881. [DOI] [PubMed] [Google Scholar]
- 25.Travis WD, Hunninghake G, King TE, Jr, Lynch DA, Colby TV, Galvin JR. Idiopathic nonspecific interstitial pneumonia report of an American Thoracic Society project [published correction appears in Am J Respir Crit Care. Med. 2008;178(2):211–211. doi: 10.1164/rccm.200611-1685OC. [DOI] [PubMed] [Google Scholar]
- 26.Chua F, Desai SR, Nicholson AG, Devaraj A, Renzoni E, Rice A. Pleuroparenchymal Fibroelastosis A Review of Clinical, Radiological, and Pathological Characteristics. Ann Am Thorac Soc. 2019;16(11):1351–1359. doi: 10.1513/AnnalsATS.201902-181CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JW, Lee KS, Lee HY, Chung MP, Yi CA, Kim TS. Cryptogenic organizing pneumonia serial high-resolution CT findings in 22 patients. AJR Am J Roentgenol. 2010;195(4):916–922. doi: 10.2214/AJR.09.3940. [DOI] [PubMed] [Google Scholar]
- 28.Debray MP, Borie R, Revel MP, Naccache JM, Khalil A, Toper C. Interstitial lung disease in anti-synthetase syndrome initial and follow-up CT findings. Eur J Radiol. 2015;84(3):516–523. doi: 10.1016/j.ejrad.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Vasakova M, Morell F, Walsh S, Leslie K, Raghu G. Hypersensitivity Pneumonitis Perspectives in Diagnosis and Management. Am J Respir Crit Care Med. 2017;196(6):680–689. doi: 10.1164/rccm.201611-2201PP. [DOI] [PubMed] [Google Scholar]
- 30.Salisbury ML, Myers JL, Belloli EA, Kazerooni EA, Martinez FJ, Flaherty KR. Diagnosis and Treatment of Fibrotic Hypersensitivity Pneumonia Where We Stand and Where We Need to Go. Am J Respir Crit Care Med. 2017;196(6):690–699. doi: 10.1164/rccm.201608-1675PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morisset J, Johannson KA, Jones KD, Wolters PJ, Collard HR, Walsh SLF. Identification of Diagnostic Criteria for Chronic Hypersensitivity Pneumonitis An International Modified Delphi Survey. Am J Respir Crit Care Med. 2018;197(8):1036–1044. doi: 10.1164/rccm.201710-1986OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira CA, Gimenez A, Kuranishi L, Storrer K. Chronic hypersensitivity pneumonitis. J Asthma Allergy. 2016;9:171–181. doi: 10.2147/JAA.S81540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raghu G, Remy-Jardin M, Ryerson CJ, Myers LJ, Kreuter M, Vasakova M, et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline [published correction appears in Am J Respir Crit Care Med. 2021 Jan 1;203(1):150-151] Am J Respir Crit Care Med. 2020;202(3):e36–e69. doi: 10.1164/rccm.202005-2032ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnett J, Molyneaux PL, Rawal B, Abdullah R, Hare SS, Vancheeswaran R. Variable utility of mosaic attenuation to distinguish fibrotic hypersensitivity pneumonitis from idiopathic pulmonary fibrosis. Eur Respir J. 2019;54(1):1900531–1900531. doi: 10.1183/13993003.00531-2019. [DOI] [PubMed] [Google Scholar]
- 35.Chung MH, Edinburgh KJ, Webb EM, McCowin M, Webb WR. Mixed infiltrative and obstructive disease on high-resolution CT differential diagnosis and functional correlates in a consecutive series. J Thorac Imaging. 2001;16(2):69–75. doi: 10.1097/00005382-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Elicker BM, Jones KD, Henry TS, Collard HR. Multidisciplinary Approach to Hypersensitivity Pneumonitis. J Thorac Imaging. 2016;31(2):92–103. doi: 10.1097/RTI.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 37.Wallace B, Vummidi D, Khanna D. Management of connective tissue diseases associated interstitial lung disease a review of the published literature. Curr Opin Rheumatol. 2016;28(3):236–245. doi: 10.1097/BOR.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung JH, Cox CW, Montner SM, Adegunsoye A, Oldham JM, Husain AN. Features of the Usual Interstitial Pneumonia Pattern Differentiating Connective Tissue Disease-Associated Interstitial Lung Disease From Idiopathic Pulmonary Fibrosis. AJR Am J Roentgenol. 2018;210(2):307–313. doi: 10.2214/AJR.17.18384. [DOI] [PubMed] [Google Scholar]
- 39.Walkoff L, White DB, Chung JH, Asante D, Cox CW. The Four Corners Sign A Specific Imaging Feature in Differentiating Systemic Sclerosis-related Interstitial Lung Disease From Idiopathic Pulmonary Fibrosis. J Thorac Imaging. 2018;33(3):197–203. doi: 10.1097/RTI.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 40.Criado E, Sánchez M, Ramírez J, Arguis P, de Caralt TM, Perea RJ. Pulmonary sarcoidosis typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics. 2010;30(6):1567–1586. doi: 10.1148/rg.306105512. [DOI] [PubMed] [Google Scholar]
- 41.Roach HD, Davies GJ, Attanoos R, Crane M, Adams H, Phillips S. Asbestos: when the dust settles an imaging review of asbestos-related disease. Radiographics. 2002;22 Spec No:S167–S184. doi: 10.1148/radiographics.22.suppl_1.g02oc10s167. [DOI] [PubMed] [Google Scholar]
- 42.Akira M, Yamamoto S, Inoue Y, Sakatani M. High-resolution CT of asbestosis and idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 2003;181(1):163–169. doi: 10.2214/ajr.181.1.1810163. [DOI] [PubMed] [Google Scholar]
- 43.Raghu G, Remy-Jardin M, Myers J, Richeldi L, Wilson KC. The 2018 Diagnosis of Idiopathic Pulmonary Fibrosis Guidelines Surgical Lung Biopsy for Radiological Pattern of Probable Usual Interstitial Pneumonia Is Not Mandatory. Am J Respir Crit Care Med. 2019;200(9):1089–1092. doi: 10.1164/rccm.201907-1324ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK. Interstitial lung disease guideline the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society [published correction appears in. Thorax. 2008;63(11):1029–1029. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 45.Flaherty KR, Travis WD, Colby TV, Toews GB, Kazerooni EA, Gross BH. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164(9):1722–1727. doi: 10.1164/ajrccm.164.9.2103074. [DOI] [PubMed] [Google Scholar]
- 46.Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD. Predicting survival across chronic interstitial lung disease the ILD-GAP model. Chest. 2014;145(4):723–728. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 47.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS. A A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 48.Ley B, Elicker BM, Hartman TE, Ryerson CJ, Vittinghoff E, Ryu JH. Idiopathic pulmonary fibrosis CT and risk of death. Radiology. 2014;273(2):570–579. doi: 10.1148/radiol.14130216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chahal A, Sharif R, Watts J, Andrade J, Luckhardt T, Kim YI. Predicting Outcome in Idiopathic Pulmonary Fibrosis Addition of Fibrotic Score at Thin-Section CT of the Chest to Gender, Age, and Physiology Score Improves the Prediction Model. Radiol Cardiothorac Imag. 2019;1(2):e180029. doi: 10.1148/ryct.2019180029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomassetti S, Ryu JH, Poletti V. Staging systems and disease severity assessment in interstitial lung diseases. Curr Opin Pulm Med. 2015;21(5):463–469. doi: 10.1097/MCP.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 51.Hansell DM, Goldin JG, King TE, Jr, Lynch DA, Richeldi L, Wells AU. CT staging and monitoring of fibrotic interstitial lung diseases in clinical practice and treatment trials a position paper from the Fleischner Society. Lancet Respir Med. 2015;3(6):483–496. doi: 10.1016/S2213-2600(15)00096-X. [DOI] [PubMed] [Google Scholar]
- 52.Walsh SLF, Devaraj A, Enghelmayer JI, Kishi K, Silva RS, Patel N. Role of imaging in progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150):180073–180073. doi: 10.1183/16000617.0073-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walsh SLF, Calandriello L, Silva M, Sverzellati N. Deep learning for classifying fibrotic lung disease on high-resolution computed tomography a case-cohort study. Lancet Respir Med. 2018;6(11):837–845. doi: 10.1016/S2213-2600(18)30286-8. [DOI] [PubMed] [Google Scholar]
- 54.Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 55.Kolb M, Bondue B, Pesci A, Miyazaki Y, Song JW, Bhatt NY. Acute exacerbations of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150):180071–180071. doi: 10.1183/16000617.0071-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


