Abstract
This review discusses the techniques available for detecting and inactivating of pathogens in municipal wastewater, landfill leachate, and solid waste. In view of the current COVID-19 pandemic, SARS-CoV-2 is being given special attention, with a thorough examination of all possible transmission pathways linked to the selected waste matrices. Despite the lack of works focused on landfill leachate, a systematic review method, based on cluster analysis, allows to analyze the available papers devoted to sewage sludge and wastewater, allowing to focalize the work on technologies able to detect and treat pathogens. In this work, great attention is also devoted to infectivity and transmission mechanisms of SARS-CoV-2. Moreover, the literature analysis shows that sewage sludge and landfill leachate seem to have a remote chance to act as a virus transmission route (pollution-to-human transmission) due to improper collection and treatment of municipal wastewater and solid waste. However due to the incertitude about virus infectivity, these possibilities cannot be excluded and need further investigation. As a conclusion, this paper shows that additional research is required not only on the coronavirus-specific disinfection, but also the regular surveillance or monitoring of viral loads in sewage sludge, wastewater, and landfill leachate. The disinfection strategies need to be optimized in terms of dosage and potential adverse impacts like antimicrobial resistance, among many other factors. Finally, the presence of SARS-CoV-2 and other pathogenic microorganisms in sewage sludge, wastewater, and landfill leachate can hamper the possibility to ensure safe water and public health in economically marginalized countries and hinder the realization of the United Nations' sustainable development goals (SDGs).
Keywords: COVID-19, SARS-CoV-2, Wastewater, Sewage sludge, Landfill leachate, Pathogenic microorganisms, Biohazards, Waste, Sustainable development goals, Pollution-to-human transmission mechanism
1. Introduction
Coronavirus disease (COVID-19) is the third major pandemic caused by a virus in the past two decades through zoonotic transmission after the severe acute respiratory syndrome coronavirus (SARS) in 2003 and middle east respiratory syndrome coronavirus (MERS-CoV) in 2012 (Nour and Houssam, 2019). Initially classified as “pneumonia of unknown etiology”, this infection was classified as Coronavirus on January 12, 2020, by World Health Organization (WHO) and later declared as COVID-19 (Niu et al., 2020). Infection with COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Poon and Peiris, 2020). Researchers discovered that SARS-CoV-2 is capable of rapid human-to-human transmission (Hui et al., 2020a, b), primarily through direct contact or sneezing, coughing, or talking (Anand et al., 2021a, b, c). In this frame it is important to remember that the possible sources of virus spread were also attributed to pollution (Bontempi, 2020b), meteorological (Anand et al., 2021a, b, c), and socio-economic factors (as for example trade exchanges (Bontempi et al., 2021a) (Bontempi and Coccia, 2021).
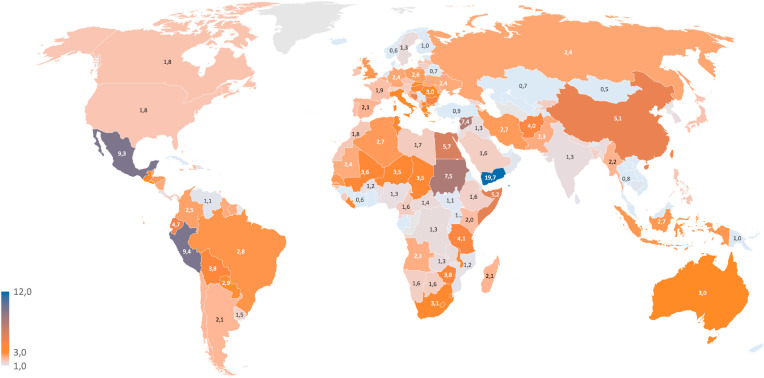
Being contagious, COVID-19 is a major concern due to its transmission at the global scale, repetitive emergence, mortality rate, and multiplicative effectiveness in susceptible groups (Field et al., 2020). The pandemic has resulted in major loss of human life globally and has laid down the unprecedented challenge to the economy, ecosystem, and health sector. As per WHO (WHO, 2021), there are 179,686,071 confirmed cases of COVID-19, including 3,899,172 deaths worldwide as noted on June 24, 2021 and the number is increasing rapidly. Fig. 1 below shows the % of deaths evaluated by considering the number of confirmed deaths and the corresponding infection cases until June 24, 2021 (data were sourced from the COVID-19 Data Repository by the Center for Systems Science and Engineering at Johns Hopkins University). The data show that the percentage of death is generally higher than 1 %, and less than 3 % for Europe. On the contrary in other countries this value results higher than 5 %, like in China, Peru, Yemen, and Mexico.
Fig. 1.
Percent of death, calculated by considering the cumulative COVID-19 confirmed deaths versus cases on June 24, 2021 (data sourced from the COVID-19 Data Repository by the Center for Systems Science and Engineering at Johns Hopkins University).
Since 2019, COVID-19 pandemic has presented a global public health emergency with scientists across the world racing against time to develop effective treatments for this virus. Although the United States Food and Drug Administration has endorsed several vaccines against COVID-19 (Anand, 2021b), they still pose some questions (Sahin et al., 2021). Though these vaccines have shown a lot of promise, they also raise concerns for their safety and effectiveness (Kelly et al., 2021). In fact, many patients in India have tested positive for COVID-19 following their second dose of vaccines. According to the Indian Council of Medical Research, more than 21,000 people tested positive following their first dose of Covishield or Covaxin, while over 5500 people tested positive following their second dose (https://news.abplive.com/2021).
Almost all the countries around the globe ordered complete or partial lockdown for weeks or longer, which were enforced through cancellation of mega-events, reduced transportation, lesser industrial activities, and lesser human to human exposure. This allowed a major improvement in the air quality index in almost all megacities of the world (Mostafa et al., 2021). Lokhandwala and Gautam (2020) have found a reduction of about 85 % in particulate matter levels in the most polluted city of India. The NO2 emission has dropped by 30–60 % in many European countries, including Rome, Paris, Madrid, Milan, and Barcelona (https://www.eea.europa.eu/highlights/air-pollution-goes-down-as). In the United States (US), the levels of NO2 have declined by 25.5 % during this COVID-19 period compared to previous years (Jesse and Keita, 2020). The lesser gaseous emissions during this period are also supported by the fact that the oil demand has dropped down to 4,350,000 barrels in the first three months of 2020 compared to the same period in 2019 (https://www.iea.org/articles/global-energy-review-co2-emissions-in-2020). Major industrial sources of pollution have stopped completely or partly (Zanoletti, 2021), which has enhanced the water quality of the rivers (Singhal and Matto, 2020). Travel restrictions have also resulted in less air or road traffic, which has reduced the noise pollution.
Despite that the current pandemic situation has in some cases reduced the environmental load, serious health issues have emerged majorly because of its contagious nature and unavailability of effective medical treatment (Coccia, 2021). It is a challenge for the public health officials right from diagnosing to the treatment of COVID-19 patients (Wolfel et al., 2020). Moreover, as it is a pandemic disease and a big population is affected, many countries are facing issues of insufficient testing capacities, inadequate hospitalization facilities, including beds, intensive care units (ICUs), doctors and medical staff (Bontempi, 2021). One additional complication is that many asymptomatic cases are also reported (Anand et al., 2021a, b, c). The history of such pandemics reveals that monitoring sewage for traces of a pathogen provides a sensitive signal of the presence of the pathogen in entire communities and can also predict whether the transmission is increasing or declining (Larsen and Wigginton, 2020). The science of wastewater-based epidemiology (WBE) by monitoring viral RNA in wastewater provides opportunities to assess the COVID-19 prevalence and spread in defined populations, proving beneficial for informing related public health policy (Wu et al., 2021). WBE can be processed to obtain an unbiased surveillance system and reflect community health (Scott et al., 2021; Yaniv et al., 2021; Tomasino et al., 2021). It is scalable, economical and provides rapid results for emergent and re-emergent pathogens (Polo et al., 2020; Randazzo et al., 2020; Tiwari et al., 2021).
The information gained from sewage can be used to refine the response to the pandemic and help the authorities to make strategies (Kumar et al., 2021b). Possible recommendations may be towards lockdown or quarantine. Researchers in Australia, the Netherlands, Sweden, and the USA have already detected SARS-CoV-2 in wastewater (Li et al., 2021a, b; Malapatty, 2020; Medema et al., 2020a, b). During the early week of the outbreak of COVID-19 in New Haven (Connecticut) several cases were tracked and declared positive by confirming the presence of SARS-CoV-2 RNA in primary sewage sludge from local wastewater treatment plants (Peccia et al., 2020). More importantly, as sewer systems continuously receive human excreta with viral particles irrespective of the symptomatology status of the patient (symptomatic/asymptomatic/subclinical/presymptomatic), WBE approach can provide a real-time and unbiased surveillance for the outbreak (Kordatou et al., 2020). Even during air travel or cruises, the wastewater provides an additional diagnostic tool of the prevalence of SARS-CoV-2 infections among passengers onboard (Ahmed et al., 2020a, b, c, d). At the same time, the virus persistence in wastewater opens other issues, related to its spread.
The mechanism for the detection of virus in sewage initiates by dilution of virus from feces and other shedding sources in toilet water, then in other municipal wastewater including grey water and some cases industrial and storm waters. Subsequently, the virus travels along with its RNA through a complex sewage system where it is exposed to different physical, chemical, and biological factors. Moreover, the detection of the virus is related to its concentration methods like polyethylene glycol (PEG) method for precipitation of viral material and ultrafiltration at molecular weight level (Aviles et al., 2021). However, the persistence and survival of the coronavirus in water systems depend on several factors like temperature, organic content, light familiarity, and the existence of antagonistic organisms (Bilal et al., 2020; Wigginton et al., 2015). At present, data about virus persistence in different regions and climatic conditions seem to be highly variable (Hart et al., 2020). Significant gaps in the knowledge of the wastewater contribution to the SARS-CoV-2 spread exist. Virus survival in the environment, including the soil, the air, water bodies, and sewage and landfill leachate, is still poorly known (Kitajima et al., 2020). Experimental data about SARS-CoV-2 inactivation and/or removal by sewage and the most suitable and verified treatment processes is scarce (Kitajima et al., 2020).
The enveloped coronavirus is considered unstable in the environment due to its higher susceptibility to oxidants like chlorine and is inactivated much faster in water than non-enveloped human enteric viruses (Rosa et al., 2020). Researchers have also tried a quantitative microbial risk assessment (QMRA) framework for estimation of SARS-CoV-2 in natural water bodies through dose-response model and Poisson's distribution (Kumar et al., 2021). However, despite the high number of papers devoted to sewage (mainly concerning wastewater surveillance to track COVID-19), considering it as an important tool in early diagnosis and prevention of its outbreak in urban areas, only few works were devoted to sewage sludge (Yang et al., 2020) and no works were devoted to landfill leachate as additional possible sources of virus spread in the environment. This study reviews the SARS-CoV-2 infectivity, surveillance, and inactivation as biohazard, that can be found in sewers, wastewater treatment plants, and landfills. Due to the possibility to use the reviewed technologies for surveillance and inactivation to pathogens in general, this work may be also used to have an overview of available possibilities to manage other outbreaks. Also the SARS-CoV-2 infectivity and reported transmission mechanisms may be useful to study or support similar behaviours for other pathogens.
2. Study design
To provide a comprehensive assessment of the surveillance, infectivity, and inactivation of SARS-CoV-2, the available literature was investigated by a systematic review.
COVID-19 pandemic has attracted great attention in literature: SCOPUS database shows the existence of more than 210,000 papers devoted to this argument (in July 2021). The present review paper is dedicated to wastewater, landfill leachate, and sewage sludge. However, as reported in the introduction, no works expressly dedicated to landfill leachate and the current pandemic were found, then the bibliographic search was devoted to “sewage sludge”, “wastewater”, and COVID-19 (or SARS-CoV-2). SCOPUS database allowed to identify about 200 papers containing these keywords in all available document fields.
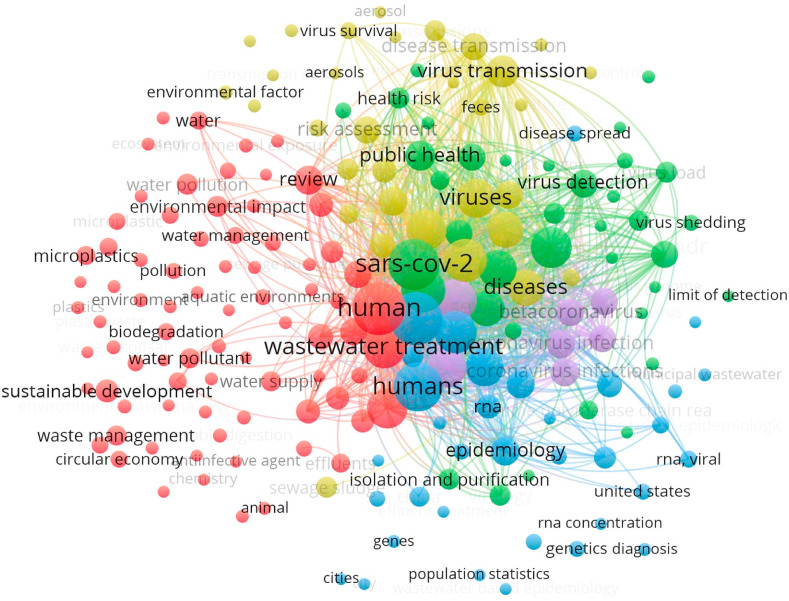
The downloaded information derived from the SCOPUS platform included for all articles title, authors, abstract, keywords, and references. In particular, an analysis of keywords co-occurrence network of papers devoted to “sewage sludge”, wastewater, and COVID-19 (or SARS-CoV-2) allowed to perform a cluster analysis of the literature (see Fig. 2 ) to propose a suitable and structured review. The study design (with connected literature) was updated on July 14, 2021. Literature data analysis was performed by “VOSviewer version 1.6.16,” 2020.
Fig. 2.
The keywords co-occurrence network of papers devoted to “sewage sludge”, wastewater, and COVID-19 (or SARS-CoV-2). The study design was updated on July 14, 2021. Data analysis was performed by “VOSviewer version 1.6.16,” 2020.
The results (see Fig. 2) show that the already available papers can be grouped in 5 clusters. The first one (79 items), represented by red bubbles, is mainly devoted to wastes generated during the pandemic and their toxicity, with attention to their management and treatment. In this cluster there are several papers treating wastewater and sewage sludge. Some interests related to the sustainable development can be also found. The second cluster (37 items), highlighted by green bubbles, concerns public health and virus characteristics and detection. The third cluster (34 items) is mainly centered on wastewater and the virus monitoring aspects (blue bubbles). The fourth cluster (42 items) is addressed to SARS-CoV-2 transmission mechanisms and sewage sludge and wastewater treatments (yellow bubbles). Finally, the last cluster (7 items) is more general: it is centered on coronavirus and pandemic (violet bubbles).
Based on the results of cluster analysis, the paper was conceived in some categories (developed in the Results and discussion, section 3), devoted to presence and risks associated to SARS-CoV-2 (3.1), its potential transmission routes (3.2), and its detection and treatment (3.3). Finally, based on the literature results, attention is also dedicated to clean water and sanitation for a sustainable future (3.4).
Due to the lack of papers devoted to “landfill leachate” and COVID-19, general literature concerning this waste was checked.
3. Results and discussion
In this era of COVID-19, inadequate wastewater treatment and management (especially in economically marginalized countries), whether at the urban, rural, domestic, or industrial level, pose a high risk and major challenge due to the massive amount of biomedical waste and wastewater that finds its way to wastewater treatment plants. Biomedical waste generated by hospitals is typically incinerated or autoclaved before disposal. However, in some countries, such treatment facilities are insufficient due to poor infrastructure, and as a result, raw waste containing living pathogens may enter water systems, jeopardizing the environment (Iyer at al., 2021). For example, in some cities in Bangladesh, many residents' septic tanks are directly connected to open drains, allowing their waste to be released into the environment without their knowledge (Jeffrey, 2018). Moreover, landfill leachate generated during this pandemic may contain pathogenic microorganisms and promote the SARS-CoV-2 migration in the environment, threatening society and leaving many scientific challenges.
These situations may be a hindrance to human health, especially in the ongoing pandemic situation. The invasion of SARS-CoV-2 needs to be assessed in each of the unit operations in the sewage treatment plant along with the presence of other hazardous pathogenic microbes.
Microorganisms such as pathogenic bacteria, virus, and worm eggs, as well as other physio-chemical pollutants, are commonly found in such a stream (Suresh et al., 2018, 2021). They are further divided into four categories: bacteria, viruses, protozoa, and helminths. The presence of many pathogenic or non-pathogenic (E. coli) bacteria (>1010/g) in septic tank sludge and wastewater sludge could greatly affect human health conditions.
Excreta from SARS-CoV-2-infected humans contains such organisms and finds way into sewage, which might pose a serious danger. There is a potential risk of SARS-CoV-2 transmission among people due to the dumping of wastewater or sewage into perennial streams or rivers (Ducoli et al., 2021). Despite such an ominous possibility, the current opinion, especially of most water microbiologists, is that wastewater does not appear to be a significant mode of SARS-CoV-2 transmission (Ahmed et al., 2021). Recently, SARS-CoV-2 highlighted the presence of risks in septic tank sludge and sewage sludge (Zambrano-Monserrate et al., 2020; Anand et al., 2021a, b, c). Coronavirus is entering into sewage stream from different sources like restroom, public toilets, hospitals, schools, and office buildings (Daughton, 2020; Heller et al., 2020; Usman et al., 2020). Diarrhea, typhoid, cholera, paratyphoid, and other types of diseases occur due to water-borne pathogenic organisms detected in wastewater/sewage. In addition, sewage sludge also contains viruses and pathogenic protozoa which comes from mainly infected humans, i.e., human adenoviruses, enteroviruses (e.g., polioviruses), diarrhea-causing viruses (e.g., rotavirus), hepatitis-A virus and reoviruses, Balantidium coli, Entamoeba histolytica and Giardia lamblia, helminths (parasitic worms) (Bibby and Peccia, 2013a, b; Carr and Strauss, 2001).
On another side, solid waste landfills or leachate contains pathogenic microbes (fecal coliforms, salmonellae, human enteroviruses, human noroviruses, and protozoan parasites), which mainly comes from food waste, pet feces, human disposable hygiene products, and biosolids from sewage plants. In particular, an increase of plastic use derived from the health dispositives (as for example from disposable facemasks), has increased the amount of waste destined to landfilling, with a risen concern about the possibility of SARS-CoV-2 migration in the leachate (Ragazzi et al., 2020).
Typical indicator bacteria (total coliforms, fecal coliforms, and fecal streptococci) were estimated in the early 19th century. However, despite that the first publications indicated that these pathogens are not a major concern in solid waste landfills or leachate (Peterson, 1974; Haas, 1996), more recent reports from the United States Environmental Protection Agency (USEPA) identified different pathogenic bacteria and viruses present in the solid waste landfills or leachates, with increasing risks to the human health (Gerba et al., 2011; USEPA, 2008; USEPA, 2009).
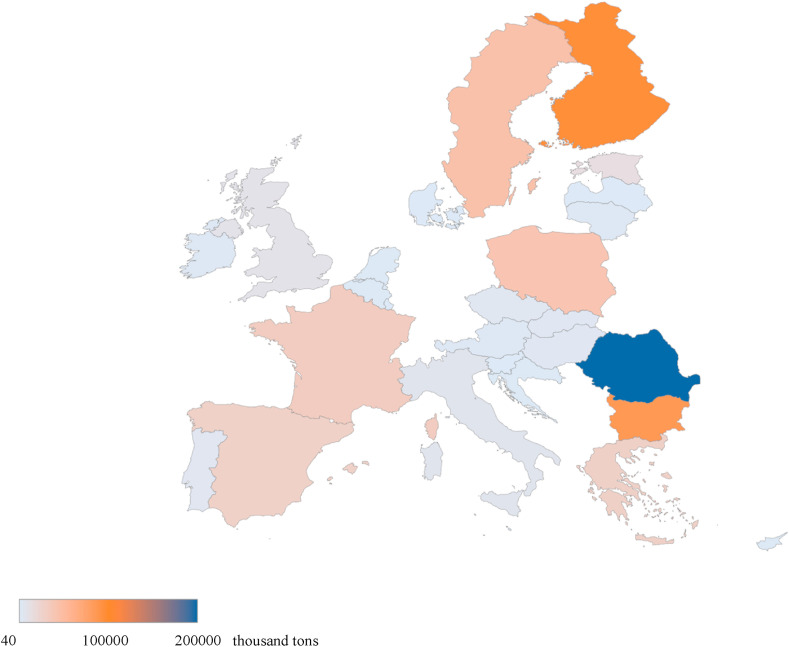
Fig. 3 shows the total amount of waste landfilled in EU in 2019. More recent data, reporting also the expected increase of landfilled waste due to pandemic, are still not available. However, it is estimated that landfilled waste is globally increased more than 3500 thousand tons in the first pandemic year, only due to facemasks disposal (Silva et al., 2021). It is evident that several countries will have a huge problem in waste management, with high consequent risk derived from possible pollution due to leachate, and possibility of virus spread (Hantoko et al., 2021).
Fig. 3.
Total amount of waste landfilled in 2019 (data are reported in thousand tons) (Eurostat, 2021).
3.1. Presence and potential risks of SARS-CoV-2 in sewage sludge and landfill leachate
Nghiem et al. (2020) examined the impact of the COVID-19 pandemic on waste and wastewater services. The lifespan of SARS-CoV-2 varies depending on the material, ranging from 3 h in aerosols to 2–3 days in sewage/solid feces. As a result of their long periods of survival, anyone who comes into contact with contaminated materials during that period is likely to become infected (Nghiem et al., 2020).
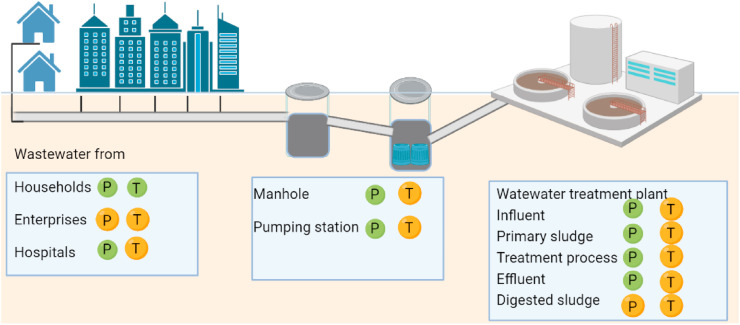
SARS-CoV-2 has spherical particle with spike proteins on its surface and positive-sense single-stranded RNA within the lipid envelope (Zhu et al., 2020). SARS-CoV-2 virions can be transmitted via air as part of aerosols with its nature of size (Anand et al., 2021a, b, c). COVID-19 patients can excrete feces and sputum with high loads of SARS-CoV-2 that eventually enter the wastewater. Several research groups, for example Australia, the Netherlands, Sweden, and the USA, have already reported detecting traces of SARS-CoV-2 in wastewater (Ahmed et al., 2020a, b, c, d) (Fig. 4 ). Randazzo et al. (2020) reported the finding of SARS-CoV-2 RNA in six wastewater treatment plants (WWTPs) serving the major municipalities in the region of Murcia in Spain. The water samples from the primary and the secondary treatments were both positive, while that from the tertiary treatment was negative. SARS-CoV-2 RNA concentrations in primary sewage sludge were linked to COVID-19 cases in New Haven, Connecticut (Peccia et al., 2020), and Ottawa (D'Aoust et al., 2021). The presence of SARS-CoV-2 RNA in untreated wastewater and sludge from WWTPs from various studies around the world is shown in Table 1 and Fig. 4. Few reports show virus in the sewage sludge or leachates, like SARS-CoV (Gorbalenya et al., 2020; Cheng et al., 2007).
Fig. 4.
Presence and transmission risks of SARS-CoV-2 in wastewater system. Green and orange dots labeled with ‘P’ are for confirmed presence and potential presence, respectively. The transmission risk of SARS-CoV-2 to human is marked with green and orange dots labeled with ‘T’, for confirmed transmission and potential transmission from wastewater, respectively.(For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
SARS-CoV-2 RNA detected in untreated wastewater/sludge from WWTPs from various studies.
| Sample locations | Sample types | Sample storage/treatment before analysis | Target genes analyzed | Reference |
|---|---|---|---|---|
| France [Paris] | Wastewater samples from WWTPs. | 4 °C; <24 h | RdRp | Wurtzer et al. (2021s) |
| India [Chennai] | Samples of hospital wastewater (HWW) | Ice cooled | N1, N2 | Chakraborty et al. (2021) |
| India [Ahmedabad] | Treated wastewater samples for receiving water bodies | 4 °C | ORF1ab, N and S | Kumar et al. (2021) |
| Israel (different Cites] | Wastewater in its raw state from various WWTPs | −80 °C or − 20 °C | E | Bar-Or et al. (2021) |
| Italy [Milan and Monza] | Effluent from three WWTPs and receiving rivers, both raw and treated | No information | ORF1ab, N, E | Rimoldi et al. (2020) |
| Italy [Milan and Rome] | Raw wastewater from three WWTPs | −20 °C | ORF1ab, S | La Rosa et al. (2020) |
| Japan [Yamanashi] | Wastewater from a single WWTP that was treated | Ice; <6 h | N1, N2 | Haramoto et al. (2020) |
| Netherlands [ Different Cities] | Raw samples from WWTPs in five cities and one airport | Melting ice; <24 h | N1, N2, N3, E | Medema et al. (2020a), b |
| Southeastern Queensland (Australia) | Raw wastewater from a pumping station and two WWTPs | −80 °C; <24 h | N | Ahmed et al. (2020a) |
| Spain [Murcia] | Sewage from six WWTPs, both raw and treated | 4 °C; <24 h | N1, N2, N3 | Randazzo et al. (2020) |
| USA [Massachusetts] | Raw wastewater from a single WWTP | 4 °C/30min@90 °C | N1, N2, N3 | Wu et al. (2021) |
| USA [New Haven, Connecticut] | Primary sewage sludge from a single WWTP | −80 °C | N1, N2 | Peccia et al. (2020) |
| Spain [Ourense in north-western Spain] | Raw wastewater and sewage sludge from a single WWTP | 4 °C; <24 h | E, N, ORF1ab, RdRP and S | Balboa et al. (2021) |
| Turkey [Istanbul] | Primary sludge and waste activated sludge from different WWTPs | No information | RdRp | Kocamemi et al. (2020) |
| Ottawa [Canada] | Primary sludge from different WWTPs | 4 °C; <24 h | N1, N2 | D'Aoust et al. (2021) |
Their findings suggest that wastewater monitoring could be a useful tool for tracking COVID-19 spread. Peccia et al. (2020) took daily sludge samples, which are solids that settle during the first stages of municipal wastewater treatment. Using RT-qPCR techniques, they discovered 1.7 × 103 to 4.6 × 105 virus RNA copies per milliliter of primary sludge. They found that sludge SARS-CoV-2 RNA concentrations matched the trends of new COVID-19 cases and hospitalizations when compared to publicly available data.
Wang et al. (2005) reported RNA of SARS-COV present in the excreta of some patients. Hung (2003) reported in 2003, transmission of SARS through sewage pipes, Amoy Gardens apartment, Hong Kong. Similar SARS-CoV-2 RNA was found in the sewage sludge as SARS-CoV-2 was isolated from COVID-19 patients' feces and urines (Randazzo et al., 2020). While the presence of virus RNA does not always mean that the virus is infective, the RNA may, however, persist in the media, even though the virus itself may have been inactivated. Moreover, these viruses may survive in the wastewater or sludge. Indeed, it has also been reported that the human coronavirus had been detected in sewage sludge (Bibby and Peccia, 2013a, b) and the possibility of secondary transmission (Liu et al., 2020).
The coronavirus spread depends on specific sludge treatment and disposal (Mandal et al., 2020). A treatment plant includes transportation, mechanical maintenance, routine sampling and detecting, with high possibility of being exposed to sludge and risk of infection. The generation of aerosol is another important exposure route to a high chance of infection. In a biological sludge treatment, mechanical agitation, sludge dewatering, shearing the liquid surface leads to the release of pathogenic sludge into the air in the form of airborne pollutant (Ducoli et al., 2021). Van Doremalen et al. (2020) found SARS-CoV-2 could survive in aerosols and remain infectious for several hours. Furthermore, the authors speculate on the possibility of COVID-19 spreading through water, soil, flow to the surface, and groundwater (Anand et al., 2021a, b, c; Anand et al., 2021b). While there are yet no concrete reports on this, given the unprecedented dynamism of the SARS-CoV-2, such possibilities, we believe, cannot be summarily dismissed and further research is necessary in this area.
Some sludge treatment techniques have an important role in eliminating some virus; anaerobic digestion with mesophilic and thermophilic conditions shows positive results in virus elimination (Gundy et al., 2009).
Moreover, solvents and detergents are present in sludge that can deactivate the viral envelop with ambient temperature. In the oxidation pond with elevated pH, long retention times and proper sunlight is a very good option to treat the sludge and to destroy the pathogen (WHO, 2020). Composting of sludge could inactive male-specific coliphage (Viau and Peccia, 2009). Based on experience, sludge treatment and disposal process should maintain in a closed system to reduce the risks of viral contact with the human and ecologic environment.
The common treatment for medical waste is through incinerators, landfills, autoclaves, furnaces, and kilns etc. Zanda and Heir (2020b) reviewed safe separation, handling, and disposal of municipal solid waste, including medical wastes from the hospital and non-hospital sources in the Tehran region. They suggested substantial structural modification in waste management is required, from the separation and storage guidelines at homes and hospitals to the safety protocols for waste collection teams during the pandemic. For example, an uncorrected management of medical waste destined to landfill may generate risk of SARS-CoV-2 spread, due to the possibility that the virus could migrate from the waste in the landfill and then into the landfill leachate. This not only may represent an additional source of environmental contamination, but also a potential exposure risk to landfill workers or workers handling the leachate after it is removed from the landfill (Langone et al., 2020). Then, the current situation of the COVID-19 pandemic requires special designed treatments due to the excess flow of pathogen laden waste and personnel protective equipment. It involved high health risks for handling workers using alternative disposal of medical waste as co-incinerate in the cement plants (Ducoli et al., 2021). Automatic incinerators can reduce the human contact with pathogens in the solid waste. Furthermore, some of the operating conditions of thermal treatment should be strictly maintained to destroy any pathogens in the medical wastes (WHO, 2017). While limited evidence was found that sludge would play an essential role in SARS-CoV-2 transmission, there is still a need to understand the fate of coronaviruses outside the human host, including their persistence and inactivation mechanisms in sludge, which would help to reveal their potential risks during sludge treatment and disposal.
3.2. Virus potential transmission via sewage and landfill leachate—Routes of environmental exposure
Viruses can transmit potentially from wastewater. This could be due to the inhalation of infectious droplets generated by contaminated waste (as happens when contacting with infected persons, which has been diffusely discussed in the literature (Bontempi, 2020a), drinking, and direct contact with contaminated water (Ducoli et al., 2021)). Water is commonly recognized to be the main vehicle of virus transmission. Most of the viruses present in the human's gastrointestinal tract can be discharged into the environment through feces, resulting from possible pollution of surface water and groundwater (Paleologos et al., 2020). As a result, the drinking of contaminated water and/or the consumption of fruits or vegetables deriving from sites contaminated with such water may be the source of virus transmission that can be recognized as a pollution-to-human transmission mechanism. This is different to the well-documented human-to-human transmission mechanism due to the contact and/or proximity to infected people.
In addition, food can be an origin of transmission, considering contamination originated from sewage (García-Ávila et al., 2020). It has been recently shown that SARS-CoV-2 can bioaccumulate in mollusks and other aquatic organisms, with its consequent possible presence in seafood markets (which is also one of the suspicious primary origins of SARS-CoV-2), with a viral transmission from wastewater to surface water bodies (for example sea) in a circular contamination process (Mordecai, 2020). In this context, it is important to cite that combined sewer overflow (CSO) caused by heavy rainfalls have been reported as a possible source of COVID-19 diffusion. In particular, this possibility was attributed to overflows of sewage that are not still opportunely treated (that may contain human wastes mixed with stormwater runoffs). This risk may occur in urban spaces and in water bodies (Han and He, 2021), with several possible negative consequences.
Although only few papers have proposed the SARS-CoV-2 infectivity due to its persistence in the municipal sewage (Bivins et al., 2020), several papers have already demonstrated that SARS-CoV-2 remains active when it is present in the feces and urine of infected people (Jeong et al., 2020; Sun et al., 2020). The inhalation of polluted water droplets can happen in all the environments where an infected sewage source can be present (Bogler et al., 2020; Kang et al., 2020; Bogler et al., 2020; Kang et al., 2020). For example, several researchers reported that the virus presence in air samples of wastewater treatment plants strongly suggest that wastewater aerosols can be a source of SARS-CoV-2 spread (Kitajima et al., 2020). Some studies developed a quantitative microbial risk analysis that showed a relatively high risk of infection deriving from bioaerosols exposition (Gholipour et al., 2021). However, it is important to highlight that the lack of a SARS-CoV-2 model for dose-response is a critical limitation for conducting quantitative microbial risk analysis for this virus (Gholipour et al., 2021). It is also important to underline that some safety measures (that may have different social acceptance (Bontempi et al., 2020; Bontempi, 2021), such as facemask wearing, can contribute to decreasing occupational health risk also in wastewater treatment plants. On the contrary, the SARS-CoV-2 transmission through the fecal-oral way has been only postulated (García-Ávila et al., 2020, Yeo et al., 2020). Information concerning contamination from urine samples needs further and in-depth investigation because the reported results are inconsistent (Anayah et al., 2021).
Finally, consideration must also be specifically addressed to the biosolid derived as by-products of wastewater treatment plants processes that may also contain human viruses (Brisolara, 2021), with the consequence of their possible transfer in wastewater systems and/or soil (Fitzmorris et al., 2021). Indeed, it is fundamental to underline that land application of biosolids may result in another potential way to generate aerosol (Siddique et al., 2020). This may be considered as another SARS-CoV-2 transmission pathway connected to pollution-to-human transmission through droplets and aerosols (Bontempi, 2020b). This can cause concerns because of pandemic, needing to re-consider the land applications of biosolids under the conditions. Finally, untreated sewage may also be used for soil penetration or exfiltrate accidently to underground soils. However, the SARS-CoV-2 survival in the soil is not still supported by solid literature (Wiktorczyk-Kapischke et al., 2021), and then it is difficult to predict its possibility of transmission to humans.
3.2.1. Sewage as a vector of SARS-CoV-2 and infectivity
Sewage that can also be defined as domestic wastewater or municipal wastewater is the untreated wastewater produced by a human community. In addition to water, it mainly consists of organic matter, viruses, bacteria, heavy metals, and inorganic salts. Due to its direct discharge in water bodies (rivers and streams) by many countries, sewage is often considered the oldest form of pollution. In particular, surface water, including rivers, oceans, estuary, and lakes, can be contaminated by viruses even after wastewater treatment due to the fact that sewage treated effluents often still contain some pathogens that could be discharged into surface water bodies (Okoh et al., 2010). Sewage, for example, may contain pieces of SARS-CoV-2 RNA. This is essentially caused by the feces of infected people or other shedding sources like sputum that end up in the sewage. It is extremely important to highlight that the presence of SARS-CoV-2 RNA does not directly mean that sewage can represent a way to transmit the contagious (Adelodun et al., 2020; Arslan et al., 2020) due to the possibility of virus inactivation. Indeed, there are several literature debating about the sewage ability to spread COVID-19 (Randazzo et al., 2020) due to the current lack of evidence. Moreover, SARS-CoV-2 RNA was already detected in sewage and wastewater in many countries (Davó et al., 2021; Weidhaas et al., 2021). Some research (e.g., Paleologos et al., 2020; Anayah et al., 2021) showed that residence time and temperature are the most significant factors that can be correlated with the diffusion/spread of COVID-19 in water.
3.2.2. Release of pathogens through landfill leachate
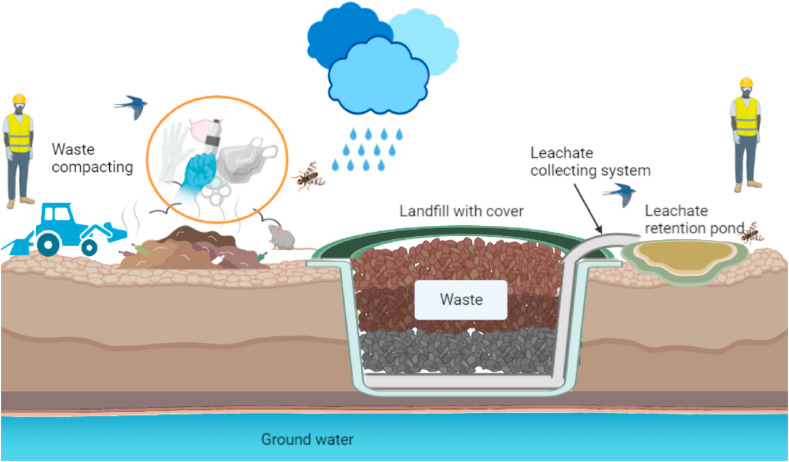
Non-suitable waste management, mainly in low-income countries, has opened several problems of possible pathogens propagation through waste (UN report, 2017). In this frame, the effect of the pandemic in the change of waste management strategies (mainly in developed counties) has been extensively treated in literature (Sharma et al., 2020; Zand and Heir, 2021), reporting the change in the composition and quantity of solid wastes due to COVID-19. On the contrary, the emerging challenges in waste management practices have been rarely addressed (Zand and Heir, 2020). Concerning the waste discarded in landfilling sites that may contain a large quantity of infected wastes, such as facemasks, gloves, plastics, and tissues contaminated with sputum, new suggested issues are connected with the possibility of generating liquid pollutants (Fig. 5 ). The persistence of pathogenic bacteria in leachate landfill can be dependent on temperature, salt content, pH, and predation with other pathogenic bacteria (Madigan et al., 2006). SARS-CoV-2 remained viable on different surfaces as cardboard, copper, stainless steel and plastic from 15 to 72 h under laboratory conditions (Van Doremalen et al., 2020). Infectious SARS-CoV-2 was also detected from tissue papers after 3 h, cloths after 2 days, glass and banknote for 4 days and a surgical mask for 7 days (Chin et al., 2020). In landfill site, waste compacting is commonly applied to minimize the size of waste before landfill. The persistence of SARS-CoV-2 on solid wastes thus poses transmission risks during waste compact and landfill process for on-site workers. Furthermore, most of the waste compacting process happens on open sites, attracting animals such as birds, bugs, or mice, which potentially become vectors for the transmission of SARS-CoV-2 to human (Fig. 5).
Fig. 5.
Generalized overview diagram of the waste compacting and landfill process.
Waste compacting coupled with the high moisture content of other collected wastes and precipitation mostly result in the landfill leachate (Zand and Heir, 2020). This liquid residue is a complex waste that may have different chemical compositions, depending on generating controlled nature and typologies of waste, among other factors (Iskander et al., 2018). Some sites collect the leachate into a tank or a retention pond for further treatment (Renou et al., 2008). The leachate in the leachate ponds can infiltrate in the soil, which can also occur due to the leaching and washing by rainfalll can also generate groundwater pollution, which can be considered a secondary way of SARS-CoV-2 spread in the environment (Langone et al., 2020). Then, secondary contagion sources of SARS-CoV-2, for example deriving from landfill leachate, is due to improper waste management that is more likely occurring in low-income countries, where the control and legislation about solid waste are lacking. During the pandemic, the leachate of landfills (also known as percolate) should be carefully processed, with the aim not only to avoid secondary pollution and virus spread but also to avoid the generation of aerosol that may occur during flushing or aeration processes in the treatment plant (Vaverková et al., 2020). It was published that the virus of avian influenza (H6N2) can be inactivated by landfill leachate, also due to the non-neutral pH of this waste (Graiver et al., 2009). However, the inactivation time was reported to be in the range of 30 and 600 days. Moreover, direct information regarding SARS-CoV-2 is lacking due to the lack of research about its fate in landfill leachate. It is thus essential to perform in-depth studies to investigate the potential virus presence in landfill leachate and its possibility to diffuse in the environment.
3.3. Detection and treatment of SARS-CoV-2
3.3.1. RT-qPCR methods of detecting SARS-CoV-2
The wastewater-based epidemiology (based on the principle of SARS-CoV-2 genetic material detection in wastewater) has been proposed as a promising approach to monitoring the pandemic status in real-time (Bivins et al., 2020). This is based on establishing a way for the surveillance of people infected within an area (that may be a city of a region) that excrete viral genomes into wastewater. WBE has the great advantage to have samples containing excrement from both symptomatic and asymptomatic people, returning a more real situation about contamination. Indeed, the current detection system is often not able to map a significant per cent of asymptomatic individuals that, on the contrary, can be accounted for by sewage analysis. Many works have reported SARS-CoV-2 molecular detection worldwide in wastewater in the USA, Australia, the Netherlands, and France (see, for example, Ahmed et al., 2020a, b, c, d; Lodder et al., 2020; Medema et al., 2020a, b). Generally, three SARS-CoV-2 genes (ORF1ab, S, and N) have been detected in the wastewater. Some of the studies depicted in the table (Table 2 ) focused on the presence of SARS-CoV-2 in various urban environments. Nevertheless, it was also reported that a step to ensure SARS-CoV-2 concentration is mandatory to increase the chance of subsequent virus detection, even if the analysis concerns untreated wastewater (Ahmed et al., 2020a, b, c, d; Lodder et al., 2020).
Table 2.
SARS-CoV-2 RNA detection methods used for untreated wastewater and sludge from WWTPs.
| Environmental samples | Methods | Primers or probes [providers]. | References |
|---|---|---|---|
| Wastewater | RT-qPCR | N_Sarbeco [Tib-Molbiol (Berlin, Germany)] NIID_2019-nCOV_N [TOYOBO, Japan] |
Ahmed et al. (2020b) |
| Wastewater Sewage sludge |
RT-qPCR | RdRp; N-gene; E-gene [Bio-Rad, Hercules, CA] | Balboa et al. (2020) |
| Wastewater | RT-PCR | 2019-nCoV_N1–F • 2019-nCoV_N1-R • 2019-nCoV_N1–P [CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel] | Medema et al. (2020a), b |
| Wastewater | RT-PCR | Fast Start Universal Probe Master, forward and reverse primers including, TaqMan probe [Sigma-Aldrich] | Bar-Or et al. (2021) |
| Raw wastewater | RT-qPCR | nucleocapsid N gene [CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel] | Wu et al. (2020) |
| Raw wastewater | RT-qPCR | E_Sarbeco_F • E_Sarbeco_P1 • E_Sarbeco_R [Tib-Molbiol (Berlin Germany)] | Wurtzer et al. (2021) |
Moreover, even if a lot of methods were developed to concentrate viruses in wastewater, it was shown that standard concentration methods, used for the most abundant viruses that can be found in environmental water samples, which have some specific structural and physical properties, are inefficient to recover coronavirus (Ye et al., 2016). The persistence of SARS-CoV-2 and the methods that may be used for its concentration have been firstly investigated by using surrogates, such as other coronaviruses and bacteriophage Φ6 (Silverman et al., 2020).
Some of these results, even if sometimes controversial (Kitajima et al., 2020), also support the idea that using a filter for virus concentration may have great potentialities to remove SARS-CoV-2 from sewage and water. Recent studies performed in the USA, Australia, the Netherlands, and France, have shown that RNA extraction of SARS-CoV-2 was possible by using several concentration methods, such as electronegative membrane adsorption, PEG precipitation, and ultrafiltration (Ahmed et al., 2020a, b, c, d; Medema et al., 2020a, b). In addition, the concentration of water volumes is a key factor that can affect the SARS-CoV-2 detection. The first studies treating SARS-CoV-2 molecular detection investigated samples up to 200 mL of raw wastewater (Ahmed et al., 2020a, b, c, d; Medema et al., 2020a, b). However, it is evident that in situations where the virus may be less prevalent, larger sewage volume in comparison to the standard used for other analysis may be necessary for the detection.
Concerning the SARS-CoV-2 detection, it primarily relies, at present, on reverse transcription-polymerase chain reaction (RT-PCR) and a quantitative assay (RT-qPCR) (see, for example, García-Ávila et al., 2020), being N protein gene the most reported target gene for these assays (Shirato et al., 2020). For example, the last developed assays based on this protein were found to be able to detect few RNA copies per reaction (Shirato et al., 2020; Corman et al., 2020). These results are supported by a recent work (Nalla et al., 2020) that shows comparative results about the performance of the RT-qPCR assay concerning the genes that are targeted (N, E, and RdRp).
It is also important to remember that RT-qPCR assays that are currently available at laboratory levels were developed for clinical diagnosis. Then their application in the environmental analysis may involve some unexpected problems and produce conflicting results (Ahmed et al., 2020a, b, c, d). To overcome this obstacle, it is strongly suggested to perform sequencing analysis or PCR pre-amplification, followed by sequencing, if RT-qPCR detects the virus RNA in wastewater samples (Ahmed et al., 2020a, b, c, d). In the future, new improvements or new techniques are foreseen. For example, it is expected that technologies will be improved to allow SARS-CoV-2 RNA detection more easily and rapidly, especially when laboratory facilities are not available. To limit the use of thermal cycler, essential to perform PCR, loop-mediated isothermal amplification methods are needed to be improved (Matsuyama et al., 2020). The use of digital RT-PCR can also introduce some advantages in detecting SARS-CoV-2 RNA in wastewater samples, such as higher sensitivity and accuracy in detection and quantification than RT-qPCR (Dong et al., 2021; Suo et al., 2020). Another critical issue of RNA analysis with molecular-based method is the inhibition, which requires process controls (Haramoto et al., 2018) to avoid the necessity to re-analyses the samples.
The conventional RT-PCR techniques used for analysis have some drawbacks, such as their unavailability in remote regions. They also are labor–intensive and requires high resources. Biosensors, some of them based on nanoparticles, were also explored as an alternative to RT-qPCR for the SARS-CoV-2 detection (Tetteh et al., 2020; Hui et al., 2020a, b). Nevertheless, for the integration of WBE into a conventional SARS-CoV-2 detection and surveillance context, other points need to be opportunely addressed, like the incidence of PCR false-positives and negatives (that may be due to unsuitable primers/probe or virus mutation in the targeted genome region), the SARS-CoV-2 persistence in wastewater and other environmental matrices (Medema et al., 2020a, b; Polo et al., 2020). SARS-CoV-2 RNA detection in sewage plants need to be investigated more accurately (Street et al., 2020; Gwenzi, 2020), and the proposed assays need to be studied and tested by multiple laboratories, also to better evaluate their sensitivities and viability.
Many research groups all over the world are monitoring wastewater for SARS-CoV-2 RNA. However, potential impacts of temporal and spatial variables on the correlation of RNA and human infection prevalence have been posed (Bivins et al., 2020; Li et al., 2021b). It is also important to investigate these relationships in different contexts, such as in low-income areas and rural locations (with decentralized infrastructure for wastewater management) and/or in urban centralized wastewater facilities. International collaborative activities must be done to assess the uncertainty and variation of the diverse settings, using systematic methodologies for the validation and the harmonization of the methodologies. Efforts in data sharing, the introduction of appropriate standards, suitable quantitative controls, and coordinated actions must also be encouraged. Efforts must also be devoted to suggesting suitable strategies for sample storage, virus concentration and extraction requirements.
In addition, efforts must also be made to recommend appropriate strategies for sampling, surveillance, and the reduction of infectivity as well as the decay of SARS-COV-2. An overall study design should be considered (Michael-Kordatoua et al., 2020). All these actions will help to ensure high quality, data defensibility, quality control, high-quality publications, and cross-laboratory comparability, to reach solid robustness of resulting data sets making able the scientific community to analyze transmission dynamics also considering various temporal and spatial levels (Bivins et al., 2020). Some concerns about sewage monitoring may include privacy implications. A protocol should be standardized to SARS-CoV-2 recovery and detection from environmental water samples. The protocol should include several of the described issues, such as concentration methods, detection assays, and process controls (Kitajima et al., 2020) and sampling and preserving methods.
3.3.2. Treatment technologies for the inactivation of SARS-CoV-2
Environmental persistence accounts for the time a pathogen can survive outside the human body. The virus is more likely to cause infection the longer it survives (Lahrich et al., 2021). The virus persistence is dependent on the type of environment (for example natural water or sewage) and by the environment chemical, physical and biological properties (for example, pH, temperature, and humidity) (Lahrich et al., 2021). The demonstrated capability of some viruses in comparison to bacteria to travel for long distances in the soil and probably into groundwater sources is attributed to their persistence that may occur for a considerable period (up to 17 days in the environment). Their limited size makes their removal extremely hard and the risk of contamination very high (Paleologos et al., 2020). Enveloped viruses were found able to survive for 6–7 days in sewage (Casanova et al., 2009), while it was suggested that SARS-CoV-2 might persist for periods ranging from 4 to 72 h on the surfaces, depending on the environmental conditions and the material surface nature (van Doremalen et al., 2020). This evidence supports the hypothesis that the SARS-CoV-2 virus may be present in water bodies, resulting in reservoirs for human diseases. As a consequence, virus inactivation is a fundamental factor that can allow to control virus fate and transport in the environment. By inactivation, a virus is destroyed or rendered non-pathogenic. This usually can be realized by altering the environment around the virus, that in turn produces an irreversible disruption and denaturation of the viral structure. It can be obtained by using physical, chemical, and/or energetic methods (Sakudo et al., 2011).
The EPA communicated that standard wastewater treatment and disinfection are expected to be effective for SARS-CoV-2 (EPA, 2020a, b). Similar considerations have been suggested by other water utilities in some European countries, such as Poland and Czech Republic.
On the contrary, China has proposed increased use of chlorine to improve its disinfection efficiency to reduce the possibilities of SARS-CoV-2 circulation by wastewater (Taleb et al., 2020), not evaluating the possibility that high concentration and high dose of disinfectants may promote the evolution toward antimicrobial resistance (Chen et al., 2021). On April 23, 2020, WHO guidance (WHO, 2020) has informed about the role of temperature, pH, and sunlight in reducing the virus persistence. However, it highlighted the possible occurrence of problems for wastewater treatment plants not optimized for the removal of viruses. It must also be considered that even if the available and most used treatment technologies show high performances for chemical, physical, and some microbiological contaminants (Adelodun et al., 2021), the removal of some human viruses (like enteric viruses) in the sewage is still unsatisfactory. In addition, regulatory standard procedures for the treatment of sewage-contaminated with viruses have not been introduced at this time. Despite that, a list of some active disinfectants against SARS-CoV-2 has been published (EPA, 2020b), essentially for surface use. On the contrary, no standard guideline exists related to sewage treatments.
SARS-CoV-2 is an enveloped virus with a fragile outer lipid membrane surrounding a protein capsule consisting of protein and glycoprotein, susceptible to chemical inactivation (García-Ávila et al., 2020) or other disinfectant solutions (Kitajima et al., 2020). For example, if chlorine is used, when it penetrates the lipid membrane, its reaction with the internal proteins leads to SARS-CoV-2 inactivation. Then, wastewater disinfection with chlorine dioxide or free chlorine has been reported to be effective (Tetteh et al., 2020). Wang et al. (2005) investigated the SARS-CoV persistence in hospital sewage, domestic sewage, urine, feces, wastewater and tap water. They also studied the role of contact time for the coronavirus inactivation in sewage with different chlorine and chlorine dioxide concentrations. Results showed that coronavirus can persist for a longer time at lower temperatures (at 4 °C in comparison to 20 °C) in domestic sewage, hospital sewage, and dechlorinated tap water. At 20 °C, coronavirus persisted in some fecal and urine samples for some days. They also showed that free chlorine resulted more effective inactivation in comparison to chlorine dioxide (Wang et al., 2005). However, a more recent work seems to be in contrast with the reported results, showing that disinfection seems less effective than other processes, as for example adsorption and coagulation for SARS-CoV-2 removal (Kumar et al., 2021c), highlighting the need of technological improvement (Ardito et al., 2021; Coccia and Watts, 2020; Coccia, 2019).
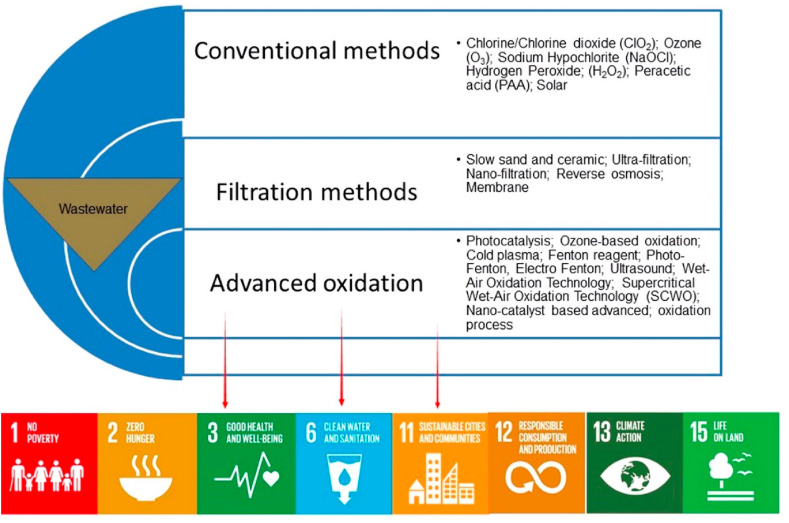
Other proposed disinfection strategies are based on advanced oxidation process, including ultrasonic process, Fenton and photo-Fenton, photocatalysis, UV irradiation, ozone combined with catalysts, and ozone combined with hydrogen peroxide (Tetteh et al., 2020). Several studies have experimented with hybrid methods where two or more technologies can be integrated to increase treatment efficiency and performance. Also, there are other strategies using solar and UV irradiation (Polo et al., 2015), coagulation and microfiltration (Matsushita et al., 2013), and ceramic water filtration (Farrow et al., 2013). A hybrid process consisting of several steps involving ozonation, coagulation, and ceramic membrane separation, has also been proposed to abate water contamination (Im et al., 2018). Several of the proposed technologies can be implemented at the plant scale in the treatment of municipal wastewater (Fig. 6 ).
Fig. 6.
Methods for inactivating microorganisms in wastewater treatment, and the impact of SARS-CoV-2 presence in wastewater on the United Nations Sustainable Development Goals.
Moreover, they are relevant only for large cities where suitable treatment plants are already in place. However, some studies propose advanced and expensive technologies that cannot be applied for low-income countries or when the implementation should be on a large scale, like the use of Ag for wastewater decontamination, that results in an advantage for photocatalytic degradation, ceramic pot filtration, and adsorption column (Liga et al., 2011). For wastewater remediation, magnetic nanomaterials have also been proposed as a promising wastewater treatment technology. In this case, contaminant-loaded magnetic particles can be selectively removed from the original solution using a magnetic field (Tetteh et al., 2020).
Membrane-coupled bioreactors are an effective system to remove SARS-CoV-2 (Lesimple et al., 2020). Literature generally assumes wastewater treatment technologies to be effective also for SARS-CoV-2, even though no experimental investigation was done. On the other hand, even if their effectiveness for SARS-CoV-2 must be investigated in detail, there are some simple existing technologies for virus decontamination, e.g., ceramic water filter (Farrow et al., 2014) due to their applicability in low-income countries and/or rural areas. Some of these simple technologies may also be improved and re-designed for application in these areas.
Finally, it is also extremely important to consider that excessive disinfectants and drug use of such may produce some effects on the environment (García-Ávila et al., 2020; Kuroda et al., 2021; Islam et al., 2020) that are not still considered or suitably evaluated. For example, in the environment, chlorine can be transformed into substances that can be highly dangerous for aquatic organisms. If it reacts with organic matter, it can also produce haloacetic acids (HAA) that are toxic for aquatic species can also be persistent for a long time in the environment (Li et al., 2019). Some research highlighted the overuse of antimicrobial agents as a possible reason for the increase in antimicrobial resistance (Koch et al., 2021 Anand, 2021a)
3.4. Pandemic and the water-related United Nations Sustainable Development Goals (SDGs) - clean water and sanitation for a sustainable future
COVID-19 is wreaking havoc on people's lives, destabilizing the global economy, and upsetting the lives of billions of people worldwide. The pandemic is a historic wake-up call, exposing profound inequalities and highlighting precisely the failures addressed in the 2030 Agenda for Sustainable Development and the Paris Climate Agreement (Sustainable Development Goals, 2021).
Despite that lockdown policies have generally produced a reduction of atmospheric emissions of several pollutants (Parida et al., 2021), the pandemic has caused negative effects on water, as discussed in the previous sections. Specifically, in connection with water pollution, pandemic has a high impact on several SDGs. Fig. 6 shows the proposed remediation technologies (discussed in section 3.3.2), that are strictly related to SDGs 3 and 6. However, in conventional methods, the addition of disinfectant chemicals frequently results in the formation of disinfection by-products (DPBs), which are again harmful and pose health risks. As a result, safe and environmentally friendly removal of microorganisms from wastewater is required to meet the United Nations SDGs. Viruses cannot be removed by microfiltration as they are too small. Most water filters will remove contaminants that are on the micron scale, however, viruses are only nanometers (1–100 nm) in size and will break through. Advanced oxidation processes (APOs) are convenient methods for degrading and detoxifying contaminants in water systems. The combination of systems such as UV radiation with ozone, hydrogen peroxide, and ferrous and titanium oxide are used for micro-pollutant removal. Viruses can be inactivated by oxidizing their DNA/RNA and protein envelope structures.
It also shows the SDGs that can be directly correlated with water management due to COVID-19 crisis. In particular, the possibility of virus survival on aquatic media can also impact SDG 2. Concerning food production, it is interesting to highlight that lockdown may promote the development of local food supply chains, that requires less transport and packaging in comparison to conventional food supply chains (Marusak et al., 2021; Thilmany et al., 2021), offering a better alternative for responsible consumption and production (SDG 12). However, sustainable food systems need to acquire food security that can be guaranteed by suitable irrigation and fertilizers. Therefore, the problem concerns not only water but also sewage sludge management that cannot be always reused as fertilizer (Ducoli et al., 2021). At urban level wastewater treatment management need particular attention, to limit the possibility of virus spread during the treatment (see section 3.2 with impact on SDG 11. In addition, it was shown that in several countries, wastes generated by personal disposal used because of pandemic (as for example facemask) are often discarded in the environment and consequently found in water bodies, as for example in wastewater gullies, inland waterways, and drainage canals. Also, this practice strongly impacts SG 11 (Benson et al., 2021) and SDG 15.
In developing countries, the food insecurity can cause the rise in poverty levels (SDG 1); the lack of safe water can lead to search for water far from home. The installation of piped water near the home promotes better economic development (Bontempi et al., 2021b). With large populations relying on forests for survival, land degradation, makes population more vulnerable to climate change that can exacerbate existing inequalities and problems related to safe water supply.
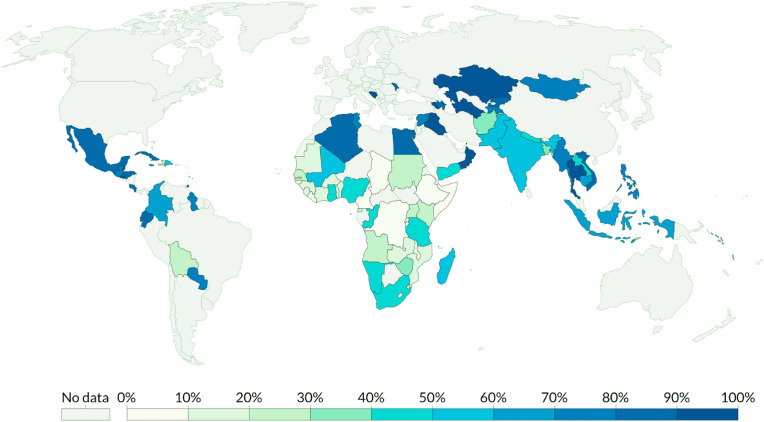
The sixth United Nations SDGs focus on the availability of clean water and better sanitation to all communities across the world. This is especially pertinent in the context of low-income countries since accessibility to these basic life necessities are the lowest in these countries. The pandemic has had a negative impact on water management strategies across the world in general, but low-income countries have faced tougher challenges in ensuring the availability of safe drinking water due to several infrastructural handicaps (Fig. 7 ). In particular, the proximity of the pit-latrine sanitation system and groundwater source is a major concern in many low-income countries while also open defecation close to the surface water is also a common practice (Adelodun et al., 2020). In these areas, most of the population, especially the population living in rural communities, largely rely on the surface and groundwater resources for the daily water consumption need. This not only can represent a local sanitary problem, but also can increase the risk of COVID-19 disease spread. In addition, the pandemic has raised the water supply-demand, for example, for the increase of the personal hygiene measures. In this context it is important also to highlight that, during the last century, water use is globally increasing, with more than twice the rate of population growth (TLGH, Editorial, 2020).
Fig. 7.
The proportion of the population with access to basic handwashing facilities, (SOURCE: World Health Organization (WHO) - United Nations Children's Fund (UNICEF) 2017, https://ourworldindata.org/sanitation).
Consequently, measures devoted to water resources protection and suitable treatment and discharge of wastewater are urgently needed. Improved sanitation facilities are necessary to ensure hygienic procedures to assure the separation of human excreta (avoiding direct human contact) from water sources destined to humans. COVID-19 pandemic has highlighted the extent of the existing gap in safe water availability worldwide and stressed the need to reach the SDG 6, addressed to guarantee water availability and sustainable management and sanitation world population by 2030. To reach SDG 6, more efforts are needed to develop and diffuse cost-effective technologies for the detection and disinfection of sewage. Reuse of wastewater for agricultural purposes to recover nutrients must be carefully considered and controlled. The potential for SARS-CoV-2 airborne transmission during sewage management due to aerosolisation of the virus must be carefully evaluated. However, the impressive global disruption due to pandemic offers an opportunity to proceed, react, and increase people's resilience. With a change of global vision and a revision of the priorities, we can achieve a more sustainable world by accelerating the process mainly concerning SGD 6 and addressing future environmental and health challenges.
4. Conclusions and future perspectives
This review paper concerns the surveillance, infectivity, and inactivation of SARS-CoV-2, addressed to wastewater, landfill leachate, and sewage sludge. Despite the lack of papers specifically devoted to landfill leachate, a systematic literature analysis allowed to provide some fundamental information. Also thanks to the recent advances in molecular biology SARS-CoV-2 (and other pathogenic organisms) has been detected in municipal wastewater, sewage sludge, and solid waste, imposing high risks of potential circulation and transmission. In particular, based on data (sometimes contrasting) about its fate and decay in urban wastewater and environment, SARS-CoV-2 has been reported to only induce a remote chance of pollution-to-human transmission.
However, this review paper shows that the knowledge of SARS-CoV-2 transmission routes and environmental fates still needs to be improved and that the effective surveillance and deactivation are critical, calling for significant efforts in establishing standardized and quantitative detection methods.
As a result, it is fundamental to conduct proper monitoring of wastewater for any pathogenic organism that has been scientifically identified. Natural water systems that receive untreated or inadequately treated wastewaters poses an even higher risk of transmitting infectious diseases. Change of wastewater and solid waste management will be critical in alleviating the problems and ensure UN SDGs to be achieved worldwide, especially in developing countries under the impact of COVID-19.
This study presents some limits. One of the main limitations of this research is due to the data source, which was confined to articles collected from SCOPUS till a certain data (on July 14, 2021). The information about COVID-19 and “sewage sludge” and wastewater obtained from the following periods may provide additional data that are not considered in this work. Another limitation of this work concerns the lack of data about COVID-19 and “landfill leachate”. This make necessary to use already published literature, concerning similar pathogens, but not directly related to SARS-CoV-2.
Credit author statement
Uttpal Anand: Conceptualization, Literature survey/mining, Writing-the major original draft preparation, Manuscript structure, Retrieved and arranged the references; Xuan Li: Reviewing, Editing, Figures preparation, Visualization; Kumari Sunita: Reviewing, Visualization, References collection; Snehal Lokhandwala: Review & editing, Revision, Response, Supervision; Pratibha Gautam: Writing – review & editing, Visualization, Retrieved and arranged the references; S. Suresh: Writing – review & editing, Visualization, Suggestions; Hemen Sarma: Critical review & editing, Figures and tables preparation, Suggestions, Revision; Balachandar Vellingiri: Suggestions, Response, Revision; Abhijit Dey: Suggestions, Response, Revision; Elza Bontempi: Conceptualization, Literature survey/mining, Investigation, Writing – review & editing, Formal analysis, Figure preparation, Manuscript structure, Formal interpretation, Validation, Project supervision; Guangming Jiang: Critical review & editing, Data curation, Suggestions, Visualization, Final draft, Project supervision
Funding
Dr Xuan Li is supported by the Australian Research Council Discovery project (DP190100385). Elza Bontempi acknowledges Regione Lombardia for the partial support of this research in the frame of the FANGHI project (call HUB Ricerca e Innovazione).
Declaration of competing interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are thankful to their respective departments/institutes/universities for providing space and other necessary facilities, which helped to draft this manuscript. Authors expresses their gratitude to Servier Medical ART for designing some figures.
References
- Adelodun B., Ajibade F.O., Ibrahim R.G., Bakare H.O., Choi K.S. Snowballing transmission of COVID-19 (SARS-CoV-2) through wastewater: any sustainable preventive measures to curtail the scourge in low-income countries? Sci. Total Environ. 2020;742:140680. doi: 10.1016/j.scitotenv.2020.140680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelodun B., Odedishemi Ajibade F., Ighalo J.O., Odey G., Gbemisola Ibrahim R., Yusuff Kareem K., Olalekan Bakare H., Olatunji Tiamiyu A., Ajibade T.F., Sholagberu Abdulkadir T., Akanni Adeniran K., Sook Choi K. Assessment of socioeconomic inequality based on virus-contaminated water usage in developing countries: a review. Environ. Res. 2021;192:110309. doi: 10.1016/j.envres.2020.110309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., Brien O., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Angel N., Bibby K., Bivins A., Dierens L., Edson J., Ehret J., Gyawali P., Hamilton K., Hosegood I., Hugenholtz P., Jiang G., Kitajima M., Sichani T., Shi J., Shimko K.M., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Zaugg J., Mueller J.F. Detection of SARS-CoV-2 RNA in commercial passenger aircraft and cruise ship wastewater: a surveillance tool for assessing the presence of COVID-19 infected travellers. J. Trav. Med. 2020;116:2020. doi: 10.1093/jtm/taaa116. taaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Koraikic A., Riddell S., Sherchan S.P., Simpson S.L., Sirikanchana K., Symonds E.M., Verhagen R., Vasan S.S., Kitajima M., Bivins A. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191:110092. doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bibby K., D'Aoust P.M., Delatolla R., Gerba C.P., Haas C.N., Hamilton K.A., Hewitt J., Julian T.R., Kaya D., Monis P. Differentiating between the possibility and probability of SARS-CoV-2 transmission associated with wastewater: empirical evidence is needed to substantiate risk. FEMS Microbes. 2021;2 doi: 10.1093/femsmc/xtab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U., Cabreros C., Mal J., Ballesteros F., Sillanpää M., Tripathi V., Bontempi E. Novel coronavirus disease 2019 (COVID-19) pandemic: from transmission to control with an interdisciplinary vision. Environ. Res. 2021;2019:111126. doi: 10.1016/j.envres.2021.111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand Potential environmental and human health risks caused by Antibiotic-Resistant Bacteria (ARB), Antibiotic Resistance Genes (ARGs) and Emerging Contaminants (ECs) from Municipal Solid Waste (MSW) landfill. Antibiotics. 2021;10 doi: 10.3390/antibiotics10040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand Potential therapeutic targets and vaccine development for SARS-CoV-2/COVID-19 pandemic management: A review on the recent update. Front. Immunol. 2021 doi: 10.3389/fimmu.2021.658519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U., Adelodun B., Pivato A., Suresh S., Indari O., Jakhmola S., Jha H.C., Jha P.K., Tripathi V., Di Maria F. A review of the presence of SARS-CoV-2 RNA in wastewater and airborne particulates and its use for virus spreading surveillance. Environ. Res. 2021:110929. doi: 10.1016/j.envres.2021.110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U., Bianco F., Suresh S., Tripathi V., Núñez-Delgado A., Race M. SARS-CoV-2 and other viruses in soil: an environmental outlook. Environ. Res. 2021:111297. doi: 10.1016/j.envres.2021.111297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anayah F., Al-Khatib I.A., Hejaz B. Assessment of water and sanitation systems at Palestinian healthcare facilities: pre-and post-COVID-19. Environ. Monit. Assess. 2021;193(1):1–22. doi: 10.1007/s10661-020-08791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardito L., Coccia M., Messeni Petruzzelli A. Technological exaptation and crisis management: evidence from COVID‐19 outbreaks. R&D Management. RADM. 2021:12455. doi: 10.1111/radm.12455. [DOI] [Google Scholar]
- Arslan M., Xu B., El-Din M.G. Transmission of SARS-CoV-2 via fecal-oral and aerosols–borne routes: environmental dynamics and implications for wastewater management in underprivileged societies. Sci. Total Environ. 2020;743:140709. doi: 10.1016/j.scitotenv.2020.140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilés P.C., Andrade I.M., Reyes J.C. Approaches applied to detect SARS-CoV-2 in wastewater and perspectives post-COVID-19. Journal of Water Process Engineering. 2021;40:101947. doi: 10.1016/j.jwpe.2021.101947. ISSN 2214-7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboa S., Mauricio-Iglesias M., Rodriguez S., Martínez-Lamas L., Vasallo F.J., Regueiro B., Lema J.M. The fate of SARS-COV-2 in WWTPS points out the sludge line as a suitable spot for detection of COVID-19. Sci. Total Environ. 2021;772:145268. doi: 10.1016/j.scitotenv.2021.145268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or Itay, Weil M, Indenbaum V, Bucris E, Bar-Ilan D, Elul M, Levi N, Aguvaev I, Cohen Z, Shirazi R, Erster O, et al. Detection of SARS-CoV-2 variants by genomic analysis of wastewater samples in Israel. Sci. Total Environ. 2021 doi: 10.1016/j.scitotenv.2021.148002. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson N.U., Fred-Ahmadu O.H., Bassey D.E., Atayero A.A. COVID-19 pandemic and emerging plastic-based personal protective equipment waste pollution and management in Africa. Journal of Environmental Chemical Engineering. 2021;9(3):105222. doi: 10.1016/j.jece.2021.105222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Peccia J. Prevalence of respiratory adenovirus species B and C in sewage sludge. Environ Sci Process Impacts. 2013;15(2):336–338. doi: 10.1039/c2em30831b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environ. Sci. Technol. 2013;47:1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal M., Nazir M.S., Rasheed T., Parra-Saldivar R., Iqbal H.M. Water matrices as potential source of SARS-CoV-2 transmission–An overview from environmental perspective. Case Studies in Chemical and Environmental Engineering. 2020:100023. doi: 10.1016/j.cscee.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J., Buttiglieri G., Calabro V., Carducci A., Castiglioni S., Cetecioglu Gurol Z., Chakraborty S., Costa F., Curcio S., de los Reyes F.L., Delgado Vela J., Farkas K., Fernandez-Casi X., Gerba C., Gerrity D., Girones R., Gonzalez R., Haramoto E., Harris A., Holden P.A., Islam M.T., Jones D.L., Kasprzyk-Hordern B., Kitajima M., Kotlarz N., Kumar M., Kuroda K., La Rosa G., Malpei F., Mautus M., McLellan S.L., Medema G., Meschke J.S., Mueller J., Newton R.J., Nilsson D., Noble R.T., van Nuijs A., Peccia J., Perkins T.A., Pickering A.J., Rose J., Sanchez G., Smith A., Stadler L., Stauber C., Thomas K., van der Voorn T., Wigginton K., Zhu K., Bibby K. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020;54(13):7754–7757. doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Bogler A., Packman A., Furman A., Kushmaro A., Ronen A., Dagot C., Hill C., Vaizel-Ohayon D., Morgenroth E., Bertuzzo E., Wells G., Kiperwas H.D., Horn H., Negev I., Zucker I., Bar-Or I., Moran-Gilad J., Balcazar J.L., Bibby K., Elimelech M., Weisbrod N., Nir O., Sued O., Gillor O., Alvarez P.J., Crameri S., Amon S., Walker S., Yaron S., Nguyen T.H., Berchenko Y., Hu Y., Ronen Z., Bar-Zeevm E. Rethinking wastewater risks and monitoring in light of the COVID-19 pandemic. Nature Sustainability. 2020;3:981–990. doi: 10.1038/s41893-020-00605-2. [DOI] [Google Scholar]
- Bontempi E. Commercial exchanges instead of air pollution as possible origin of COVID-19 initial diffusion phase in Italy: more efforts are necessary to address interdisciplinary research. Environ. Res. 2020;188:109775. doi: 10.1016/j.envres.2020.109775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. First data analysis about possible COVID-19 virus airborne diffusion due to air particulate matter (PM): the case of Lombardy (Italy) Environ. Res. 2020;186:109639. doi: 10.1016/j.envres.2020.109639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E. The europe second wave of COVID-19 infection and the Italy “strange” situation. Environ. Res. 2021;193:109639. doi: 10.1016/j.envres.2020.110476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Coccia M. International trade as critical parameter of COVID-19 spread that outclasses demographic, economic, environmental, and pollution factors. Environ. Res. 2021;201:111514. doi: 10.1016/j.envres.2021.111514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Vergalli S., Squazzoni F. Understanding COVID-19 diffusion requires an interdisciplinary, multi-dimensional approach. Environ. Res. 2020;188:109814. doi: 10.1016/j.envres.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Coccia M., Vergalli S., Zanoletti A. Can commercial trade represent the main indicator of the COVID-19 diffusion due to human-to-human interactions? A comparative analysis between Italy, France, and Spain. Environ. Res. 2021;201:111529. doi: 10.1016/j.envres.2021.111529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Sorrentino G.P., Zanoletti A., Alessandri I., Depero L.E., Caneschi A. Sustainable materials and their contribution to the sustainable development goals (SDGs): a critical review based on an Italian example. Molecules. 2021;26(5):1407. doi: 10.3390/molecules26051407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisolara Assessing and managing SARS-CoV-2 occupational health risk to workers handling residuals and biosolids. Sci. Total Environ. 2021;774 doi: 10.1016/j.scitotenv.2021.145732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr R., Strauss M. In: Water Quality: Guidelines, Standards and Health; Assessment of Risk and Risk Management for Water-Related Infectious Disease. Fewtrell L., Bartram J., editors. International Water Association (IWA) behalf of the World Health Organization; London: 2001. Excreta-related infections and the role of sanitation in the control of transmission; pp. 89–113. [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P., Pasupuleti M., Jai Shankar M.R., Bharat G.K., Krishnasamy S., Dasgupta S.C., Sarkar S.K., Jones K.C., et al. First surveillance of SARS-CoV-2 and organic tracers in community wastewater during post lockdown in Chennai, South India: Methods, occurrence and concurrence. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Guo J., Jiang Y., Shao Y. High concentration and high dose of disinfectants and antibiotics used during the COVID-19 pandemic threaten human health. Environ. Sci. Eur. 2021;33(1):1–4. doi: 10.1186/s12302-021-00456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng V.C.C., Lau S.K.P., Woo P.C.Y., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20(4):660. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W., Chu J.T., Perera M.R., Hui K.P., Yen H.L., Chan M.C., Poon L.L. Stability of SARS-CoV-2 in different environmental conditions. The Lancet Microbe. 2020;1(1):e10. doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. The theory of technological parasitism for the measurement of the evolution of technology and technological forecasting. Technol. Forecast. Soc. Change. 2019;141:289–304. doi: 10.1016/j.techfore.2018.12.012. [DOI] [Google Scholar]
- Coccia M. Pandemic prevention: lessons from COVID-19. Encyclopedia. 2021;1(2):433–444. doi: 10.3390/encyclopedia1020036. [DOI] [Google Scholar]
- Coccia M., Watts J. A theory of the evolution of technology: technological parasitism and the implications for innovation magement. J. Eng. Technol. Manag. 2020;55:101552. doi: 10.1016/j.jengtecman.2019.11.003. [DOI] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G.J.C., Haagmans B.L., van der Veer B., van den B., Sharon W.L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P.G., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide COVID-19: the present and future. Sci. Total Environ. 2020;736:139631. doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davó L., Seguí R., Botija P., Beltrán M.J., Albert E., Torres I., López-Fernández P.Á., Ortí R., Maestre J.F., Sánchez G., Navarro D. Early detection of SARS-CoV-2 infection cases or outbreaks at nursing homes by targeted wastewater tracking. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.02.003. Article (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Zhou J., Niu C., Wang Q., Pan Y., Sheng S., Wang X., Zhang Y., Yang J., Liu M., Zhao Y., Zhang X., Zhu T., Peng T., Xie J., Gao Y., Wang D., Dai X., Fang X. Highly accurate and sensitive diagnostic detection of SARS-CoV-2 by digital PCR. Talanta. 2021;224:121726. doi: 10.1016/j.talanta.2020.121726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducoli S., Zacco A., Bontempi E. Incineration of sewage sludge and recovery of residue ash as building material: a valuable option due to as a consequence of the COVID-19 pandemic. J. Environ. Manag. 2021;282:111966. doi: 10.1016/j.jenvman.2021.111966. [DOI] [PubMed] [Google Scholar]
- D'Aoust P.M., Mercier E., Montpetit D., Jia J.J., Alexandrov I., Neault N., et al. Quantitative analysis of SARS-CoV-2 RNA from wastewater solids in communities with low COVID-19 incidence and prevalence. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116560. Article 116560, 10.1016/j.watres.2020.116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA 2020. https://www.epa.gov/coronavirus/do-wastewater-treatment-plants-treat-covid-19
- EPA 2020. https://www.epa.gov/pesticide-registration/list-n-disinfectants-coronavirus-covid-19
- Eurostat 2021. https://ec.europa.eu/eurostat
- Farrow C., McBean E., Salsali H. Virus removal efficiency of ceramic water filters: effects of bentonite turbidity. Water Supply. 2014;14(2):304–311. doi: 10.2166/ws.2013.206. [DOI] [Google Scholar]
- Field R.I., Orlando A.W., Rosoff J.R. Genetics and COVID-19: how to protect the susceptible. Trends Genet. 2021;37:106–108. doi: 10.1016/j.tig.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmorris B.K., Maal-Bared R., Sobsey M.D., Reimers R.S., Rubin A., Bastian R.K., Gerba C., Smith J.E., Bibby K., Kester G., Brown S. Assessing and managing SARS-CoV-2 occupational health risk to workers handling residuals and biosolids. Sci. Total Environ. 2021;774:145732. doi: 10.1016/j.scitotenv.2021.145732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ávila F., Valdiviezo-Gonzales L., Cadme-Galabay M., Gutiérrez-Ortega H., Altamirano-Cárdenas L., Zhindón-Arévalo C., del Pino L.F. Considerations on water quality and the use of chlorine in times of SARS-CoV-2 (COVID-19) pandemic in the community. Case Studies in Chemical and Environmental Engineering. 2020:100049. doi: 10.1016/j.cscee.2020.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C.P., Tamimi A.H., Pettigrew C., Weisbrod A.V., Rajagopalan V. Sources of microbial pathogens in municipal solid waste landfills in the United States of America. Waste Manag. Res. 2011;29(8):781–790. doi: 10.1177/0734242X10397968. [DOI] [PubMed] [Google Scholar]
- Gholipour S., Mohammadi F., Khazeni A., Sahbaei Z., Mousavi S.M., Mirhendi H. COVID-19 infection risk from exposure to aerosols of wastewater treatment plants. Chemosphere. 2021:129701. doi: 10.1016/j.chemosphere.2021.129701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., Coronaviridae V. Study, Group of the International Committee on Taxonomy of, the species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graiver D.A., Topliff C.L., Kelling C.L., Bartelt-Hunt S.L. Survival of the avian influenza virus (H6N2) after land disposal. Environ. Sci. Technol. 2009;43(11):4063–4067. doi: 10.1021/es900370x. [DOI] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2008;1(1):10. doi: 10.1007/s12560-008-9001-6. [DOI] [Google Scholar]
- Gwenzi W. Leaving no stone unturned in light of the COVID-19 faecal-oral hypothesis? A water, sanitation and hygiene (WASH) perspective targeting low-income countries. Sci. Total Environ. 2020;753:141751. doi: 10.1016/j.scitotenv.2020.141751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas Monte Carlo assessment of microbial risk associated with landfilling of fecal material. Water Environ. Res. 1996;68 https://www.jstor.org/stable/25044821 [Google Scholar]
- Han J., He S. Urban flooding events pose risks of virus spread during the novel coronavirus (COVID-19) pandemic. Sci. Total Environ. 2021;755:142491. doi: 10.1016/j.scitotenv.2020.142491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantoko D., Li X., Pariatamby A., Yoshikawa K., Horttanainen M., Yan Mi. Challenges and practices on waste management and disposal during COVID-19 pandemic. J. Environ. Manag. 2021;286:112140. doi: 10.1016/j.jenvman.2021.112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral trans- mission: are we asking the right questions? Sci. Total Environ. 2020;729:138919. doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., I Azhar E., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis.: IJID: official publication of the International Society for Infectious Diseases. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui Q., Pan Y., Yang Z. Paper-based devices for rapid diagnostics and testing sewage for early warning of COVID-19 outbreak. Case Studies in Chemical and Environmental Engineering. 2020;2:100064. doi: 10.1016/j.cscee.2020.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Lee Shiu. The SARS epidemic in Hong Kong: what lessons have we learned? J. R. Soc. Med. 2003;96(8):374–378. doi: 10.1258/jrsm.96.8.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im D., Nakada N., Fukuma Y., Kato Y., Tanaka H. Performance of combined ozonation, coagulation and ceramic membrane process for water reclamation: effects and mechanism of ozonation on virus coagulation. Separ. Purif. Technol. 2018;192:429–434. doi: 10.1016/j.seppur.2017.10.044. [DOI] [Google Scholar]
- Iskander S.M., Zhao R., Pathak A., Gupta A., Pruden A., Novak J.T., He Z. A review of landfill leachate induced ultraviolet quenching substances: sources, characteristics, and treatment. Water Res. 2018;145:297–311. doi: 10.1016/j.watres.2018.08.035. [DOI] [PubMed] [Google Scholar]
- Islam N.F., Sarma H., Prasad M.N. Disinfection By-Products in Drinking Water. Elsevier; 2020. Emerging disinfection by-products in water: novel biofiltration techniques; pp. 109–135. [DOI] [Google Scholar]
- Iyer M., Tiwari S., Renu K., Pasha M.Y., Pandit S., Singh B., Raj N., Krothapalli S., Kwak H.J., Balasubramanian V., Jang S., Bin G., D K., Uttpal A., Narayanasamy A., Kinoshita M., Subramaniam M.D., Nachimuthu S.K., Roy A., Valsala Gopalakrishnan A., Ramakrishnan P., Cho S.G., Vellingiri B. Environmental survival of SARS-CoV-2 – a solid waste perspective. Environ. Res. 2021;197:111015. doi: 10.1016/j.envres.2021.111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey N. The devastating impact of poor wastewater management. Water and Sanitation for the Urban Poor (WSUP) November 2018 https://www.wsup.com/blog/thedevastating-imp act-of-poor-wastewater-management/ [Google Scholar]
- Jeong H.W., Kim S.M., Kim H.S., Kim Y.I., Kim J.H., Cho J.Y., Kim S.H., Kang H., Kim S.G., Park S.J., Kim E.H. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin. Microbiol. Infect. 2020;26(11):1520–1524. doi: 10.1016/j.cmi.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesse B., Keita E. Changes in U.S. air pollution during the COVID-19 pandemic. Sci. Total Environ. 2020;739:139864. doi: 10.1016/j.scitotenv.2020.139864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Wei J., Yuan J., Guo J., Zhang Y., Hang J., Qu Y., Qian H., Zhuang Y., Chen X., Peng X. Probable evidence of fecal aerosol transmission of SARS-CoV-2 in a high-rise building. Ann. Intern. Med. 2020;173(12):974–980. doi: 10.7326/M20-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly H., Sokola B., Abboud H. Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients. J. Neuroimmunol. 2021;356:577599. doi: 10.1016/j.jneuroim.2021.577599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocamemi B.A., Kurt H., Sait A., Sarac F., Saatci A.M., Pakdemirli B. 2020. SARS-CoV-2 Detection in Istanbul Wastewater Treatment Plant Sludges, medRxiv. [DOI] [Google Scholar]
- Koch N., Islam N.F., Sonowal S., Prasad R., Sarma H. Environmental antibiotics and resistance genes as emerging contaminants: methods of detection and bioremediation. Current Research in Microbial Sciences. 2021;2:100027. doi: 10.1016/j.crmicr.2021.100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordatou M.I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. Journal of environmental chemical engineering. 2020;8(5):104306. doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Alamin M., Kuroda K., Dhangar K., Hata A., Yamaguchi H., Honda R. Potential discharge, attenuation and exposure risk of SARS-CoV-2 in natural water bodies receiving treated wastewater. npj Clean Water. 2021;4(2021):8. doi: 10.1038/s41545-021-00098-2. [DOI] [Google Scholar]
- Kumar M., Joshi M., Shah A.V., Srivastava V., Dave S. Wastewater surveillance-based city zonation for effective COVID-19 pandemic preparedness powered by early warning: a perspectives of temporal variations in SARS-CoV-2-RNA in Ahmedabad, India. Sci. Total Environ. 2021;792:148367. doi: 10.1016/j.scitotenv.2021.148367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Kuroda K., Joshi M., Bhattacharya P., Barcelo D. First comparison of conventional activated sludge versus root-zone treatment for SARS-CoV-2 RNA removal from wastewaters: statistical and temporal significance. Chem. Eng. J. 2021;425:130635. doi: 10.1016/j.cej.2021.130635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Li C., Dhangar K., Kumar M. Predicted occurrence, ecotoxicological risk and environmentally acquired resistance of antiviral drugs associated with COVID-19 in environmental waters. Sci. Total Environ. 2021:145740. doi: 10.1016/j.scitotenv.2021.145740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa Giuseppina, Iaconelli M., Mancini P., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahrich S., Laghrib F., Farahi A., Bakasse M., Saqrane S., El Mhammedi M.A. Review on the contamination of wastewater by COVID-19 virus: impact and treatment. Sci. Total Environ. 2021;751:142325. doi: 10.1016/j.scitotenv.2020.142325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langone M., Petta L., Cellamare C.M., Ferraris M., Guzzinati R., Mattioli D., Sabia G. SARS-CoV-2 in water services: presence and impacts. Environ. Pollut. 2020;268:115806. doi: 10.1016/j.envpol.2020.115806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen D.A., Wigginton K.R.( Tracking COVID-19 with wastewater. Nat. Biotechnol. 2020;38:1151–1153. doi: 10.1038/s41587-020-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesimple A., Jasim S.Y., Johnson D.J., Hilal N. The role of wastewater treatment plants as tools for SARS-CoV-2 early detection and removal. J. Water Process. Eng. 2020;38:101544. doi: 10.1016/j.jwpe.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liu X., Huang Z., Hu S., Wang J., Qian Z., Feng J., Xian Q., Gong T. Occurrence and ecological risk assessment of disinfection byproducts from chlorination of wastewater effluents in East China. Water Res. 2019;157:247–257. doi: 10.1016/j.watres.2019.03.072. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang S., Shi J., Luby S.P., Jiang G. Uncertainties in estimating SARS-CoV-2 prevalence by wastewater-based epidemiology. Chem. Eng. J. 2021:129039. doi: 10.1016/j.cej.2021.129039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kulandaivelu J., Zhang S., Shi J., Sivakumar M., Mueller J., Luby S., Ahmed W., Coin L., Jiang G. Data-driven estimation of COVID-19 community prevalence through wastewater-based epidemiology. Sci. Total Environ. 2021;789:147947. doi: 10.1016/j.scitotenv.2021.147947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liga M.V., Bryant E.L., Colvin L.V., Li Q. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011;45:535–544. doi: 10.1016/j.watres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Liu D., Thompson J.R., Carducci A., Bi X. Potential secondary transmission of SARS-CoV-2 via wastewater. Sci. Total Environ. 2020;749:142358. doi: 10.1016/j.scitotenv.2020.142358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., Roda Husmanm A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhandwala S., Gautam P. Indirect impact of COVID-19 on environment: a brief study in Indian context. Environ. Res. 2020;188:109807. doi: 10.1016/j.envres.2020.109807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M.T., Martino J.M., Brock B.T.D. Pearson Prentice Hall; 2006. Biology of Microorganisms. [Google Scholar]
- Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580:176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- Mandal P., Gupta A.K., Dubey B.K. A review on presence, survival, disinfection/removal methods of coronavirus in wastewater and progress of wastewater-based epidemiology. Journal of Environmental Chemical Engineering. 2020;8(5):104317. doi: 10.1016/j.jece.2020.104317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak A., Sadeghiamirshahidi N., Krejci C.C., Mittal A., Beckwith S., Cantu J., Morris M., Grimm J. Resilient regional food supply chains and rethinking the way forward: key takeaways from the COVID-19 pandemic. Agric. Syst. 2021;190:103101. doi: 10.1016/j.agsy.2021.103101. [DOI] [Google Scholar]
- Matsushita T., Shirasaki N., Tatsuki Y., Matsui Y. Investigating norovirus removal by microfiltration, ultrafiltration, and precoagulation–microfiltration processes using recombinant norovirus virus-like particles and real-time immuno-PCR. Water Res. 2013;47(15) doi: 10.1016/j.watres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Nao N., Shirato K., Kawase M., Takeda M. Proceedings of the National Academy of Sciences Mar. 2020;117(13):7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Been F., Heijnen L., Petterson S. Implementation of environmental surveillance for SARS-CoV-2 virus to support public health decisions: opportunities and challenges. Curr. Opin. Environ. Sci. Health. 2020;17:49–71. doi: 10.1016/j.coesh.2020.09.006. https://doi.org/10.1016/j.coesh.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. Water Res. 181, 115942. [DOI] [PubMed] [Google Scholar]
- Michael-Kordatoua I., Karaoliaa P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. Journal of Environmental Chemical Engineering. 2020;8:104306. doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai Coronaviruses in the Sea. Front. Microbiol. 2020;24 doi: 10.3389/fmicb.2020.01795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa M.K., Gamal G., Wafiq A. The impact of COVID 19 on air pollution levels and other environmental indicators - a case study of Egypt. J. Environ. Manag. 2021;277:111496. doi: 10.1016/j.jenvman.2020.111496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalla A.K., Casto A.M., Huang M.L.W., Perchetti G.A., Sampoleo R., Shrestha L., Wei Y., Zhu H., Jerome K.R., Greninger A.L. Comparative performance of SARS-CoV-2 detection assays using seven different primer-probe sets and one assay kit. J. Clin. Microbiol. 2020;58(6) doi: 10.1128/JCM.00557-20. e00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Studies in Chemical and Environmental Engineering. 2020;1:100006. doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour R., Houssam S. Middle East respiratory syndrome coronavirus (MERS-CoV): a review. Germs. 2019;9:35–42. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoh A.I., Sibanda T., Gusha S.S. Inadequately treated wastewater as a source of human enteric viruses in the environment. Int. J. Environ. Res. Publ. Health. 2010;7(6):2620–2637. doi: 10.3390/ijerph7062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleologos E.K., O'Kelly B.C., Tang C.S., Cornell K., Rodríguez-Chueca J., Abuel-Naga H., Koda E., Farid A., Vaverková M.D., Kostarelos K., Goli V.S.N.S. Post Covid-19 water and waste water management to protect public health and geoenvironment. Environmental Geotechnics. 2020;40:1–15. doi: 10.1680/jenge.20.00067. [DOI] [Google Scholar]
- Parida B.R., Bar S., Roberts G., Mandal S.P., Pandey A.C., Kumar M., Dash J. Improvement in air quality and its impact on land surface temperature in major urban areas across India during the first lockdown of the pandemic. Environ. Res. 2021;199:111280. doi: 10.1016/j.envres.2021.111280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Warren J.L., Weinberger D.M., Arnold W., Omer S.B. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38:1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo D., García-Fernández I., Fernández-Ibáñez P., Romalde J.L. Solar water disinfection (SODIS): impact on hepatitis A virus and on a human norovirus surrogate under natural solar conditions. Int. Microbiol. 2015;18:41–49. doi: 10.2436/20.1501.01.233. [DOI] [PubMed] [Google Scholar]
- Peterson Soiled disposable diapers: a potential source of viruses. Am. J. Publ. Health. 1974;64 doi: 10.2105/ajph.64.9.912. https://ajph.aphapublications.org/doi/pdf/10.2105/AJPH.64.9.912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo D., Baluja M.Q., Corbishley A., Jones D.L., Singer A.C., Graham D.W., Romalde J.L. Making waves: wastewater-based epidemiology for COVID-19 –approaches and challenges for surveillance and prediction. Water Res. 2020;186:116404. doi: 10.1016/j.watres.2020.116404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon L.L.M., Peiris M. Emergence of a novel human coronavirus threatening human health. Nat. Med. 2020;26(3):317–319. doi: 10.1038/s41591-020-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragazzi M., Rada E.C., Schiavon M. Municipal solid waste management during the SARS-COV-2 outbreak and lockdown ease: lessons from Italy. Sci. Total Environ. 2020;745:141159. doi: 10.1016/j.scitotenv.2020.141159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Ferrando F.C., Simon P., Allende A., Sanchez G.S. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renou S., Givaudan J., Poulain S., Dirassouyan F., Moulin P. Landfill leachate treatment: review and opportunity. J. Hazard Mater. 2008;150(3):468–493. doi: 10.1016/j.jhazmat.2007.09.077. [DOI] [PubMed] [Google Scholar]
- Rimoldi Sara, Stefani F, Gigantiello A, Polesello S, Comandatore F, Mileto D, Maresca M, Longobardi C, Mancon A, Romeri F, Pagani C., et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa G.L., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - a scoping review. Water Res. 2020;179:115899. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin Tutak A., Söylemez F., Konuk H.B., Çakmak E., Karakaya B., Doğan A., Sayiner H.S., Aksöz S., Alev M. A patient presenting with ARDS after COVID-19 vaccination: a COVID-19 case report. Journal of Infection and Public Health. 2021 doi: 10.1016/j.jiph.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakudo A., Onodera T., Tanaka Y. 2011. Inactivation of Viruses. Sterilization and Disinfection by Plasma: Sterilization Mechanisms, Biological and Medical Applications (Medical Devices and Equipment) pp. 49–56. [Google Scholar]
- Scott L.C., Aubee A., Babahaji L., Vigil K., Tims S., Aw T.G. Targeted wastewater surveillance of SARS-CoV-2 on a University Campus for COVID-19 outbreak detection and mitigation. Environ. Res. 2021;200:111374. doi: 10.1016/j.envres.2021.111374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H.B., Vanapalli K.R., Cheela V.S., Ranjan V.P., Jaglan A.K., Dubey B., Goel S., Bhattacharya J. Challenges, opportunities, and innovations for effective solid waste management during and post COVID-19 pandemic. Resour. Conserv. Recycl. 2020;162:105052. doi: 10.1016/j.resconrec.2020.105052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F., Katoh H., Sakata M., Nakatsu Y., Mori Y., Kageyama T., Matsuyama S., Takeda M. Development of genetic diagnostic methods for detection for novel coronavirus 2019 (nCoV-2019) in Japan, Japanese. JID (J. Infect. Dis.) 2020;73(4):304–307. doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- Siddique A., Shahzad A., Lawler J., Mahmoud K.A., Lee D.S., Ali N., Bilal M., Rasool K. Unprecedented environmental and energy impacts and challenges of COVID-19 pandemic. Environ. Res. 2021;193:110443. doi: 10.1016/j.envres.2020.110443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.L.P., Prata J.C., Duarte A.C., Barcelò D., Rocha-Santos T. An urgent call to think globally and act locally on landfill disposable plastics under and after covid-19 pandemic: pollution prevention and technological (Bio) remediation solutions. Chem. Eng. J. 2021:131201. doi: 10.1016/j.cej.2021.131201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman A.I., Boehm A.B. Systematic review and meta-analysis of the persistence and disinfection of human coronaviruses and their viral surrogates in water and wastewater. Environ. Sci. Technol. Lett. 2020;7(8):544–553. doi: 10.1021/acs.estlett.0c00313. [DOI] [PubMed] [Google Scholar]
- Singhal and Matto . 2020. Down to Earth.https://www.downtoearth.org.in/blog/covid-19-lockdown-aventilator-for-rivers-70771 Retrieved from. [Google Scholar]
- Street R., Malema S., Mahlangeni N., Mathee A. Wastewater surveillance for COVID-19: an African perspective. Sci. Total Environ. 2020;743:140719. doi: 10.1016/j.scitotenv.2020.140719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhu A., Li H., Zheng K., Zhuang Z., Chen Z., Shi Y., Zhang Z., Chen S.B., Liu X., Dai J. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Microb. Infect. 2020;9(1):991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo T., Liu X., Feng J., Guo M., Hu W., Guo D., Ullah H., Yang Y., Zhang Q., Wang X., Sajid M., Huang Z., Deng L., Chen T., Liu F., Xu K., Liu Y., Zhang Q., Liu Y., Xiong Y., Chen G., Lan K., Chen Y. ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg. Microb. Infect. 2020;9(1):1259–1268. doi: 10.1080/22221751.2020.1772678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh S., Srivastava V.C., Mishra I.M. Equilibrium modeling of ternary adsorption of phenols onto modified activated carbon. Theor. Found. Chem. Eng. 2018;52(2):271–285. [Google Scholar]
- Suresh S., Srivastava V.C., Mishra I.M., Singh A.P. Multicomponent Column Optimization of ternary adsorption based removal of phenolic compounds using Modified Activated Carbon. J. Environ. Chem. Eng. 2021;9(1):104843. [Google Scholar]
- Taleb M.A., Mowafi S., El-Sayed H. Utilization of keratin or sericin-based composite in detection of free chlorine in water. J. Mol. Struct. 2020;1202:127379. doi: 10.1016/j.molstruc.2019.127379. [DOI] [Google Scholar]
- Tetteh E.K., Amankawa M.O., Armah E.K., Rathila S. Fate of COVID-19 occurrences in wastewater systems: emerging detection and treatment technologies—a review. Water. 2020;12(10):2680. doi: 10.3390/w12102680. [DOI] [Google Scholar]
- The Sustainable Development Goals: Our Framework for COVID-19 Recovery – United Nations Sustainable Development. Accessed on May 25, 2021. https://www.un.org/sustainabledevelopment/sdgs-framework-for-covid-19-recovery/.
- Thilmany D., Canales E., Low S.A., Boys K. Local food supply chain dynamics and resilience during COVID‐19. Appl. Econ. Perspect. Pol. 2021;43(1):86–104. [Google Scholar]
- Tiwari S.B., Gahlot P., Tyagi V.K., Zhang L., Zhou Y., Kazmi A.A., Kumar M. Surveillance of wastewater for early epidemic prediction (SWEEP): environmental and health security perspectives in the post COVID-19 anthropocene. Environ. Res. 2021;195:110831. doi: 10.1016/j.envres.2021.110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TLGH Editorial Water and sanitation in a post-COVID world. Lancet. 2020;8:E1101. doi: 10.1016/S2214-109X(20)30368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasino M.P., Semedo M., Vieira P., Ferraz E., Rocha A., Carvalho M.F., Mucha A.P. SARS-CoV-2 RNA detected in urban wastewater from Porto, Portugal: method optimization and continuous 25-week monitoring. Sci. Total Environ. 2021;792:148467. doi: 10.1016/j.scitotenv.2021.148467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN report . 2017. United Nations World Water Development Report; p. 2017.https://www.unwater.org/publications/world-water-development-report-2017 [Google Scholar]
- USEPA (US Environmental Protection Agency) US EPA, Office of Solid Waste; Washington, DC: 2008. Municipal Solid Waste in the United States; p. 5306. [Google Scholar]
- USEPA (US Environmental Protection Agency) US EPA; Washington, DC: 2009. Municipal Solid Waste Generation, Recycling, and Disposal in the United States: Facts and Figures for 2008. EPA-530-F-009-021. [Google Scholar]
- Usman M., Farooq M., Hanna K. Existence of SARS-CoV-2 in wastewater: implications for its environmental transmission in developing communities. Environ. Sci. Technol. 2020;54(13):7758–7759. doi: 10.1021/acs.est.0c02777. [DOI] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaverková M.D., Paleologos E.K., Dominijanni A., Koda E., Tang C.S., Małgorzata W., Li Q., Guarena N., Mohamed A.M.O., Vieira C.S., Manassero M. Municipal solid waste management under COVID-19: challenges and recommendations. Environmental Geotechnics. 2020;40:1–16. [Google Scholar]
- Viau E., Peccia J. Evaluation of the enterococci indicator in biosolids using cul- ture-based and quantitative PCR assays. Water Res. 2009;43:4878–4887. doi: 10.1016/j.watres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Wang X.W., Li J., Jin S., Zhen M., Kong B., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y., Guo B.Z., Liu C., Ou G.R., Wang M.N., Fang T.Y., Chao F.H., Li J.W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol Methods. 2005;126(1–2):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., Hoffman K., Jamal R., Heck P., Zhang Y., Torgersen K. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775:145790. doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 2020. https://apps.who.int/iris/bitstream/handle/10665/331846/WHO-2019-nCoV-IPC_WASH-2020.3-eng.pdf
- WHO . 2021. WHO Corona Virus Dash Board.https://covid19.who.int/ Retrieved from. [Google Scholar]
- Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ. Sci.: Water Research & Technology. 2015;1(6):735–746. [Google Scholar]
- Wiktorczyk-Kapischke N., Grudlewska-Buda E., Wałecka-Zacharska E., Kwiecińska-Piróg J., Radtke L., Gospodarek-Komkowska E., Skowron K. SARS-CoV-2 in the environment—non-droplet spreading routes. Sci. Total Environ. 2021;770:145260. doi: 10.1016/j.scitotenv.2021.145260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brunink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu J., Wang Z., Lin Y., Zhang L., Chen J., Li P., Yang K. Technical framework for wastewater-based epidemiology of SARS-CoV-2. Sci. Total Environ. 2021;791:148271. doi: 10.1016/j.scitotenv.2021.148271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Waldman P., Ferrier-Rembert A., Frenois-Veyrat G., Mouchel J.M., Boni M., Maday Y., Marechal V., Moulin L. Several forms of SARS-CoV-2 RNA can be detected in wastewaters: implication for wastewater-based epidemiology and risk assessment. Water Res. 2021;198:117183. doi: 10.1016/j.watres.2021.117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Cai C., Dai X. The potential exposure and transmission risk of SARS-CoV-2 through sludge treatment and disposal. Resour. Conserv. Recycl. 2020;162:105043. doi: 10.1016/j.resconrec.2020.105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv K., Shagan M., Lewis Y.E., Kramarsky-Winter E., Weil M., Indenbaum V., Kushmaro A. City-level SARS-CoV-2 sewage surveillance. Chemosphere. 2021;283:131194. doi: 10.1016/j.chemosphere.2021.131194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater 2016. Environ. Sci. Technol. 2016;50(10):5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? The Lancet Gastroenterol. Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano-Monserrate M.A., Ruano M.A., Sanchez-Alcalde L. Indirect effects of COVID-19 on the environment. Sci. Total Environ. 2020;728:138813. doi: 10.1016/j.scitotenv.2020.138813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zand A.D., Heir A.V. Emerging challenges in urban waste management in Tehran, Iran during the COVID-19 pandemic. Resour. Conserv. Recycl. 2020;162:105051. doi: 10.1016/j.resconrec.2020.105051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zand A.D., Heir A.V. Environmental impacts of new Coronavirus outbreak in Iran with an emphasis on waste management sector. J. Mater. Cycles Waste Manag. 2021;23(1):240–247. doi: 10.1007/s10163-020-01123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoletti A post-pandemic sustainable scenario: What actions can be pursued to increase the raw materials availability? Environ. Res. 2021;202 doi: 10.1016/j.envres.2021.111681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]