Abstract
Introduction
The epidemic of coronavirus disease 2019 (COVID-19) rapidly spread worldwide, and the various infection control measures have a significant influence on the spread of many infectious diseases. However, there have been no multicenter studies on how the number of hospitalized children with various infectious diseases changed before and after the outbreak of COVID-19 in Japan.
Methods
We conducted a multicenter, prospective survey for hospitalized pediatric patients in 18 hospitals in Hokkaido Prefecture, Japan, from July 2019 to February 2021. We defined July 2019 to February 2020 as pre-COVID-19, and July 2020 to February 2021 as post-COVID-19. We surveyed various infectious diseases by sex and age.
Results
In total, 5300 patients were hospitalized during the study period. The number of patients decreased from 4266 in the pre-COVID-19 period to 701 (16.4%) post-COVID-19. Patients with influenza and RSV decreased from 308 to 795 pre-COVID-19 to zero and three (0.4%) post-COVID-19. However, patients with adenovirus (respiratory infection) only decreased to 60.9% (46–28) of pre-COVID levels. Patients with rotavirus, norovirus, and adenovirus gastroenteritis decreased markedly post-COVID-19 to 2.6% (38–1), 27.8% (97–27) and 13.5% (37–5). The number of patients with UTIs was similar across the two periods (109 and 90). KD patients decreased to 31.7% (161–51) post-COVID-19.
Conclusions
We suggest that current infection control measures for COVID-19 such as wearing masks, washing hands, and disinfecting hands with alcohol are effective against various infectious diseases. However, these effects vary by disease.
Keywords: COVID-19, Infection, Virus, Bacteria, Hospitalization, Child
1. Introduction
The most common conditions seen in the field of pediatrics are infectious diseases, and there are a wide variety of epidemic diseases that are often difficult to manage. Infectious disease outbreaks vary greatly depending on the season, year, region and country, and it is important to understand the epidemiology. In Japan, the National Institute of Infectious Diseases (NIID) surveys the national trends in infectious diseases [1]. National surveillance in Hokkaido Prefecture reports weekly on infectious diseases including influenza, respiratory syncytial virus (RSV) and infectious gastroenteritis [2]. However, these data do not provide information on trends in patients who require hospitalization.
From July 1, 2019, we have run a survey called Hokkaido Pediatric Infectious Diseases Surveillance (HPIDS), to gather data about inpatient children with infectious diseases in the 18 hospitals in Hokkaido Prefecture. The survey's purpose is to share information on severe infectious diseases among hospitals and use this to alert and provide information to medical staff and patients.
The epidemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), rapidly spread worldwide shortly after the survey started. In Hokkaido Prefecture, Japan, COVID-19 spread from mid-February 2020. Primary, junior high, and high schools and special support schools all closed from 28 February to May 31, 2020, to prevent the spread of COVID-19 among children [3,4]. In pediatrics, we know that various infection control measures have a significant influence on the spread of many infectious diseases. However, to our knowledge, there have been no multicenter studies on how the number of hospitalized children with various infectious diseases changed before and after the outbreak of COVID-19. This paper therefore reports changes in the number and age distribution of pediatric patients with various infectious diseases, drawing on the results of surveillance up to February 28, 2021.
2. Materials and methods
2.1. Study period and institutions
We aggregated data from patients aged 0–15 years who were newly admitted to the 18 hospitals in Hokkaido Prefecture from July 1, 2019 to February 28, 2021. We defined the 8 months from July 1, 2019 to February 28, 2020 as “pre-COVID-19”, and from July 1, 2020 to February 28, 2021 as “post-COVID-19”. The staff of each hospital input the data every week using Microsoft Excel and emailed it to the central staff.
2.2. Diseases
We surveyed for measles, rubella, varicella, mumps, pertussis, influenza A, influenza B, respiratory syncytial virus (RSV), human metapneumovirus (hMPV), mycoplasma pneumoniae, adenovirus (respiratory infection), lower respiratory tract infection (LRTI), rotavirus gastroenteritis, norovirus gastroenteritis, adenovirus gastroenteritis, other gastroenteritis, aseptic meningitis, bacterial meningitis, encephalitis/encephalopathy, complex febrile seizure, urinary tract infection (UTI), and Kawasaki disease (KD). We aggregated each disease by sex and age (0–3 months, 4–6 months, 7–12 months, 1 year old, 2 years old, 3–6 years old, 7–12 years old and 13–15 years old).
2.3. Inclusion and exclusion criteria
For influenza, RSV, hMPV, adenovirus (respiratory infection), rotavirus gastroenteritis, norovirus gastroenteritis, and adenovirus gastroenteritis, we included patients with positive rapid antigen detection tests. For mycoplasma pneumoniae, we included patients with mycoplasma deoxyribonucleic acid (DNA) or antibodies in the blood, or positive rapid antigen tests. We excluded patients whose symptoms or epidemiological associations suggested that they might be infected, but who either had a negative rapid test or were not tested. If the items overlapped, we aggregated both. For example, when RSV was detected in a patient with pneumonia, the patient was counted for both RSV and LRTI.
2.4. Data analysis
The data was only analyzed in a descriptive manner. Comparisons of number of patients between pre-COVID-19 and post-COVID-19 are reported as relative differences in percentages.
Ethical approval
This study was approved by the Ethics Committee of Sapporo Medical University (Institutional ethical clearance number: 312–3349).
3. Results
In total, 5300 patients were hospitalized during the study period (July 1, 2019 to February 28, 2021) (Table 1 ). Of these, 4266 were pre-COVID-19 and 701 post-COVID-19, or just 16.4% of the pre-COVID numbers. From 1 March to June 31, 2020, 333 patients were hospitalized. No diseases increased post-COVID-19.
Table 1.
Comparison of number of patients pre- and post-COVID-19.
|
Whole study period |
pre-COVID-19 |
March to June 2020 |
post-COVID-19* |
||
|---|---|---|---|---|---|
| (20 months) | (8 months) | (4 months) | (8 months) | ||
| Total patients | 5300 | 4266 | 333 | 701 | (16.4%) |
| Measles | 0 | 0 | 0 | 0 | (−) |
| Rubella | 0 | 0 | 0 | 0 | (−) |
| Varicella | 9 | 6 | 1 | 2 | (33.3%) |
| Mumps | 1 | 0 | 0 | 1 | (−) |
| Pertussis | 18 | 16 | 2 | 0 | (0.0%) |
| Influenza A | 271 | 269 | 2 | 0 | (0.0%) |
| Influenza B | 45 | 39 | 6 | 0 | (0.0%) |
| RSV | 804 | 795 | 6 | 3 | (0.4%) |
| hMPV | 210 | 201 | 9 | 0 | (0.0%) |
| Mycoplasma penumoniae | 164 | 158 | 4 | 2 | (1.3%) |
| Adenovirus (respiratory infection) | 88 | 46 | 14 | 28 | (60.9%) |
| LRTI | 2439 | 1984 | 121 | 334 | (16.8%) |
| Rotavirus gastroenteritis | 44 | 38 | 5 | 1 | (2.6%) |
| Norovirus gastroenteritis | 134 | 97 | 10 | 27 | (27.8%) |
| Adenovirus gastroenteritis | 48 | 37 | 6 | 5 | (13.5%) |
| Other gastroenteritis | 396 | 232 | 37 | 127 | (54.7%) |
| Aseptic meningitis | 17 | 14 | 2 | 1 | (7.1%) |
| Bacterial meningitis | 3 | 0 | 0 | 3 | (−) |
| Encephalitis/Encephalopathy | 27 | 22 | 3 | 2 | (9.1%) |
| Complex febrile seizure | 73 | 42 | 7 | 24 | (57.1%) |
| UTI | 239 | 109 | 40 | 90 | (82.6%) |
| KD | 270 | 161 | 58 | 51 | (31.7%) |
*Number in parentheses is the percentage of patients post-COVID-19 compared to pre-COVID-19.
The number of patients with influenza, RSV, hMPV, and mycoplasma pneumoniae, which cause respiratory symptoms, all decreased drastically by more than 98%. Diseases that decreased moderately (50–70%) were adenovirus (respiratory infection), other gastroenteritis, complex febrile seizure, and KD. UTIs decreased relatively little to 82.6% of pre-COVID-19 levels.
3.1. Influenza A and B
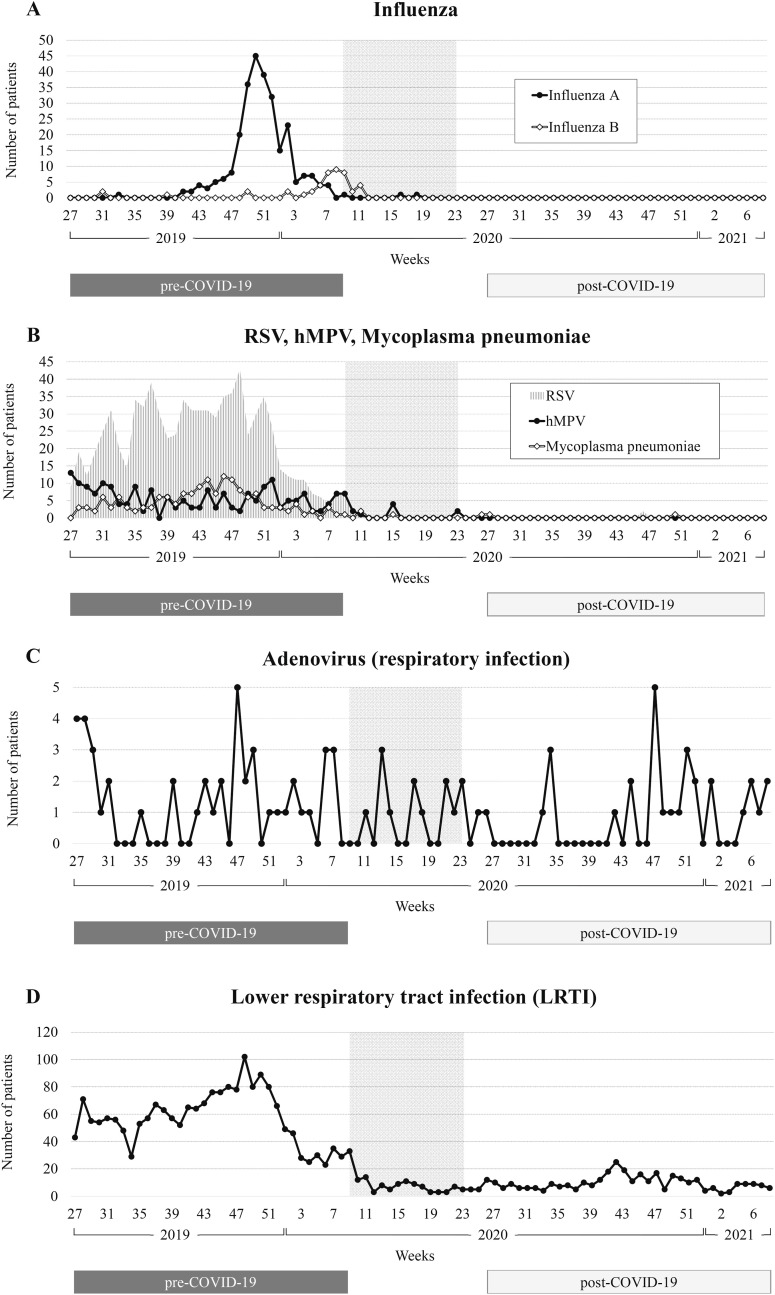
Pre-COVID-19, there were 269 patients with influenza A and 39 with influenza B. No patients were hospitalized post-COVID-19 (Table 1). Influenza A increased from the end of November 2019, and the largest cluster of cases was at the beginning of December (Fig. 1 ). After that, incidence decreased sharply, and no patients were observed after May 2020. Influenza B peaked in mid-February 2020, when influenza A cases tended to converge, but the number of hospitalizations was not as high as at the peak of influenza A. Influenza B also decreased during the school closure period (28 February to May 31, 2020), and there were no patients after April 2020.
Fig. 1.
Trends in the number of patients per week over the study period. A: influenza. B: RSV, hMPV, mycoplasma pneumoniae. C: adenovirus (respiratory infection). D: lower respiratory tract infection (LRTI). The vertical axis shows the number of patients, and the horizontal axis weeks. The shaded area indicates the school closure period from 28 February to May 31, 2020.
3.2. RSV, hMPV and mycoplasma pneumoniae
In total, 795 patients had RSV, 201 hMPV and 158 mycoplasma pneumoniae pre-COVID-19. This decreased drastically to three (0.4%), zero and two (1.3%) post-COVID-19 (Table 1). RSV and mycoplasma pneumoniae increased from June to November 2019. About five to ten patients with hMPV were detected every week from July 2019 to the end of the year. RSV, hMPV and mycoplasma pneumoniae all tended to decrease from the end of 2019 and were hardly seen after the school closure period (28 February to May 31, 2020) until February 28, 2021 (Fig. 1).
3.3. Adenovirus (respiratory infection)
There were 46 patients with adenovirus (respiratory infection) hospitalized pre-COVID-19 and 28 post-COVID-19, or 60.9% of the pre-COVID figure (Table 1). The number of patients with adenovirus (respiratory infection) was small in August–September but increased around November in both 2019 and 2020 (Fig. 1). Unlike the other respiratory infectious diseases surveyed, the incidence of adenovirus (respiratory infection) did not decrease drastically during the school closure period.
3.4. Gastroenteritis (rotavirus, norovirus, adenovirus and others)
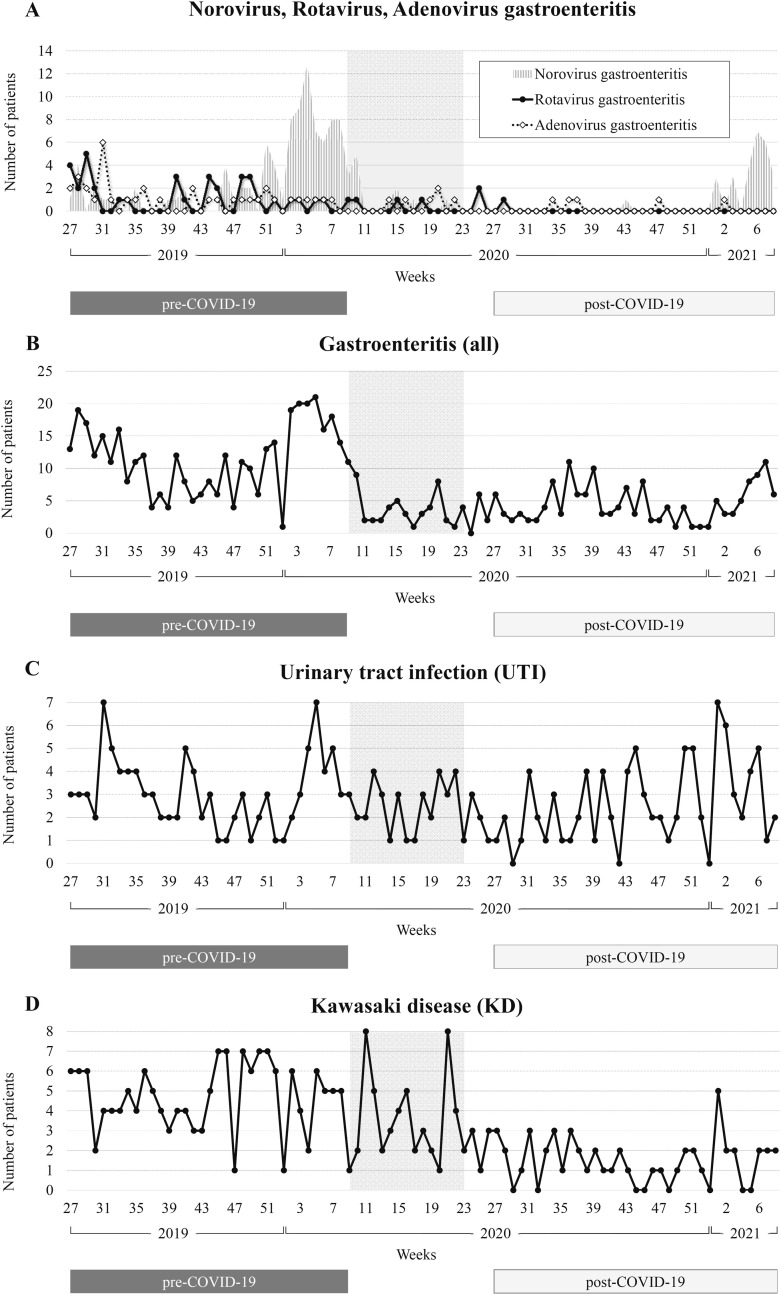
There were 38, 97, 37, and 232 patients with rotavirus, norovirus, adenovirus, and other gastroenteritis pre-COVID-19. This decreased to one, 27, five, and 127 post-COVID-19 (Table 1). The number of patients with rotavirus, norovirus, and adenovirus gastroenteritis decreased markedly post-COVID-19 to 2.6%, 27.8% and 13.5%. However, other forms of gastroenteritis decreased relatively little to 54.7%. Around 10–20 patients with gastroenteritis were hospitalized every week in July–August 2019, and rotavirus and adenovirus gastroenteritis were observed in about five patients/week during that period (Fig. 2 ). The incidence tended to decrease slightly toward September–October 2019, then increased again from December during an epidemic of norovirus gastroenteritis. It decreased drastically after the school closure period, and almost never exceeded 10 patients/week until the end of 2020. From January 2021, the number of patients with norovirus gastroenteritis tended to increase, but remained lower than the same period in 2020.
Fig. 2.
Trends in the number of patients per week over the study period. A: norovirus, rotavirus and adenovirus gastroenteritis. B: gastroenteritis (all). C: urinary tract infection (UTI). D: Kawasaki disease (KD). The vertical axis shows the number of patients, and the horizontal axis weeks. The shaded area indicates the school closure period from 28 February to May 31, 2020.
3.5. Urinary tract infections (UTIs)
Patients with UTIs were hospitalized during almost all weeks (96.6%, 85/88 weeks) during the study period, and the number of patients did not vary greatly pre- and post-COVID-19 (109 and 90 patients) (Fig. 2 and Table 1).
3.6. Kawasaki disease (KD)
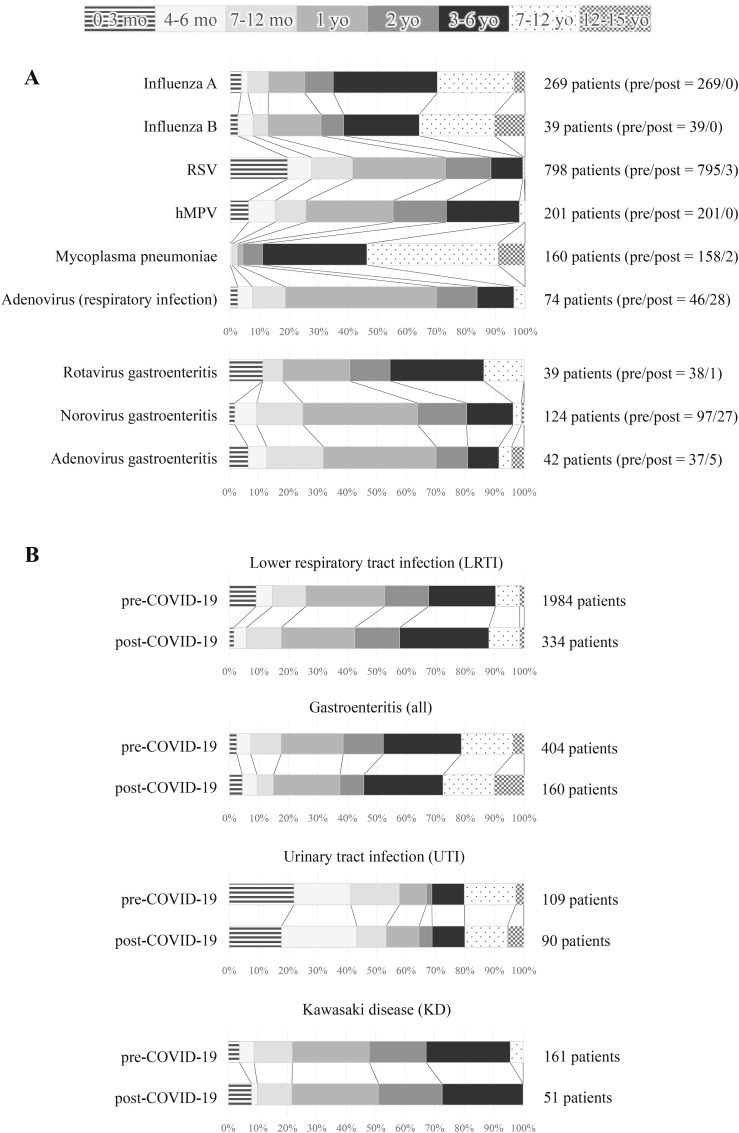
Pre-COVID-19, 161 patients were hospitalized with KD, decreasing to 51 (31.7%) post-COVID-19 (Table 1). The number of patients with KD increased from November 2019, and more than five patients were hospitalized almost every week before the school closure period (28 February to May 31, 2020) (Fig. 2). The number of patients did not decrease markedly during the school closure period. However, there were rarely more than three patients/week hospitalized after the school closure period and this decreased further toward the end of the survey. Among all patients, almost all patients were ≤6 years old (97.4%, 263/270), and the number of patients under 1 year old, 1 year old, and 2 years old was almost the same (23.0%, 62/270; 25.2%, 68/270; and 21.9%, 59/270) (Fig. 3 ). There were no deaths among anyone with KD and KD-like symptoms during the study period.
Fig. 3.
Age distribution of each disease. The percentages of patients aged 0–3 months, 4–6 months, 7–12 months, 1 year old, 2 years old, 3–6 years old, 7–12 years old and 13–15 years old for each disease are shown by color. A: For influenza A/B, RSV, hMPV, mycoplasma pneumoniae, and rotavirus/norovirus/adenovirus gastroenteritis, total number of patients during the study period is listed on the right side of the bars, and the number of patients pre- and post-COVID-19 are in parentheses. B: For LRTI, gastroenteritis, UTI and KD, upper and lower rows show pre- and post-COVID-19, and the number of patients are listed on the right side of the bars.
4. Discussion
To the best of our knowledge, this is the first multicenter survey to comprehensively describe the trends in various infectious diseases among hospitalized children before and after the COVID-19 epidemic in Japan. We found that the number of children who needed hospitalization for infectious diseases decreased more than 80% post-COVID-19 in Hokkaido Prefecture, Japan. The figures for some diseases decreased more than 98%, but some decreased by less than half. SARS-CoV-2 is primarily transmitted between people through droplets, aerosols or contact, and the importance of wearing a mask, securing physical distance from others, washing hands, and disinfecting hands with alcohol has become well known [[5], [6], [7], [8]]. Nowadays in Japan, we wear masks when going out, and the installation and use of alcohol disinfectants is widespread in both homes and public places [9]. It seems that these infection control measures have affected not only COVID-19 but also many other infectious diseases.
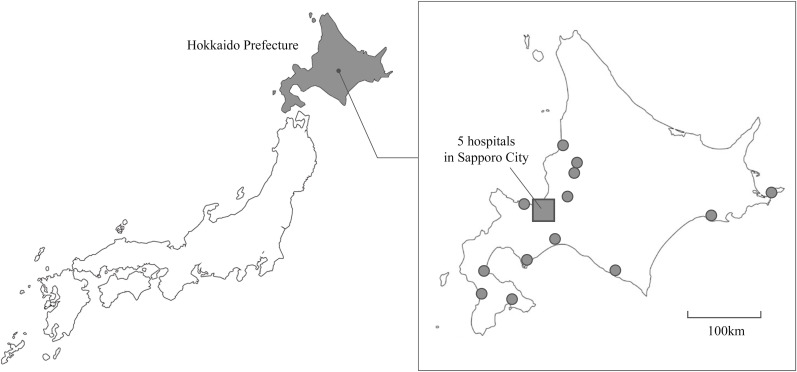
Hokkaido Prefecture, which we surveyed in this study, is located in the northernmost part of Japan (Fig. 4 ). The population of Hokkaido Prefecture is about 5.4 million, around 4.2% of the total population of Japan, and 11.4% of population is aged <15 years old [10]. According to information about infectious diseases from the national surveillance in Japan and Hokkaido Prefecture, the patterns of infectious disease outbreaks seen in Hokkaido Prefecture are similar to those elsewhere, although RSV is most often seen nationwide around September, whereas there has been no fixed epidemic period for the past few years in Hokkaido Prefecture [1,2].
Fig. 4.
Location of Hokkaido Prefecture and the 18 hospitals in this study. The shaded area is Hokkaido Prefecture. Filled circles indicate each hospital. The filled square is Sapporo City, where five hospitals are located.
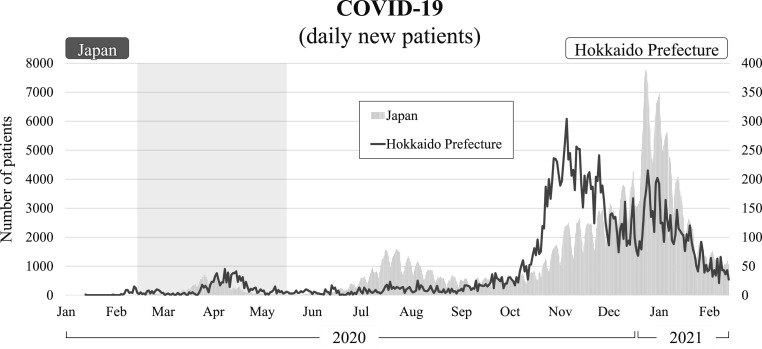
In December 2019, an outbreak of COVID-19-associated pneumonia was reported in Wuhan, China, and soon spread all over the world [11]. In Hokkaido Prefecture, Japan, the first COVID-19 patient was recognized on January 28, 2020, and the number of patients increased rapidly from around 20 February as shown in Fig. 5 [3]. Since then, two major peaks in infection have been recognized in April and November 2020. We used this information about COVID-19 in Hokkaido Prefecture to evaluate trends in other infectious diseases.
Fig. 5.
Daily number of new patients with COVID-19 in 2020. The vertical axis shows the number of patients, and the horizontal axis days. The shaded area indicates the school closure period from 28 February to May 31, 2020. Figures for both Japan and Hokkaido Prefecture are shown.
Respiratory infectious diseases such as influenza, RSV, hMPV and mycoplasma pneumoniae decreased drastically during the school closure period (28 February to May 31, 2020). Additionally, almost no patients with these diseases were hospitalized after the school closure period until February 28, 2021. In Hokkaido Prefecture, there is usually a higher incidence of influenza A during December to February, influenza B in February to April, hMPV in March to June, and mycoplasma pneumoniae in October to December [2]. RSV in Hokkaido Prefecture has been seen almost year-round since 2017. Considering the trends in these diseases over the past few years, the period post-COVID-19 was extremely unusual.
Infectious gastroenteritis also decreased markedly post-COVID-19. In Hokkaido Prefecture, the largest number of cases of infectious gastroenteritis have generally been seen in March to May in the last few years, and the numbers have decreased from August to October, then gradually increased toward winter [2]. Rotavirus gastroenteritis accounts for most of the March–May increase, and the increase in the number of patients in winter may be related to norovirus gastroenteritis. In our study, the number of patients with gastroenteritis overall decreased remarkably during the school closure period, and there was no increase in March to May. Even after the school closure period, the number of patients remained at about half those seen in the same period in 2019, and few patients were hospitalized with rotavirus, norovirus, and adenovirus gastroenteritis.
However, the decrease in the number of patients with infectious gastroenteritis was smaller than that for respiratory infectious diseases (overall gastroenteritis decreased to 39.6% post-COVID-19, while influenza, RSV, hMPV and mycoplasma pneumoniae decreased to almost none). This may be because of the different transmission routes for infectious gastroenteritis and respiratory infectious diseases. Influenza, RSV, hMPV and mycoplasma pneumoniae are mainly transmitted by droplets in the air from a person who coughs or sneezes [[12], [13], [14], [15], [16], [17]]. Infectious gastroenteritis including rotavirus, norovirus and adenovirus are mainly transmitted through the fecal–oral route [[18], [19], [20]]. This suggests that the current infection control measures for COVID-19 such as wearing a mask, securing physical distance from others, washing hands, and disinfecting hands with alcohol are more effective for droplet transmission than fecal–oral transmission. Another reason why respiratory infectious diseases decreased markedly might be that children who had even slight respiratory symptoms (e.g., coughing or sneezing) were not sent to schools or nurseries, because of the possibility of COVID-19 infection.
Adenovirus (respiratory infection) decreased after the school closure period. However, the figures never dropped as low as for the other respiratory diseases. One reason may be that the adenovirus (respiratory infection) is spread by both droplet infection and by touching surfaces or objects that an infected person has touched [21]. It is also possible to be an asymptomatic carrier of adenovirus, and its incubation period is relatively long, at 2 days to 2 weeks [22,23]. Children might therefore have gone to nursery without knowing they were infected and spread the infection by touching other children, desks, doors, or toys. Another reason might be that adenovirus is a non-enveloped virus. SARS-CoV-2 is an enveloped virus and is susceptible to alcohol disinfectants. The importance and effectiveness of disinfection using alcohol is now widely known [[24], [25], [26]]. Influenza, RSV, and hMPV are also enveloped viruses and can be expected to be inactivated by alcohol [27]. However, adenovirus, as a non-enveloped virus, is generally considered to be more resistant to disinfectants including alcohol [27,28]. It may therefore be difficult to control adenovirus (respiratory infection) with the current infection control measures for COVID-19.
Urinary tract infections (UTIs) are common bacterial infections in childhood. The majority of febrile UTI results from the ascent of bacteria from the periurethral area, migrating in a retrograde fashion through the urethra to reach the upper urinary tract, which is often related to the congenital anomalies of the kidney and urinary tract [29]. Almost all patients with UTIs might have required inpatient treatment regardless of the COVID-19 epidemic, so our survey (HPIDS) may have shown the number of patients with UTIs. Unlike other diseases, the number of patients with UTIs was similar pre- and post-COVID-19 (109 and 90 patients) (Table 1). This suggests that UTIs were not affected by the current infection control measures, and that they are not spread directly from child to child.
The cause of KD is still unknown, but epidemiology strongly suggests the involvement of infectious factors in triggering its onset [30]. In Japan, the number of patients peaks in January every year, decreases in February, increases from spring to summer, and decreases again in September–October [31]. A similar trend was seen from HPIDS during the study period, but the number of hospitalizations after the school closure period in 2020 was lower than during the same period in 2019 (Fig. 2). Many infectious diseases decreased after the school closure period suggesting that infectious diseases may indeed be involved in triggering the onset of KD.
During the COVID-19 epidemic worldwide, there have been several reports of critically ill patients with symptoms like KD [[32], [33], [34]]. This was described and understood as a disease different from KD, called multisystem inflammatory syndrome in children (MIS-C) [35]. Compared with KD, MIS-C often presents with severe abdominal pain and myocardial dysfunction, and requires intensive care [36,37]. The median age for MIS-C was 9–10 years. This is in marked contrast to KD, which occurs predominately in children ≤5 years old [35,37]. KD is often seen in Asian children, including in Japan, but MIS-C is rarely reported in Asian children, and is recorded predominantly as an issue among African children [35]. Not all the 270 patients with KD in this study underwent COVID-19 tests, but there were no reports of the severe symptoms or characteristics associated with suspected MIS-C. There had been almost no reports of MIS-C in Japan, despite many worldwide. However, Fukuda et al. reported the first case of MIS-C in a Japanese boy in April 2021 [38]. The Japanese Pediatric Society and Japanese Society of Kawasaki Disease also published notification that there have been a few patients who may be MIS-C in Japan from mid-February 2021 [39]. It is therefore likely that MIS-C may become apparent in Japan when the number of children with COVID-19 has increased.
We have therefore found that many infectious diseases decreased after the outbreak of COVID-19. We targeted patients who needed to be hospitalized, so is likely to show relatively little effect of patients refraining from seeing a medical institution for fear of being infected with COVID-19. Doctors may also have performed rapid tests (e.g., influenza, RSV, and rotavirus/norovirus/adenovirus gastroenteritis) more actively on admission than among outpatients, to improve infection control. Our study is therefore considered to be able to detect rapid test positive patients more accurately. Our findings may not only reflect changes in awareness and behavior associated with COVID-19. For example, viral interference, which is a phenomenon where one virus out-competes and suppresses the replication of other co-infecting viruses, may also be happening [[40], [41], [42]]. Certainly, the decrease in the number of children with many infectious diseases post-COVID-19 is surprising.
Several limitations of this study should be highlighted. First, we started the HPIDS survey from July 1, 2019, so we have only 8 months of data pre-COVID-19. It is therefore not possible to compare the post-COVID-19 period with data from several years before the pandemic. Second, to compare the number of patients between pre-COVID-19 and post-COVID-19 in the same months, we omitted patients from March to June 2020, the period shortly after COVID-19 spread. Third, for several diseases such as influenza, mycoplasma, and rotavirus/norovirus/adenovirus gastroenteritis, we included patients with positive rapid tests. Rapid antigen tests or antibody tests have problems of sensitivity and specificity, so may not be reliable for diagnosis. We also did not specify rapid tests and the 18 hospitals may therefore not have used the same products. This could have resulted in some detection bias.
In conclusion, the number of children who needed hospitalization for infectious diseases decreased markedly after the COVID-19 epidemic in Japan. We suggest that current infection control measures for COVID-19 such as wearing masks, washing hands, and disinfecting hands with alcohol are effective against various infectious diseases. However, these effects vary by disease and the result of our survey may not only reflect infection control measures for COVID-19. Further surveys are required to evaluate whether the outbreak of many infectious diseases can be suppressed in the long term.
5. Authorship statement
All authors meet the ICMJE authorship criteria.
Author contributions
Yuya Fukuda drafted the manuscript and contributed to the data analysis. Takeshi Tsugawa prepared the final draft, critically revised the article for important intellectual content. Yukihiko Kawasaki critically revised the article for important intellectual content. Tomohiro Nawa, Atsuo Togashi, Jun Kunizaki, Satoshi Hirakawa, Junya Iida, Toju Tanaka, Toshitaka Kizawa, Dai Yamamoto, Ryoh Takeuchi, Yoshiyuki Sakai, Masayoshi Kikuchi, Kazushige Nagai, Hirofumi Asakura, Rina Tanaka, Masaki Yoshida, and Ryo Hamada acquired or interpreted data. All authors contributed to the writing of the final manuscript and gave final approval for the article to be published.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
The authors declare that they have no conflicts of interest.
Acknowledgement
We would like to thank all the pediatricians who participated in this survey. We thank Melissa Leffler, MBA, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript. We thank Shiro Hinotsu for supervising data analysis of this manuscript.
References
- 1.National Institute of Infectious Diseases Japan. Infectious diseases weekly report. https://www.niid.go.jp/niid/en/idwr-e.html Accessed.
- 2.Hokkaido Infectious Disease Surveillance Center Sentinel weekly reporting diseases. https://www.iph.pref.hokkaido.jp/kansen/weekunitinfection.html (in Japanese) Accessed.
- 3.Hokkaido government Data for COVID-19 in Hokkaido prefecture. http://www.pref.hokkaido.lg.jp/ss/dtf/opendata/covid19.htm (in Japanese) Accessed.
- 4.Hokkaido Government Board of Education Information about COVID-19. http://www.dokyoi.pref.hokkaido.lg.jp/hk/ktk/corona.htm (in Japanese) Accessed.
- 5.World Health Organization Transmission of SARS-CoV-2: implications for infection prevention precautions. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions Accessed.
- 6.Centers for Disease Control and Prevention Science brief: SARS-CoV-2 and potential airborne transmission. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html Accessed.
- 7.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19). How to protect yourself & others. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html Accessed.
- 8.Gandhi Monica, Beyrer Chris, Goosby Eric. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063–3066. doi: 10.1007/s11606-020-06067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of Health, Labout and Welfare, Japan Prevention measures against coronavirus disease 2019 (COVID-19) https://www.mhlw.go.jp/content/10900000/000597148.pdf Accessed.
- 10.Statistics Bureau of Japan Final report of 2015 “Population and households of Japan”. https://www.stat.go.jp/english/data/kokusei/2015/final_en/pdf/s01.pdf Accessed.
- 11.Centre for Health Protection of the Hong Kong Special Administrative Region Government CHP closely monitors cluster of pneumonia cases on Mainland. Dec 31, 2019. https://www.info.gov.hk/gia/general/201912/31/P2019123100667.htm Accessed.
- 12.Weber Thomas, Stilianakis Nikolaos. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J Infect. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paynter S. Humidity and respiratory virus transmission in tropical and temperate settings. Epidemiol Infect. 2015;143:1110–1118. doi: 10.1017/S0950268814002702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths Cameron, Drews Steven, Marchant David. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30:277–319. doi: 10.1128/CMR.00010-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vinci Alexandra, Lee Paul, Leonard Krilov. Human metapneumovirus infection. Pediatr Rev. 2018;39:623–624. doi: 10.1542/pir.2017-0213. [DOI] [PubMed] [Google Scholar]
- 16.Uche Ifeanyi, Guerrero-Plata Antonieta. Interferon-mediated response to human metapneumovirus infection. Viruses. 2018;10:505. doi: 10.3390/v10090505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter Nicholas, Grant Gavin, Bandy Utpala, Alexander Nicole, Winchell Jonas, Jordan Hannah, et al. Community outbreak of mycoplasma pneumoniae infection: school-based cluster of neurologic disease associated with household transmission of respiratory illness. J Infect Dis. 2008;198:1365–1374. doi: 10.1086/592281. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein David. Rotavirus overview. Pediatr Infect Dis J. 2009;28:S50–S53. doi: 10.1097/INF.0b013e3181967bee. [DOI] [PubMed] [Google Scholar]
- 19.Robilotti Elizabeth, Deresinski Stan, Pinsky Benjamin. Norovirus. Clin Microbiol Rev. 2015;28:134–164. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanaei Dashti Anahita, Ghahremani Pedram, Hashempoor Tayebeh, Karimi Abdollah. Molecular epidemiology of enteric adenovirus gastroenteritis in under-five-year-old children in Iran. Gastroenterol Res Pract. 2016;2016:2045697. doi: 10.1155/2016/2045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charles S. Dela Cruz, Pasnick Susan, Gross Jane, Keller Jon, Carlos Graham, Cao Bin, et al. Adenovirus infection and outbreaks: what you need to know. Am J Respir Crit Care Med. 2019;199:13–14. doi: 10.1164/rccm.1997P13. [DOI] [PubMed] [Google Scholar]
- 22.Joseph P. Lynch, III, Kajon Adriana E. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin Respir Crit Care Med. 2016;37:586–602. doi: 10.1055/s-0036-1584923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galanti M., Birger R., Ud-Dean M., Filip I., Morita H., Comito D., et al. Rates of asymptomatic respiratory virus infection across age groups. Epidemiol Infect. 2019;147:e176. doi: 10.1017/S0950268819000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asselah Tarik, Durantel David, Pasmant Eric, Lau George, Schinazi Raymond. COVID-19: discovery, diagnostics and drug development. J Hepatol. 2021;74:168–184. doi: 10.1016/j.jhep.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kratzel Annika, Todt Daniel, V’kovski Philip, Steiner Silvio, Gultom Mitra, Tran Thi Nhu Thao, et al. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020;26:1592–1595. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Hand hygiene recommendations. Guidance for healthcare providers about hand hygiene and COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/hand-hygiene.html Accessed.
- 27.Kampf G. Efficacy of ethanol against viruses in hand disinfection. J Hosp Infect. 2018;98:331–338. doi: 10.1016/j.jhin.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uzuner H., Karadenizli A., Er D.K., Osmani A. Investigation of the efficacy of alcohol-based solutions on adenovirus serotypes 8, 19 and 37, common causes of epidemic keratoconjunctivitis, after an adenovirus outbreak in hospital. J Hosp Infect. 2018;100:30–36. doi: 10.1016/j.jhin.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Leung Alexander K.C., Wong Alex H.C., Leung Amy A.M., Hon Kam L. Urinary tract infection in children. Recent Pat Inflamm Allergy Drug Discov. 2019;13:2–18. doi: 10.2174/1872213X13666181228154940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowley A.H., Shulman Stanford T. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018;11:374. doi: 10.3389/fped.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Japan Kawasaki Disease Research Center 25th nationwide survey on Kawasaki disease, 1 January 2017 to 31 December 2018. https://www.jichi.ac.jp/dph/wp-dph/wp-content/uploads/2019/09/1bb34be7b6c9f852c1df45cc2ac4152c-1.pdf (in Japanese) Accessed.
- 32.Toubiana Julie, Poirault Clément, Corsia Alice, Bajolle Fanny, Fourgeaud Jacques, Angoulvant François, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldstein Leora, Rose Erica, Horwitz Steven, et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdoni Lucio, Mazza Angelo, Gervasoni Annalisa, Martelli Laura, Ruggeri Maurizio, Ciuffreda Matteo, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowley A.H. Multisystem inflammatory syndrome in children and Kawasaki disease: two different illnesses with overlapping clinical features. J Pediatr. 2020;224:129–132. doi: 10.1016/j.jpeds.2020.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakra Natasha A., Blumberg Dean A., Herrera-Guerra Angel, Lakshminrusimha Satyan. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. 2020;7:69. doi: 10.3390/children7070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esposito Susanna, Nicola Principi Multisystem inflammatory syndrome in children related to SARS-CoV-2. Paediatr Drugs. 2021;23:119–129. doi: 10.1007/s40272-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukuda Sayaka, Kaneta Mio, Miyake Mayuko, Ohya Takashi, Miyakawa Kei, Iwamoto Mari, et al. A case of multisystem inflammatory syndrome in children in a Japanese boy: with discussion of cytokine profile. Mod Rheumatol Case Rep. 2021 Apr 27:1–11. doi: 10.1080/24725625.2021.1920140. [DOI] [PubMed] [Google Scholar]
- 39.About severe pediatric cases of Covid-19 in Japan Japanese pediatric society. https://www.jpeds.or.jp/modules/guidelines/index.php?content_id=129 (in Japanese) Accessed.
- 40.Wu Anchi, Mihaylova Valia T., Landry Marie L., Foxman Ellen F. Interference between rhinovirus and influenza A virus: a clinical data analysis and experimental infection study. Lancet Microbe. 2020;1:e254–e262. doi: 10.1016/S2666-5247(20)30114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nickbakhsh Sema, Mair Colette, Matthews Louise, Reeve Richard, Johnson Paul C.D., Thorburn Fiona, et al. Virus–virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci Unit States Am. 2019;116:27142–27150. doi: 10.1073/pnas.1911083116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dee Kieran, Goldfarb Daniel M., Haney Joanne, Amat Julien A.R., Herder Vanessa, Stewart Meredith, et al. Human rhinovirus infection blocks severe acute respiratory syndrome coronavirus 2 replication within the respiratory epithelium: implications for COVID-19 epidemiology. J Infect Dis. 2021;224:31–38. doi: 10.1093/infdis/jiab147. [DOI] [PMC free article] [PubMed] [Google Scholar]