Abstract
Objectives
Galectin-3 is β-galactoside-binding lectin with several roles in immune-inflammatory response. To date, there is no evidence of Galectin-3 role as a prognostic biomarker in COVID-19 disease. The aim of this study is to clarify the prognostic role of Galectin-3 in patients with COVID 19 acute respiratory failure.
Methods
We enrolled 156 consecutive patients with COVID-19 disease. Routine laboratory test, arterial blood gas, chest X-ray or Computed Tomography and Galectin-3 dosage were performed. The primary outcome was to assess Galectin-3 predictive power for 30-day mortality. Secondary outcomes were 30-day Intensive Care Unit admission and Acute Respiratory Distress Syndrome stratification according to Galectin-3 dosage. We performed Mann-Whitney U and Kruskal-Wallis tests for continuous variables comparison. Fisher's exact test or Chi-square test were used for categorical variables analysis. Receiver Operating Characteristic curves estimated Galectin-3 predictive power for the endpoints. With a fixed cut-off of 35.3 ng/ml, Kaplan-Meier with Log-Rank test and Cox Regression were performed to assess mortality and Intensive Care Unit admission risk.
Results
Galectin-3 correlated with many other prognostic predictors tested in our analysis. Moreover, patients with serum levels of Galectin-3 above 35.3 ng/ml had increased risk for mortality, Intensive Care Unit admission and severe Acute Respiratory Distress Syndrome.
Conclusions
Our study demonstrates the role of Galectin-3 as a predictor of mortality, Intensive Care Unit access and ARDS stratification in patients with COVID 19 acute respiratory failure.
Keywords: Inflammation, Lung injury, Viral pneumonia, ARDS, ICU
Abbreviations
- COronaVIrus Disease 19
(COVID-19)
- Severe Acute Respiratory Syndrome-COronaVirus 2
(SARS-COV2)
- Acute Respiratory Distress Syndrome
(ARDS)
- Intensive Care Unit
(ICU)
- Receiver Operating Characteristic
(ROC)
- Area Under the Curve
(AUC)
- Lactate DeHydrogenase
(LDH)
- N-terminal pro-Brain Natriuretic Peptide
(NT-pro-BNP)
- C-Reactive Protein
(CRP)
- Procalcitonin
(PCT)
- Sequential Organ Failure Assessment
(SOFA)
- Physiology And Chronic Health Evaluation II
(APACHE II)
- Angiotensin-Converting Enzyme 2
(ACE2)
1. Introduction
Since its first description in 2019, COronaVIrus Disease 19 (COVID-19) has demonstrated a predilection for the lungs along with dramatic systemic impact, albeit a wide spectrum of clinical phenotypes [1]. Considering the variability in clinical presentation and complexity in management, numerous factors have been evaluated to identify fragile patients and to stratify their risk of adverse outcomes [2]. At this regard, serum inflammatory markers play an important role, as they are a sign of disease worsening and progression [[3], [4], [5]]. Increased serum concentrations of C-Reactive Protein (CRP), Interleukin 6/Interferon γ ratio (IL6/INF γ) and Interleukin 10 (IL10) were frequently encountered in patients with a higher severity degree of COVID-19 pneumonia [6]. Moreover, White Blood Cells (WBC) count, Lactate DeHydrogenase (LDH) and D-dimer blood levels were also accounted for a predictive role for unfavorable outcome in severe COVID-19 disease [6]. Recently, Galectin-3, a β-galactoside-binding lectin, has raised some interest as a potential marker of lung damage and a possible therapeutic target in COVID-19 disease [7,8]. Galectin-3 has pleiotropic effects on the immune response: it modulates immune cells lifecycle, angiogenesis and reparative response after lung injury [9,10]. Galectin-3 is highly expressed on fibroblast, endothelial and alveolar macrophages [11,12]. Furthermore, alveolar epithelial cells surface show an increased exposure of Galectin-3 after a lung injury, probabily as consequence of the re-epithelization process of the damaged lung [13]. During viral infections, Galectin-3 can be a binding site for viruses, easing viral entrance into immune cells [14]. It is well known that Severe Acute Respiratory Syndrome-COronaVirus2 (SARS-COV2) bond with Angiotensin-Converting Enzyme 2 (ACE2) is crucial for virus entry in host cells [15]. Indeed, Galectin-3 is not only structurally close to the N-terminal domain of Coronaviruses spike protein subunit 1 [16] but is also able to bind ACE receptor, which has a similar structure to ACE2 receptor [17]. Galectin-3 has also a central role in innate immunity regulation [18], modulating cytokines production and release. Considering the increased levels of Galectin-3 in immune cells infected by SARS-COV2 [19], it has also been assumed that Galectin-3 can enhance cytokine release during SARS-COV2 infection, leading to a cytokine storm syndrome [7,8]. In fact, patients with higher blood levels of Galectin-3 and SARS-COV2 infection tend to show a more severe degree of the disease [[20], [21], [22]]. Despite these interesting findings in scientific literature, no specific data are present about the role of Galectin-3 as a prognostic biomarker in COVID-19 disease. The aim of this study is to clarify the role of Galectin-3 in patients SARS-COV2 infection admitted to our respiratory intensive care unit due to acute respiratory failure.
2. Materials and methods
In this single-center retrospective observational study, from September 2020 to March 2021 we enrolled 156 consecutive patients admitted to our respiratory intensive care unit of “Policlinico” University Hospital of Bari, Italy, with a diagnosis of COVID-19 disease and acute respiratory failure. At the emergency department, a nose-throat swab with Real Time-Polymerase Chain Reaction has been performed to confirm SARS-COV2 infection. In our ward, blood samples, arterial blood gas analysis and thoracic imaging (chest X-ray or Computed Tomography-scan) were collected within 48 h from admission. Similarly, demographic, anamnestic and clinical data were recorded and reported in a database along with serum dosages of Galectin-3 and other inflammation markers. Plasma samples were collected and stored at −80 °C before the analysis. Then, plasma levels of Galectin-3 were measured with chemiluminescence immunoassay kits. Exclusion criteria in our study were the follows: age <18 years, no blood samples collection within 48 h, no thoracic imaging performed at the admission. Finally, 140 patients met all the inclusion criteria and were considered for statistical analysis. Acute Respiratory Distress Syndrome (ARDS) severity stratification was performed according to the Berlin definition, since it is currently considered the best standard for ARDS diagnosis [23]. The primary outcome of this study was to assess 30-day mortality according to Galectin-3 serum levels. Secondary outcomes were the assessment of 30-day Intensive Care Unit (ICU) admission and ARDS stratification. The study was approved by the Institutional Review Board of our hospital (Ethical Committee number: 6717). The present study was conducted in accordance with the Helsinki Declaration of 1975 and following the standards of Good Clinical Practice. We verified the non-normal distribution of data with the Shapiro-Wilk test, considering medians and interquartile ranges for statistical purposes. Consequently, Mann-Whitney U test was used to compare continuous variables, whereas Kruskal-Wallis test was performed in our ARDS severity stratification. Categorical variables were compared using Fisher's exact test or Chi-square test. To estimate the predictive power of Galectin-3 for the outcomes, we carried out Receiver Operating Characteristic (ROC) curves, estimating the area under the curve (AUC) of our predictors. Then, Kaplan-Meier analysis with log-rank test was performed using Galectin-3 to stratify our patients according to different outcomes. Moreover, risk factors for 30-day mortality were assessed using a univariate Cox proportional hazard regression model. Finally, statistically significant predictors were used to generate a multivariate model of Cox regression analysis, whose accuracy was tested using a ROC curve. All statistical analysis were realized using SPSS 26.0 (SPSS Inc, Chicago, Ill) and Prism 8.0.1 (Graphpad Software, La Jolla, Calif). A p-value level <0.05 was considered to be statistically significant.
3. Results
3.1. Population analysis
Anamnestic, clinical and laboratory characteristics of our population are described in Supplemental Table 1. In our cohort, 95% of patients had ARDS according to Berlin definition. During the hospitalization, 12 patients underwent a worsening of their respiratory condition and were transferred in ICU.
3.2. Survival vs non-survival group
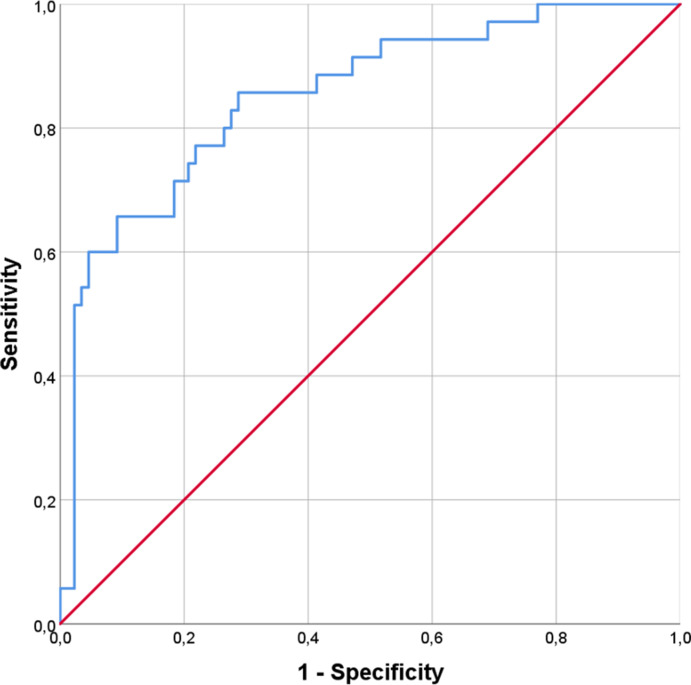
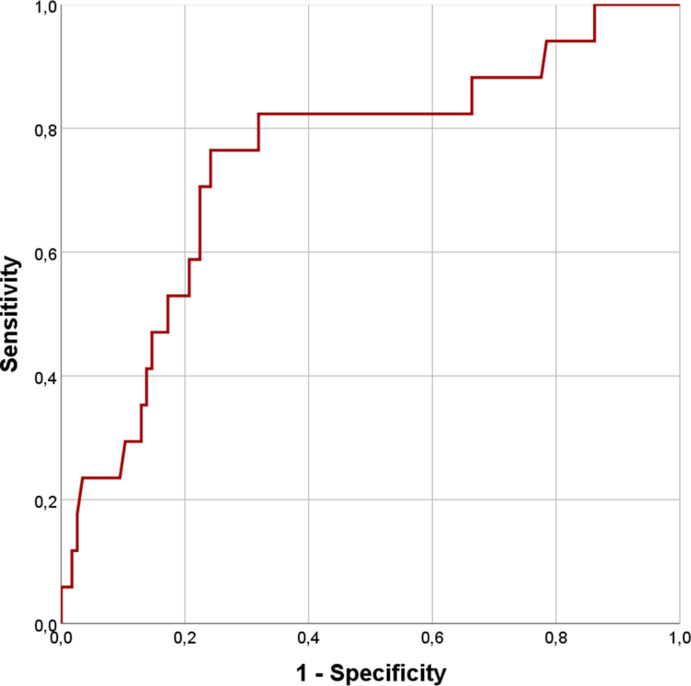
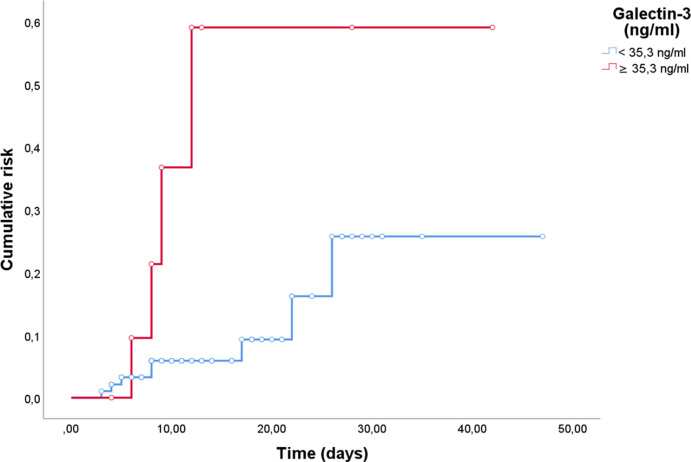
Non survivor patients (27.9%) were found to have higher median age (p < 0.0001) and worse prognostic scores (p < 0.0001), developing more frequently severe ARDS (p < 0.0001) and requiring ICU admission (p = 0.04). As for laboratory variables, increased levels of serum lactate (p = 0.006), IL-6 (p < 0.0001), creatinine (p < 0.0001), LDH (p < 0.0001), N-terminal pro-Brain Natriuretic Peptide (NT-pro-BNP, p < 0.0001), CRP (p < 0.0001), Procalcitonin (PCT, p < 0.0001), D-dimer (p < 0.0001), presepsin (p < 0.0001) and Galectin-3 (p < 0.0001) were found. On the contrary, lower levels of platelets (PLT, p = 0.01) and Vitamin D (p = 0.001) were also reported (Table 1 ). Considering various anamnestic, clinical and laboratory parameters, different ROC curves were constructed (see Supplemental table 2). A Galectin-3 cut-off of 35.3 ng/ml was fixed to achieve a sensitivity of 80% and a specificity of 92.1% for mortality prediction, with an AUC value of 0.906 (Figs. 1 and 95% CI 0.85–0.96, p < 0.0001). According to this cut-off, 39 patients had serum Galectin-3 concentrations above 35.3 ng/ml. Sensitivity, specificity, accuracy, negative likelihood ratio, positive and negative predictive values are reported in Supplemental Table 3. Therefore we tested our hypothesis with Log-Rank analysis and Kaplan-Meier curves, finding a greater death risk with Galectin-3 serum levels above 35.3 ng/ml (Fig. 2, χ2 = 70.4, p < 0.0001). To complete our survival analysis, we performed a univariate Cox regression (Supplemental Table 4). Factors associated with higher risk of mortality were age (HR = 1.061, p < 0.0001), number of comorbidities (HR = 1.55, p < 0.0001), PaO2/FiO2 at the admission (HR = 0.99, p = 0.005), IL-6 (HR = 1.005, p < 0.0001), PLT (HR = 1, p = 0.04), creatinine (HR = 1.54, p < 0.0001), LDH (HR = 1.003, p < 0.0001), CPK (HR = 1, p = 0.021), CRP (HR = 1.008, p < 0.0001), PCT (HR = 1.089, p < 0.0001), presepsin (HR = 1, p < 0.0001), vitamin D (HR = 0.97, p = 0.02), SOFA score (HR = 1.5, p < 0.0001) and Galectin-3 (HR = 1.023, p < 0.0001). After adjusting for confounding factors, our multivariate Cox regression model (Table 2 ) identified only the number of total comorbidities (HR = 1.75, p = 0.001), PaO2/FiO2 at the admission (HR = 0.99, p = 0.05), IL-6 (HR = 1.003, p = 0.04), CRP (HR = 1.010, p < 0.0001) and Galectin-3 (HR = 1.027, p < 0.03) as statistically significant for mortality prediction. Finally, we created a ROC curve to test the accuracy of our model, finding an AUC of 0.853 (p < 0.0001).
Table 1.
Comparison of clinical and laboratory data according to survival status.
| Survivors | Non-survivors | P-value | |
|---|---|---|---|
| Patients (%, n) | 72.1% (101) | 27.9% (39) | |
| Sex (Male/Female, n, %) | 76.9%/23.1% (30/9) | 67.3%/32.7% (68/33) | |
| Age (years, mean, SD#) | 63 [53.25–72] | 81 [71–86] | <0.0001 |
| ARDS* (PaO2/FiO2 < 300) (%, n) Mild (300 <PaO2/FiO2≤ 200) (%, n) Moderate (200<PaO2/FiO2≤100) (%, n) Severe (PaO2/FiO2<100) (%, n) No ARDS (%, n) |
93.1% (94) 26.6% (25) 69.1% (65) 4.3% (4) 6.9% (7) |
100% (39) 15.4% (6) 51.3% (20) 33.3% (13) 0 |
<0.0001 |
| SOFA score ll (Median, IQR§) | 3 [2–4] | 6 [4–7] | <0.0001 |
| PaO2†/FiO2‡ admission (Median, IQR) PaO2/FiO2 discharge (Median, IQR) |
161 [132–224.5] 211 [166.5–267.5] |
117 [89–164] 80 [55–115] |
<0.0001 <0.0001 |
| Patients with comorbidity (%, n) Cardiovascular disease Chronic kidney disease Diabetes type II Neurological disease Psychiatric disease Malignancy COPD Asthma Total comorbidities (n, Median, IQR) |
66.3% (67) 76.1% (51) 10.4% (7) 25.4% (17) 13.4% (9) 3% (2) 4.5% (3) 17.9% (12) 6% (4) 1 [0–2] |
94.9% (37) 81.1% (30) 21.6% (8) 32.4% (12) 29.7% (11) 10.8% (4) 18.9% (7) 10.8% (4) 5.4% (2) 2 [2–3] |
0.0004 |
|
Serum levels (Median, IQR) - Lactate (mmol/L) - Interleukin-6 (pg/ml) - WBC** (x10^3/μL) - Platelets (x10^3/μL) - Creatinine (mg/dL) - Total bilirubine (mg/dL) - LDH†† (U/L) - CPK‡‡ (U/L) - NT-pro-BNP §§ (pg/mL) - C-Reactive Protein (mg/L) - Procalcitonin (ng/mL) - D-dimer (ug/L) - Fibrinogen (mg/dL) - Presepsin (pg/ml) - Vitamin D (ng/ml) - Galectin-3 (ng/ml) |
1 [0.8–1.6] 21.3 [8.3–51.6] 8.7 [6.5–11.8] 283 [189.5–375] 0.8 [0.7–1] 0.6 [0.4–0.7] 303 [238.5–350] 61 [32–108.5] 166 [88–468] 49.2 [20.3–94.5] 0.08 [0.06–0.18] 849 [424–1585] 424 [328.5–548] 383 [281.3–541.3] 25 [16.25–34] 21.9 [17.6–27.5] |
1.4 [1.1–1.9] 84.3 [23.6–124.1] 8.8 [6.3–12.1] 221 [155–290] 1.2 [0.8–2] 0.6 [0.4–0.8] 425 [331–575] 77 [39–314] 746 [474–3190] 95.4 [58.9–127] 0.3 [0.15–1.45] 2398 [984–5248] 438 [374–547] 899 [633–1795] 16 [9–25] 43.8 [36.2–59] |
0.006 <0.0001 0.01 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 0.001 <0.0001 |
| Days of hospitalization (Median, IQR) ICU ll ll admission (%, n) |
12 [8–18] 5% (5) |
9 [7–11] 17.9% (7)ù |
0.008 0.04 |
#SD, Standard Deviation.
*ARDS, Acute Respiratory Distress Syndrome.
†PaO2, Pressure of Arterial Oxygen.
‡FiO2, Fraction of Inspired Oxygen.
§ IQR, InterQuartile Range.
ll SOFA, Sequential Organ Failure Assessment.
**WBC, White Blood (cell) Count.
†† LDH, Lactate DeHydrogenase.
‡‡ CPK, Calcium-dependent Protein Kinase.
§§ NT-pro-BNP, N-Terminal pro-Brain-type Natriuretic Peptide.
ll ll ICU, Intensive Care Unit.
Table 2.
Multivariate survival Cox regression for clinical and laboratory data.
| Paramethers | HR# | IC 95%** | P value |
|---|---|---|---|
| Age | 1.03 | 0.992–1.07 | |
| Comorbidities | 1.754 | 1.261–2.439 | 0.001 |
| †PaO2/‡FiO2 admission | 0.994 | 0.987–1 | 0.05 |
| Interleukin-6 (pg/ml) | 1.003 | 1–1.006 | 0.04 |
| Platelets (x10^3/μL) | 0.99 | 0.995–1.002 | |
| Creatinine (mg/dL) | 1.11 | 0.793–1.55 | |
| †† LDH (U/L) | 1 | 0.998–1.002 | |
| ‡‡ CPK (U/L) | 1 | 1–1.001 | |
| C-Reactive Protein (mg/L) | 1.010 | 1.005–1.015 | <0.0001 |
| Procalcitonin (ng/ml) | 0.896 | 0.798–1.006 | |
| Presepsin (pg/ml) | 1 | 1–1.001 | |
| Vitamin D (ng/ml) | 0.988 | 0.961–1.015 | |
| Galectin-3 (ng/ml) | 1.027 | 1.003–1.051 | 0.03 |
| ll SOFA score | 1.014 | 0.751–1.37 |
#HR, Hazard Ratio.
**CI, Confidence Interval.
†PaO2, Pressure of Arterial Oxygen.
‡FiO2, Fraction of Inspired Oxygen.
†† LDH, Lactate DeHydrogenase.
‡‡ CPK, Calcium-dependent Protein Kinase.
ll SOFA, Sequential Organ Failure Assessment.
3.3. Statistical analysis for ICU admission
Concerning ICU admission, we considered only 108 patients, as for the rest of them there were contraindications for endotracheal intubation and invasive ventilation in ICU. Among these patients, 12 were transferred in ICU due to worsening of gas exchange. To predict ICU admission using serum levels of Galectin-3, a ROC curve was performed, obtaining an AUC of 0.7 (p = 0.02). With the same cut-off of 35.3 ng/ml fixed for mortality analysis, we found a statistically significant result concerning risk of ICU admission after Log-Rank test (Fig. 3, χ2 = 6.5, p = 0.01). Subsequently, a univariate Cox regression was performed to evaluate hazard ratios for ICU access (Supplemental Table 5). Creatinine (HR = 1.52, p = 0.001), LDH (HR = 1.004, p = 0.001), CPK (HR = 1.001, p = 0.01), CRP (HR = 1.014, p = 0.002), PCT (HR = 1.15, p = 0.002), fibrinogen (HR = 1.003, p = 0.009), presepsin (HR = 1, p = 0.026), SOFA score (HR = 1.54, p < 0.0001) and Galectin-3 (HR = 1.037, p < 0.0001) were associated with an increased risk for this outcome. However, after adjusting for confounding factors, none of these parameters remained statistically significant (Supplemental Table 6).
3.4. Statistical analysis for ARDS degree
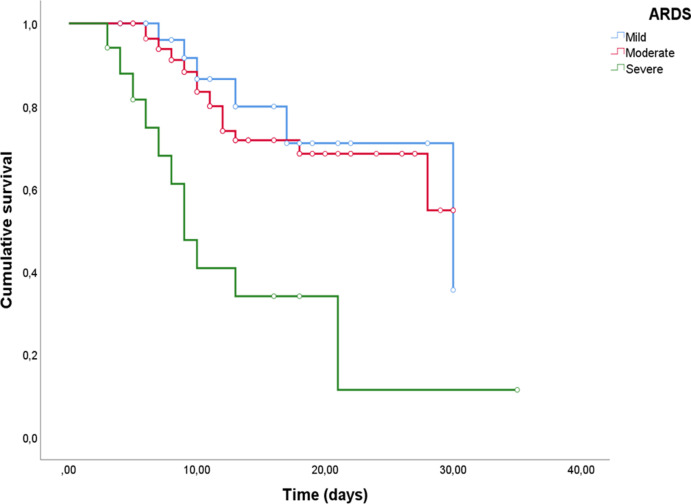
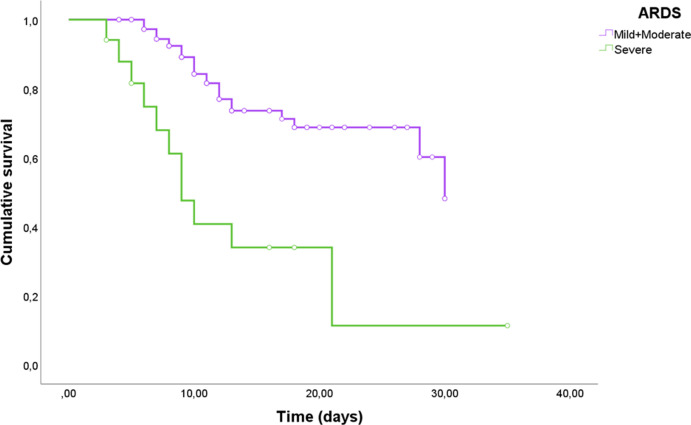
A comparison of clinical and laboratory data in patients with different degrees of ARDS severity is reported Supplemental table 7. Patients with severe ARDS shows higher levels of SOFA score and increased serum concentrations of LDH (p = 0.01), NT-pro-BNP (p = 0.01), CRP (p = 0.002), presepsin (p = 0.006) and Galectin-3 (p = 0.004). Moreover, death percentages tend to increase according to a worse severity of the ARDS (p < 0.0001). To verify the power of Galectin-3 for ARDS severity stratification, 3 different ROC curves were performed (see Supplemental Figure 3, 4 and 5). Although we did not find remarkable results for mild and moderate ARDS, Galectin-3 has shown good diagnostic power for severe ARDS (see Supplemental Table 8, AUC 0.75, p = 0.001). In this case, using the fixed cut-off of 35.3 ng/ml, we found a sensitivity of 70.6% and a specificity of 78% for the outcome. Interestingly, similar results were found in Kaplan-Meier analysis (Supplemental Figure 2, χ2 = 20.51, p < 0.0001).Whereas no differences were found between mild and moderate ARDS (see Supplemental Figure 6, χ2 = 0.19, p = 0.7), severe ARDS was significantly stratified according to our fixed cut-off (Fig. 4, χ2 = 19.8, p < 0.0001).
4. Discussion
This is the first study in scientific literature assessing the prognostic role of Galectin-3 in acute respiratory failure secondary to COVID-19 disease. Patients with higher serum levels of Galectin-3 tend to develop a more severe degree of ARDS with a worse prognosis. It is well known that SARS-COV2 infection can lead to the so called “cytokine storm” in some susceptible patients [24]. For instance, our non-survivors group shows increased blood levels of various inflammation markers, which are frequently associated with negative outcomes in COVID-19 disease [2,25]. Nevertheless, only IL-6, CRP and Galectin-3 remain statistically significant in our multivariate regression model. This finding is not surprising for IL-6 and CRP, which were previously reported as important prognostic markers in COVID-19 disease [[26], [27], [28]]. On the contrary, this is the first study addressing this role for Galectin-3. Furthermore, among the explored parameters, Galectin-3 shows the best AUC curve in ROC analysis, suggesting a better predictive power for mortality outcome. We suggest that since Galectin-3 is expressed both in alveolar epithelial and endothelial cells, it can specifically reflect both parenchymal and vascular damage occurring during COVID-19 disease. As stated before, hyperinflammation can trigger Galectin-3 release from a wide range of host cells [29]. Furthermore, increased blood concentrations of Galectin-3 have also been described in heart failure and chronic kidney disease [30,31]. Although having higher serum levels in our non-survivor group, both NT-pro-BNP and creatinine did not predict a higher death risk in our multivariate models. For this reason, we speculate that during SARS-COV2 infection, Galectin-3 release can be specifically associated with lung damage, earlier predicting the clinical worsening of this disease. Indeed, our analysis on ARDS stratification seems to confirm this hypothesis, as Galectin-3 can properly identify severe ARDS secondary to COVID-19 disease with good sensitivity and specificity. Similarly, Xu el al have already explained the role of Galectin-3 as a prognostic factor in ARDS [32]. However, this study was not performed during COVID-19 pandemic, taking into consideration patients suffering from severe pneumonia, burns, aspiration or gastrointestinal lesions. Moreover, unlike our study, the ROC analysis was performed considering a combined model with Galectin-3, Acute Physiology And Chronic Health Evaluation II (APACHE II) score and PaO2/FiO2, in order to gain a sufficient predictive power for the outcome.
Concerning the ICU admission, our multivariate analysis did not find any significant predictor for this outcome. It has to be said that our cohort of patients undergoing ICU transfer was very limited. Since our ward was deputed to manage patients requiring non-invasive ventilation, ICU admission was allowed only for patients requiring endotracheal intubation or extracorporeal membrane oxygenation (ECMO). For this reason, 32 patients were excluded from this analysis, as they were considered neither eligible for these treatments nor for ICU admission. By contrast, Kaplan-Meier and ROC analysis did result in a statistically significant assessment of risk for ICU admission using our Galectin-3 cut-off. For this reason, we believe that a larger cohort of patients should elucidates this point. For mortality, ICU admission and ARDS severity assessments, we decided to use the same cut-of value of 35.3 ng/ml, as it guarantees us the best compromise in terms of sensitivity and specificity. Furthermore, this cut-off only discriminates severe ARDS from mild-moderate ones and can be really considered in prognostic terms the “edge of the cliff”. Patients with Galectin-3 serum levels above 35.3 ng/ml, in fact, were not only more prone to develop severe ARDS, but also markedly at higher risk of ICU admission or death. Our study has several limitations. Firstly, as previously mentioned, the sample size is limited for a complete statistical analysis. Moreover, the choice of a fixed cut-off has to be validated in a larger cohort, since its diagnostic performance can exceed its real clinical impact in small populations. Secondly, we performed a single-center retrospective study, while a cohort study with prospective design would be advisable to obtain further prognostic information. Thirdly, we only assessed serum levels of Galectin-3 at the admission, without monitoring its plasma concentrations during the hospitalization. Another important limitation is the lack of a non-COVID ARDS control group. Although no important differences seem to characterize this two types of ARDS [33,34], many pathophysiological aspects have to be clarified and a direct confront of Galectin-3 serum levels in these two groups could be interesting to better understand this point. Finally, since our study had only prognostic purposes, we did not collect any brochoalveolar lavage or pulmonary tissue sample for Galectin-3 detection. Future studies should verify how Galectin-3 concentrations in these samples could affect COVID-19 ARDS development and prognosis.
5. Conclusions
In conclusion, our study demonstrates the importance of Galectin-3 as a prognostic factor in COVID-19 disease. Galectin-3 can predict mortality and ARDS severity of patients with ARF secondary to COVID-19 disease. Moreover, higher levels of this marker seem to be correlated with an increased risk for ICU admission, although further studies are needed to clarify this issue.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Andrea Portacci: participated in the design of the study, in the sequence of alignment, in the statistical analysis and drafted the manuscript. Fabrizio Diaferia: participated in the literature research, in data acquisition and drafted the manuscript. Carla Santomasi: participated in the design of the study, in the interpetation of the results and drafted the manuscript. Silvano Dragonieri: participated in the in the literature research, in data acquisition and in the final revision ot the manuscript. Esterina Boniello: participated in literetaure research, in data collection and interpretation and in the final revision ot the manuscript. Francesca Di Serio: analyzed all the biological samples, collected data and revised critically the final draft of the manuscript. Giovanna Elisiana Carpagnano: participated participated in the design of the study, in the interpetation of the results and in the final revision ot the manuscript, All authors read and approved the final manuscript. All the authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of competing interest
None.
Acknowledgements
We thank all the physicians, nurses and healthcare professionals of our Respiratory Intensive Care Unit team for their great support during the study and the whole COVID-19 pandemic.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2021.106556.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
fig S1.
fig S2.
fig S3.
fig S4.
fig S5.
fig S6.
fig S7.
fig S8.
References
- 1.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallo Marin B., Aghagoli G., Lavine K., Yang L., Siff E.J., Chiang S.S., et al. Predictors of COVID-19 severity: a literature review. Rev. Med. Virol. 2021 doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. JAMA - J Am Med Assoc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., et al. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet, (S0140673620305663), (101016/S0140-6736(20)30566-3)). Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallo Marin B., Aghagoli G., Lavine K., Yang L., Siff E.J., Chiang S.S., et al. vol. 31. John Wiley and Sons Ltd; 2021. Predictors of COVID-19 severity: a literature review [Internet] (Reviews in Medical Virology). [cited 2021 Apr 12]. pp. 1–10. Available from:/pmc/articles/PMC7855377/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velpula K.K., Caniglia J.L., Asuthkar S., Tsung A.J., Guda M.R. 2020. Immunopathology of Galectin-3: an Increasingly Promising Target in COVID-19. F1000Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caniglia J.L., Guda M.R., Asuthkar S., Tsung A.J., Velpula K.K. PeerJ; 2020. A Potential Role for Galectin-3 Inhibitors in the Treatment of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elola M.T., Ferragut F., Méndez-Huergo S.P., Croci D.O., Bracalente C., Rabinovich G.A. Galectins: multitask signaling molecules linking fibroblast, endothelial and immune cell programs in the tumor microenvironment. Cell. Immunol. 2018 doi: 10.1016/j.cellimm.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Nangia-Makker P., Hogan V., Raz A. Galectin-3 and cancer stemness. Glycobiology. 2018 doi: 10.1093/glycob/cwy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Díaz-Alvarez L., Ortega E. 2017. The many roles of galectin-3, a multifaceted molecule, in innate immune responses against pathogens. Mediators of Inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacKinnon A.C., Gibbons M.A., Farnworth S.L., Leffler H., Nilsson U.J., Delaine T., et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012 doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasper M., Hughes R.C. Immunocytochemical evidence for a modulation of galectin 3 (Mac-2), a carbohydrate binding protein, in pulmonary fibrosis. J. Pathol. 1996 doi: 10.1002/(SICI)1096-9896(199607)179:3<309::AID-PATH572>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 14.Wang W.H., Lin C.Y., Chang M.R., Urbina A.N., Assavalapsakul W., Thitithanyanont A., et al. The role of galectins in virus infection - a systemic literature review. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell [Internet]. 2020 Apr 16 [cited 2021 Jul 10];181(2):271. Available from:/pmc/articles/PMC7102627/. [DOI] [PMC free article] [PubMed]
- 16.Behloul N., Baha S., Shi R., Meng J. 2020 Sep 1. Role of the GTNGTKR motif in the N-terminal receptor-binding domain of the SARS-CoV-2 spike protein. Virus Res [Internet] [cited 2021 Jul 10];286:198058. Available from:/pmc/articles/PMC7282740/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Revilla J., Deierborg T., Venero J.L., Boza-Serrano A. Hyperinflammation and fibrosis in severe COVID-19 patients: galectin-3, a target molecule to consider. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato S., St-Pierre C., Bhaumik P., Nieminen J. Galectins in innate immunity: dual functions of host soluble β-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol. Rev. 2009 doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Zhu A., He J., Chen Z., Liu L., Xu Y., et al. 2020 May 26. Single-Cell Analysis Reveals Macrophage-Driven T Cell Dysfunction in Severe COVID-19 Patients.https://www.medrxiv.org/content/10.1101/2020.05.23.20100024v1 medRxiv [Internet] [cited 2021 Jul 9];2:2020.05.23.20100024. Available from: [Google Scholar]
- 20.De Biasi S., Meschiari M., Gibellini L., Bellinazzi C., Borella R., Fidanza L., et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020 doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020 doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kazancioglu S., Yilmaz F.M., Bastug A., Ozbay B.O., Aydos O., Yücel Ç., et al. Assessment of galectin-1, galectin-3, and PGE2 levels in patients with COVID-19. Jpn. J. Infect. Dis. 2021 doi: 10.7883/yoken.JJID.2021.020. https://www.jstage.jst.go.jp/article/yoken/advpub/0/advpub_JJID.2021.020/_article [Internet] Mar 31 [cited 2021 Apr 9]; Available from: [DOI] [PubMed] [Google Scholar]
- 23.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA, J. Am. Med. Assoc. 2012 doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 24.Ragab D., Salah Eldin H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tjendra Y., Al Mana A.F., Espejo A.P., Akgun Y., Millan N.C., Gomez-Fernandez C., et al. vol. 144. Archives of Pathology and Laboratory Medicine. College of American Pathologists; 2020. https://pubmed.ncbi.nlm.nih.gov/32818235/ (Predicting Disease Severity and Outcome in COVID-19 Patients: A Review of Multiple Biomarkers). [Internet] [cited 2021 Apr 12]. pp. 1465–74. Available from: [DOI] [PubMed] [Google Scholar]
- 26.Galván-Román J.M., Rodríguez-García S.C., Roy-Vallejo E., Marcos-Jiménez A., Sánchez-Alonso S., Fernández-Díaz C., et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: an observational study. J. Allergy Clin. Immunol. 2021 Jan 1 doi: 10.1016/j.jaci.2020.09.018. https://pubmed.ncbi.nlm.nih.gov/33010257/ [Internet] [cited 2021 Apr 12];147(1):72-80.e8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z., McGoogan J.M. vol. 323. JAMA - Journal of the American Medical Association. American Medical Association; 2020. https://pubmed.ncbi.nlm.nih.gov/32091533/ (Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention). [Internet] [cited 2021 Apr 12]. pp. 1239–42. Available from: [DOI] [PubMed] [Google Scholar]
- 28.Luo X., Zhou W., Yan X., Guo T., Wang B., Xia H., et al. 2020 Oct 15. Prognostic value of C-reactive protein in patients with coronavirus 2019.https://pubmed.ncbi.nlm.nih.gov/32445579/ Clin Infect Dis [Internet] [cited 2021 Apr 12];71(16):2174–9. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ten Oever J., Giamarellos-Bourboulis E.J., Van De Veerdonk F.L., Stelma F.F., Simon A., Janssen M., et al. Circulating galectin-3 in infections and non-infectious inflammatory diseases. Eur. J. Clin. Microbiol. Infect. Dis. 2013 doi: 10.1007/s10096-013-1919-4. [DOI] [PubMed] [Google Scholar]
- 30.Chen H., Chen C., Fang J., Wang R., Nie W. vol. 25. Heart Failure Reviews. Springer; 2020. https://pubmed.ncbi.nlm.nih.gov/31641977/ (Circulating Galectin-3 on Admission and Prognosis in Acute Heart Failure Patients: a Meta-Analysis). [Internet] [cited 2021 Apr 12]. pp. 331–41. Available from: [DOI] [PubMed] [Google Scholar]
- 31.Tuegel C., Katz R., Alam M., Bhat Z., Bellovich K., de Boer I., et al. GDF-15, galectin 3, soluble ST2, and risk of mortality and cardiovascular events in CKD. 2018 Oct 1. https://pubmed.ncbi.nlm.nih.gov/29866459/ Am J Kidney Dis [Internet] [cited 2021 Apr 12];72(4):519–28. Available from: [DOI] [PMC free article] [PubMed]
- 32.Xu Z., Li X., Huang Y., Mao P., Wu S., Yang B., et al. 2017 Mar 1. The Predictive Value of Plasma Galectin-3 for Ards Severity and Clinical Outcome.https://journals.lww.com/00024382-201703000-00011 Shock [Internet] [cited 2021 Apr 20];47(3):331–6. Available from. [DOI] [PubMed] [Google Scholar]
- 33.Brault C., Zerbib Y., Kontar L., Fouquet U., Carpentier M., Metzelard M., et al. vol. 202. American Thoracic Society; 2020. COVID-19- versus Non-COVID-19-related Acute Respiratory Distress Syndrome: Differences and Similarities [Internet] (American Journal of Respiratory and Critical Care Medicine). [cited 2021 Apr 12]. pp. 1301–4. Available from:/pmc/articles/PMC7605202/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grieco D.L., Bongiovanni F., Chen L., Menga L.S., Cutuli S.L., Pintaudi G., et al. 2020 Aug 28. Respiratory Physiology of COVID-19-Induced Respiratory Failure Compared to ARDS of Other Etiologies.https://pubmed.ncbi.nlm.nih.gov/32859264/ Crit Care [Internet] [cited 2021 Apr 12];24(1). Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.