Abstract
A spectrum of neurological disease associated with COVID-19 is becoming increasingly apparent. However, the mechanisms behind these manifestations remain poorly understood, significantly hindering their management. The present review subsequently attempts to address the evolving molecular, cellular and systemic mechanisms of NeuroCOVID, which we have classified as the acute and long-term neurological effects of COVID-19. We place particular emphasis on cerebrovascular, demyelinating and encephalitic presentations, which have been reported. Several mechanisms are presented, especially the involvement of a “cytokine storm”. We explore the genetic and demographic factors that may predispose individuals to NeuroCOVID. The increasingly evident long-term neurological effects are also presented, including the impact of the virus on cognition, autonomic function and mental wellbeing, which represent an impending burden on already stretched healthcare services. We subsequently reinforce the need for cautious surveillance, especially for those with predisposing factors, with effective clinical phenotyping, appropriate investigation and, if possible, prompt treatment. This will be imperative to prevent downstream neurological sequelae, including those related to the long COVID phenotypes that are being increasingly recognised.
Keywords: COVID-19, SARS-CoV-2, Neurology, NeuroCOVID
1. Introduction
The COVID-19 pandemic is now established as the most severe pandemic since that of Influenza in 1918, with more than 183 million infected cases and 3.9 million deaths worldwide as of July 2021 [1]. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) represents the seventh coronavirus known to infect humans, with presenting symptoms that are largely respiratory in nature. More recently there has been a growing interest into potential neurological complications, which can present before, during or after the onset of respiratory symptoms [2]. Whilst mechanistic and epidemiological studies are upcoming, it remains unclear which patients are predisposed to having such features. Although these complications may be rare, given the vast number of patients affected worldwide, the cumulative morbidity and mortality from them may be significant. Hence, our review aims to explore the short- and long-term neurological complications of COVID-19, which we together here term “NeuroCOVID”, highlighting potential mechanisms and predisposing factors to provide more insight into this diverse and ever-expanding topic.
2. Methodology of literature search
We searched PubMed for articles written in the English language that were published between March 2020 and early April 2021, which detailed clinical information on patients with neurological disorders associated with SARS-CoV-2 infection. Further papers were retrieved from respective references and additional information included from other important resources.
3. Challenges in investigation
Studying the neurovirulence of COVID-19 has continued to pose a challenge. Moriguchi and colleagues provided evidence of SARS-CoV-2 RNA in the cerebrospinal fluid (CSF) of a patient with meningitic and encephalitic signs [3]. Puelles et al. (2020) and Matschke et al. (2020) also recently detected SARS-CoV-2 RNA in post-mortem brain tissue samples of confirmed COVID-19 patients [4,5]. These studies potentially demonstrate some degree of neurotropic potential of the virus, however more large scale investigations have been unable to replicate such findings in patients with suspected NeuroCOVID [2]. The inconsistency in evidence for SARS-CoV-2 neurotropism have led many to suggest that, whilst changes on brain parenchyma and cerebral vasculature are confirmed with COVID-19 infection, these are instead more likely related to subsequent inflammatory changes and effects on the blood-brain and blood-CSF barriers [6,7]. One could additionally argue the reported neurological syndromes may be coincidental to COVID-19 infection and the establishment of a causal link requires further examination. However, a recent article using the principles of Bradford Hill concluded that a pathophysiological relationship between COVID-19 and its proposed neurological manifestations are biologically plausible and coherent [8]. Reported neurological manifestations to SARS-CoV-1 and Middle East Respiratory Syndrome (MERS-CoV), to which COVID-19 is genetically similar, also implies cautious surveillance and further investigation of the neurotropic effects of SARS-CoV-2 are warranted [9].
4. Entry into the nervous system
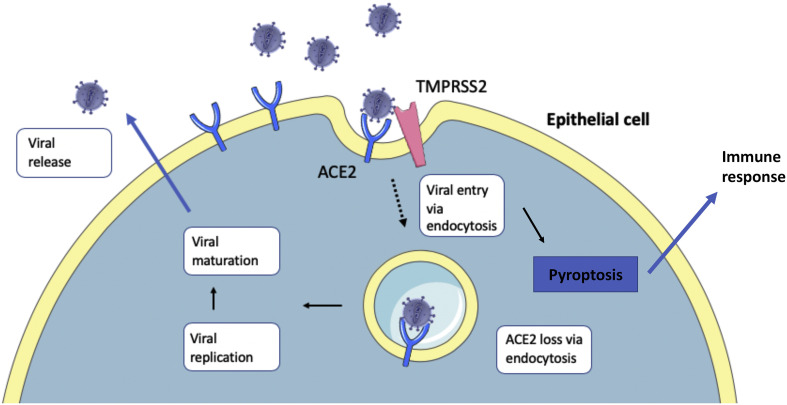
If indeed neurotropic, SARS-CoV-2 entry into the central nervous system (CNS) would likely be driven by the use of its spike (S) protein, which binds the Angiotensin-Converting Enzyme 2 (ACE2) receptor (see Fig. 1 ) [10]. Alongside many other bodily tissues, the ACE2 receptor is shown to be expressed in several neurological tissue types, including endothelial and arterial smooth muscle cells, neurons, various brain nuclei and glial cells [11]. The binding is facilitated by transmembrane serine protease 2 (TMPRSS2), which primes the S protein, to enable entry of the virus into its host cell [10].
Fig. 1.
Epithelial entry of SARS-CoV-2. SARS-CoV-2 entry into respiratory epithelial cells is thought to be analogous to entry into cells of the central nervous system (CNS). After entering the airways, SARS-CoV-2 uses its spike (S) protein to bind the Angiotensin-Converting Enzyme 2 (ACE2) receptor on the cell surface of alveolar type II cells. Transmembrane serine protease 2 (TMPRSS2) primes the S protein to facilitate endocytosis of the bound ACE2 receptor [10]. The viral life cycle continues within the cell, culminating in viral replication and release after pyroptosis. The release of viral particles and cell death triggers downstream immune response cascades.
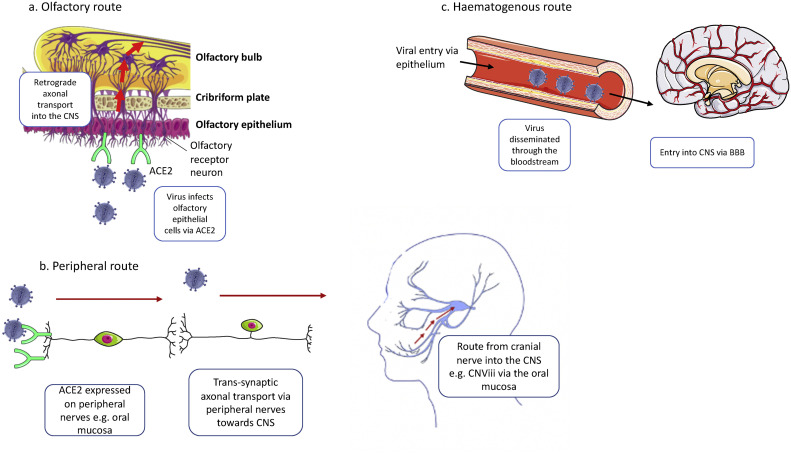
The method by which SARS-CoV-2 arrives at ACE2 receptors in CNS tissue, however, remains unclear (see Fig. 2 ). The virus may breach the CNS via the olfactory route. The olfactory nerves and bulb are efficiently organized to allow retrograde neuronal transport of the virus between the nasal epithelium and CNS [9]. Findings that show viral infection and replication occur in the apical layer of the olfactory mucosa, alongside studies showing ACE2 is widely expressed in the olfactory neuroepithelium (particularly the sustentacular cells), further support this [12,13]. MRI has also confirmed olfactory bulb atrophy in patients with prolonged COVID-19 infection [14]. On the contrary, one would expect if COVID-19 neurotropism was facilitated through the olfactory route, a much greater number of patients would have presented with neurological manifestations of the disease, particularly those that present with anosmia but ultimately do not suffer further complications. Hence, another mechanism of CNS penetration could be through dissemination of the virus into the circulation and spread through the haematogenous route [9]. Associated cytokine production would then increase permeability of the blood brain barrier to further enhance viral penetrance of the CNS. Finally, the virus could also first invade peripheral nerve terminals and subsequently enter the CNS via trans-synaptic pathways. The oral mucosa, for example, has been shown to express the ACE2 receptor, which would allow viral spread through its associated cranial nerves [15].
Fig. 2.
Routes into the central nervous system. SARS-CoV-2 may reach the CNS in a number of ways: a) Olfactory route: SARS-CoV-2 reaches the nasal epithelium through the upper respiratory airways and binds to ACE2 receptors on sustentacular and basal olfactory epithelial cells [12]. Following entry into these cells, the virus travels toward the CNS via retrograde transport along olfactory nerves through the cribriform plate to cells of the olfactory bulb. b) Peripheral route: ACE2 expression at peripheral nerve endings facilitates trans-synaptic spread of the virus into the CNS. ACE2 receptor expression in the oral mucosa, for example, would allow spread along the mandibular division of the trigeminal nerve (CNViii) into the CNS [15]. c) Haematogenous route: SARS-CoV-2 enters the bloodstream following infection, replication and release from epithelial cells, most typically alveolar and respiratory epithelial cells. The viremia allows spread to multiple tissues. At the blood brain barrier (BBB), circulating cytokines may also increase permeability to allow passage of the virus and immune cells into the CNS [18].
5. Pathophysiology of neurological complications
The neurological complications of COVID-19 were first described by Mao et al. (2020), with 36% of their inpatient cohort developing neurological symptoms [16]. These included CNS (headache, dizziness, impaired consciousness, ataxia, stroke and epilepsy), peripheral nervous system (hypogeusia, hyposmia, neuralgia) and muscular symptoms. In the early stages of the pandemic it was unclear if these complications were due to one or a combination of hypoxia, sepsis, intensive care support (e.g. ventilation and extracorporeal membrane oxygenation) or a result of direct viral injury, hypercoagulability and/or immune hyperstimulation [17].
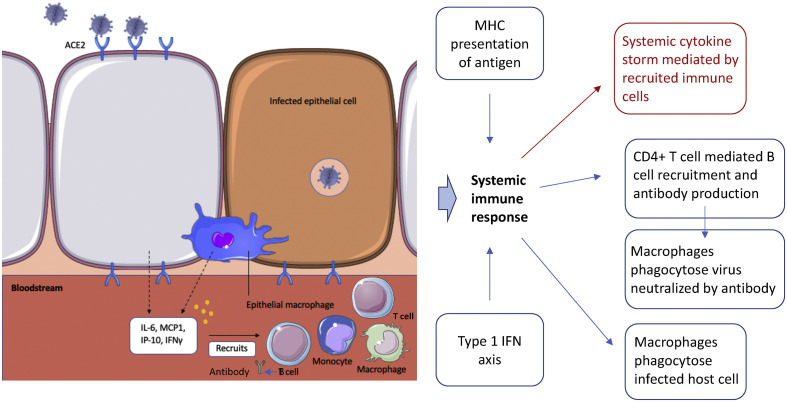
A rapid overproduction of cytokines and immune cell hyperactivation, together termed the cytokine storm (see Fig. 3 ), has particularly been implicated in the link between COVID-19 and severe infection, including the development of its neurological manifestations. This phenomenon has previously been associated with several other triggers, such as cancers, autoimmune conditions, monogenic disorders and various therapies [18]. However, there remains no widely accepted definition for what the cytokine storm fully entails. Previous cytokine storms induced by viral triggers are suggested to be secondary to host defects in pathogen detection, effector and regulatory mechanisms or resolution of inflammation. The COVID-19 storm may therefore be similar, either relating to immune hyperactivity or a failure to resolve the initial inflammatory response as the virus is consistently replicating [18,19]. There have been previous suggestions of a cytokine storm in SARS-CoV-1 patients who demonstrated worse outcomes, which further supports this [20]. On the contrary, demonstrating the role of a cytokine storm to NeuroCOVID specifically has continued to prove difficult, although has shown success more recently. A recent study by Perrin et al. (2021) showed that CSF samples of patients with NeuroCOVID contain elevated levels of interleukin-6 (IL-6) and hyperalbuminorrhachia, implying cytokine dysregulation and increased blood brain barrier permeability [21]. Espíndola et al. (2021) discovered further interleukins and chemokine ligands were abnormally high in the CSF of their NeuroCOVID cohort [22]. Remsik et al. (2021) additionally showed cancer patients presenting with NeuroCOVID possessed elevated CSF interferon-γ, chemokine and matrix metalloproteinase-10 levels [23]. However, there still remain other studies, which have been unable to replicate such findings, indicating further work is needed to validate the role of a potential cytokine storm to NeuroCOVID [24].
Fig. 3.
Tissue-mediated immune response to SARS-CoV-2 infection. Following infection of cells, active replication and viral release generates a local immune response from neighbouring cells and resident macrophages, which release cytokines, such as Interleukin-6 (IL-6), Monocyte Chemoattractant Protein-1 (MCP1), Interferon-inducible Protein-10 (IP-10) and Interferon-gamma (IFNγ) [20,30]. Further inflammatory cells, such as monocytes, T cells and macrophages are subsequently recruited to the site of infection, which can phagocytose infected cells and viral particles. Recruited dendritic cells also carry antigen to lymphoid organs, leading to major histocompatibility complex (MHC) presentation of antigen and activation of T cells. Further secretion of pro-inflammatory cytokines leads to a ‘cytokine storm’ at the systemic level, causing multi-organ failure. Permeability of the blood-brain-barrier is also enhanced, allowing entry of activated immune cells from the periphery into the CNS, hence additionally activating CNS immune cells [18]. Further recruitment of T cells triggers the humoral immune response, in which viral antigen recognition results in B cell-mediated antibody production and subsequently neutralization of the virus.
The brain, unlike the rest of the body, possesses its own innate immune system comprising astrocytes and microglial cells, which can recruit further inflammatory cells, such as macrophages and lymphocytes, through cytokine production [25]. In COVID-19 there appears to be over-recruitment of these inflammatory cells, which may lead to several of the described neurological sequelae [17]. This is supported by neuropathological studies, which confirm the accumulation of T cells and macrophages both in perivascular spaces, but also the brain parenchyma of NeuroCOVID patients [26,27]. The reported expansion of dedifferentiated monocytes and exhausted T cells in the CSF of NeuroCOVID patients may suggest many of these recruited cells are additionally ineffective to adequately control the virus [28]. Data correlating accumulations of inflammatory cells with presenting neurological symptoms however remains sparse, hence further investigation is needed to better understand this. Preclinical models suggest microglia and type 1 astrocytes are particularly responsible for the cytokine storm associated with COVID-19 [25]. This is again supported by histopathological data, which show NeuroCOVID patients display activated microglia and hypertrophilic astrocytes in the cerebral perivascular spaces and brainstem [26,27]. Furthermore, type 1 astrocytes are shown to produce IL-1α, IL-1β, IL2, IL13, IL15, IL17, interferons and tumour necrosis factor in response to coronaviruses, whereas microglia induce the production of IL-6, interferons and tumour necrosis factor [29]. Young healthy adults may be able to balance this, producing a network of anti-inflammatory cytokines such as IL-4, IL-5, IL-9 and IL-10 to limit effects of the virus [30]. With ageing, this anti-inflammatory response becomes further impaired, providing a potential contributing factor to explain worse outcomes with COVID-19 in older patients.
Mitochondrial dysfunction may also be important to the pathophysiology of NeuroCOVID. The intracellular inflammatory response triggered by the virus leads to the production of reactive oxygen species at the level of the mitochondria, further driving cytokine production and inflammation [31]. This is worsened by the limited mitochondrial release of antiviral factors, such as interferons [28]. A recent study additionally showed monocytes produced in response to COVID-19 pneumonia possess abnormal mitochondrial ultrastructure, less capable of performing an oxidative burst [32]. Furthermore, the virus may elicit effects on metabolism and energy production within cells of the brain. The hypoxia, for example, secondary to COVID-19 pulmonary disease would lead to increased anaerobic metabolism in the mitochondria of brain cells, causing acid accumulation, cerebral vasodilation, interstitial oedema and obstruction of cerebral blood flow [33]. The impaired ability for mitochondria to perform oxidative phosphorylation has additionally been linked to T-cell exhaustion in cancer, which may be synonymous with the T cell exhaustion suggested in COVID-19 [34,35].
6. Genetic predispositions
Polymorphisms in genes encoding ACE2 and TMPRSS2 suggestively relate to COVID-19 susceptibility. The distribution of ACE2 variants, as well as observed patterns in gene expression, differs amongst ethnic populations and may contribute to the variation of COVID-19 severity and susceptibility between ethnicities [[36], [37], [38]]. In particular, a recent study identified ACE2 variants rs73635825 and rs143936283 to interact with the SARS-CoV-2 spike protein, which may be especially damaging to host cells [39,40]. This may also confer a greater susceptibility to neurological disease, given ACE2 receptors additionally act as the entry point to the CNS. Moreover, male-associated susceptibility to COVID-19 may partially be explained by ACE2 being localised to Xp22.2 [37,38,41].
The COVID-19 Host Genetics Initiative showed that a cluster of risk alleles at locus 3p21.31 confers the most significant risk to severe disease after infection and hospitalisation [36]. The locus comprises six major candidate genes involved in normal immunity, ACE2 function and LZTFL1 [42]. LZTFL1 is widely expressed and regulates the ciliary trafficking important to epithelial cells. Replication in another study of critically ill patients confirmed this [43]. Homozygous carriers of the identified risk allele cluster were typically younger, potentially contributing to the paradoxical number of young patients developing severe disease [36]. The proportion of allelic carriers differs amongst ethnicities, which could again partially account for the varied risk of severe disease amongst different races [44].
The role of genetics specifically with regards to susceptibility to NeuroCOVID, however, remains unstudied. Using other viral infections as exemplars, it has been demonstrated that Herpes Simplex 1 (HSV-1) encephalitis can result from inborn mutations in the TLR3 pathway, leading to a dysregulated CNS immune response to HSV-1 [45]. It may be possible that analogous genetic mechanisms are also responsible for the neuroinflammatory manifestations of COVID-19. For example, a recent study showed that individuals with loss-of-function variants of the X-chromosomal TLR7 gene develop worse outcomes with the SARS-CoV-2 virus, even in young healthy patients [46]. COVID-19 susceptibility and severe disease have additionally been associated with a deficient antiviral host response and type 1 interferon signalling, although immune-related genes, such as the HLA genes, are shown not to be related [36,43].
Shared genes between severe COVID-19 and established neurological diseases may additionally point to shared pathogenic mechanisms, such as the OAS1 gene in Alzheimer's Disease [47]. Importantly, TMPRSS2 has also been found to be upregulated in several tissues, including the CNS, in Down's Syndrome (DS) patients, which may partly explain why these individuals appear to be more susceptible to COVID-19 infection [48]. Additionally, this may suggest DS patients would be more susceptible to neurological involvement if infected with the virus. An upregulation of IFNAR genes involved in the pro-inflammatory interferon signalling and CXCL10 gene have also been demonstrated in DS. The association between COVID-19 susceptibility and schizophrenia spectrum disorders, as well as the clinical improvement observed with aripiprazole, additionally implies there may be shared susceptibility genes between NeuroCOVID and schizophrenia [49,50]. However, many of these aforementioned genetic associations to NeuroCOVID require further study to validate or dismiss potential links.
7. Mechanisms underlying specific manifestations
7.1. Cerebrovascular syndromes
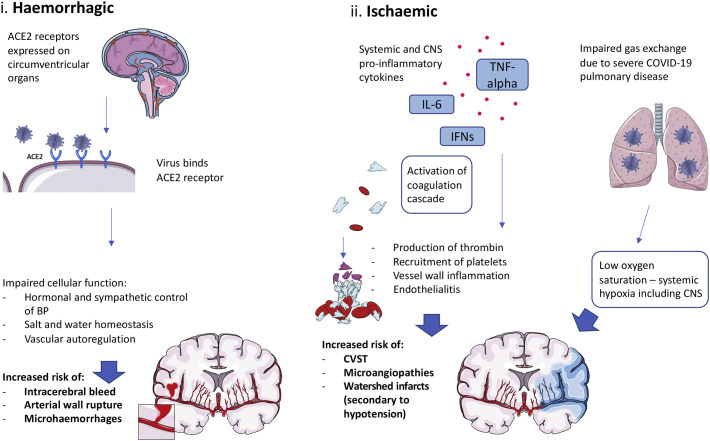
Amongst its neurological manifestations, COVID-19 has been shown to predispose patients to multiple large vessel ischaemic strokes, even in its early stages, as well as cryptogenic strokes (see Fig. 4 ) [27,51,52]. The mechanisms underlying this remain unclear, complicated further by the confounding variable that many patients may already possess risk factors for cerebrovascular disease before becoming infected, such as hypertension, diabetes and coronary artery disease [53]. Reduced perfusion to the brain may partly be driven by systemic hypotension secondary to the cardiovascular compromise caused by COVID-19. This is supported by findings of ischaemic lesions in the borderzone areas of patients who died of the infection [54]. Other virally induced cardiovascular complications may also occur, including myocarditis, atrial fibrillation and decompensated heart failure to further increase risk of ischaemic stroke [55]. Cytokine production could additionally cause endothelial and mononuclear cell activation, with the virus also directly infecting brain endothelial cells to produce a diffuse endothelialitis and further endothelial dysfunction [56]. This would shift the vascular equilibrium toward vasoconstriction, leading to organ ischaemia and inflammation. Downstream of this, activation of the coagulation cascade, production of thrombin and recruitment of platelets leads to thrombosis and clotting propensity [57]. Elevated antiphospholipid antibody levels found in some patients may further enhance the thrombotic risk [58]. The hypercoagulable state of COVID-19 is exemplified by elevated D-dimer levels, fibrin degradation products and increased clot strength [57]. This explains the higher-than-expected incidence of thrombotic complications elsewhere in the body, ranging from pulmonary emboli and deep vein thromboses to microangiopathies. Rarer neurovascular complications, such as cerebral venous sinus thromboses (CVST), have additionally been described [59]. Importantly, such studies include subjects without significant inherited or acquired risk factors for developing CVST, further implying the virus directly drives hypercoagulability rather than exacerbating pre-existing risk factors for developing thromboses [60].
Fig. 4.
Mechanisms of vascular manifestations. Cerebrovascular manifestations can be separated into haemorrhagic and ischaemic diseases: i) Binding the ACE2 receptor on epithelial cells of the circumventricular organs leads to dysregulation of blood pressure homeostasis in the cerebral circulation, causing aneurysmal rupture and intracerebral bleeds [65]. ii) Impaired gas exchange due to COVID-19 effects on the respiratory system may result in poor oxygen delivery to the CNS. Cardiovascular compromise may also cause systemic hypotension and decreased perfusion to the CNS. Furthermore, circulating pro-inflammatory cytokines lead to activation of the coagulation cascade and vessel wall inflammation in the periphery and cerebrovasculature to increase the risk of various thrombotic complications [58].
Intracerebral haemorrhage has also been reported in COVID-19 patients and has been shown to be a common cause of COVID-related death, particularly in younger patients [61]. A proposed mechanism is that the virus interacts with ACE2 receptors expressed in the circumventricular organs and cerebrovascular endothelial cells [62]. As these cells usually regulate cerebral blood flow by hormonal and sympathoadrenal control, salt and water homeostasis and vascular autoregulation, interaction with the virus may lead to their damage and functional impairment. Consequently, erratic changes in cerebral perfusion pressure may cause arterial wall rupture, although this is yet to be formally confirmed in infected patients. The thrombocytopenia associated with COVID-19 may additionally increase risk of bleeding, with the coagulopathy appearing similar but not perfectly matched to disseminated intravascular coagulation [58]. Bleeding risk would be further enhanced with anticoagulants, which infected patients often receive to prevent the development of thromboses. The disruption in haemostasis may also increase risk of haemorrhagic transformation of ischaemic strokes, especially if anticoagulation has been administered [55,63]. Another theory suggests cytokine imbalances may lead to instability of existing intracranial aneurysms, which is shown to occur with other viruses such as Influenza [64]. Systemic inflammation caused by COVID-19 may additionally elevate levels of substances, such as matrix metalloproteinases, to cause breakdown of collagen within basal arterial wall membranes resulting in aneurysmal rupture [65]. Post-mortem high resolution magnetic resonance imaging and histopathological examination has suggested injury on the microvascular level as well, with accumulating inflammatory cells in the perivascular spaces of infected patients causing a thinning of the endothelial wall, subsequently leading to fibrinogen leakage and microhaemorrhages [26,27].
7.2. Central and peripheral demyelinating syndromes
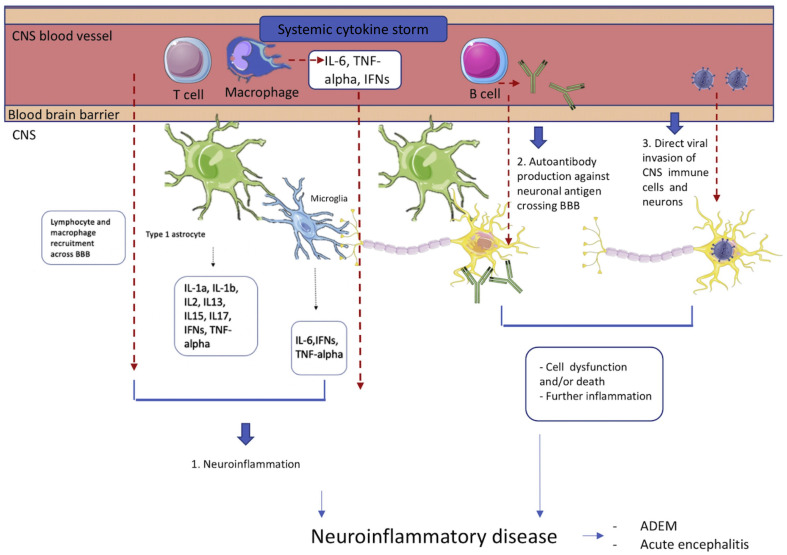
The virus is also postulated to cause demyelination of the nervous system, which may be due to the cytokine storm and excessive activation of glial cells (see Fig. 5 ). Alternatively, COVID-19 may induce the production of autoantibodies against glial cells [66]. Histopathological data have indeed confirmed a demyelinating process with the infection, showing haemorrhagic white matter lesions with surrounding axonal injury and macrophage accumulation [6]. Numerous publications have particularly reported an association between COVID-19 and acute demyelinating encephalomyelitis (ADEM). This is not unexpected since the syndrome has been frequently described following vaccination or infection with Influenza, Enterovirus and Epstein-Barr virus, with a particular preference toward affecting children [67]. Paterson et al. (2020) recently described 9 cases of ADEM associated with COVID-19, supported by imaging or, in one of their cases, brain biopsy [2]. The authors emphasized that this case number was found over a 5-week period in their hospital when, in usual circumstances, it would take approximately 5 months to accrue this number of cases. Hence, an association between COVID-19 and ADEM appeared likely, although the virus could not be identified in any CSF or neuropathological samples. Other studies have shown that whilst ADEM tends to affect patients with severe COVID-19 pulmonary infection, often on ventilation, it may additionally present in infected patients with mild or occasionally no respiratory symptoms [[68], [69], [70], [71]]. Karapanayiotides et al. (2020) also reported an association between COVID-19 and acute haemorrhagic leukoencephalitis using MRI, which showed haemorrhagic demyelination in a pattern encountered in Balo's concentric sclerosis [72]. This pattern has previously been attributed to a combination of hypoxia-induced tissue preconditioning and abnormally high proinflammatory cytokine levels, which may similarly explain the mechanism here [73]. The authors also found a symmetric distribution of leukoencephalopathy affecting both external capsules, which they suggested may be related to a clustering of ACE2 expression. However, ACE2 clusters have not been reported to be abundantly expressed in these regions in other studies [11].
Fig. 5.
Mechanisms of immune-mediated manifestations. Circulating T cells and macrophages release pro-inflammatory cytokines, such as Interleukin-6 (IL-6), tumour necrosis factor alpha (TNFα) and interferons (IFNs) in response to SARS-CoV-2 infection [20]. In severely ill patients, the immune cells and cytokines may cross the blood brain barrier (BBB), further activating microglia and astrocytes [25]. This causes inflammation within the CNS, resulting in a spectrum of neuroinflammatory diseases [29]. In addition, circulating B cells may produce autoantibodies to either surface or intracellular neuronal antigens, which cross the BBB into the CNS, binding to neurons and leading to cell dysfunction and death [66,82]. There is additional evidence that, in some cases, the virus directly gains access to the CNS via the ACE2 receptor to infect CNS immune cells and neurons, subsequently causing cell damage, death and CNS inflammation [11].
Reports of Guillain-Barre Syndrome (GBS) and Miller-Fisher Syndrome with the virus additionally suggested SARS-CoV-2 may utilize molecular mimicry to lead to antibody-mediated destruction against cranial and peripheral nerve components [[74], [75], [76]]. More recently the association between COVID-19 and GBS was contradicted by a UK epidemiological study, which refuted a causal link between the two [77]. The authors conversely showed a reduced incidence of GBS during the first wave of the pandemic when compared to previous years. Lunn et al. (2020), further suggested the increase in GBS and implied association between COVID-19 and GBS identified in the Northern Italian population was likely to be smaller than thought and could be attributed to chance rather than a causal link [75,78]. Another study looking at hospitals in Liguria during the period of lockdown showed their total incidence of GBS cases was similar to numbers identified the year prior [79]. This was despite the risk of C. jejuni transmission, which is the infection most commonly associated with GBS, likely being much lower than normal. Moreover, only one of their six GBS patients who also tested positive for COVID-19 had preceding symptoms of diarrhoea, which would usually be indicative of C. jejuni infection, leaving the rest as idiopathic cases that could be linked to COVID-19. The conflicting findings therefore highlight that the relationship between COVID-19 and GBS remains unclear and warrants further investigation.
7.3. Encephalitic syndromes
Encephalitis is another rare neurological complication of COVID-19, reported both in children and adults [2,80]. The encephalitis may be autoimmune driven and it remains unclear if it is facilitated by direct or indirect effects of the virus on the brain, although the reported improvement with immunotherapy implies an immune-related mechanism likely exists [2,81]. Studies correlating sites of ACE2 expression to posed locations of encephalitic lesions remain scarce, however imaging findings associated with encephalitis show abnormalities in subcortical and cortical areas, including the temporal and frontal lobes [82,83]. Chen et al. (2021) confirmed ACE2 is highly expressed in the temporal lobe, particularly the medial temporal gyrus, whilst only a minority of patients show ACE2 expression in the frontal lobe [11]. The relationship between ACE2 expression and COVID-19 encephalitis therefore requires further exploration. Recent identification of various serum and CSF anti-neuronal autoantibodies in patients with NeuroCOVID additionally implicated molecular mimicry mechanisms to the pathology [66]. Conversely, one study of nine suspected cases of autoimmune encephalitis (AE) related to COVID-19 found no evidence of the antibodies typically associated with AE, including those to N-methyl-d-aspartate receptor (NMDAR), Myelin oligodendrocyte glycoprotein (MOG), Aquaporin 4 (AQP4), Leucine-rich glioma inactivated 1 (LGI1) and Glutamic Acid Decarboxylase (GAD) [2]. The additional absence of SARS-CoV-2 RNA in the CSF of these patients also implied indirect mechanisms more likely drive the encephalitis, again perhaps through cytokine imbalances, although there have since also been isolated reports of encephalitis with CSF-confirmed COVID-19 [82,84]. Alternatively, this may imply the virus is largely intracellular within astrocytes, neuronal and endothelial cells, but can on occasions also present in the CSF. Crunfli et al. (2021) recently suggested using histopathology that intracellular astrocytic infection with SARS-CoV-2 may take place, but this remains a heavily controversial and under validated theory [85].
8. Paediatric neurological involvement
A further poorly researched complication of COVID-19 is its hindrance on neurodevelopment, particularly within the paediatric population who are at greatest risk of developmental insult. A similar spectrum of NeuroCOVID is observed in children as in adults, including thrombo-ischemic disease, which follows stereotyped patterns according to acute, post-acute and post-infectious phases [86]. Brain manifestations are typically ADEM-like in presentation affecting the brain and spinal cord, particularly showing MRI signal changes in the splenium of the corpus callosum [80,87]. In the largest paediatric NeuroCOVID study thus far, although most children had recovered upon follow-up, nine out of 38 retained mild neurological deficits, such as ataxia, limb weakness and encephalopathy [80]. A further two children had very poor outcomes, which included quadriplegia following myelitis and ventilation-dependence following anti-NMDAR encephalitis. Four children also died. It is clear that neurological deficits will prevent children from being able to acquire and develop skills at critical ages. Furthermore, patients who demonstrate a more severe profile and disease trajectory requiring longer hospital admission with persistent physical disability may experience a more significant impact on neurodevelopment [87]. With the majority of paediatric reports existing as single case studies, the effects on neurodevelopment are difficult to interpret equally across patients, warranting the need for larger longitudinal studies.
9. Long-term sequelae
It is becoming apparent the longer-term symptoms of COVID-19 (also termed long COVID), which are largely defined as symptoms persisting beyond 4–12 weeks of their onset, are widespread in patients previously assumed to have recovered from the acute illness [88]. Similar to acute COVID-19, long COVID is also a multisystem disorder. An Italian study reported 87% of patients discharged from hospital had ongoing symptoms after 60 days, including fatigue, cough, dyspnoea, myalgia and chest heaviness [89]. Chronic neurological symptoms, such as cognitive impairment, dysautonomia, prolonged anosmia, psychiatric disturbances including anxiety, have also been widely reported [[90], [91], [92]]. This extends to patients who do not require hospitalisation during the acute phase of their illness, although those with severe infection particularly requiring intensive care possess the greatest risk of suffering substantial chronic neurological and psychiatric complications [93,94]. A UK study reported that patients with COVID-19 achieved significantly worse scores in a multi-organ assessment at 2–3 months when compared to unaffected controls [91]. The severity of chronic symptoms appears to be heterogeneous amongst patients and symptoms may additionally present in a remitting and relapsing manner [90,95].
The COVID Symptom Study identified several risk factors for persisting symptoms, such as obesity, female sex, age and having 5 or more listed symptoms in the first week of illness [[96], [97], [98]]. Coincidental illness, such as asthma and vitamin D deficiency, have been associated with persistent symptoms [97,99]. Disease severity, hospital admission and abnormal auscultation at symptom onset also increase the risk of long COVID [100]. Moreover, polymorphisms in the ACE gene have been suggested to play a role in the link between COVID-19 and chronic fatigue syndrome [101]. It remains vital for subsequent studies, with the increasing incidence of long COVID, to identify further genetic and demographic risk factors as this may reveal components to its pathophysiology and improve surveillance of affected patients.
The prevalence of neurological and neuropsychiatric sequelae is particularly concerning, as SARS-CoV-1 patients reported a high incidence of depression, fatigue, post-traumatic stress disorder and panic disorder even several years after the acute phase of their illness [102,103]. Although these may partly be driven by the effects of hospitalisation rather than the disease itself, if COVID-19 follows a similar trajectory this could pose a major burden on available healthcare services in the coming years. Recent studies show that 55.7% of patients, one month after being discharged from hospital with COVID-19, self-report psychiatric symptoms and impaired ability to carry out daily, work and social activities, further indicating this may be an impending problem [90,104].
It is particularly important, therefore, to first enable future studies to be readily accessible for long COVID patients, for example through patient advocacy and support groups, to facilitate their further contribution to this field [88]. Secondly, the use of functional assessment tools to promptly identify and manage such chronic symptoms are highly encouraged. As with acute COVID-19 infection, rigorous clinical neurological assessment dissecting the multiple presenting symptoms is also essential. This will be supported by investigations, including imaging studies, blood and CSF examination for immune markers, neurocognitive assessment and neurophysiological studies that are now being utilised in COVID-19 follow-up studies [88]. Management protocols may then follow rationally to prevent the development of further complications [91].
10. Conclusions and limitations
Here, we explore potential pathophysiological links between COVID-19 and its suggested neurological manifestations, additionally taking into account genetic susceptibilities. We want to emphasize the importance of recognizing and addressing the long-term effects of COVID-19, which are becoming increasingly apparent, including its impact on mental wellbeing as well as neurodevelopment.
We appreciate the obvious limitations to our work. In particular, studies exploring the neurological manifestations of COVID-19 are rapidly emerging and an inclusive and up-to-date summary is challenging if not impossible. Studies exploring mechanisms behind these manifestations additionally remain sparse, hence many of our interpretations were derived from non-uniform study types with differing methodologies. Furthermore, whilst it is an exciting and growing area of COVID-19 research at the moment, we chose not to study treatment options in this review, given this has been reviewed thoroughly elsewhere. This includes the effects of the recently developed vaccines, which show promising potential in limiting the acute and long-term complications of the virus. Finally, future studies are encouraged to validate contemporary findings with, as the disease persists and time goes on, a special emphasis on thorough pursuit of longer follow-up periods to improve our understanding of long COVID and its potential features that need monitoring, treatment and prevention.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
MSZ has received honoraria for lecturing from Eisai and UCB Pharma.
SW – Declarations of interest: none.
AJ – Declarations of interest: none.
FM – Declarations of interest: none.
HM – Declarations of interest: none.
Acknowledgements
All authors helped to draft, critically appraise and approve this final version of the manuscript. They agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MSZ is supported by the NIHR UCL/UCLH Biomedical Research Centre and National Brain Appeal. SW is supported by the Ministry of Science, Research and the Arts of Baden-Württemberg and the European Social Fund (ESF) of Baden-Württemberg (31-7635 41/67/1).
References
- 1.COVID-19 Map Johns Hopkins Coronavirus Resource Center. 2021. https://coronavirus.jhu.edu/map.html Accessed January 26, 2021.
- 2.Paterson R.W., Brown R.L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2021 doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moriguchi T., Harii N., Goto J., et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matschke J., Lütgehetmann M., Hagel C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2011400. Published online May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. (Berl). 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellul M., Varatharaj A., Nicholson T.R., et al. Defining causality in COVID-19 and neurological disorders. J. Neurol. Neurosurg. Psychiatry. 2020;91(8):811–812. doi: 10.1136/jnnp-2020-323667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y., Xu X., Chen Z., et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen R., Wang K., Yu J., Chen Z., Wen C., Xu Z. 2020. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in Human and Mouse Brain. Published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M., Shen W., Rowan N., et al. Elevated ACE2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Biorxiv Prepr. Serv. Biol. 2020 doi: 10.1101/2020.05.08.084996. Published online May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DosSantos M.F., Devalle S., Aran V., et al. Neuromechanisms of SARS-CoV-2: a review. Front. Neuroanat. 2020;14 doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsivgoulis G., Fragkou P.C., Lachanis S., et al. Olfactory bulb and mucosa abnormalities in persistent COVID-19-induced anosmia: a magnetic resonance imaging study. Eur. J. Neurol. 2021;28(1):e6–e8. doi: 10.1111/ene.14537. [DOI] [PubMed] [Google Scholar]
- 15.Xu H., Zhong L., Deng J., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. Published online April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J. Clin. Neurosci. Off J Neurosurg Soc Australas. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horby P., Lim W.S., et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. Published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang K., Su I., Theron M., et al. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrin P., Collongues N., Baloglu S., et al. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur. J. Neurol. 2021;28(1):248–258. doi: 10.1111/ene.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espíndola O.M., Gomes Y.C.P., Brandão C.O., et al. Inflammatory cytokine patterns associated with neurological diseases in coronavirus disease 2019. Ann. Neurol. 2021 doi: 10.1002/ana.26041. Published online February 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remsik J., Wilcox J.A., Babady N.E., et al. Inflammatory leptomeningeal cytokines mediate COVID-19 neurologic symptoms in cancer patients. Cancer Cell. 2021;39(2):276–283. doi: 10.1016/j.ccell.2021.01.007. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia M.A., Barreras P.V., Lewis A., et al. Cerebrospinal fluid in COVID-19 neurological complications: no cytokine storm or neuroinflammation. medRxiv. 2021 doi: 10.1101/2021.01.10.20249014. Published online January 12. 2021.01.10.20249014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavi E., Cong L. Type I astrocytes and microglia induce a cytokine response in an encephalitic murine coronavirus infection. Exp. Mol. Pathol. 2020;115:104474. doi: 10.1016/j.yexmp.2020.104474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M.-H., Perl D.P., Nair G., et al. Microvascular injury in the brains of patients with COVID-19. N. Engl. J. Med. 2021;384(5):481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thakur K.T., Miller E.H., Glendinning M.D., et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021 doi: 10.1093/brain/awab148. awab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heming M., Li X., Räuber S., et al. Neurological manifestations of COVID-19 feature T cell exhaustion and dedifferentiated monocytes in cerebrospinal fluid. Immunity. 2021;54(1):164–175. doi: 10.1016/j.immuni.2020.12.011. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y., Fu L., Gonzales D.M., Lavi E. Coronavirus Neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J. Virol. 2004;78(7):3398–3406. doi: 10.1128/JVI.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meftahi G.H., Jangravi Z., Sahraei H., Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of “inflame-aging”. Inflamm. Res. 2020;69(9):825–839. doi: 10.1007/s00011-020-01372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saleh J., Peyssonnaux C., Singh K.K., Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibellini L., De Biasi S., Paolini A., et al. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol. Med. 2020;12(12) doi: 10.15252/emmm.202013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdennour L., Zeghal C., Dème M., Puybasset L. Interaction brain-lungs. Ann. Fr. Anesth. Reanim. 2012;31(6):e101–e107. doi: 10.1016/j.annfar.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Vardhana S.A., Hwee M.A., Berisa M., et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat. Immunol. 2020;21(9):1022–1033. doi: 10.1038/s41590-020-0725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang S. Mitochondrial oxidative phosphorylation is linked to T-cell exhaustion. Aging. 2020;12(17):16665–16666. doi: 10.18632/aging.103995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LoPresti M., Beck D.B., Duggal P., Cummings D.A.T., Solomon B.D. The role of host genetic factors in coronavirus susceptibility: review of animal and systematic review of human literature. medRxiv. 2020 doi: 10.1101/2020.05.30.20117788. Published online June 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou Y., Zhao J., Martin W., et al. New insights into genetic susceptibility of COVID-19: an ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020;18(1):216. doi: 10.1186/s12916-020-01673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao Y., Li L., Feng Z., et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain M., Jabeen N., Raza F., et al. Structural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein. J. Med. Virol. 2020 doi: 10.1002/jmv.25832. Published online April 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Cruz J.O., IMCA Conceição, SMB Sousa, Luizon M.R. Functional prediction and frequency of coding variants in human ACE2 at binding sites with SARS-CoV-2 spike protein on different populations. J. Med. Virol. 2020 doi: 10.1002/jmv.26126. Published online June 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asselta R., Paraboschi E.M., Mantovani A., Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging. 2020;12(11):10087–10098. doi: 10.18632/aging.103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaser A. Genetic risk of severe Covid-19. N. Engl. J. Med. 2020;383(16):1590–1591. doi: 10.1056/NEJMe2025501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pairo-Castineira E., Clohisey S., Klaric L., et al. Genetic mechanisms of critical illness in Covid-19. Nature. 2021;591(7848):92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 44.Zeberg H., Pääbo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature. 2020;587(7835):610–612. doi: 10.1038/s41586-020-2818-3. [DOI] [PubMed] [Google Scholar]
- 45.Zhang S.-Y., Casanova J.-L. Inborn errors underlying herpes simplex encephalitis: from TLR3 to IRF3. J. Exp. Med. 2015;212(9):1342–1343. doi: 10.1084/jem.2129insight4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Made C.I., Simons A., Schuurs-Hoeijmakers J., et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.13719. Published online July 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salih D.A., Bayram S., Guelfi S., et al. Genetic variability in response to amyloid beta deposition influences Alzheimer’s disease risk. Brain Commun. 2019;1(fcz022) doi: 10.1093/braincomms/fcz022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Toma I., Dierssen M. Network analysis of down syndrome and SARS-CoV-2 identifies risk and protective factors for COVID-19. Sci. Rep. 2021;11(1):1930. doi: 10.1038/s41598-021-81451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nemani K., Li C., Olfson M., et al. Association of Psychiatric Disorders with Mortality among Patients with COVID-19. JAMA Psychiatry. 2021 doi: 10.1001/jamapsychiatry.2020.4442. Published online January 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crespo-Facorro B., Ruiz-Veguilla M., Vázquez-Bourgon J., et al. Aripiprazole as a candidate treatment of COVID-19 identified through genomic analysis. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.646701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y., Li M., Wang M., et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc. Neurol. 2020 doi: 10.1136/svn-2020-000431. Published online July 2. svn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katsanos A.H., Palaiodimou L., Zand R., et al. The impact of SARS-CoV-2 on stroke epidemiology and care: a meta-analysis. Ann. Neurol. 2021;89(2):380–388. doi: 10.1002/ana.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shahjouei S., Naderi S., Li J., et al. Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaunmuktane Z., Mahadeva U., Green A., et al. Microvascular injury and hypoxic damage: emerging neuropathological signatures in COVID-19. Acta Neuropathol. (Berl.) 2020 doi: 10.1007/s00401-020-02190-2. Published online July 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsivgoulis G., Palaiodimou L., Zand R., et al. COVID-19 and cerebrovascular diseases: a comprehensive overview. Ther. Adv. Neurol. Disord. 2020;13 doi: 10.1177/1756286420978004. 1756286420978004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gąsecka A., Borovac J.A., Guerreiro R.A., et al. Thrombotic complications in patients with COVID-19: pathophysiological mechanisms, diagnosis, and treatment. Cardiovasc. Drugs Ther. 2020 doi: 10.1007/s10557-020-07084-9. Published online October 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y., Xiao M., Zhang S., et al. Coagulopathy and Antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hemasian H., Ansari B. First case of Covid-19 presented with cerebral venous thrombosis: a rare and dreaded case. Rev. Neurol. (Paris) 2020;176(6):521–523. doi: 10.1016/j.neurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mowla A., Shakibajahromi B., Shahjouei S., et al. Cerebral venous sinus thrombosis associated with SARS-CoV-2; a multinational case series. J. Neurol. Sci. 2020;419:117183. doi: 10.1016/j.jns.2020.117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet Lond. Engl. 2020;395(10241) doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharifi-Razavi A., Karimi N., Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dogra S., Jain R., Cao M., et al. Hemorrhagic stroke and anticoagulation in COVID-19. J. Stroke Cerebrovasc. Dis. 2020;29(8):104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muhammad S., Petridis A., Cornelius J.F., Hänggi D. Letter to editor: severe brain haemorrhage and concomitant COVID-19 infection: a neurovascular complication of COVID-19. Brain Behav. Immun. 2020;87:150–151. doi: 10.1016/j.bbi.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheruiyot I., Sehmi P., Ominde B., et al. Intracranial hemorrhage in coronavirus disease 2019 (COVID-19) patients. Neurol. Sci. 2020:1–9. doi: 10.1007/s10072-020-04870-z. Published online November 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Franke C., Ferse C., Kreye J., et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.12.022. Published online December 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tenembaum S., Chitnis T., Ness J., Hahn J.S., International Pediatric MS Study Group Acute disseminated encephalomyelitis. Neurology. 2007;68(16 Suppl 2):S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 68.Novi G., Rossi T., Pedemonte E., et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflam. 2020;7(5) doi: 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Utukuri P.S., Bautista A., Lignelli A., Moonis G. Possible acute disseminated encephalomyelitis related to severe acute respiratory syndrome coronavirus 2 infection. Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6714. Published online July 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCuddy M., Kelkar P., Zhao Y., Wicklund D. Acute demyelinating encephalomyelitis (ADEM) in COVID-19 infection: a case series. Neurol. India. 2020;68(5):1192–1195. doi: 10.4103/0028-3886.299174. [DOI] [PubMed] [Google Scholar]
- 71.Langley L., Zeicu C., Whitton L., Pauls M. Acute disseminated encephalomyelitis (ADEM) associated with COVID-19. BMJ Case Rep. CP. 2020;13(12) doi: 10.1136/bcr-2020-239597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karapanayiotides T., Geka E., Prassopoulos P., et al. Concentric demyelination pattern in COVID-19-associated acute haemorrhagic leukoencephalitis: a lurking catastrophe? Brain J. Neurol. 2020;143(12) doi: 10.1093/brain/awaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takai Y., Misu T., Nishiyama S., et al. Hypoxia-like tissue injury and glial response contribute to Balo concentric lesion development. Neurology. 2016;87(19):2000–2005. doi: 10.1212/WNL.0000000000003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Virani A., Rabold E., Hanson T., et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filosto M., Piccinelli S.C., Gazzina S., et al. Guillain-Barré syndrome and COVID-19: an observational multicentre study from two Italian hotspot regions. J. Neurol. Neurosurg. Psychiatry. 2020 doi: 10.1136/jnnp-2020-324837. Published online November 6. [DOI] [PubMed] [Google Scholar]
- 76.Gutiérrez-Ortiz C., Méndez A., Rodrigo-Rey S., et al. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000009619. Published online April 17. [DOI] [PubMed] [Google Scholar]
- 77.Keddie S., Pakpoor J., Mousele C., et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barre syndrome. medRxiv. 2020 doi: 10.1101/2020.07.24.20161471. Published online July 24. 2020.07.24.20161471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lunn M.P., Cornblath D.R., Jacobs B.C., et al. COVID-19 vaccine and Guillain-Barré syndrome: let’s not leap to associations. Brain J. Neurol. 2020 doi: 10.1093/brain/awaa444. Published online December 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garnero M., Del Sette M., Assini A., et al. COVID-19-related and not related Guillain-Barré syndromes share the same management pitfalls during lock down: the experience of Liguria region in Italy. J. Neurol. Sci. 2020;418:117114. doi: 10.1016/j.jns.2020.117114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindan C.E., Mankad K., Ram D., et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2020 doi: 10.1016/S2352-4642(20)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao A., Rohaut B., Le Guennec L., et al. Severe COVID-19-related encephalitis can respond to immunotherapy. Brain. 2020;143(12):e102. doi: 10.1093/brain/awaa337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pilotto A., Masciocchi S., Volonghi I., et al. SARS-CoV-2 encephalitis is a cytokine release syndrome: evidences from cerebrospinal fluid analyses. Clin. Infect. Dis. 2021:ciaa1933. doi: 10.1093/cid/ciaa1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamal Y.M., Abdelmajid Y., Madani A.A.R.A. Cerebrospinal fluid confirmed COVID-19-associated encephalitis treated successfully. BMJ Case Rep. CP. 2020;13(9) doi: 10.1136/bcr-2020-237378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang Y.H., Jiang D., Huang J.T. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav. Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crunfli F., Carregari V.C., Veras F.P., et al. SARS-CoV-2 infects brain astrocytes of COVID-19 patients and impairs neuronal viability. medRxiv. 2021 doi: 10.1101/2020.10.09.20207464. Published online February 7. 2020.10.09.20207464. [DOI] [Google Scholar]
- 86.Mirzaee S.M.M., Gonçalves F.G., Mohammadifard M., Tavakoli S.M., Vossough A. Focal cerebral Arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297(2):E274–E275. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdel-Mannan O., Eyre M., Löbel U., et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2687. Published online July 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nalbandian A., Sehgal K., Gupta A., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Halpin S.J., McIvor C., Whyatt G., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J. Med. Virol. 2020 doi: 10.1002/jmv.26368. Published online July 30. [DOI] [PubMed] [Google Scholar]
- 91.Raman B., Cassar M.P., Tunnicliffe E.M., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. medRxiv. 2020 doi: 10.1101/2020.10.15.20205054. Published online October 18. 2020.10.15.20205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rogers J.P., Chesney E., Oliver D., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Augustin M., Schommers P., Stecher M., et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health – Eur. 2021;6 doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gousseff M., Penot P., Gallay L., et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J. Inf. Secur. 2020 doi: 10.1016/j.jinf.2020.06.073. Published online June 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sudre C., Lee K., Ni Lochlainn M., et al. 2020. Symptom Clusters in COVID-19: A Potential Clinical Prediction Tool from the COVID Symptom Study App. Published online June 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sudre C.H., Murray B., Varsavsky T., et al. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv. 2020 doi: 10.1101/2020.10.19.20214494. Published online December 19. 2020.10.19.20214494. [DOI] [Google Scholar]
- 98.Cirulli E., Schiabor Barrett K., Riffle S., et al. 2020. Long-Term COVID-19 Symptoms in a Large Unselected Population. Published online October 11. [DOI] [Google Scholar]
- 99.Report: What Does COVID-19 Recovery Actually Look Like? Patient Led Research; 2021. https://patientresearchcovid19.com/research/report-1/ Accessed December 30, 2020. [Google Scholar]
- 100.Carvalho-Schneider C., Laurent E., Lemaignen A., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.09.052. Published online October 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vladutiu G.D., Natelson B.H. Association of medically unexplained fatigue with ACE insertion/deletion polymorphism in gulf war veterans. Muscle Nerve. 2004;30(1):38–43. doi: 10.1002/mus.20055. [DOI] [PubMed] [Google Scholar]
- 102.Law R.K.Y., Lee E.W.C., Poon P.Y.H., Lau T.C.H., Kwok K.M.L., Chan A.C.M. The functional capacity of healthcare workers with history of severe acute respiratory distress syndrome (SARS) complicated with avascular necrosis--case report. Work Read Mass. 2008;30(1):17–26. [PubMed] [Google Scholar]
- 103.Tansey C.M., Louie M., Loeb M., et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch. Intern. Med. 2007;167(12):1312–1320. doi: 10.1001/archinte.167.12.1312. [DOI] [PubMed] [Google Scholar]
- 104.Mazza M.G., De Lorenzo R., Conte C., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]